Abstract
Background
Antidepressants are commonly prescribed medications in the elderly, but their relationship with incident mild cognitive impairment (MCI) and probable dementia is unknown.
Methods
The study cohort included 6,998 cognitively healthy, postmenopausal women, aged 65–79 years, who were enrolled in a hormone therapy clinical trial and had baseline depressive symptoms and antidepressant use history assessments at enrollment, and at least one postbaseline cognitive measurement. Participants were followed annually and the follow-up averaged 7.5 years for MCI and probable dementia outcomes. A central adjudication committee classified the presence of MCI and probable dementia based on extensive neuropsychiatric examination.
Results
Three hundred and eighty-three (5%) women were on antidepressants at baseline. Antidepressant use was associated with a 70% increased risk of MCI, after controlling for potential covariates including the degree of depressive symptom severity. Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) were both associated with MCI (SSRIs: hazard ratios (HR), 1.78 [95% CI, 1.01–3.13]; TCAs: HR, 1.78 [95% CI, 0.99–3.21]). Depressed users (HR, 2.44 [95% CI, 1.24–4.80]), non-depressed users (HR, 1.79 [95% CI, 1.13–2.85]), and depressed non-users (HR, 1.62 [95% CI, 1.13–2.32]) had increased risk of incident MCI. Similarly, all three groups had increased risk of either MCI or dementia, relative to the control cohort.
Conclusions
Antidepressant use and different levels of depression severity were associated with subsequent cognitive impairment in a large cohort of postmenopausal women. Future research should examine the role of antidepressants in the depression–dementia relationship and determine if antidepressants can prevent incident MCI and dementia in individuals with late-life depression subtypes with different levels of severity.
Keywords: antidepressants, selective serotonin reuptake inhibitors, late-life depression, cognitive decline, mild cognitive impairment, dementia
Introduction
The societal impact of late-life depression (LLD) is enormous since it is associated with poorer outcomes of comorbid medical disorders, accelerated cognitive and functional decline, and increased mortality risk (Alexopoulos, 2005; Almeida et al., 2010). LLD increases the future incidence of mild cognitive impairment (MCI) and probable dementia, including Alzheimer’s disease (AD), in a subset of older adults (Ownby et al., 2006; Goveas et al., 2011). In the Women’s Health Initiative Memory Study (WHIMS), depressive symptoms were independently associated with incident MCI and probable dementia, after a mean follow-up of 5.4 years (Goveas et al., 2011). While several studies have provided evidence of the detrimental effects of depression on future cognitive function, the relationship between antidepressant use and subsequent cognitive decline, including MCI and probable dementia, is less clear.
Antidepressants are among the most widely prescribed medications in older adults. Antidepressants are efficacious in the acute and maintenance therapy of LLD, thus decreasing disability and improving the quality of life of depressed elderly persons (Alexopoulos, 2005; Rajji et al., 2008; Smoller et al., 2009). However, antidepressants frequently cause adverse effects in elderly persons and are associated with increased risk of incident falls, lower bone mineral density and fractures, hyponatremia, cardiovascular morbidity, subclinical and clinical strokes, and all-cause mortality (Smoller et al., 2009; Woolcott et al., 2009; Wright et al., 2009; Haney et al., 2010; Coupland et al., 2011; Mark et al., 2011). Furthermore, despite some studies reporting that the cognitive deficits seen in depressed older adults may improve with antidepressant treatment, especially when taking selective serotonin reuptake inhibitors (SSRIs), these findings are not universal (Knegtering et al., 1994; Lee et al., 2007; Bhalla et al., 2009; Culang et al., 2009). Moreover, the magnitude of cognitive improvement seen in patients with LLD is greater in treatment responders (Culang et al., 2009). Depressed patients who have persisting deficits in the memory and executive function domains after effective treatment may be at higher risk for conversion to dementia (Steffens et al., 2004).
Prospective population-based studies that evaluate the association between depressive symptoms, antidepressant use, and the future incidence of MCI and probable dementia are essential because certain antidepressants, mainly SSRIs, reportedly have neuroprotective properties (Nelson et al., 2007). Furthermore, antidepressant treatment may reduce cognitive decline and even improve cognition in older persons with MCI and AD, though these results are controversial (Mossello et al., 2008; Ravaglia et al., 2008; Weintraub et al., 2010). Therefore, in the WHIMS, a large, multisite study, we examined the prospective relationship of antidepressant use and specific antidepressant class on cognition, in healthy postmenopausal women. We also investigated the association between antidepressant use and subsequent cognitive decline based on the baseline depressive symptom status.
