ABSTRACT
Pancreatic islet transplantation is being extensively researched as an alternative treatment for type 1 diabetic patients. This treatment is currently limited by temporal mismatch, between the availability of pancreas and isolated islets from deceased organ donor, and the recipient's need for freshly isolated islets. To solve this issue, cryopreservation of islets may offer the potential to bank islets for transplant on demand. Cryopreservation, however, introduces an overwhelmingly harsh environment to the ever-so-fragile islets. After exposure to the freezing and thawing, islets are usually either apoptotic, non-functional, or non-viable. Several studies have proposed various techniques that could lead to increased cell survival and function following a deep freeze. The purpose of this article is to critically review the techniques of islet cryopreservation, with the goal of highlighting optimization parameters that can lead to the most viable and functional islet upon recovery and/or transplant.
KEYWORDS: cryopreservation, cryoprotectant, pancreatic islets, transplantation, islet freezing, islet thawing, diabetes, Islet cell biology/physiology
Introduction
The idea of solely transplanting the endocrine component of the pancreas has been around since the 19th century, however, it was not until the mid-1980s when the first human islet transplant occurred at Washington University.1 In 1990, the University of Pittsburgh successfully performed human islet allografts with prolonged insulin-independence, due to improvements in immunosuppression.1 In the year 2000, the Edmonton protocol was established under which 7 consecutive type 1 diabetic patients underwent islet transplantations and became euglycemic for three to five years.2 In the years following the Edmonton protocol, the total number of islet allotransplants has been 1,011,3 and continues to increase yearly. In a study comparing pre-and post-Edmonton protocols for cryopreserved islet recovery and survival, they found a 19.3% higher survival rate at 24 hours (50.1% vs 69.4% respectively), and an overall higher rate at each time point for a total of 7 days.4 Although this is an improvement over previous techniques, there needs to be a better protocol to make long-term islet cryopreservation feasible.
As islet transplantation technology evolves and becomes more realistic, the organ donor shortage is further exacerbated due to the necessity of using islets from multiple donors.5 During human islet isolation, donor islets undergo great stress which begins during harvest and continues until the moment of transplantation.6 Although a human pancreas has nearly a million islets, the isolation process causes a 15–50% reduction in islet mass and function.5 Therefore, an integral component of pancreatic islet isolation is the ability to preserve the cells once obtained from the donor. Currently, the gold standard for islet transplantation is to procure the cells from a deceased donor and rapidly transfer them to the recipient in a 4° Celsius preservation solution.7,8 Inherent to this process is a donor-recipient mismatch, that is, it is difficult to match the cocktail of islets from several recently deceased donors to a surgically-ready recipient. With each improvement in islet cryopreservation, the utility of clinical islet transplantations becomes more feasible for type 1 diabetic patients. Preserving highly functional islets for an indefinite period of time would not only allow islet transplantations in remote areas to be possible, but also would permit more successful transplantations. The purpose of improving current methods of islet cryopreservation is to minimize the challenge of time and bridge the gap between donor and recipient, thus improving clinical outcome and overall utility of islet transplantation in type 1 diabetic patients.
Islet preservation options include the following: conventionally culturing at 37°C, cold culturing at ∼ 4°C, and cryopreservation at −80 to −196°C.9 The different temperatures of preservation each have their own strengths and weaknesses regarding islet viability and function after the preservation period. For example, in one study, islets preserved in the 37°C group showed better function and less tissue death than the ones in the −80°C group at day 1, but at day 7, they were significantly less functional and more apoptotic.9 It is important to seek the technique which provides the highest islet viability and function after extended preservation periods. This would make it possible to bank islets indefinitely and use them only when they are needed for transplant. In this paper, we describe the history of islet cryopreservation, and the various parameters associated with successful function after the freeze and thaw periods.
History of islet cryopreservation
In the late 19th century, the first cryopreservation experiments were conducted on spermatozoa and red blood cells. Researchers found that they could freeze human spermatozoa and later show functional recovery. The problem was, the results were inconsistent and when the spermatozoa were used for fertilization, there was early embryonic cell death.10 This was due to the lack of cryoprotectants and the technique of instantaneously freezing and thawing the cells.10 It was not until the 1920s when James Lovelock first explained that red blood cells experience osmotic stress during freezing which leads to cellular death.11 In 1948, Polge, Smith, and Parkes accidentally discovered the cryoprotective effect of glycerol, which they unintentionally used during the successful cryopreservation of avian spermatozoa.12 After this discovery, Smith successfully used glycerol to cryopreserve human red blood cells.13 At this point, researchers realized that the cryoprotectant along with the freezing and thawing rate were two important parameters which affected cell function after recovery.14,15 Mazur et al explained that both freezing and thawing should occur slowly and in a controlled matter so that cellular equilibrium is reached at each incremental moment, so that neither ice crystals nor high solute levels would damage the cells.14 When the cell is frozen too quickly, water is not able to flow out of the cell quick enough, leading to ice within the cell. Currently, a common cryoprotectant is dimethyl sulfoxide (DMSO), which is used in 10%, 2 M concentrations and added to cells prior to the freezing stage.16 This makes the membrane porous allowing for water to flow out of the cell more easily. Likewise, another method used to prevent ice crystal formation is nucleation (Fig. 1).17 This is done by touching the meniscus of a −7.5°C test tube with a metal rod pre-dipped in liquid nitrogen, thus allowing the latent heat of fusion to be released causing a more uniform temperature within the test tube.17 Furthermore, a shift from entire organ to tissue cryopreservation is also occurring due to the challenges of preventing ice crystal damage when cryopreserving entire organs.
Figure 1.
