ABSTRACT
Atrial natriuretic peptide (ANP) increases during exercise in the heat wherein heat loss responses of sweating and cutaneous vasodilatation are activated. Hence ANP might be involved in the regulation of sweating and cutaneous vasodilatation. However, whether ANP directly mediates sweating and cutaneous vasodilatation needs to be clarified. Also, muscarinic receptor activation induces sweating and cutaneous vasodilatation, however, it remains to be determined whether ANP modulates these responses. In this study, in 11 young males (25 ± 5 years), cutaneous vascular conductance and sweat rate were assessed at intradermal microdialysis sites that were continuously perfused with either lactated Ringer (Control) or 3 different concentrations of ANP (0.1, 1, 10 µM). All 4 sites were co-administrated with methacholine, a muscarinic receptor agonist, in a dose-dependent fashion (0.0125, 0.25, 5, 100, and 2000 mM, 25 min for each). ANP at all concentrations did not increase sweat rate and cutaneous vascular conductance as compared with pre-ANP infusion values (all P > 0.05). Methacholine increased both sweat rate and cutaneous vascular conductance (all P ≤ 0.05). However, the responses were unaffected by co-administration of ANP relative to methacholine only, even as assessed in context of the methacholine concentration required to elicit 50% of the maximal response (EC50) (all P > 0.05). We show that exogenous ANP administration intradermally does not directly modulate sweating and cutaneous vasodilatation under room temperature conditions in resting young adults. Further, there is no effect of ANP on muscarinic sweating and cutaneous vasodilatation.
KEYWORDS: thermoregulation, heat loss responses, microcirculation
Introduction
Atrial natriuretic peptide (ANP) is secreted from atrial muscle cells when the atrial wall is stretched due to increases in right atrial pressure that can occur with increases in blood pressure1 and blood volume.2 This hormone is also known to increase in response to exercise performed in non-heat stress3,4 and high heat stress conditions5,6 possibly due to increases in right atrial pressure as a result of increased venous return associated with active muscle contraction. The activation of heat loss responses of cutaneous vasodilatation and sweating are necessary to prevent potentially dangerous increases in body core temperature especially during exercise in the heat. Impairments in the body's ability to dissipate heat such as occurs with aging for example, can cause a greater level of hyperthermia even during a brief 3-hour passive heat exposure.7 Elucidating whether ANP modulates the regulation of cutaneous vasodilatation and sweating is paramount to provide insight into if ANP can be a target in improving heat loss thereby preventing extreme hyperthermia during heat stress.
It has been shown that nerves innervating eccrine sweat glands are immunoreactive to ANP.8 Also, the ANP receptor guanylate cyclase A exists in the eccrine sweat glands.9 Taken together, these observations lend support to the possibility that a functional role of ANP in the regulation of sweat secretion. However, one preliminary study demonstrated that intravenous infusion of ANP under moderate-to-high ambient temperature conditions (i.e., 30–39°C) did not increase sweating.10 Sweating during heat stress is almost exclusively mediated via muscarinic receptor activation achieved by endogenous acetylcholine released from cholinergic nerves.11-13 In this context, the aforementioned study by Yamashida et al.10 demonstrated that sweating induced by intradermal injection of methacholine, an acetylcholine mimetic, was greater with co-injection of ANP in comparison with methacholine only. Their findings support a modulatory role of ANP in muscarinic sweating. However, while their observations provide evidence suggesting a possible link between ANP and muscarinic sweating, they should be interpreted with caution as the authors did not report the number of subjects or sweat rate data. Therefore, further work is required to confirm this response.
ANP is a powerful vasodilator as evidenced by increased forearm blood flow.14,15 However, results related to the regulation of cutaneous blood flow have been mixed with studies showing an increase,16-18 or no increase19,20 in cutaneous blood flow in response to ANP. Cutaneous vasodilatation during heat stress is partly mediated by muscarinic receptor activation,11,21 and muscarinic contribution to cutaneous vasodilatation can be assessed using the administration of methacholine.22-25 As with the regulation of sweating, it may be that ANP augments muscarinic cutaneous vasodilatation via methacholine, though this possibility has yet to be investigated.
The purpose of the present study was to evaluate the effect of intradermal administration of ANP on sweating and cutaneous vascular responses in humans in vivo. We hypothesized that ANP does not directly induce sweating and cutaneous vasodilatation, but it augments muscarinic sweating and cutaneous vasodilatation under a resting state with room temperature.
Materials and methods
The University of Ottawa Health Sciences and Science Research Ethics Board approved the current study in accordance with the guidelines set forth by the Declaration of Helsinki. Verbal and written informed consent was obtained from all volunteers before participating in this study.