Materials and methods
WHIMS study design and sample
WHIMS, an ancillary study to the Women’s Health Initiative randomized clinical trials (WHI-CT), was designed to examine the risks and benefits of postmenopausal hormone therapy on cognition and memory in healthy women aged 65 years and older at baseline. Thirty-nine of the 40 WHI clinical centers participated in the WHIMS and a total of 7,479 community-dwelling postmenopausal women were enrolled beginning in May 1996. Written informed consent was obtained, and the National Institutes of Health (NIH) and institutional review boards of the participating institutions approved the WHI-CT and WHIMS protocols. The study design, eligibility criteria, recruitment procedures, and the results from the WHI-CT have been published previously (Shumaker et al., 1998; 2004). Annual follow-up of the WHIMS participants continued after WHI-CT termination. Women gave consent for extended follow-up, which continued until September 2007. Results from initial WHIMS enrollment to the end of extended follow-up are presented here; these show a higher number of incident MCI and dementia cases than previously reported (Shumaker et al., 2004). The analytic cohort for this study included the WHIMS participants whose history of current antidepressant use was available, had completed the eight-item Burnam depression screen (Borhani et al., 1991), and had attended at least one follow-up visit. After excluding 481 participants who did not meet the above criteria, 6,998 women were included in the analysis.
Assessment of medication use
Participants were asked to bring all current medications to their baseline interview in their original bottles. Medication information was directly entered from the containers into a medications database that assigned codes using the Master Drug Data Base (MDDB; Medi-Span, Indianapolis, IN, USA). Women were classified into current antidepressant users or non-users based on the baseline medication use. Use of antidepressants was classified into the four mutually exclusive categories of SSRIs, tricyclic antidepressants (TCAs), other/multiple antidepressants, or none.
Depression measurement
Depressive symptoms were measured using the Burnam screening algorithm (Borhani et al., 1991). This consists of six items from the 20-item Center for Epidemiologic Studies Depression Scale (CES-D) and two items from the National Institute of Mental Health’s Diagnostic Interview Schedule (DIS), as described elsewhere (Borhani et al., 1991; Goveas et al., 2011). The Burnam screen, initially developed for the National Study of Medical Care Outcomes, uses a logistic regression algorithm and provides a composite score between 0 and 1. A score of 0.06 or higher on the Burnam screen, which has shown excellent sensitivity and specificity for detecting depressive disorder during the past month, was used to define the presence of depressive symptoms.
In order to fully explore the joint relationship between depressive symptoms and antidepressant use and its potential impact on cognitive decline, we created a composite variable consisting of four groups: (1) depressed users (i.e. women with depressive symptoms and antidepressant use, likely representative of undertreated women and poor responders); (2) depressed non-users (i.e. women with depressive symptoms and no antidepressant use, reflective of the untreated group); (3) non-depressed users (i.e. women on antidepressants but without depressive symptoms, which is the remitted depression group); and (4) non-depressed, non-users (i.e. women without depressive symptoms and antidepressant use, which is the reference cohort). This approach permits a more in-depth examination of these groups regarding risk of future cognitive decline for data exploration and hypothesis-generation.
Other covariates
Baseline data including demographic information, medical history (including history of cardiovascular and cerebrovascular disease), and lifestyle variables (including alcohol use and smoking history) were primarily obtained by self-report and clinical measurements using standardized study forms as detailed elsewhere (Shumaker et al., 1998; Goveas et al., 2011). Body mass index (BMI) was calculated as weight (kg) / height2 (m2). Physical exercise was defined as moderate or strenuous activity based on 20 minutes or more duration with the metabolic equivalent score of at least 4.0. Cognitive function was measured using the Modified Mini-Mental State (3MS) Examination at baseline and annually, by centrally trained and certified technicians (Shumaker et al., 1998; Goveas et al., 2011).
