ABSTRACT
Temperature is one of the main environmental factors that affect plant metabolism. Considering that plants are sessile, their survival depends on the efficient activation of resistance responses to thermal stress. In this comprehensive review, we discuss recent work on rapid biochemical and physiological adjustments, herein referred to as those occurring during the first few hours or a few days after the beginning of the change in the ambient temperature. The short-term metabolic modulation after plant exposure to heat and cold, including chilling and freezing, is discussed. Effects on photosynthesis, cell membranes, antioxidant system, production of heat shock proteins and nitric oxide, as well as an overview of signaling events to heat or cold stress are presented. In addition, we also discuss the acclimation process that occurs when the plant acquires resistance to an increase or decrease in temperature, adjusting its homeostasis and steady-state physiology to the new temperatures. Finally, we present studies with tropical plants that aim at elucidating the effects of temperature and the identification of the resilience levels of these plants to the expected climate changes, and which seek the development of techniques for germplasm conservation of endangered species.
KEYWORDS: abiotic stress, cold stress, C-repeat binding factor, heat stress, membrane lipids, oxidative stress, phytohormone, stress signaling, thermotolerance, in vitro conservation
Introduction
Temperature is the main factor that determines the geographic distribution of organisms, both in the context of latitudinal and altitudinal gradients of thermal niches occupation.1 The thermal range of the Biosphere is broad and varies from +400°C of the hydrothermal winds operating at the bottom of the oceans2 to -94,3°C of the surface air of the Dome Argus – Antarctica.3
No organism has the capacity to withstand the full range of Biosphere temperatures. Thus, life is limited to a much narrower temperature range, from −20 to +122°C.4,5 Specifically, the existence of plants is limited to an approximate thermal range of −10 to +60°C, defined by the freezing point of intracellular water and the temperature of protein denaturation.6–8 This survival range can be exemplified by the woody trees of regions such as Alaska, northern Canada, Europe and Asia, which are highly adapted to intracellular water freezing8; and by plants from arid regions such as cacti and agaves, which tolerate high diurnal temperatures that exceed 60°C.9
The importance of temperature as a physical factor on the distribution of organisms is a consequence of its direct influence on molecular (DNA, proteins) or supramolecular (membranes, chromosomes) structures, which results from merely a thermodynamic effect.10,11 These changes are usually fast; therefore, changes in the ambient temperature can be quickly detected by cell organelles, triggering specific pathways of biochemical and molecular responses in each of these cell compartments and making up an integrated cell response to temperature changes.10
Based on the ability to occupy thermal niches, organisms can be classified into: psychrophiles, which live and reproduce at temperatures below +15°C, some of which maintain metabolic activities at temperatures up to -20°C; mesophiles, which live comfortably between +15 and +40°C; and thermophiles, which have their best performance from +50 to +60°C (moderate thermophiles). The term hyperthermophiles (or extreme thermophiles) has been used for organisms with optimal growth rates above +80°C.12–15 A schematic representation of this classification is shown in Fig. 1. This classification is based mainly on studies of microorganisms that live in environments with extremely low and high temperatures (e.g. Antarctica and geothermal regions, respectively). Therefore, the thermal limits of each class may vary according to the temperatures that different groups of organisms can tolerate.
Figure 1.
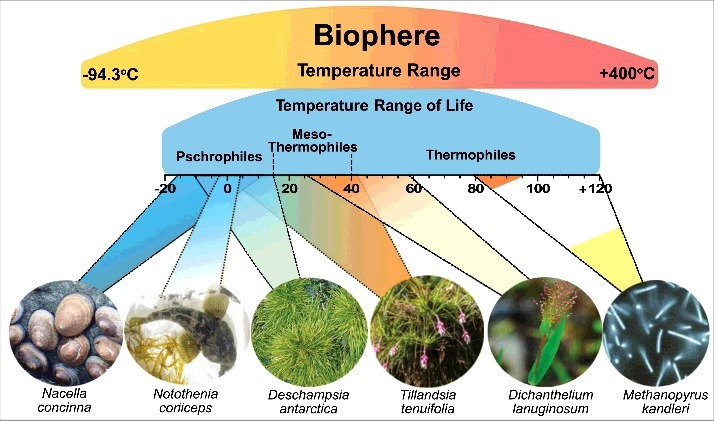
Schematic representation of the main thermal niches of the Biosphere. The gastropod Nacella concinna (Strebel, 1908), the fish Notothenia neglecta Nybelin and the vascular plant Deschampsia antarctica E. Desv. are adapted to the extreme low temperature conditions of Antarctica. The bromeliad Tillandsia tenuifolia L. is adapted to a thermal amplitude from 5 to 46°C. The grass Dichanthelium lanuginosum (Elliott) Gould grows and reproduces in thermophilic soils. The extreme thermophile microorganism Methanopyrus kandleri Kurr et al. 1992 is able to colonize deep ocean geothermal areas. Photo credits: N. concinna and N. neglecta: Edson Rodrigues; D. antarctica: Robert W. Hernandez; T. tenuifolia: Suzana Ehlin Martins; D. lanuginosum: Shoryl Pollack; M. kandleri: K.O. Stetter, R. Huber and R. Rachel.
A classification that focuses on the plant kingdom, alongside algae and fungi, was proposed by Levitt (1980)13 and more recently by Źróbek-Sokolnik (2012).7 These authors describe more restricted thermal limits for psychrophiles and mesophiles, and a lower minimum limit for thermophiles compared to the classification described previously. According to this, psychrophiles have their growth limited from 0 to 15/20°C. In this group, we find mainly algae and fungi and some species of plants from Polar Regions, such as the grass Deschampsia antarctica E. Desv. found in Antarctica, whose optimum for photosynthetic activity is 10°C (Fig. 1).16 Most higher plants are classified as mesophiles, whose optimal growth range is from 10 to 30°C.7,13 Many of the main agricultural species are included in this group.17 According to the classification of Levitt (1980)13 and Źróbek-Sokolnik (2012)7, thermophiles develop at temperatures above 30°C. Both authors report that the maximum survival limit for thermophilic higher plants is approximately 65°C, considering species from warmer regions. For example, the grass Dichanthelium lanuginosum (Elliott) Gould grows on heated soils adjacent to geysers and hot springs in the Yellowstone National Park (USA), where rhizosphere temperature ranges between 40–57°C (Fig. 1).18 However, many native plants of wide geographic distribution can tolerate broad thermal amplitudes, possibly resulting from adaptation to diverse environments. For example, the epiphytic bromeliad Tillandsia tenuifolia L., which is distributed from Cuba to south Argentina, is adapted to temperatures from 5 to 46°C (Fig. 1).19 The most extreme thermophiles of photosynthetic organisms are cyanobacteria, which can survive in hot springs under maximum temperatures of 73–74°C.20
Within the thermal niche of an organism, there is an optimum temperature range that allows its growth and physiological functioning to occur at the highest rate, which is delimited by a minimum and maximum temperature that completely interrupt biological processes.6 When temperature or other environmental factors exceed the optimal limit for a given species, changes in its metabolism and functioning occur, which are defined as stress.21 These changes can be reversible or even irreversible and may lead to the death of the organism, depending directly on the intensity and duration of the stressful conditions.13 Because plants are sessile organisms, they cannot move like animals to avoid environmental stress. Thus, they should use different adaptation mechanisms to withstand and survive under adverse conditions for optimal growth.22 Resistance mechanisms to environmental stress in plants are divided into two types: (i) avoidance, which includes strategies that prevent the external stress factor from triggering responses that modify plant functioning; and (ii) tolerance, developed through the activation or even modification of physiological mechanisms that allow the plant to either resist stress without the onset of injuries or repair the damage. Thus, the level of resistance of a plant to a given stress will depend on its ability to activate tolerance mechanisms and also on the presence of adaptations for avoidance, which is directly related to its habitat.21 Table 1 shows the variety of tolerance mechanisms, mainly biochemical, which are present in species from different climatic regions. Plant resistance mechanisms to stress are often activated by changes in gene expression. Therefore, environmental stressors are recognized first by the plant and then distributed through the cells by signaling pathways. Finally, these pathways activate changes in the level of gene expression that lead to adjustments in plant metabolism and development, aiming at reaching homeostasis under stressful conditions.23
Table 1.
Tolerance mechanisms in plants native from distinct areas of the globe in response to heat or cold stress conditions.
| Species | Family | Native location268 | Stress | Thermal treatment/conditions | Tolerance mechanisms | References |
|---|---|---|---|---|---|---|
| Arabis paniculata Franch. | Brassicaceae | Temperate/alpine – Tibet, China, Nepal and West Himalaya | Heat | Growth chamber: 35°C. Duration: 0–22 d. | Increased lipid saturation, HSP101 and HSP70 expression, and soluble sugar content. | Tang et al. (2016)68 |
| Dichanthelium lanuginosum var. sericeum (Schmoll) Spellenb. | Poaceae | Temperate/geothermally heated soil – North America | Heat | Growth chamber: 45/35°C (day/night) and 25/20°C (control). Duration: 4–8 w. | Increased number and thickness of leaf trichomes. Roughened cuticular waxes in leaves. Reduced leaf thickness. Higher content of silica in leaves. | Banowetz et al. (2008)269 |
| Cabbage (Brassica oleracea L. var. capitata L.) | Brassicaceae | Temperate – France, Great Britain, Spain | Heat | Growth chamber: exposure to 40, 50 or 60°C during 20 min every 24 h. Control – constant exposure to 25°C. Duration: 5 d. | Increased isothiocyanate and glucosinolates (secondary metabolites), ROS scavenging capacity, ascorbic acid, total phenolics content and antioxidant enzymes activities (SOD, GPX, CAT, POD) | Yang et al. (2016)110 |
| Rhazya stricta Decne. | Apocynaceae | Arid – South and Southwestern Asia. | Heat | Diurnal analysis at the native habitat: max. leaf temperature of 43°C at 2 PM. | Maintenance of net photosynthesis, carboxylation capacity of Rubisco, and leaf water content. Stomatal closure and reduced transpiration at mid-day. Increased photoprotective mechanisms (NPQ and photorespiration). | Lawson et al. (2014)270 |
| Native habitat: leaf temperature of 40–42.4°C between 1–2:30 PM. | Increased gene expression of HSPs, chaperones and aquaporins | Obaid et al. (2016)271 | ||||
| Cordeauxia edulis Hemsl. | Fabaceae | Arid and semi-arid – East Africa | Heat | Growth chamber: 32/23°C (day/night), 37/27°C, 42/31°C or 27/19°C (control). Duration: 7, 14 or 15 d. | Maintenance of net photosynthesis. Increased emission rate of isoprenoids and total phenolics content in leaves. | Egigu et al. (2014)103 |
| Eupatorium odoratum L. | Asteraceae | Tropical – Central and South America | Heat | Growth chamber: 25 (control), 30, 35, 38 and 42°C. Duration: 24 h in each temperature, subsequently. | Gradual increase in activities of antioxidant enzymes (SOD, CAT, POD, APX, GR, MDAR and DHAR). | Lu et al. (2008)272 |
| Sugarcane (Saccharum officinarum L.) | Poaceae | Tropical – New Guinea | Heat | Growth chamber: 40/35°C (day/night) and 28/23°C (control). Duration: 72 h. | Gradual accumulation of free proline, glycinebetaine and soluble sugars. Recovery of leaf water content and osmotic potential to pre-stress values after 24 h. | Wahid and Close (2007)117 |
| Cabbage (Brassica oleracea L. cv. Banchurisou) | Brassicaceae | Temperate – France, Great Britain, Spain | Cold | Growth chamber: 5°C. Duration: 10 d. | Increased SS and SPS activity and soluble sugars content. | Sasaki et al. (2001)187 |
| Growth chamber: after 3, 7 and 10 days at 5°C, leaf discs were transferred to – 4 or -6°C for 1 h. | Increased soluble sugars (sucrose, glucose and fructose) and starch content. | Sasaki et al. (1996)273 | ||||
| Peach (Prunus persica L. Batsch cv. Yulu) | Polyganeace | Temperate – China North-Central | Cold | Stored at 0 and 5°C. Duration: 28 d. | Maintenance of cell membrane stability, increased soluble sugars content (sucrose) and SPS transcripts. | Wang et al. (2013)274 |
| Tomato (Lycopersicon esculentum Mill. cv. Moneymaker) |
Solanaceae | Temperate/Subtropical/Tropical – North, South and Central America | Cold | Phytotron: 4°C. Duration: 24 h. | Increased NR activity, NR relative expression, NO, ABA and polyamines content. | Diao et al. (2017)275 |
| Cucumber (Cucumis sativus L.) | Cucurbitaceae | Subtropical/Tropical – Assam, Bangladesh, China, East Himalaya, Myanmar, Nepal, Thailand, West Himalaya | Cold | Growth chamber: 4°C. Duration: 48 h. | Increased antioxidant enzymes activities (POD, APX GR and SOD), soluble sugar, protein and chlorophyll content. | Liu et al. (2011)276 |
| Hevea brasiliensis (Willd. ex A.Juss.) Müll.Arg.s Muell. Arg. | Euphorbiaceae | Tropical – Bolivia, Brazil, Colombia, French Guiana, Peru, Venezuela | Cold | Growth chamber: 10 and 28°C (control). Duration: 4, 24, 96 or 192 h. | Increased antioxidant enzymes activities (APX, DHAR, GR and SOD) along with the induction of antioxidant gene expression. | Mai et al. (2009)157 |
| Vriesea inflata (Wawra) Wawra | Bromeliaceae | Tropical – Southeast and South Brazil | Cold | Growth chamber: 15 and 28°C (control). Duration: 24 m. | Increased cell number of aquiferous parenchyma and maintenance of chlorophyll content. | Pedroso et al. (2010)240 |
| Nidularium minutum Mez | Bromeliaceae | Tropical – Southeast Brazil | Cold | Growth chamber: 10, 15 and 25°C (control). Duration: 3 or 6 m. | Increased thickness of aquiferous parenchyma, reducing sugars and pectin content. | Carvalho et al. (2013)237 |
Abbreviations: ABA – abscisic acid, APX – ascorbate peroxidase, CAT – catalase, DHAR – dehydroascorbate reductase, d – days, GPX – glutathione peroxidase, GR – glutathione reductase, h – hours, HSP – heat shock protein, m – months, MDAR – monodehydroascorbate reductase, NO – nitric oxide, NPQ – non-photochemical quenching, NR – nitrate reductase, POD – guaiacol peroxidase, ROS – reactive oxygen species, SOD – superoxide dismutase, SPS – sucrose phosphate synthase, SS – sucrose synthase, w – weeks.
As previously mentioned, changes in ambient temperature can cause cold and heat effects on the plant, between the optimum growth range and the lethal thermal limit. When compared to other types of stress (e.g. water, nutritional), thermal stress causes symptoms in plants quickly and in the short term, i.e. in a few minutes to a few hours after exposure.13,21 Thus, plants should display a rapid defense response to variations in ambient temperature, which can occur frequently during the day.10 According to Levitt (1980),13 the stress due to temperature changes is resisted by plants through tolerance and not avoidance because plants are not able to keep their cells at the optimum constant temperature as the homeotherms, thus, the cold/heat might lead to an unavoidable fall/rise in plant tissue temperature.6,13 Nevertheless, certain morphological adaptations of some plant species such as bromeliads, e.g. presence of epidermal structures such as hairs and cuticles, and the vertical positioning of leaves in relation to sunlight, may function as strategies that prevent the heating of tissues caused by the incidence of sunlight.24 These characteristics are found mainly in species from warm and arid regions.13,25 However, most of the resistance processes described in plants are referred to the physiological and biochemical mechanisms that allow them to tolerate the effects of temperature changes.
In this review, we will describe the resistance responses to thermal stress classified as tolerance, especially the rapid responses that occur soon after the heat and/or cold exposure,13 herein referred to as those occurring during the first few hours up to a few days after the beginning of the change in the ambient temperature.
Plants can be tolerant or sensitive to cold or heat, depending on the thermal niche where they are found and the adaptations of each species. Through the evolutionary process, species that have emerged from colder regions are naturally sensitive to heat, and others from warmer regions are tolerant to heat and sensitive to cold.7 Recently, for example, O'Sullivan et al. (2016)26 studied the maximum temperatures for photosynthesis and respiration of trees from seven world´s biomes. These authors observed that species in the Arctic region of Alaska had a thermal upper limit of nine degrees Celsius lower than species in the rainforests of Peru. However, the main agricultural species are considered sensitive to both hot and cold temperature extremes because they have a very narrow optimal thermal limit, particularly during the reproductive period.17 This fact is possibly due to the process of domestication and the consequent low genetic variability of selected strains with higher productivity for commercial cultivation.27,28
The acquisition of thermotolerance occurs when a plant is exposed previously to low or high temperatures for a short period of time, until a limit in which no fatal injuries occur (a process often called “hardening” or “priming”). Thereafter, the plant “is able” to withstand the increase or reduction in temperature that was previously considered harmful.13,29–31 In this case, the homeostasis and steady-state physiology of the plant is adjusted, enabling it to withstand the new temperature conditions.23 This process is called acclimation. For example, trees from temperate regions become hardened to severe winter during the fall season, when low temperatures are milder, through the gradual activation of various resistance mechanisms that ultimately allow survival to freezing during the winter.21 The acclimation capacity has also been reported for species in hot regions, such as the succulent Agave deserti Engelm., which is able to tolerate temperatures between 63–67°C for one hour when previously exposed to 50/40°C (day/night), and it is also able to tolerate exposure to -10°C for one hour when treated earlier at 10/0°C (day/night).9 These and other biochemical processes of thermal tolerance acquisition will be described in further detail throughout this review.
The impact that changes in temperature could cause to terrestrial life has been addressed in several studies.32–38 In this context, understanding plant responses to thermal changes can contribute to unveil the resilience capacity of an ecosystem. It is important to note that plants are suppliers of the primary energy source to heterotrophic organisms by converting the solar energy into organic matter through photosynthesis.27 In addition, forests, for example, are key for global ecological and climatic balance, especially in the case of tropical forests such as the Amazon, which reduce the atmospheric temperature due to high rates of evapotranspiration and carbon sequestration. Thus, deforestation of tropical forests, for example, would pose a great threat to the maintenance of terrestrial temperature.39,40 Regarding agricultural species, not only the climatic factor but also the global population increase have motivated scientists to concentrate their efforts on the development of techniques for optimizing cultivation under stressful conditions and in marginal lands, in order to supply the growing demand for food, fiber, wood, paper, and ornamental plants.27,41,42 The same concept applies to the study of strategies for the conservation of native plant species threatened by climate change, which can be potential sources of various products and substances not yet explored.43,44
All of the aspects mentioned previously on the importance of plants reinforce the urgency and need for research on the effects of thermal changes on plants, which in turn intensify the effects of other abiotic stresses globally.41 Therefore, reporting the advances in the knowledge of the plant physiological responses to the cold and the heat can help understanding the effects of climate change. These effects include the more rapid establishment of intense hot periods that can be long and often associated with water scarcity, as well as the occurrence of sudden and more extreme cold periods than registered in the last century.45–47
Thus, in this review we aim to present the physiological and biochemical aspects involved in plant tolerance to cold and heat by summarizing recent studies focusing on responses that occur in the first few hours up to a few days of thermal adjustment – a crucial period that defines the survival or not of plants to stress –, whose greater understanding is relevant to predict plant responses to the steep temperature variations of the future climatic scenario. Finally, we will also present a perspective on the physiological response to temperature changes of native species from tropical regions that harbor biomes with great plant biodiversity.48
Limit of heat tolerance in plants
When the limits of tolerance and adaptation to heat are exceeded in a plant, several changes occur at the molecular, physiological and cellular levels, which can have negative effects on growth and development that may trigger plant death.21,49,50
Nevertheless, the effects that the increase in temperature can cause to plants depend on several factors like the temperature level; the period of exposure to high temperature; if the temperature rise occurs gradually or acutely (i.e., “heat shock”); whether the plant had a pre-acclimation process at non-lethal high temperatures prior to the exposure to extreme heat; whether the heat increase occurs associated with other stresses, such as the excess of luminosity and water scarcity (frequently observed in drier regions of tropical, subtropical and temperate zones); and also the tolerance level of the plant that is subjected to high temperature.6,8,25,30,49 This set of factors may have a direct influence on the metabolic response of the species and its ability to acclimatize to heat stress.
