ABSTRACT
Fusion protein and monoclonal antibody-based tumor necrosis factor (TNF) inhibitors represent established treatment options for a range of inflammatory diseases. Regulatory authorities have outlined the structural characterization and clinical assessments necessary to establish biosimilarity of a new biotherapeutic product with the innovator biologic drug. Biologic products that would not meet the minimum World Health Organization's standard for evaluation of similar biotherapeutic products are available in some countries; in some cases relevant data to assess biosimilarity and appropriate regulatory approval pathways are lacking.
Batches of seven intended copy (IC) products for etanercept (Enbrel®) were subjected to a subset of test methods used in the routine release and heightened characterization of Enbrel®, to determine key attributes of identity, quality, purity, strength, and activity. While a number of quality attributes of the IC lots tested met the release specifications for Enbrel®, none fell within these limits across all methods performed, and there were no IC lots that satisfied the criteria typically applied by the innovator to support comparability with Enbrel®. Although the consequences of these differences are largely unknown, the potential for unanticipated clinical outcomes should not be overlooked.
KEYWORDS: biosimilar, characterization, comparability, etanercept, intended copy, quality attribute
Abbreviations
- ACR20
20% response in American College of Rheumatology criteria
- AE
adverse event
- AEX
anion exchange chromatography
- ASAS40
40% response in Assessment of SpondyloArthritis international Society criteria
- AU
absorbance units
- BU
binding units
- CHO
Chinese hamster ovary
- DAS28
Disease Activity Score in 28 joints
- DMARD
disease-modifying antirheumatic drug
- DS
drug substance
- dSE-HPLC
denaturing size exclusion HPLC
- ELISA
enzyme-linked immunosorbent assay
- HIC
hydrophobic interaction chromatography
- HCP
host-cell protein
- HMW
high molecular weight
- HPLC
high-performance liquid chromatography
- IC
intended copy
- IEF
isoelectric focusing
- JIA
juvenile idiopathic arthritis
- LMW
low molecular weight
- MTX
methotrexate
- MS
mass spectrometric
- PD
pharmacodynamic
- PK
pharmacokinetic
- PRI
process-related impurities
- RA
rheumatoid arthritis
- RS
reference standard
- SE
size-exclusion
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- TNFR
tumor necrosis factor receptor
- UV
ultraviolet
Introduction
Etanercept (Enbrel®) is a recombinant human fusion protein used in the treatment of rheumatoid arthritis (RA) and other autoimmune diseases,1 including plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis,2 and juvenile idiopathic arthritis (JIA; including the JIA categories extended oligoarthritis, enthesitis-related arthritis, and psoriatic arthritis).3 Etanercept is a technically complex protein expressed by modified Chinese hamster ovary (CHO) cells in culture, and produced as a covalent dimer.4 Each subunit of the dimer comprises the extracellular domain sequence of tumor necrosis factor receptor II (p75 or TNFRII) linked to an analog human Fc portion of a type 1 immunoglobulin G. The fusion protein is denoted as TNFR:Fc.
Biosimilars are biologic drugs designed to be highly similar to the approved reference innovator biologic therapeutic agent; they have undergone rigorous analytical, nonclinical, and clinical evaluations to demonstrate similarity to the approved biologic.5–7 Biologic products that claim biosimilarity, but have not undergone the rigorous characterization and testing as described in World Health Organization (WHO) guidelines, are available in certain countries.8 These products may have unintentional structural or chemical differences, and therefore are not biosimilars; they are termed intended copies (ICs), biomimics,9 or non-comparable biotherapeutic products.10
ICs of Enbrel® are being marketed in some countries as biosimilars of etanercept,8 despite little information being available on the extent of the biochemical differences with respect to innovator etanercept and their potential effects on clinical efficacy and safety.8 Several publications11 have claimed a degree of structural and biochemical comparability between ICs and innovator etanercept.12–16 However, a critique of one of these publications has suggested that the analyses conducted were insufficient to make a comprehensive evaluation of biochemical similarity,17 while another publication demonstrated differences in the primary amino acid sequence in one IC compared with innovator etanercept.16
In this article, the results for the analytical assessments of multiple lots of six ICs and a single lot of one IC are reported against the licensed etanercept innovator product (Enbrel®). All of these ICs have received marketing approval in various global markets.
Results
The following IC products were assessed: Yisaipu (n = 5 batches); Etanar® (n = 5); Etacept (n = 6); Infinitam (n = 3); Altebrel™ (n = 1); Intacept (n = 3); and Qiangke (n = 2). Their origin and status is listed in Table 1. Biochemical comparability was assessed according to the criteria Pfizer would apply for release testing and heightened characterization of Enbrel®. Results for the analysis of each IC are summarized in Table 2.
Table 1.
Origin and status of IC products assessed.
| Product | Market Authorization Holder | Country | Status |
|---|---|---|---|
| Yisaipu | Shanghai CP Guojian Pharmaceutical Co, Ltd | China | Marketed as a recombinant human tumor necrosis factor-α receptor II – IgG Fc fusion protein.18 |
| Etanar® | Lafrancol (Laboratorio Franco-Colombiano SA) | Colombia | Described as a new biologic type rhTNFR:Fc.19 |
| Etacept | Cipla | India | Marketed as a biosimilar of etanercept.20 |
| Infinitam | Probiomed | Mexico | Marketed as a biosimilar of Enbrel.21 |
| Altebrel™ | Aryogen | Iran | Marketed as a biosimilar for etanercept.22 |
| Intacept | Intas | India | Marketed as a biosimilar for etanercept.23 |
| Qiangke | Shanghai Celgen Biopharma | China | Marketed as a new biologic.24 |
Table 2.
Results for the analysis of each IC, expressed as a range for number of lots evaluateda.
| Brand name (no. of batches tested) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Method (parameter) | Enbrel® specification | Yisaipu (n = 5) | Etanar® (n = 5) | Etacept (n = 6) | Infinitam (n = 3) | Altebrel™ (n = 1) | Qiangke (n = 2) | Intacept (n = 3) |
| Peptide map (identity) | Profile | Comparable | Comparable | Comparable | Comparable | Not comparableb | Not comparableb | Not comparableb |
| Hydrophobic interaction | Peak 1: ≤5%c | 0 | 0 | 0–2 | 3–4 | 3 | 0 | 1–2 |
| chromatography (purity) | Peak 2: ≥70%c | 99–100 | 99–100 | 88–100 | 82–95 | 81 | 100 | 80–87 |
| Peak 3: ≤28%c | 0 | 0 | 0–10 | 2–13 | 16 | 0 | 12–19 | |
| Profile | Not comparabled | Not comparabled | Not comparabled | Comparable | Comparable | Not comparabled | Not comparable | |
| Size exclusion HPLC (purity) | Peak A+A’: ≥94.0%c | 91.8–95.7 | 91.0–97.7 | 92.3–95.5 | 97.6–98.8 | 95.9 | 95.3–96.3 | 98.3–98.5 |
| Peak B: ≤6.0%c | 4.3–8.2e | 2.3–9.0e | 4.5–7.6e | 1.2–2.4 | 4.1 | 3.7–4.7 | 1.5–1.7 | |
| Profile | Comparable | Comparable | Comparable | Comparable | Comparable | Comparable | Comparable | |
| N-linked oligosaccharide map | Neutral %c | 50–59 | 52–58 | 47–56 | 69–74 | 87 | 79–86 | 69–72 |
| (quality) | Sialylated %c | 41–50 | 42–48 | 44–53 | 26–31 | 13 | 13–21 | 28–31 |
| Profile | Comparable | Comparable | Comparable | Not comparablef | Not comparablef | Not comparablef | Not comparablef | |
| HCP ELISA (purity) | ≤60 ppm | 23–61g | 40–105g | 17–66g | ND | 32 | ND | 1 |
| Protein A ELISA (purity) | ≤40 ppm | 2–5 | 2–52h | 1–3 | ND–1 | 28 | 5–13 | <1 |
| Charge distribution IEF & AEX (characterization) | N/A | Less negatively chargedi | Less negatively chargedi | Less negatively chargedi | Similar charge distribution | Less negatively chargedj | Less negatively chargedi | Less negatively chargedj |
| SDS-PAGE Coomassie scanning | Monomer ≥88% | 92–94 | 91–94 | 92–95 | 90–97 | 93 | 92–95 | 95–96 |
| densitometry | HMW component ≤6% | 3–5 | 3–5 | 3–5 | 2–4 | 3 | 3–6 | 2 |
| LMW component ≤8% | 2–3 | 2–5 | 1–3 | 1–6 | 5 | 2 | 2–3 | |
| Sialic acid content | 70–130% of RS | 78–92 | 76–89 | 63–91 | 91–105 | 84 | 70–79 | 75–76 |
| Receptor binding (TNF by ELISA) | 70–150 BU | 112–128 | 81–91 | 92–121 | 98–138 | 100 | 124–135 | 109–144 |
| U937 cell-based potency (inhibition of TNF-induced apoptosis) | 1.0–2.9 × 106 U/mg (IU) | 1.7–2.1 | 1.5–1.9 | 1.7–2.2 | 1.7–1.9 | 1.4 | 1.9–2.1 | 1.5–1.8 |
Results outside the specified range are indicated in bold;
presence of additional peaks, and absence of expected peaks compared with Enbrel® RS (Fig. 1);
relative % peak area;
atypical profile (Peak 1 [clips] and Peak 3 [aggregate] not detected) but species known to be present by orthogonal testing;
batches (Yisaipu [n = 3]; Etanar® [n = 3]; Etacept [n = 3]) failed release specification (levels of aggregate exceeded);
atypical profile (presence of new glycan species identified (Fig. 2C) and reduced content of sialylated N-linked oligosaccharides);
above specification for HCP (Yisaipu [1/5], Etanar® [4/5], Etacept [1/6];
above specification for residual protein A (Etanar® [2/5]);
due to greater abundance of more neutrally charged species;
due to fewer negatively charged species. BU, binding units; ELISA, enzyme-linked immunosorbent assay; HCP, host-cell protein; HMW, high molecular weight; HPLC, high-performance liquid chromatography; IC, intended copy; LMW, low molecular weight; N/A, not applicable; ND, not detected; RS, reference standard; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; TNF, tumor necrosis factor.
