ABSTRACT
Heatstroke results from a failure to dissipate accumulated heat during exposure to hot environments, or during strenuous physical exercise under heat stress. It is characterized by core body temperatures > 41°C, with central nervous system dysfunction. Functional morphology and thermoregulatory effectors differences between dogs and humans may require special heatstroke protective adaptations in dogs, however, the risk factors for developing heatstroke are similar in both. In dogs, these include hot, especially highly humid environments, excessive physical activity, obesity, large (>15 kg) body weight, being of certain breed (e.g., Labrador retrievers and brachycephalic breeds), upper airway obstruction and prolonged seizures. Lack of acclimation to heat and physical fitness decreases the survival of heat stroked dogs. At the systemic level, blood pooling within the large internal organs (e.g., spleen, liver) is a major contributor to the development of shock and consequent intestinal ischemia, hypoxia and endothelial hyperpermeability, commonly occurring in heatstroke patients. Evoked serious complications include rhabdomyolysis, acute kidney injury, acute respiratory distress syndrome and ultimately, sepsis and disseminated intravascular coagulation. The most common clinical signs in dogs include acute collapse, tachypnea, spontaneous bleeding, shock signs and mental abnormalities, including depression, disorientation or delirium, seizures, stupor and coma. In such dogs, presence of peripheral blood nucleated red blood cells uniquely occurs, and is a highly sensitive diagnostic and prognostic biomarker. Despite early, appropriate body cooling, and intensive supportive treatment, with no available specific treatment to ameliorate the severe inflammatory and hemostatic derangements, the mortality rate is around 50%, similar to that of human heatstroke victims. This review discusses the pathophysiology of canine heatstroke from a veterinarian's point of view, integrating new and old studies and knowledge.
KEYWORDS: acute kidney injury, canine, disseminated intravascular coagulation, heat shock protein
Most of the data on heatstroke have been derived from experimentally induced models of heatstroke in rodents, baboons and dogs, and from clinical studies in humans.1-12 Only a single study of naturally occurring heat-induced illness in 42 dogs has been published until year 2006.13 Since then, different aspects of this devastating syndrome have been investigated in dogs with naturally occurring heatstroke, admitted to the Hebrew University Veterinary Teaching Hospital, and are presented in this review. This review integrates the unique risk factors, complications, biomarkers, organ and system damage in dogs, from the veterinary perspective.
Epidemiology
Factors predisposing to heatstroke
Heat-related illness is a major, worldwide cause of morbidity and mortality.14-21 Recently, changes in global climate and weather patterns have led to unprecedented deaths due to heatstroke.22-25 In 2003, a prolonged heat wave in Western Europe has led to 45,000 deaths of people, of which a third has been attributed to heatstroke.3,22,26-29 The concept of ‘One Health’ suggests that it would be prudent for veterinary medicine to anticipate a similar rise in heat-related illness in domestic animal species, sharing our homes and lifestyle habits. The time to start developing a more complete understanding of the pathophysiology of heatstroke in dogs, and search for its optimal treatment is now.
Several predisposing risk factors for heatstroke in dogs have been identified, including obesity, a high body weight (>15 kg), being of certain breed (e.g., Labrador retriever), lack of acclimation to heat stress, lack of fitness and exposure to a hot, often highly humid environment.30-32 These are presented in several examples below.
In a study of 54 dogs diagnosed with naturally occurring heatstroke by Bruchim et al.,the median body weight of the dogs was 31 kg, supporting a higher susceptibility of large breed dogs to heat stress.30 Likewise, several large breed dogs, such as Golden and Labrador retrievers, brachychephalic breeds (e.g., English bulldog) and military working dogs (mostly Belgian and Dutch shepherds) were significantly over represented in large-scale studies of canine heatstroke.30,33,34
Brachycephalic dogs are at risk for heat related illness due to their poor ineffective evaporative ability (i.e., stenotic nares, elongated soft palate and hypoplastic trachea), and their tendency to develop laryngeal edema.35 The over representation of military working dogs (e.g., Belgian malinios) in past studies of naturally occurring heatstroke is very likely due to their frequent exposure to intensive training and work under harsh environmental heat stress, especially if insufficiently trained and acclimated, rather than because of their larger body weight.33,36
Obesity and an active playful character, even under environmental heat stress conditions, likely account for the over representation of Golden and Labrador retrievers in our past studies, most of which had sustained exertional heatstroke.34,37,38 Hereditary exercise-induced collapse (EIC), characterized by episodic limb weakness, ataxia and collapse, has been described in Labrador retriever dogs, and might thus account for some heatstroke cases in this breed.39-41 It was reported that a canine Dynamin 1 (DNM1) mutation is highly associated with the syndrome of EIC.42 Dynamin 1 belongs to a family of catalytic enzymes involved in synaptic vesicle endocytosis and neurotransmission during sustained neural stimulation, such as during exercise.39,42 EIC was reported in Border collie working dogs, and in racing greyhounds.43,44 Notably, although a genetic basis in the Border collie is suspected, all 13 Border collies with clinical presentation of EIC were negative for the known DNM1 mutation, as well as for the malignant hyperthermia gene mutation, suggesting an additional mutation.41
Another reported syndrome in dogs, potentially predisposing them to heatstroke, is exercise induced malignant hyperthermia in English Springer spaniels, in which moderate exercise results in collapse, dyspnea, hyperthermia and hyperlactatemia.45 Interestingly, the caffeine/halothane test was positive in these dogs, indicating a role of a calcium homeostasis defect, due to ryanodine receptor (RYR) calcium release dysfunction, as described in humans and pigs.45-49
Pathogenesis
Systemic outlook
Heatstroke is a highly fatal syndrome, caused by elevated core body temperature, where intrinsic and extrinsic heat production exceeds the capability of the heat dissipation mechanisms. In dogs it is characterized by core temperatures above 41°C (105.8ºF), with central nervous system dysfunction.1,15,50 It results from exposure to a hot, often highly humid environment (classical or environmental heatstroke), due to excessive voluntary strenuous physical exercise and/or prolonged uncontrolled muscle tremors or seizures (exertional heatstroke).4,36,51
In dogs, under normal environmental conditions, more than 70% of the total body heat is dissipated through radiation and convection from body surfaces. During the initial stages of exposure to heat stress, the cardiac output increases, as a result of splenic contraction and an increase in systemic vascular resistance (SVR) in major internal organs (i.e., spleen, liver and gastrointestinal tract [GIT]), redistributing blood flow to the skin to increase heat dissipation.52-54
As environmental temperature increases further, approaching the body temperature, heat dissipation by convection and radiation through the skin diminishes, and evaporation, primarily through panting, becomes the major heat dissipation mechanism.1
In dogs, the nasal turbinates provide a large surface area for water evaporation, from the moist mucous membranes, thereby playing an important role in heat dissipation through panting, while hypersalivation increases the evaporative efficiency through evaporation from the oral mucous membranes and the tongue. With high environmental temperatures and increased relative humidity (>35%), panting becomes progressively less effective for dissipating excessive body heat, and when the relative humidity is >80%, this body cooling mechanism in dogs is negated.1,55 In addition, with a progressive increase in core body temperature, metabolic derangement ensues, cardiac output decreases, leading to heat dissipation failure, aggravating body heat accumulation.52,56,57 When hyperthermia, combined with dehydration, progresses, cutaneous and splanchnic blood pooling results in decreased circulating blood volume and hypotension.52,53,58-61 It has been suggested that one of the mechanisms linked with splanchnic vasodilatation involves nitric-oxide overproduction, leading to the uncontrolled splanchnic dilation preceding vascular collapse during heatstroke.61,62 (For detailed discussion see in Gastrointestinal lesions and bacterial translocation). Pooling of blood within the large internal organs (e.g., spleen and liver) is a major contributor to the development of shock and the consequent intestinal ischemia, hypoxia and endothelial hyperpermeability that commonly occur in heatstroke patients.15,35,63 Shapiro et al.,64 demonstrated in experimentally-induced heatstroke mongrel dogs that the rectal temperature should exceed 43°C for 40 minutes for development of clinical signs of heatstroke. The severity of the heatstroke was positively correlated to the maximal rectal temperature, as well as to its duration.64 However, our experience with highly trained military working dogs, that although they oftentimes sustain hyperthermia of >42°C following strenuous physical activity, did not result in any clinical or laboratory signs of heatstroke, suggesting that acclimation is an important factor in preventing overt heatstroke in dogs.