Injury and cell death caused by exposure to severe heat stress may occur within a few minutes due to rapid protein denaturation. However, if a plant is exposed to a moderate temperature increase (mild/moderate heat stress), injury or cell death will only be observed after a longer period of exposure (e.g. hours or days) due to the disruption of metabolic processes.7
Naturally, the time at which the onset of symptoms of heat stress occurs varies according to the plant species and its natural environment. This can be verified comparing the response of desert plants with the response of plants from temperate regions. For example, herbaceous plants from shaded locations of temperate regions show leaf injuries when exposed to a minimum of 30 minutes to temperatures of 40–45°C.21 Meanwhile, desert plants such as cacti and agaves require temperatures above 60°C, or even 65°C, for injuries begin to arise, which can take from 30 minutes to one hour.9,21 The high tolerance of cacti and agaves to high temperatures comes from several morphological adaptations and some highly specialized biochemical mechanisms, such as the photosynthetic pathway of the crassulacean acid metabolism (CAM). In this pathway, the opening of the stomata and uptake of CO2 from the atmosphere occur only at night, avoiding excessive water losses during the day due to high temperatures, and allowing high efficiency of water use in hot environments.9 In general, the CO2 absorbed overnight is fixated in oxaloacetate through the reaction catalyzed by phosphoenolpyruvate carboxylase. The oxaloacetate is then converted mainly into malate by malate dehydrogenase, and stored in the cell vacuoles.51,52 However, CO2 can also be fixated and stored in the form of other organic acids such as citrate, isocitrate, fumarate and succinate.53,54 During the daytime, when the stomata are closed, the organic acids stored in the vacuole are released into the cytosol and decarboxylated, releasing CO2 that is converted into carbohydrates by the Calvin-Benson (C3) cycle.51,52 In this review, this type of photosynthetic metabolism will be addressed on some occasions because it is activated in response to several stresses associated or not with thermal changes.55
In general, the morphological damage observed in vascular plants in response to heat stress include leaf and branch burn, foliar senescence and abscission, inhibition of shoot and root growth, discoloration and fruit damage.56 These symptoms are often observed in species of economic importance, causing decreases in productivity.13,56 Considering native species in their natural environment, morphological changes due to temperature increases can negatively affect forest ecosystems. In a study performed in a region of temperate deciduous forest of Canada, Filewod and Thomas (2014)57 observed that a three-day heat wave (31-33°C) during the spring caused a 25% reduction in the number of leaves of the dominant tree (Acer saccharum Marshall), and induced the appearance of new leaves. Consequently, there was a decrease in 64% of leaf area of the forest during this period, which lead the authors to conclude that even short periods of high temperature have severe impacts on temperate forests, especially during the spring period of leaf expansion.
Among the physiological processes that are affected by heat, photosynthesis is the first metabolic mechanism influenced by thermal variation, showing a decrease in rates when there is an increase in temperature above the optimum for a given species.58,59 This decrease in photosynthetic activity is perceived as a decrease in CO2 fixation and in its conversion to carbohydrates through the C3 cycle, primarily due to the decrease in the activity of the main enzyme of this cycle, the Rubisco (Ribulose bisphosphate carboxylase oxygenase).60 In some cases, stomata can be closed, preventing CO2 entry and further reducing the rate of photosynthesis.50,58 The general decrease in reactions of the C3 cycle in response to heat comes from the decrease in the photochemical stage of photosynthesis, that is, the reactions that use light energy to provide adenosine triphosphate and NADPH (nicotinamide adenine dinucleotide phosphate) used during the C3 cycle. This is due to the high sensitivity of the photosystem II (PSII) to temperature increase.21,58 The PSII is formed by a complex of integral proteins, and is crucial for the electron transport that occurs during the photochemical stage of photosynthesis.21,61 PSII damage can be observed after a few minutes to a few hours of exposure to heat. For example, Havaux (1993)62 observed an irreversible decrease in the photochemical efficiency of PSII after 90 minutes of exposure of potato plants (Solanum tuberosum L.) to 39.5°C, while Camejo et al. (2005)63 found that a variety of heat-sensitive tomato (Lycopersicon esculentum Mill. cv. Campbell-28) exposed to 45°C for two hours suffered significant reductions in both Rubisco activity and PSII performance. The inhibition of PSII may derive from the damage to the thylakoids caused by heat stress, which are highly responsive to increases in temperature.58 In winter wheat (Triticum aestivum L.) plants, damage to thylakoid membranes has also been associated with a reduction in chlorophyll content in response to heat stress after eight-16 days of exposure to 36/30°C (day/night).64 This pigment is located in the antenna complexes of the photosystems, and captures the light energy used for the synthesis of adenosine triphosphate and NADPH.8 However, heat resistance mechanisms can be activated when the exposure to high temperature does not exceed the tolerance limit of a plant, resulting in the recovery of the PSII efficiency and consequent maintenance of the photosynthetic process. Indeed, Mathur and Jajoo (2014)65 observed that wheat plants (T. aestivum cv. Lok-1) exposed to a hot summer day reduced the efficiency of PSII during the period of higher temperature (from 11 AM to 3 PM, at temperatures of 40–41°C), but recovered this efficiency at the beginning of the night (7 PM, 30°C).
As previously mentioned for thylakoids, cell membranes are generally one of the first structures that are affected by the increase in temperature.7,10,56,66 Heat accelerates the movement of lipids and leads to denaturation of the protein components of cell membranes. These effects cause an increase in membrane fluidity, allowing ions to leak and making these structures more prone to rupture, which ultimately leads to the inhibition of various cellular (e.g. transport of ions and metabolites) and physiological (e.g. photosynthesis) processes.7,8,10,56,67 In plants of Arabidopsis thaliana (L.) Heynh. exposed to 35°C for 22 days, it was observed a reduction in the membrane lipid content, and an increase in lipid degradation during the late period of stress exposure (18-22 days).68 Likewise, wheat plants (T. aestivum) exposed to high temperatures (35/24°C, day/night) for 12 days presented damage to thylakoid membranes due to reductions in lipid components.69 These authors also found a relationship between lipid composition and oxidative stress, since a higher content of malondialdehyde (MDA; a reaction product of the lipid oxidation by reactive oxygen species or ROS) and a greater formation of “ox-lipids” (i.e. lipids containing oxidized fatty acids) by the endoplasmic reticulum were observed after heat exposure.69 In fact, heat may lead to an increase in ROS formation mainly in the chloroplasts, mitochondria, and peroxisomes.70–72 These ROS are more reactive forms of oxygen in their basal state (O2), such as singlet oxygen (1O2), superoxide radical (•O2−), hydroxyl radical (HO•) and hydrogen peroxide (H2O2), which are formed naturally during highly energetic processes such as photosynthesis and respiration.70 ROS can cause oxidative damage to molecules such as nucleic acids, proteins, and lipids, which ultimately affect metabolic activities and the integrity of organelles.73,74 Among the main damages caused by ROS is lipid peroxidation (LPO), a complex process of oxidation of polyunsaturated fatty acids that occurs both in animals and plants, and leads to the formation and propagation of lipid radicals, the rearrangement of double bonds of unsaturated lipids and the potential destruction of membrane lipids.75,76 Under favorable environmental conditions for plant metabolism and growth, the excess of these oxidative damages is avoided because ROS production is counterbalanced by antioxidant mechanisms that eliminate these compounds. In general, the plant antioxidant system includes non-enzymatic components such as ascorbate, glutathione, tocopherols (vitamin E) and carotenoids; and a complex enzyme system containing, for example, superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol peroxidase, glutathione reductase (GR) and catalase (CAT).70,73,77,78 SOD is the first enzyme to perform ROS detoxification, converting the superoxide radical into H2O2, which is converted into H2O by CAT, APX and guaiacol peroxidase enzymes.79 The APX also acts in the ascorbate-glutathione cycle along with GR and several other enzyme components to regenerate ascorbate and reduced glutathione.80
In the case of exposure to environmental stresses such as heat stress, the balance between ROS production and elimination is rapidly disturbed, leading to an increase in ROS content.70 According to heat intensity, ROS production can be continuously increased until detoxification by plant antioxidants is no longer possible and leads to irreversible oxidative damage (known as oxidative stress), which can cause cell death and affect plant survival.73 Thus, the tolerance of a plant species to heat stress is directly related to its ability to control ROS production and redox homeostasis.31 For example, Zou et al. (2017)81 found that a heat-tolerant cultivar of the Chinese cabbage (Brassica campestris L.) presented less damage to the photosynthetic apparatus and less accumulation of ROS when exposed to 40/30°C (day/night) for five days compared to the heat-sensitive cultivar. The authors attributed these responses to increased activities of the antioxidant enzymes CAT, SOD and guaiacol peroxidase. In a study with two blueberry cultivars (Vaccinium spp.), it was observed that the Jersey cultivar had higher heat tolerance than the Diana cultivar, which was associated with lower oxidative content (H2O2 and superoxide radical content) and higher transcriptional levels for antioxidant enzymes and oxidative protein-repairing genes in the tolerant cultivar after exposure at 48°C for 18 hours.82
Heat exposure may also lead to increased production of reactive nitrogen species (RNS), which include the gaseous nitric oxide radical (NO) and several other compounds that result from their reaction with different molecules, such as peroxynitrite (ONOO−), peroxynitrous acid (HONO2), and the nitrogen dioxide radical (•NO2).83,84 Similarly to ROS, when stress exposure leads to deregulated synthesis or exacerbated production of RNS, these molecules can have toxic effects on cells because they can react with various cellular components such as thiols, enzyme cofactors, proteins, nucleotides, and lipids, which finally result in the so-called nitrosative stress.83,85 Specifically for lipids, RNS can cause LPO like ROS, and thus affect the membrane structure through nitration reactions caused mainly by NO derivatives such as •NO2 and ONOO−.86,87 NO and related molecules may also perform post-translational modifications through various mechanisms such as S-nitrosylation, nitration and binding to metal centers, targeting tyrosine residues of proteins, thiols, DNA and lipids88,89, affecting metabolism and gene expression.90 Studies with sunflower plants (Helianthus annuus L.) have shown that heat stress (exposure to 30°C for one hour, followed by 35°C for one hour and 38°C for four hours) causes an increase in the content of S-nitrosylation and nitration reaction products caused by RNS.91,92 In addition, the authors found that enzymes with tyrosine residues such as carbonic anhydrase and ferredoxin-NADP reductase, which are important components of the photosynthetic process, had significantly reduced activities when affected by nitration. In citrus plants (Citrus aurantium L.), it was observed that the exposure at 42°C for five hours caused a significant increase in the NO content associated with visible leaf damage and increased rate of electrolyte leakage, suggesting damage to membranes.93
However, it is important to note that, despite the deleterious effect of ROS and RNS accumulation over a long-term period of heat exposure, the rapid increase in the content of these molecules during the initial period of stress (known as oxidative and nitrosative burst for ROS and RNS, respectively) can act as part of the signaling pathways for activating acclimation mechanisms in species tolerant to high temperatures, in order to allow their survival under such conditions.94,95
Resisting heat – rapid physiological and biochemical mechanisms of tolerance to high temperature
Plant species considered to be tolerant to high temperatures have acclimation mechanisms (Table 1) that can be activated by transcriptome, proteome and metabolome adjustments in the plant cell when exposed to heat.66 One of the first acclimation responses to heat is the change in the composition and integrity of the cell membranes.7,10,21,49
Resistant species exposed to heat show an increase in the proportion of saturated fatty acids, which allow the increase of their thermal melting level, avoiding the fluidization of the membranes under conditions of high temperatures and thus increasing their resistance to heat.7 In a study carried out with cool-season turf grasses (two varieties of the hybrid Poa arachnifera Torr. x Poa pratensis L., and Festuca arundinacea Schreb.) from the US central region with different levels of heat stress tolerance, it was observed that the exposure to temperatures of 35/25°C (14/10 hours light/dark) for 36 days followed by 27 days at 40/30°C (14/10 hours light/dark) led to an increase in the lipid saturation rate in the more tolerant grasses, showing that lipid saturation is associated with the difference in tolerance between the three grasses.96 It has also been reported that changes in the lipid composition aiming at increasing the heat resistance of the membranes may occur during a short exposure to high temperatures. Higashi et al. (2015)97 assessed the changes in the lipidome of A. thaliana exposed to high temperatures (30, 34 and 38°C) for one day. These authors found that heat stress induced a reduction in the amount of polyunsaturated fatty acids chloroplast membrane, that is, the membrane of this organelle became more resistant to temperature increase by enhancing the proportion of saturated lipids. Similarly, Tang et al. (2016)68 found that Arabis paniculata Franch. increased the membrane lipid saturation after two days of exposure to 35°C. This pattern was maintained throughout the long-term exposure to stress (22 days), suggesting that the thermotolerance of A. paniculata resulted from the maintenance of the membrane lipid composition so it does not fluidize during exposure to heat.68
A highly consolidated mechanism of heat acclimation and an example of rapid response in plants is the production of heat shock proteins (HSPs).98 HSPs are highly preserved proteins among eukaryotes and prokaryotes, which are key for thermotolerance acquisition.98 When a plant is exposed abruptly to a temperature rise of 5°C or more above its optimum growth temperature, a reduction in the synthesis of regular proteins may be coupled with an increase in the production of HSPs.23 Induction of HSPs expression may occur a few seconds after the temperature increase, and the maximum level of transcripts may be observed within one to two hours of exposure, when it begins to decline.30 HSPs can be classified as HSP100, HSP90, HSP70, HSP60, and small HSPs (sHSPs) according to their molecular mass, and act as molecular chaperones in the quality control of other proteins.99 The specific functions of each HSP are still quite obscure, but it is known that these chaperones assist in various processes involved in the maintenance of heat responses.23 A recent study demonstrated that the acquired thermotolerance of the wild genotype of A. thaliana occurred when the exposure to temperature increase was gradual, i.e. they were first exposed to 37°C for 90 minutes, followed by recovery at 22°C for 90 minutes, and then at the higher temperature of 44°C for 45 minutes.100 At the end of this process, HSP21 expression in the plants was higher, allowing recovery and greater tolerance to the new exposure to 44°C for 90 minutes compared to plants that were not subjected to a gradual increase in temperature. These results clearly show the importance of HSP for the acquisition of heat tolerance in A. thaliana.100
Secondary metabolites are essential compounds for plant survival to environmental changes, including to heating.101 Some studies showed accumulation of these substances, mainly phenolic and terpenoid compounds, when tolerant plants are exposed to high temperatures.102–105 Heating tolerance was also related to an increase in steroids (e.g. brassinosteroids).56,106 In a screening study for tomato (Solanum lycopersicum L.) genotypes tolerant to heat, Zhou et al. (2015)105 observed that the most tolerant genotype had higher accumulation of total phenolic compounds and carotenoids when exposed to temperatures of 36/28°C for seven days compared to the most sensitive genotype. Some studies also reported the importance of secondary metabolites in plant response to short periods of exposure to high temperatures.107–110 In a study on the emission of isoprenes from the canopy of oak trees,107 the authors exposed the canopy of the plants to hot and cold light alternately every 20 seconds in order to induce a change in leaf temperature for approximately 20 minutes. The alternation of foliar temperature (30.5–35.7°C) was accompanied by a similar fluctuation in isoprene emission, where the increase and decrease in temperature were followed by higher and lower isoprene content, respectively. The authors concluded that the emission of isoprenes is rapidly activated as a tolerance response to the short periods of high temperature in the leaves of this tree.107 The increase in content of total phenolic compounds was also observed in cabbage plants (B. oleracea L.) exposed for 20 minutes to 40, 50 or 60°C every 24 hours for five days when compared to plants that were not subjected to heat.110 In addition, phenolic content was higher in plants exposed to 60°C compared to other thermal conditions, evidencing the involvement of these molecules in the initial periods of acclimation to intense heat.
The accumulation of phenolics and isoprenoids in response to heating may aid in heat tolerance because they have an antioxidant action and protect the photosynthetic apparatus.101,106,111 This effect was clearly observed for anthocyanins in mutants of A. thaliana deficient in the synthesis of these phenolics exposed to high temperatures (35-45°C) for 30 minutes.109 In this study, the activity of antioxidant enzymes and the scavenging ability of the 1.1-diphenyl-2-picrylhy.drazyl radical were lower than in the wild variety, and the content of H2O2 and membrane leakage were higher in the mutants than in wild plants, which maintained the anthocyanin synthesis. Finally, the brassinosteroids, recently defined as phytohormones that are involved in plant growth and development, are also related to tolerance to stresses such as heat.101,112 For instance, studies show that the exogenous application of brassinosteroids in plants prior to high-temperature exposure induce thermotolerance mechanisms such as increased HSP expression and antioxidant capacity during heat stress (for reviews see.113,114).
The raise in temperature often leads to a reduction in the water content of plant tissues. In this case, the increased synthesis of substances such as compatible osmolytes may help enhance the osmotic potential of cells, allowing the recapture of soil water and maintenance of the plant's water potential.22,115 Compatible osmolytes are substances that have no adverse effects on plant metabolism even at high concentrations and include soluble sugars, polyols, proline and glycine betaine.56,116 Wahid and Close (2007)117 observed that exposing sugarcane (Saccharum officinarum L.) plants to temperatures of 40/35°C (day/night) led to a decrease in water content during the first 12 hours, which was recovered after 24 hours. These authors observed that water state recovery after 24 hours in sugarcane was associated with the accumulation of proline, glycine betaine and soluble sugars, which indicated that the osmotic adjustment occurs rapidly through the increase in osmolyte content after exposure to heat stress. Similarly, Ramani et al. (2017)118 found that exposing a heat-tolerant variety of wheat plant to 45°C for four hours resulted in a smaller decrease in leaf water content than in the heat-sensitive variety, which was associated with higher proline and glycine betaine content in the heat-tolerant variety. In addition, it has been reported that compatible osmolytes also act as buffers of redox potential and prevent oxidative damage.119 Such effects could be elucidated especially in studies with exogenous application of osmolytes before heat exposure (for a review, see50). For instance, Rasheed et al. (2011)120 observed that the immersion of sugarcane nodal buds in solutions of 20 mM proline and glycine betaine for eight hours before treatment at high temperature (42°C for up to 48 hours) led to a reduction in ROS production concomitantly to an increase in the concentrations of compatible osmolytes.