Outcome measures
The WHIMS protocol for detecting MCI and probable dementia has been described previously (Shumaker et al., 1998; 2004). Centrally trained and certified technicians who were re-certified semi-annually collected the WHIMS-related data. The protocol was divided into four phases. In phase 1, 3MS was administered to all participants as a screening assessment of global cognitive functioning during baseline and then at annual follow-up visits. Women who advanced to phases 2 and 3 were scheduled for completion of these phases within three months of phase 1. In phase 2, the certified technician administered the modified Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery, standardized interviews to assess for acquired cognitive and mental disorders, and a standardized set of 36 questions to assess the participant’s acquired cognitive and behavioral changes. In phase 3, a local physician (geriatrician, neurologist, or geriatric psychiatrist) with experience in diagnosing dementia evaluated the participants. After reviewing all available data and completing a structured medical history, and a physical and neuropsychiatric examination, the participants were classified as having probable dementia, MCI, or no dementia, based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria. MCI was diagnosed based on the accepted criteria at the time when the WHIMS was initiated, and was operationally defined as poor performance (10th or lower percentile based on CERAD norms on at least one CERAD test, some decline in instrumental activities of daily living (IADL) due to cognitive impairment, intact basic activities of daily living (ADL) reported by a reliable informant, no evidence of medical or psychiatric disorders that can explain the decline in cognitive function, and the absence of adjudicated dementia). For the diagnosis of probable dementia, functional impairment had to be secondary to cognitive impairment and could not be explained by medical etiologies. Women who were suspected of having probable dementia underwent phase 4 evaluation that included a non-contrast computed tomography (CT) brain scan and necessary laboratory blood tests to rule out possible reversible causes of cognitive decline and dementia. Subsequently, the physician was required to provide the most probable etiology of dementia based on the DSM-IV criteria for AD, vascular dementia, and other dementia-related classifications. Finally, the clinical and test data were transmitted to the WHIMS clinical coordinating center for review and central adjudication of MCI and probable dementia. The central adjudication process is described in detail elsewhere (Shumaker et al., 1998; 2004).
Statistical analysis
Women classified as antidepressant users at baseline versus non-users were compared with respect to demographic, lifestyle, cognitive, and clinical characteristics at the time of randomization to the WHI Hormone Therapy (HT) trials using χ2 tests for categorical variables and t tests for continuous variables. Cox proportional hazards regression was conducted separately for our three outcomes of interest: time until (1) MCI, (2) probable dementia, and (3) either MCI or probable dementia. Time to event was defined as the number of days from randomization into the WHI trials until the date of the 3MS that triggered the referral for additional cognitive testing resulting in the first postrandomization diagnosis of MCI or probable dementia. Participants without an event were censored at the time of their last 3MS testing.
The predictors of primary interest included baseline antidepressant use, a composite variable jointly representing antidepressant use and depression, and antidepressant use by class. Although we chose to treat depressive symptoms as a dichotomous variable, we also used the underlying score as a continuous variable to characterize and compare depression levels among the four groups using median scores and non-parametric tests (Wilcoxon rank-sum and Kruskal–Wallis tests). We fitted unadjusted Cox models focused on our single predictors of interest and adjusted for potential confounders (all baseline variables in Table 1 including baseline depressive symptoms). The effect of antidepressant use was assessed with unadjusted and adjusted hazard ratios (HR) and nominal 95% confidence intervals, and significance with asymptotic Wald tests. In each adjusted model, we examined the interaction between antidepressant use and baseline depressive symptoms, 3MS, and age. We plotted cumulative hazard functions to depict incidence over time by the predictors of primary interest. All statistical analyses were performed with SAS statistical software, version 9.2 (SAS Institute Inc., Cary, NC).
Table 1.