Body hyperthermia initiates a myriad of inflammatory, hemostatic and tissue damage processes of varying severity and progression rate in dogs. Activation of the inflammatory and hemostasis cascades initiates a systemic inflammatory response syndrome (SIRS), often progressing to multiple organ dysfunction syndrome (MODS).37,65-68 Combination of the direct heat insult, severe hypovolemic, distributive shock, metabolic acidosis, neurologic dysfunction, endotoxemia and disseminated intravascular coagulation (DIC) result in decreased organ perfusion, tissue necrosis and hemorrhagic diathesis, as recorded at necropsy in fatal cases of naturally occurring heatstroke in dogs.56,58 The serious complications of heatstroke in dogs include rhabdomyolysis, neurological damage and dysfunction, acute kidney injury (AKI), acute respiratory distress syndrome (ARDS), hepato-billiary damage, sepsis, acute pancreatitis and DIC.34,69-71 It seems that the main causes of death in dogs with heatstroke are systemic hemodynamic deterioration and pulmonary lesions, as determined from postmortem studies of dogs that sustaibned naturally occurring heatstroke.69
Neurological dysfunction and abnormalities
Neurological abnormalities are invariably present in dogs with clinical heatstroke, including coma (40%), seizures (35%) and stupor (33%).30 Mild cases may show milder central nervous system (CNS) signs, such as disorientation or ‘delirium-like’ behavior. Extreme hyperthermia leads to cerebral hypoperfusion due to respiratory alkalosis and shock.72 This metabolic derangement is combined with the direct hyperthermic effects resulting in vascular damage, cerebral edema, hemorrhage and multifocal vascular thrombosis and infraction.72 Brain histopathology in fatal heatstroke cases in dogs has recorded cerebral edema, hemorrhage, hyperemia, and neuronal necrosis.34 The brain damage in heatstroke has been previously investigated in other mammals, including humans, and has been mainly attributed to a direct brain tissue thermal injury.73 Conversely, it has been shown that the canine brain has intrinsic thermal resistance, protecting it from direct thermal injury.72 It is therefore unlikely that direct thermal brain injury per se is the major factor in the pathogenesis of CNS lesions and abnormalities in dogs, although it might play a more minor part in the pathogenesis. The CNS abnormalities in dogs with heatstroke probably occur mostly secondary to shock and multi-organ dysfunction, including metabolic derangement, alkalosis or acidosis, hypoxia, hypoglycemia, bleeding and formation of microthrombi.34,72
Muscle damage and rhabdomyolysis
Rhabdomyolysis is a prominent feature of heatstroke in dogs, occurring during and following the heat insult, and is exacerbated during the first 24 hrs of hospitalization due to skeletal and cardiac muscular hypoperfusion, resulting from hypovolemic, distributive shock and microthromboses, secondary to developing DIC.37,74 Heatstroke in dogs is invariably reflected by increased muscle leakage enzymes activity.75 The severity of this increase reflects the extent of cellular muscular damage and the direct thermal injury to myocardial and skeletal muscle myocytes.74 In humans, rhabdomyolysis is confirmed through measurement of serum and urinary myoglobin concentration, however, in dogs, the human-based myoglobin immunoassays are insensitive, and currently no myoglobin immunoassay has been validated for use in dogs. Nevertheless, since oftentimes creatine kinase (CK) activity is markedly increased (median 17,000 U/L, >5 to 400-fold its upper reference limit) in dogs presented with heatstroke, it is reasonable to assume that rhabdomyolysis does occur in dogs with heatstroke, as described in humans victims of heatstroke.74
Hemostatic derangement
Thermal endothelial cellular injury leads to diffuse vascular damage and activation of the coagulation cascade, resulting in hypercoagulability, formation of multiple microthrombi, and subsequently, to diffuse microvascular thrombosis.76 In addition, multi-organ cellular necrosis further activates coagulation.65 The terminal result is DIC, which might be initially subclinical, but with time, more often progresses to overt DIC, a major factor in the morbidity and mortality of heatstroke patients.37,77 The injured endothelium releases tissue thromboplastin, kinines, kalikrein and activated factor XII, thereby initiating activation of the intrinsic coagulation and the complement cascades, inducing SIRS and widespread, overt DIC.65,78 Hepatic injury and dysfunction due to hypoperfusion, microembolism and direct hyperthermic hepatocellular damage may exacerbate the hemostatic derangment.77 In vitro studies have shown that high body temperatures (>42°C) increase platelet aggregation and activation of the coagulation cascade and enhance fibrinolysis.36,65,70,76,79 Normalization of the body temperature decrease fibrinolysis, but does not decrease the hypercoagulable state, which occurs due to activation of the coagulation cascade or the increased platelet aggregation.79 In a retrospective study of 54 dogs with naturally-occurring heatstroke, 50% were diagnosed with DIC during the disease course.30 In necropsy of fatal cases, microscopic examination invariably showed severe bleeding and widespread microthromboses and characteristic of hemorrhagic diathesis.58 As DIC may become clinically overt only hours to days after the initial thermal insult, dogs with heatstroke should be monitored closely for hemostatic abnormalities and clinical signs of DIC (i.e., petechiae, ecchymoses, melena, hematochezia and hematuria) for at least 24 to 48 hours after the thermal insult had occurred.37 In a recent study of dogs with naturally occurring heatstroke, in which serial monitoring of hemostatic analytes was done over the first 36 hours of hospitalization, the results of hemostatic tests at presentation to the hospital were not associated with death. However, prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT) at 12 to 24 hours post-presentation to the hospital, a lower total protein C activity at 12 hrs post-presentation and hyperfibrinogenemia at 24 hrs post-presentation were significantly associated with death (Fig. 1). These results exemplify the importance of intensive serial monitoring of hemostasis during hospitalization, which allow for timely therapeutic intervention. Increased D-dimer concentration and decreased antithrombin activity were common at all time-points, in dogs with heatstroke, but both these abnormalities were unassociated with death (Fig. 1).37
Figure 1.
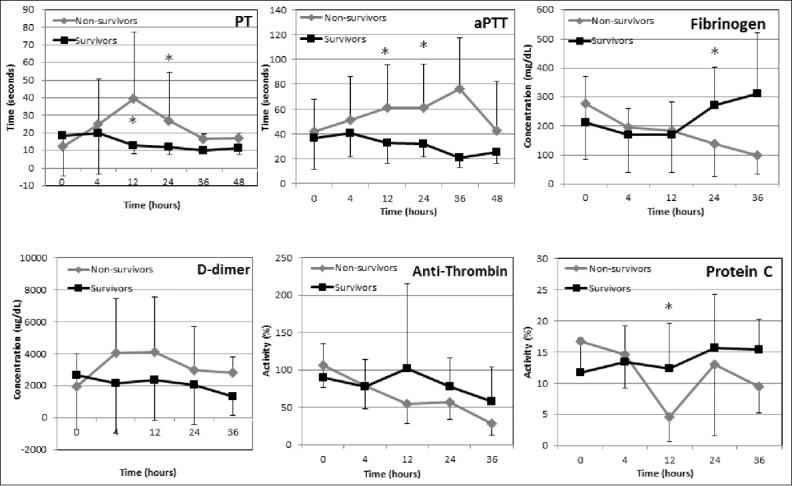
Trends of hemostatic analytes during hospitalization of 30 dogs with naturally-occurring heatstroke. The survivors (n = 18) are depicted by solid black line, while the non-survivors by solid gray lines. *, significant difference between the survivors and the non survivors; PT, prothrombin time; aPTT, activated partial thromboplastin time. Data are presented as mean ±SD. (From Bruchim et al. JVECCS 2016).37 © Wiley-Blackwell. Reproduced by permission of Wiley-Blackwell. Permission to reuse must be obtained from the rightsholder.
Acute kidney injury
AKI in dogs occurs secondary to numerous and variable conditions, but whatever its cause is, it is invariably associated with sudden onset of renal parenchymal injury.80 The pathogenesis of heatstroke-associated AKI is likely multifactorial. Such factors include direct renal thermal injury, decreased renal perfusion due to hypovolemic, distributive shock and dehydration, endotoxemia, myoglobulinemia secondary to rhabdomyolysis, release of cytokines and vasoactive mediators and microthromboses, associated with DIC.36,81,82 Kidney injury may be mild and go unnoticed, especially at presentation for veterinary care, but often, overt failure of the kidneys to meet the excretory, metabolic and endocrine body demands ensues.83 In dogs with naturally-occurring heatstroke, the occurrence of AKI at any time-point during hospitalization, as well as recording of a serum creatinine concentration >1.5 mg/dL (the upper reference limit is 1/3 mg/dL) at 12 and at 24 hours post-presentation, are independent risk factors for death (Table 1).30,75,83 However, early AKI, which is invariably present early in dogs with naturally-occurring heatstroke, is often unapparent when dogs are presented, when using the routine available kidney function tests (i.e., serum creatinine), because such tests, being renal functional ones, lack sensitivity and specificity for diagnosing AKI before dysfunction has already developed.83 However, novel, recently investigated biomarkers of kidney injury have been developed in humans, and are being increasingly investigated and used in dogs.84,85 Such markers, most of which are measured in the urine, detect AKI hours to days before serum creatinine increases above its upper reference limit. In a recent study, we have prospectively assessed kidney injury urinary biomarkers in dogs with naturally occurring heatstroke, including, urinary neutrophil gelatinase-associated lipocalin (NGAL), urinary retinol-binding protein and urinary C-reactive protein (CRP).83 This study has shown that AKI occurs invariably in dogs with heatstroke, and that the urinary concentrations of all these three biomarkers were significantly higher among dogs with heatstroke, compared to healthy controls.83 These novel biomarkers were increased even in dogs in which serum creatinine concentration was within its reference interval. The study also confirmed that both a renal tubular damage and a glomerular damage coexist in dogs with naturally-occurring heatstroke.83
Table 1.
