Abstract
There is growing appreciation for the role that the extracellular environment plays in regulating cell behavior. Mechanical, structural, and compositional cues, either alone or in concert, can drastically alter cell function. Biomaterials, and particularly hydrogels, have been developed and implemented to present defined subsets of these cues for investigating countless cellular processes towards understanding morphogenesis, aging, and disease. While most scientists concede that standard cell culture materials (tissue culture plastic and glass) do a poor job of recapitulating native cellular milieus, there is currently a knowledge barrier for many researchers towards the application of hydrogels for cell culture. Here, we introduce hydrogels to those who may be unfamiliar with procedures to culture and study cells with these systems, with a particular focus on commercially available hydrogels.
Introduction: why hydrogels for cell culture?
Our collective understanding of many cell-based processes is derived from experiments performed on flat, unphysiologically stiff materials such as polystyrene and glass. While the simplicity of these platforms is attractive, cells cultured in these environments tend to display aberrant behaviors: flattened shape, abnormal polarization, altered response to pharmaceutical reagents, and loss of differentiated phenotype (Fig. 1). Furthermore, these culture systems are typically two dimensional1, whereas cells in the body are likely to receive signals not just at their ventral surface, but in all three dimensions. Culture systems that better mimic the biological milieu are needed to bridge the gap between conventional cultures and complex native in vivo environments.
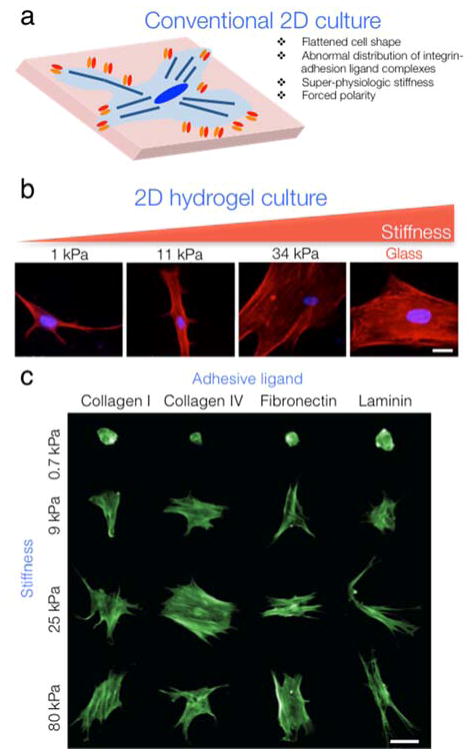
Figure 1. Cell culture atop 2D hydrogels.
(a) Conventional 2D culture on super-physiologically stiff plastic or glass substrates leads to cells displaying aberrant phenotypes. (b) Culturing cells on 2D hydrogel films has some of the same disadvantages as conventional methods, but permits user-defined control of the substrate stiffness and adhesive ligand presentation. Human mesenchymal stem cells (MSCs) cultured on increasingly stiff 2D substrates display increasing spread area. From left: 1 kPa polyacrylamide (PA), 11 kPa PA, 34 kPa PA, and glass (~ GPa). Scale bar: 10 μm. Images modified from 65 with permission. (c) Substrate stiffness (y-axis) and adhesive ligand type (x-axis) combine to regulate MSC morphology. Human MSCs spread more with increasing stiffness, but cells on laminin-coated hydrogels are smaller compared to other ECM protein coatings. Images modified from 64 with permission. Scale bar: 50 μm.
The field of biomaterials continues to advance the introduction of such complexity into cell culture systems, providing ways to control mechanical, compositional, and structural cues and thus more accurately represent features of native tissues2. A range of biomaterial systems have been developed towards this goal, for example patterned glass substrates, elastomeric films, hydroxyapatite ceramics and fibrillar foams. However, hydrogels - water-swollen networks of polymers - have emerged as the most promising for cell culture (Fig. 2) since they mimic salient elements of native extracellular matrices (ECMs), have mechanics similar to many soft tissues, and can support cell adhesion and protein sequestration3.
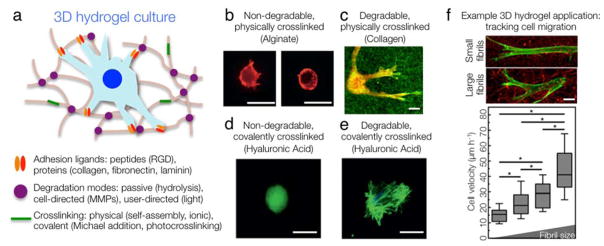
Figure 2. 3D hydrogels for cell culture.
(a) 3D hydrogels can be engineered to present a more realistic microenvironment to cells. Hydrogel design variables are indicated. (b) Mouse MSCs cultured in 3D alginate hydrogels display rounded morphology regardless of substrate stiffness. Left panel: 5 kPa, right panel: 110 kPa. Images modified from 61 with permission. (c) Bovine dermal fibroblasts encapsulated in 3D collagen hydrogels spread at low stiffness (< 1 kPa). Image modified from 96 with permission. (d) Human MSCs cultured in a hyaluronic acid (HA) hydrogel are restricted from spreading regardless of substrate stiffness (shown here ~ 4 kPa). Image modified from 97 with permission. (e) Human MSCs cultured within a HA hydrogel with equivalent stiffness to (d) but crosslinked with MMP-degradable crosslinkers permits cells to locally remodel their environment, generate tractions, and spread. Image modified from 97 with permission. (f) Human foreskin fibroblast spreading and migration speed is influenced by collagen fibril size. Image modified from 44 with permission. Scale bars: 10 μm.
Hydrogels have proven useful in a range of cell culture applications, revealing fundamental phenomena regulating cell behavior and providing tools for the expansion and directed differentiation of various cell types in ways not possible with conventional culture substrates. It would be impossible to cover all of these advances and is not the intent of this review. As one interesting example, seminal work from the Bissell lab demonstrated that healthy mammary epithelial cells exhibit tumorigenic potential in conventional monolayer culture but assemble into multicellular spherical structures resembling healthy acini when encapsulated in a 3D basement membrane-derived hydrogel4. In separate experiments, embryonic stem cells (ESCs) typically spontaneously differentiate within a few days in conventional culture but show a more complex response in hydrogel culture: pluripotency markers can be maintained through control over hydrogel mechanics in the absence of leukemia inhibitory factor supplementation5, or over hydrogel chemistry with the introduction of hyaluronic acid (HA)6 and other glycosaminoglycans7.
In studies using hydrogels as models for drug screening, cells grown on stiff, collagen-rich substrates show greater resistance to chemotherapies compared to cells on softer substrates8. Or, in studies of mechanical influence on cell behavior, lung fibroblasts grown on stiff substrates undergo myofibroblast differentiation and retain a contractile phenotype even when later moved to soft substrates9, implying that cells retain memory of past mechanical environments10, which may bias subsequent experiments. Although these are just a few examples where hydrogel culture platforms have been used, it is clear that cellular outcomes can be quite different than in standard culture.