Eleven relatively healthy active young males (21–36 years) participated in this study. Mean (± standard deviation) body mass, height, and age of the subjects were 77.2 ± 8.2 kg, 1.78 ± 0.07 m, and 25 ± 5 years, respectively. They were free of cystic fibrosis transmembrane conductance regulator mutations, skindisorders, hypertension, heart disease, diabetes, autonomic disorders, cigarette smoking, and prescription medications. In this study, we examined responses in young males only to avoid age-related26 and sex-related24,27,28 differences in the regulation of cutaneous vascular and sweating responses.
All subjects were instructed to abstain from taking over-the-counter medications including non-steroidal anti-inflammatory agents and vitamins for at least 48 hours prior to the experiment. Alcohol and caffeine consumption was avoided at least 12 hours prior. Subjects avoided performing intense exercise the day before the study. On the day of the experiment, subjects refrained from consuming food 2 hours prior to and throughout the experiment. Upon arrival, the subjects voided their bladder, thereafter body mass was measured using a digital weight scale platform (Model CBU150X, Mettler Toledo Inc., OH, USA). The subject's body height was measured using an eye-level physician stadiometer (Model 2391, Detecto Scale Company, Webb City, MO, USA). Subjects were then seated in a semi-recumbent position in a thermoneutral room (∼23–24ºC) and instrumented with 4 intradermal microdialysis fibers (30 kDa cutoff, 10 mm membrane) (MD2000, Bioanalytical Systems, West Lafayette, IN, USA). The fibers were implanted in the dermal layer of the left dorsal forearm. Using an aseptic technique, a 25 gauge needle was first introduced into the unanesthetized skin, then a microdialysis fiber was threaded through the lumen of the needle. The needle was subsequently withdrawn, leaving only the fiber in place. The entry and exit points for the fibers were separated by ∼2.5 cm. Each fiber was separated by ∼4.0 cm. All fibers were secured with surgical tape.
Approximately 10 min after completing fiber insertion, perfusion of lactated Ringer (Baxter, Deerfield, IL, US) was initiated at each site at a rate of 4.0 µl min−1 for a minimum of 60 min. The constant infusion rate was achieved by a microinfusion pump (Model 4004, CMA Microdialysis, Solna, Sweden). Thereafter, a 10-min baseline measurement was taken while the 4 microdialysis sites were perfused with lactated Ringer solution (defined as Baseline). Thereafter they were perfused with 1) lactated Ringer (Control) or 3 different does of ANP (molecular weight: 3,080 Da) (Sigma-Aldrich, St. Louis, MO, USA): 2) 0.1 µM, 3) 1 µM, or 4) 10 µM. ANP concentration of 0.1 µM is approximately 6 times higher than that observed in plasma during exercise in the heat.5 We employed this higher dose to compensate for the possible dilution of ANP which may occur prior to reaching the target end-organs (i.e., sweat glands and skin vessels) as ANP is introduced into the interstitial fluid via the microdialysis membrane. To greatly evoke an ANP effect and assess dose-dependent response, we also employed higher doses of ANP (1 and 10 µM ANP). All ANP infusions were performed at a rate of 4.0 µl min−1 and lasted for 40 min, the last 5 min of which was defined as ANP Baseline. Thereafter, methacholine chloride (molecular weight: 195.69 Da), a muscarinic receptor agonist (Sigma-Aldrich), was co-infused at all 4 sites in a step-wise fashion (0.0125, 0.25, 5, 100, 2000 mM; each 25 min) at a rate of 4.0 µl min−1 in combination with the site-specific solutions. These methacholine concentrations were based on our previous study.27 For human safety and avoiding any bacterial degradation of ANP, we sterilized all pharmacological solutions with syringe filters (Corning Inc., Corning, NY, USA) and microdialysis fibers with H2O2. In addition, we stored ANP at −20°C and thawed it on the day of the experiment to minimize potential degradation. After completion of the last methacholine infusion (2000 mM), 50 mM sodium nitroprusside (Sigma-Aldrich) was administered to each microdialysis site at a rate of 6.0 µl min−1 that persisted for 20–30 min to obtain a maximal cutaneous blood flow (i.e., stable high cutaneous blood flow averaged over ≥ 3 min). In some cases, maximal cutaneous blood flow was observed during methacholine administration as was observed in previous work.22,29 In that case, the highest cutaneous blood flow achieved during methacholine administration was considered as maximal cutaneous blood flow.
A sweat capsule specifically designed for use with an intradermal microdialysis probe30 was placed directly over the center of each microdialysis membrane. The area covered by the capsule was 1.1 cm2. The sweat capsules were attached to the skin with adhesive rings and topical skin glue (Collodion HV, Mavidon Medical products, Lake Worth, FL, USA). Dry compressed air in gas tanks located in the thermoneutral room (∼23–24°C) was supplied to each capsule at a rate of 0.20 l min−1, while water content of the effluent air from the sweat capsule was measured with high-precision dew point mirrors (Model 473, RH systems, Albuquerque, NM, USA). Long vinyl tubes were used for connections between the gas tank and the sweat capsule (inlet), and between the sweat capsule and the dew point mirror (outlet). Local forearm sweat rate was measured continuously (5 s sampling rate) and calculated from the difference in water content between influent and effluent air multiplied by the flow rate and normalized for the skin surface area under the capsule (mg min−1 cm−2).