Identity based on mass spectrometric (MS) tryptic peptide mapping with high-performance liquid chromatography (HPLC) was confirmed for Yisaipu, Etanar®, Etacept, and Infinitam. The peptide map results for Altebrel™, Intacept, and Qiangke were not comparable with that of Enbrel® reference standard (RS) (illustrated for a single representative batch of each of these products in Fig. 1). Altebrel™ demonstrated a shift in retention time (∼1 min) for peptides T19, T20, T22, and T23 (and consequently the partially digested T21/T22 and T23/T24 peptides). Intacept demonstrated an atypical peptide map due to the presence of multiple unidentified peaks at 34, 39, 43, 46, 56, 58, and 88 minutes, and absence of a peak at 51 minutes. The peptide map for Qiangke was atypical due to an absence of two expected peptides in a single peak at approximately 74 minutes (T13G and T29T33), and presence of additional unidentified peaks at 26, 27, and 72 minutes. The exact cause of these shifts in the peptide maps was not identified. The likely shifts observed appear to be related to a combination of factors, including possible amino acid substitutions, unidentified impurities, or resulting from changes in glycosylation. Additional MS/MS studies would be required to fully understand the nature of the observed differences.
Figure 1.
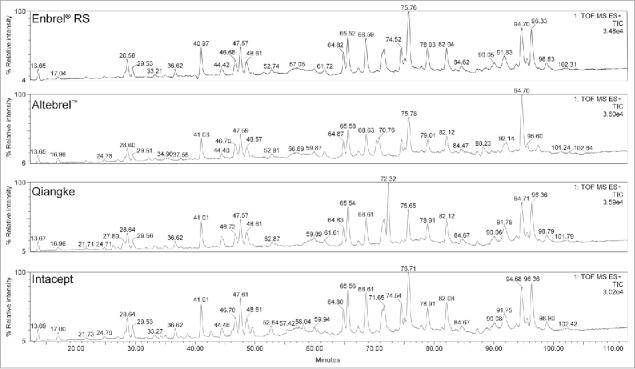
Stacked Tryptic peptide map HPLC profile for single batches of Altebrel™, Qiangke, and Intacept versus Enbrel® RS. (Presence of additional peaks, and absence of expected peaks versus Enbrel® RS). HPLC, high-performance liquid chromatography; RS, reference standard.
Purity based on hydrophobic interaction chromatography (HIC) showed atypical chromatography profiles for Yisaipu, Etanar®, Etacept, and Qiangke. The expected resolution of Peak 1 (clipped species) and Peak 3 (misfolded and aggregated species) from Peak 2 (dimeric etanercept) was not observed in these four ICs (illustrated for a single representative batch of each of these products in Fig. 2A). This was unexpected and is not currently understood, as orthogonal testing by size-exclusion (SE)-HPLC (Fig. 2B) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) Coomassie analyses (Table 3) demonstrated the presence of both clipped and aggregated species at elevated levels for each of these products. The level of aggregated species (SE-HPLC, Peak B) observed was above the Enbrel® specification limit for Yisaipu (3/5 batches), Etanar® (3/5 batches), and Etacept (3/6 batches). Aggregated species (SE-HPLC, Peak B) were within specification for each batch of Qiangke. Based on the combination of HIC and SE-HPLC analyses, the purity profiles for Yisaipu, Etanar®, Etacept, and Qiangke were therefore considered not comparable with that of Enbrel® RS. Intacept also demonstrated atypical chromatography by HIC analysis; however, this was due to reduced levels of clipped species (Peak 1) and misfolded/aggregate (Peak 3) as supported by orthogonal results for SE-HPLC and SDS/PAGE Coomassie analyses.
Figure 2.
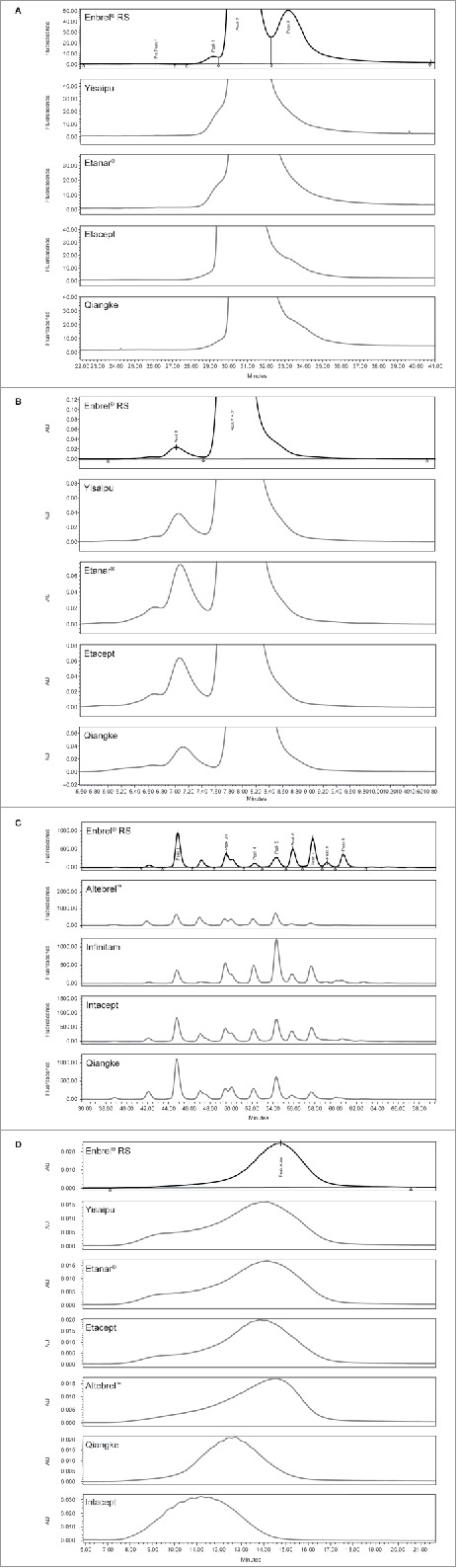
(A) Stacked HIC profiles for single batches of Yisaipu, Etanar®, Etacept, and Qiangke versus Enbrel® RS. (Similarly atypical profiles were obtained for all batches of each product and are not comparable to Enbrel® RS). (B) Stacked SE-HPLC profiles for single batches of Yisaipu, Etanar®, Etacept, and Qiangke versus Enbrel® RS. (Higher levels of aggregate were observed in all batches of each IC). (C) Stacked N-linked oligosaccharide HPLC profiles for Altebrel™, Infinitam, Intacept, and Qiangke versus Enbrel® RS. (Atypical profiles with new N-glycan species observed). (D) Stacked anion exchange HPLC profiles for overall negative charge heterogeneity of Yisaipu, Etanar®, Etacept, Altebrel™, Qiangke, and Intacept. (Shift in overall charge profiles versus Enbrel® RS). AU, absorbance units; HIC, hydrophobic interaction chromatography; HPLC, high-performance liquid chromatography; IC, intended copy; RS, reference standard; SE, size-exclusion.