The objective of this review is to provide a guide for those interested in using hydrogels for cell culture, but who may not be sufficiently familiar with the technology to implement such studies. Although some hydrogels, in particular natural materials like collagen and Matrigel, are being explored by biologists, there are many other hydrogel options that can be used depending on the biological questions being asked. We will present an overview of various hydrogels that can be used for cell culture, ranging from simple 2D films (where cells sit atop a substrate) to more complex 3D systems (where cells are embedded within a hydrogel), as well as discuss advantages and disadvantages of such systems. We provide basic instruction on hydrogel formation and assessment and direct the user to references for commercially available systems that may be of particular interest. We caution that not all hydrogels are equal and hope this review provides guidance to similarities and differences between them. Our hope is that this review can serve as a primer to hydrogels for non-experts and provide a roadmap towards applying such culture systems to their cell studies.
Hydrogel fabrication and characterization
Fabricating hydrogels
Forming hydrogels for cellular experiments typically involves either encapsulation of viable cells within the material or fabrication of substrates using molds that are later seeded with cells. Hydrogel formation involves the transition of liquid precursor solutions into solid materials, which can be performed using either physical (non-covalent) or chemical (covalent) crosslinking to assemble the hydrogel components. The majority of peptide or protein-based systems are formed through self-assembly by physical crosslinking processes; in collagen hydrogels, for example, interactions between solubilized fibrils lead to fiber and network assembly over time. Peptide hydrogels are often engineered with amphiphilic or other complementary sequences that can self-assemble into supramolecular structures such as β-sheets during gelation11. Other natural materials may assemble through charge interactions; for instance, divalent cations induce gelation of anionic alginate polymers. Synthetic polymers have also been modified with various functional groups to enable physical crosslinking12.
Chemical crosslinking of polymers can also be used for hydrogel formation. Chain-growth polymerizations may be initiated with one of various stimuli (e.g., redox initiation, photoinitiation) to induce the covalent reaction of reactive groups (e.g., acrylates, methacrylates, acrylamides) for rapid hydrogel formation13. For the use of such preparations with cells, it is important to keep polymerization times short and use non-toxic initiators (e.g. I2959 or lithium acylphosphinate salt for photopolymerization), to minimize cell death and maintain overall cellular function. Free radicals generated through photopolymerization have been reported to damage cells, especially sensitive primary cell types14; thus, it is important to investigate the compatibility of crosslinking procedures for any cell types of interest. Alternatively, step-growth polymerizations occur when two or more hydrogel precursors are combined that react directly upon mixing. Perhaps the most common reaction of this type for hydrogel formation is the Michael-type addition reaction between multi-functional monomers and crosslinkers. Again, it is important that the polymerization time and reagents are designed so that cell encapsulation occurs in a cytocompatible manner. In the examples included later in this review, procedures optimized for encapsulated cell viability are typically reported. In both examples of chemical crosslinking, gelation needs to occur fast enough to prevent the settling of cells during the encapsulation process.
Characterization of hydrogel properties
There are a variety of hydrogel properties that may be of interest to characterize, including mechanics, swelling, mesh size, and degradation. When purchasing commercial kits or following specific hydrogel recipes, these may already be known and will not need to be characterized by every user. However, it is important to understand how these features are characterized and how they may influence the utility of hydrogels for cell culture applications.
Hydrogel mechanical properties are important towards the stability of the material in culture and may also influence cellular mechanotransduction (the conversion of mechanical information from the microenvironment into biochemical signaling), which in turn has consequences for cellular behaviors like spreading, migration, and stem cell differentiation15. Hydrogel mechanical properties are typically reported as either their shear modulus (G) or elastic modulus (E), which are related to each other as a function of the material’s Poisson’s ratio (v) as shown by the equation:
Most hydrogels are assumed to have a Poisson’s ratio of around 0.45–0.5, meaning that E ~ 3G. 2D hydrogel film mechanics are typically assessed by atomic force microscopy (AFM), perhaps the most suitable technique for measuring substrate mechanical properties on a cellular scale due to the micron-sized cantilever probes used to indent the sample. Techniques such as compressive/tensile testing, which provide bulk mechanical properties by pushing or pulling a material, respectively, or other indentation methods, may be used to characterize the mechanics of 3D hydrogels. The hydrogel elastic modulus is calculated from the applied stress and the resultant strain on the material within the linear elastic region of deformation. Indentation testing is well-suited for many of the viscoelastic and poroviscoelastic natural materials used for tissue engineering, due to the minimal sample preparation requirements and the ability to assay material properties at multiple length scales16. Time-dependent properties such as gelation time and shear modulus are measured using rheology, where shear forces are applied in order to characterize the rate of hydrogel formation or the ability of a material to relax after gelation. For a more comprehensive review of hydrogel mechanical characterization techniques the reader is referred elsewhere17.
Another important hydrogel property is swelling, defined as the amount of water or buffer taken up into the hydrogel. This is a straightforward property to measure and is an indicator of the polymer network hydrophilicity, as well as the relative crosslinking density, where stiffer networks typically exhibit lower swelling. The swelling properties can be useful as an indicator of batch-to-batch variations and consistency in hydrogel fabrication properties, as well as to understand if the hydrogel mechanics are changing over time. The swelling ratio is measured by first allowing hydrogels to reach equilibrium swelling (typically by incubating at 37°C for at least 24 h), blotting to remove excess solvent, and then weighing to obtain the wet weight (Mw). The hydrogel is then dried to determine the dry weight (Md). The mass-swelling ratio (Qm) is typically defined as the ratio of wet weight to dry weight (Mw/Md), while the volumetric swelling ratio (Qv) is calculated from the mass swelling ratio and the densities of the hydrogel polymer (ρp) and solvent (ρs) using the following equation:
The mesh size or molecular porosity of the hydrogel is typically on the nanometer scale and can influence nutrient flux throughout the matrix. It is correlated to the hydrogel swelling behavior and mechanical properties, since lower swelling and higher modulus indicates a smaller mesh size in the hydrogel. While imaging techniques such as scanning electron microscopy (SEM) are commonly used to assess hydrogel microstructure, these techniques are inherently flawed since the sample must be dried before analysis, which alters the native hydrogel structure. Alternatively, fluorescence recovery after photobleaching (FRAP)18, DNA electrophoresis19, or simply measuring the diffusion of fluorescently-tagged polymers entrapped within the hydrogel can be used to characterize mesh size and molecular transport. The mesh size of step-growth hydrogels can also be estimated using theoretical approaches20. More details on characterizing hydrogel swelling ratio and mesh size can be found elsewhere21.
Hydrogel degradation can lead to changes in mechanics and swelling over time, which in turn affects cell behaviors such as motility, spreading, and traction force generation22; whether or not hydrogel degradation is desirable depends on the goal of the study. Hydrogels typically degrade through either hydrolytic or enzymatic mechanisms, where hydrolysis occurs throughout the entire hydrogel and enzymatic degradation is local to the presented enzyme. Hydrolysis occurs with inclusion of hydrolytically unstable chemical bonds and the rate of hydrolysis can be tuned by altering parameters such as the crosslinking density, which is controlled through the polymer concentration or extent of crosslinking during material fabrication. More advanced hydrogels have used external triggers such as ultraviolet (UV) light23 to control degradation.