Local cutaneous blood flow was assessed from cutaneous red blood cell flux (expressed in perfusion units) measured by laser-Doppler flowmetry (PeriFlux System 5000, Perimed, Stockholm, Sweden) using integrated laser-Doppler flowmetry probes with a 7-laser array (Model 413, Perimed). The data sampling rate was 32 Hz. The integrated laser-Doppler flowmetry probes were housed in the center of each sweat capsule over each microdialysis fiber for simultaneous measurement of both local forearm sweat rate and cutaneous red blood cell flux. Manual auscultation blood pressures were obtained every 10–15 min using a mercury column sphygmomanometer (Baumanometer Standby Model, WA Baum Co, Copiague, NY, USA). Mean arterial pressure was calculated as diastolic arterial pressure plus one-third the difference between systolic and diastolic pressures (i.e., pulse pressure). Cutaneous vascular conductance was evaluated as cutaneous red blood cell flux divided by mean arterial pressure.
Cutaneous vascular conductance data were presented relative to maximal absolute cutaneous vascular conductance (%max). Sweat rate and cutaneous vascular conductance values used for data analysis were obtained by averaging values over the last 5 min of each stage (i.e., Baseline, ANP Baseline, 0.0125, 0.25, 5, 100, 2000 mM of methacholine).
The methacholine concentration required to elicit 50% of the maximal sweating and cutaneous vasodilatation (EC50, in mM) was evaluated using a nonlinear regression with 4 parameters as was reported previously31 using commercially available software (GraphPad Prism 6.0; GraphPad Software, La Jolla, CA). In this analysis, sweat rate and cutaneous vascular conductance were normalized within each site by defining the smallest value as 0% and largest value as 100%.
All statistical tests were performed using the software package SPSS 24 for Windows (IBM, Armonk, NY, USA). Sweat rate (mg min−1 cm−2) and cutaneous vascular conductance (%max) were analyzed using a 2-way repeated-measures analysis of variance with the factors of treatment sites (4 intradermal sites) and stage (Baseline, ANP Baseline and 5 doses of methacholine). One-way repeated measures analysis of variance was also employed to analyze EC50 for sweat rate and cutaneous vascular conductance as well as maximal absolute cutaneous vascular conductance (perfusion units mmHg−1) with a factor of treatment site (4 intradermal sites). When a significant interaction or main effect was detected, post hoc multiple comparisons were performed with a modified Bonferroni correction (i.e., Holm procedure], to adjust P values used for Student's paired 2-tailed (for between-site comparisons) or one-tailed (Baseline vs. ANP Baseline and ANP Baseline vs. each methacholine dose) t-tests. The level of significance for all analyses was set at P ≤ 0.05. All values are reported with a mean ± standard deviation.
Results
No increases in sweat rate (Fig. 1) and cutaneous vascular conductance (Fig. 2) were measured for ANP administration as compared to Baseline (all P > 0.27). Methacholine administration of 0.25 mM and greater increased sweat rate (Fig. 1), whereas all doses of methacholine increased cutaneous vascular conductance (Fig. 2) from ANP Baseline at all 4 sites (all P ≤ 0.05). Sweat rate (Fig. 1) and cutaneous vascular conductance (Fig. 2) did not differ between sites throughout the experiment including absolute maximal cutaneous vascular conductance (Table 1) (all P > 0.16 for a main effect of treatment site and/or an interaction between treatment site and stage). There were no between-site differences in EC50 (mM) for sweat rate (Control: 49 ± 49, 0.1 µM ANP: 32 ± 35, 1 µM ANP: 32 ± 30, 10 µM ANP: 18 ± 20, P = 0.08 for a main effect of treatment site) and cutaneous vascular conductance (Control: 10 ± 23, 0.1 µM ANP: 10 ± 29, 1 µM ANP: 2 ± 2, 10 µM ANP: 6 ± 15, P = 0.61 for a main effect of treatment site).
Figure 1.