Table 3.
Product parameters and test methods applied to analysis of all batches of each IC.
| Product parameter | Test method | Purpose |
|---|---|---|
| Identity | Tryptic peptide map, HPLC, UV, and MS detection | Identification test to evaluate similar peptides and examine for new different species |
| Characterization of peptide masses, and assess intact N- and C-terminal amino acid residues, distribution of O-linked glycans on specific peptides | ||
| SDS-PAGE silver stain (reducing /non-reducing) | Identification test to evaluate principal bands for monomer, dimer, aggregates, and clips | |
| Strength | UV spectrophotometric scan | Strength test to measure protein concentration |
| Purity and impurities | HIC | Purity test to evaluate hydrophobicity and assess level of misfolded species |
| HCP ELISA | Impurities derived from CHO cells are quantified by ELISA | |
| Protein A ELISA | Impurities derived from leached Protein A column are quantified | |
| SE-HPLC | Purity test to quantify aggregated species | |
| SDS-PAGE (Coomassie) scanning densitometry | Purity test to quantify protein aggregate and clips | |
| Potency | Apoptosis bioassay | Cell-based bioassay based on potency to prevent induced apoptosis in cells (in vitro neutralization of TNF) |
| Quality | Sialic acid | Test to quantify total N- and O-linked sialic acid content |
| Receptor-binding assay | Test for strength of binding to target molecule, TNF | |
| N-linked oligosaccharide map | HPLC test for presence and relative % of specific N-linked glycansa | |
| Charge heterogeneity | IEF | Characterization test to evaluate charge heterogeneity |
| Purity | dSE-HPLC | Characterization test to quantify specific low-molecular-weight clipped species |
| Negative charge heterogeneity | AEX | Characterization test to evaluate negative charge heterogeneity |
Each etanercept monomer has three sites for the addition of N-linked oligosaccharide structures. Each of the three N-linked oligosaccharides added to etanercept can be any one of multiple characterized species.
AEX, anion-exchange chromatography; dSE-HPLC, denaturing size exclusion HPLC; ELISA, enzyme-linked immunosorbent assay; HCP, host-cell protein; HIC, hydrophobic interaction chromatography; HPLC, high-performance liquid chromatography; IC, intended copy; IEF, isoelectric focusing; MS, mass spectrometry; PRI, process-related impurities; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; SE-HPLC, size exclusion HPLC; TNF, tumor necrosis factor; UV, ultraviolet.
The composition and distribution of N-linked oligosaccharide species observed for Infinitam, Altebrel™, Qiangke, and Intacept were not comparable with those of Enbrel® RS. Each batch of these products displayed the presence of new peaks in the N-linked oligosaccharide chromatograms, which indicate N-glycan species that were not resolved for Enbrel®. Each batch of these ICs also demonstrated an overall reduced content of sialylated N-linked oligosaccharide structures (lower abundance of Peaks 6–9) relative to Enbrel® (shown in Fig. 2C for a single batch of each product). As such, Infinitam, Altebrel™, Intacept, and Qiangke are not considered comparable with Enbrel® RS with respect to N-linked oligosaccharides.
Host-cell protein (HCP) content was found to be elevated in all batches of Yisaipu, Etanar®, and Etacept compared with expected results for Enbrel®. The results for analysis of HCP were above the Enbrel® specification limit for Yisaipu (1/5 batches), Etanar® (4/5 batches, with two batches being almost 2-fold higher than the specification limit), and Etacept (1/6 batches, with a further two batches just below the specification limit). HCP from CHO cells were below the limit of detection for all three batches of Infinitam, and for the two batches of Qiangke, but were detected at low levels in all three batches of Intacept. Yisaipu, Etanar®, and Etacept were therefore not considered comparable with Enbrel® due to consistently elevated levels of HCP across all batches tested and multiple results above the Enbrel® specification limit. Results for all batches of each IC, based on SDS-PAGE Coomassie scanning densitometry (purity), sialic acid content (quality), TNF receptor binding (quality), and cell-based inhibition of TNF-induced apoptosis (potency) were within the specified ranges for Enbrel®.
Isoelectric focusing (IEF) gels of multiple ICs compared with Enbrel® RS are shown in Fig. 3. Infinitam displayed a similar charge distribution to Enbrel® (not shown). The IEF gel profiles of all other ICs demonstrated an overall less negative charge. Yisaipu, Etanar®, and Etacept demonstrated an increased abundance of more neutrally charged species compared with Enbrel®. Altebrel™ and Qiangke demonstrated reduced abundance of negatively charged species, and an increased abundance of neutrally charged species. Intacept demonstrated decreased abundance of both negatively charged and neutral species. This overall reduction in negative charge distribution for Yisaipu, Etanar®, Etacept, Altebrel™, Qiangke, and Intacept was also evidenced in the results for anion exchange chromatography (AEX) (Fig. 2D).
Figure 3.
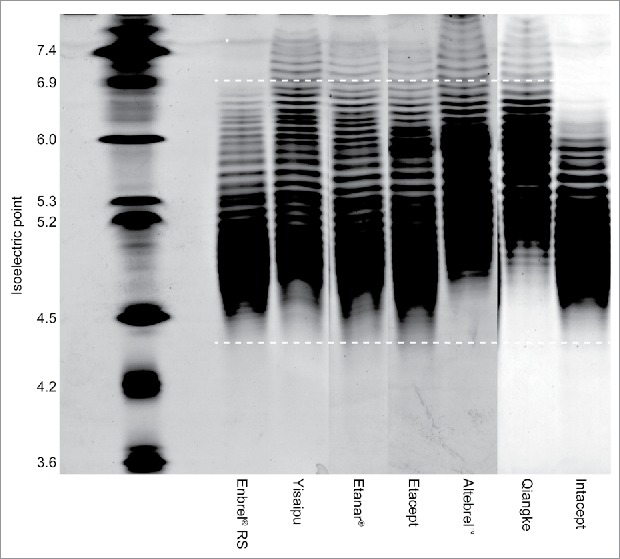
Isoelectric focusing gel of multiple intended copies versus Enbrel® RS. Image is a composite of three individual gels assembled for illustrative purposes. White bars indicate isoelectric point range for principal etanercept species. RS, reference standard.
The in vitro biological activity of all batches of the ICs tested, based on receptor binding using enzyme-linked immunosorbent assay (ELISA), and functional potency as determined by inhibition of TNF-mediated apoptosis in histiocytic lymphoma U937 cells, fell within the release specification for Enbrel®.
Discussion
Results for each IC were evaluated against licensed specifications for Pfizer-manufactured Enbrel®, and against Enbrel® RS for the additional characterization test results. The test methods used for analysis of the ICs are a subset of those used for routine release testing of etanercept drug substance (DS) together with additional methods applied for heightened product characterization studies and process validation activities. These methods were selected based on their utility in assessing the identity, quality, purity, strength, and activity of the ICs relative to Enbrel®.