Plants have a complex signaling system that trigger the responses to high temperature throughout the organism, thus reaching acclimation to the heat stress (Fig. 2). It is already widely supported that high-temperature signaling pathways are activated by the increase in fluidity of the plasma membrane.66 The alteration of the membrane fluidity activates the channels that mediate the entrance of calcium ions (Ca2+) into the cell. Ca2+ triggers several downstream events that lead to the acquisition of thermotolerance, such as the activation of heat shock transcription factors that induce changes in the expression of several heat-responsive genes.66,99 Saidi et al. (2010)121 demonstrated that the increase of Ca2+ in the cell cytoplasm begins in less than five minutes after transferring mosses from 22 to 35°C, and reaches a maximum at 15 minutes, which in turn triggers higher levels of HSP genes expression. In this work, these authors also verified that a previous exposure to 32°C led to lower Ca2+ influx and HSP induction after a new exposure at 35°C. The authors associated this result with the higher rigidity of the membranes caused by the higher saturation level of the lipids induced during the initial acclimation period, supporting the hypothesis of the importance of the membrane in the signaling of heat responses.
Figure 2.
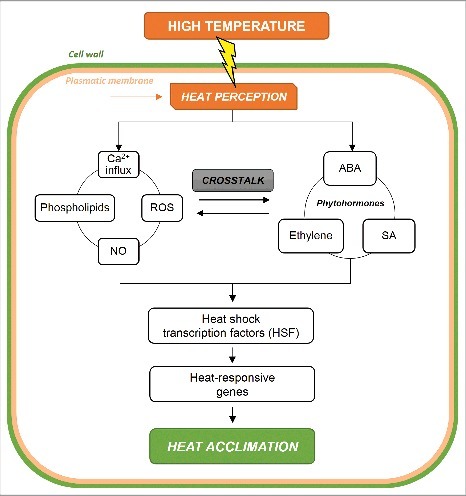
Schematic representation of the high-temperature perception by the plant cell and components of the signaling pathway for the activation of acclimation mechanisms to heat stress. ABA: abscisic acid, Ca2+: calcium, NO: nitric oxide, ROS: reactive oxygen species, SA: salicylic acid.
Other important signaling molecules include NO and ROS, which, according to some studies, may be integrated with Ca2+ in the signaling transduction network that causes the activation of transcription factors and heat acclimation genes (Fig. 2).71,122,123 Regarding ROS, the exposure to abiotic stresses such as heat leads to the induction of two subsequent peaks of ROS synthesis in the apoplast through the respiratory burst oxidase homolog D protein during the first minutes of exposure. These peaks are said to be propagated to other plant tissues, carrying a systemic signal over long distances.71 NO synthesis is also induced a short time after heat exposure. When exposing A. thaliana plants to 45°C, the NO synthesis began to increase after 10 minutes, reaching a maximum between 60-70 minutes of stress, which seems to be related to the heat shock protein transcript 18.2 (HSP18.2).124 Another type of molecule involved in stress signaling are phospholipids, such as phosphatidyl inositol 4.5-bisphosphosphate and phosphatidic acid (PA).125 It was observed that the synthesis of these signaling lipids is induced in less than five minutes after the transfer of suspension-cultured tobacco (Nicotiana tabacum L.) BY-2 cells to 40°C.126
Heat responses can also be activated by phytohormone-dependent signaling pathways, such as abscisic acid (ABA), salicylic acid and ethylene (for a review, see114,127,128). Suzuki et al. (2013)72 using different mutants of A. thaliana showed that exposure to high temperature (37°C) increases ROS synthesis by respiratory burst oxidase homolog D after five minutes, which is required for a peak in ABA synthesis at 10 minutes, demonstrating a crosstalk relationship between the reactive species and hormones in heat response signaling. For mustard plants (Sinapis alba L.), exposure to 45°C led to a 400% increase in total salicylic acid levels after one hour, which was associated with increased antioxidant activity during the next five hours of treatment.129 Ethylene activates heat-specific transcription factors that lead to the expression of genes that increase plant tolerance, as revised in.130 There is still much to explore in terms of heat hormone signaling, especially considering that there is a complex crosstalk system between hormones and other signaling molecules such as ROS, NO and Ca2+ that make up the stress response (Fig. 2),131,132 and that the signal transduction pattern may be highly specific to the plant organ and type of thermotolerance.114
The knowledge of heat effects on plant physiology is of great importance if we consider that we are living under a progressive process of global warming, which began in the 19th century45 and may directly affect productivity and ecosystems. The global temperature showed the greatest increase in the last 30 years, and 2015 was the year in which the average global temperature reached for the first time an increase of 1°C compared to the end of the 19th century.133 Thus, temperature may be considered one of the major climatic factors influencing plant physiology in the subsequent years. In fact, if anthropogenic gas emissions are not efficiently controlled, the thermal increase over the 21st century may be greater than 2°C by 2100.45 In addition, there is strong evidence showing that periods of extreme heat will be more frequent and longer while the average global temperature continues to increase.45,134 Consequently, with a warmer Earth, the drought rate in dry climate regions may be exacerbated because the higher temperature increases plant evapotranspiration and water evaporation from the soil.135 Therefore, the increase in global temperature will most likely be associated with more intense and longer drought periods,46 exposing plants to thermal and water stress simultaneously, requiring a great plasticity from plants to tolerate such conditions and survive.
It is predicted that a rise in temperature above 1°C for the next 100 years may directly affect the biodiversity on Earth.32,38,45,136,137 Some studies have already reported changes in the geographical distribution of plant species, which are moving towards the poles and places of higher altitude due to the temperature increase in the environments where these plants originally inhabited.138–140 In addition, the mortality of trees worldwide due to heat stress and the decrease in water availability resulting from global warming is becoming more frequent.141 Many cultivated species may also reduce their productivity due to global temperature increase, including wheat, corn, rice, and soybean, which may threaten global food supply.37,142,143 Thus, it is increasingly relevant to evaluate and study plant responses to temperature increase, which may help to preserve native species potentially under threat of extinction due to climate change, as well as the development of cultivated species with increased tolerance to heat stress.
Resistance and injury by cold
Despite the predicted global warming scenario for the next decades, models of climate simulation reveal that cold events will persist in the 21st century and may be more intense than in the 20th century.47 These events occur in both tropical and temperate regions and usually have a short duration, from 24 hours to a few days.47,144–146 Depending on the intensity, the temperature of these events may be low but above 0°C that is called chilling, or if it is below 0°C is defined as freezing, in which ice formation may occur within the tissues.147,148 In this section, we present the responses of plants to both chilling and freezing stresses.
Exposure of plants to low temperatures is one of the major environmental factors that influence their growth and development.149 In fact, approximately 57% of the world's land areas and 26% of rural areas are affected by cold stress.150 Plants that are sensitive to cooling can suffer damage at temperatures close to 15°C, while tolerant plants survive at temperatures just below 5°C.147,151 Among the plants susceptible to cooling are most of the tropical plants, whereas among the tolerant plants there are species of subtropical and temperate plants, as well as mosses, lichens, and trees of arctic areas.152
The impact of cooling on plants depends on the chilling rate, exposure time, and other associated stresses.153 Depending on the tolerance of the species, the damage caused by low temperatures (chilling injury) may occur in a few hours or days,21 determining the degree of sensitivity of a given species. When there is a sudden cold, the injury can occur after a few minutes and is called a “cold shock”.13,154
One of the plant damages caused by chilling is the reduction of photosynthetic rates, since the cold affects both electronic transport in the thylakoids and carbon fixation.155 In addition, cold can inhibit photosynthesis by reduced availability of free phosphate in the chloroplast due to the low utilization rate and consequently, the accumulation of trioses-phosphate, reducing phosphate availability for photosynthesis.8,156 In plants susceptible to low temperatures, photosynthesis is interrupted just above the freezing point mainly due to the susceptibility of thylakoids to cold, while in tolerant plants, photosynthesis can be maintained even at negative temperatures until the freezing point of cellular fluids.21 Photosynthetic damage can occur within a few hours of cold exposure, as demonstrated for rubber plants (Hevea brasiliensis Muell. Arg.), which presented injuries after four hours of exposure to 10°C.157 However, these plants showed to be tolerant to cold and after their transference to 28°C, they returned to their previous values of photosynthetic rates in less than 24 hours. In chicory plants (Cichorium intybus L.), the lowest maximum photosynthetic rate found in plants maintained at 4°C was related to changes in the activity of enzymes involved in CO2 fixation and the availability of this gas for photosynthesis.158 A reduction in the content of photosynthetic pigments induced by cold may also contribute to the decrease in photosynthesis. For example, cold sensitive genotypes of corn (Zea mays L.) grown at 14/12°C (day/night) showed reductions in the content of the photosynthetic pigments and, consequently, in the photosynthetic rates when compared to tolerant genotypes.159
The efficiency and regulation of the photosynthetic process can be assessed by the chlorophyll a fluorescence analysis.160–162 During the irradiation period, the light absorbed by the chlorophyll is used in the photochemical processes of photosynthesis and the excess of energy can be dissipated as heat or be re-emitted as light, a process known as chlorophyll a fluorescence.163 These three processes are competitive and changes in photosynthetic rates and heat dissipation will cause additional changes in chlorophyll a fluorescence, resulting in lower fluorescence values. Due to its relevance, this parameter has already been used as a tool for the selection of cold tolerant maize lines.164 A reduction in the values of chlorophyll a fluorescence can be observed after a few hours of cooling, as related for pea (Pisum sativum L. cv. Ranen) which was maintained for eight hours at 4°C and the bromeliad Aechmea blanchetiana (Baker) LB Sm. after four hours at 10°C.165,166 Nevertheless, an increase in the rates of chlorophyll a fluorescence, which would indicate a recovery of the photosynthetic process, may occur if the thermal conditions return to the optimum of the species.157,165
Another damage related to cold is the change in the cell membranes fluidity. This damage can also be caused by heat, as mentioned in the previous section of this review. However, under cold exposure membranes become rigid, while under heat they become more fluid. Chilling may cause a reduction in membrane fluidity due to the transition of the lipid components from a fluid-crystalline state to a solid-gel state, leading to dysfunctions such as increased permeability, water and intracellular solutes loss, and inactivation of transport channels.21,153
The membrane lipids can move within one of the layers, and rotate around their own axis, and these movements are thermodynamically directed and temperature dependent.10 These movements are reduced when temperature decreases, making the membrane more rigid. In most chilling-sensitive plants, the temperature at which the transition phase begins is close to 10°C. In addition, differences in the structure of membrane lipids can influence chilling tolerance. Sensitive plants usually have a higher content of saturated fatty acid residues in the lipids, while the more resistant ones show an increase in the desaturation of these molecules.152
The loss of membrane fluidity is one of the main signals for the activation of the cold response pathways and may lead to an enhance in ROS and RNS synthesis, causing an imbalance between the production of these reactive species and the protection of the antioxidant defense system, generating injuries like LPO.76 MDA is one of the main products of LPO and is considered a good indicator of structural integrity of membranes exposed to low temperature.167 Two hybrid strains of sweet sorghum (Sorghum bicolor (L.) Moench), M81-E and Roma, were evaluated for cold tolerance. Lower values of MDA and higher content of unsaturated fatty acids were verified in the membranes of the hybrid M81-E after 24 hours at 10°C. This suggests that the high degree of unsaturation would protect the photosynthetic system during the initial stress exposure, ensuring a higher cold tolerance for this hybrid compared to Roma.168 Interestingly, in soybean plants (Glycine max (L), Merr. cv. Essor) maintained at 1°C for up to eight days, higher MDA values were observed in the roots than in the hypocotyl, suggesting that this organ is more susceptible to injury caused by low temperature.169 The authors related these results to the increase in the antioxidant enzymes activity in the hypocotyl, indicating a greater resistance to the effects of ROS damage in this tissue.
In order to control the increase of ROS and thus contribute to cooling tolerance, several species use the antioxidant system. For example, in watermelon plants (Citrullus lanatus (Thomb.) Mansf. cv. dulce maravilla) cultivated at 10°C for 30 days, the lowest cold tolerance was related to the higher H2O2 content and reduction in the activity of APX, CAT and GR when compared to plants grown at 35°C.170 Interestingly, the comparison of cold tolerance in leaves of female and male plants of the Chinese Populus cathayana Rehd., showed an increase in H2O2 and APX values in both sexes after cultivation at 4°C for 14 days, but SOD and GR levels were higher in male plants, suggesting that male plants of this species are more tolerant to chilling than females.171 The increase in the enzyme activities in response to cold may be related to adjustments in gene expression. It has been reported that rubber plants grown at 10°C have a 2.5-fold increase in APX gene expression after 24 hours, and the activity levels of this enzyme also increased after one hour of cold exposure, demonstrating that responses of the antioxidant system may occur rapidly.157
Together with ROS, there is also evidence of the involvement of RNS in response to cold stress. For example, in pea plants (P. sativum cv. Lincoln) subjected to different stresses, such as chilling, heat and high light intensity, an enhancement in NO and its derived molecule RSNO (S-nitrosothiol) levels were verified under low temperature (8°C for 48 hours).172 Furthermore, it is reported that the activity of the antioxidant enzyme system can be increased or inhibited due to NO-induced post-translational modifications,90,173 suggesting that RNS has a dual role both as a pro-oxidant and as an antioxidant. This role is thought to be dose-dependent, i.e., it stimulates the antioxidant system and promotes cell survival under low concentrations and causes injury under high concentrations.85,174 Proteomic studies have identified that NO can target the antioxidant enzymes SOD, APX, GR and CAT causing S-nitrosylation and nitration in these molecules.90,173,175,176 Additionally, these post-translational modifications occur in about 30% of the cold responsive signaling-related proteins.177
Particularly, cold is considered one of the abiotic stresses with greater efficiency in the activation of NO synthesis.178 Although the molecular mechanism responsible for the synthesis of NO in plants is still controversial, L-arginine and nitrite are considered the main NO precursors.179 In mammals, the major pathway of NO production is the NADPH-dependent oxidation of L-arginine to L-citrulline, catalyzed by nitric oxide synthase, although no mammalian gene homologous to this enzyme was found in plants.89 In plants, the nitrate reductase (NR) catalyzes the reduction of nitrite in NO.180,181 NR is the key enzyme in the nitrogen metabolism and participates in the reduction of nitrate in nitrite, which is subsequently reduced to ammonium by the enzyme nitrite reductase and the ammonium formed is used in the amino acids production.153 Thus, to produce NO, NR changes nitrate, its higher affinity substrate, by nitrite, generating NO.89,179 This regulation appears to be at the concentration level of the substrate, therefore high levels of nitrite are required to competitively inhibit the reduction of nitrate.182 In plants maintained under cold conditions, this is considered one of the main pathways for the production of NO.93,183,184 The induction of an increased NO production by cold occurred in A. thaliana after 20 minutes of exposure to 4°C compared to plants maintained at 22°C. NO production continued to increase up to four hours of cold exposure and tests with NR inhibitors showed that this enzyme was responsible for the NO generation.184
The low temperatures, as well as heat, can also reduce water absorption by plants due to the decrease of their water potential, which can lead to dehydration.13 Thus, many plants regulate their osmotic potential through the accumulation of solutes in the interior of their cells, such as compatible osmolytes.116,153 Examples of these osmolytes are sugars, being sucrose, glucose and fructose one of the most important that are accumulated during cold stress.185 In addition, studies show that enzymes involved in sucrose metabolism can be regulated by temperature.186,187 An increase in the activity of the enzyme sucrose-phosphate synthase, one of the enzymes of sucrose synthesis, was verified in A. thaliana after cultivation at 4°C for 24 hours188 and in cabbage (B. oleracea) at 5°C for 72 hours.187 This increase in this enzyme shows an important role in sugar accumulation and acquisition of cold tolerance.
Sugars can also be related to antioxidant defense.185,189 The antioxidant role of glucose might be related to its function in the pentose phosphate pathway, increasing the production of NADPH, one of the major cofactors of the ROS-scavenging enzymes, and thus contributing to decrease the formation of this molecule in the cells.185 Fructose also plays a relevant role as an antioxidant, since this sugar was twice more effective than glucose in removing •O2− and therefore, it was associated with a key role in defense during chilling.190 In cold acclimated plants, raffinose might be involved in the stabilization of PSII.191 As mentioned previously, membrane stabilization is an important mechanism of cold resistance, and carbohydrates play a key role in this process.192 Sucrose, fructose, and oligosaccharides from the raffinose family can be inserted between the lipid polar groups decreasing membrane permeability,193,194 which might be increased due to the membrane damage induced by cold.
Exposure to cold also leads to a reduction in plant growth, as demonstrated for Cistus albidus L. and Quercus ilex L.195 This growth decrease was associated with the reduction of the starch: soluble sugars ratio, suggesting that photoassimilation under these conditions was deficient for maintaining growth since the stock components were remobilized and not re-stocked.195 Different varieties of chicory also showed a growth reduction when transferred from 16 to 4°C, which was associated with changes in the photosynthesis during this period.158
Lower nutrient uptake can affect plant growth during exposure to low temperature. The nitrate uptake is temperature sensitive and depends not only on the temperature at the root but also on the temperature at the shoots, in which its absorption is generally lower at chilling.196 In relation to ammonium absorption, a study with sensitive (L. esculentum Mill. cv. T-5) and cold resistant (L. hirsutum LAI778) tomato plants showed the absorption inhibition of this ion up to seven hours after a four-hour exposure to a temperature gradient from 15 to 5°C in susceptible plants, whereas the reabsorption in resistant plants occurred soon after the increase in temperature.197
Cold acclimation – signaling and acquisition of freezing tolerance
There are more than 1,000 cold-induced genes and among them, about 170 genes encode transcription factors and presumably, all these factors might reconfigure the transcriptome at low temperatures.198 Thus, signaling responses to cooling are complex and often involve similar stages to those reported for plants exposed to heat. Efficient signaling that ensures survival during exposure to low temperature can lead to cold acclimation, a stage for the acquisition of freezing stress. Fig. 3 shows the major signaling pathways described in this section.
Figure 3.