Natural hydrogels like collagen and fibrin degrade by cell-mediated proteases such as matrix metalloproteinases (MMPs). Synthetic hydrogels are increasingly being engineered with peptide crosslinkers24 that can be tailored to degrade in response to these same MMPs. Degradation kinetics are usually tracked by incubating hydrogels in buffer, collecting samples for analysis every few hours to several days, depending on anticipated degradation profiles, and monitoring degradation byproducts (e.g., uronic acid for HA or soluble collagen). Degradation can also be quantified by labeling hydrogels with fluorophores and tracking the fluorescence of degradation byproducts or through monitoring changes in mechanical properties. It is important to note that even hydrogels that would be considered non-degradable on the time scale of most cell experiments, such as photocrosslinked poly(ethylene glycol), may eventually degrade; however, their stability under useful timescales for these studies makes them attractive for use.
Hydrogel sterilization
Hydrogels may be sterilized prior to cell culture using gamma or germicidal UV irradiation, ethylene oxide exposure, ethanol treatment of already formed hydrogels, or dense carbon dioxide gas sterilization25. For cell encapsulation, the precursor solutions must be sterilized prior to hydrogel formation. This can be accomplished either through filtering (if solutions are not too viscous) or through germicidal UV irradiation of the solution or dry polymer. While all of these approaches are effective, one must be careful to choose a technique that will not degrade, denature, or otherwise alter hydrogel physical properties. For example, extended UV treatment can denature collagen and promote peptide degradation within functionalized hydrogels26, while gamma irradiation can degrade alginate27. Commercial hydrogel kit components are typically provided pre-sterilized or may include specific sterilization instructions.
Characterizing cell outcomes in hydrogel cultures
Isolating cells from hydrogels
Just as for cells cultured on conventional surfaces, cells cultured on or within hydrogels often need to be harvested to propagate the culture or to carry out molecular or cellular analyses. RNA and protein can be isolated from cells grown on 2D hydrogel substrates in a manner similar to cells seeded on plastic or glass, although cell scraping may be more difficult. Sample preparation from hydrogel-embedded cells presents more technical challenges. Techniques used on tissue samples, including mechanical and/or enzymatic disruption, can be useful for liberating embedded cells but must be done with great care to maintain the integrity of intracellular components, while still generating sufficient yields. The specific properties of the hydrogel must be considered as well. For example, RNA isolation from polysaccharide matrices using guanidinium thiocyanate-based methods common in the Qiagen Mini-kits results in inferior RNA yields and quality compared to Trizol and cationic surfactants like cetyltrimethylammonium bromide (CTAB)28. It may also be desirable to isolate intact cells for applications such as flow cytometry or when using hydrogels for cell expansion. Enzymatic degradation of naturally-derived materials such as collagen (collagenase), fibrin (nattokinase), and hyaluronic acid (hyaluronidase) permits cell liberation, although care must be taken not to disrupt cell surface receptors through extended enzyme treatment. Once isolated, cells can be split and re-encapsulated in new hydrogels for further culture and expansion. More advanced materials and techniques (e.g., photodegradation) are also being applied to enable cell isolation from hydrogels where cell release is not currently possible29.
Visualizing cells and biomolecules in hydrogels
Rather than extracting cells for analysis, some hydrogel studies will require in situ cell imaging. Hydrogel films intended for microscopy are usually fabricated on glass coverslips to enable high-resolution imaging. Cells cultured on these 2D hydrogels can often be processed for immunohistochemistry in the same fashion as cells cultured conventionally, although care must be taken not to disturb the hydrogel attachment to coverslips during the staining and washing steps. Coverslip silanization to permit covalent attachment of the hydrogel directly to the coverslip surface is a common way to prevent this30. When preparing 3D hydrogels for imaging, standard immunostaining protocols can likely be used, although incubation steps should be lengthened and/or include mechanical agitation to encourage adequate diffusion of the reagents into the hydrogel31. Many hydrogels are also optically transparent - including those specifically mentioned later in this review - and permit imaging using confocal microscopy.
While hydrogels are usually processed for histology in a manner similar to soft tissue samples, this may not be ideal: the dehydration and heating steps in paraffin embedding can result in hydrogel deformation and folding during sectioning32, although careful processing can ameliorate these concerns33. Sugar-based solvents used in cryosectioning result in brittle embedding blocks that are difficult to cut due to elevated water content in hydrogels compared to that in many tissues. Recent evidence suggests that using alternatives to sucrose such as polyvinyl alcohol (PVA) and Optimum Cutting Temperature (OCT) compound during cryosectioning could improve histological assessment of hydrogels34. Other studies have used plastic resins such as glycol methacrylate as the embedding medium35. These materials tend to more faithfully preserve hydrogel structure but typically need to be stained whole mount before sectioning.
Selecting a hydrogel for cell culture
Hydrogels can be broadly classified as either natural or synthetic materials with each classification containing a distinct set of advantages and disadvantages. In this section we will discuss seven commonly used hydrogels: three naturally-derived (collagen, fibrin, alginate), two synthetic (polyacrylamide, polyethylene glycol), and two hybrid materials that combine elements of synthetic and natural polymers (hyaluronic acid, polypeptides). We summarize commercial vendors and advantages/disadvantages of using each material (Table 1), and include references for cell studies using these materials (Supplementary Table 1). Although other hydrogels have been used for cell culture (e.g., chitosan36, silk37, PVA38, dextran39), they involve polymer synthesis prior to hydrogel formation; we focus here only on systems where kits or hydrogel precursors can be directly purchased from vendors. Additionally, although beyond the scope of this review, many of these hydrogels have applicability for in vivo studies including cell delivery and soft tissue engineering40.
Table 1.
Representative hydrogels that can be used for cell culture studies.