Although a degree of structural similarity and binding activity with Enbrel® was observed for each of the ICs analyzed, a number of significant structural and biochemical differences were noted. None of the products analyzed met the combination of release specification and additional characterization criteria typically applied to support a comparability assessment with innovator etanercept DS. The clinical impact of the differences identified here for each IC is unknown compared with Enbrel®.
The standards set out by the WHO are defined such that biosimilarity of the biological product to the reference product is established following rigorous preclinical analytical and clinical evaluation.5 These biosimilarity standards are not being uniformly applied by all regulatory agencies, particularly in some emerging markets. Therefore, clinicians may be uncertain about the distinctions between truly biosimilar products (i.e., those approved via a regulatory pathway that is aligned with WHO, U.S. Food and Drug Administration [FDA], or European Medicines Agency [EMA] guidance) from those that are not (i.e., ICs). Moreover, the scarcity of post-marketing pharmacovigilance programs in countries where ICs are available has contributed to a lack of clarity on their quality, efficacy, and safety. The WHO has recognized the need to address biologic products licensed through a pathway for generics or small molecule drugs, or with limited analytical, nonclinical and/or clinical data, and has set out recommended steps for regulatory risk assessment of such products.25
In this analysis, the assessment of similarity with etanercept DS was based on the specification limits for batch release testing of Enbrel®. As indicated, none of the ICs met the criteria across all assays to indicate conformity with release specification for Enbrel®. Moreover, since the quality range determined by the EMA7 and FDA6 for analytical biosimilarity is based on multiple batches of the reference product, these limits are narrower than the specifications applied for release testing. As such, a failure to meet the release specifications for Enbrel® brings into question the development strategy applied to these ICs, and advocates for use of a pathway consistent with the approach outlined by the WHO.5
Here, we documented for the first time that, by analytical comparison, the ICs Infinitam, Yisaipu, Etanar®, Etacept, Altebrel™, Qiangke, and Intacept would not satisfy the characterization criteria and release specifications typically applied to support a comparability assessment with etanercept DS. Such tests represent a subset of the methods employed to evaluate the non-clinical analytical characteristics of two products in an assessment of biosimilarity. The regulatory pathways for biosimilars that apply in the EU and US rely on a totality of evidence approach, comprising a stepwise pathway that shows structural similarity and functional equivalence between the proposed biosimilar and the innovator biologic, coupled with clinical pharmacokinetic (PK) and pharmacodynamic (PD) equivalence studies to confirm similar efficacy, safety, and immunogenicity.26 The pathways that apply in the EU and US are aligned with WHO guidance for the evaluation of similar biotherapeutic products,5 and none of the ICs described here has been subjected to such a stringent regulatory assessment. In countries where they are available, many patients who were previously receiving Enbrel® may have been switched to an IC (often for nonclinical reasons), and therefore the potential risk that lack of efficacy, immunogenicity, or allergic manifestations may arise from these differences in characteristics cannot be overlooked.27 For instance, additional testing, including clinical data, would be needed to fully understand the effect of differences in the N-glycan profiles for Infinitam, Altebrel™, Qiangke, and Intacept. While recent publications have described the presence of multiple N-glycan species in Enbrel®,28-31 the relative abundance of several minor species are not quantified in the routine test applied for release testing of Enbrel®, but have been characterized historically. The extent and pattern of glycosylation in the Fc region of fusion protein and monoclonal antibody-based therapies can play a critical role in the various effector functions, such as antibody-dependent cell-mediated cytotoxicity, antibody-dependent cell-mediated phagocytosis, or complement-dependent cytotoxicity.32 While such modes of action are considered critical for the function of intact anti-TNF monoclonal antibodies, they have not been found to play a role in the efficacy of etanercept in the approved disease indications.33 Differences in N-glycan profile may alter binding with Fc receptors, can potentially influence the PK and PD profiles, and the production of auto-antibodies or potentially neutralizing antibodies. The PK parameters of Enbrel® are well established34 and contribute to a dosing recommendation that offers flexibility (25 mg twice weekly or 50 mg once weekly) across all indications.1 Uncertainty surrounding the PK properties of the ICs also raises questions as to whether these products can offer the same dosing options.
Published data are available from only one comparative clinical trial between Enbrel® and one of the seven ICs described in this analysis.15 Conducted in 59 patients with moderate to severe RA, this study used a double-blind, randomized, three-arm design to evaluate the PD effect of Infinitam compared with innovator etanercept.15 All patients received concomitant methotrexate (MTX) treatment. In two of the treatment arms, patients received Infinitam for 24 weeks, whereas in the third arm, patients were switched from innovator etanercept after 12 weeks to receive Infinitam. Although the authors claim comparable biological effects between treatments based on the change in a number of markers of inflammatory response (e.g., B-cell activating factor protein, erythrocyte sedimentation rate, C-reactive protein, TNF, rheumatoid factor) and disease parameters (Disease Activity Score in 28 joints [DAS28]), the study was not designed or powered to show bioequivalence.15 In this same publication, the authors presented data on the physicochemical and biological comparability of Infinitam and innovator etanercept. However, the N-glycan profile of one batch of innovator etanercept presented was markedly different from the other batches, and contained glycan species that were not resolved in the other batches. Moreover, the profile was distinct from those of historical batches (>2,000) and with the specification reported here, raising questions as to the validity of the physicochemical similarity exercise between the products.35
Comparable safety and efficacy of Altebrel™ and innovator etanercept, based on a non-inferiority, randomized, double-blind, parallel clinical trial in patients with active RA (n = 128), has been described36; however, full details of this study have not been published in the peer-reviewed literature.
In an observational single-arm study37 of Etanar® over 20 weeks in patients (n = 110) with active RA despite disease-modifying antirheumatic drug (DMARD) therapy, significant improvements from baseline in disease activity (DAS28) and patient functioning (Health Assessment Questionnaire) were reported. However, details on the statistical power of the study were not provided and there was no comparator (placebo or active).37 Patients were also receiving a variety of concomitant antirheumatic drug regimens. Results of an observational cohort study19 conducted in Colombia, of patients with active RA (n = 105) despite treatment with DMARDs and receiving Etanar®, have also been described. After 12 months of follow-up, a high proportion of patients showed significant clinical improvement in disease control based on 20% response in American College of Rheumatology criteria (ACR20) and DAS28 responses.19 Marked superiority of response over innovator etanercept was claimed based on comparison with historical 12-month data. The open-label design, few details on the patient disease characteristics, and lack of a comparator arm limits meaningful comparison of Etanar® with innovator etanercept and raises questions about the validity of the authors' assertion that Etanar® should be considered a distinct biologic drug rather than an IC of innovator etanercept.38 Etanar® has also been compared with adalimumab and infliximab in a cross-sectional cohort study39 in patients (n = 158) with established RA (mean disease duration, 11 years) in Colombia, where it was stated to be as effective as the other biologics in controlling disease activity, with fewer adverse events (AEs).
Etacept and Intacept are marketed in India. The efficacy and safety of Etacept (25 mg for subcutaneous injection) was assessed in an open-label, prospective, non-comparative, multicenter study40 in patients with moderate-to-severe, active RA (n = 98), who had shown inadequate response to DMARDs. While the Etacept Prescribing Information reports patients achieved an ACR20 response of 76%41 in this study, the results remain unpublished. Intacept was studied in an open-label comparative study, where patients with active RA were randomized to receive innovator etanercept (n = 25) and Intacept (n = 87) (25 mg) by subcutaneous injection twice weekly.42 ACR20 responses of 84% were achieved in both treatment arms at the end of the 12-week treatment period,43 although the data are unpublished. Intacept is marketed in India in both 25 mg and 50 mg dosage strengths. The clinical experience of treatment of children with JIA with Etacept and Intacept compared with innovator etanercept over 6 months from a single center in India has recently been reported.44 Although the efficacy and safety of Etacept and Intacept were considered to be comparable with one another and with innovator etanercept, the study was not powered to show equivalence. A single-arm study45 on the short-term clinical experience with Etacept in patients with axial spondyloarthritis (n = 25) showed improvements in measures of disease outcome (40% response in Assessment of SpondyloArthritis international Society criteria [ASAS40]) over 12 weeks of treatment; however, no conclusions can be drawn on biosimilarity with innovator etanercept due to the absence of a comparator arm.