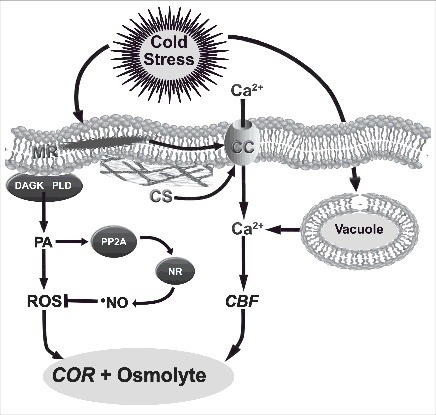
Diagram illustrating the cold signaling in plants via gene activation and accumulation of compatible osmolytes. The association between PA, PP2A and NR is hypothetical, and there may exist other forms of NR activation during cold exposure. CBF: C-repeat binding factor gene, CC: calcium channel, COR: cold responsive gene, CS: cytoskeleton, DAGK: diacylglycerol kinase, MR: membrane rigidity, NO: nitric oxide, NR: nitrate reductase, PA: phosphatidic acid, PLD: phospholipase D, PP2A: protein phosphatase 2A, ROS: reactive oxygen species.
The modification in the plasma membrane fluidity induced by cold acts as a biological thermometer and as a signal of various physiological processes in plants.199-201 Similarly, the cold perception by prokaryotic and mammalian cells also occurs by modifying the fluidity of biological membranes.202 The higher rigidity of the plasma membrane, induced by cold, might modulate the enzymes activity on membrane lipids, such as diacylglycerol kinase and phospholipase D, and thus increase the concentration of PA.203,204 PA formation occurs rapidly, starting in less than two minutes after exposing Arabidopsis plants to 0 or 10°C.205 The increase of PA in the cells can trigger an increase in ROS production, which has a regulatory effect at low concentrations.203 The accumulation of NO during the cold can contribute to the maintenance of this low concentration of ROS since NO can signal the activation of antioxidant enzymes through post-translational regulation.90,95 The main source of NO during the cold is via NR,93,183,184 however, the mechanisms regulating the activity of this enzyme are still unclear.206 Yaneva et al. (2002)207 demonstrated that cold stimulation in NR activation does not occur via de novo synthesis of the enzyme, but through the post-translational NR regulation via protein phosphatases, being protein phosphatase 2A one of the most important in this process. One of the pathways for this phosphatase activation by cold is related to the increase in intracellular PA level,208 suggesting a possible relationship between PA increase via membrane lipid catabolism and the consequent increase in NO synthesis via NR (Fig. 3). The accumulation of ROS and NO can induce COR (cold responsive) genes and the accumulation of compatible osmolytes, activating tolerance responses to cold.203,209 Among ROS, H2O2 was responsible for the induction of nearly 60% of the tolerance genes for chilling, and most gene alterations started within 24 hours after exposure to 10°C in Japonice rice.210
As in the case of heat, cold-induced membrane rigidity may increase intracellular Ca2+ concentration which is caused by an influx of this ion through the plasma membrane via a calcium channel and/or the release of the calcium stored inside the vacuole.199 Another activating mechanism of calcium channels by cold is via depolymerization of microtubules and microfilaments. In this case, the temperature drop might induce the reorganization of microfilaments, which somehow might be connected to calcium channels, thus dissipating the tensile force that kept them closed and allowing them to open.10,211
The induction of CBF (C-repeat binding factor) genes by calcium occurred within 15 minutes after transferring A. thaliana plants to 4°C.198 These activated CBF genes generate transcription factors that in turn activate the COR genes, which finally lead to cold acclimation.11 Among these transcription factors are CBF proteins, which are activated by binding to C-repeat/dehydration-responsive elements, which is one of the main regulation processes of plant response to cold.116 It is considered that approximately 12% of the COR genes are controlled by these transcription factors.201 As a result, the increase in cytoplasmic calcium, together with membrane fluidity, are important indicators of plant responses to cold.10,201
Some studies reported the role of ABA in signaling, although others indicated its absence or a small role of this hormone. Because of this controversy, many authors suggested that there are two pathways: one that is dependent on ABA and another independent, which might result in the expression of COR genes.201,212,213 It is generally considered that ABA increase is not enough to induce all genes related to cold tolerance.212 Furthermore, Rihan et al. (2017)201 observed that the concentration of this hormone must be constantly regulated according to environmental and physiological changes in order to guarantee effective signaling and thus modulate physiological responses to cold. These authors addressed the actions of other hormones such as salicylic and jasmonic acids during cold exposure, which might also promote cold tolerance, and of gibberellins that might activate regulating proteins, usually reducing growth during chilling.
On the other hand, signaling responses of plant acclimation to low temperatures may be a process of freezing tolerance acquisition. In temperate regions, for example, woody plants are able to become hardened, i.e. they resist harsh winter in which temperatures are below the freezing point of the water.21 The process of acclimation of these species occurs during fall, when exposed to warmer temperatures that are slightly above 0°C. This process occurs progressively according to the drop in temperature until the trees become completely resistant to temperatures between -5 and -15°C that are characteristic of winter. During acclimation, growth is interrupted and several resistance mechanisms are activated such as the accumulation of soluble carbohydrates and unsaturated lipids of cell membranes (Table 1). However, if there are extremely cold winters or even sudden periods of cold during spring or fall, these plants may suffer damage because they were exposed at a stage when they were not yet acclimated.21
In order to survive the harsh winter, plants must cope with the two major freezing damages: cell dehydration and membrane damage.213 Formation of intracellular ice (known as intracellular freezing) can lead to cytoplasm destruction.21 Often formation of ice crystals occur first in the vessels and spread to other parts of the plant, but the ice remains outside the cell and does not produce a direct damage to the protoplasm.147 However, extracellular ice, which is formed in the intercellular spaces and between the cell wall and the protoplasm, has a very low water potential and can reduce cell volume, leading to membrane damage, with a similar effect to dehydration.21,153
As mentioned previously for plants exposed to heat and low temperature, the accumulation of compatible osmolytes acts on the cell osmotic adjustment and allows the maintenance of water content, reducing stress by dehydration.116 This accumulation of osmolytes can also delay water freezing in the tissue by decreasing the freezing point, since the freezing temperature of the protoplasm becomes the same of the solute.21 In addition to this mechanism, ice formation can be avoided with the process of super cooling, in which water remains in the liquid state even at temperatures below the freezing point.21,147,214 Besides that, plants and others living beings can produce ice-binding proteins that bind to ice crystals surface making them smaller.215 The freeze resistance mechanisms may also be phenological, e.g. leaves fall in autumn and higher growth occurs in spring, when thermal conditions are more favorable.214
The ability of plant cells to tolerate freezing was the basis for the plant preservation technique known as cryopreservation, which consists of storing cells, tissues, organs or seeds at very low temperatures, usually in liquid nitrogen at -196°C or in its vapor phase at -150°C.216 Under these conditions, the cells do not divide and the metabolic activity would be virtually zero, which would guarantee a theoretical conservation with no time limit.217 This technique is well established for species propagated vegetatively (asexual multiplication of cells, tissues or plant organs) as tubers, fruit trees, ornamental and cultivated plants of tropical or temperate origin, which present a generally high survival to the process (close to 100%) and have a rapid and direct regeneration.217
This technique is particularly useful for the conservation of endangered plants. For example, in the case of Androcalva perlaria CF Wilkins, an endangered species from Australia, and the palm tree Phoenix reclinata Jacq., which both presented a regeneration capacity of 70% after cryopreservation.218,219 Touchell and Dixon (1994)220 also showed that it is possible to preserve some seeds of the Australian flora at a temperature of -20/-80°C. These kind of studies can serve as a basis for the establishment of cryopreservation banks, which would preserve plant genetic resources of endangered or economically important native species.
Engelmann (2004)217 in a review on cryopreservation listed examples of germplasm banks around the world: the National Center for Genetic Resources Preservation (Fort Collins, CO), which retains a total of 360,629 seeds especially of rare and threatened species and/or with limited longevity; the National Bureau for Plant Genetic Resources (New Delhi, India), which maintains 1,200 accesses of 50 different species, mainly of medicinal use; and the Indian Institute for Horticultural Research (Bangalore), which has a pollen collection of 600 accesses belonging to 40 species from 15 different families, some of which have been stored for more than 15 years. Considering the recent climate changes, which may lead to species extinction, the formation of germplasm banks using this technique might be used as a conservation measure. However, it should be noted that for some species, whose seeds do not support the effects of freezing, other methods should be employed.
Tropical plants: Conservation and temperature changes
Tropical forests are an essential component of the global climate system, providing habitats for a myriad of plant and animal species.221 Our knowledge about most of the biogeochemical functions that operate in tropical forest ecosystems is still scarce, so these environments have great potential for the study by future generations of scientists. The current lack of knowledge is particularly worrying given that tropical forests have unrestricted access to land use and conversion for other purposes.221 This situation may be worsened if we consider the vulnerability of tropical plants to the expected climate change. Tropical plants have developed under narrow temperature limits, suggesting a limited ability to adapt to high heating changes and sudden cooling.222 Cunningham and Read (2002)223 showed the vulnerability of tropical species to temperature changes. These authors observed that temperate tree species have a higher photosynthetic rate at lower temperatures than tropical species, and maintain higher photosynthetic rates in a higher thermal range (12-16°C) than tropical plants (9-11°C). According to these authors, data were consistent with the highest seasonal and daily temperature variation for temperate climate compared to the tropical climate. Similarly, Rao (2004)151 reports that plants from temperate climate tend to be more tolerant to low temperatures and have high survival during exposure to thermal variations between 0 and 5°C than tropical ones, which may suffer at temperatures below 10°C.
Feeley et al. (2012)224 referred that the vulnerability of tropical plants to temperature changes may lead them to migrate to more suitable places for survival. However, it is still uncertain if tropical forest plant species can migrate rapidly enough to keep pace with climatic changes, which could gravely endanger such species. These authors mention that such uncertainty derives from the fact that most studies on climate change and conservation are focused primarily on North America and Europe, with only a few on tropical systems – despite the fact that the tropics retain the highest biodiversity of the planet.48,224
Although the vulnerability referred for tropical plants, the great biodiversity in tropical environments suggests the existence of various tolerance mechanisms to temperature stress, especially in plants species that are known to survive beyond the thermal range commonly described for the tropical region. For example, Cunningham and Read (2006)225 found that leaves of tropical tree species tolerated higher temperatures (51-55°C) than temperate ones (48-51°C). Furthermore, the fact that many tropical trees are more than a century old suggests that they have tolerated climate fluctuations in the past.224 However, according to Feeley (2015),226 tropical plants are poorly represented in studies that investigate and predict the impacts of climate change. In this sense, investigations about the effects of temperature on plants become an important indicator of the impact that climate change might cause to tropical biodiversity.
Physiological and/or biochemical analyses can be important tools for predicting plant resilience to thermal changes, including for tropical species. In fact, many tropical biomes show great thermal amplitude. For example, regions of the Cerrado (Brazil) have extremely low temperatures during certain times of the year, reaching values close to or below zero during the winter;227 and the region of Serra dos Órgãos, located in the state of Rio de Janeiro (Brazil), where the average temperature is 19°C, with an amplitude of 5 to 40°C during the day.228
These data show that tropical species can be interesting study objects of tolerance mechanisms to thermal changes by both cold and heat. Since 2004, our work group has studied the physiological and biochemical aspects of species from the Bromeliaceae, whose representatives inhabit several thermal ranges present in the tropics. Members of Bromeliaceae (commonly known as bromeliads) are distributed in the Neotropical region, from the state of Virginia to Texas in the USA, throughout Central and South America, being only one species, Pitcairnia feliciana (A. Chevalier) Harms & Mildbraed, reported in the East African coast.24 Bromeliads are represented by approximately 58 genera and 3,200 species229 and can be found in regions that extend from sea level to the Andes, inhabiting humid places such as the Atlantic Rainforest to dry regions such as the Caatinga.230
The broad distribution of bromeliads indicates a great plasticity in the occupancy of a variety of environments and the presence of multiple adaptations to stressful conditions such as temperatures ranging from 0 to 40°C, drought, intense sun, and nutrient shortage.24 In Brazil, about 40% of the total species and 73% of the genera of bromeliads are present, being 80% found in the Atlantic Forest.231 Fig. 4 shows examples of bromeliads of different habits, demonstrating the high adaptability of the family to the environment: Tillandsia tenuifolia L. (Fig. 4A) and Vriesea inflata (Wawra) Wawra (Fig. 4B) are epiphytes; Acanthostachys strobilacea (Schult f.) Link, Klotzsch & Otto (Fig. 4C) is an epiphyte or saxicolous; Alcantarea imperialis (Carrière) Harms (Fig. 4D) is rupicolous, while Nidularium minutum Mez (Fig. 4E) is terricolous. These species can survive to mean temperatures ranging from 9 to 29°C, while some of them survive even when the thermal sensation is equivalent to 0°C, as is the case of the imperial bromeliad (Fig. 4D).
Figure 4.

Natural populations and ornamental characteristics of bromeliads from the Brazilian Atlantic rainforest. (A) Tillandsia tenuifolia L. growing in the State Park of Intervales (Ribeirão Grande, São Paulo), where minimum and maximum average annual temperatures are approximately 9 and 29°C, respectively.277 (B) Vriesea inflata (Wawra) Wawra growing in the Biological Reserve of “Alto da Serra de Paranapiacaba” (Santo André, São Paulo), where temperatures may vary from 2 to 32°C.278 (C) Acanthostachys strobilacea (Schult. f.) Link, Klotzsch & Otto growing in the Biological Reserve and Experimental Station of “Mogi-Guaçu” (Mogi-Guaçu, São Paulo), where the minimum and maximum average annual temperatures are approximately 11 and 30°C, respectively.277 This species also occurs in Cerrado areas. (D) Alcantarea imperialis (Carrière) Harms growing in an inselberg at the National Park of “Serra dos Órgãos” (Teresópolis, Rio de Janeiro). Temperatures at this site may vary from -5 up to 38°C.279 (E) In situ conservation of Nidularium minutum Mez in the Biological Reserve of “Alto da Serra de Paranapiacaba”. (F) A specimen of Vriesea hieroglyphica (Carrière) E. Morren with a tank formed by overlapping of broad leaves that allows water storage from the rain. (G) Ornamental aspect of the foliage of tank-forming bromeliads. Photo credits: (A) Suzana Ehlin Martins; (B) Camila Freitas; (C) Victória Carvalho; (D) Luciana Mollo; (E) Camila P. Carvalho; (F, G) Catarina C. Nievola.
Recently, Cach-Pérez et al. (2014)232 published a review referring that epiphytic bromeliads are suitable for studies on global climate change, due to their high dependence on water availability in the form of rain, fog or dew.233–236 According to the same review, the fact that bromeliads present small sizes and are easy to handle both in the field and in the laboratory makes them suitable for research on rapid physiological responses to environmental changes232 that occur at daily intervals.
In vitro cultivation is a tool that can be applied to the study of variations in environmental factors on growth and physiology, which was already applied to bromeliads in several of our studies.228,237–241 This technique allows the cultivation of seed or plant tissues (explants) under controlled temperature and light (Fig. 5A) in a nutrient medium under aseptic conditions,242 guaranteeing disease-free plant production243 (Fig. 5B to D). In the case of studies on the influence of temperature, plants grown in vitro can be maintained in Biochemical Oxygen Demand incubators that allow easy adjustment and control of temperature and photoperiod (Fig. 5E). Thus, the maintenance of plants in culture flasks allows their easy transfer to different temperature conditions, in order to simulate the temperature variations of the natural environment. In addition, plants under in vitro conditions may also be subsequently removed from the flasks and maintained in substrates to adapt to ex vitro growth, i.e. acclimatization process (Fig. 5F to J). This stage is considered critical and often limiting for in vitro cultivation, mainly due to the fact that the plants are transferred from an environment with high relative humidity, low light intensity and external energy supply (carbohydrates in the culture medium) to an environment with opposite conditions, which requires the plants’ own production of carbohydrates through higher photosynthetic activity, and increased transpiration rate due to reduced environmental humidity.244
Figure 5.

Methods of plant cultivation (A to J) and conservation of bromeliad species (K and L). Controlled light period and temperature for plant growth can be obtained in a culture room (A). In vitro cultivation can be performed with meristematic tissues activity such as nodal segments (B) or from seeds germination (C) for the propagation of a large number of plants (D). Biochemical Oxygen Demand incubators with controlled environmental conditions can be used in experimental studies about temperature influence on plants survival and growth (E). In vitro cultured plants may be acclimatized in soil and maintained in a culture room for later use in experimental studies (F). A plastic sack may be used as a cover to prevent moisture loss and dehydration during acclimatization (G). Detail of juvenile bromeliads in seedling trays during acclimatization (H). Species obtained from their natural environments and allocated in the Bromeliaceae collection of the Research Center on Ornamental Plants – “Instituto de Botânica”, São Paulo, Brazil (ex situ conservation; more information at http://botanica.sp.gov.br/) (K and L) were used as seed sources for in vitro conservation and propagation studies. Photo credits: (A-C, E-H, J) Victória Carvalho; (D) Camila P. Carvalho; (I, K, L) Catarina C. Nievola.
Thus, the acclimatization process allows the evaluation of plant growth recovery after the in vitro period. It is worth mentioning that in vitro cultivation and acclimatization also allow the rapid and numerous production of plants for use in ex vitro physiological studies.245–247
We used the in vitro technique for evaluating the influence of the low temperature on the in vitro growth of the bromeliad N. minutum,237 as shown in Fig. 4E. Our results showed that changes in the carbohydrate metabolism of this species were associated with cold tolerance. It was observed a higher glucose content in the leaves of this bromeliad when maintained at 10°C for six months compared to those grown at 25°C, thus attributing to these carbohydrates a cryoprotection function. In the case of sucrose amount, it was lower in plants cultivated at 10°C than in those maintained at 25°C. Similar results were observed for the bromeliad A. imperialis, also cultivated in vitro (Fig. 4D).228
Mollo et al. (2011)228 found that myo-inositol, glucose, fructose and sucrose were found predominantly under high temperatures (30°C), while under low temperatures (15°C), sucrose was apparently replaced by trehalose, raffinose and stachyose. Starch content was highest in plants grown under high temperatures. The lowest starch content was found under low temperatures, suggesting its conversion into soluble carbohydrates to protect plants from cold. Despite the fact that Guy et al. (1992)186 observed that sucrose is accumulated in largest quantities during cold exposure, a low proportion of this carbohydrate was detected compared to other hexoses when this bromeliad was maintained at lower temperatures.228 These results indicated that chilling delayed the growth of A. imperialis and increased sugar levels, mainly trehalose, thus suggesting that these sugars can be involved in cold tolerance, as referred previously in this review (see section on mechanisms of cold tolerance).