| Material | Example Vendors | Notable Material Features |
|---|---|---|
| Natural Materials | ||
| Collagen | BD BioSciences, Advanced BioMatrix (PureCol, FibriCol), Vitrogen, Flexcell (Thermacol, Collagel) | Typically sourced from rat tail tendon or bovine skin/tendon; Usually purchased in pepsin or acid solubilized form and stored at low pH and temperature; Enzymatically degradable; Exhibits structural and mechanical properties reminiscent of native tissues; Presents native cell adhesion ligands |
| Fibrin | Baxter (Tisseel, Artiss), Johnson & Johnson (Evicel), Sigma | Typically sourced from human plasma; Enzymatically degradable; Provides good substrate for studying wound healing phenomena in vitro; low mechanics limit utility |
| Alginate | NovaMatrix-3D, PRONOVA (FMC BioPolymer) | Derived from brown algae; Must be modified with adhesive ligands for cell attachment; Ionic crosslinking with divalent cations enables easy cell encapsulation and recovery; Additional covalent crosslinking often needed for strength |
| Synthetic Materials | ||
| Polyacrylamide (PA) | Sigma | Wide range tuning of substrate mechanics; Probably the most standardized material as far as protocols for making hydrogels and using for culture; Suitable for 2D cell culture only |
| Polyethylene glycol (PEG) | QGel Inc (QGel), Sigma, Cellendes (3-D Life Dextran-PEG or PVA-PEG) BioTime Inc (PEG-gel) | “Blank slate” synthetic material enables a wealth of user modifications; Pre-modified and various molecular weights are readily available; Can be engineered to present different adhesive ligands and to degrade via passive, proteolytic, or user-directed modes |
| Hybrid Materials | ||
| Hyaluronic acid (HA) | Lifecore (Corgel BioHydrogel), BioTime Inc (HyS-tem), BRTI Life Sciences (Cell-Mate3D) | Usually produced via bacterial fermentation, but can also be sourced from animal products; Wide variety and high degree of potential chemical modification enables considerable tunability; Interacts with cell receptors but must be modified with adhesive ligands to permit cell attachment |
| Polypeptides | Corning (PuraMatrix), PepGel LLC (PGmatrix), Sigma (HydroMatrix) | Typically formed by self-assembly; Useful in soft tissue applications and in conjunction with other materials; Protein engineering enables great design flexibility |
Many factors should be considered when selecting a hydrogel; the most important for the typical biologist are adhesivity to cells and whether this occurs in native or modified gel, stability in culture, and biophysical properties such as hydrogel elastic modulus. Some materials, including collagen and fibrin, are typically used without further modification. Others, including PEG and HA, are often chemically modified to support specific crosslinking mechanisms; modified polymers can be purchased commercially or prepared in house. Additionally, some materials interact with cells through integrin-ligand interactions (e.g., collagen, fibrin, polypeptides) or other cell surface receptors (e.g., HA), while others are considered more inert (e.g., PEG, polyacrylamide). These and other material-specific design considerations will be discussed in the subsequent sections.
Beyond hydrogel selection, it is important to identify whether culturing cells in 2D (atop a hydrogel film) or in 3D (encapsulated within a hydrogel) is most appropriate. While this choice will primarily be influenced by the individual user’s application of interest, there are additional considerations. In general, cells are less constrained in 2D when compared to 3D hydrogel environments (Fig. 1). 3D hydrogels may more accurately model the architecture of some tissues and present milieus that lead to more realistic cellular responses, especially in the context of pathophysiological environments41 (Fig. 2). However, this again may vary depending on the specific application; cells such as epithelial cells or endothelial cells may naturally interact with more 2D-like substrates within their native environments. Nevertheless, in comparison to conventional culture surfaces, 2D hydrogels offer control over crucial environmental factors such as stiffness and presentation of adhesive ligands. While most hydrogels discussed here are suitable for either 2D or 3D cultures, materials like polyacrylamide are only usable in 2D due to toxicity of the precursor components.
Collagen
Collagen is the primary organic constituent of native tissues; about 90% of the 29 identified types of collagen in the human body are fibrillar. Type I collagen is the most common type and as such is the major structural component of many tissues42. This ubiquity makes collagen an attractive material for cell studies. Collagen hydrogels are mostly composed of type I collagen, although types II, III, and other constituents such as glycosaminoglycans can also be incorporated. Gelatin, the amorphous form of collagen with the same amino acid sequence but lacking the triple helical character, is also a common hydrogel material. Collagen used in hydrogels is usually derived from solutions of acid or pepsin-solubilized type I collagen, is often sourced from rat tail tendon, and is readily available from numerous vendors including BD Biosciences, Advanced BioMatrix, and Flexcell. These low pH solutions are stored at low temperature to prevent spontaneous fibrillogenesis and gelation. Hydrogels are typically formed by raising the temperature and the pH to initiate collagen fibril self-assembly, which can occur in the presence of cells or culture media43. Gelation occurs in about 30 minutes under physiological conditions and in shapes that can be flexibly molded. Temperature can critically affect hydrogel architecture, with lower gelation temperatures leading to the formation of larger fibrils44. These changes in hydrogel microstructure can critically influence cell behavior with fibroblasts showing less elongation and greater migration velocities in collagen matrices with larger fibrils44 (Fig. 2F).
The primary advantage of collagen is its biomimetic properties; collagen hydrogels are cytocompatible, amenable to cell adhesion without modification, and present a native viscoelastic environment to resident cells (Fig. 2C). Collagen hydrogels have a rich history of use as model cellular microenvironments for studies on topics ranging from MSC differentiation45 to carcinoma cell reprogramming46. Collagen suffers from some important drawbacks, shared by other natural materials, including low stiffness, limited long-term stability, and batch-to-batch variability. Collagen hydrogel mechanics are dictated by the collagen concentration, but this is coupled to changes in the adhesive ligand density, which limits independent control of these features. It is also difficult to produce collagen hydrogels with higher stiffnesses (> 1 kPa) without extensive chemical crosslinking, which fundamentally alters the degradability of collagen fibrils. As a result, culturing cells in collagen hydrogels for long times results in significant contraction of the matrix, although this phenomenon can also be leveraged for studies ranging from modular tissue assembly47 to measurement of cell contractile forces48. Despite some drawbacks, collagen is an excellent choice for in vitro studies of cell behavior such as migration41, 44 (Fig. 2F) in a tissue-like environment.
Other collagen-containing hydrogels have been used in cell studies, most notably Matrigel. Matrigel is a basement membrane-derived preparation extracted from Engelbreth-Holm-Swarm (EHS) mouse sarcoma tumors that is primarily composed of laminin, type IV collagen, and entactin, with various other constituents including proteoglycans and growth factors49. Corning sells Matrigel as a frozen protein solution that is diluted to a working concentration in PBS (~ 3–4 mg ml−1) and self-assembles into a hydrogel at physiological temperatures. Matrigel offers many of the advantages of collagen and other natural hydrogels and has been used to study cell migration, angiogenesis, and tumor development49. Significant drawbacks include Matrigel’s tumorigenic origin, diverse composition, and batch-to-batch variability in terms of mechanical and biochemical properties, which in turn brings a significant level of uncertainty to cellular experiments50. For these reasons, the more well-defined and tunable hydrogels discussed here may be more suitable for cell culture.
Fibrin
Fibrin is a natural polymer formed during wound coagulation51. Selective cleavage of the dimeric glycoprotein fibrinogen by the serine protease thrombin results in the formation of fibrin molecules that interact through a series of disulfide bonds52. Additional fibrin crosslinking is provided by Factor XIIIa, a form of Factor XIII also activated by thrombin. The wide use of fibrin sealants in the medical community provides several options for acquiring fibrin material for cell culture applications (typically sourced from human plasma), including Tisseel and Artiss (Baxter), as well as Evicel (Johnson & Johnson). The individual hydrogel components fibrinogen and thrombin can also be purchased separately from Sigma and other vendors to provide the user with greater design flexibility. For example, increased thrombin content relative to fibrinogen results in fibrin hydrogels with thinner fibrils and smaller pores53. Fibrin’s role as a natural matrix critically involved in hemostasis and wound healing make it useful as an in vitro tool to study these and related phenomena, including angiogenesis54 and platelet mechanosensing55. Fibrin has also been used as a temporary scaffold to guide cell-mediated collagenous tissue assembly56, 57. However, fibrin’s extreme susceptibility to protease-mediated degradation limits the use of fibrin for long-term cell cultures.