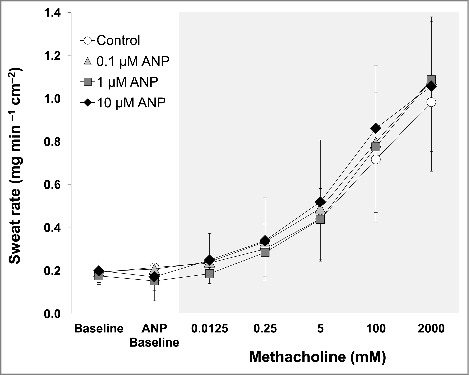
Sweat rate during baseline before any drug administration (Baseline), during atrial natriuretic peptide (ANP) infusion (ANP Baseline), and during co-administration of each dose of methacholine in the presence of ANP. Data are presented as mean ± standard deviation (n = 11). The 4 intradermal forearm sites were perfused with either lactated Ringer (Control) or 3 doses of ANP (0.1, 1, 10 µM). ANP did not increase sweat rate relative to Baseline (all P > 0.05). There were no between-site differences in sweat rate at any time points (all P > 0.05).
Figure 2.
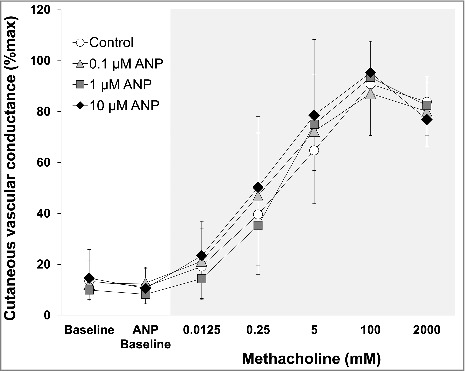
Cutaneous vascular conductance during baseline before any drug administration (Baseline), during atrial natriuretic peptide (ANP) infusion (ANP Baseline), and during co-administration of each dose of methacholine in the presence of ANP. Data are presented as mean ± standard deviation (n = 11). The 4 intradermal forearm sites were perfused with either lactated Ringer (Control) or 3 doses of ANP (0.1, 1, 10 µM). ANP did not increase cutaneous vascular conductance relative to Baseline (all P > 0.05). There were no between-site differences in cutaneous vascular conductance at any time points (all P > 0.05).
Table 1.
Absolute maximal cutaneous vascular conductance at each skin site.
| |
(perfusion units mmHg−1) |
| Control | 1.70 ± 0.74 |
| 0.1 µM ANP | 1.88 ± 0.86 |
| 1 µM ANP | 1.55 ± 0.76 |
| 10 µM ANP | 2.14 ± 0.76 |
Values are expressed as mean ± standard deviation (n = 11). ANP, atrial natriuretic peptide. There were no between-site differences (P > 0.16 for a main effect of treatment site).
Discussion
We evaluated for the first time whether intradermal administration of ANP modulates sweating and cutaneous vasodilator responses in young adults under resting conditions with room temperature. Our results show that intradermal infusion of ANP does not directly induce sweating and cutaneous vasodilatation. Further, it does not affect muscarinic sweating and cutaneous vasodilatation.
We demonstrate that ANP does not directly increase sweat secretion under room temperature resting conditions (Fig. 1). In line with this, a previous study showed that infusing ANP intravenously did not influence sweating measured under moderate-to-high heat stress conditions (i.e., exposure to ambient temperature ranging from 30–39°C) at rest.10 This early study also reported that intradermal injection of ANP augmented muscarinic sweating induced by methacholine;10 a response we were unable to replicate in the present study (Fig. 1). We showed that sweating elicited by methacholine was unaffected by co-infusion of ANP at all doses (Fig. 1). This is also true when we evaluated muscarinic sweating based on the EC50. Thus, our results suggest that ANP has no role in modulating muscarinic sweating, providing new information pertaining to the mechanisms underpinning sweat secretion in humans in vivo. However, given the very limited information provided in the report by Yamashida and colleagues (i.e., no information provided on participants or sweat rate data10), it is difficult to identify the underlying reasons for this disparate response (i.e., effect of ANP on muscarinic sweating). However, their study employed intradermal injection10 that accompanied the release of vasodilators such as substance P and calcitonin gene-related peptide; the latter of which can augment muscarinic sweating.32
As with sweating response, the current study shows that ANP does not directly mediate cutaneous vasodilatation (Fig. 2). This is consistent with previous work using intravenous infusion of ANP on cutaneous blood flow.19,20 These findings however, contrast the observation of Webb et al.16 who showed that arterial infusion of ANP greatly increased cutaneous blood flow. The underlying reasons for why arterial infusion of ANP was able to stimulate cutaneous vasodilatation but intradermal infusion of ANP was not remains unclear. Arterial infusion of ANP increases intravascular ANP availability and as a consequence would most likely activate endothelial cells. On the other hand, intradermal infusion of ANP increases its availability in interstitial fluid, which may primarily influence vascular smooth muscle cells, as ANP must diffuse further into the intravascular space to activate endothelial cells. Hence, while speculative, it is possible that ANP induces cutaneous vasodilatation mainly through endothelium-dependent mechanisms. Further study is warranted to assess this possibility. In addition, we also found that methacholine-induced cutaneous vasodilatation is not influenced by ANP irrespective of the dose employed (i.e., 0.1–10 µM) (Fig. 2). This remains true when assessing responses using EC50. Accordingly, our results show that intradermal infusion of ANP does not modulate muscarinic cutaneous vasodilatation. Our results provide important insights into whether ANP is physiologically important in the regulation of cutaneous blood flow.