Research from our group also performed with A. imperialis cultivated in vitro showed that the periodic thermal condition of hot days and cold nights (30/15°C) might be related to the ability of this species to modulate the CAM metabolism in the absence of water (unpublished data). This type of metabolism allows the maintenance of photosynthetic activity and growth even under adverse environmental conditions,55,248,249 such as extreme temperatures. Lüttge (2004)249 pointed out that low nocturnal and high diurnal temperatures are favorable to CAM induction. Likewise, Haag-Kerwer et al. (1992)250 verified that CAM induction is enhanced when day/night temperature differences are increased, being this transition less sensitive to constant temperatures. Dodd et al. (2002)251 reported that the induction of CAM metabolism may be related to phytohormone signaling. Nievola et al. (2005)252 verified the influence of low nocturnal temperatures over the induction to CAM by ABA hormone in the bromeliad Ananas comosus (L.) Merr. cultivated in vitro. Results showed that plants exposed to a thermoperiod of 28/15°C (light/dark) for four months induced CAM activity and presented higher amounts of ABA compared to plants kept at constant 28°C light and dark, which consequently maintained C3 photosynthesis. In another work performed with A. comosus plants under the same cultivation and temperature conditions of the previous study, it was shown that the cytokinin levels decreased in terms of hours in CAM-expressing plants (under 28/15°C, light/dark) when compared to plants utilizing C3 photosynthesis (under constant 28°C).253 These rapid variations may be essential for the control of metabolic activities related to day/night thermal adjustment. The functioning of CAM metabolism is described previously in this review (see the section on heat effects).
Finally, studies were also carried out maintaining bromeliads in vitro at high temperatures in order to identify their thermal limits in relation to heat exposure. In fact, the concern with the possible effects of global warming impels research in order to identify heat-tolerant species. The survival of A. strobilacea (Fig. 4C) was maintained under high rates for up to 45 days under 35°C (light/dark) in conjunction with water deficit conditions simulated by the addition of polyethylene glycol 6000 in nutrient medium (reaching a water potential of -0.8 MPa),254 although the plants showed significant growth reduction. Fig. 6A and B show the effects of exposure to a high temperature (32°C) to a seven-day period over the leaf temperature of A. strobilacea through infrared thermograph images. This technique allows non-contact temperature measurements and can be useful to detect the level of the stress response in the tissues.255–258 The influence of cooling on the tissues of the bromeliad N. minutum was also verified using thermographic images. This species, when exposed to 10°C for 10 days, showed a clear drop in temperature mainly in the leaves when compared to plants maintained at 25°C (Fig. 6C to F).
Figure 6.
Morphological (A, C and E) and infrared thermograph images (B, D and F) of bromeliads exposed to varying temperatures. (A and B) Acanthostachys strobilacea (Schult. f.) Link, Klotzsch & Otto plants of approximately one month old exposed to 32°C for seven days. (C-F) Nidularium minutum Mez plants of five months old exposed to 25°C (C and D) and 10°C (E and F) for 10 days. Thermographs taken with an infrared camera allows the qualitative assessment of surface temperature of leaves and roots. Overall, it is possible to verify that plants under exposure to higher temperatures (25-32°C) were able to keep roots with lower temperatures than the leaves. Meanwhile, N. minutum plants under cold stress (10°C) kept roots with higher temperatures than the leaves. All thermographs were obtained with a Testo 875-1i infrared camera (Lenzkirch, Germany). Bars: (A) 2 cm, (C and E) 3 cm. Photo credits: (A, B) William T. Hagiwara; (C-F) Camila P. Carvalho.
In addition to the great applicability of in vitro culture for the study of tolerance mechanisms to thermal stress, as demonstrated previously, this technique has been considered an efficient strategy to propagate genetic material of rare and endangered species, aiming to ensure long-term survival of this material in nature.259 In this sense, the use of low culture temperatures, aiming to slow down growth, has been considered for those collections aimed at conserving germplasm in small spaces.260
Seed cultivation allows the maintenance of the genetic variability of the species, which is required in conservation programs and is considered an alternative to seed banks, especially for recalcitrant seeds that lose viability when stored or frozen.261 Under slow growth, the need for subcultures (replacement of culture medium) is decreased and the expenses related to cultivation are reduced. The maintenance of in vitro collections using low temperatures has been applied for several species, and the thermal range used may vary. Examples are the apple cultivation at 4°C,262 Drosophyllum lusitanicum (L.) Link and Phoenix dactylifera L. at 5°C,263,264 Xanthosoma spp. at 9°C,265 and Podophyllum peltatum L. at 10°C.266
In the case of bromeliads, many species are considered vulnerable, including in Brazil.267 This is largely due to the widespread use of bromeliads as ornamental plants and their illegal extraction (Fig. 4F and G).42 In fact, most bromeliads have overlapping leaf sheaths that form a structure known as tank or phytotelma,24 as observed in Vriesea hieroglyphica (Carrière) E. Morren (Fig. 4F), a species that is much appreciated in landscaping.230 Because of this, it is important to develop strategies of germplasm conservation for endangered bromeliads, including the maintenance of slow growing in vitro collections. We cultivated and maintained in vitro collections of the bromeliads V. inflata240 and A. imperialis228 at 15°C, a temperature that is considered low for tropical environments.151 We also observed that this cultivation temperature could still be reduced, since some bromeliads like N. minutum and A. strobilacea survived for up to three months at 10°C,237,238 being transferred successfully to ex vitro cultivation and restoring growth at 25°C.
Therefore, considering the thermal plasticity of members of the Bromeliaceae, our research group aims to investigate the resistance of several species, vulnerable or not, currently present in the scientific collections of the Research Centre on Ornamental Plants of “Instituto de Botânica”, São Paulo, Brazil (Fig. 5K and L), which is composed of 160 species and 1,158 specimens (more information at http://botanica.sp.gov.br/).
In view of the scenario of global warming, even if controlled, it is necessary to increase our knowledge of resilience mechanisms of organisms to this condition. Regarding plants, it is important to estimate the survivability of the existing vegetation to heat stress, as well as to discover sources of genes related to heat tolerance, aiming at biotechnological application e.g. the development of resilient crops. Tropical plants have been considered to be more vulnerable to climate change.222 However, the responses of these species to temperature stress in general still requires further research in order for us to better evaluate the capacity of survival of tropical plants to potential climate changes. Certainly this knowledge will contribute significantly to ecosystem conservation, creating sustainable alternatives for humanity.
Funding Statement
The work that the authors cited in this review was supported by “Fundação de Amparo à Pesquisa do Estado de São Paulo” (FAPESP) under grant 2013/25047-0; “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES); and “Conselho Nacional de Pesquisa e Desenvolvimento” (CNPq) under grant 132909/2014-6.
Abbreviations
- ABA
abscisic acid
- APX
ascorbate peroxidase
- C3
Calvin-Benson cycle
- CAM
crassulacean acid metabolism
- CAT
catalase
- CBF
C-repeat binding factor
- COR
cold responsive genes
- cv
cultivar
- GR
glutathione reductase
- HSP
heat-shock protein
- LPO
lipid peroxidation
- MDA
malondialdehyde
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- NR
nitrate reductase
- PA
phosphatidic acid
- PSII
photosystem II
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
About the authors

Dr. Catarina C. Nievola is a scientific researcher at the “Instituto de Botânica de São Paulo” and since 2004 studies the effects of temperature and water availability on the physiology and biochemistry of tropical plants, with emphasis on the conservation of endangered species, especially of Bromeliaceae members.

Dr. Camila P. Carvalho and MSc. Victória Carvalho, under the supervision of Dr. Nievola, conduct research on the use of low culture temperatures for in vitro preservation of bromeliads and assess the biochemical and physiological aspects involved in drought tolerance and thermal changes in tropical bromeliads.


Dr. Edson Rodrigues has experience in the biochemistry of Antarctic organisms and participates in the “National Institute for Science and Technology Antarctic Environmental Research” investigating the impact of natural and anthropic factors on biochemical biomarkers. In 2013, he started a partnership with Dr. Nievola to study the effect of cold on the antioxidant system of bromeliads.
References
- 1.Hochachka PW, Somero GN. Biochemical adaptation: mechanism and process in physiological evolution. New York: Oxford University Press; 2002. [Google Scholar]
- 2.Jupp T, Schultz A. A thermodynamic explanation for black smoker temperatures. Nature. 2000;403(6772):880–883. doi: 10.1038/35002552. [DOI] [PubMed] [Google Scholar]
- 3.Scambos T. NASA-USGS Landsat 8 Satellite pinpoints coldest spots on Earth. https://www.nasa.gov/content/goddard/nasa-usgs-landsat-8-satellite-pinpoints-coldest-spots-on-earth (accessed Jun 8, 2017).
- 4.Deming JW. Psychrophiles and polar regions. Curr Opin Microbiol. 2002;5(3):301–309. doi: 10.1016/s1369-5274(02)00329-6. [DOI] [PubMed] [Google Scholar]
- 5.Takai K, Nakamura K, Toki T, Tsunogai U, Miyazaki M, Miyazaki J, Hirayama H, Nakagawa S, Nunoura T, Horikoshi K. Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci. 2008;105(31):10949–10954. doi: 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitter A, Hay R. Environmental Physiology of Plants, 3rd ed London, San Diego: Academic Press; 2002. [Google Scholar]
- 7.Źróbek-Sokolnik A. Temperature stress and responses of plants. In: Environmental adaptations and stress tolerance of plants in the era of climate change. Ahmad P, Prasad MN V, editors. New York, NY: Springer New York; 2012. p. 113–134. [Google Scholar]
- 8.Taiz L, Zeiger E. Plant physiology and development, 6th ed Sinauer Associates, Inc; 2015. [Google Scholar]
- 9.Nobel PS. Environmental biology of agaves and cacti. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 10.Ruelland E, Zachowski A. How plants sense temperature. Environ Exp Bot. 2010;69(3):225–232. doi: 10.1016/j.envexpbot.2010.05.011. [DOI] [Google Scholar]
- 11.Knight MR, Knight H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol. 2012;195(4):737–751. doi: 10.1111/j.1469-8137.2012.04239.x. [DOI] [PubMed] [Google Scholar]
- 12.Counts JA, Zeldes BM, Lee LL, Straub CT, Adams MWW, Kelly RM. Physiological, metabolic and biotechnological features of extremely thermophilic microorganisms. Wiley Interdiscip Rev Syst Biol Med. 2017;9(3):e1377. doi: 10.1002/wsbm.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitt J. Responses of plants to environmental stress, Volume 1: Chilling, freezing, and high temperature stresses. New York, London, Toronto, Sydney, San Francisco: Academic Press; 1980. [Google Scholar]
- 14.Dalmaso G, Ferreira D, Vermelho A. Marine extremophiles: a source of hydrolases for biotechnological applications. Mar Drugs. 2015;13(4):1925–1965. doi: 10.3390/md13041925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pikuta E V., Hoover RB, Tang J.. Microbial extremophiles at the limits of life. Crit Rev Microbiol. 2007;33(3):183–209. doi: 10.1080/10408410701451948. [DOI] [PubMed] [Google Scholar]
- 16.Xiong FS, Ruhland CT, Day TA. Photosynthetic temperature response of the Antarctic vascular plants Colobanthus quitensis and Deschampsia antarctica. Physiol Plant. 1999;106(3):276–286. doi: 10.1034/j.1399-3054.1999.106304.x. [DOI] [Google Scholar]
- 17.Luo Q. Temperature thresholds and crop production: A review. Clim Change. 2011;109(3–4):583–598. doi: 10.1007/s10584-011-0028-6. [DOI] [Google Scholar]
- 18.Stout RG, Summers ML, Kerstetter T, McDermott TR. Heat- and acid-tolerance of a grass commonly found in geothermal areas within Yellowstone National Park. Plant Sci. 1997;130(1):1–9. doi: 10.1016/s0168-9452(97)00205-7. [DOI] [Google Scholar]
- 19.Smith LB, Downs RJ. Tillandsioideae (Bromeliaceae). Fl Neotrop Monogr. 1977;14(2):663–1492. [Google Scholar]
- 20.Seckbach J, Oren A. Oxygenic photosynthetic microorganisms in extreme environments. In: Algae and cyanobacteria in extreme environments. Dordrecht: Springer Netherlands; 2007. p. 3–25. [Google Scholar]
- 21.Larcher W. Ecofisiologia vegetal. São Carlos: RiMa; 2004. [Google Scholar]
- 22.Iba K. Acclimative response to temperature stress in higher plants: Approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol. 2002;53(1):225–245. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- 23.Shinozaki K, Uemura M, Bailey-Serres J, Bray EA, Weretilnyk E. Responses to abiotic stress. In: Biochemistry and molecular biology of plants. Buchanan BB, Gruissem W, Vickers K, Jones RL, editors. Hoboken, USA: John Wiley & Sons; 2015. [Google Scholar]
- 24.Benzing DH. Bromeliaceae. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 25.Rewald B, Eppel A, Shelef O, Hill A, Degu A, Friedjung A, Rachmilevitch S. Hot desert environments. In: Life at extremes – Environments, organisms and strategies for survival. Bell EM, editor New York: CABI Publishing; 2012. p. 196–218. [Google Scholar]
- 26.O'sullivan OS, Heskel MA, Reich PB, Tjoelker MG, Weerasinghe LK, Penillard A, Zhu L, Egerton JJG, Bloomfield KJ, Creek D, et al. Thermal limits of leaf metabolism across biomes. Glob Chang Biol. 2016;23(1):209–223. doi: 10.1111/gcb.13477. [DOI] [PubMed] [Google Scholar]
- 27.Raven PH, Evert RF, Eichhorn SE. Biology of plants. New York and Basingstoke: W.H. Freeman; 2005. [Google Scholar]
- 28.Chaudhary B. Plant domestication and resistance to herbivory. Int J Plant Genomics. 2013;2013:1–14. doi: 10.1155/2013/572784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guy C. Molecular responses of plants to cold shock and cold acclimation. J Mol Microbiol Biotechnol. 1999;1(2):231–242. [PubMed] [Google Scholar]
- 30.Larkindale J, Mishkind M, Vierling E. Plant responses to high temperature. In: Plant Abiotic Stress. Jenks M, Hasegawa PM, editors Oxford Ames Carlton: Blackwell Publishing; 2005. p. 100–134. [Google Scholar]
- 31.De Pinto MC, Locato V, Paradiso A, De Gara L. Role of redox homeostasis in thermo-tolerance under a climate change scenario. Ann Bot. 2015;116(4):487–496. doi: 10.1093/aob/mcv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas CD, Thomas CD, Cameron A, Cameron A, Green RE, Green RE, Bakkenes M, Bakkenes M, Beaumont LJ, Beaumont LJ, et al. Extinction risk from climate change. Nature. 2004;427(6970):145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- 33.Gottfried M, Pauli H, Futschik A, Akhalkatsi M, Barančok P, Alonso JLB, Coldea G, Dick J, Erschbamer B, Kazakis G, et al. Continent-wide response of mountain vegetation to climate change. Nat Clim Chang. 2012;2(2):111–115. doi: 10.1038/nclimate1329. [DOI] [Google Scholar]
- 34.Sunday JM, Bates AE, Dulvy NK. Thermal tolerance and the global redistribution of animals. Nat Clim Chang. 2012;2(9):686–690. doi: 10.1038/nclimate1539. [DOI] [Google Scholar]
- 35.Anderegg WRL, Kane JM, Anderegg LDL. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat Clim Chang. 2013;3(1):30–36. doi: 10.1038/nclimate1635. [DOI] [Google Scholar]
- 36.Muhlfeld CC, Kovach RP, Jones LA, Al-Chokhachy R, Boyer MC, Leary RF, Lowe WH, Luikart G, Allendorf FW. Invasive hybridization in a threatened species is accelerated by climate change. Nat Clim Chang. 2014;4(7):620–624. doi: 10.1038/nclimate2252. [DOI] [Google Scholar]
- 37.Challinor AJ, Koehler A-KA-K, Ramirez-Villegas J, Whitfield S, Das B. Current warming will reduce yields unless maize breeding and seed systems adapt immediately. Nat Clim Chang. 2016;6(10):954–958. doi: 10.1038/nclimate3061. [DOI] [Google Scholar]
- 38.O'Neill BC, Oppenheimer M, Warren R, Hallegatte S, Kopp RE, Pörtner HO, Scholes R, Birkmann J, Foden W, Licker R, et al. IPCC reasons for concern regarding climate change risks. Nat Clim Chang. 2017;7(1):28–37. doi: 10.1038/nclimate3179. [DOI] [Google Scholar]
- 39.Betts RA, Cox PM, Collins M, Harris PP, Huntingford C, Jones CD. The role of ecosystem-atmosphere interactions in simulated Amazonian precipitation decrease and forest dieback under global climate warming. Theor Appl Climatol. 2004;78(1):157–175. doi: 10.1007/s00704-004-0050-y. [DOI] [Google Scholar]
- 40.Bonan GB. Forests and climate Change: forcings, feedbacks, and the climate benefits of forests. Science. 2008;320(5882):1444–1449. doi: 10.1126/science.1155121. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad P, Rasool S. Emerging technologies and management of crop stress tolerance: Volume 2-A sustainable approach. San Diego, London, Waltham: Academic Press; 2014. [Google Scholar]
- 42.Negrelle RRB, Anacleto A, Mitchell D. Bromeliad ornamental species: conservation issues and challenges related to commercialization. Acta Sci Biol Sci. 2012;34(1):91–100. doi: 10.4025/actascibiolsci.v34i1.7314. [DOI] [Google Scholar]
- 43.Guerrant EO, Havens K, Maunder M. Ex situ plant conservation: supporting species survival in the wild. Washington: Island Press; 2004. [Google Scholar]
- 44.Cruz-Cruz C, González-Arnao M, Engelmann F. Biotechnology and conservation of plant biodiversity. Resources. 2013;2(2):73–95. doi: 10.3390/resources2020073. [DOI] [Google Scholar]
- 45.IPCC. Climate Change 2014: Synthesis Report. Contribution of working groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed The Core Writing Team, Pachauri RK, Meyer LA, editors Geneva: IPCC; 2015. [Google Scholar]
- 46.Trenberth KE, Dai A, van der Schrier G, Jones PD, Barichivich J, Briffa KR, Sheffield J. Global warming and changes in drought. Nat Clim Chang. 2014;4(1):17–22. doi: 10.1038/nclimate2067. [DOI] [Google Scholar]
- 47.Kodra E, Steinhaeuser K, Ganguly AR. Persisting cold extremes under 21st-century warming scenarios. Geophys Res Lett. 2011;38(8): doi: 10.1029/2011gl047103. [DOI] [Google Scholar]