Baseline characteristics based on antidepressant use (N = 6,998)
| VARIABLE | BASELINE ANTIDEPRESSANT USE N = NUMBER (%) |
P VALUEa | |
|---|---|---|---|
|
| |||
| NO N = 6,615 (95) |
YES N = 383 (5) |
||
| Age in years mean, standard deviation | 70.9, 3.8 | 71.0, 3.9 | 0.8996 |
| Ethnicity, n (%) | 0.0528b | ||
| White | 5,777 (87) | 350 (91) | |
| African American | 464 (7) | 16 (4) | |
| Hispanic | 142 (2) | 9 (2) | |
| Asian | 116 (2) | 2 (1) | |
| American Indian | 21 (0) | 2 (1) | |
| Other/unknown | 95 (1) | 4 (1) | |
| Education, n (%) | 0.2967 | ||
| < high school grad/GED | 479 (7) | 34 (9) | |
| High school grad/GED | 1,449 (22) | 89 (23) | |
| Some college | 2,665 (40) | 158 (41) | |
| College graduate | 2,014 (30) | 101 (26) | |
| Family income, $, n (%) | 0.0044 | ||
| <20,000 | 1,586 (24) | 100 (26) | |
| 20,000–34,999 | 1,942 (29) | 127 (33) | |
| 35,000–49,999 | 1,302 (20) | 64 (17) | |
| 50,000+ | 1,406 (21) | 59 (15) | |
| Unreported | 379 (6) | 33 (9) | |
| Marital status, n (%) | 0.6941 | ||
| Never married | 222 (3) | 13 (3) | |
| Divorced/separated | 815 (12) | 49 (13) | |
| Widowed | 2,010 (30) | 126 (33) | |
| Married/marriage-like relationship | 3,553 (54) | 194 (51) | |
| Smoking, n (%) | 0.0236 | ||
| Never | 3,527 (54) | 177 (47) | |
| Past | 2,567 (39) | 171 (45) | |
| Current | 434 (7) | 30 (8) | |
| Alcohol use, n (%) | <0.0001 | ||
| Non-drinker | 875 (13) | 33 (9) | |
| Past drinker | 1,240 (19) | 116 (31) | |
| Current drinker | 4,445 (68) | 230 (61) | |
| Episodes per week of moderate and strenuous physical activity, n (%) | <0.0001 | ||
| None | 1,146 (17) | 95 (25) | |
| Some of limited duration | 2,971 (45) | 176 (46) | |
| 2–3 | 1,061 (16) | 61 (16) | |
| 4+ | 1,429 (22) | 51 (13) | |
| Body mass index, n (%) | 0.0514 | ||
| <25 | 1,930 (29) | 97 (26) | |
| 25–29 | 2,389 (36) | 129 (34) | |
| 30+ | 2,262 (34) | 153 (40) | |
| Prior hormone therapy, n (%) | <0.0001 | ||
| No | 3,641 (55) | 155 (40) | |
| Yes | 2,972 (45) | 228 (60) | |
| Hypertension, n (%) | <0.0001 | ||
| None | 3,376 (51) | 156 (41) | |
| Current/controlled | 1,047 (16) | 87 (23) | |
| Current/uncontrolled | 2,192 (33) | 140 (37) | |
| History of diabetes, n (%) | <0.0001 | ||
| No | 6,092 (92) | 326 (85) | |
| Yes | 514 (8) | 57 (15) | |
| High cholesterol requiring pills, n (%) | 0.0007 | ||
| No | 5,366 (82) | 285 (75) | |
| Yes | 1,166 (18) | 94 (25) | |
| History of cardiovascular diseasec, n (%) | <0.0001 | ||
| No | 5,730 (87) | 296 (77) | |
| Yes | 885 (13) | 87 (23) | |
| History of stroke or transient ischemic attack, n (%) | 0.0125 | ||
| No | 6,381 (96) | 360 (94) | |
| Yes | 234 (4) | 23 (6) | |
| Hormone therapy assignment, n (%) | 0.5600 | ||
| Active | 3,262 (49) | 183 (48) | |
| Placebo | 3,353 (51) | 200 (52) | |
| 3MS (mean, standard deviation) | 95.3, 4.2 | 94.6, 4.5 | 0.0021 |
| Depression status, n (%) | <0.0001 | ||
| No | 6,159 (93) | 291 (76) | |
| Yes | 456 (7) | 92 (24) | |
P value for categorical variables is based on χ2 test and for continuous variables, t test.
Based on collapsing to three categories (White, African American, and other).
Based on self-report of prior myocardial infarction, angina, coronary revascularization, cardiac arrest, congestive heart failure, cardiac catheterization, carotid endarterectomy, atrial fibrillation, or aortic aneurysm.
Results
Of the total sample of 6,998 participants, 5% (N = 383) were on antidepressants at baseline: 170 (44%) were on SSRIs; 160 (42%) were on TCAs; and 53 (14%) were on other/multiple antidepressants. Use of trazodone, which is typically prescribed adjunctively in low doses to promote sleep, was not an exclusion criterion for the SSRI and TCA categories: six women were taking an SSRI with trazodone, and none were taking a TCA with trazodone.
Compared to non-users, women on antidepressants were more likely to be depressed and to have lower family income and global cognitive function, have a past history of smoking and alcohol use, and were less likely to exercise. In addition, users tended to have a higher number of vascular risk factors, a history of cardiovascular and cerebrovascular disease, and a prior history of hormone therapy (Table 1).