ANP is a peptide that has a relatively greater molecular weight of 3,080 Da. While one may be led to the conclusion that our observation of a lack of an effect of ANP may be due to the fact that the large molecular weight of ANP is too large to pass through the pores of the microdialysis membrane, the molecular weight cut-off for the microdialysis probe in the presence of continuous perfusion of the membrane is ∼6,000–7,000 Da (see technical report by Bioanalytical Systems.33 As such, given that this value is still larger than the molecular weight of ANP, we believe that ANP was able to adequately pass through the membrane and reach the interstitial space. Moreover, as outlined in the technical report,33 insulin which has a molecular weight of ∼6,000 Da (nearly double of that for ANP) has been shown to pass through the membrane. Additionally, studies show that the use of intradermal microdialysis membranes with 6,000–7,000 Da cut-off for the administration of peptides that have similar or even greater molecular weight compared to ANP including endothelin-1 (2,491 Da), vasoactive intestinal peptide (3,325 Da), calcitonin-gene related peptide (3,789 Da), and pituitary adenylate cyclase activating peptide 38 (4,534 Da) have been shown to successfully modulate cutaneous blood flow.34-38
While as outlined above ANP likely crossed the microdialysis membrane, it is important to highlight the fact that ANP infused in the skin would be diluted by interstitial fluid, reducing the actual concentration of ANP targeting the sweat glands and skin vessels. Further, it is important to note that as molecular weight of the agent increases, the amount of agent crossing the membrane decreases thereby decreasing the available agent. While it may be surmised that using a larger cut-off membrane (thus a membrane with large pores) would increase the delivery of the agent, this could actually have a deleterious effect as the use of a larger pore size can easily reach a size that would allow water to cross the membrane, which would ultimately change interstitial fluid conditions. Hence using a larger pore is not necessarily the optimal or correct solution. To circumvent the potential dilution effects, we employed relatively high concentrations of ANP (0.1–10 µM). According to the study by Nose et al.5, ANP in the plasma can increase up to 100 pg mL−1. In the present study, the highest dose of ANP employed (10 µM) is equivalent to 60 µg mL−1 that is ∼600 times higher than the aforementioned values observed during exercise in the heat. Also, the use of intradermal microdialysis permits the continuous perfusion of ANP which ensures a constant infusion of ANP to the target end organs (sweat glands, cutaneous vasculature). Thus, we believe ANP in the skin was sufficiently elevated in the present study.
It has been shown that ANP increases during exercise in the heat,5 and the physiological significance of this elevated ANP remains largely unknown. As for this, our results do not support a role of ANP in sweating during heat stress. Further, while the present study refuted a role of ANP in muscarinic cutaneous vasodilatation, muscarinic mechanisms only partially mediate cutaneous vasodilatation during whole-body heating.11 A recent study reported that other co-transmitters such as vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide also appear to be involved in the control of cutaneous vasodilatation during heat stress.38 Therefore, we cannot exclude the possibility that the modulation of ANP on cutaneous vasodilatation elicited by the aforementioned co-transmitter(s).
In contrast to the observed increases in ANP during exercise in the heat,5 no increases in ANP has been observed during a passively-induced state of hyperthermia.39 Hence, exercise per se and not the associated exercise-induced increase in core temperature, and therefore level of hyperthermia, may be responsible for increases in ANP production. However, isolating these separate factors poses obvious challenges. One approach may be to clamp core temperature during exercise through the use of water immersion or a water perfusion suit. By clamping core temperature, and therefore controlling the level of hyperthermia, it would be possible to assess the separate influences of exercise and level of hyperthermia on ANP production and its subsequent influence on the regulation of heat loss responses. Further, ANP is known to be affected by changes in blood volume.39 Hence altered heat loss responses secondary to changes in hydration status40-42 may be in part explained by ANP. Further studies are required to evaluate the aforementioned possibilities.
In order to elucidate the role of endogenous ANP in the regulation of heat loss responses during exercise in the heat, the use of ANP receptor (guanylate cyclase A) antagonist would be a more robust approach as compared with the use of an agonist as employed in the current study. However, to the best of our knowledge, there is currently no ANP receptor antagonist available that can be safely used for in vivo human study.
In conclusion, we show that intradermal administration of ANP does not directly mediate sweating and cutaneous vasodilatation in young adults under room temperature conditions at rest. Further, we show that ANP administration does not affect sweating and cutaneous vasodilatation in response to muscarinic receptor activation.