A preliminary analysis46 of an observational report of patients with rheumatic diseases treated with Infinitam or Etanar®, or the rituximab IC, Kikuzubam, in four hospitals in Mexico and Colombia, found a significant proportion of patients experienced grade 3/4 AEs with a very short time to onset. Kikuzubam was widely marketed in Mexico47 before its license was subsequently revoked by the Mexican Federal Commission for Protection Against Health Risks in 2014, due to concerns over the safety of the product.9
Yisaipu has been available in China for some time,9 but comparative data with innovator etanercept that would allow assessment of issues of sustainability of clinical response are currently lacking. The prescribing information for Yisaipu48 describes two 12-week, MTX-controlled, multicenter clinical trials, one conducted in patients with moderate to severe active RA (n = 238) and the other in patients with moderate to severe plaque psoriasis (n = 144). A third study was conducted in patients with active ankylosing spondylitis (n = 141) in which they were randomized to receive Yisaipu or placebo in a double-blind manner for 6 weeks, followed by open-label treatment with Yisaipu for a further 6 weeks. At 6 weeks, 68% of the 71 patients in the Yisaipu group were reported to have achieved an ASAS20 response, compared with 28% of those in the placebo arm,49 but otherwise, the results of these studies are unpublished and no comparative efficacy studies of Yisaipu and innovator etanercept have been described. The manufacturer of Yisaipu also produced the DS for production of Etanar® and Etacept for marketing in Colombia and India, respectively.8 This common source for these products is reflected in the similarity in analytical and biochemical characteristics reported above, and highlights the wider scientific challenges to be met, in terms of the safety and clinical efficacy demonstrated by the products.
A publication on TuNEX™, an IC that was not part of our analysis, indicated that the primary sequence at residues 376 and 378 differs from that for innovator etanercept.16 Although this product is undergoing assessment for regulatory approval in Taiwan,50 it does not conform to FDA51 or EMA52 guidelines for asserting biosimilarity, which specify identity in primary amino acid sequence with the innovator product as one of the requirements.
The clinical consequences of the structural differences of the ICs with respect to innovator etanercept or ICs of other biologic compounds are unknown. However, as the clinical outcomes of a particular compound are stringently related to the molecular structure, there are potentially unforeseen outcomes. On the other hand, the long-term benefits of innovator etanercept are well established, with over 4.5 million patient-years of post-marketing experience accumulated since the initiation of clinical trials in 1993.53 In all of these populations, etanercept (with or without MTX) effectively reduced the signs and symptoms of disease, disease activity and disability, and improved health-related quality of life, with these benefits being sustained during long-term treatment.1 Moreover, data from a long-term prospective study,54 with some patients receiving >10 years of treatment, indicate that etanercept is safe and effective as a long-term, continuous therapy for the treatment of both patients with early RA and long-standing RA, and the risk/benefit ratio of continuous long-term treatment remains favorable.54
In conclusion, we have highlighted differences in product quality characteristics between the etanercept innovator product and ICs that are currently available to patients in some markets. None of the batches for the ICs tested (n = 24) met the criteria that would be typically applied to establish comparability with the innovator product. The combination of the results from these analyses and the limited clinical data available indicate that Yisaipu, Etanar®, Etacept, Infinitam, Altebrel™, Qiangke, and Intacept should not be considered biosimilar to etanercept as per the standards outlined in WHO, FDA, or EMA guidance.
The findings reported here reinforce the view that demonstration of biosimilarity between etanercept and proposed biosimilars should follow a stepwise approach, comprising rigorous analytical, non-clinical, and clinical evaluation aligned with WHO, EMA, and FDA standards. For patients already receiving etanercept, rheumatologists and all prescribing clinicians should be aware of issues regarding switching to, and interchangeability with, ICs.
Materials and methods
The tests used for this analysis of the ICs are shown in Table 3. These methods represent a subset of characterization tests typically applied to support etanercept evaluations that were performed to assess key parameters of individual ICs. The key methods are described below. Details of the SDS-PAGE Coomassie analysis and cell-based bioassay (neutralization of TNF-mediated apoptosis in U937 cells) used in this study have been described previously.55
Tryptic peptide mapping
Tryptic peptide mapping is used as an identification procedure to confirm the peptide profile of etanercept DS by HPLC with ultraviolet (UV) detection. Results for selected peaks are reported as a percentage of Enbrel® RS analyzed in the same assay occasion. MS detection (using a Waters Acquity UPLC with Waters MALDI Q-ToF Premier Micromass MS detection) is additionally applied to characterize the mass of each peptide, assess for intact N- and C-terminal amino acid residues, and evaluate the distribution of O-linked glycans on specific peptides.
Guanidine-tris(hydroxymethyl)aminomethane buffer solution, pH 8.3 (500 µL) and dithiothreitol (7 µL of a 154-g/L solution) were added to an aqueous solution of the test sample (200 µL of a 15-mg/mL solution). The mixture was incubated at 65°C for 15 minutes, and then cooled in an ice-bath for 5–10 minutes. A freshly prepared solution of iodoacetamide (15.4 µL of a 185-g/L solution) was added and the mixture was allowed to stand for 10 minutes, while protected from light. Dithiothreitol (1.4 µL of a 154-g/L solution) was added and the mixture allowed to stand protected from light for a further 10 minutes.
0.1M tris-hydrochloride buffer solution, pH 7.5 (903 µL) was added to the reduced test solution (97 µL). A 500,000-U/mL solution of peptide N-glycosidase F (9.6 µL) (New England Biolabs, 704L: Purified from Flavobacterium meningosepticum, free of proteases and Endo F activities) was added and the mixture incubated at 37°C for 1 hour. Trypsin for peptide mapping (Trypsin Sequencing Grade, modified, Roche Molecular Biochemicals, 1047841) (40 µL of a 1-mg/mL solution) was added and the mixture incubated at 37°C for 5 hours. Following heating of the mixture at 95°C for 5 minutes and cooling in ice for 5 minutes, the mixture was adjusted to pH 2 with trifluoroacetic acid (approximately 30 µL of a 150-g/L solution).
The product was analyzed by reverse-phase HPLC (Kromasil C18 by Phenomenex/5.0 µm with a pore size of 10 nm) on an analytical column (3.2 mm × 250 mm) at 30–35°C. Components were separated by gradient elution of 2%–95% of mobile phase B (mobile phase A, 0.15% trifluoroacetic acid in water; mobile phase B, 0.1% trifluoroacetic acid in water/acetonitrile [Fisher: #A996-400] [1/4, v/v]) at a flow rate of 0.5 mL/min. Monitoring was by UV (220 nm) and MS detection. Results for selected peaks were reported as a percentage of Enbrel® RS analyzed in the same assay occasion. MS detection was additionally applied to characterize the mass of each peptide to assess for intact N- and C-terminal amino acid residues, and to evaluate the distribution of O-linked glycans on specific peptides.
Hydrophobic interaction chromatography
HIC is performed to assess the purity of etanercept DS and the related product species present. The method is used to resolve etanercept into three variants, which differ in biological activity: Peak 1 is predominantly clipped species; Peak 2 is homogenous etanercept; Peak 3 consists of misfolded species, aggregates, etanercept fragments, and other process-related impurities. Results are expressed as relative percentage peak area.
Each sample (5 µL; 2 mg/mL) was injected onto a TSKgel Butyl-NPR (Tosoh Corporation, 14947) (2.5 µm) analytical column (4.6 mm × 35 mm) at 35°C, connected to an HPLC system. Product-related impurities were separated by gradient elution over 50 minutes using mobile phase A (ammonium sulfate [475.9 g] and anhydrous disodium hydrogen phosphate [28.4 g] in 1,950 mL water for chromatography, adjusted to pH 7.0 with phosphoric acid and diluted to 2,000 mL with water for chromatography), and mobile phase B (anhydrous disodium hydrogen phosphate [28.4 g] in water for chromatography [J.T. Baker Ultra-Resi Analyzed Water; #JT4219-3) and diluted to 1,950 mL with water, adjusted to pH 7.0 with phosphoric acid and diluted to 2,000 mL with water). The flow rate was 1.0 mL/min, and chromatography was monitored by fluorescence detection (278 nm for excitation; 350 nm for emission). Elution of the protein molecules occurred in order of increasing hydrophobicity, as the salt concentration decreased throughout the run, separating the sample into HIC Peak 1, HIC Peak 2, and HIC Peak 3, which were integrated and reported as relative %Peak 1, %Peak 2, and %Peak 3.