A total of 331 women (4.7%) developed MCI, 216 women (3.1%) developed probable dementia, and 471 participants (6.7%) developed MCI/probable dementia (76 women who initially developed MCI converted to probable dementia during follow-up). Follow-up time averaged 7.6 years each for MCI and probable dementia, and 7.5 years for MCI/probable dementia. After adjusting for multiple covariates, baseline antidepressant use was associated with incident MCI (HR, 1.70 [95% CI, 1.14–2.54]), and incident MCI/probable dementia (HR, 1.55 [95% CI, 1.09–2.20]). Anti-depressant use was not significantly associated with incident probable dementia (Table 2). There were no significant interactions between antidepressant use and depressive symptoms (p = 0.37), baseline 3MS scores (p = 0.71) or age (p = 0.15). Table S1, available as supplementary material attached to the electronic version of this paper at www.journals.cambridge.org/jid_IPG, includes scores on the CERAD measures for antidepressant users and non-users grouped by incident MCI, probable dementia, and MCI/probable dementia.
Table 2.
Hazard ratio (95% CI) of developing adverse cognitive outcomes with antidepressant use versus none
| COGNITIVE OUTCOME | BASELINE ANTIDEPRESSANT USE |
UNADJUSTED HR (95% CI) p-VALUE |
ADJUSTED HR (95% CI) p-VALUE* |
|
|---|---|---|---|---|
|
| ||||
| YES (N = 383) | NO (N = 6,615) | |||
| Mild cognitive impairment | 1.97 (1.36–2.85) | 1.70 (1.14–2.54) | ||
| Number (%) of cases | 31 (8) | 300 (5) | 0.0003 | 0.009 |
| Probable dementia | 1.55 (0.93–2.58) | 1.24 (0.71–2.17) | ||
| Number (%) of cases | 16 (4) | 200 (3) | 0.09 | 0.45 |
| Mild cognitive impairment or probable dementia | 1.69 (1.21–2.35) | 1.55 (1.09–2.20) | ||
| Number (%) of cases | 38 (10) | 433 (7) | 0.002 | 0.02 |
Fully adjusted for age, ethnicity, education, family income, and marital status, smoking, alcohol use, baseline depressive symptoms, physical activity, body mass index, prior hormone therapy, high cholesterol requiring medication, hypertension, diabetes mellitus (treated or not), history of cardiovascular disease (based on self-report of prior myocardial infarction, angina, coronary revascularization, cardiac arrest, congestive heart failure, cardiac catheterization, carotid endarterectomy, atrial fibrillation, or aortic aneurysm), stroke or transient ischemic attack, hormone therapy assignment in Women’s Health Initiative Memory Study, and baseline Modified Mini-Mental State Examination score.
Abbreviations: HR = hazard ratio; CI = confidence interval.
In the fully adjusted analysis, use of SSRIs was associated with incident MCI (HR, 1.78 [95% CI, 1.01–3.13]), when compared with no antidepressant use. The TCA use was associated with incident MCI (HR, 1.78 [95% CI, 0.99–3.21]) and MCI/probable dementia (HR, 1.75 [95% CI, 1.05–2.91]) (Table 3). Although TCAs appear to show an increased risk for incident dementia compared to SSRIs, these findings were not statistically significant. Among women treated with multiple/other antidepressants, there were no significant associations (Table 3). In both unadjusted and adjusted analyses, statistically significant association between antidepressant use and incident MCI and MCI/probable dementia persisted even after using depression scores as a continuous covariate.
Table 3.