This manuscript was submitted through the Accelerated Track.
Abbreviations
- ANP
atrial natriuretic peptide
- EC50
concentration required to elicit 50% of the maximal response
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all the volunteers for taking their time to participate in this study. We thank Sarah Y. Zhang and Mercy O. Danquah for their help in conducting this study.
Funding
This study was supported by the Natural Sciences and Engineering Research Council of Canada (Discovery grant, RGPIN-06313–2014 and Discovery Grants Program – Accelerator Supplement, RGPAS-462252–2014; funds held by Dr. Glen Kenny). G. Kenny is supported by a University of Ottawa Research Chair Award. N. Fujii is supported by the Human and Environmental Physiology Research Unit. The current affiliation of N. Fujii is the University of Tsukuba, Institute of Health and Sport Sciences, Tsukuba City, Japan. B. D. McNeely is supported by a Queen Elizabeth II Graduate Scholarship in Science and Technology.
References
- [1].Imada T, Takayanagi R, Inagami T. Changes in the content of atrial natriuretic factor with the progression of hypertension in spontaneously hypertensive rats. Biochem Biophys Res Commun. 1985;133(2):759-765. doi: 10.1016/0006-291X(85)90969-6. PMID:2935149 [DOI] [PubMed] [Google Scholar]
- [2].Chappell D, Bruegger D, Potzel J, Jacob M, Brettner F, Vogeser M, Conzen P, Becker BF, Rehm M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014;18(5):538. PMID:25497357. doi: 10.1186/s13054-014-0538-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nishikimi T, Kohno M, Matsuura T, Akioka K, Teragaki M, Yasuda M, Oku H, Takeuchi K, Takeda T. Effect of exercise on circulating atrial natriuretic polypeptide in valvular heart disease. Am J Cardiol. 1986;58(11):1119-1120. PMID:2946217. doi: 10.1016/0002-9149(86)90131-1 [DOI] [PubMed] [Google Scholar]
- [4].Mandroukas A, Metaxas TI, Heller J, Vamvakoudis E, Christoulas K, Riganas CS, Sendelides T, Stefanidis P, Kotoglou K, Karamouzis I, Mandroukas K. The effect of different exercise-testing protocols on atrial natriuretic peptide. Clin Physiol Funct Imaging. 2011;31(1):5-10. PMID:20831660. doi: 10.1111/j.1475-097X.2010.00971.x [DOI] [PubMed] [Google Scholar]
- [5].Nose H, Takamata A, Mack GW, Kawabata T, Oda Y, Hashimoto S, Hirose M, Chihara E, Morimoto T. Right atrial pressure and ANP release during prolonged exercise in a hot environment. J Appl Physiol. 1994;76(5):1882-1887. PMID:8063645 [DOI] [PubMed] [Google Scholar]
- [6].Follenius M, Candas V, Bothorel B, Brandenberger G. Effect of rehydration on atrial natriuretic peptide release during exercise in the heat. J Appl Physiol (1985). 1989;66(6):2516-2521. PMID:2545660 [DOI] [PubMed] [Google Scholar]
- [7].Kenny GP, Poirier MP, Metsios GS, Boulay P, Dervis S, Friesen BJ, Malcolm J, Sigal RJ, Seely AJ, Flouris AD. Hyperthermia and cardiovascular strain during an extreme heat exposure in young versus older adults. Temperature. 2017;4(1):79-88. PMID:28349096. doi: 10.1080/23328940.2016.1230171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tainio H, Vaalasti A, Rechardt L. The distribution of substance-P-like, Cgrp-like, galanin-like and Anp-like immunoreactive nerves in human sweat glands. Histochemical Journal. 1987;19(6–7):375-380. PMID:2444569. doi: 10.1007/BF01680455 [DOI] [PubMed] [Google Scholar]
- [9].Spreca A, Simonetti S, Rambotti MG. Atrial natriuretic peptide and guanylin-activated guanylate cyclase isoforms in human sweat glands. Histochem J. 2000;32(12):725-731. PMID:11254088. doi: 10.1023/A:1004149010623 [DOI] [PubMed] [Google Scholar]
- [10].Yamashida Y, Ogawa T, Sugenoya J, Ohnishi N, Natsume K, Imamura R. Effects of atrial natriuretic peptide on human sweating activity. J Auton Nerv Syst. 1990;31(3):252-253. doi: 10.1016/0165-1838(90)90205-W [DOI] [Google Scholar]
- [11].Kellogg DL Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995;77(6):1222-1228. PMID:7586235. doi: 10.1161/01.RES.77.6.1222 [DOI] [PubMed] [Google Scholar]
- [12].Machado-Moreira CA, McLennan PL, Lillioja S, van Dijk W, Caldwell JN, Taylor NAS. The cholinergic blockade of both thermally and non-thermally induced human eccrine sweating. Exp Physiol. 2012;97(8):930-942. PMID:22496503. doi: 10.1113/expphysiol.2012.065037 [DOI] [PubMed] [Google Scholar]