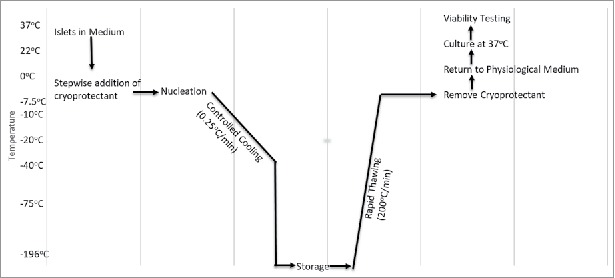
Flowchart of Cryopreservation. This chart describes the range of temperature, rate of temperature change, and the procedure involved during cryopreservation (adapted from ref#17).
Since discovery, the focus of cryopreservation has been to freeze spermatozoa and oocytes. Beginning in the late 1970s, protocols for the optimal cryopreservation of islets were written because of successful preservation of rat islets.18 Some of the original parameters included the effective freezing/thawing rate and cryoprotectants.19–21 In 1977, Rajotte et al transplanted cryopreserved rat islets through the portal vein into the rat liver. They froze the islets using DMSO, and after thawing and removing the DMSO, transplanted the islets into the liver. They noted hyperglycemia which was present 6 weeks after transplant, but then normalized by the 13th week. Similarly, the rat no longer had glycosuria, had improved vitality and showed weight gain.22 This study, although promising, was limited by its sample size of 1, and a relatively short 16-week follow-up period. In 1981, Nakagawara saw similar results when using 10–20% DMSO, and 1–2°C cooling per minute to −80°C.23 He explains that islet isolation for small rodents is possible, but those for large animals and humans is largely unsuccessful due to being unable to isolate large enough numbers of islets.23 In fact, a study examining the outcome of the auto transplantation of cryopreserved porcine islets reported inconsistent blood glucose homeostasis after transplant, and normoglycemia in only four of the twelve total pigs, indicating the need for better isolation and cryopreservation techniques.24
Within the last five years, many new islet cryopreservation techniques are being studied to optimize cell survival and function after thawing. This includes analyzing the effects of the freezing duration, cryogenic oxygen environments, cryoprotectants, and freezing the islets as a single cell or as a cluster of cells. In the next section, we will evaluate each of the above parameters individually.
Advancements in cell survival following cryopreservation
Freezing and thawing
Background
The rate of freezing and thawing during cryopreservation is important for islet function and morphology. The slower the islet is frozen, the more time is allowed for the liquid in the cell to reach equilibrium with the outside of the cell, thus, preventing destructive ice crystal formation inside. However, when the tissue is frozen slowly (<1°C/min), more immunostimulatory cells such as macrophages, lymphocytes, and dendritic cells survive, thus making them more susceptible to rejection after transplant.25,26 It is important to define the optimal rate of freezing such that the islet recovery and function remains high, while immunostimulatory molecules remain low. Foreman et al conducted one of the first studies in 1992 comparing the effects of three rates of freezing on islets. Namely, slow cooling (0.3°C /min) (Fig. 2), and rapid cooling (20°C /min, and 70°C /min) (Figs. 3 and 4). The results of the study showed that if the islets were cultured in 1 M DMSO for thirty minutes at room temperature, followed by 2 M DMSO for ten minutes at 0°C, then the in vitro insulin secretory ability of the rapidly cooled islets was improved after thawing.27 They found that the in vitro insulin secretory ability for the fast and slow cooling groups was similar, given they were cultured with 1 M DMSO for thirty minutes at room temperature, followed by 2 M DMSO for ten minutes at 0°C. Other studies had similar findings, and determined the rate of cooling becomes irrelevant if the islets have enough time to equilibrate with the cryoprotectant.28,29
Figure 2.
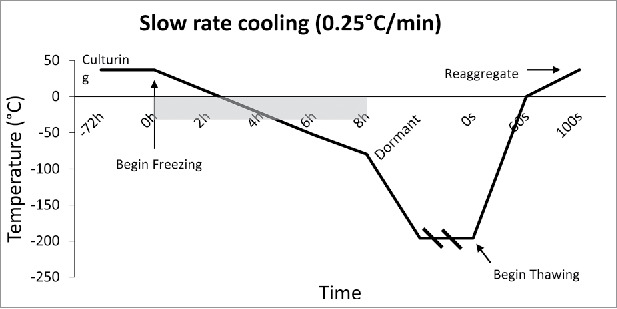
Slow Rate Cooling Temperature Diagram: Temperature Diagram showing the current standard in cooling rate, which is 0.25°C/min. This slow rate allows the tissue to reach thermal equilibrium, and allows time for water displacement, thus preventing intracellular ice crystal formation. Outlined is the burden of time which occurs due to such slow rates of cooling.
Figure 3.
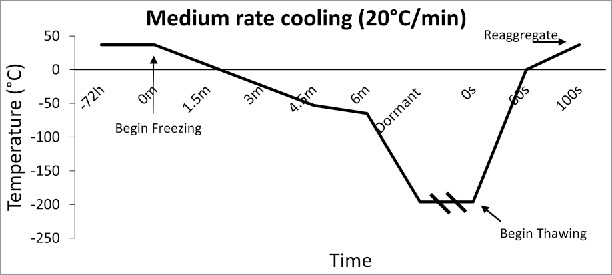
Medium Rate Cooling Temperature Diagram: Temperature diagram showing the significant difference in time when cooling at a rate of 20°C/min. This fast rate of cooling, although untraditional, was found to arrest immunostimulatory agents better than slow freezing.
Figure 4.
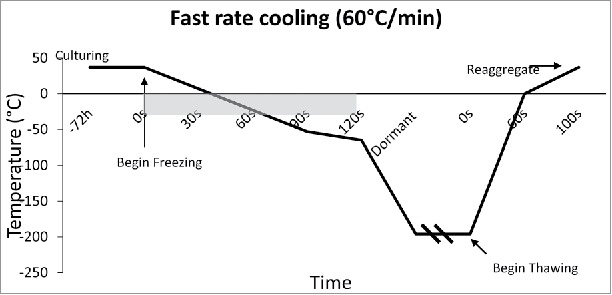
Fast Cooling Temperature Diagram: Temperature diagram showing the significant difference in time when cooling at a rate of 60°C/min. This fast rate of cooling, although untraditional, was found to arrest immunostimulatory agents better than slow freezing.