Alginate
Alginate is a polysaccharide derived from brown algae that has been applied in industries as varied as food, textiles, printing, and pharmaceuticals58. Alginate consists of β-D-mannuronic acid M units and α-L-guluronic acid G units59 assembled as block copolymers with regions composed either homogeneously of M or G units or with alternating G and M units58, 60. Unlike collagen and fibrin, alginate must be modified with an adhesive ligand such as RGD to enable cell attachment.
Alginate is notable for its ability to form hydrogels via ionic crosslinking, making it easily amenable to cell encapsulation, as well as cell recovery for downstream applications. Alginate’s ionic crosslinks are formed using divalent cations such as calcium, magnesium, or barium to promote the formation of ionic bridges between alginate G units58. Although the formation of alginate hydrogels is possible through covalent crosslinking60, most commercial approaches, such as the NovaMatrix-3D cell culture system, use ionic crosslinking. The NovaMatrix kit consists of an air-dried alginate foam disk preformed inside well plates of varying sizes and lyophilized alginate that can be mixed with cells and culture media. The alginate/cell solution is then added to the foam disk, where residual cations in the disk promote ionic crosslinking and gelation. Ion chelation with an isotonic solution later allows facile hydrogel dissolution and cell harvesting. Alginate solutions can also be purchased from vendors such as PRONOVA. The ability of alginate to be easily dissolved for cell recovery makes it attractive for studying cell-material interactions in 3D. However, cells normally remain rounded, as they cannot degrade the matrix61 (Fig. 2B).
Polyacrylamide
Polyacrylamide (PA) is a synthetic polymer with a rich history of use in molecular biology and, more recently, cell culture applications62. PA hydrogels are produced by reacting acrylamide monomer and bis-acrylamide crosslinker, usually in the presence of ammonium persulfate (APS) and tetramethylethylenediamine (TEMED). These components are readily available from Sigma and other commercial vendors. APS serves as a source of free radicals, while TEMED is a catalyst to initiate redox radical polymerization of the PA. PA hydrogels are typically fabricated as thin films bound to coverslips functionalized with aminosilanes, which can be prepared in house or purchased from vendors (e.g., Schott). Protein conjugation to the PA hydrogel surface to enable cell attachment is usually achieved using a bifunctional crosslinker such as sulfo-SANPAH, although other approaches including hydrazine modification of polyacrylamide amide groups for coupling to aldehyde or ketone groups on oxidized proteins have been used63. Detailed protocols on functionalizing coverslips, tuning monomer/crosslinker amounts to produce specific hydrogel mechanics, and conjugating adhesive ligands to the surface of the hydrogels have been published elsewhere30.
The appeal of PA hydrogels for cell culture is the existence of well-established protocols for the fabrication of hydrogels with tunable stiffness and coupling of proteins30, 62. The ability to independently modulate hydrogel stiffness and adhesive ligand presentation can lead to more complete understanding of complex cell responses to these inputs and is difficult to accomplish with natural materials. For example, many cell types such as human MSCs spread and proliferate more with increasing stiffness, but MSCs on laminin-coated hydrogels spread less compared to other ECM protein coatings, illustrating how the combination of hydrogel stiffness and adhesive ligand presentation can be engineered to modify cell response64 (Fig. 1C). PA hydrogels do not inherently interact with cell surface receptors or integrins, permitting user-defined control of these interactions. One major disadvantage is that PA cannot be used to encapsulate cells in 3D, due to toxicity of the hydrogel precursors. In our view, PA is ideally suited for mechanobiology studies where hydrogel stiffness needs to be finely controlled. This has been exemplified by elegant studies that illustrate the influence of substrate stiffness on cell motility62, spreading62, 65, and differentiation65, 66. The simple fabrication procedure also makes PA hydrogels an excellent choice for investigators without much experience in hydrogel substrates.
Polyethylene glycol
The synthetic polymer polyethylene glycol (PEG) is advantageous due to its hydrophilicity and relative inertness; PEG is often referred to as a “blank slate” material. It shows relatively low protein adsorption and is thus amenable to user-defined crosslinking chemistry and presentation of ligands to cells. Like other materials discussed in this review, PEG can be modified with a wealth of different functional groups and hydrogels can be formed using a variety of chain-growth, step-growth, or mixed mode polymerization techniques13, giving the user more design flexibility than most of the other polymers discussed in this review. The utility of PEG has been demonstrated in a diverse set of cell culture applications23, 24, 67, 68 including studies of stem cell differentiation, mechanobiology, and angiogenesis. In particular, PEG is an excellent choice for photoencapsulation experiments69, 70.
PEG can be purchased from common vendors like Sigma in a variety of molecular weights and with several chemical modifications, including (meth)acrylates that are needed for crosslinking. In addition to its use as a base hydrogel, chemically modified PEG is often used to crosslink other polymeric materials. For example, Cellendes sells hydrogel kits based on PEG-thiol crosslinkers and maleimide-functionalized dextran or PVA; maleimides react rapidly with thiols under physiological conditions to form hydrogels. The PEG-crosslinkers are available in either non-degradable or MMP-degradable forms. Other more specialized PEG hydrogel kits exist, including the QGel assay kit for drug screening. This kit includes PEG (functionalized with or without the adhesive ligand RGD) and peptide crosslinkers containing sequences that degrade via MMPs. Hydrogels are formed by simply mixing the components together24. As PEG is very cytocompatible and the reaction conditions are mild, cells for encapsulation can also be introduced at this step. PEG hydrogels have also been formed with cells through photoencapsulation techniques, including with osteoblasts69 and chondrocytes70. BioTime Inc. sells a photopolymerizable kit called PEGgel that includes PEG-diacrylate and photoinitiator. The PEG-diacrylate, photoinitiator, and cells are mixed together and gelled via UV light exposure for a prescribed time.
Hyaluronic acid
Hyaluronic acid (HA) is a non-sulfated glycosaminoglycan composed of a repeating disaccharide unit of glucuronate and N-acetyl glucosamine71. HA is distributed throughout many tissues, including skin, cartilage, and brain, and is known to play an important role in development, wound healing, and disease72. Although HA can be isolated from animal tissue such as rooster combs, animal-free production of HA can be achieved via microbial fermentation in Escherichia coli. Notable HA vendors include Lifecore and BioTime Inc. HA can be purchased from Lifecore as a sodium hyaluronate, permitting user modification of the structure before forming a hydrogel. Lifecore also markets an HA hydrogel kit called Corgel BioHydrogel, which includes a tyramine-substituted HA that is enzymatically crosslinked using peroxidase to form a quick-setting hydrogel73. BioTime sells a line of HyStem HA hydrogel kits composed of thiolated HA and PEG-diacrylate crosslinker. In addition to these components, HyStem kits can also be purchased with collagen (to permit cell adhesion) and/or heparin (for sequestration of growth factors and other therapeutic proteins). BRTI Life Sciences offers a simple system (Cell-Mate3D) composed of HA and chitosan where the hydrogel assembles through electrostatic interactions between negatively charged carboxyl groups on HA and positively charged amino groups on chitosan. Cells can be mixed and encapsulated directly into the hydrogel. Notably, the BRTI Life Sciences website includes protocols for RNA isolation, protein extraction, and microscopy in conjunction with this kit.