XXXX
Acute respiratory distress syndrome
Thermal and biochemical injury to the pulmonary endothelium may lead to acute non-cardiogenic pulmonary edema, also known as ARDS.58,86 Histopathologic pulmonary lesions in fatal heatstroke cases in dogs include infarcts, marked alveolar hemorrhage or edema.58 Therefore, positive pressure ventilation is indicated, both for cases suffering from hypoventilation (e.g., due to cerebral edema) as well as for those with primary pulmonary failure due to inadequate diffusion, oxygenation and perfusion (e.g., pulmonary thromboembolism).
Myocardial damage and cardiac arrhythmia
Several extra-cardiac mechanisms were proposed as contributing processes to myocardial damage and development of cardiac arrhythmias in heatstroke, in both humans and animals, including myocardial hypoperfusion and injury, lactic acidosis, hypoxemia, electrolyte imbalance and possibly, direct thermal injury.87-89 Serum cardiac troponins I and T concentrations are increased in naturally occurring exertional and environmental heatstroke in both human patients and dogs, as well as in rats induced with heatstroke.63,87,90,91 In the latter, cardiac troponin concentration accurately predicted the severity of the heatstroke.87 Necropsy findings in dogs with fatal naturally occurring heatstroke invariably showed mild to severe subendocardial, myocardial and epicardial hemorrhages and hyperemia,58 suggesting that DIC has a pivotal role in the pathogenesis of heatstroke-associated cardiac arrhythmias. Antiarrhythmic therapy should be considered, depending on the severity of the arrhythmia, especially if the patient has related clinical signs.
Gastrointestinal tract lesions and bacterial translocation
In humans with naturally occurring heatstroke, as well as in animal experimentallyinduced with heatstroke, markedly increased core body temperatures are associated with blood flow redistribution, characterized by cutaneous vasodilatation, with concurrent decreased GIT blood flow.15,62 This splanchnic vasoconstriction may cause intestinal ischemia, and limits the local vascular heat exchange, thereby promoting intestinal tissue hyperthermia and, in-turn, excessive reactive oxygen species production and circulatory intestinal barrier dysfunction.62,92,93 Overproduction of nitric oxide (NO), yielded by NO synthase (NOS), an important factor controlling the required splanchnic blood flow for normal stress tolerance, may contribute to the uncontrolled splanchnic dilatation that precedes the vascular collapse seen in heatstroke patients.62 These structural and functional changes increase intestinal permeability, thereby facilitating intestinal luminal bacterial and endotoxin translocation into the bloodstream, which are normally harmless when contained within the intestinal lumen.62,93 These vicious processes subsequently worsen the SIRS condition in heatstroke, potentially progressing to sepsis, MODS and death.62,93,94 Gastrointestinal bacterial translocation has not been confirmed to date in dogs with naturally-occurring heatstroke; however given the massive hemorrhagic diarrhea and hematemesis that rapidly ensue in dogs with severe heatstroke,58 it is reasonable to assume that it is an important contributing factor to SIRS, sepsis and MODS and death in such cases.
Clinical signs upon presentation
58delirium, stupor, coma and seizures).30 The median systolic and diastolic blood pressures upon presentation to medical care in 30 dogs with naturally occurring heatstroke were mostly unremarkable (>90 mmHg and >60 mmHg, respectively).75 This is in contrast to hypotension, documented in both experimental animal models of heatstroke and in human patients.61,95 The absence of hypotension in dogs with naturally occurring heatstroke is probably because such cases are often presented for care in a state of compensatory shock. The neurohormonal compensatory mechanisms to maintain blood pressure react fast, and therefore hypotension at presentation is uncommon.75
Diagnosis
In contrast to heatstroke in humans, where its diagnosis is more often clear based on the anamnesis, in dogs with naturally-occurring heatstroke, the history may be vague. Dogs owners may be unaware of any particular extraordinary event of a heat insult. In fact, dogs may collapse acutely during playful activities. In dogs kept in open premises, under harsh environmental heat stress, with limited access to water and shade, the owners may discover the animal collapsed, with no knowledge of the heat insult. Therefore, although the diagnosis of heatstroke in dogs should always be based on the history obtained from their owners, heatstroke should never be disregarded or excluded in absence of a clear history of physical exertion or exposure to heat stress.58 he following categories: 1) Evidence of rhabdomyolysis and muscle damage, including increased activity of CK, aspartate transaminase (AST) and alanine transaminase (ALT), and possibly, presence of myoglobinuria; 2) Hematologic abnormalities; metarubricytosis (i.e., increased counts of nucleated red blood cells [nRBCs]) in examination of stained blood smears, in absence of anemia and polychromasia (i.e., pathologic metarubricytosis); increased hematocrit (resulting from hemoconcentration, secondary to splenic contraction, compensatory shock and dehydration; 3) hepatobilliary damage. This is evident by increased activity of serum alkaline phosphatase (ALP), ALT, AST and γ-glutamyl transpeptidase (GGT) activities.
Due to the thermal injury and advanced shock with hypoperfusion and ischemic muscle damage, virtually all dogs with heatstroke, whether exertional or environmental, show increased serum muscle enzymes activity, often much higher than their upper reference limits. In 40 dogs with naturally occurring heatstroke, median CK activity at presentation to the hospital was 17,000 U/L, with a maximal value of 345,000 U/L (The upper reference limit is 400 U/L). Nevertheless, serum CK activity was not found to be significantly higher among more the severely affected animals (i.e., non-survivors) compared to the milder cases (i.e., survivors).75
Marked pathologic metarubricytosis (termed normoblasthemia in human medicine) is a rather specific phenomenon in canine heatstroke, and should point to heatstroke when a history of the heat insult is unclear.34 This pathologic metarubricytosis occurs in absence of anemia or polychromasia, mostly in face of increased hematocrit, red blood cell count and hemoglobin concentration.34 Therefore, presence of nRBCs in the peripheral blood, combined with increased serum muscle enzyme activity (i.e., CK) is an important tool to diagnose heatstroke in cases in which history is vague and when the dogs are normothermic or hypothermic at presentation.34
Prognosis
Several significant risk factors for death were identified in dogs with heatstroke, including obesity, prolonged (>90 min) time-lag from the occurrence of the heat insult to presentation for veterinary care, hypoglycemia (glucose <47 mg/dL) at presentation, azotemia (serum creatinine concentration >1.5 mg/dL) at 24 hours post presentation, and occurrence of DIC, AKI, ventricular arrhythmia, seizures and abnormal mental status (Table 1).13 Pathologic metarubricytosis at presentation to the hospital was significantly more intense (i.e., the nRBC count was higher) in non-surviving dogs compared to the survivors of heatstroke, and was an excellent prognostic marker for prediction of secondary complications as well as survival (Fig. 2).
Figure 2.
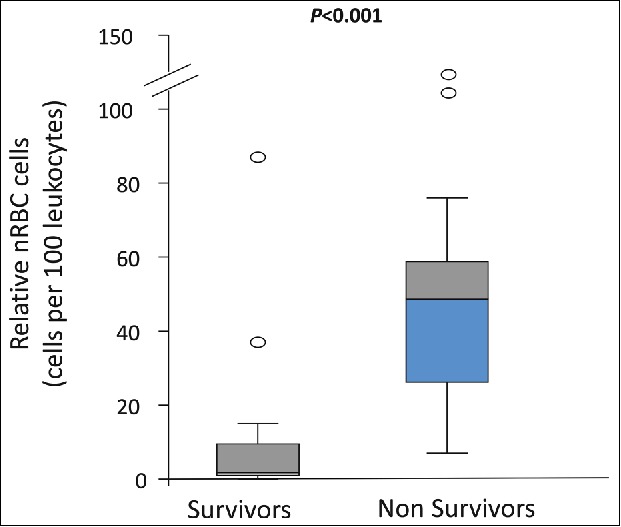
The relative nucleated red blood cell count (nRBC) in 41 dogs with naturally occurring heatstroke upon admission to the hospital. Non-survivors had significantly (P = 0.001) higher nRBCs compared to the survivors (median 55 nRBC/100 leukocytes vs. median 5 nRBC/100 leukocytes, respectively). (From Aroch et al. 2009).34 © Wiley-Blackwell. Reproduced by permission of Wiley-Blackwell. Permission to reuse must be obtained from the rightsholder.
Although several risk factors for death have been identified in dogs with naturally-occurring heatstroke, it is often difficult to accurately use these as single prognostic markers in individual cases.38 We have therefore recently developed a novel severity scoring system, using a stepwise regression model, for dogs with heatstroke, to improve the prediction of complications and the overall outcome (i.e., survival vs. death), when such dogs are presented for veterinary care. Individual parameters that were significantly associated with the outcome were included and used in the final severity score model, including the PT, aPTT and hemoglobin concentration, as well as the occurrence of petechiae (pin-point body surface bleeding), AKI, shock, DIC and seizures.38 The receiver operator (ROC) area under the curve (AUC) of the final predictive score for predicting the final outcome of heatstroke in dogs was 0.92 (95% confidence interval 0.86-0.99), with an optimal cutoff point of 35.0, corresponding to sensitivity and specificity of 93% and 86%, respectively (Fig. 3).38
Figure 3.