Vitrification
Although the use of permeating cryoprotectants (CPAs) such as DMSO and ethylene glycol (EG) have made islets more viable and robust to the cryopreservation process, they have also been toxic due to the high concentrations required to prevent ice crystal formation. There are two current methods to combat this toxicity, the first is slow cooling and second is by vitrification. During the vitrification process, the CPAs prevent ice formation inside the islets themselves, and instead to allow ice to form solely on the outside causing the islets to be arrested in a vitreous, glass-like phase.30 This occurs because the CPA permeates the cellular membrane, and allows water to travel extracellularly during the freezing process.17 The toxicity of a CPA is temperature dependent, and each CPA has a different osmotic load which it imposes on the cell membrane. To protect the islets from the toxic effects of the CPAs, researchers have successfully added the CPAs in a stepwise fashion as the temperature decreases, thus reducing the toxic effects to the cells.31
Thawing
The rate of thawing dictates whether the islet can recrystallize while it warms up. The current standard of thawing is to rapidly agitate the samples in a 37°C H2O bath which cools at a rate of 150–200°C/min. Next, the sample is spun at 1500 RPM, and the supernatant is removed. Following the removal of supernatant, a 0.75 M sucrose buffer is added, and the sample is kept at 0°C for 30 minutes to remove the CPA.32 This protocol was established nearly 28 years ago and is still considered the thawing standard.
Cryoprotectant additives
There are two types of CPAs, permeating, and non-permeating. Historically, EG and DMSO have been the permeating gold standard, while various sugars such as trehalose and raffinose have been considered the gold-standard non-permeating CPAs for cryopreservation.33 The difference between the two is simple: permeating CPAs enter the cell while non-permeating do not.34 Historically, permeating CPAs have been shown to be more effective, but they also have the potential to be more toxic.34 Although effective, CPAs do not have cellular protective abilities. For example, oxidative stress reduces islet survival due to the islet's poor defense mechanisms.35 Likewise, other mechanisms, such as glucotoxicity,36 and endoplasmic reticulum strain36 due to secretory insulin demands lead to islet apoptosis. In the last ten years, many researchers have been experimenting with different CPAs which could also protect the islet from cryopreservation induced apoptosis.
Taurine: As an antioxidant, taurine has the ability to scavenge for free radicals and also protect the membrane from oxidative damage37
Hardikar et al have shown the protective effects of adding taurine prior to cryopreservation.37 They found increased islet viability when adding 0.3 mM and 3.0 mM taurine prior to cryopreservation. The viabilities were (91.9±2.3% for the 0.3 mM, and 94.6±1.58% for the 3.0 mM).37 Likewise, they showed a reduction in lipid peroxidation, and a normal glucose clearance after transplantation into BALB/c mice. Hyperglycemia was reached once the graft was removed, showing that the islets were functional.37
Metformin
Metformin is a prescribed anti-diabetic drug that causes insulin sensitivity.38 It has been shown to have beneficial effects on islet survival,39 and thus, was further researched by Chandravanshi et al.40 As part of the experiment, they added 100 µg/ml of metformin to the cryopreservation solution of mice islets. They tested the functionality of the islets after a low (4°C) and ultra-low temperature (−196°C) storage period of 15 and 30 days respectively. The islets treated with metformin and stored at the low temperature for 15 days secreted insulin (8 ng/ml) and had a stimulation index greater than 5 indicating a high functionality and glucose sensitivity.40 They found a similar result with metformin after a 30-day storage period at the very low temperature. Similarly, they found reduced levels of reactive oxygen species (ROS) in both circumstances, when compared to islets not treated with metformin.40
Gamma aminobutyric acid (GABA)
GABA is the chief inhibitory neurotransmitter in the central nervous system which has also been shown to act as a neuroprotective agent.41 Soltani et al have demonstrated the protective effects of GABA on β cells caused by the membrane depolarization which activates survival pathways.42 This has also been shown on human β cells.43 Chandrayanshi et al hypothesized that the effects of GABA would also aid in the process of cryopreservation. After 15 days in low temperature, islets treated with 100 µM GABA secreted 5.5 ng/ml, and after 30 days in very low temperature, secreted 20 ng/ml.40 Similarly, they found reduced levels of reactive oxygen species (ROS) in both circumstances, when compared to islets not treated with GABA.40
Eicosapeexeanoic acid (EPA) and docosahexanoic acid (DHA)
EPA and DHA are polyunsaturated fatty acids which are associated with dietary intake of omega 3 fatty acids.44 They have been shown to have anti-inflammatory effects on humans in diseases such as asthma, inflammatory bowel syndrome, and arthritis.45 Chandrayanashi et al were the first researchers to investigate the cryoprotective effects of EPA and DHA on islet cells. They added 1 µM of EPA+DHA to the islet solution listed above. The results of the Glucose stimulated insulin secretion (GSIS) was 11 ng/ml and 20 ng/ml after 15 days at low temperature and after 30 days of very low temperature storage respectively. There was also a significant reduction in the levels of ROS.40 The greatest insulin release following glucose challenge was achieved with a solution that had a combination of EPA, DHA, and metformin.
Sericin
Sericin is a silk protein produced by silkworm (Bombyx mori). It was tested in 2012 by Ohnishi et al for its ability to replace fetal bovine serum (FBS) as an additive for DMSO.46 FBS has been scrutinized due to recent animal health problems such as bovine spongiform encephalopathy and viral infections. Using sericin as a replacement for FBS, Ahnishi et al showed no significant differences between GSIS results between the FBS+DMSO and the Sericin+DMSO groups. Although this needs more testing, it allows the researcher to use a non-animal product alternative to FBS.