- [13].Shibasaki M, Crandall CG. Mechanisms and controllers of eccrine sweating in humans. Front Biosci (Schol Ed). 2010;2:685-696. PMID:20036977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schmitt M, Broadley AJ, Nightingale AK, Payne N, Gunaruwan P, Taylor J, Lee L, Cockcroft J, Struthers AD, Frenneaux MP. Atrial natriuretic peptide regulates regional vascular volume and venous tone in humans. Arterioscler Thromb Vasc Biol. 2003;23(10):1833-1838. PMID:12842844. doi: 10.1161/01.ATV.0000084826.86349.1D [DOI] [PubMed] [Google Scholar]
- [15].Doorenbos CJ, Blauw GJ, van Brummelen P. Arterial and venous effects of atrial natriuretic peptide in the human forearm. American J Hypertens. 1991;4(4 Pt 1):333-340. doi: 10.1093/ajh/4.4.333 [DOI] [PubMed] [Google Scholar]
- [16].Webb DJ, Benjamin N, Allen MJ, Brown J, O'Flynn M, Cockcroft JR. Vascular responses to local atrial natriuretic peptide infusion in man. Br J Clin Pharmacol. 1988;26(3):245-251. PMID:2972307. doi: 10.1111/j.1365-2125.1988.tb05273.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bidiville J, Waeber G, Porchet M, Nussberger J, Biollaz J, Gomez H, Callahan L, Waeber B, Brunner HR. Hemodynamic, renal, and endocrine effects of 4-h infusions of human atrial natriuretic peptide in normal volunteers. Fundam Clin Pharmacol. 1988;2(5):413-429. PMID:2976727. doi: 10.1111/j.1472-8206.1988.tb01007.x [DOI] [PubMed] [Google Scholar]
- [18].Jansen TL, Tan AC, Wollersheim H, Benraad TJ, Thien T. Age-dependent vasodilation of the skin microcirculation by atrial natriuretic factor. J Cardiovasc Pharmacol. 1991;18(4):622-630. PMID:1724541. doi: 10.1097/00005344-199110000-00020 [DOI] [PubMed] [Google Scholar]
- [19].Rolleke T, Berke B, Arndt JO. Atrial natriuretic peptide alters neither capillary filtration nor vascular compliance of both skin and skeletal muscle of humans. Basic Res Cardiol. 1994;89(2):192-205. PMID:8074642 [DOI] [PubMed] [Google Scholar]
- [20].Sharara AM, Higham MA, Spanevello A, Ind PW. Effects of intradermal injection of atrial natriuretic peptide. Br J Clin Pharmacol. 1995;40(3):283-285. PMID:8527294. doi: 10.1111/j.1365-2125.1995.tb05787.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wong BJ, Hollowed CG. Current concepts of active vasodilation in human skin. Temperature. 2017;4(1):41-59. PMID:28349094. doi: 10.1080/23328940.2016.1200203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee K, Mack GW. Role of nitric oxide in methacholine-induced sweating and vasodilation in human skin. J Appl Physiol. 2006;100(4):1355-1360. PMID:16239618. doi: 10.1152/japplphysiol.00122.2005 [DOI] [PubMed] [Google Scholar]
- [23].Patik JC, Christmas KM, Hurr C, Brothers RM. Impaired endothelium independent vasodilation in the cutaneous microvasculature of young obese adults. Microvasc Res. 2016;104:63-68. PMID:26631530. doi: 10.1016/j.mvr.2015.11.007 [DOI] [PubMed] [Google Scholar]
- [24].Gagnon D, Crandall CG, Kenny GP. Sex-differences in post-synaptic sweating and cutaneous vasodilation. J Appl Physiol. 2013;114(3):394-401. PMID:23154992. doi: 10.1152/japplphysiol.00877.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stapleton JM, Fujii N, McGinn R, McDonald K, Carter M, Kenny GP. Age-related differences in postsynaptic increases in sweating and skin blood flow postexercise. Physiol Rep. 2014;2(7):e12078. PMID:25347861. doi: 10.14814/phy2.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Larose J, Boulay P, Wright-Beatty HE, Sigal RJ, Hardcastle S, Kenny GP. Age-related differences in heat loss capacity occur under both dry and humid heat stress conditions. J Appl Physiol (1985). 2014;117(1):69-79. PMID:24812643. doi: 10.1152/japplphysiol.00123.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fujii N, Halili L, Singh MS, Meade RD, Kenny GP. Intradermal administration of ATP augments methacholine-induced cutaneous vasodilation but not sweating in young males and females. Am J Physiol Regul Integr Comp Physiol. 2015;309:R912-R919. PMID:26290105. doi: 10.1152/ajpregu.00261.