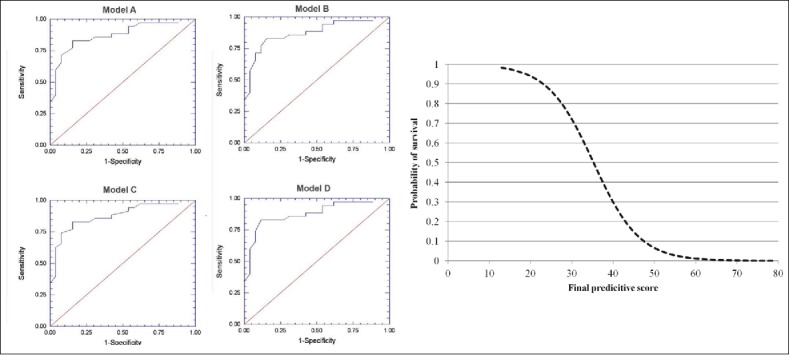
A novel severity scoring system for dogs with heatstroke upon admission to the hospital; In the left side, 4 receiver operator (ROC) analyses curves for 4 models done evaluate the performance of the proposed scoring system in 30 dogs with naturally occurring heatstroke upon admission to the hospital. The area under the ROC curve (AUC) for the final predictive score using model A as a predictor of the outcome (i.e., survival vs. death) was 0.92 (95% confidence interval (0.86-0.99), with an optimal cutoff point of 35.0, corresponding to sensitivity and specificity of 93% and 86%, respectively. In the right side, the graph depicts the probability of survival against the predictive score upon admission to the hospital. The probability of survival is 50% with a predictive score of 35.0. * Receiver Operator Characteristic- implies for the best sensitivity and specificity at various threshold points. (From Segev et al. JVECCS 2016).38 © Wiley-Blackwell. Reproduced by permission of Wiley-Blackwell. Permission to reuse must be obtained from the rightsholder.
Novel molecular and biochemical biomarkers in canine heatstroke
Heat shock protein 72 (HSP72)
The heat shock response, or the stress response, is a highly conserved cellular adaptive, rapid molecular cytoprotective mechanism, protecting cells and tissues from excessive heat and other noxious stimuli, such as hypoxia, inflammation and ischemic reperfusion injuries, and involves heat shock proteins (HSPs) production, of which HSP72 is considered a most sensitive one.3,31,96-98 Serum HSP72 (eHSP72) has recently been evaluated as a potential biomarker of heat tolerance in experimental heatstroke in monkeys and in clinical heatstroke cases in humans.36,67,95,99 In monkeys experimentally induced with heatstroke, eHSP72 concentration positively correlated hepatic, myocardial and skeletal muscle necrosis, and was lower in the survivors than in the non-survivors.95 In human exertional heatstroke patients, immediately after a 14-km running event, occurrence of early heatstroke-associate neurological signs (e.g., confusion) was associated with increased eHSP72 concentration.75,99,100 Recently, we have shown that the dynamics of eHSP72 U-shape profiles during hospitalization are predictive of survival in dogs with naturally-occurring heatstroke, and positively correlated the severity of their hemostatic derangement and lactic acidosis.75 In the survivors group, eHSP72 had recovered 24 hrs post the heatstroke event, while in the non-survivor dogs, no recovery of eHSP72 concentration was noted. The increase of eHSP72 at the 24-hr time point might have been associated with enhanced immunological activity and protection.75 There was a positive correlation between the eHSP72 profile and the occurrence of acidosis.75 eHSP72 concentration is known to increase in human patients with AKI, and its urinary concentration (uHSP72) is a sensitive marker of AKI, and a death-associated complication also in heatstroke.101 Our preliminary results showed that uHSP72 is increased in dogs with AKI as well (Fig. 4), and therefore, might also serve as an early, useful and specific biomarker of AKI of various etiologies, including heatstroke.102
Figure 4.
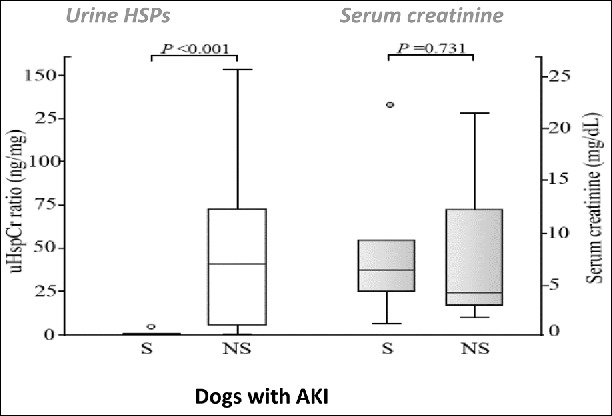
Urine heat shock protein 72 (uHSP72) concentrations in 6 survivors (S) and 6 non-survivors (NS) dogs sustaining acute kidney injury (AKI). The uHSP72/urine creatinine ratio was significantly (P > 0.001) higher among the non-survivors compared to the survivors; serum creatinine did not differ among the groups. (From Bruchim et al. Vet J 2017).102 © Elsevier. Reproduced by permission of Elsevier. Permission to reuse must be obtained from the rightsholder.
Serum histones
Histones are highly conserved alkaline, positively charged proteins, serving as the basic unit structure block of chromatin, namely the nucleosome. Histone deficiency leads to genomic DNA disorganization and ineffective structure.103,104 There are five different histones, divided into core (H2A, H2B, H3, and H4) and linker (H1 and H5) histones.105 Emerging studies indicate that histones leak from damaged and activated cells into the extracellular space, exhibiting toxic, proinflammatory and procoagulant properties.103 Studies in animals and humans suggest that the dynamics of serum histones concentration over time during the disease course serve as a potential biomarker of disease severity, stress and the overall outcome, and as a potential novel therapeutic target in several inflammatory diseases.103,106,107 We have recently, evaluated serum histone concentration in a preliminary study in 16 dogs with naturally occurring heatstroke and in 7 healthy control dogs.106 Total serum histone concentration was markedly increased in the dogs with heatstroke compared to the healthy controls, and among the dogs with heatstroke, it was significantly higher in the non-survivors compared to survivors (Fig. 5).108 The dogs with heatstroke, which showed markedly increased total serum histone concentration also showed concurrent severe hemostatic derangement, suggesting that serum histones play a role in the inflammatory and hemostatic derangements. Nevertheless, this suggestion should be accepted cautiously, since the cohort size was limited, and no direct cause and effect was proven between serum histone concentration and inflammation or hemostatic derangement. Therefore, larger scale studies are warranted, with investigation of other concurrent markers of inflammation. Based on this recent preliminary study, total serum histone concentration has diagnostic, prognostic and therapeutic potentials in canine heatstroke. It is noteworthy that histones may become an attractive therapeutic target, as they can be pharmacologically targeted and manipulated by specific antibodies, activated protein C, albumin or heparin.103
Figure 5.
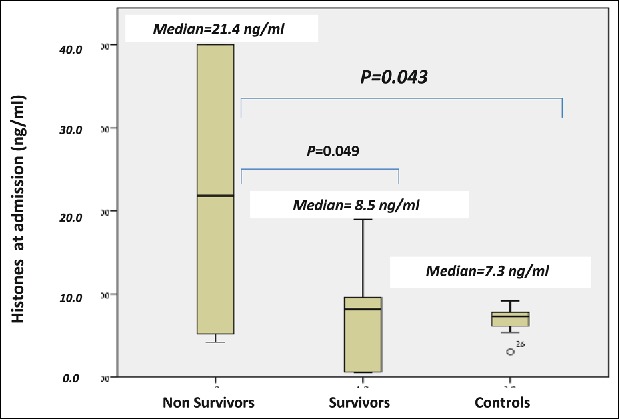
Median total serum histones concentration at presentation to the hospital in 10 non- survivors and 6 survivor dogs of naturally-occurring heatstroke and 7 healthy control dogs. Median total serum histones are significantly (P = 0.043) increased in all dogs with naturally occurring heatstroke compared the 7 healthy controls. Median total serum histones in the non-survivors was significantly (P = 0.049) higher compared to survivors of heatstroke (21.3 ng/ml vs. 8.5 ng/ml, respectively). (From Bruchim et al. Cell Stress & Chaperon 2017).108 © Springer. Reproduced by permission of Springer. Permission to reuse must be obtained from the rightsholder.
Preconditioning against heatstroke
Two endogenous adaptive mechanisms are directly invoked to combat heat stress: physical fitness, heat acclimation and the rapid heat shock response.31,96 Heat acclimation induces adaptive physiological and behavioral changes, improving the individual's ability to cope with extreme environmental heat.31,32 Acclimation is a time-dependent process, leading to a dynamic expansion of the body temperature regulatory range due to left and right shifts in the temperature threshold for heat dissipation and thermal injury, respectively.32 The heat shock response is a rapid molecular cytoprotective mechanism, involving heat shock protein (HSPs) production.106 When subjected to sub-lethal heat stress, the body enhances HSPs transcription and synthesis, to increase HSP72 cellular reserves, thereby providing cytoprotection.96 The correlation between eHSP72, heat acclimation and physical performance in military working dogs has been recently prospectively evaluated.36 During the study, 3 consecutive physical performance tests (PPTs) were performed, over a 2-year training period. eHSP72 concentration and lymphocyte HSP72, and its corresponding mRNA level, were measured before and after each PPT. The results showed that together with the profound enhancement of aerobic power and physical performance, HSP72 mRNA was induced, and progressively increased, and there was also a significant rise in basal and peak eHSP72 post-PPT concentrations (Fig. 6).36 As we learn from studies on military working dogs, the majority of heatstroke events among such dogs have occurred during their first year of training (immediately after importation from Northern Europe, which characterized by cool temperature, to Israel, which is characterized by intense heat stress during summer), indicating a role of acclimation and adequate training in their adaptation to heat stress.30,36
Figure 6.