Size-exclusion HPLC
SE-HPLC is performed to assess the purity of etanercept DS by separation of the high-molecular-weight component from homogenous dimeric etanercept and low-molecular-weight component (clipped species) based on molecular size, and resolves etanercept into two principal peaks: Peak B = high-molecular-weight component; Peak A + A’ = etanercept dimer + low-molecular-weight component. Results are expressed as relative percentage peak area.
Each sample (14 µL; 2.5 mg/mL) was injected onto an analytical HPLC column (8 mm × 300 mm) containing hydrophilic silica gel (5 μm with a pore size of 30 nm and of a grade suitable for fractionation of globular proteins in the relative molecular mass range of 10,000–1,000,000) at 25°C. Monitoring was performed by UV detection (λ = 220 nm). A mobile phase consisting of 220 mL of solution A (sodium dihydrogen phosphate [15.6 g] and of sodium chloride [8.8 g] in 1,000 mL water for chromatography and 780 mL of solution B (of anhydrous disodium hydrogen phosphate [14.2 g] and of sodium chloride [8.75 g] in 1,000 mL water for chromatography), adjusted to pH 7.2, was used. The flow rate was 1.0 mL/min. Etanercept was resolved into two principal peaks based on molecular size: Peak B = high-molecular-weight component; Peak A + A’ = etanercept dimer + low-molecular-weight component.
N-linked oligosaccharide mapping
N-linked oligosaccharides are post-translational modifications that are added to the polypeptide during the production in cell culture. Each etanercept monomer has three sites for the addition of N-linked oligosaccharide structures. Each of the three N-linked oligosaccharides added to etanercept can be any one of multiple characterized N-linked species. Nine of the principal N-linked oligosaccharide species are routinely quantified for every batch of etanercept DS and must meet stringent acceptance ranges. N-linked glycans are chromatographically resolved into various neutral (Peaks 1–5) and sialylated (Peaks 6–9) species after enzymatic cleavage from the protein. Each peak is controlled within a specified range.
0.25 M sodium phosphate, pH 7.5 (3 µL), and a 500,000-U/mL solution of peptide N-glycosidase F (2 µL), were added to the test solution (4 µL of 25 mg/mL solution). The mixture was incubated at 37°C for 20–24 hours. The released N-glycans were labeled with 2-aminobenzamide (2-AB Labeling Kit: Glyko Inc. #K404). The labeled N-glycans were resuspended or diluted in water (100 µL) and separated by reverse-phase HPLC (GlycoSepN HPLC column from Prozyme/5.0 µm) on an analytical column (4.6 mm × 250 mm) at 35°C. Components were separated by gradient elution from 80% to 20% of mobile phase B (mobile phase A, 0.5% formic acid in water [adjusted to pH with ammonia]; mobile phase B, acetonitrile) at a flow rate of 0.4 mL/min. Monitoring was by fluorescence detection (330 nm for excitation; 420 nm for emission). N-linked glycans are resolved into various neutral (Peaks 1–5) and sialylated (Peaks 6–9) species after enzymatic cleavage from the protein.
HCP enzyme-linked immunosorbent assay
The HCP ELISA employs polyclonal antibodies to quantitate HCP impurities from CHO cells (null vector) in purified etanercept DS (the reagents used are proprietary to Pfizer). The units are specific to this assay and expressed as ppm.
Protein A ELISA
The purification of etanercept includes affinity chromatography on protein A sepharose. Leached protein A can remain bound to etanercept and be present in the DS. The protein A ELISA ensures product purity, consistency, and quality by quantitation of the leached protein A, expressed as ppm (the reagents used are proprietary to Pfizer).
Isoelectric focusing
IEF is used to separate proteins based on their isoelectric point, which is the pH for which that protein demonstrates no net charge. The various bands observed on the IEF gel are indicative of the charge heterogeneity of the etanercept molecule, which is primarily attributable to the heterogeneity of the sialylated oligosaccharides attached. There are no specifications for Enbrel® RS; however, a visual comparison of the sample profile is made with respect to the IEF gel profile and isoelectric point range of Enbrel® RS.
Anion exchange chromatography
Etanercept is a complex and heterogeneous recombinant protein composed of multiple isoforms. AEX is used to assess negative charge heterogeneity of etanercept. This heterogeneity results in a wide elution range of etanercept isoforms in the AEX assay.
Each sample (200 µL; 250 µg/mL in mobile phase A) was injected onto a non-porous quaternary amine (3 µm); Anion Exchange Column (4.6 mm × 50 mm) at 30°C connected to an HPLC system. The negative charge distribution was evaluated over 34 minutes by gradient elution using mobile phase A (imidazole [4.18 g] in 1,500 mL water for chromatography [pH 6.2], and mobile phase B (imidazole [4.18 g] and sodium chloride [81.816 g] in 1,500 mL water for chromatography and diluted to 2,000 mL with water [pH 6.2]). The flow rate was 0.8 mL/min, and chromatography was monitored by UV detection (280 nm).
The heterogeneity of the multiple isoforms in etanercept results in a wide elution range and broad peak.
Sialic acid content
Test samples (5 mg/mL in water) were diluted to a concentration of 1 mg/mL with dilution buffer (prepared from L-arginine [Sigma-Aldrich: #A5006] [8.71 g] and 1% polysorbate 80 [Sigma-Aldrich; #P8074] [0.5 mL] in water [40 mL], adjusted to pH 7.3 with 85% phosphoric acid [Sigma-Aldrich; #43808-1], and diluted to a volume of 250 mL with water). N-acetylneuraminic acid [Sigma-Aldrich: #A2751/A2388] (40 μL of 1 mg/mL solution) and bovine serum albumin (Peribo Science UK, Ltd.) (40 μL of 1 mg/mL solution) were added to dilution buffer (120 μL). 8 M acetic acid (Fisher: #A/0400/PB17) (50 µL) was added to each preparation (N-acetylneuraminic acid solution [50 µL] and test sample [50 µL]) and the mixture incubated at 90°C ± 5°C on a heat block for 65 ± 2 minutes. On cooling, the sample was briefly centrifuged, and then vacuum centrifuged until dry. 7.0 mM 1,2-diamino-4,5-methyleneoxybenzene (DMB) label (15 µL; prepared by dissolving DMB dihydrochloride in water, containing 1.0 M β-mercaptoethanol and 18 mM sodium hydrosulfite) was added to each vial of dried sample. Components were separated by reverse-phase HPLC (Beckman Ultrasphere C18/5.0 µm) on an analytical column (4.6 mm × 250 mm) at 35°C, by elution (mobile phase prepared from methanol [Fisher: #A412-400] [500 mL] and acetonitrile [8 mL] in water [1820 mL]) at a flow rate of 1.0 mL/min. Monitoring was by fluorescence detection (374 nm for excitation; 448 nm for emission). Sialic acid content in each sample was calculated based on an N-acetylneuraminic acid standard calibration curve.
Receptor binding
The receptor binding assay used was a quantitative solid-phase ELISA. Etanercept (TNFR:Fc) in samples and standards binds to TNF adsorbed to microplate wells and were detected using a horseradish peroxidase-conjugated antibody. Binding activities are calculated based on the ratio of the ED50 values of the calibration curve relative to the control or sample curves.
Funding details
This work was funded by Pfizer.