Hazard ratio (95% CI) of developing adverse cognitive outcomes according to antidepressant classes versus no antidepressant
| COGNITIVE OUTCOME | ANTIDEPRESSANT USE BY CLASS | |||
|---|---|---|---|---|
|
| ||||
| SSRI (N = 170) |
TCA (N = 160) |
OTHER/MULTIPLE ANTIDEPRESSANTS (N = 53) |
NO ANTIDEPRESSANTS (N = 6,615) |
|
| Mild cognitive impairment | ||||
| Number (%) of cases | 15 (9) | 13 (8) | 3 (6) | 300 (5) |
| Unadjusted HR (95% CI)/p-value | 2.18 (1.30–3.66)/0.003 | 1.93 (1.11–3.37)/0.02 | 1.40 (0.45–4.36)/0.56 | |
| Adjusted HR (95% CI)/p-value* | 1.78 (1.01–3.13)/0.05 | 1.78 (0.99–3.21)/0.05 | 1.23 (0.39–3.90)/0.73 | |
| Probable dementia | ||||
| Number (%) of cases | 6 (4) | 8 (5) | 2 (4) | 200 (3) |
| Unadjusted HR (95% CI)/p-value | 1.32 (0.59–2.98)/0.50 | 1.82 (0.90–3.69)/0.10 | 1.43 (0.36–5.77)/0.61 | |
| Adjusted HR (95% CI)/p-value* | 0.96 (0.38–2.41)/0.93 | 1.77 (0.85–3.67)/0.13 | 0.75 (0.17–3.40)/0.71 | |
| Mild cognitive impairment or probable dementia | ||||
| Number (%) of cases | 17 (10) | 17(11) | 4(8) | 433 (7) |
| Unadjusted HR (95% CI)/p-value | 1.73 (1.06–2.80)/0.03 | 1.78 (1.09–2.88)/0.02 | ||
| Adjusted HR (95% CI)/p-value* | 1.50 (0.89–2.53)/0.13 | 1.75 (1.05–2.91)/0.03 | 1.30 (0.49–3.48)/0.60 | 1.12 (0.41–3.05)/0.82 |
Fully adjusted for age, ethnicity, education, family income, and marital status, smoking, alcohol use, baseline depressive symptoms, physical activity, body mass index, prior hormone therapy, high cholesterol requiring medication, hypertension, diabetes mellitus (treated or not), history of cardiovascular disease (based on self-report of prior myocardial infarction, angina, coronary revascularization, cardiac arrest, congestive heart failure, cardiac catheterization, carotid endarterectomy, atrial fibrillation, or aortic aneurysm), stroke or transient ischemic attack, hormone therapy assignment in Women’s Health Initiative Memory Study, and baseline Modified Mini-Mental State Examination score.
Abbreviations: HR = hazard ratio; CI = confidence interval; SSRI = selective serotonin reuptake inhibitors; TCA = tricyclic antidepressants.
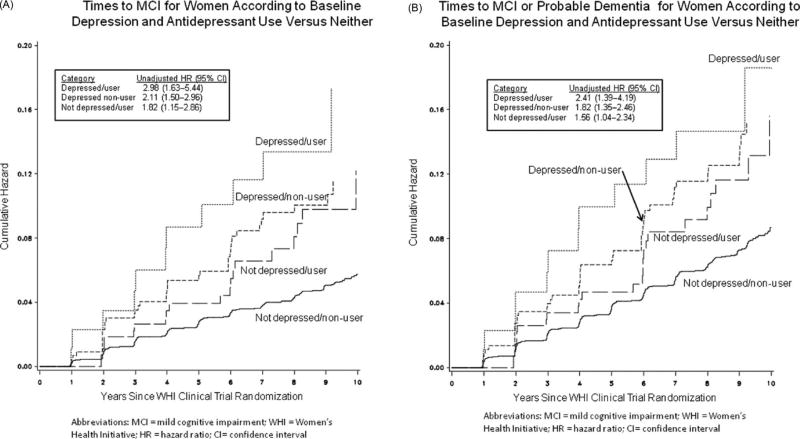
Depressed users were found to have a higher median level of depressive symptom severity than the depressed non-users (median values: 0.355 versus 0.213; p = 0.004). Likewise, not depressed users reported a higher medial level of depressive symptoms than not depressed non-users (median values: 0.0022 versus 0.0017; p < 0.0001). In a fully adjusted model, all three groups were associated with incident MCI (depressed user: HR, 2.44 [95% CI, 1.24–4.80]; non-depressed user: HR, 1.79 [95% CI, 1.13–2.85]; depressed non-user: HR, 1.62 [95% CI, 1.13–2.32]) and MCI/probable dementia (depressed user: HR, 2.34 [95% CI, 1.27– 4.32]; non-depressed user: HR, 1.60 [95% CI, 1.06–2.41]; depressed non-user: HR, 1.65 [95% CI, 1.20–2.26]), relative to the reference cohort (Table 4 and Figure 1). The risk of probable dementia associated with the three groups did not reach statistical significance.
Table 4.