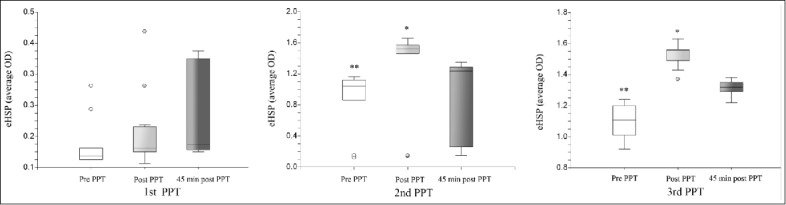
1, Physical Performance Test; – pre-PPT; – immediately post PPT; – 45 min post PPT. eHSP72 in serum of the studied dogs under basal conditions, at the end of the PPT and 45 min post-PPT; Figure 6a- the first year of the study. Figure 6b- the second year of the study; Figure 6c- the third year of the study. Each box represents Median (range); *, significant increase in eHSP72 immediately post-PPT at the second and third PPTs; **- significant increase in the basal eHSP72 at the second and third PPTs. (From Bruchim et al. JAP 2014).36 © American Physiological Society. Reproduced by permission of American Physiological Society. Permission to reuse must be obtained from the rightsholder.
Conclusions
Heatstroke in dogs is a life-threatening condition, resulting in serious secondary complications such as rhabdomyolysis, cardiac arrhythmia, DIC, AKI and ARDS, and has a high mortality rate, despite appropriate intensive, supportive and symptomatic treatment. Despite differences in the species-specific evaporative cooling effector and splenic function in dogs, similarity exists in heatstroke-induced derangements between dogs and other mammals. Hence, the knowledge gained in dogs with heatstroke, both in experimental models and in naturally occurring cases, may serve as an additional guide of the pathogenesis, diagnosis, prognostication and therapy of heatstroke in general.
Early admission, diagnosis and intensive treatment, along with prompt whole body cooling by dog owners and caregivers are important for survival. The diagnosis of canine heatstroke should not rely exclusively on presence of hyperthermia or neurological abnormalities upon admission, but should rather be based on the combination of the history, clinical signs and laboratory results. Treatment and monitoring of heatstroke should be intensive and prolonged, since most of its complications often have delayed onsets, and most are serious risk factors for death.
Biographies
About the authors
Dr. Yaron Bruchim: Yaron Bruchim DVM, IVMS, Dip-ACVECC, Dip-ECVECC is a Senior Lecturer of Veterinary Medicine, Small Animal Emergency and Critical Care Unit, Koret School of Veterinary Medicine (KSVM), The Hebrew University of Jerusalem. Dr. Bruchim has gained his diploma in veterinary medicine in 1999, from the KSVM. He is a board certified specialist in veterinary emergency and critical care, of both the American and European Colleges of Veterinary Emergency and Critical Care (2012 and 2014, respectively). Dr. Bruchim has a strong interest in heatstroke and heat related illness, and is currently investigating the pathophysiology of heatstroke and acclimation in dogs. Our studies focus on heat shock proteins (HSP72) as biomarkers of acclimation and physical performance and on total and H3 serum histones as diagnostic and prognostic biomarkers in several inflammatory and immune-mediated diseases, including heatstroke.

Professor Michal Horowitz Michal Horowitz is Professor Emeritus in Physiology at the Hebrew University of Jerusalem, Israel. Her research focusses on problems associated with heat stress and heat acclimation dynamics, with emphasis on molecular-physiological linkage yielding Heat Acclimation mediated Cross-Tolerance or its Interference, and Heat Acclimation memory as an epigenetic phenomenon. Her International academic activities included member/co-chair of IUPS Commissions in Environmental Physiology and Thermal Physiology. She is EIC of JBCPP and in the Editorial Board of Temperature.

Professor Itamar Aroch Itamar Aroch, DVM, Dip ECVIM-CA (Internal Medicine is an Associate Professor at the KSVM, The Hebrew University of Jerusalem. He has gained his diploma in veterinary medicine in 1990, from the KSVM. He is a board-certified specialist in companion animal internal medicine of the European College of Veterinary Internal Medicine – Companion Animals (2001). He is interested in diagnostic and prognostic laboratory markers of disease in dogs and cats, with special interest, focusing on inflammatory, immune-mediated and infectious diseases, including heatstroke, pancreatitis, spirocercosis, immune-mediated hemolytic anemia and acute kidney injury.

Abbreviations
- AKI
Acute kidney injury
- ALP
alkaline phosphatase
- ALT
alanine transaminase
- aPTT
Activated partial thromboplastin time
- ARDS
Acute respiratory distress syndrome
- AST
aspartate transaminase
- CK
creatine kinase
- CNS
Central nervous system
- DIC
Disseminated intravascular coagulation
- eHSP72
Serum heat shock protein 72
- EIC
Hereditary exercise-induced collapse
- GIT
Gastrointestinal tract
- GGT
γ-glutamyl transpeptidase
- HSP72
Heat shock protein 72
- MODS
Multiple organ dysfunction syndrome
- nRBCs
Nucleated red blood cells
- PT
Prothrombin time
- SIRS
Systemic inflammatory response syndrome
- uHSP72
Urinary heat shock protein 72
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Hemmelgarn C, Gannon K. Heatstroke: thermoregulation, pathophysiology, and predisposing factors. Compendium. 2013;35:E4. PMID:23677841 [PubMed] [Google Scholar]
- [2].Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015;10:93. doi: 10.1186/s13023-015-0310-1. PMID:26238698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Misset B, De Jonghe B, Bastuji-Garin S, Gattolliat O, Boughrara E, Annane D, Hausfater P, Garrouste-Orgeas M, Carlet J. Mortality of patients with heatstroke admitted to intensive care units during the 2003 heat wave in France: a national multiple-center risk-factor study. Critical Care Med. 2006;34:1087-1092. doi: 10.1097/01.CCM.0000206469.33615.02 [DOI] [PubMed] [Google Scholar]
- [4].Shibolet S, Lancaster MC, Danon Y. Heat stroke: a review. Aviation Space Environ Med. 1976;47:280-301 [PubMed] [Google Scholar]
- [5].Hsu SF, Chao CM, Chang CP, Lin MT, Cheng BC. Heat shock protein 72 may improve hypotension by increasing cardiac mechanical efficiency and arterial elastance in heatstroke rats. Int J Cardiol. 2016;219:63-69. doi: 10.1016/j.ijcard.2016.05.004. PMID:27288968 [DOI] [PubMed] [Google Scholar]
- [6].Dehbi M, Uzzaman T, Baturcam E, Eldali A, Ventura W, Bouchama A. Toll-like receptor 4 and high-mobility group box 1 are critical mediators of tissue injury and survival in a mouse model for heatstroke. PloS One. 2012;7:e44100. doi: 10.1371/journal.pone.0044100. PMID:22962600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Roberts GT, Ghebeh H, Chishti MA, Al-Mohanna F, El-Sayed R, Al-Mohanna F, Bouchama A. Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arteriosclerosis Thrombosis Vascular Biol. 2008;28:1130-1136. doi: 10.1161/ATVBAHA.107.158709 [DOI] [PubMed] [Google Scholar]
- [8].Adato B, Dubnov-Raz G, Gips H, Heled Y, Epstein Y. Fatal heat stroke in children found in parked cars: autopsy findings. European J Pediatr. 2016;175:1249-1252. doi: 10.1007/s00431-016-2751-5 [DOI] [PubMed] [Google Scholar]
- [9].Romanovsky AA, Blatteis CM. Naltrexone modifies thermoregulatory symptoms and lessens the severity of heat stroke in guinea pigs. Annals N York Acad Sci. 1997;813:548-552. doi: 10.1111/j.1749-6632.1997.tb51745.x [DOI] [PubMed] [Google Scholar]
- [10].King MA, Leon LR, Mustico DL, Haines JM, Clanton TL. Biomarkers of multiorgan injury in a preclinical model of exertional heat stroke. J Applied Physiol. 2015;118:1207-1220. doi: 10.1152/japplphysiol.01051.2014 [DOI] [PubMed] [Google Scholar]
- [11].King YT, Lin CS, Lin JH, Lee WC. Whole-body hyperthermia-induced thermotolerance is associated with the induction of heat shock protein 70 in mice. J Exp Biol. 2002;205:273-278. PMID:11821493 [DOI] [PubMed] [Google Scholar]