Three-Dimensional structure
Islets in their native form are multicellular tissues containing 1,000–10,000 cells.47 They are complex structures which have an average diameter of 150µm.48 For reasons similar to entire organs, islets pose a great difficulty to cryopreserve due to differential ice crystal formation which occurs because of non-uniform temperature changes.49 Essentially, as the islet is cooled, the cells on the outside experience a greater rate of temperature change than the cells on the inside. Because of this differential temperature gradient, ice crystals can form on the inside of the cells, leading to cellular death.50 Researchers have proposed the prospect of freezing the islets as individual cells, and then reconstituting them into their natural spherical form after thawing.51
In vitro human islet experience
Rawal et al tested the differences in function, insulin release, and viability of human islets which were either cryopreserved as single cells or as native tissue.51 Native islet spheroids were broken down into single cells using their previous protocol.52 They then were dispersed in 10% DMSO, cooled at 1°C/min, and frozen at −196°C.51 The native islet tissue was prepared using a modified Lakey protocol including stepwise DMSO addition during freezing until −196°C.16 After the single cells were thawed, they reformed into spheroids at 37°C and compared with the native islets in terms of structure and function.51 After 4 weeks of cryopreservation, both the single cells and the native islets were compared. There was no significant different between the average spheroid diameter or the volume. They found a significant difference in viability between the two groups, with an 80% cell death of native islets compared to only a 25% cell death for the reaggregated islet cells.51 Using GSIS, they were unable to detect any insulin secretion from the intact native tissue. The single islet cells, however, secreted insulin and showed moderate levels of insulin sensitivity.
In vivo rat islet
The same group of researchers tested the in vivo effects of islet cryopreservation in the native vs cellular forms. They isolated rat islets and cryopreserved them for 1–4 months in either their native islet form, or in their cellular form. They compared these two groups to transplanting fresh islet tissue in diabetic rats with an average pre-transplantation blood glucose of 390 mg/dl. When they transplanted 5000–8500 islet equivalents/kg (IEQ) of fresh islets, they were unable to return normoglycemia averaging a blood glucose of 330 mg/dL at one month. Transplanting the same IEQ/kg ratio of cryopreserved islet cells yielded far better results: the rats were normoglycemic within 24 hours of the transplant and maintained an average blood glucose of 150mg/dL one month after. They continued to be normoglycemic for 10 months after transplant. The group of rats receiving the cryopreserved native islets were unable to reverse hyperglycemia.51
Oxygen environment
The composition of the air around the islets during both culturing and cryopreservation can affect their viability. One of the mechanisms for islet death is ATP depletion, and this becomes increasingly significant during the rewarming process, as the cytoplasmic enzymes regain their function.53 Since ATP could be made aerobically through oxidative phosphorylation, Komatsu et al hypothesized that the ATP depletion during rewarming could be reduced by using increased oxygen in the chamber around the islets during cryopreservation.54
Human islets
Komatsu et al tested this hypothesis using human islets in two groups, a low oxygen group (21%), and high oxygen group (50%).54 500 IEQ of human islets were cryopreserved using the islet cryopreservation solution (ICS) for a period of at least three months. For oxygenated thawing, the islets were equilibrated under 50% O2, 45% N2, and 5% CO2. Once thawed, they were placed in a 24 well plate, at 250 IEQ/well on ice, and transferred to a 50% oxygen incubator.54 The islets were incubated for a total of 90 minutes, the first 45 of which at 22°C, and the second 45 minutes at 37°C. They then were analyzed for islet volume and compared to their volume prior to the cryopreservation. They defined this as short-term recovery rate. For long-term recovery rate, they evaluated the islet's volumes after 1 or 2 days in the incubator, and compared the results to the short-term, and pre-cryopreservation periods using both the hyperoxic group, and the regular oxygen group. Other tests they conducted were RNA analysis, and GSIS from the pre-cryopreserved and post-thaw/rewarmed groups. They noted several results for the short-term recovery group. First, there was no significant difference of oxygenated thawing/rewarming on short-term islet recovery rate based on the islet volume ratio. However, there was a significant reduction in the amount of inflammatory genes that were expressed. The long-term recovery group showed a reduction in volume loss in the oxygenated thawing/rewarming group. The results of the GSIS for each of the groups showed no significant differences.54
Cryopreservation duration
The purpose of cryopreservation is to metabolically arrest the islets for as long as possible. The question becomes, “How long is too long?” specifically to prevent cellular death and to preserve islet function. Although studies have been performed for a three month, and a two-year period of cryopreservation,55,56 the most informative was the one conducted by Fox et al.57 In this study, they tested both the in vivo and in vitro effects of using human islets which were cryopreserved for a mean period of 18 years. In this study, they examined cryopreserved human islets from 43 human donors using the Rajotte protocol using 2 M DMSO.58 The mean age of the islet donors for cryopreservation was 40.9 ± 2.0 years, with 47% male vs 53% female, while the fresh tissue donors were 60.5 ± 3.0 years, with 56% male vs 43% female.57
In vitro human islets
After rapid thawing at a rate of 150°C/min to 4°C, the DMSO was removed with a sucrose buffer. The in vivo tests performed compared the fresh tissue group to the cryopreserved group of islets. Using dithizone staining, they found no significant decrease in purity between the two groups. Immunofluorescence staining showed expression of insulin positive beta cells and glucagon positive alpha cells, indicative of a healthy islet. Apoptosis was similar in both groups. Using a patch clamp apparatus, they determined that the cryopreserved group retained ion channel function and had exocytotic responsiveness like the fresh tissue group. GSIS results were similar between the two groups, with no correlation between cryopreserved time and insulin responsiveness.