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Inoue Y, Ichinose-Kuwahara T, Funaki C, Ueda H, Tochihara Y, Kondo N. Sex differences in acetylcholine-induced sweating responses due to physical training. J Physiol Anthropol. 2014;33(1):13. PMID:24887294. doi: 10.1186/1880-6805-33-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fujii N, McGinn R, Paull G, Stapleton JM, Meade RD, Kenny GP. Cyclooxygenase inhibition does not alter methacholine-induced sweating. J Appl Physiol (1985). 2014;117(9):1055-1062. PMID:25213633. doi: 10.1152/japplphysiol.00644.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Meade RD, Louie JC, Poirier MP, McGinn R, Fujii N, Kenny GP. Exploring the mechanisms underpinning sweating: The development of a specialized ventilated capsule for use with intradermal microdialysis. Physiol Rep. 2016;4(6):e12738. PMID:27033452. doi: 10.14814/phy2.12738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wenner MM, Wilson TE, Davis SL, Stachenfeld NS. Pharmacological curve fitting to analyze cutaneous adrenergic responses. J Appl Physiol. 2011;111(6):1703-1709. PMID:21868682. doi: 10.1152/japplphysiol.00780.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schlereth T, Dittmar JO, Seewald B, Birklein F. Peripheral amplification of sweating–a role for calcitonin gene-related peptide. J Physiol. 2006;576(Pt 3):823-832. PMID:16931551. doi: 10.1113/jphysiol.2006.116111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bioanalytical systems. Tech Notes: What is the molecular weight cut-off of BASi's microdialysis probes? Do you have probes with large cut-offs for sampling small proteins? https://www.basinc.com/assets/library/TechNotes/BASi_TN_1013.pdf [Google Scholar]
- [34].Halili L, Singh MS, Fujii N, Alexander LM, Kenny GP. Endothelin-1 modulates methacholine-induced cutaneous vasodilation but not sweating in young human skin. J Physiol. 2016;594(12):3439-3452. PMID:26846374. doi: 10.1113/JP271735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fujii N, Amano T, Halili L, Louie JC, Zhang SY, McNeely BD, Kenny GP. Intradermal administration of endothelin-1 attenuates endothelium-dependent and -independent cutaneous vasodilation via Rho kinase in young adults. Am J Physiol Regul Integr Comp Physiol. 2017;312(1):R23-R30. PMID:27881399. doi: 10.1152/ajpregu.00368.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wilkins BW, Wong BJ, Tublitz NJ, McCord GR, Minson CT. Vasoactive intestinal peptide fragment VIP10-28 and active vasodilation in human skin. J Appl Physiol. 2005;99(6):2294-2301. PMID:16109832. doi: 10.1152/japplphysiol.00500.2005 [DOI] [PubMed] [Google Scholar]
- [37].Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J Appl Physiol. 2004;97(4):1291-1298. PMID:15155712. doi: 10.1152/japplphysiol.00366.2004 [DOI] [PubMed] [Google Scholar]
- [38].Kellogg DL Jr, Zhao JL, Wu Y, Johnson JM. VIP/PACAP receptor mediation of cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 2010;109(1):95-100. PMID:20395540. doi: 10.1152/japplphysiol.01187.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vogelsang TW, Marving J, Crandall CG, Wilson C, Yoshiga CC, Secher NH, Hesse B, Kjaer A, Atrial natriuretic peptide and acute changes in central blood volume by hyperthermia in healthy humans. Open Neuroendocrinol J. 2012;5:1-4. PMID:28018493. doi: 10.2174/1876528901205010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fujii N, Honda Y, Hayashi K, Kondo N, Nishiyasu T. Effect of hypohydration on hyperthermic hyperpnea and cutaneous vasodilation during exercise in men. J Appl Physiol. 2008;105(5):1509-1518. PMID:18787094. doi: 10.1152/japplphysiol.01206.2007 [DOI] [PubMed] [Google Scholar]
- [41].Fortney SM, Nadel ER, Wenger CB, Bove JR. Effect of blood volume on sweating rate and body fluids in exercising humans. J Appl Physiol Respir Environ Exerc Physiol. 1981;51(6):1594-1600. PMID:7319888 [DOI] [PubMed] [Google Scholar]
- [42].Ikegawa S, Kamijo YI, Okazaki K, Masuki S, Okada Y, Nose H. Effects of hypohydration on thermoregulation during exercise before and after 5-day aerobic training in a warm environment in young men. J Appl Physiol. 2011;110(4):972-980. PMID:21310891. doi: 10.1152/japplphysiol.01193.2010 [DOI] [PubMed] [Google Scholar]