- [12].Chen ZC, Wu WS, Lin MT, Hsu CC. Protective effect of transgenic expression of porcine heat shock protein 70 on hypothalamic ischemic and oxidative damage in a mouse model of heatstroke. BMC Neurosci. 2009;10:111. doi: 10.1186/1471-2202-10-111. PMID:19725984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Drobatz KJ, Macintire DK. Heat-induced illness in dogs: 42 cases (1976-1993). J Am Veterinary Medical Association. 1996;209:1894-1899 [PubMed] [Google Scholar]
- [14].Leon LR, Bouchama A. Heat stroke. Comprehensive Physiol. 2015;5:611-647. doi: 10.1002/cphy.c140017 [DOI] [PubMed] [Google Scholar]
- [15].Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978-1988. doi: 10.1056/NEJMra011089. PMID:12075060 [DOI] [PubMed] [Google Scholar]
- [16].Veltmeijer MT, Eijsvogels TM, Barteling W, Verbeek-Knobbe K, van Heerde WL, Hopman MT. The impact of exercise-induced core body temperature elevations on coagulation responses. J Sci Med Sport. 2017;20:202-207. doi: 10.1016/j.jsams.2016.01.007. PMID:27036711 [DOI] [PubMed] [Google Scholar]
- [17].de' Donato FK, Leone M, Scortichini M, De Sario M, Katsouyanni K, Lanki T, Basagaña X, Ballester F, Åström C, Paldy A, et al.. Changes in the effect of heat on mortality in the Last 20 years in nine European cities. Results from the PHASE Project. Int J Environmental Res Public Health. 2015;12:15567-15583. doi: 10.3390/ijerph121215006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Morignat E, Perrin JB, Gay E, , Vinard JL, Calavas D, Hénaux V. Assessment of the impact of the 2003 and 2006 heat waves on cattle mortality in France. PloS One. 2014;9:e93176. doi: 10.1371/journal.pone.0093176. PMID:24667835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Horowitz M. Lessons from gold mines. Temperature. 2017;4:107-108. doi: 10.1080/23328940.2017.1290571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Roberts WO. Exertional heat stroke and the evolution of field care: A physician's perspective. Temperature. 2017;4:101-103. doi: 10.1080/23328940.2017.1316352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Armed Forces Health Surveillance B Update: Heat illness, active component, U.S. Armed Forces, 2016. Msmr. 2017;24:9-13 [Google Scholar]
- [22].Poumadere M, Mays C, Le Mer S, Blong R. The 2003 heat wave in France: dangerous climate change here and now. Risk Anal. 2005;25:1483-1494. doi: 10.1111/j.1539-6924.2005.00694.x. PMID:16506977 [DOI] [PubMed] [Google Scholar]
- [23].Martiello MA, Baldasseroni A, Buiatti E, Giacchi MV. Health effects of heat waves. Igiene E Sanita Pubblica. 2008;64:735-772. PMID:19219085 [PubMed] [Google Scholar]
- [24].Grundstein AJ, Duzinski SV, Dolinak D, Null J, Iyer SS. Evaluating infant core temperature response in a hot car using a heat balance model. Forensic Sci Med Pathol. 2015;11:13-19. doi: 10.1007/s12024-014-9619-7. PMID:25332172 [DOI] [PubMed] [Google Scholar]
- [25].Saleem SG, Ansari T, Ali AS, Fatima S, Rizvi MH, Samad MA. Risk factors for heat related deaths during the June 2015 heat wave in Karachi, Pakistan. J Ayub Med Coll Abbottabad. 2017;29:320-324. PMID:28718257 [PubMed] [Google Scholar]
- [26].Vandentorren S, Bretin P, Zeghnoun A, Mandereau-Bruno L, Croisier A, Cochet C, Ribéron J, Siberan I, Declercq B, Ledrans M. August 2003 heat wave in France: risk factors for death of elderly people living at home. Eur J Public Health. 2006;16:583-591. doi: 10.1093/eurpub/ckl063. PMID:17028103 [DOI] [PubMed] [Google Scholar]
- [27].Ledrans M, Pirard P, Tillaut H, Pascal M, Vandentorren S, Suzan F, Salines G, Le Tertre A, Medina S, Maulpoix A, et al.. The heat wave of August 2003: what happened?. La Revue Du Praticien. 2004;54:1289-1297. PMID:15461047 [PubMed] [Google Scholar]
- [28].Centers for Disease C , Prevention. Impact of heat waves on mortality–Rome, Italy, June-August 2003. MMWR Morbidity and Mortality Weekly Report. 2004;53:369-371. PMID:15129195 [PubMed] [Google Scholar]
- [29].Stott PA, Stone DA, Allen MR. Human contribution to the European heatwave of 2003. Nature. 2004;432:610-614. doi: 10.1038/nature03089. PMID:15577907 [DOI] [PubMed] [Google Scholar]
- [30].Bruchim Y, Klement E, Saragusty J, Finkeilstein E, Kass P, Aroch I. Heat stroke in dogs: A retrospective study of 54 cases (1999-2004) and analysis of risk factors for death. J Vet Intern Med. 2006;20:38-46. PMID:16496921 [DOI] [PubMed] [Google Scholar]
- [31].Horowitz M. Heat acclimation: Heat acclimation: phenotypic plasticity and cues to the underlaying molecular mechanism. J Therm Biol. 2001;26:357-363. doi: 10.1016/S0306-4565(01)00044-4 [DOI] [Google Scholar]
- [32].Horowitz M. Heat acclimation, epigenetics, and cytoprotection memory. Comprehensive Physiol. 2014;4:199-230. doi: 10.1002/cphy.c130025 [DOI] [PubMed] [Google Scholar]
- [33].Andress M, Goodnight ME. Heatstroke in a military working dog. US Army Med Dep J. 2013:34-37. PMID:23277443 [PubMed] [Google Scholar]
- [34].Aroch I, Segev G, Loeb E, Bruchim Y. Peripheral nucleated red blood cells as a prognostic indicator in heatstroke in dogs. J Vet Intern Med. 2009;23:544-551. doi: 10.1111/j.1939-1676.2009.0305.x. PMID:19422468 [DOI] [PubMed] [Google Scholar]
- [35].Trappler M, Moore K. Canine brachycephalic airway syndrome: pathophysiology, diagnosis, and nonsurgical management. Compendium. 2011;33:E1-4; quiz E5 [PubMed] [Google Scholar]
- [36].Bruchim Y, Aroch I, Eliav A, Abbas A, Frank I, Kelmer E, Codner C, Segev G, Epstein Y, Horowitz M. Two years of combined high-intensity physical training and heat acclimatization affect lymphocyte and serum HSP70 in purebred military working dogs. J Applied Physiol. 2014;117:112-118. doi: 10.1152/japplphysiol.00090.2014 [DOI] [PubMed] [Google Scholar]
- [37].Bruchim Y, Kelmer E, Cohen A, Codner C, Segev G, Aroch I. Hemostatic abnormalities in dogs with naturally occurring heatstroke. J Veterinary Emerg Critical Care. 2017;27(3):315-324. doi: 10.1111/vec.12590 [DOI] [PubMed] [Google Scholar]
- [38].Segev G, Aroch I, Savoray M, Kass PH, Bruchim Y. A novel severity scoring system for dogs with heatstroke. J Vet Emerg Critical Care. 2015;25:240-247. doi: 10.1111/vec.12284 [DOI] [PubMed] [Google Scholar]
- [39].Ferguson SM, Brasnjo G, Hayashi M, Wölfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, et al.. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570-574. doi: 10.1126/science.1140621. PMID:17463283 [DOI] [PubMed] [Google Scholar]
- [40].Pedersen N, Liu H, Theilen G, Sacks B. The effects of dog breed development on genetic diversity and the relative influences of performance and conformation breeding. J Anim Breed Genet. 2013;130:236-248. doi: 10.1111/jbg.12017. PMID:23679949 [DOI] [PubMed] [Google Scholar]
- [41].Taylor SM, Shmon CL, Adams VJ, Mickelson JR, Patterson EN, Shelton GD. Evaluations of labrador retrievers with exercise-induced collapse, including response to a standardized strenuous exercise protocol. J Am Animal Hospital Association. 2009;45:3-13. doi: 10.5326/0450003 [DOI] [PubMed] [Google Scholar]
- [42].Patterson EE, Minor KM, Tchernatynskaia AV, Taylor SM, Shelton GD, Ekenstedt KJ, Mickelson JR. A canine DNM1 mutation is highly associated with the syndrome of exercise-induced collapse. Nat Genetics. 2008;40:1235-1239. doi: 10.1038/ng.224. PMID:18806795 [DOI] [PubMed] [Google Scholar]
- [43].Dickinson PJ, Sullivan M. Exercise induced hyperthermia in a racing greyhound. Veterinary Record. 1994;135:508. doi: 10.1136/vr.135.21.508. PMID:7871692 [DOI] [PubMed] [Google Scholar]
- [44].Taylor S, Shmon C, Su L, Epp T, Minor K, Mickelson J, Patterson E, Shelton GD. Evaluation of dogs with border collie collapse, including response to two standardized strenuous exercise protocols. J Am Animal Hospital Association. 2016;52:281-290. doi: 10.5326/JAAHA-MS-6361 [DOI] [PubMed] [Google Scholar]