In vivo human islets in mice
The same group then transplanted 2,000–4,000 IEQ which were cryopreserved for a mean of 16 years into the kidney capsule of STZ-induced diabetic mice. The transplantation of the islets could reduce the circulating blood glucose levels of the mice; however, they were not able to restore normoglycemia. Of a total of 11 mice receiving the cryopreserved islets, only one could achieve similar glucose levels as the mice with fresh islet transplants. This reduction in the insulin secreting ability of the cryopreserved islets in vivo could be proportional to the duration of the extended cryopreservation period. This phenomenon has also been shown in vitro.59
Other methods
Hollow fiber vitrification (HFV)
Although commonly used for the cryopreservation of embryos,60 HFV has only been recently studied for the pancreatic islet model. The reason HFV is an desirable method of preserving islets is because it allows for a reduced amount of CPA use, which in turn, could lead to purer and more viable islets after the preservation period.61 In 2016, Nagaya et al compared their HFV protocol to the open pulled straw method,62 and to Sasamoto et al's protocol.63 It was shown that the new HFV method yielded the highest viability of the three protocols in vitro.61 When they transplanted HFV islets into STZ mice, they were all euglycemic within 4–8 days, and returned to hyperglycemia once the kidney graft was removed after 30 days.61
Adenosine
The addition of adenosine to UW solution during regular non-cryopreservation has been shown to increase yield, viability, purity, and insulin release when compared to regular UW solution alone.64 The addition of adenosine to the cryoprotective solution as a method of better islet outcome has not been conducted. There needs to be more research done with this substance to see if it has similar protective effects during cryopreservation.
Novel combinations of EG + DMSO
Traditionally, the standard protocol for cryopreserving islets is to use 2 M DMSO. Lakey et al tested the in vitro and in vivo differences between using 2 M DMSO, 1 M DMSO + 1 M EG, and 1 M DMSO + 0.5 M EG. They intraperitoneally implanted rat islets into a total of 20 streptozotocin (STZ) induced diabetic mice. After transplantation, the 1 M DMSO + 0.5 M EG group had a time to normoglycemia (<200mg/dL) of 6 days, compared to 4 days for fresh islets, and 18 days for 2 M DMSO islets. Likewise, they demonstrated a higher islet yield and viability when using 1 M DMSO + 0.5 M EG when compared to 2 M DMSO. They did not find any statistical difference between the GSIS values of either of the three groups after a 48-hour culturing period (Unpublished data).
Alginate encapsulated cryopreservation
Encapsulating islets with 1.75% alginate prior to cryopreservation was shown to decrease the time to normoglycemia for STZ induced diabetic nude mice. The time to normoglycemia for fresh islets was 4 days compared to 5 days for the 1.75% alginate encapsulated islets (unpublished data from Lakey et al). Unencapsulated cryopreserved islets took 18 days to return the mice to normoglycemia, showing that there was a significant difference in islet function when they were encapsulated with 1.75% alginate. (unpublished data from Lakey et al)
Conclusion
Islet cryopreservation has come a long way in the last forty years. Many parameters of cryopreserving islets are being actively researched because of the high demand for long term storage. Currently, entire organ cryopreservation is not entirely feasible, and is only shown possible after a few hours of storage.65 Based on all the experiments, we have outlined new generation parameters with the goal of optimizing islet function after cryopreservation (Table 1). We hope that with a standardized cryopreservation protocol, islet banking would be more feasible, and ultimately, transplantation would no longer be throttled by the donor-recipient mismatch.
Table 1.
Cryopreservation Parameters and Suggested Methods: A quick summary of the parameters which had the best outcome for the islets during cryopreservation. Assessment methods include islet viability, glucose sensitivity, and GSIS values after thawing.
| Parameter | Method | Reference Number |
|---|---|---|
| Cryoprotectant | EPA+DHA+Metformin | 40 |
| Cooling Rate | Rapid (50–70°C/min) | 27–29 |
| Thawing Rate | Rapid (150–200°C/min) | 32 |
| Oxygen Environment | 50% during thawing | 54 |
| 3D Structure | Freeze as individual cells, re-aggregate into spheroids after thaw | 51 |
| Encapsulation | 1.75% Alginate encapsulation prior to cryopreservation | Unpublished data (Lakey J, et al.) |
Declaration of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors gratefully acknowledge the Department of Surgery and the Department of Biomedical Engineering at University of California Irvine and the Sue and Bill Gross Stem Cell Research Center for all their support in the writing of this article.
References
- 1.Ricordi C, Strom TB. Clinical islet transplantation: Advances and immunological challenges. Nat Rev Immunol. 2004;4(4):259–268. doi: 10.1038/nri1332. PMID:15057784 [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. PMID:10911004 [DOI] [PubMed] [Google Scholar]