- 48.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 49.Hasanuzzaman M, Nahar K, Fujit M. Extreme temperature responses, oxidative stress and antioxidant defense in plants. In: Abiotic Stress – Plant Responses and Applications in Agriculture. Vahdat K, Leslie C, editors InTech; 2013. p. 169–205. [Google Scholar]
- 50.Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci. 2013;14(5):9643–9684. doi: 10.3390/ijms14059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osmond CB. Crassulacean acid metabolism: a curiosity in context. Annu Rev Plant Physiol. 1978;29(1):379–414. [Google Scholar]
- 52.Winter K, Smith JAC. Crassulacean acid metabolism: current status and perspectives. In: Crassulacean acid metabolism. Biochemistry, ecophysiology and evolution. Winter K, Smith JAC, editors Berlin Heidelberg: Springer; 1996. p. 389–426. [Google Scholar]
- 53.Ting IP. Crassulacean acid metabolism. Annu Rev Plant Physiol. 1985;960(90):596–622. doi: 10.1146/annurev.pp.36.060185.003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gawronska K, Niewiadomska E. Participation of citric acid and isocitric acid in the diurnal cycle of carboxylation and decarboxylation in the common ice plant. Acta Physiol Plant. 2015;37(61):1–8. doi: 10.1007/s11738-015-1807-x. [DOI] [Google Scholar]
- 55.Cushman JC, Borland AM. Induction of Crassulacean acid metabolism by water limitation. Plant, Cell Environ. 2002;25(2):295–310. doi: 10.1046/j.0016-8025.2001.00760.x. [DOI] [PubMed] [Google Scholar]
- 56.Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: An overview. Environ Exp Bot. 2007;61(3):199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- 57.Filewod B, Thomas SC. Impacts of a spring heat wave on canopy processes in a northern hardwood forest. Glob Chang Biol. 2014;20(2):360–371. doi: 10.1111/gcb.12354. [DOI] [PubMed] [Google Scholar]
- 58.Mathur S, Agrawal D, Jajoo A. Photosynthesis: Response to high temperature stress. J Photochem Photobiol B Biol. 2014;137(January):116–126. doi: 10.1016/j.jphotobiol.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 59.Berry J, Björkman O. Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol. 1980;31(1):491–543. doi: 10.1146/annurev.pp.31.060180.002423. [DOI] [Google Scholar]
- 60.Crafts-Brandner SJ, Salvucci ME. Analyzing the impact of high temperature and CO2 on net photosynthesis: biochemical mechanisms, models and genomics. F Crop Res. 2004;90(1):75–85. doi: 10.1016/j.fcr.2004.07.006. [DOI] [Google Scholar]
- 61.Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth Res. 2008;98(1–3):541–550. doi: 10.1007/s11120-008-9331-0. [DOI] [PubMed] [Google Scholar]
- 62.Havaux M. Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant, Cell Environ. 1993;16(4):461–467. doi: 10.1111/j.1365-3040.1993.tb00893.x. [DOI] [Google Scholar]
- 63.Camejo D, Rodríguez P, Angeles Morales M, Miguel Dell'Amico J, Torrecillas A, Alarcón JJ. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J Plant Physiol. 2005;162(3):281–289. doi: 10.1016/j.jplph.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 64.Ristic Z, Bukovnik U, Prasad PVV. Correlation between heat stability of thylakoid membranes and loss of chlorophyll in winter wheat under heat stress. Crop Sci. 2007;47(5):2067–2073. doi: 10.2135/cropsci2006.10.0674. [DOI] [Google Scholar]
- 65.Mathur S, Jajoo A. Alterations in photochemical efficiency of photosystem II in wheat plant on hot summer day. Physiol Mol Biol Plants. 2014;20(December):527–531. doi: 10.1007/s12298-014-0249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mittler R, Finka A, Goloubinoff P. How do plants feel the heat? Trends Biochem Sci. 2012;37(3):118–125. doi: 10.1016/j.tibs.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Prasad PV V, Staggenborg SA, Ristic Z. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In: Response of crops to limited water: Understanding and modeling water stress effects on plant growth processes. Madison: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America; 2008. p. 301–355. [Google Scholar]
- 68.Tang T, Liu P, Zheng G, Li W. Two phases of response to long-term moderate heat: Variation in thermotolerance between Arabidopsis thaliana and its relative Arabis paniculata. Phytochemistry. 2016;122:81–90. doi: 10.1016/j.phytochem.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Narayanan S, Tamura PJ, Roth MR, Prasad PVV, Welti R. Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant, Cell Environ. 2016;39(4):787–803. doi: 10.1111/pce.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55(1):373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 71.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. ROS signaling: the new wave? Trends Plant Sci. 2011;16(6):300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo X, Shah J, Schlauch K, et al. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell. 2013;25(9):3553–3569. doi: 10.1105/tpc.113.114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48(12):909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki N, Koussevitzky S, Mittler R, Miller G. ROS and redox signalling in the response of plants to abiotic stress. Plant, Cell Environ. 2012;35(2):259–270. doi: 10.1111/j.1365-3040.2011.02336.x. [DOI] [PubMed] [Google Scholar]
- 75.Hariyadi P, Parkin KL. Chilling-induced oxidative stress in cucumber fruits. Postharvest Biol Technol. 1991;1(1):133–45. doi: 10.1093/jxb/erh001. [DOI] [Google Scholar]
- 76.Repetto M, Semprine J, Boveris A. Lipid peroxidation: chemical mechanism, biological implications and analytical determination. In: Lipid Peroxidation. Catala A, editor Rijeka: InTech; 2012. p. 3–30. [Google Scholar]
- 77.Asada K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50(1):601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 78.Hajiboland R. Reactive oxygen species and photosynthesis. In: Oxidative damage to plants: antioxidant networks and signaling. Ahmad P, editor San Diego, London, Waltham: Academic Press; 2014. p. 1–63. [Google Scholar]
- 79.Gupta DK, Palma JM, Corpas FJ. Redox state as a central regulator of plant-cell stress responses. Cham: Springer International Publishing; 2016. [Google Scholar]
- 80.Anjum NA, Chan M-T, Umar S. Ascorbate-glutathione pathway and stress tolerance in plants. Dordrecht: Springer Netherlands; 2010. [Google Scholar]
- 81.Zou M, Yuan L, Zhu S, Liu S, Ge J, Wang C. Effects of heat stress on photosynthetic characteristics and chloroplast ultrastructure of a heat-sensitive and heat-tolerant cultivar of wucai (Brassica campestris L.). Acta Physiol Plant. 2017;39(1):30. doi: 10.1007/s11738-016-2319-z. [DOI] [Google Scholar]
- 82.Yu K, Zhu K, Ye M, Zhao Y, Chen W, Guo W. Heat tolerance of highbush blueberry is related to the antioxidative enzymes and oxidative protein-repairing enzymes. Sci Hortic (Amsterdam, Neth). 2016;198:36–43. doi: 10.1016/j.scienta.2015.11.018. [DOI] [Google Scholar]
- 83.Corpas FJ, Leterrier M, Valderrama R, Airaki M, Chaki M, Palma JM, Barroso JB. Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci. 2011;181(5):604–611. doi: 10.1016/j.plantsci.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 84.Baudouin E. The language of nitric oxide signalling. Plant Biol. 2011;13(2):233–242. doi: 10.1111/j.1438-8677.2010.00403.x. [DOI] [PubMed] [Google Scholar]
- 85.Groß F, Durner J, Gaupels F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front Plant Sci. 2013;4(October):419. doi: 10.3389/fpls.2013.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rubbo H, Trostchansky A. Nitro-fatty acids: synthesis, properties, and role in biological system. In: Nitric oxide in plants: metabolism and role in stress physiology. Khan MN, Mobin M, Mohammad F, Corpas FJ, editors Cham: Springer International Publishing; 2014. p. 153–162. [Google Scholar]
- 87.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288(2):481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 88.Pugin A, Wendehenne D, Besson-Bard A, Pugin A, Wendehenne D. New insights into nitric oxide signaling in plants. Annu Rev Plant Biol. 2008;59:21–39. doi: 10.1146/annurev.arplant.59.032607.092830. [DOI] [PubMed] [Google Scholar]
- 89.Khan MN, Mobin M, Mohammad F, Corpas FJ. Nitric oxide in plants: metabolism and role in stress physiology. Cham: Springer International Publishing; 2014. [Google Scholar]
- 90.Begara-Morales JC, Sánchez-Calvo B, Chaki M, Mata-Pérez C, Valderrama R, Padilla MN, López-Jaramillo J, Luque F, Corpas FJ, Barroso JB. Differential molecular response of monodehydroascorbate reductase and glutathione reductase by nitration and S-nitrosylation. J Exp Bot. 2015;66(19):5983–5996. doi: 10.1093/jxb/erv306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chaki M, Valderrama R, Fernández-Ocaña AM, Carreras A, Gómez-Rodríguez MV, López-Jaramillo J, Sánchez-Calvo B, Luque F, Leterrier M, et al. High temperature triggers the metabolism of S-nitrosothiols in sunflower mediating a process of nitrosative stress which provokes the inhibition of ferredoxin-NADP reductase by tyrosine nitration. Plant, Cell Environ. 2011;34(11):1803–1818. doi: 10.1111/j.1365-3040.2011.02376.x. [DOI] [PubMed] [Google Scholar]
- 92.Chaki M, Carreras A, López-Jaramillo J, Begara-Morales JC, Sánchez-Calvo B, Valderrama R, Corpas FJ, Barroso JB. Tyrosine nitration provokes inhibition of sunflower carbonic anhydrase (β-CA) activity under high temperature stress. Nitric Oxide. 2013;29(December 2012):30–33. doi: 10.1016/j.niox.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 93.Ziogas V, Tanou G, Filippou P, Diamantidis G, Vasilakakis M, Fotopoulos V, Molassiotis A. Nitrosative responses in citrus plants exposed to six abiotic stress conditions. Plant Physiol Biochem. 2013;68(April 2013):118–126. doi: 10.1016/j.plaphy.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 94.Suzuki N, Mittler R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol Plant. 2006;126:45–51. doi: 10.1111/j.1399-3054.2005.00582.x. [DOI] [Google Scholar]
- 95.Fancy NN, Bahlmann A-K, Loake GJ. Nitric oxide function in plant abiotic stress. Plant Cell Environ. 2017;40(4):462–472. doi: 10.1111/pce.12707. [DOI] [PubMed] [Google Scholar]
- 96.Su K, Bremer DJ, Jeannotte R, Welti R, Yang C. Membrane lipid composition and heat tolerance in cool-season turfgrasses, including a hybrid bluegrass. J Am Soc Hortic Sci. 2009;134(5):511–520. [Google Scholar]
- 97.Higashi Y, Okazaki Y, Myouga F, Shinozaki K, Saito K. Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci Rep. 2015;5(1):10533. doi: 10.1038/srep10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vierling E. The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol. 1991;42(1):579–620. doi: 10.1146/annurev.pp.42.060191.003051. [DOI] [Google Scholar]
- 99.Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017;22(1):53–65. doi: 10.1016/j.tplants.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 100.Sedaghatmehr M, Mueller-Roeber B, Balazadeh S. The plastid metalloprotease FtsH6 and small heat shock protein HSP21 jointly regulate thermomemory in Arabidopsis. Nat Commun. 2016;7(d):12439. doi: 10.1038/ncomms12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bartwal A, Mall R, Lohani P, Guru SK, Arora S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J Plant Growth Regul. 2013;32(1):216–232. doi: 10.1007/s00344-012-9272-x. [DOI] [Google Scholar]
- 102.Rivero RM, Ruiz JM, García PC, López-Lefebre LR, Sánchez E, Romero L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001;160(2):315–321. doi: 10.1016/s0168-9452(00)00395-2. [DOI] [PubMed] [Google Scholar]
- 103.Egigu MC, Ibrahim MA, Riikonen J, Yahya A, Holopainen T, Julkunen-Tiitto R, Holopainen JK. Effects of rising temperature on secondary compounds of yeheb (Cordeauxia edulis Hemsley). Am J Plant Sci. 2014;5(5):517–527. doi: 10.4236/ajps.2014.55066. [DOI] [Google Scholar]
- 104.Jansen K, Du B, Kayler Z, Siegwolf R, Ensminger I, Rennenberg H, Kammerer B, Jaeger C, Schaub M, Kreuzwieser J, et al. Douglas-fir seedlings exhibit metabolic responses to increased temperature and atmospheric drought. PLoS One. 2014;9(12):1–21. doi: 10.1371/journal.pone.0114165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou R, Yu X, Kjær KH, Rosenqvist E, Ottosen C, Wu Z. Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Environ Exp Bot. 2015;118(May):1–11. doi: 10.1016/j.envexpbot.2015.05.006. [DOI] [Google Scholar]
- 106.Wahid A, Farooq M, Hussain I, Rasheed R, Galani S. Responses and management of heat stress in plants. In: Environmental adaptations and stress tolerance of plants in the era of climate change. New York, NY: Springer New York; 2012. p. 135–157. [Google Scholar]
- 107.Singsaas EL, Sharkey TD. The regulation of isoprene emission responses to rapid leaf temperature fluctuations. Plant, Cell Environ. 1998;21(11):1181–1188. doi: 10.1046/j.1365-3040.1998.00380.x. [DOI] [Google Scholar]
- 108.Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL, Molecular P, Program CB, et al. Exploring the temperature-stress metabolome. Plant Physiol. 2004;136(December):4159–4168. doi: 10.1104/pp.104.052142.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shao L, Shu Z, Sun SL, Peng CL, Wang XJ, Lin ZF. Antioxidation of anthocyanins in photosynthesis under high temperature stress. J Integr Plant Biol. 2007;49(9):1341–1351. doi: 10.1111/j.1744-7909.2007.00527.x. [DOI] [Google Scholar]
- 110.Yang R, Guo L, Wang J, Wang Z, Gu Z. Heat shock enhances isothiocyanate formation and antioxidant capacity of cabbage sprouts. J Food Process Preserv. 2016;41(4):1–10. doi: 10.1111/jfpp.13034. [DOI] [Google Scholar]
- 111.Smirnoff N. Ascorbate, Tocopherol and carotenoids: metabolism, pathway engineering and functions. In: Antioxidants and reactive oxygen species in plants. Smirnoff N, editor Oxford, UK: Blackwell Publishing Ltd; 2007. p. 53–86. [Google Scholar]
- 112.Hayat S, Ahmad A. Brassinosteroids: a class of plant hormone. Dodrecht: Springer Science & Business Media; 2010. [Google Scholar]
- 113.Mazorra LM. Brassinosteroid action and its relation with heat stress mechanisms in plants. In: Brassinosteroids: a class of plant hormone. Hayat S, Ahmad A, editors Dordrecht: Springer Netherlands; 2011. p. 289–307. [Google Scholar]
- 114.Ahammed GJ, Yu J, Lado J, Manzi M, Sainz MM, Sotelo M, Zacarías L. Plant hormones under challenging environmental factors. Ahammed GJ, Yu J-Q, editors Dordrecht: Springer Netherlands; 2016. [Google Scholar]
- 115.Hare PD, Cress WA, Van Staden J. Dissecting the roles of osmolyte accumulation during stress. Plant, Cell Environ. 1998;21(6):535–553. doi: 10.1046/j.1365-3040.1998.00309.x. [DOI] [Google Scholar]
- 116.Ruelland E, Vaultier M-N, Zachowski A, Hurry V. Cold signalling and cold acclimation in plants. Adv Bot Res. 2009;49(8):36–150. doi: 10.1016/s0065-2296(08)00602-2. [DOI] [Google Scholar]
- 117.Wahid A, Close TJ. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol Plant. 2007;51(1):104–109. doi: 10.1007/s10535-007-0021-0. [DOI] [Google Scholar]
- 118.Ramani H, Mandavia M, Dave R, Bambharolia R, Silungwe H, Garaniya N. Biochemical and physiological constituents and their correlation in wheat (Triticum aestivum L.) genotypes under high temperature at different development stages. Int J Plant Physiol Biochem. 2017;9(1):1–8. doi: 10.5897/ijppb2015.0240. [DOI] [Google Scholar]
- 119.Anwar Hossain M, Hoque MA, Burritt DJ, Fujita M. Proline protects plants against abiotic oxidative stress. In: Oxidative damage to plants. Ahmad P, editor San Diego, London, Waltham: Elsevier; 2014. p. 477–522. [Google Scholar]
- 120.Rasheed R, Wahid A, Farooq M, Hussain I, Basra SMA. Role of proline and glycinebetaine pretreatments in improving heat tolerance of sprouting sugarcane (Saccharum sp.) buds. Plant Growth Regul. 2011;65(1):35–45. doi: 10.1007/s10725-011-9572-3. [DOI] [Google Scholar]
- 121.Saidi Y, Peter M, Finka A, Cicekli C, Vigh L, Goloubinoff P. Membrane lipid composition affects plant heat sensing and modulates Ca(2+)-dependent heat shock response. Plant Signal Behav. 2010;5(12):1530–1533. doi: 10.4161/psb.5.12.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Khan MN, Mobin M, Abbas ZK. Nitric oxide and high temperature stress: a physiological perspective. In: Nitric oxide action in abiotic stress responses in plants. Khan MN, Mobin M, Mohammad F, Corpas FJ, editors Cham, Heidelberg, New York Dordrecht, London: Springer; 2015. p. 77–93. [Google Scholar]
- 123.Niu L, Liao W. Hydrogen peroxide signaling in plant development and abiotic responses: crosstalk with nitric oxide and calcium. Front Plant Sci. 2016;7(March):1–14. doi: 10.3389/fpls.2016.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xuan Y, Zhou S, Wang L, Cheng Y, Zhao L. Nitric oxide functions as a signal and acts upstream of AtCaM3 in thermotolerance in Arabidopsis seedlings. Plant Physiol. 2010;153(4):1895–1906. doi: 10.1104/pp.110.160424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qu A-L, Ding Y-F, Jiang Q, Zhu C. Molecular mechanisms of the plant heat stress response. Biochem Biophys Res Commun. 2013;432(2):203–207. doi: 10.1016/j.bbrc.2013.01.104. [DOI] [PubMed] [Google Scholar]
- 126.Mishkind M, Vermeer JEM, Darwish E, Munnik T. Heat stress activates phospholipase D and triggers PIP2 accumulation at the plasma membrane and nucleus. Plant J. 2009;60(1):10–21. doi: 10.1111/j.1365-313x.2009.03933.x. [DOI] [PubMed] [Google Scholar]
- 127.Kotak S, Larkindale J, Lee U, von Koskull-Döring P, Vierling E, Scharf K-D. Complexity of the heat stress response in plants. Curr Opin Plant Biol. 2007;10(3):310–316. doi: 10.1016/j.pbi.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 128.Bita CE, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci. 2013;4(July):273. doi: 10.3389/fpls.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dat JF, Foyer CH, Scott IM. Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol. 1998;118(4):1455–1461. doi: 10.1104/pp.118.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Müller M, Munné-Bosch S. Ethylene response factors: a key regulatory hub in hormone and stress signaling. Plant Physiol. 2015;169(1):32–41. doi: 10.1104/pp.15.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Farnese FS, Menezes-Silva PE, Gusman GS, Oliveira JA. When bad guys become good ones: the key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front Plant Sci. 2016;7(April):471. doi: 10.3389/fpls.2016.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xia X-J, Zhou Y-H, Shi K, Zhou J, Foyer CH, Yu J-Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot. 2015;66(10):2839–2856. doi: 10.1093/jxb/erv089. [DOI] [PubMed] [Google Scholar]
- 133.NASA Goddard Institute for Space Studies. NASA, NOAA analyses reveal record-shattering global warm temperatures in 2015 https://www.giss.nasa.gov/research/news/20160120/ (accessed Feb 20, 2017).