Hazard ratio (95% CI) of developing adverse cognitive outcomes according to baseline depression and antidepressant use versus neither
| COGNITIVE OUTCOME | BASELINE DEPRESSION/ANTIDEPRESSANT USE | |||
|---|---|---|---|---|
|
| ||||
| DEPRESSED/USER (N = 92) |
DEPRESSED/NON-USER (N = 456) |
NOT DEPRESSED/USER (N = 291) |
NOT DEPRESSED/NON-USER (REFERENCE GROUP) (N = 6159) |
|
| Mild cognitive impairment | ||||
| Number (%) of cases | 11 (12) | 38 (8) | 20 (7) | 262 (4) |
| Unadjusted HR (95% CI)/p-value | 2.98 (1.63–5.44)/0.0004 | 2.11 (1.50–2.96)/<0.0001 | 1.82 (1.15–2.86)/0.01 | |
| Adjusted HR (95% CI)/p-value* | 2.44 (1.24–4.80)/0.01 | 1.62 (1.13–2.32)/0.009 | 1.79 (1.13–2.85)/0.01 | |
| Probable dementia | ||||
| Number (%) of cases | 5 (5) | 20 (4) | 11 (4) | 180 (3) |
| Unadjusted HR (95% CI)/p-value | 1.92 (0.79–4.66)/0.15 | 1.62 (1.02–2.57)/0.04 | 1.50 (0.82–2.76)/0.19 | |
| Adjusted HR (95% CI)/p-value* | 1.21 (0.43–3.43)/0.72 | 1.50 (0.92–2.44)/0.10 | 1.45 (0.78–2.72)/0.24 | |
| Mild cognitive impairment or probable dementia | ||||
| Number (%) of cases | 13 (14) | 48 (11) | 25 (9) | 385 (6) |
| Unadjusted HR (95% CI)/p-value | 2.41 (1.39–4.19)/0.002 | 1.82 (1.35–2.46) /<0.0001 | 1.56 (1.04–2.34) /0.0306 | |
| Adjusted HR (95% CI)/p-value* | 2.34 (1.27–4.32)/0.006 | 1.65 (1.20–2.26)/0.002 | 1.60 (1.06–2.41)/0.03 | |
Fully adjusted for age, ethnicity, education, family income, and marital status, smoking, alcohol use, physical activity, body mass index, prior hormone therapy, high cholesterol requiring medication, hypertension, diabetes mellitus (treated or not), history of cardiovascular disease (based on self-report of prior myocardial infarction, angina, coronary revascularization, cardiac arrest, congestive heart failure, cardiac catheterization, carotid endarterectomy, atrial fibrillation, or aortic aneurysm), stroke or transient ischemic attack, hormone therapy assignment in Women’s Health Initiative Memory Study, and baseline Modified Mini-Mental State Examination score.
Abbreviations: HR = hazard ratio; CI = confidence interval.
Figure 1.
Adverse cognitive outcomes according to baseline depression and antidepressant use. (A) Incidence of mild cognitive impairment; (B) incidence of mild cognitive impairment/probable dementia.
Discussion
This is the largest prospective study to examine the association between antidepressant use and incident cognitive impairment in elderly women. First, we found a significant relationship between antidepressant use and incident cognitive impairment. In comparison to women not on antidepressants, users had a 70% increased risk of MCI, after controlling for several potential covariates. Second, the risk of incident MCI and MCI/probable dementia was similarly increased in both SSRI and TCA users, with no specific antidepressant class showing a greater risk. Finally, depressed antidepressant users (undertreated depressed cohort) showed the highest risk for incident cognitive impairment, followed by non-depressed users (remitted depression group) and depressed non-users (untreated depressed women).
Antidepressants, regardless of the class, were associated with an increased risk of MCI and MCI/probable dementia in this study, suggesting a direct link between antidepressant use and incident cognitive impairment in postmenopausal women. Several possible mechanisms may explain the relationship between antidepressants and cognitive decline. The presence of baseline depressive symptoms, lower cognitive function, vascular risk factors, or cardiovascular and cerebrovascular disease may increase the risk of subsequent cognitive impairment (Alexopoulos, 2005; Ownby et al., 2006; Steffens et al., 2006; Goveas et al., 2011). The severity of vascular risk factors, poor baseline cognitive performance, and increased subclinical cerebrovascular disease predicts poor antidepressant response in LLD (Sheline et al., 2010). However, the WHIMS cohort was cognitively healthy at baseline and the detrimental effects of antidepressants persisted even after controlling for the above-mentioned confounding variables. Also, the relationship between antidepressant exposure and incident cognitive impairment persisted even after controlling for the level of depressive symptoms. Another possibility is that antidepressants may increase the risk of subclinical cerebrovascular disease, thus accelerating cognitive decline. In the Cardiovascular Health Study (CHS), while individuals on TCAs showed greater worsening of white matter disease, those on serotonergic antidepressants had an insignificant worsening of subclinical cerebrovascular disease (Steffens et al., 2008). Also, the antiplatelet aggregating activity of serotonergic antidepressants may increase the risk of hemorrhagic stroke in postmenopausal women (Smoller et al., 2009).