- 3.Center CC. 9th annual report of the clinical islet transplant registry. Rockville, MD: Clinical Islet Transplant Registry, 2016. [Google Scholar]
- 4.Miranda PM, Mohan V, Ganthimathy S, Anjana RM, Gunasekaran S, Thiagarajan V, Churchill TA, Kin T, Shapiro AM, Lakey JR. Human islet mass, morphology, and survival after cryopreservation using the edmonton protocol. Islets. 2013;5(5):188–195. doi: 10.4161/isl.26304. PMID:24759005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Adra DP, Gill RS, Imes S, O'Gorman D, Kin T, Axford SJ, Shi X, Senior PA, Shapiro AM. Single-donor islet transplantation and long-term insulin independence in select patients with type 1 diabetes mellitus. Transplantation. 2014;98(9):1007–1012. doi: 10.1097/TP.0000000000000217. PMID:24911037 [DOI] [PubMed] [Google Scholar]
- 6.Kin T. Islet isolation for clinical transplantation. Adv Exp Med Biol. 2010;654:683–710. doi: 10.1007/978-90-481-3271-3_30. PMID:20217520 [DOI] [PubMed] [Google Scholar]
- 7.Kin T, Shapiro AM. Surgical aspects of human islet isolation. Islets. 2010;2(5):265–273. doi: 10.4161/isl.2.5.13019. PMID:21099323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricordi C, Mazzeferro V, Casavilla A, Scotti C, Pinna A, Tzakis A, Starzl TE. Pancreas procurement from multiorgan donors for islet trasplantation. Diabetes Nutr Metab. 1992;5(S1):39–41. PMID:2157294724944122 [PMC free article] [PubMed] [Google Scholar]
- 9.Liu F, Tian W, Yang Y, Zhang Q, Zhu M, Yang L, Yang L, Li J, Liu J, Wu P, et al.. Optimal method for short-term or long-term islet preservation: Comparison of islet culture, cold preservation and cryopreservation. J Artif Organs. 2014;17(4):337–343. doi: 10.1007/s10047-014-0777-x. PMID:24944122 [DOI] [PubMed] [Google Scholar]
- 10.Parkes AS. Preservation of spermatozoa at low temperatures. Br Med J. 1945;2(4415):212–213. doi: 10.1136/bmj.2.4415.212. PMID:20786227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovelock J. Gaia: The living earth. Nature. 2003;426(6968):769–770. doi: 10.1038/426769a. PMID:14685210 [DOI] [PubMed] [Google Scholar]
- 12.Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164(4172):666. doi: 10.1038/164666a0. PMID:18143360 [DOI] [PubMed] [Google Scholar]
- 13.Smith AU. Prevention of haemolysis during freezing and thawing of red blood-cells. Lancet. 1950;2(6644):910–911. doi: 10.1016/S0140-6736(50)91861-7. PMID:14795743 [DOI] [PubMed] [Google Scholar]
- 14.Mazur P, Leibo SP, Chu EH. A two-factor hypothesis of freezing injury. Evidence from chinese hamster tissue-culture cells. Exp Cell Res. 1972;71(2):345–355. doi: 10.1016/0014-4827(72)90303-5. PMID:5045639 [DOI] [PubMed] [Google Scholar]
- 15.Lovelock JE, Bishop MW. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature. 1959;183(4672):1394–1395. doi: 10.1038/1831394a0. PMID:13657132 [DOI] [PubMed] [Google Scholar]
- 16.Lakey JR, Anderson TJ, Rajotte RV. Novel approaches to cryopreservation of human pancreatic islets. Transplantation. 2001;72(6):1005–1011. doi: 10.1097/00007890-200109270-00005. PMID:11579292 [DOI] [PubMed] [Google Scholar]
- 17.Rajotte RV, Lakey J, Warnock GL. Adult islet cryopreservation In: Ricordi C, editor. Methods in cell transplantation. Austin, TX: R. G. Landes Company, 1995. p. 517–524. [Google Scholar]
- 18.Bank HL. Cryobiology of isolated islets of langerhans circa 1982. Cryobiology. 1983;20(2):119–128. doi: 10.1016/0011-2240(83)90001-9. PMID:6406148 [DOI] [PubMed] [Google Scholar]
- 19.Andersson A, Sandler S. Viability tests of cryopreserved endocrine pancreatic cells. Cryobiology. 1983;20(2):161–168. doi: 10.1016/0011-2240(83)90005-6. PMID:6342950 [DOI] [PubMed] [Google Scholar]
- 20.Bretzel RG, Schneider J, Dobroschke J, Schwemmle K, Pfeiffer EF, Federlin K. Islet transplantation in experimental diabetes of the rat. Vii. Cryopreservation of rat and human islets. Preliminary results. Horm Metab Res. 1980;12(6):274–275. doi: 10.1055/s-2007-996265. PMID:6773869 [DOI] [PubMed] [Google Scholar]
- 21.Bank HL, Davis RF, Emerson D. Cryogenic preservation of isolated rat islets of langerhans: Effect of cooling and warming rates. Diabetologia. 1979;16(3):195–199. doi: 10.1007/BF01219798. PMID:372038 [DOI] [PubMed] [Google Scholar]
- 22.Rajotte RV, Stewart HL, Voss WA, Shnitka TK. Viability studies on frozen–thawed rat islets of langerhans. Cryobiology. 1977;14(1):116–120. doi: 10.1016/0011-2240(77)90130-4. PMID:402252 [DOI] [PubMed] [Google Scholar]
- 23.Nakagawara G, Kojima Y, Mizukami T, Ono S, Miyazaki I. Transplantation of cryopreserved pancreatic islets into the portal vein. Transplant Proc. 1981;13(2):1503–1507. PMID:67877603161702 [PubMed] [Google Scholar]
- 24.Wise MH, Gordon C, Johnson RW. Intraportal autotransplantation of cryopreserved porcine islets of langerhans. Cryobiology. 1985;22(4):359–366. doi: 10.1016/0011-2240(85)90183-X. PMID:3161702 [DOI] [PubMed] [Google Scholar]
- 25.Taylor MJ, Bank HL. Function of lymphocytes and macrophages after cryopreservation by procedures for pancreatic islets: Potential for reducing tissue immunogenicity. Cryobiology. 1988;25(1):1–17. doi: 10.1016/0011-2240(88)90014-4. PMID:3280245 [DOI] [PubMed] [Google Scholar]