- [45].Rand JS, O'Brien PJ. Exercise-induced malignant hyperthermia in an English springer spaniel. J Am Veterinary Medical Association. 1987;190:1013-1014 [PubMed] [Google Scholar]
- [46].O'Brien PJ, Pook HA, Klip A, Britt BA, Kalow BI, McLaughlin RN, Scott E, Elliott ME. Canine stress syndrome/malignant hyperthermia susceptibility: calcium-homeostasis defect in muscle and lymphocytes. Res Veterinary Sci. 1990;48:124-128 [PubMed] [Google Scholar]
- [47].Voermans NC, Snoeck M, Jungbluth H. RYR1-related rhabdomyolysis: A common but probably underdiagnosed manifestation of skeletal muscle ryanodine receptor dysfunction. Revue Neurologique. 2016;172:546-558. doi: 10.1016/j.neurol.2016.07.018. PMID:27663056 [DOI] [PubMed] [Google Scholar]
- [48].Zhao X, Song Q, Gao Y. Hypothesis: exertional heat stroke-induced myopathy and genetically inherited malignant hyperthermia represent the same disorder, the human stress syndrome. Cell Biochem Biophysics. 2014;70:1325-1329. doi: 10.1007/s12013-014-0059-5 [DOI] [PubMed] [Google Scholar]
- [49].Carsana A. Exercise-induced rhabdomyolysis and stress-induced malignant hyperthermia events, association with malignant hyperthermia susceptibility, and RYR1 gene sequence variations. Scientific World J. 2013;2013:531465. doi: 10.1155/2013/531465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kalaiselvan MS, Renuka MK, Arunkumar AS. A retrospective study of clinical profile and outcomes of critically ill patients with heat-related illness. Indian J Anaesthesia. 2015;59:715-720. doi: 10.4103/0019-5049.170030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Epstein Y, Roberts WO. The pathopysiology of heat stroke: an integrative view of the final common pathway. Scand J Med Sci Sports. 2011;21:742-748. doi: 10.1111/j.1600-0838.2011.01333.x. PMID:21635561 [DOI] [PubMed] [Google Scholar]
- [52].Miki K, Morimoto T, Nose H, Itoh T, Yamada S. Circulatory failure during severe hyperthermia in dog. Japanese J Physiol. 1983;33:269-278. doi: 10.2170/jjphysiol.33.269 [DOI] [PubMed] [Google Scholar]
- [53].Horowitz M, Samueloff S. Cardiac output distribution in thermally dehydrated rodents. Am J Physiol. 1988;254:R109-116. PMID:3276223 [DOI] [PubMed] [Google Scholar]
- [54].Wong BJ, Hollowed CG. Current concepts of active vasodilation in human skin. Temperature. 2017;4:41-59. doi: 10.1080/23328940.2016.1200203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hemmelgarn C, Gannon K. Heatstroke: clinical signs, diagnosis, treatment, and prognosis. Compendium. 2013;35:E3. PMID:23894763 [PubMed] [Google Scholar]
- [56].Zurovski Y, Eckstein L, Horowitz M. Heat stress and thermal dehydration: lactacidemia and plasma volume regulation. J Applied Physiol. 1991;71:2434-2439 [DOI] [PubMed] [Google Scholar]
- [57].Lin MT, Liu HH, Yang YL. Involvement of interleukin-1 receptor mechanisms in development of arterial hypotension in rat heatstroke. Am J Physiol. 1997;273:H2072-2077. PMID:9362278 [DOI] [PubMed] [Google Scholar]
- [58].Bruchim Y, Loeb E, Saragusty J, Aroch I. Pathological findings in dogs with fatal heatstroke. J Comp Pathol. 2009;140:97-104. doi: 10.1016/j.jcpa.2008.07.011. PMID:19111315 [DOI] [PubMed] [Google Scholar]
- [59].Sils II, Szlyk-Modrow PC, Tartarini KA, Matthew CB, Francesconi RP. Effect of nitric oxide synthase inhibition on regional blood flow during hyperthermia. J Therm Biol. 2001;26:1-7. doi: 10.1016/S0306-4565(00)00017-6. PMID:11070338 [DOI] [PubMed] [Google Scholar]
- [60].Horowitz M, Sugimoto E, Okuno T, Morimoto T. Changes in blood volume and vascular compliance during body heating in rats. Pflugers Archiv Eur J Physiol. 1988;412:354-358. doi: 10.1007/BF01907551 [DOI] [PubMed] [Google Scholar]
- [61].Quinn CM, Audet GN, Charkoudian N, Leon LR. Cardiovascular and thermoregulatory dysregulation over 24 h following acute heat stress in rats. Am J Physiol Heart Circulatory Physiol. 2015;309:H557-564. doi: 10.1152/ajpheart.00918.2014 [DOI] [PubMed] [Google Scholar]
- [62].Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circulatory Physiol. 2001;280:H509-521 [DOI] [PubMed] [Google Scholar]
- [63].Quinn CM, Duran RM, Audet GN, Charkoudian N, Leon LR. Cardiovascular and thermoregulatory biomarkers of heat stroke severity in a conscious rat model. J Applied Physiol. 2014;117:971-978. doi: 10.1152/japplphysiol.00365.2014 [DOI] [PubMed] [Google Scholar]
- [64].Shapiro Y, Rosenthal T, Sohar E. Experimental heatstroke. A model in dogs. Arch Internal Med. 1973;131:688-692. doi: 10.1001/archinte.1973.00320110072010 [DOI] [PubMed] [Google Scholar]
- [65].Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. J Applied Physiol. 2010;109:1980-1988. doi: 10.1152/japplphysiol.00301.2010 [DOI] [PubMed] [Google Scholar]
- [66].Leon LR, Helwig BG. Role of endotoxin and cytokines in the systemic inflammatory response to heat injury. Frontiers Biosci. 2010;2:916-938. doi: 10.2741/s111 [DOI] [PubMed] [Google Scholar]
- [67].Lee BJ, Clarke ND, Hankey J, Thake CD. Whole body precooling attenuates the extracellular HSP72, IL-6 and IL-10 responses after an acute bout of running in the heat. J Sports Sci. 2017;5:1-8 [DOI] [PubMed] [Google Scholar]
- [68].King MA, Leon LR, Morse DA, Clanton TL. Unique cytokine and chemokine responses to exertional heat stroke in mice. J Applied Physiol. 2017;122:296-306. doi: 10.1152/japplphysiol.00667.2016 [DOI] [PubMed] [Google Scholar]
- [69].Gerner C, Vejda S, Gelbmann D, Bayer E, Gotzmann J, Schulte-Hermann R, Mikulits W. Concomitant determination of absolute values of cellular protein amounts, synthesis rates, and turnover rates by quantitative proteome profiling. Mol Cell Proteomics. 2002;1:528-537. doi: 10.1074/mcp.M200026-MCP200. PMID:12239281 [DOI] [PubMed] [Google Scholar]
- [70].Bouchama A, Roberts G, Al Mohanna F, El-Sayed R, Lach B, Chollet-Martin S, Ollivier V, Al Baradei R, Loualich A, Nakeeb S. Inflammatory, hemostatic, and clinical changes in a baboon experimental model for heatstroke. J Applied Physiol. 2005;98:697-705. doi: 10.1152/japplphysiol.00461.2004 [DOI] [PubMed] [Google Scholar]
- [71].Ramanathan M, Pedersen M, Ramsey R, Seetharam A. Diagnostic value of coagulation factor and intracranial pressure monitoring in acute liver failure from heat stroke: case report and review of the literature. Transplantation Proc. 2015;47:817-819. doi: 10.1016/j.transproceed.2015.02.007 [DOI] [PubMed] [Google Scholar]
- [72].Oglesbee MJ, Alldinger S, Vasconcelos D, Diehl KA, Shinko PD, Baumgärtner W, Tallman R, Podell M. Intrinsic thermal resistance of the canine brain. Neuroscience. 2002;113:55-64. doi: 10.1016/S0306-4522(02)00159-8. PMID:12123684 [DOI] [PubMed] [Google Scholar]
- [73].Sminia P, van der Zee J, Wondergem J, Haveman J. Effect of hyperthermia on the central nervous system: a review. Int J Hyperthermia. 1994;10:1-30. doi: 10.3109/02656739409009328. PMID:8144981 [DOI] [PubMed] [Google Scholar]
- [74].Alzeer AH, el-Hazmi MA, Warsy AS, Ansari ZA, Yrkendi MS. Serum enzymes in heat stroke: prognostic implication. Clin Chem. 1997;43:1182-1187. PMID:9216454 [PubMed] [Google Scholar]
- [75].Bruchim Y, Segev G, Kelmer E, Codner C, Marisat A, Horowitz M. Hospitalized dogs recovery from naturally occurring heatstroke; does serum heat shock protein 72 can provide prognostic biomarker? Cell Stress Chaperones. 2016;21:123-130. doi: 10.1007/s12192-015-0645-5. PMID:26441274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Diehl KA, Crawford E, Shinko PD, Tallman RD Jr, Oglesbee MJ. Alterations in hemostasis associated with hyperthermia in a canine model. Am J Hematol. 2000;64:262-270. doi:. PMID:10911378 [DOI] [PubMed] [Google Scholar]
- [77].Krau SD. Heat-related illness: a hot topic in critical care. Critical Care Nursing Clin N Am. 2013;25:251-262. doi: 10.1016/j.ccell.2013.02.012 [DOI] [PubMed] [Google Scholar]