HA has several important advantages as a hydrogel platform, including its biological relevance and chemical tunability. The ability to modify HA to present functional groups enabling a range of crosslinking chemistries is perhaps its biggest advantage and has been reviewed elsewhere74. This permits formation of a range of hydrogel systems that can be processed into 2D films, 3D free-swelling hydrogels, nanofibers, and injectable materials. The versatility of crosslinking chemistries available with HA has also enabled the fabrication of hydrogels with a wide range of mechanics suitable for cell studies as with hydrogels such as PA and PEG. In particular, HA hydrogels whose mechanics can be changed in a user-directed manner have proven useful for cellular mechanotransduction investigations22, 75. Tuning HA hydrogel degradability with the incorporation of MMP-cleavable crosslinks showed that a degradable environment was required for traction force generation, as well as stem cell spreading and osteogenic differentiation in a covalently crosslinked hydrogel environment22 (Fig. 2D,E). Although unmodified HA does not support integrin-mediated cell adhesion, HA interacts with numerous cell surface markers including CD44 and RHAMM (CD168)76. This can be a double-edged sword as HA’s cellular interactions and important role in numerous physiological and pathological processes may convolute experimental results.
Polypeptides
Advances in the design and synthesis of custom peptide sequences and non-natural amino acids provide an intriguing tool kit for the design of synthetic hydrogels with user-defined properties77. There are many methods to fabricate peptide-based hydrogels, but perhaps the most common involves peptide self-assembly into supramolecular nanostructures. Several polypeptide hydrogel formulations are commercially available, including PuraMatrix (Corning), PGmatrix (PepGel LLC)78, and HydroMatrix (Sigma). PuraMatrix is composed of an amphiphilic 16 residue peptide containing a repeating arginine-alanine-aspartate-alanine sequence (RADARADARADARADA). This peptide self assembles into β-sheets in the presence of monovalent cations and forms a stable network with fibril diameter and pore size on the order of tens of nanometers, analogous to native ECM. Other common peptide-derived hydrogels have applied a similar assembly principle including EAK16 (AEAEAKAKAEAEAKAK)79 and KLD12 (KLDLKLDLKLDL)80.
Polypeptide hydrogels are intriguing for cell culture applications in the sense that they are synthetic materials with tunable properties that can be engineered to exhibit many of the advantages of naturally-derived polymers, including interactions with cells, assembly into hierarchical structures reminiscent of native proteins81, and degradability, while also being able to decouple the effects of these parameters (unlike with natural matrices). A critical disadvantage of peptide-based materials at this point is expense; cost is prohibitive for large-scale culture systems. As with naturally-derived protein materials, it is difficult to form gels that remain for long periods of time and that exhibit mechanics for high cell traction.
Future Perspective
Although the systems described in this review are already much more complex than standard tissue culture plastic or glass, the properties of hydrogels are constantly evolving in an effort to match the complexity of native tissues. The approaches discussed here, including most commercially available systems, are primarily macroscale constructs with static properties, but there has been tremendous progress in the design of dynamic hydrogels that are responsive and/or instructive to resident cells82. Thermoresponsive hydrogels such as poly-N-isopropylacrylamide (pNIPAm) have been used to efficiently harvest cell populations83. Additionally, the convergence of microscale technologies for cell culture84 with tunable hydrogel designs has enabled diverse studies including the investigation of cell migration in microfluidic hydrogels85 and high-throughput screening platforms for probing cell-material interactions86. Of particular interest for the mechanobiology community are a range of mechanically dynamic hydrogels that can be stiffened22, 75, softened23, or reversibly stiffened and softened87 to probe cellular responses. These mechanically dynamic substrates enable investigations of the influence of mechanical dosing on cell behavior in a manner similar to what has been performed with soluble factors for decades.
Beyond dynamic properties, fibrous88, 89 and viscoelastic90, 91 hydrogels that more accurately recreate the complex structural and mechanical milieus found in tissues are emerging as useful cell culture substrates. This also includes spatial patterning, where numerous biochemical and biophysical signals can be introduced in a heterogeneous manner to control populations of single cell types or co-cultures92, 93. Techniques are also advancing for engineering heterogeneity and including multiple cell types within 3D constructs. This includes newly developed methods where hydrogels act as bio-inks to print cells either in a layer-by-layer manner from a 2D substrate94 or directly in 3D space within another hydrogel95. As these types of platforms progress, they will likely become available to a wider audience. But in the meantime, it is important to maintain an open, collaborative dialogue between cell biologists and materials scientists and engineers so that the next generation of hydrogel systems will be equipped to tackle important challenges of increasing biological complexity.
Supplementary Material
Acknowledgments
The authors would like to acknowledge funding from the National Institutes of Health (F32 DK103463 to SRC and R01EB008722/R01AR056624 to JAB).
Key terms
- Biomimetic
imitate or replicate specific properties or features of natural tissues
- Chain-growth polymerization
polymerization mode where monomers are sequentially added onto the active site of a growing polymer chain
- Mesh size
the space between crosslinks in a polymer network, essentially a molecular porosity of the network
- Poisson’s ratio
negative ratio of the transverse to axial strain, where strain is the change in material length in response to an applied force divided by the original length
- RGD
peptide often used in hydrogels to facilitate cell attachment containing the adhesive sequence arginine-glycine-aspartic acid found in fibronectin
- Rheology
study of flow and deformation of materials in response to an applied force
- Step-growth polymerization
polymerization mode where multifunctional monomers and/or crosslinkers react gradually to form progressively higher molecular weight polymers
Footnotes
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Baker BM, Chen CS. Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues. Journal of Cell Science. 2012;125:3015–3024. doi: 10.1242/jcs.079509. This review elegantly discusses the critical factors such as adhesion, mechanics, and nutrient transport that make 3D cultures different from cell experiments on 2D substrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 3.Tibbitt MW, Anseth KS. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnology and bioengineering. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury F, et al. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS ONE. 2010;5:e15655. doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerecht S, et al. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proceedings of the National Academy of Sciences. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. This paper uses a 3D hydrogel platform based on hyaluronic acid to expand human embryonic stem cells over long-term cultures while maintaining them in an undifferentiated state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musah S, et al. Glycosaminoglycan-Binding Hydrogels Enable Mechanical Control of Human Pluripotent Stem Cell Self-Renewal. ACS Nano. 2012;6:10168–10177. doi: 10.1021/nn3039148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen TV, Sleiman M, Moriarty T, Herrick WG, Peyton SR. Sorafenib resistance and JNK signaling in carcinoma during extracellular matrix stiffening. Biomaterials. 2014;35:5749–5759. doi: 10.1016/j.biomaterials.2014.03.058. [DOI] [PubMed] [Google Scholar]