- 26.Taylor MJ, Bank HL, Benton MJ. Selective destruction of leucocytes by freezing as a potential means of modulating tissue immunogenicity: Membrane integrity of lymphocytes and macrophages. Cryobiology. 1987;24(2):91–102. doi: 10.1016/0011-2240(87)90011-3. PMID:3568745 [DOI] [PubMed] [Google Scholar]
- 27.Foreman J, Moriya H, Taylor MJ. Effect of cooling rate and its interaction with pre-freeze and post-thaw tissue culture on the in vitro and in vivo function of cryopreserved pancreatic islets. Transpl Int. 1993;6(4):191–200. doi: 10.1111/j.1432-2277.1993.tb00646.x. PMID:8347264 [DOI] [PubMed] [Google Scholar]
- 28.Ishihara K, Taniguchi H, Hara Y, Ejiri K, Baba S. Effect of cooling rate on insulin release from frozen-thawed dispersed rat islet cells. Diabetes Res Clin Pract. 1989;6(4):243–246. doi: 10.1016/0168-8227(89)90063-6. PMID:2666062 [DOI] [PubMed] [Google Scholar]
- 29.Taylor MJ, Benton MJ. Interaction of cooling rate, warming rate, and extent of permeation of cryoprotectant in determining survival of isolated rat islets of langerhans during cryopreservation. Diabetes. 1987;36(1):59–65. doi: 10.2337/diab.36.1.59. PMID:3098610 [DOI] [PubMed] [Google Scholar]
- 30.Taylor MJ, Baicu S. Review of vitreous islet cryopreservation: Some practical issues and their resolution. Organogenesis. 2009;5(3):155–166. doi: 10.4161/org.5.3.9812. PMID:20046679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakey JR, Rajotte RV, Fedorow CA, Taylor MJ. Islet cryopreservation using intracellular preservation solutions. Cell Transplant. 2001;10(7):583–589. PMID:11714192 [PubMed] [Google Scholar]
- 32.Rajotte RV, Warnock GL, Kneteman NM, Erickson C, Ellis DK. Optimizing cryopreservation of isolated islets. Transplant Proc. 1989;21(1 Pt 3):2638–2640. PMID:2495654 [PubMed] [Google Scholar]
- 33.Elliott TF, Das GC, Hammond DK, Schwarzkopf RJ, Jones LB, Baker TL, Love JE. Screening and identification of cryopreservative agents for human cellular biotechnology experiments in microgravity. Gravit Space Biol Bull. 2005;18(2):83–84. PMID:16038096 [PubMed] [Google Scholar]
- 34.Sztein JM, Noble K, Farley JS, Mobraaten LE. Comparison of permeating and nonpermeating cryoprotectants for mouse sperm cryopreservation. Cryobiology. 2001;42(1):28–39. doi: 10.1006/cryo.2001.2300. PMID:11336487 [DOI] [PubMed] [Google Scholar]
- 35.Modak MA, Parab PB, Ghaskadbi SS. Pancreatic islets are very poor in rectifying oxidative DNA damage. Pancreas. 2009;38(1):23–29. doi: 10.1097/MPA.0b013e318181da4e. PMID:18695629 [DOI] [PubMed] [Google Scholar]
- 36.McKenzie MD, Jamieson E, Jansen ES, Scott CL, Huang DC, Bouillet P, Allison J, Kay TW, Strasser A, Thomas HE. Glucose induces pancreatic islet cell apoptosis that requires the bh3-only proteins bim and puma and multi-bh domain protein bax. Diabetes. 2010;59(3):644–652. doi: 10.2337/db09-1151. PMID:19959756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardikar AA, Risbud MV, Remacle C, Reusens B, Hoet JJ, Bhonde RR. Islet cryopreservation: Improved recovery following taurine pretreatment. Cell Transplant. 2001;10(3):247–253. doi: 10.3727/000000001783986756. PMID:11437070 [DOI] [PubMed] [Google Scholar]
- 38.Korenaga M, Kawaguchi K, Korenaga K, Uchida K, Sakaida I. insulin sensitizer–anti-diabetic drugs, metformin and pioglitazone that can improve insulin resistance]. Nihon Rinsho. 2006;64(6):1157–1164. PMID:16768125 [PubMed] [Google Scholar]
- 39.Sasnoor LM, Kale VP, Limaye LS. Prevention of apoptosis as a possible mechanism behind improved cryoprotection of hematopoietic cells by catalase and trehalose. Transplantation. 2005;80(9):1251–1260. doi: 10.1097/01.tp.0000169028.01327.90. PMID:16314793 [DOI] [PubMed] [Google Scholar]
- 40.Chandravanshi B, Dhanushkodi A, Bhonde R. High recovery of functional islets stored at low and ultralow temperatures. Rev Diabet Stud. 2014;11(3–4):267–278. doi: 10.1900/RDS.2014.11.267. PMID:26177487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franklin IK, Wollheim CB. Gaba in the endocrine pancreas: Its putative role as an islet cell paracrine-signalling molecule. J Gen Physiol. 2004;123(3):185–190. doi: 10.1085/jgp.200409016. PMID:14769848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soltani N, Qiu H, Aleksic M, Glinka Y, Zhao F, Liu R, Li Y, Zhang N, Chakrabarti R, Ng T, et al.. Gaba exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci U S A. 2011;108(28):11692–11697. doi: 10.1073/pnas.1102715108. PMID:21709230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian J, Dang H, Chen Z, Guan A, Jin Y, Atkinson MA, Kaufman DL. Gamma-aminobutyric acid regulates both the survival and replication of human beta-cells. Diabetes. 2013;62(11):3760–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bjerve KS, Brubakk AM, Fougner KJ, Johnsen H, Midthjell K, Vik T. Omega-3 fatty acids: Essential fatty acids with important biological effects, and serum phospholipid fatty acids as markers of dietary omega 3-fatty acid intake. Am J Clin Nutr. 1993;57(5 Suppl):801S–805S; discussion 805S-806. PMID:8475898 [DOI] [PubMed] [Google Scholar]
- 45.Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6(6):461–467. doi: 10.1007/s11883-004-0087-5. PMID:15485592 [DOI] [PubMed] [Google Scholar]
- 46.Ohnishi K, Murakami M, Morikawa M, Yamaguchi A. Effect of the silk protein sericin on cryopreserved rat islets. J Hepatobiliary Pancreat Sci. 2012;19(4):354–360. doi: 10.1007/s00534-011-0415-4. PMID:21678022 [DOI] [PubMed] [Google Scholar]