- 134.Yao Y, Luo Y, Huang J, Zhao Z. Comparison of monthly temperature extremes simulated by CMIP3 and CMIP5 models. J Clim. 2013;26(19):7692–7707. doi: 10.1175/jcli-d-12-00560.1. [DOI] [Google Scholar]
- 135.Dai AG. Increasing drought under global warming in observations and models. Nat Clim Chang. 2013;3(1):52–58. doi: 10.1038/nclimate1633. [DOI] [Google Scholar]
- 136.Rull V, Vegas-Vilarrúbia T. Unexpected biodiversity loss under global warming in the neotropical Guayana Highlands: A preliminary appraisal. Glob Chang Biol. 2006;12(1):1–9. doi: 10.1111/j.1365-2486.2005.001080.x. [DOI] [Google Scholar]
- 137.Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, Raven PH, Roberts CM, Sexton JO. The biodiversity of species and their rates of extinction, distribution, and protection. Science. 2014;344(6187):1246752. doi: 10.1126/science.1246752. [DOI] [PubMed] [Google Scholar]
- 138.Lenoir J, Svenning JC. Climate-related range shifts – a global multidimensional synthesis and new research directions. Ecography (Cop). 2015;38(1):15–28. doi: 10.1111/ecog.00967. [DOI] [Google Scholar]
- 139.Walther GR, Berger S, Sykes MT. An ecological “footprint” of climate change. Proc R Soc B. 2005;272(1571):1427–1432. doi: 10.1098/rspb.2005.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bertrand R, Lenoir J, Piedallu C, Riofrío-Dillon G, de Ruffray P, Vidal C, Pierrat J-C, Gégout J-C. Changes in plant community composition lag behind climate warming in lowland forests. Nature. 2011;479(V):517–520. doi: 10.1038/nature10548. [DOI] [PubMed] [Google Scholar]
- 141.Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manage. 2010;259(4):660–684. doi: 10.1016/j.foreco.2009.09.001. [DOI] [Google Scholar]
- 142.Teixeira EI, Fischer G, Van Velthuizen H, Walter C, Ewert F. Global hot-spots of heat stress on agricultural crops due to climate change. Agric For Meteorol. 2013;170:206–215. doi: 10.1016/j.agrformet.2011.09.002. [DOI] [Google Scholar]
- 143.Uprety DC, Reddy V. Crop responses to global warming. Singapore: Springer Singapore; 2016. [Google Scholar]
- 144.Marengo J, Cornejo A, Satyamurty P, Nobre C, Sea W. Cold surges in tropical and extratropical South America: the strong event in June 1994. Mon Weather Rev. 1997;125(11):2759–2786. doi:. [DOI] [Google Scholar]
- 145.Barroso JB, Corpas FJ, Carreras A, Sandalio LM, Valderrama R, Palma JM, Lupiáñez JA, del Río LA. Localization of nitric-oxide synthase in plant peroxisomes. J Biol Chem. 1999;274(51):36729–36733. doi: 10.1074/jbc.274.51.36729. [DOI] [PubMed] [Google Scholar]
- 146.Petoukhov V, Semenov VA. A link between reduced Barents-Kara sea ice and cold winter extremes over northern continents. J Geophys Res Atmos. 2010;115(21):1–11. doi: 10.1029/2009jd013568. [DOI] [Google Scholar]
- 147.Chen THH. Plant adaptation to low temperature stress. Can J Plant Pathol. 1994;16(3):231–236. doi: 10.1080/07060669409500760. [DOI] [Google Scholar]
- 148.Puyaubert J, Baudouin E. New clues for a cold case: Nitric oxide response to low temperature. Plant, Cell Environ. 2014;37(12):2623–2630. doi: 10.1111/pce.12329. [DOI] [PubMed] [Google Scholar]
- 149.Janská A, Marsík P, Zelenková S, Ovesná J. Cold stress and acclimation – what is important for metabolic adjustment? Plant Biol. 2010;12(3):395–405. doi: 10.1111/j.1438-8677.2009.00299.x. [DOI] [PubMed] [Google Scholar]
- 150.Cramer GR, Urano K, Delrot S, Pezzotti M, Shinozaki K. Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol. 2011;11(1):163. doi: 10.1186/1471-2229-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rao NK. Plant genetic resources: Advancing conservation and use through biotechnology. Afr J Biotechnol. 2004;3(2):136–145. doi: 10.5897/ajb2004.000-2025. [DOI] [Google Scholar]
- 152.Ahmad P, Prasad MNV. Environmental adaptations and stress tolerance of plants in the era of climate change. New York, Dordrecht, Heidelberg, London: Springer; 2012. [Google Scholar]
- 153.Buchanan BB, Gruissem W, Jones RL. Biochemistry & molecular biology of plants, 2nd ed West Sussex, UK: Wiley Blackwell; 2015. [Google Scholar]
- 154.Phadtare S, Alsina J, Inouye M. Cold-shock response and cold-shock proteins. Curr Opin Microbiol. 1999;2(2):175–180. doi: 10.1016/s1369-5274(99)80031-9. [DOI] [PubMed] [Google Scholar]
- 155.Allen DJ, Ort DR. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001;6(1):36–42. doi: 10.1016/s1360-1385(00)01808-2. [DOI] [PubMed] [Google Scholar]
- 156.Huner NPA, Öquist G, Hurry VM, Krol M, Falk S, Griffith M. Photosynthesis, photoinhibition and low temperature acclimation in cold tolerant plants. Photosynth Res. 1993;37(1):19–39. doi: 10.1007/bf02185436. [DOI] [PubMed] [Google Scholar]
- 157.Mai J, Herbette S, Vandame M, Kositsup B, Kasemsap P, Cavaloc E, Julien JL, Améglio T, Roeckel-Drevet P. Effect of chilling on photosynthesis and antioxidant enzymes in Hevea brasiliensis Muell. Arg. trees – Struct Funct. 2009;23(4):863–874. doi: 10.1007/s00468-009-0328-x. [DOI] [Google Scholar]
- 158.Devacht S, Lootens P, Rold?n-Ruiz I, Carlier L, Baert J, Van Waes J, Van Bockstaele E. Influence of low temperatures on the growth and photosynthetic activity of industrial chicory, Cichorium intybus L. partim. Photosynthetica. 2009;47(3):372–380. doi: 10.1007/s11099-009-0058-8. [DOI] [Google Scholar]
- 159.Haldimann P. How do changes in temperature during growth affect leaf pigment composition and photosynthesis in Zea mays genotypes differing in sensitivity to low temperature? J Exp Bot. 1999;50(333):543–550. doi: 10.1093/jxb/50.333.543. [DOI] [Google Scholar]
- 160.Lichtenthaler HK, Miehé JA. Fluorescence imaging as a diagnostic tool for plant stress. Trends Plant Sci. 1997;2(8):316–320. doi: 10.1016/s1360-1385(97)89954-2. [DOI] [Google Scholar]
- 161.Oliveira ET, Crocomo OJ, Farinha TB, Gallo LA. Large-scale Micropropagation of Aloe vera. HortScience. 2009;44(6):1675–1678. [Google Scholar]
- 162.Li X, Gong B, Xu K. Interaction of nitric oxide and polyamines involves antioxidants and physiological strategies against chilling-induced oxidative damage in Zingiber officinale Roscoe. Sci Hortic (Amsterdam, Neth). 2014;170:237–248. doi: 10.1016/j.scienta.2014.03.026. [DOI] [Google Scholar]
- 163.Maxwell K. Chlorophyll fluorescence-a practical guide. J Exp Bot. 2000;51(345):659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 164.Fracheboud Y, Haldimann P, Leipner J, Stamp P. Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). J Exp Bot. 1999;50(338):1533–1540. doi: 10.1093/jxb/50.338.1533. [DOI] [Google Scholar]
- 165.Georgieva K, Lichtenthaler HK. Photosynthetic response of different pea cultivars to low and high temperature treatments. Photosynthetica. 2006;44(4):569–578. doi: 10.1007/s11099-006-0073-y. [DOI] [Google Scholar]
- 166.Chaves CJN, Leal BSS, de Lemos-Filho JP. Temperature modulation of thermal tolerance of a CAM-tank bromeliad and the relationship with acid accumulation in different leaf regions. Physiol Plant. 2015;154(4):500–510. doi: 10.1111/ppl.12295. [DOI] [PubMed] [Google Scholar]
- 167.Jouve L, Engelmann F, Noirot M, Charrier A. Evaluation of biochemical markers (sugar, proline, malonedialdehyde and ethylene) for cold sensitivity in microcuttingsof two coffee species. Plant Sci. 1993;91(1):109–116. doi: 10.1016/0168-9452(93)90194-5. [DOI] [Google Scholar]
- 168.Guo Y, Liu S, Yang Z, Tian S, Sui N. Responses of unsaturated fatty acid in membrane lipid and antioxidant enzymes to chilling stress in sweet sorghum (Sorghum bicolor (L.) Moench) seedling. J Agric Sci. 2016;8(9):71. doi: 10.5539/jas.v8n9p71. [DOI] [Google Scholar]
- 169.Posmyk MM, Bailly C, Szafrańska K, Janas KM, Corbineau F. Antioxidant enzymes and isoflavonoids in chilled soybean (Glycine max (L.) Merr.) seedlings. J Plant Physiol. 2005;162:403–412. doi: 10.1016/j.jplph.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 170.Rivero RM, Ruiz JM, García PC, López-Lefebre LR, Sánchez E, Romero L. Response of oxidative metabolism in watermelon plants subjected to cold stress. Funct Plant Biol. 2002;29(5):643. doi: 10.1071/pp01013. [DOI] [PubMed] [Google Scholar]
- 171.Zhang S, Jiang H, Peng S, Korpelainen H, Li C. Sex-related differences in morphological, physiological, and ultrastructural responses of Populus cathayana to chilling. J Exp Bot. 2011;62(2):675–686. doi: 10.1093/jxb/erq306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Corpas FJ, Chaki M, Fernández-Ocaña A, Valderrama R, Palma JM, Carreras A, Begara-Morales JC, Airaki M, Del Río LA, Barroso JB. Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol. 2008;49(11):1711–1722. doi: 10.1093/pcp/pcn144. [DOI] [PubMed] [Google Scholar]
- 173.Begara-Morales JC, Sánchez-Calvo B, Chaki M, Valderrama R, Mata-Pérez C, López-Jaramillo J, Padilla MN, Carreras A, Corpas FJ, Barroso JB. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. J Exp Bot. 2014;65(2):527–538. doi: 10.1093/jxb/ert396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, et al. The chemical biology of nitric oxide: Implications in cellular signaling. Free Radic Biol Med. 2008;45(1):18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Clark D, Durner J, Navarre DA, Klessig DF. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol Plant Microbe Interact. 2000;13(12):1380–1384. doi: 10.1094/mpmi.2000.13.12.1380. [DOI] [PubMed] [Google Scholar]
- 176.Lin A, Wang Y, Tang J, Xue P, Li C, Liu L, Hu B, Yang F, Loake GJ, Chu C. Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol. 2012;158(1):451–464. doi: 10.1104/pp.111.184531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sehrawat A, Gupta R, Deswal R. Nitric oxide-cold stress signalling cross-talk, evolution of a novel regulatory mechanism. Proteomics. 2013;13(12–13):1816–1835. doi: 10.1002/pmic.201200445. [DOI] [PubMed] [Google Scholar]
- 178.Mioto PT, Freschi L, Mercier H. Phytohormones and nitric oxide interactions during abiotic stress responses. In: Nitric oxide in plants: metabolism and role in stress physiology. Khan MN, Mobin M, Mohammad F, Corpas FJ, editors Cham: Springer International Publishing; 2014. p. 211–224. [Google Scholar]
- 179.Salgado I, Carmen Martínez M, Oliveira HC, Frungillo L. Nitric oxide signaling and homeostasis in plants: a focus on nitrate reductase and S-nitrosoglutathione reductase in stress-related responses. Braz J Bot. 2013;36(2):89–98. doi: 10.1007/s40415-013-0013-6. [DOI] [Google Scholar]
- 180.Lesch M, Kaiser WM, Cantrel C, Vazquez T, Puyaubert J, Reze N, Dutilleul C, Guillas I, Zachowski A, Baudouin E. Nitric oxide participates in cold-responsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana. 2011;1(2010):415–427. doi: 10.1111/j.1469-8137.2010.03500.x. [DOI] [PubMed] [Google Scholar]
- 181.Chamizo-Ampudia A, Sanz-Luque E, Llamas A, Galvan A, Fernandez E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017;22(2):163–174. doi: 10.1016/j.tplants.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 182.Kaiser WM, Huber SC. Post-translational regulation of nitrate reductase: mechanism, physiological relevance and environmental triggers. J Exp Bot. 2001;52(363):1981–1989. doi: 10.1093/jexbot/52.363.1981. [DOI] [PubMed] [Google Scholar]
- 183.Zhao M-G, Chen L, Zhang L-L, Zhang W-H, Oa AW, Zhao M-G, Chen L, Zhang L-L, Zhang W-H, Oa AW. Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol. 2009;151(2):755–767. doi: 10.1104/pp.109.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cantrel C, Vazquez T, Puyaubert J, Rezé N, Lesch M, Kaiser WM, Dutilleul C, Guillas I, Zachowski A, Baudouin E. Nitric oxide participates in cold-responsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana. New Phytol. 2011;189(2):415–427. doi: 10.1111/j.1469-8137.2010.03500.x. [DOI] [PubMed] [Google Scholar]
- 185.Couée I, Sulmon C, Gouesbet G, El Amrani A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot. 2006;57(3):449–459. doi: 10.1093/jxb/erj027. [DOI] [PubMed] [Google Scholar]
- 186.Guy CL, Huber JL, Huber SC. Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant Physiol. 1992;100:502–508. doi: 10.1104/pp.100.1.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sasaki H, Ichimura K, Imada S, Yamaki S. Sucrose synthase and sucrose phosphate synthase, but not acid invertase, are regulated by cold acclimation and deacclimation in cabbage seedlings. J Plant Physiol. 2001;158(7):847–852. doi: 10.1078/0176-1617-00391. [DOI] [Google Scholar]
- 188.Nägele T, Kandel BA, Frana S, Meißner M, Heyer AG. A systems biology approach for the analysis of carbohydrate dynamics during acclimation to low temperature in Arabidopsis thaliana. FEBS J. 2011;278(3):506–518. doi: 10.1111/j.1742-4658.2010.07971.x. [DOI] [PubMed] [Google Scholar]
- 189.Demidchik V. Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot. 2015;109:212–228. doi: 10.1016/j.envexpbot.2014.06.021. [DOI] [Google Scholar]
- 190.Bogdanović J, Mojović M, Milosavić N, Mitrović A, Vucinić Z, Spasojević I. Role of fructose in the adaptation of plants to cold-induced oxidative stress. Eur Biophys J. 2008;37(7):1241–1246. doi: 10.1007/s00249-008-0260-9. [DOI] [PubMed] [Google Scholar]
- 191.Knaupp M, Mishra KB, Nedbal L, Heyer AG. Evidence for a role of raffinose in stabilizing photosystem II during freeze – thaw cycles. Planta. 2011;234:477–486. doi: 10.1007/s00425-011-1413-0. [DOI] [PubMed] [Google Scholar]
- 192.Tarkowski ŁP, Van den Ende W. Cold tolerance triggered by soluble sugars: a multifaceted countermeasure. Front Plant Sci. 2015;6(April):203. doi: 10.3389/fpls.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Strauss G, Hauser H. Stabilization of lipid bilayer vesicles by sucrose during freezing. Proc Natl Acad Sci. 1986;83(8):2422–2426. doi: 10.1073/pnas.83.8.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hincha DK, Zuther E, Heyer AG. The preservation of liposomes by raffinose family oligosaccharides during drying is mediated by effects on fusion and lipid phase transitions. Biochim Biophys Acta – Biomembr. 2003;1612(2):172–177. doi: 10.1016/s0005-2736(03)00116-0. [DOI] [PubMed] [Google Scholar]
- 195.Oliveira G, Peñuelas J. Effects of winter cold stress on photosynthesis and photochemical efficiency of PSII of the Mediterranean Cistus albidus L. and Quercus ilex L. Plant Ecol. 2004;175:179–191. doi: 10.1007/s11258-005-4876-x. [DOI] [Google Scholar]
- 196.Bose B, Srivastava HS. Absorption and accumulation of nitrate in plants: Influence of environmental factors. Indian J Exp Biol. 2001;39(2):101–110. [PubMed] [Google Scholar]
- 197.Smart DR, Bloom AJ. Influence of root NH4+ and NO3− content on the temperature response of net NH4+ and NO3− uptake in chilling sensitive and chilling resistant Lycopersicon taxa. J Exp Bot. 1991;42(236):331–338 [Google Scholar]
- 198.Thomashow MF. Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiol. 2010;154(2):571–577. doi: 10.1104/pp.110.161794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Plieth C, Hansen UP, Knight H, Knight MR. Temperature sensing by plants: The primary characteristics of signal perception and calcium response. Plant J. 1999;18(5):491–497. doi: 10.1046/j.1365-313x.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- 200.Browse J, Xin Z. Temperature sensing and cold acclimation. Curr Opin Plant Biol. 2001;4(3):241–246. doi: 10.1016/s1369-5266(00)00167-9. [DOI] [PubMed] [Google Scholar]
- 201.Rihan HZ, Al-Issawi M, Fuller MP. Advances in physiological and molecular aspects of plant cold tolerance. J Plant Interact. 2017;12(1):143–157. doi: 10.1080/17429145.2017.1308568. [DOI] [Google Scholar]
- 202.Vigh L, Nakamoto H, Landry J, Gomez-Munoz A, Harwood JL, Horvath I. Membrane regulation of the stress response from prokaryotic models to mammalian cells. Ann N Y Acad Sci. 2007;1113:40–51. doi: 10.1196/annals.1391.027. [DOI] [PubMed] [Google Scholar]
- 203.Gupta KJ, Hincha DK, Mur LAJ. No way to treat a cold. New Phytol. 2011;189:360–363. doi: 10.1111/j.1469-8137.2010.03586.x. [DOI] [PubMed] [Google Scholar]
- 204.Hou Q, Ufer G, Bartels D. Lipid signalling in plant responses to abiotic stress. Plant, Cell Environ. 2016;39(5):1029–1048. doi: 10.1111/pce.12666. [DOI] [PubMed] [Google Scholar]
- 205.Ruelland E, Cantrel C, Gawer M, Kader JC, Zachowski A. Activation of phospholipases C and D is an early response to a cold exposure in Arabidopsis suspension cells. Plant Physiol. 2002;130(2):999–1007. doi: 10.1104/pp.006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lillo C. Signalling cascades integrating light-enhanced nitrate metabolism. Biochem J. 2008;415(1):11–19. doi: 10.1042/bj20081115. [DOI] [PubMed] [Google Scholar]