- 9.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol (Camb) 2012;4:410–421. doi: 10.1039/c2ib00149g. This work shows that lung myofibroblast phenotype is influenced by microenvironmental mechanical history with extended culture on stiff surfaces promoting sustained myofibroblast activity, even when cells are moved to soft materials. [DOI] [PubMed] [Google Scholar]
- 10.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13:645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggeli A, et al. Responsive gels formed by the spontaneous self-assembly of peptides into polymeric [beta]-sheet tapes. Nature. 1997;386:259–262. doi: 10.1038/386259a0. [DOI] [PubMed] [Google Scholar]
- 12.Appel EA, del Barrio J, Loh XJ, Scherman OA. Supramolecular polymeric hydrogels. Chemical Society Reviews. 2012;41:6195–6214. doi: 10.1039/c2cs35264h. [DOI] [PubMed] [Google Scholar]
- 13.Lin CC, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26:631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen A, Davis BH. UV irradiation activates JNK and increases alphaI(I) collagen gene expression in rat hepatic stellate cells. J Biol Chem. 1999;274:158–164. doi: 10.1074/jbc.274.1.158. [DOI] [PubMed] [Google Scholar]
- 15.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb Journal. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 16.Oyen ML, Cook RF. A practical guide for analysis of nanoindentation data. Journal of the Mechanical Behavior of Biomedical Materials. 2009;2:396–407. doi: 10.1016/j.jmbbm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Oyen ML. Mechanical characterisation of hydrogel materials. International Materials Reviews. 2013;59:44–59. [Google Scholar]
- 18.Branco MC, Pochan DJ, Wagner NJ, Schneider JP. Macromolecular diffusion and release from self-assembled beta-hairpin peptide hydrogels. Biomaterials. 2009;30:1339–1347. doi: 10.1016/j.biomaterials.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stellwagen NC. Apparent pore size of polyacrylamide gels: comparison of gels cast and run in Tris-acetate-EDTA and Tris-borate-EDTA buffers. Electrophoresis. 1998;19:1542–1547. doi: 10.1002/elps.1150191004. [DOI] [PubMed] [Google Scholar]
- 20.Canal T, Peppas NA. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. Journal of Biomedical Materials Research. 1989;23:1183–1193. doi: 10.1002/jbm.820231007. [DOI] [PubMed] [Google Scholar]
- 21.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. European Journal of Pharmaceutics and Biopharmaceutics. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 22.Khetan S, et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12:458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutolf MP, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proceedings of the National Academy of Sciences. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. This paper integrates molecular engineering into hydrogel design to enable the fabrication of synthetic hydrogels amenable to cell-mediated proteolysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karajanagi SS, et al. Application of a dense gas technique for sterilizing soft biomaterials. Biotechnology and Bioengineering. 2011;108:1716–1725. doi: 10.1002/bit.23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huebsch N, Gilbert M, Healy KE. Analysis of sterilization protocols for peptide-modified hydrogels. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2005;74B:440–447. doi: 10.1002/jbm.b.30155. [DOI] [PubMed] [Google Scholar]
- 27.Lee DW, et al. Effect of γ-Irradiation on Degradation of Alginate. Journal of Agricultural and Food Chemistry. 2003;51:4819–4823. doi: 10.1021/jf021053y. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Stegemann JP. Extraction of high quality RNA from polysaccharide matrices using cetyltrimethylammonium bromide. Biomaterials. 2010;31:1612–1618. doi: 10.1016/j.biomaterials.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin DS, et al. Photodegradable Hydrogels for Capture, Detection, and Release of Live Cells. Angewandte Chemie International Edition. 2014;53:8221–8224. doi: 10.1002/anie.201404323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tse JR, Engler AJ. Preparation of hydrogel substrates with tunable mechanical properties. Current protocols in cell biology / editorial board, Juan S. Bonifacino … [et al.] 2010;Chapter 10(Unit 10):16. doi: 10.1002/0471143030.cb1016s47. This chapter serves as a guide for preparing polyacrylamide hydrogels with tunable mechanics, a substrate that is commonly used for mechanotransduction studies. [DOI] [PubMed] [Google Scholar]
- 31.Wylie RG, et al. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat Mater. 2011;10:799–806. doi: 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- 32.Loebsack AB, et al. The development of an embedding technique for polylactide sponges. J Biomed Mater Res. 1999;48:504–510. doi: 10.1002/(sici)1097-4636(1999)48:4<504::aid-jbm16>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 33.Hoemann CD, et al. Chitosan–glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthritis and Cartilage. 2007;15:78–89. doi: 10.1016/j.joca.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Ruan JL, et al. An improved cryosection method for polyethylene glycol hydrogels used in tissue engineering. Tissue Eng Part C Methods. 2013;19:794–801. doi: 10.1089/ten.tec.2012.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James R, Jenkins L, Ellis SE, Burg KJL. Histological processing of hydrogel scaffolds for tissue-engineering applications. J Histotechnol. 2004;27:133–139. [Google Scholar]
- 36.Berger J, et al. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics. 2004;57:19–34. doi: 10.1016/s0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 37.Kim UJ, et al. Structure and Properties of Silk Hydrogels. Biomacromolecules. 2004;5:786–792. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
- 38.Hassan CM, Peppas NA. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv Polym Sci. 2000;153:37–65. [Google Scholar]
- 39.Van Tomme SR, Hennink WE. Biodegradable dextran hydrogels for protein delivery applications. Expert Rev Med Devices. 2007;4:147–164. doi: 10.1586/17434440.4.2.147. [DOI] [PubMed] [Google Scholar]
- 40.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 41.Kim HD, et al. Epidermal Growth Factor–induced Enhancement of Glioblastoma Cell Migration in 3D Arises from an Intrinsic Increase in Speed But an Extrinsic Matrix- and Proteolysis-dependent Increase in Persistence. Molecular Biology of the Cell. 2008;19:4249–4259. doi: 10.1091/mbc.E08-05-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walters BD, Stegemann JP. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014;10:1488–1501. doi: 10.1016/j.actbio.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doyle AD, Carvajal N, Jin A, Matsumoto K, Yamada KM. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat Commun. 2015;6 doi: 10.1038/ncomms9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615–1627. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- 46.Ali MY, Chuang CY, Saif MTA. Reprogramming cellular phenotype by soft collagen gels. Soft Matter. 2014;10:8829–8837. doi: 10.1039/c4sm01602e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bian W, Liau B, Badie N, Bursac N. Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues. Nat Protocols. 2009;4:1522–1534. doi: 10.1038/nprot.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Legant WR, et al. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proceedings of the National Academy of Sciences. 2009;106:10097–10102. doi: 10.1073/pnas.0900174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Hughes CS, Postovit LM, Lajoie GA. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 51.Weisel JW, Litvinov RI. Mechanisms of fibrin polymerization and clinical implications. Blood. 2013;121:1712–1719. doi: 10.1182/blood-2012-09-306639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown AC, Barker TH. Fibrin-based biomaterials: Modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 2014;10:1502–1514. doi: 10.1016/j.actbio.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawrie AS, McDonald SJ, Purdy G, Mackie IJ, Machin SJ. Prothrombin time derived fibrinogen determination on Sysmex CA-6000. J Clin Pathol. 1998;51:462–466. doi: 10.1136/jcp.51.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nehls V, Drenckhahn D. A Novel, Microcarrier-Based in Vitro Assay for Rapid and Reliable Quantification of Three-Dimensional Cell Migration and Angiogenesis. Microvascular Research. 1995;50:311–322. doi: 10.1006/mvre.1995.1061. [DOI] [PubMed] [Google Scholar]