- 47.Benson JD, Benson CT, Critser JK. Mathematical model formulation and validation of water and solute transport in whole hamster pancreatic islets. Math Biosci. 2014;254:64–75. doi: 10.1016/j.mbs.2014.06.003. PMID:24950195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bertram R, Pernarowski M. Glucose diffusion in pancreatic islets of langerhans. Biophys J. 1998;74(4):1722–1731. doi: 10.1016/S0006-3495(98)77883-X. PMID:9545035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunt CJ, Taylor MJ, Pegg DE. Freeze-substitution and isothermal freeze-fixation studies to elucidate the pattern of ice formation in smooth muscle at 252 k (−21 degrees c). J Microsc. 1982;125(Pt 2):177–186. [DOI] [PubMed] [Google Scholar]
- 50.Levin RL, Cravalho EG, Huggins CE. Water transport in a cluster of closely packed erythrocytes at subzero temperatures. Cryobiology. 1977;14(5):549–558. doi: 10.1016/0011-2240(77)90165-1. PMID:908192 [DOI] [PubMed] [Google Scholar]
- 51.Rawal S, Harrington S, Williams SJ, Ramachandran K, Stehno-Bittel L. Long-term cryopreservation of reaggregated pancreatic islets resulting in successful transplantation in rats. Cryobiology. 2017;76:41–50. doi: 10.1016/j.cryobiol.2017.04.010. PMID:28483491 [DOI] [PubMed] [Google Scholar]
- 52.Williams SJ, Wang Q, Macgregor RR, Siahaan TJ, Stehno-Bittel L, Berkland C. Adhesion of pancreatic beta cells to biopolymer films. Biopolymers. 2009;91(8):676–685. doi: 10.1002/bip.21196. PMID:19353639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lablanche S, Cottet-Rousselle C, Argaud L, Laporte C, Lamarche F, Richard MJ, Berney T, Benhamou PY, Fontaine E. Respective effects of oxygen and energy substrate deprivation on beta cell viability. Biochim Biophys Acta. 2015;1847(6–7):629–639. doi: 10.1016/j.bbabio.2015.04.002. PMID:25868875 [DOI] [PubMed] [Google Scholar]
- 54.Komatsu H, Barriga A, Medrano L, Omori K, Kandeel F, Mullen Y. Oxygenated thawing and rewarming alleviate rewarming injury of cryopreserved pancreatic islets. Biochem Biophys Res Commun. 2017;486(3):817–823. doi: 10.1016/j.bbrc.2017.03.134. PMID:28351620 [DOI] [PubMed] [Google Scholar]
- 55.Misler S, Dickey A, Barnett DW. Maintenance of stimulus-secretion coupling and single beta-cell function in cryopreserved-thawed human islets of langerhans. Pflugers Arch. 2005;450(6):395–404. doi: 10.1007/s00424-005-1401-y. PMID:15988591 [DOI] [PubMed] [Google Scholar]
- 56.Arata T, Okitsu T, Fukazawa T, Ikeda H, Kobayashi K, Yong C, Kosaka Y, Narushima M, Matsuoka J, Yamamoto I, et al.. Maintenance of glucose-sensitive insulin secretion of cryopreserved human islets with university of wisconsin solution and ascorbic acid-2 glucoside. Artif Organs. 2004;28(6):529–536. doi: 10.1111/j.1525-1594.2004.07296.x. PMID:15153144 [DOI] [PubMed] [Google Scholar]
- 57.Manning Fox JE, Lyon J, Dai XQ, Wright RC, Hayward J, van de Bunt M, Kin T, Shapiro AM, McCarthy MI, Gloyn AL, et al.. Human islet function following 20 years of cryogenic biobanking. Diabetologia. 2015;58(7):1503–1512. doi: 10.1007/s00125-015-3598-4. PMID:25930156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajotte RV, Evans MG, Warnock GL, Kneteman NM. Islet cryopreservation. Horm Metab Res Suppl. 1990;25:72–81. PMID:2088990 [PubMed] [Google Scholar]
- 59.Rich SJ, Swift S, Thirdborough SM, James RF, Bell PR, London NJ. Islet cryopreservation: A detailed study of total functional losses. Transplant Proc. 1994;26(2):823–824. PMID:8171677 [PubMed] [Google Scholar]
- 60.Matsunari H, Maehara M, Nakano K, Ikezawa Y, Hagiwara Y, Sasayama N, Shirasu A, Ohta H, Takahashi M, Nagashima H. Hollow fiber vitrification: A novel method for vitrifying multiple embryos in a single device. J Reprod Dev. 2012;58(5):599–608. doi: 10.1262/jrd.2011-051. PMID:22785381 [DOI] [PubMed] [Google Scholar]
- 61.Nagaya M, Matsunari H, Kanai T, Maehara M, Nakano K, Umeki I, Katsumata Y, Kasai Y, Sakai R, Kobayashi M, et al.. An effective new cryopreservation procedure for pancreatic islets using hollow fiber vitrification. Horm Metab Res. 2016;48(8):540–549. doi: 10.1055/s-0042-102628. PMID:27341475 [DOI] [PubMed] [Google Scholar]
- 62.Vajta G, Holm P, Kuwayama M, Booth PJ, Jacobsen H, Greve T, Callesen H. Open pulled straw (ops) vitrification: A new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev. 1998;51(1):53–58. doi: 10.1002/(SICI)1098-2795(199809)51:1%3c53::AID-MRD6%3e3.0.CO;2-V. PMID:9712317 [DOI] [PubMed] [Google Scholar]
- 63.Sasamoto H, Futami M, Ando Y, Nakaji S. Cryopreservation of rat islets of langerhans by vitrification. J Artif Organs. 2012;15(3):283–289. doi: 10.1007/s10047-012-0635-7. PMID:22382647 [DOI] [PubMed] [Google Scholar]
- 64.Song WQ, Fu DZ, Cheng Y, Liu YF. Influence of adenosine on preservation of porcine pancreas in islet transplantation. Genet Mol Res.2015;14(4):18293–18301. doi: 10.4238/2015.December.23.17. PMID:26782477 [DOI] [PubMed] [Google Scholar]
- 65.Giwa S, Lewis JK, Alvarez L, Langer R, Roth AE, Church GM, Markmann JF, Sachs DH, Chandraker A, Wertheim JA, et al.. The promise of organ and tissue preservation to transform medicine. Nat Biotechnol. 2017;35(6):530–542. doi: 10.1038/nbt.3889. PMID:28591112 [DOI] [PMC free article] [PubMed] [Google Scholar]