Disclosure of potential conflicts of interest
M.S. is a consultant for Boehringer Mannheim, GSK, Orygen, Pfizer, and Sanofi Genzyme. G.C-H. is a consultant for AbbVie, Bayer, Boehringer Ingelheim, Eli Lilly, Laboratorios Liomont, Laboratorios Sophia, Medix, Merck, Merck-Serono, Novartis, Pfizer, Roche, Sanofi, and Sharp and Dohme. He has participated in speakers' bureaus for AbbVie, Amgen, Astra-Zeneca, Bayer, Eli Lilly, Janssen-Cilag, Laboratorios Liomont, Laboratorios Sophia, Medix, Merck, Merck-Serono, Novartis, Pfizer, Roche, Sanofi, Sharp and Dohme, and UC. M.L. has no conflicts to declare. U.R.K.R. is a consultant for Pfizer and Sanofi. E.S., E.M., J.C., J.O., S.M.V., B.F., and B.H. are shareholders and employees of Pfizer.
Acknowledgments
Editorial/medical writing support was provided by Iain McDonald of Engage Scientific Solutions and was funded by Pfizer.
References
- 1.Scott LJ. Etanercept: a review of its use in autoimmune inflammatory diseases. Drugs. 2014;74:1379–410. doi: 10.1007/s40265-014-0258-9. PMID:25034360 [DOI] [PubMed] [Google Scholar]
- 2.Maksymowych WP, Dougados M, van der Heijde D, Sieper J, Braun J, Citera G, Van den Bosch F, Logeart I, Wajdula J, Jones H, et al.. Clinical and MRI responses to etanercept in early non-radiographic axial spondyloarthritis: 48-week results from the EMBARK study. Ann Rheum Dis. 2016;75:1328–35. doi: 10.1136/annrheumdis-2015-207596. PMID:26269397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Windschall D, Müller T, Becker I, Horneff G. Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis-related arthritis and psoriasis arthritis. Clin Rheumatol. 2015;34:61–9. doi: 10.1007/s10067-014-2744-6. PMID:25034081 [DOI] [PubMed] [Google Scholar]
- 4.Baldo BA. Chimeric fusion proteins used for therapy: indications, mechanisms, and safety. Drug Saf. 2015;38:455–79. doi: 10.1007/s40264-015-0285-9. PMID:25832756 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Guidelines on evaluation of similar biotherapeutic products (SBPs). 2010. [accessed 2016June23]. http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf.
- 6.US Food and Drug Administration Scientific considerations in demonstrating biosimilarity to a reference product. 2015. [accessed 2016December7]. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf.
- 7.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP ). Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues (last update: December18, 2014). [accessed 2016June23]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf
- 8.Azevedo VF, Galli N, Kleinfelder A, D'Ippolito J, Urbano PC. Etanercept biosimilars. Rheumatol Int. 2015;35:197–209. doi: 10.1007/s00296-014-3080-5. PMID:24980068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castañeda-Hernández G, González-Ramírez R, Kay J, Scheinberg MA. Biosimilars in rheumatology: what the clinician should know. RMD Open. 2015;1:e000010. doi: 10.1136/rmdopen-2014-000010. PMID:26509046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Federation of Pharmaceutical Manufacturers & Associations Policy Statement. Non-comparable Biotherapeutic Products. Geneva, Switzerland: IFPMA; 2014July24 [accessed 2017March28]. http://www.ifpma.org/wp-content/uploads/2016/02/Non-comparable_Biotherapeutic_Products__English__02.pdf. [Google Scholar]
- 11.Schiestl M, Stangler T, Torella C, Cepeljnik T, Toll H, Grau R. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat Biotechnol. 2011;29:310–2. doi: 10.1038/nbt.1839. PMID:21478841 [DOI] [PubMed] [Google Scholar]
- 12.Maity S, Ullanat R, Lahiri S, Shekar S, Sodhan G, Vyas A, Dyaga G, Ireni S, Nair N, Sotsios Y, et al.. A non-innovator version of etanercept for treatment of arthritis. Biologicals 2011;39:384–95. doi: 10.1016/j.biologicals.2011.08.014. PMID:21996051 [DOI] [PubMed] [Google Scholar]
- 13.Yi S, Kim SE, Park MK, Yoon SH, Cho JY, Lim KS, Shin SG, Jang IJ, Yu KS. Comparative pharmacokinetics of HD203, a biosimilar of etanercept, with marketed etanercept (Enbrel(R)): a double-blind, single-dose, crossover study in healthy volunteers. BioDrugs 2012;26:177–84. doi: 10.2165/11631860-000000000-00000. PMID:22515513 [DOI] [PubMed] [Google Scholar]
- 14.Tan Q, Guo Q, Fang C, Wang C, Li B, Wang H, Li J, Guo Y. Characterization and comparison of commercially available TNF receptor 2-Fc fusion protein products. MAbs 2012;4:761–74. doi: 10.4161/mabs.22276. PMID:23032066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miranda-Hernández MP, López-Morales CA, Perdomo-Abúndez FC, Salazar-Flores RD, Ramírez-Ibanez ND, Pérez NO, Molina-Pérez A, Revilla-Beltri J, Flores-Ortiz LF, Medina-Rivero E. New alternatives for autoimmune disease treatments: physicochemical and clinical comparability of biosimilar etanercept. J Immunol Res. 2016;2016:9697080. doi: 10.1155/2016/9697080. PMID:27382576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang LJ, Chiang CW, Lee YW, Wang TF, Fong CC, Chen SH. Characterization and comparability of stress-induced oxidation and deamidation on vulnerable sites of etanercept products. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1032:189–97. doi: 10.1016/j.jchromb.2016.05.007. PMID:27237733 [DOI] [PubMed] [Google Scholar]
- 17.Hassett B, McMillen S, Fitzpatrick B. Characterization and comparison of commercially available TNF receptor 2-Fc fusion protein products: letter to the editor. MAbs. 2013;5:624–5. doi: 10.4161/mabs.25817. PMID:23924794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shanghai CP Guojian Pharmaceutical Co Ltd Products (prescription drugs). [accessed 2017August29]. http://www.cpgj-pharm.com/en/Products.asp?id = 7
- 19.Santos-Moreno PI, Sanchez G, Gomez D, Castro C. Clinical outcomes in a cohort of Colombian patients with rheumatoid arthritis treated with Etanar, a new biologic type rhTNFR:Fc. Clin Exp Rheumatol. 2015;33:858–62. PMID:26343288 [PubMed] [Google Scholar]
- 20.Ciplamed Product Index. ETACEPT Injection (Etanercept). [accessed 2017Augustust 29]. https://ciplamed.com/content/etacept-injection. [Google Scholar]
- 21.Probiomed Divisiones. Línea RTO (Reuma/Trauma/Orto). [accessed 2017August29]. http://www.probiomed.com.mx/divisiones. [Google Scholar]
- 22.Aryogen Products. Altebrel™. [accessed 2017August29]. http://aryogen.com/English/Altebrel.html. [Google Scholar]
- 23.Intas Pharmaceuticals Ltd. , Intacept. [accessed 2017August29]. http://intacept.in/.