- 55.Qiu Y, et al. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proceedings of the National Academy of Sciences. 2014;111:14430–14435. doi: 10.1073/pnas.1322917111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bian W, Juhas M, Pfeiler TW, Bursac N. Local tissue geometry determines contractile force generation of engineered muscle networks. Tissue Eng Part A. 2012;18:957–967. doi: 10.1089/ten.tea.2011.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paxton JZ, Wudebwe UNG, Wang A, Woods D, Grover LM. Monitoring Sinew Contraction During Formation of Tissue-Engineered Fibrin-Based Ligament Constructs. Tissue Engineering Part A. 2012;18:1596–1607. doi: 10.1089/ten.TEA.2011.0535. [DOI] [PubMed] [Google Scholar]
- 58.Augst AD, Kong HJ, Mooney DJ. Alginate Hydrogels as Biomaterials. Macromolecular Bioscience. 2006;6:623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 59.Martinsen A, Skjåk-Bræk G, Smidsrød O. Alginate as immobilization material: I. Correlation between chemical and physical properties of alginate gel beads. Biotechnology and Bioengineering. 1989;33:79–89. doi: 10.1002/bit.260330111. [DOI] [PubMed] [Google Scholar]
- 60.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 61.Huebsch N, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Damljanovic V, Lagerholm BC, Jacobson K. Bulk and micropatterned conjugation of extracellular matrix proteins to characterized polyacrylamide substrates for cell mechanotransduction assays. Biotechniques. 2005;39:847–851. doi: 10.2144/000112026. [DOI] [PubMed] [Google Scholar]
- 64.Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. American Journal of Physiology - Cell Physiology. 2008;295:C1037–C1044. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 65.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 66.Wen JH, et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater. 2014;13:979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phelps EA, et al. Maleimide Cross-Linked Bioactive PEG Hydrogel Exhibits Improved Reaction Kinetics and Cross-Linking for Cell Encapsulation and In Situ Delivery. Advanced Materials. 2012;24:64–70. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moon JJ, et al. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials. 2010;31:3840–3847. doi: 10.1016/j.biomaterials.2010.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 70.Bryant SJ, Anseth KS, Lee DA, Bader DL. Crosslinking density influences the morphology of chondrocytes photoencapsulated in PEG hydrogels during the application of compressive strain. J Orthop Res. 2004;22:1143–1149. doi: 10.1016/j.orthres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Meyer K. Chemical structure of hyaluronic acid. Federation proceedings. 1958;17:1075–1077. [PubMed] [Google Scholar]
- 72.Fraser JRE, Laurent TC, Laurent UBG. Hyaluronan: its nature, distribution, functions and turnover. Journal of Internal Medicine. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 73.Darr A, Calabro A. Synthesis and characterization of tyramine-based hyaluronan hydrogels. J Mater Sci-Mater Med. 2009;20:33–44. doi: 10.1007/s10856-008-3540-0. [DOI] [PubMed] [Google Scholar]
- 74.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23:H41–56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Commun. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 76.Dicker KT, et al. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014;10:1558–1570. doi: 10.1016/j.actbio.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matson JB, Stupp SI. Self-assembling peptide scaffolds for regenerative medicine. Chemical Communications. 2012;48:26–33. doi: 10.1039/c1cc15551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang H, et al. Design of a shear-thinning recoverable peptide hydrogel from native sequences and application for influenza H1N1 vaccine adjuvant. Soft Matter. 2011;7:8905–8912. [Google Scholar]
- 79.Zhang S, Holmes T, Lockshin C, Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proceedings of the National Academy of Sciences. 1993;90:3334–3338. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kisiday J, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Webber MJ, et al. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proceedings of the National Academy of Sciences. 2011;108:13438–13443. doi: 10.1073/pnas.1016546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat Commun. 2012;3:1269. doi: 10.1038/ncomms2271. This review highlights advances in the design of hydrogels that have complex and dynamic properties, such as changing mechanics, growth factor release, and patterned adhesion. [DOI] [PubMed] [Google Scholar]
- 83.Nash ME, Healy D, Carroll WM, Elvira C, Rochev YA. Cell and cell sheet recovery from pNIPAm coatings; motivation and history to present day approaches. Journal of Materials Chemistry. 2012;22:19376–19389. [Google Scholar]
- 84.Young EWK, Beebe DJ. Fundamentals of microfluidic cell culture in controlled microenvironments. Chemical Society reviews. 2010;39:1036–1048. doi: 10.1039/b909900j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jeong G, et al. Microfluidic assay of endothelial cell migration in 3D interpenetrating polymer semi-network HA-Collagen hydrogel. Biomed Microdevices. 2011;13:717–723. doi: 10.1007/s10544-011-9541-7. [DOI] [PubMed] [Google Scholar]
- 86.Gobaa S, et al. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat Methods. 2011;8:949–955. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- 87.Stowers RS, Allen SC, Suggs LJ. Dynamic phototuning of 3D hydrogel stiffness. Proceedings of the National Academy of Sciences. 2015;112:1953–1958. doi: 10.1073/pnas.1421897112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han LH, Tong X, Yang F. Photo-crosslinkable PEG-Based Microribbons for Forming 3D Macroporous Scaffolds with Decoupled Niche Properties. Advanced Materials. 2014;26:1757–1762. doi: 10.1002/adma.201304805. [DOI] [PubMed] [Google Scholar]
- 89.Wade RJ, Bassin EJ, Rodell CB, Burdick JA. Protease-degradable electrospun fibrous hydrogels. Nat Commun. 2015;6 doi: 10.1038/ncomms7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cameron AR, Frith JE, Cooper-White JJ. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials. 2011;32:5979–5993. doi: 10.1016/j.biomaterials.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 91.Chaudhuri O, et al. Substrate stress relaxation regulates cell spreading. Nat Commun. 2015;6 doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wade RJ, Bassin EJ, Gramlich WM, Burdick JA. Nanofibrous Hydrogels with Spatially Patterned Biochemical Signals to Control Cell Behavior. Advanced Materials. 2015;27:1356–1362. doi: 10.1002/adma.201404993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.DeForest CA, Tirrell DA. A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nat Mater. 2015;14:523–531. doi: 10.1038/nmat4219. [DOI] [PubMed] [Google Scholar]
- 94.Malda J, et al. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Advanced Materials. 2013;25:5011–5028. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 95.Highley CB, Rodell CB, Burdick JA. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Advanced Materials. 2015;27:5075–5079. doi: 10.1002/adma.201501234. [DOI] [PubMed] [Google Scholar]
- 96.Ventre M, Netti AP. Controlling Cell Functions and Fate with Surfaces and Hydrogels: The Role of Material Features in Cell Adhesion and Signal Transduction. Gels. 2016;2 doi: 10.3390/gels2010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wade RJ, Burdick JA. Engineering ECM signals into biomaterials. Materials Today. 2012;15:454–459. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.