- [78].Jilma B, Derhaschnig U. Disseminated intravascular coagulation in heat stroke: a hot topic. Critical Care Med. 2012;40:1370-1372. doi: 10.1097/CCM.0b013e31823d785d [DOI] [PubMed] [Google Scholar]
- [79].Bouchama A, Bridey F, Hammami MM, Lacombe C, al-Shail E, al-Ohali Y, Combe F, al-Sedairy S, de Prost D. Activation of coagulation and fibrinolysis in heatstroke. Thrombosis Haemostasis 1996;76:909-915. PMID:8972010 [PubMed] [Google Scholar]
- [80].Langston CE. Acute uremia. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. Philadelphia: Saunders WB; 2010:1955-2115 [Google Scholar]
- [81].Lin YF, Wang JY, Chou TC, Lin SH. Vasoactive mediators and renal haemodynamics in exertional heat stroke complicated by acute renal failure. QJM. 2003;96:193-201. doi: 10.1093/qjmed/hcg029. PMID:12615983 [DOI] [PubMed] [Google Scholar]
- [82].Heled Y, Fleischmann C, Epstein Y. Cytokines and their role in hyperthermia and heat stroke. J Basic Clin Physiol Pharmacol. 2013;24:85-96. PMID:23509213 [DOI] [PubMed] [Google Scholar]
- [83].Segev G, Daminet S, Meyer E, De Loor J, Cohen A, Aroch I, Bruchim Y. Characterization of kidney damage using several renal biomarkers in dogs with naturally occurring heatstroke. Veterinary J. 2015;206:231-235. doi: 10.1016/j.tvjl.2015.07.004 [DOI] [PubMed] [Google Scholar]
- [84].Madden LA, Sandstrom ME, Lovell RJ, McNaughton L. Inducible heat shock protein 70 and its role in preconditioning and exercise. Amino Acids. 2008;34:511-516. doi: 10.1007/s00726-007-0004-7. PMID:18046502 [DOI] [PubMed] [Google Scholar]
- [85].Di Grande A, Giuffrida C, Carpinteri G, Narbone G, Pirrone G, Di Mauro A, Calandra S, Noto P, Le Moli C, Alongi B, et al.. Neutrophil gelatinase-associated lipocalin: a novel biomarker for the early diagnosis of acute kidney injury in the emergency department. Eur Rev Med Pharmacol Sci. 2009;13:197-200. PMID:19673171 [PubMed] [Google Scholar]
- [86].el-Kassimi FA, Al-Mashhadani S, Abdullah AK, Akhtar J. Adult respiratory distress syndrome and disseminated intravascular coagulation complicating heat stroke. Chest. 1986;90:571-574. doi: 10.1378/chest.90.4.571. PMID:3757568 [DOI] [PubMed] [Google Scholar]
- [87].Audet GN, Quinn CM, Leon LR. Point-of-care cardiac troponin test accurately predicts heat stroke severity in rats. Am J Physiol Regulatory Integrative Comparative Physiol. 2015;309:R1264-1272 [DOI] [PubMed] [Google Scholar]
- [88].Muniz AE. Ischemic electrocardiographic changes and elevated troponin from severe heatstroke in an adolescent. Pediatric Emergency Care. 2012;28:64-67. doi: 10.1097/PEC.0b013e31823f2557. PMID:22217892 [DOI] [PubMed] [Google Scholar]
- [89].Whiticar R, Laba D, Smith S. Exertional heat stroke in a young man with a documented rise in troponin I. Emergency Med J. 2008;25:283-284. doi: 10.1136/emj.2007.052472 [DOI] [PubMed] [Google Scholar]
- [90].Chen JH, Inamori-Kawamoto O, Michiue T, Ikeda S, Ishikawa T, Maeda H. Cardiac biomarkers in blood, and pericardial and cerebrospinal fluids of forensic autopsy cases: a reassessment with special regard to postmortem interval. Legal Med. 2015;17:343-350. doi: 10.1016/j.legalmed.2015.03.007. PMID:26052007 [DOI] [PubMed] [Google Scholar]
- [91].Hausfater P, Doumenc B, Chopin S, Le Manach Y, Santin A, Dautheville S, Patzak A, Hericord P, Mégarbane B, Andronikof M, et al.. Elevation of cardiac troponin I during non-exertional heat-related illnesses in the context of a heatwave. Critical Care. 2010;14:R99. doi: 10.1186/cc9034. PMID:20507603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Shapiro Y, Alkan M, Epstein Y, Newman F, Magazanik A. Increase in rat intestinal permeability to endotoxin during hyperthermia. Eur J Applied Physiol Occupational Physiol. 1986;55:410-412. doi: 10.1007/BF00422742 [DOI] [PubMed] [Google Scholar]
- [93].Vargas N, Marino F. Heat stress, gastrointestinal permeability and interleukin-6 signaling – Implications for exercise performance and fatigue. Temperature. 2016;3:240-251. doi: 10.1080/23328940.2016.1179380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Soares AD, Costa KA, Wanner SP, Santos RG, Fernandes SO, Martins FS, Nicoli JR, Coimbra CC, Cardoso VN. Dietary glutamine prevents the loss of intestinal barrier function and attenuates the increase in core body temperature induced by acute heat exposure. British J Nutrition. 2014;112:1601-1610. doi: 10.1017/S0007114514002608 [DOI] [PubMed] [Google Scholar]
- [95].Dehbi M, Baturcam E, Eldali A, Ahmed M, Kwaasi A, Chishti MA, Bouchama A. Hsp-72, a candidate prognostic indicator of heatstroke. Cell Stress Chaperones. 2010;15:593-603. doi: 10.1007/s12192-010-0172-3. PMID:20174993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Horowitz M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp Biochem Physiol A Mol Integr Physiol. 2002;131:475-483. doi: 10.1016/S1095-6433(01)00500-1. PMID:11867273 [DOI] [PubMed] [Google Scholar]
- [97].Sandstrom ME, Siegler JC, Lovell RJ, Madden LA, McNaughton L. The effect of 15 consecutive days of heat-exercise acclimation on heat shock protein 70. Cell Stress Chaperones. 2008;13:169-175. doi: 10.1007/s12192-008-0022-8. PMID:18759002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Leon LR. Common mechanisms for the adaptive responses to exercise and heat stress. J Applied Physiol. 2016;120:662-663. doi: 10.1152/japplphysiol.00113.2016 [DOI] [PubMed] [Google Scholar]
- [99].Ruell PA, Thompson MW, Hoffman KM, Brotherhood JR, Richards DA. Plasma Hsp72 is higher in runners with more serious symptoms of exertional heat illness. European J Applied Physiol. 2006;97:732-736. doi: 10.1007/s00421-006-0230-9 [DOI] [PubMed] [Google Scholar]
- [100].Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6:386-393. doi:. PMID:11795476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mazzei L, Docherty NG, Manucha W. Mediators and mechanisms of heat shock protein 70 based cytoprotection in obstructive nephropathy. Cell Stress Chaperones 2015;20:893-906. doi: 10.1007/s12192-015-0622-z. PMID:26228633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Bruchim Y, Avital Y, Horowitz M, Mazaki-Tovi M, Aroch I, Segev G. Urinary heat shock protein 72 as a biomarker of acute kidney injury in dogs. Veterinary J. 2017;225:32-34. doi: 10.1016/j.tvjl.2017.04.008 [DOI] [PubMed] [Google Scholar]
- [103].Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318-1321. doi: 10.1038/nm.2053. PMID:19855397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Chen R, Kang R, Fan XG, et al.. Release and activity of histone in diseases. Cell Death Dis. 2014;5:e1370. doi: 10.1038/cddis.2014.337. PMID:25118930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Gould TJ, Lysov Z, Liaw PC. Extracellular DNA and histones: double-edged swords in immunothrombosis. J Thrombosis Haemostasis. 2015;13 Suppl 1:S82-S91. doi: 10.1111/jth.12977 [DOI] [PubMed] [Google Scholar]
- [106].Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hägele H, Lichtnekert J, Hagemann JH, Rupanagudi KV, Ryu M, Schwarzenberger C, et al.. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrology. 2012;23:1375-1388. doi: 10.1681/ASN.2011111077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hoeksema M, van Eijk M, Haagsman HP, Hartshorn KL. Histones as mediators of host defense, inflammation and thrombosis. Future Microbiol. 2016;11:441-453. doi: 10.2217/fmb.15.151. PMID:26939619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bruchim Y, Ginsburg I, Segev G, Mreisat A, Avital Y, Aroch I, Horowitz M. Serum histones as biomarkers of the severity of heatstroke in dogs. Cell Stress Chaperones. 2017. doi: 10.1007/s12192-017-0817-6. PMID:28643239 [DOI] [PMC free article] [PubMed] [Google Scholar]