- 207.Yaneva IA, Hoffmann GW, Tischner R. Nitrate reductase from winter wheat leaves is activated at low temperature via protein dephosphorylation. Physiol Plant. 2002;114:65–72. doi: 10.1034/j.1399-3054.2002.1140110.x. [DOI] [PubMed] [Google Scholar]
- 208.Gao HB, Chu YJ, Xue HW. Phosphatidic acid (PA) Binds PP2AA1 to regulate PP2A activity and PIN1 polar localization. Mol Plant. 2013;6(5):1692–1702. doi: 10.1093/mp/sst076. [DOI] [PubMed] [Google Scholar]
- 209.Li PH, Palva ET. Plant cold hardiness. Boston, MA: Springer US; 2002. [Google Scholar]
- 210.Yun K-Y, Park MR, Mohanty B, Herath V, Xu F, Mauleon R, Wijaya E, Bajic VB, Bruskiewich R, de los Reyes BG. Transcriptional regulatory network triggered by oxidative signals configures the early response mechanisms of Japonica rice to chilling stress. BMC Plant Biol. 2010;10(1):16. doi: 10.1186/1471-2229-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Orvar BL, Sangwan V, Omann F, Dhindsa RS. Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity. Plant J. 2000;23(6):785–794. doi: 10.1046/j.1365-313x.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- 212.Gusta L V., Trischuk R, Weiser CJ. Plant cold acclimation: the role of abscisic acid. J Plant Growth Regul. 2005;24(4):308–318. doi: 10.1007/s00344-005-0079-x. [DOI] [Google Scholar]
- 213.Thomashow MF. Plant cold accliation: Freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- 214.Vitasse Y, Lenz A, Körner C. The interaction between freezing tolerance and phenology in temperate deciduous trees. Front Plant Sci. 2014;5(October):1–12. doi: 10.3389/fpls.2014.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bar Dolev M, Braslavsky I. Ice-binding proteins—not only for ice growth control. Temperature. 2017;4(2):112–113. doi: 10.1080/23328940.2017.1279255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Benson EE. Cryopreservation of phytodiversity: a critical appraisal of theory & practice. CRC Crit Rev Plant Sci. 2008;27(3):141–219. doi: 10.1080/07352680802202034. [DOI] [Google Scholar]
- 217.Engelmann F. Plant cryopreservation: Progress and prospects. In Vitro Cell Dev Biol: Plant. 2004;40(5):427–433. doi: 10.1079/ivp2004541. [DOI] [Google Scholar]
- 218.Ngobese NZ, Sershen , Pammenter NW, Berjak P. Cryopreservation of the embryonic axes of Phoenix reclinata, a representative of the intermediate seed category. Seed Sci Technol. 2010;38(3):704–716. doi: 10.15258/sst.2010.38.3.18. [DOI] [Google Scholar]
- 219.Whiteley SE, Bunn E, Menon A, Mancera RL, Turner SR. Ex situ conservation of the endangered species Androcalva perlaria (Malvaceae) by micropropagation and cryopreservation. Plant Cell Tissue Organ Cult. 2016;125(2):341–352. doi: 10.1007/s11240-016-0955-z. [DOI] [Google Scholar]
- 220.Touchell DH, Dixon KW. Cryopreservation for seedbanking of australian species. Ann Bot. 1994;74(5):541–546. doi: 10.1006/anbo.1994.1152. [DOI] [Google Scholar]
- 221.Köhl M, Pancel L. Tropical forestry handbook. Pancel L, Köhl M, editors Berlin, Heidelberg: Springer Berlin Heidelberg; 2016. [Google Scholar]
- 222.Cavaleri MA, Reed SC, Smith WK, Wood TE. Urgent need for warming experiments in tropical forests. Glob Chang Biol. 2015;21(6):2111–2121. doi: 10.1111/gcb.12860. [DOI] [PubMed] [Google Scholar]
- 223.Cunningham SC, Read J. Comparison of temperate and tropical rainforest tree species: Photosynthetic responses to growth temperature. Oecologia. 2002;133(2):112–119. doi: 10.1007/s00442-002-1034-1. [DOI] [PubMed] [Google Scholar]
- 224.Feeley KJ, Rehm EM, Machovina B. The responses of tropical forest species to global climate change: acclimate, adapt, migrate or go extinct? Front Biogeogr. 2012;4(2):69–84. [Google Scholar]
- 225.Cunningham SC, Read J. Foliar temperature tolerance of temperate and tropical evergreen rain forest trees of Australia. Tree Physiol. 2006;26(11):1435–1443. doi: 10.1093/treephys/26.11.1435. [DOI] [PubMed] [Google Scholar]
- 226.Feeley KJ. Moving forward with species distributions. Am J Bot. 2015;102(2):173–175. doi: 10.3732/ajb.1400545. [DOI] [PubMed] [Google Scholar]
- 227.Klein AL. Eugen Warming e o cerrado brasileiro: um século depois. Klein AL, editor São Paulo: Unesp; 2000. [Google Scholar]
- 228.Mollo L, Martins MCM, Oliveira VF, Nievola CC, L. Figueiredo-Ribeiro RDC. Effects of low temperature on growth and non-structural carbohydrates of the imperial bromeliad Alcantarea imperialis cultured in vitro. Plant Cell Tissue Organ Cult. 2011;107(1):141–149. doi: 10.1007/s11240-011-9966-y. [DOI] [Google Scholar]
- 229.Versieux LM, Wanderley MGL. Bromélias gigantes do Brasil. Natal: Capim Macio & Offset; 2015. [Google Scholar]
- 230.Nunes JVC. Bromélias. In: Sustentável Mata Atlântica: a exploração de seus recursos florestais. Simões LL, Lino CF, editors São Paulo: Senac; 2006. p. 119–132. [Google Scholar]
- 231.Leme EMC, Marigo LC. Bromélias na natureza. Rio de Janeiro: Marigo Comunicação Visual; 1993. [Google Scholar]
- 232.Cach-Pérez MJ, Andrade JL, Reyes-García C. La susceptibilidad de las bromeliáceas epífitas al cambio climático. Bot Sci. 2014;92(2):157–168. doi: 10.17129/botsci.55. [DOI] [Google Scholar]
- 233.Andrade JL. Dew deposition on epiphytic bromeliad leaves: an important event in a Mexican tropical dry deciduous forest. J Trop Ecol. 2003;19(5):479–488. [Google Scholar]
- 234.Graham EA, Andrade JL. Drought tolerance associated with vertical stratification of two co-occurring epiphytic bromeliads in a tropical dry forest. Am J Bot. 2004;91(5):699–706. doi: 10.3732/ajb.91.5.699. [DOI] [PubMed] [Google Scholar]
- 235.Mondragón D, Calvo-Irabien LM, Benzing DH. The basis for obligate epiphytism in Tillandsia brachycaulos (Bromeliaceae) in a Mexican tropical dry forest. J Trop Ecol. 2004;20(1):97–104. doi: 10.1017/s0266467403001007. [DOI] [Google Scholar]
- 236.Reyes-García C, Griffiths H, Rincón E, Huante P. Niche differentiation in tank and atmospheric epiphytic bromeliads of a seasonally dry forest. Biotropica. 2008;40(2):168–175. doi: 10.1111/j.1744-7429.2007.00359.x. [DOI] [Google Scholar]
- 237.Carvalho CP, Hayashi AH, Braga MR, Nievola CC. Biochemical and anatomical responses related to the in vitro survival of the tropical bromeliad Nidularium minutum to low temperatures. Plant Physiol Biochem. 2013;71:144–154. doi: 10.1016/j.plaphy.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 238.De Carvalho V, Dos Santos DS, Nievola CC. In vitro storage under slow growth and ex vitro acclimatization of the ornamental bromeliad Acanthostachys strobilacea. S Afr J Bot. 2014;92(MAY):39–43. doi: 10.1016/j.sajb.2014.01.011. [DOI] [Google Scholar]
- 239.Freitas C, Carvalho V, Nievola CC. Effect of sucrose concentrations on in vitro growth and subsequent acclimatization of the native bromeliad Vriesea inflata (Wawra) Wawra. Biotemas. 2015;28(3):37. doi: 10.5007/2175-7925.2015v28n3p37. [DOI] [Google Scholar]
- 240.Pedroso ANV, Lazarini RA. de M, Tamaki V, Nievola CC. In vitro culture at low temperature and ex vitro acclimatization of Vriesea inflata an ornamental bromeliad. Rev Bras Botânica. 2010;33(3):407–414. doi: 10.1590/s0100-84042010000300004. [DOI] [Google Scholar]
- 241.Santos DS dos, Cardoso-Gustavson P, Nievola CC. Stem elongation of ornamental bromeliad in tissue culture depends on the temperature even in the presence of gibberellic acid. Acta Physiol Plant. 2017;39(October):230. doi: 10.1007/s11738-017-2536-0. [DOI] [Google Scholar]
- 242.Hartmann HT, Kester DE, Davies FT Jr, Geneve RL. Plant propagation: principles and practices. Upper Saddle River: Prentice Hall; 2002. [Google Scholar]
- 243.Mercier H, Kerbauy GB. Microprogation of ornamental bromeliads (Bromeliaceae). In: High-tech and micropropagation VI. Bajaj YPS, editor Springer Berlin Heidelberg; 1997. p. 43–57. [Google Scholar]
- 244.Grattapaglia D, Machado MA. Micropropagação. In: Cultura de tecidos e transformação genética de plantas. Torres AC, Caldas LS, Buso JA, editors Brasília: Embrapa-SPI/Embrapa-CNPH; 1998. p. 183–260. [Google Scholar]
- 245.Rodrigues MA, Hamachi L, Mioto PT, Purgatto E, Mercier H. Implications of leaf ontogeny on drought-induced gradients of CAM expression and ABA levels in rosettes of the epiphytic tank bromeliad Guzmania monostachia. Plant Physiol Biochem. 2016;108:400–411. doi: 10.1016/j.plaphy.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 246.Carvalho V, Abreu ME, Mercier H, Nievola CC. Adjustments in CAM and enzymatic scavenging of H2O2 in juvenile plants of the epiphytic bromeliad Guzmania monostachia as affected by drought and rewatering. Plant Physiol Biochem. 2017;113:32–39. doi: 10.1016/j.plaphy.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 247.Pereira PN, Purgatto E, Mercier H. Spatial division of phosphoenolpyruvate carboxylase and nitrate reductase activity and its regulation by cytokinins in CAM-induced leaves of Guzmania monostachia (Bromeliaceae). J Plant Physiol. 2013;170(12):1067–1074. doi: 10.1016/j.jplph.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 248.Taybi T, Cushman JC, Borland AM. Environmental, hormonal and circadian regulation of crassulacean acid metabolism expression. Funct Plant Biol. 2002;29(6):669–678. doi: 10.1071/pp01244. [DOI] [PubMed] [Google Scholar]
- 249.Lüttge U. Ecophysiology of Crassulacean Acid Metabolism (CAM). Ann Bot. 2004;93(6):629–652. doi: 10.1093/aob/mch087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Haag-Kerwer A, Franco AC, Lüttge U. The effect of temperature and light on gas exchange and acid accumulation in the C3-CAM plant Clusia minor L. J Exp Bot. 1992;43(3):345–352. doi: 10.1093/jxb/43.3.345. [DOI] [Google Scholar]
- 251.Dodd AN, Borland AM, Haslam RP, Griffiths H, Maxwell K. Crassulacean acid metabolism: plastic, fantastic. J Exp Bot. 2002;53(369):569–580. doi: 10.1093/jexbot/53.369.569. [DOI] [PubMed] [Google Scholar]
- 252.Nievola CC, Kraus JE, Freschi L, Souza BM, Mercier H. Temperature determines the occurrence of CAM or C3 photosynthesis in pineapple plantlets grown in vitro. In Vitro Cell Dev Biol: Plant. 2005;41(6):832–837. doi: 10.1079/ivp2005694. [DOI] [Google Scholar]
- 253.Freschi L, Nievola CC, Rodrigues MA, Domingues DS, Van Sluys M-A, Mercier H. Thermoperiod affects the diurnal cycle of nitrate reductase expression and activity in pineapple plants by modulating the endogenous levels of cytokinins. Physiol Plant. 2009;137(3):201–212. doi: 10.1111/j.1399-3054.2009.01283.x. [DOI] [PubMed] [Google Scholar]
- 254.Hagiwara WT, Menezes FO, Nievola CC. Sobrevivência de plantas da bromélia Acanthostachys strobilacea (Schultz f.) Klotzsch & Otto cultivadas in vitro em alta temperatura constante e déficit hídrico. Paper presented at: 22ª Reunião Anual do Instituto de Botânica; 2015; São Paulo, Brazil. [Google Scholar]
- 255.Liu Y, Subhash C, Yan J, Song C, Zhao J, Li J. Maize leaf temperature responses to drought: Thermal imaging and quantitative trait loci (QTL) mapping. Environ Exp Bot. 2011;71(2):158–165. doi: 10.1016/j.envexpbot.2010.11.010. [DOI] [Google Scholar]
- 256.Sirault XRR, James RA, Furbank RT. A new screening method for osmotic component of salinity tolerance in cereals using infrared thermography. Funct Plant Biol. 2009;36(11):970–977. doi: 10.1071/fp09182. [DOI] [PubMed] [Google Scholar]
- 257.Wang Y, Holroyd G, Hetherington AM, Ng CKY. Seeing “cool” and “hot” – Infrared thermography as a tool for non-invasive, high-throughput screening of Arabidopsis guard cell signalling mutants. J Exp Bot. 2004;55(400):1187–1193. doi: 10.1093/jxb/erh135. [DOI] [PubMed] [Google Scholar]
- 258.Wisniewski M, Lindow SE, Ashworth EN. Observations of ice nucleation and propagation in plants using infrared video thermography. Plant Physiol. 1997;113(2):327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mercier H, Nievola CC. Obtenção de bromélia in vitro como estratégia de preservação. Vidália. 2003;1(1):57–62. [Google Scholar]
- 260.Reed BM, Gupta S, Uchendu EE. In vitro genebanks for preserving tropical biodiversity. In: Conservation of tropical plant species. Normah MN, Noor NM, Reed BM, editors Springer; 2013. p. 77–106. [Google Scholar]
- 261.Ellis RH, Hong TD, Roberts EH. An intermediate category of seed storage behaviour? J Exp Bot. 1990;41(9):1167–1174. doi: 10.1093/jxb/41.9.1167. [DOI] [Google Scholar]
- 262.Orlikowska T. Effect of in vitro storage at 4° C on survival and proliferation of two apple rootstocks. Plant Cell Tissue Organ Cult. 1992;31(1):1–7. doi: 10.1007/bf00043468. [DOI] [Google Scholar]
- 263.Bekheet SA, Taha HS, Saker MM. In vitro long-term storage of date palm. Biol Plant. 2002;45(1):121–124. doi: 10.1023/a:1015121111934. [DOI] [Google Scholar]
- 264.Gonçalves S, Romano A. In vitro minimum growth for conservation of Drosophyllum lusitanicum. Biol Plant. 2007;51(4):795–798. doi: 10.1007/s10535-007-0163-0. [DOI] [Google Scholar]
- 265.Zandvoort EA, Hulshof MJH, Staritsky G. In vitro storage of Xanthosoma spp. under minimal growth conditions. Plant Cell Tissue Organ Cult. 1994;36(3):309–316. doi: 10.1007/bf00046088. [DOI] [Google Scholar]
- 266.Lata H, Moraes RM, Bertoni B, Pereira AMS. In vitro germplasm conservation of Podophyllum peltatum L. under slow growth conditions. In Vitro Cell Dev Biol: Plant. 2009;46(1):22–27. doi: 10.1007/s11627-009-9243-5. [DOI] [Google Scholar]
- 267.Forzza RC, Costa AF, Leme EMC, Versieux LM, Wanderley MGL, Louzada RB, Monteiro RF, Judice DM, Fernandez EP, Borges RAX, et al. Bromeliaceae. In: Livro vermelho da flora do Brasil. Martinelli G, Moraes MA, editors Rio de Janeiro: Centro Nacional de Conservação da Flora; 2013. p. 315–397. [Google Scholar]
- 268.Kew Plants of the world online. http://powo.science.kew.org/ (accessed Jun 7, 2017).
- 269.Banowetz GM, Azevedo MD, Stout R. Morphological adaptations of hot springs panic grass Dichanthelium lanigunosum var sericeum (Schmoll) to thermal stress. J Therm Biol. 2008;33(2):106–116. doi: 10.1016/j.jtherbio.2007.08.006. [DOI] [Google Scholar]
- 270.Lawson T, Davey PA, Yates SA, Bechtold U, Baeshen M, Baeshen N, Mutwakil MZ, Sabir J, Baker NR, Mullineaux PM. C3 photosynthesis in the desert plant Rhazya stricta is fully functional at high temperatures and light intensities. New Phytol. 2014;201(3):862–873. doi: 10.1111/nph.12559. [DOI] [PubMed] [Google Scholar]
- 271.Obaid AY, Sabir JSM, Atef A, Liu X, Edris S, El-Domyati FM, Mutwakil MZ, Gadalla NO, Hajrah NH, Al-Kordy MA, et al. Analysis of transcriptional response to heat stress in Rhazya stricta. BMC Plant Biol. 2016;16(1):252. doi: 10.1186/s12870-016-0938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lu P, Sang WG, Ma KP. Differential responses of the activities of antioxidant enzymes to thermal stresses between two invasive Eupatorium species in China. J Integr Plant Biol. 2008;50(4):393–401. doi: 10.1111/j.1744-7909.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 273.Sasaki H, Ichimura K, Oda M. Changes in sugar content during cold acclimation and deacclimation of cabbage seedlings. Ann Bot. 1996;78(3):365–369. doi: 10.1006/anbo.1996.0131. [DOI] [Google Scholar]
- 274.Wang K, Shao X, Gong Y, Zhu Y, Wang H, Zhang X, Yu D, Yu F, Qiu Z, Lu H. The metabolism of soluble carbohydrates related to chilling injury in peach fruit exposed to cold stress. Postharvest Biol Technol. 2013;86:53–61. doi: 10.1016/j.postharvbio.2013.06.020. [DOI] [Google Scholar]
- 275.Diao Q, Song Y, Shi D, Qi H. Interaction of polyamines, abscisic acid, nitric oxide, and hydrogen peroxide under chilling stress in tomato (Lycopersicon esculentum Mill.) Seedlings. Front Plant Sci. 2017;8(February):1–16. doi: 10.3389/fpls.2017.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Liu X, Wang L, Liu L, Guo Y, Ren H. Alleviating effect of exogenous nitric oxide in cucumber seedling against chilling stress. Afr J Biotechnol. 2011;10(21):4380–4386. doi: 10.5897/ajb10.812. [DOI] [Google Scholar]
- 277.CEPAGRI – Centro de Pesquisas Meteorológicas e Climáticas Aplicadas à Agricultura Clima dos municípios paulistas http://www.cpa.unicamp.br/outras-informacoes/clima-dos-municipios-paulistas.html (accessed May 30, 2017).
- 278.Gutjahr MR, Tavares R. Clima . In: Patrimônio da Reserva Biológica do Alto da Serra de Paranapiacaba. Lopes MIMS, Kirizawa M, Melo MMRF, editors São Paulo: Instituto de Botânica; 2009. p. 39–51. [Google Scholar]
- 279.ICMBio – Instituto Chico Mendes de Conservação da Biodiversidade do Ministério do Meio Ambiente Parque Nacional Serra dos Órgãos – Clima http://www.icmbio.gov.br/parnaserradosorgaos/atributos-naturais/45-clima.html (accessed May 30, 2017).