- 24.Shanghai Celgen Biopharma SC Products. Qiangke. [accessed 2017August29]. http://www.celgenpharm.com/products. [Google Scholar]
- 25.World Health Organization Expert Committee on Biological Standardization. Regulatory Assessment of Approved rDNA-Derived Biotherapeutics. 2015. [accessed 2016June23]. http://www.who.int/biologicals/RA_for_BTP_for_WHO_web_editor_2_Nov_2015(2).pdf. [Google Scholar]
- 26.Markus R, Liu J, Ramchandani M, Landa D, Born T, Kaur P. Developing the Totality of Evidence for Biosimilars: Regulatory Considerations and Building Confidence for the Healthcare Community. BioDrugs. 2017;31:175–87. doi: 10.1007/s40259-017-0218-5. PMID:28439817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheinberg M, Castañeda-Hernández G. Anti-tumor necrosis factor patent expiration and the risks of biocopies in clinical practice. Arthritis Res Ther. 2014;16:501. doi: 10.1186/s13075-014-0501-5. PMID:25677586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho IH, Lee N, Song D, Jung SY, Bou-Assaf G, Sosic Z, Zhang W, Lyubarskaya Y. Evaluation of the structural, physicochemical, and biological characteristics of SB4, a biosimilar of etanercept. MAbs. 2016;8:1136–55. doi: 10.1080/19420862.2016.1193659. PMID:27246928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srikanth J, Agalyadevi R, Babu P. Targeted, Site-specific quantitation of N- and O-glycopeptides using 18O-labeling and product ion based mass spectrometry. Glycoconj J. 2017;34:95–105. doi: 10.1007/s10719-016-9733-8. PMID:27714477 [DOI] [PubMed] [Google Scholar]
- 30.Houel S, Hilliard M, Yu YQ, McLoughlin N, Martin SM, Rudd PM, Williams JP, Chen W. N- and O-glycosylation analysis of etanercept using liquid chromatography and quadrupole time-of-flight mass spectrometry equipped with electron-transfer dissociation functionality. Anal Chem. 2014;86:576–84. doi: 10.1021/ac402726h. PMID:24308717 [DOI] [PubMed] [Google Scholar]
- 31.DiPaola M, Li J, Stephens E. Development of Biosimilars: Analysis of Etanercept Glycosylation as a Case Study. J Bioanal Biomed. 2013;5:180–6. doi: 10.4172/1948-593X.1000096. [DOI] [Google Scholar]
- 32.Liu L. Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins. J Pharm Sci. 2015;104:1866–84. doi: 10.1002/jps.24444. PMID:25872915 [DOI] [PubMed] [Google Scholar]
- 33.US Food and Drug Administration FDA Briefing Document. Arthritis Advisory Committee Meeting, July 13, 2016. BLA 761042. GP2015, a proposed biosimilar to Enbrel. [accessed 2017August29]. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM510493.pdf.
- 34.Zhou H. Clinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion protein. J Clin Pharmacol. 2005;45:490–7. doi: 10.1177/0091270004273321. PMID:15831771 [DOI] [PubMed] [Google Scholar]
- 35.Hassett B, Vicik SM, Fitzpatrick B. Comment on “new alternatives for autoimmune disease treatments: physicochemical and clinical comparability of infinitam with etanercept innovator product” J Immunol Res 2017;(Accepted 2017 October 11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AryoGen Pharmed AltebelTM (etanercept). Health care professional information. Alborz, Iran: AryoGen Pharmed. [accessed 2016December7]. http://aryogen.com/English/docs/Altebrel%20HCP.pdf. [Google Scholar]
- 37.Rondon F, Bautista A, Salazar J, Casas N, Santos P, Vargas F, Marquez J. Etanar therapy in real-life patients with rheumatoid arthritis. Arthritis Rheum. 2010;62(Suppl 10):1811. [Google Scholar]
- 38.Scheinberg M. Is Etanar a new biologic? Clin Exp Rheumatol. 2016;34:954. PMID:27383125 [PubMed] [Google Scholar]
- 39.Santos-Moreno P, Saavedra-Martinez G, Villarreal L, Gomez D, Bello-Gualtero J, Giraldo V, Martinez P, Sanchez A, Sanchez M, Uribe E, Boon M. . Etanar – a etanercept biosimilar is as effective as adalimumab and infliximab in a cohort of real-life patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74(Suppl 2):789. doi: 10.1136/annrheumdis-2015-eular.2508. [DOI] [Google Scholar]
- 40.Clinical Trials Registry India CTRI/2009/000399. An open label, prospective, non-comparative, multicentre study to assess the safety and efficacy of Etanercept for injection 25 mg in patients with moderate to severe active rheumatoid arthritis.: Clinical Trials Registry India; 2009. [accessed 2017March28]. http://www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid = 622&EncHid = &modid = &compid = ‘,'622det’.
- 41.Ciplamed. Product Index ETACEPT Injection (Etanercept). (Last updated: March2016). [accessed 2017March28]. http://www.ciplamed.com/content/etacept-injection.
- 42.Clinical Trials Registry India CTRI/2013/003963. A Prospective, Comparative, Open Label, Randomized, Multicentric Phase III study to compare the safety and efficacy of etanercept of Intas Biopharmaceuticals Ltd against Enbrel® in patients with Active Rheumatoid Arthritis.: Clinical Trials Registry India.; 2013. [accessed 2017March28]. http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid = 7319&EncHid = &modid = &compid = ‘,'7319det’.
- 43.Intas Pharmaceuticals Ltd Intacept prescribing information. Etanercept solution for injection 25 mg/0.5 mL or 50 mg/1.0 mL. Gujarat, India: Intas Pharmaceuticals Ltd.; [accessed 2017March28]. http://intacept.in/pdfs/prescribingInfo/Prescribing%20Informatio.pdf. [Google Scholar]
- 44.Shivpuri A, Mittal S, Agarwal M, Sawhney S. A single centre experience from India on the safety and efficacy of Cipla etanercept and Intas etanercept and its comparison with reference etanercept (Enbrel) in children with JIA. Arthritis Rheumatol. 2016;68(Suppl 10):391. [Google Scholar]
- 45.Kumar A, Bansal R, Goel A, Dembla G, Abrol A. Clinical experience with etanercept biosimilar (Etacept) in axial spondyloarthritis. Int J Rheum Dis 2015;18 (Suppl 1):116. [Google Scholar]
- 46.Barile-Fabris LA, Irazoque-Palazuelos F, Hernández Vásquez R, Carrillo Vazquez S, Gúzman R. Incidence of adverse events in patients treated with intended copies of biologic therapeutic agents in Colombia and Mexico. Arthritis Rheumatol. 2014;66(suppl 10):1506. [Google Scholar]
- 47.Scheinberg MA, Kay J. The advent of biosimilar therapies in rheumatology–“O brave new world”. Nat Rev Rheumatol. 2012;8:430–6. doi: 10.1038/nrrheum.2012.84. PMID:22664834 [DOI] [PubMed] [Google Scholar]
- 48.Shanghai CP Guojian Pharmaceutical Co. Ltd Recombinant Human Tumor Necrosis Factor-αReceptor II: IgG Fc Fusion Protein for Injection— Package Insert. Shanghai, China: Shanghai CP Guojian Pharmaceutical Co. Ltd.; 2007. [accessed 2017March28]. http://www.cpgj-pharm.com/uploadfile/Yisaipu.pdf. [Google Scholar]
- 49.Huang F, Liang D-F, Guo J-H, Liao Z-T, Deng X-H, Zhang Y-M, Gu Y-R, Bao C-D, Zhang J-L, Hu D-W, Lin Z-M, Yang C-H. . Treatment of ankylosing spondylitis with a recombinant human tumor necrosis factor receptor-Fc fusion protein: a multicenter, randomized, double blind, controlled trial. Chinese J Rheumatol. 2008;12:314–20. [Google Scholar]
- 50.Mycenax Mycenax filed for marketing approval of TuNEX® (press release). May 6, 2016. [accessed 2016December7]. http://www.mycenax.com.tw/en/news.php?act = view&no = 11.
- 51.US Food and Drug Administration Quality Considerations in Demonstrating Biosimilarity of a Therapeutic Protein Product to a Reference Product. Guidance for Industry. (April 2015). [accessed 2016December7]. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm291134.pdf [Google Scholar]
- 52.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) Guideline on Similar Biological Medicinal Products. CHMP/437/04 Rev 1 (23 October 2014). [accessed 2016December7]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf. [Google Scholar]
- 53.Hassett B, Singh E, Mahgoub E, O'Brien J, Vicik SM, Fitzpatrick B. Manufacturing history of etanercept (Enbrel®): Consistency of product quality through major process revisions. MAbs (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinblatt ME, Bathon JM, Kremer JM, Fleischmann RM, Schiff MH, Martin RW, Baumgartner SW, Park GS, Mancini EL, Genovese MC. Safety and efficacy of etanercept beyond 10 years of therapy in North American patients with early and longstanding rheumatoid arthritis. Arthritis Care Res (Hoboken). 2011;63:373–82. PMID:20957659 [DOI] [PubMed] [Google Scholar]
- 55.Shannon E, Daffy J, Jones H, Paulson A, Vicik SM. Etanercept (Enbrel®) alternative storage at ambient temperature. Clin Pharmacol Adv Applications. 2017;9:87–99. doi: 10.2147/CPAA.S131832. [DOI] [PMC free article] [PubMed] [Google Scholar]