On the contrary, chronic treatment with SSRIs in non-human primates is shown to promote hippocampal neurogenesis and modulate the signal pathways involved in neuroplasticity and survival, thereby alleviating depressive symptoms and improving cognitive performance (Santarelli et al., 2003). SSRIs also reduce N-methyl-D-aspartate (NMDA) receptor function, suppress pro-inflammatory cytokine production, exhibit antithrombotic properties, and may protect against amyloid and tau pathology (Nelson et al., 2007; Hashioka et al., 2009; Aboukhatwa et al., 2010). Several studies in human primates have shown beneficial effects of SSRIs on cognitive performance, but these findings may be more pronounced in treatment responders and in individuals without baseline cognitive impairment and less vascular disease (Sheline et al., 2010). Finally, SSRI antidepressants have different anticholinergic activity. Paroxetine that has greater anticholinergic properties may cause impaired delayed verbal memory recall, whereas sertraline is associated with improved verbal fluency and delayed memory recall and citalopram with better memory consolidation, in healthy elderly volunteers (Knegtering et al., 1994). The potential benefits may have been masked by the enrollment in this study of participants on SSRIs with more anticholinergic activity.
Our findings of depressed antidepressant users showing the highest risk for incident cognitive decline and MCI are consistent with the Conselice Study of Brain Aging (CSBA) findings (Ravaglia et al., 2008). Similar to the CSBA, the indications for antidepressant use and duration and adequacy of treatment were not assessed in the WHIMS. It is also not clear if the depressed antidepressant users were undertreated or had recurrent or treatment refractory depression. The CSBA was limited by the small number of antidepressant users and incident MCI cases, predominance of TCAs (71%) among users, and exclusion of participants who subsequently developed dementia (Ravaglia et al., 2008). While a deleterious effect of SSRIs in treatment non-responders in verbal learning and psychomotor speed measures is seen, treatment responders show significant improvement in visuospatial functioning and psychomotor speed relative to non-responders (Culang et al., 2009). Regardless, our findings that older adults with a greater degree of depressive symptoms may be at highest risk for incident MCI and dementia should be a focus of future investigations.
The increased risk of cognitive impairment seen in the treatment responders was an unexpected finding. However, these results are consistent with previous mixed gender studies that found that over 40% of older adults with remitted LLD met the criteria for a cognitive disorder diagnosis, the majority of whom had MCI (Lee et al., 2007; Bhalla et al., 2009; Reynolds et al., 2011). Conversely, our findings are not consistent with results from shorter term randomized controlled trials that have shown a positive impact of antidepressants on cognitive function (Culang et al., 2009), albeit these studies did not carefully assess for a cognitive disorder diagnosis posttreatment. Since TCAs were more commonly prescribed in our remitted depression cohort (47% vs. 41%) (p=0.002), it is plausible that the differential assignment of antidepressant classes may have masked the neuroprotective effects of SSRIs in this group.
The lack of antidepressant use in the depressed women did not decrease the risk for cognitive decline, suggesting that there are complex pathophysiological mechanisms that may explain the relationship between depression and dementia. Several mechanistic links including vascular risk factors and disease, medial temporal lobe (MTL) and frontal regional atrophy, increased white matter hyperintensities, AD neuropathological lesion load, and APOE ε4 status are proposed and require rigorous investigation (Steffens et al., 2000; 2006; Butters et al., 2008; Irie et al., 2008).
The strengths of the study include: a large cohort of postmenopausal women; medication ascertainment; a thorough baseline assessment of several confounding variables, including depression screen; and a rigorous protocol to diagnose incident MCI and dementia cases, including extensive neuropsychiatric examination and a central adjudication process. This is the first prospective study to show a significant relationship between depressive symptoms, antidepressant use, and incident cognitive impairment in healthy, ethnically diverse, older postmenopausal women.
However, the study has limitations. The indication and duration of antidepressant use, and the precise dosages and medication compliance history were not available. While we are perplexed by the significantly higher number of antidepressant users being prescribed tricyclics (42%), this percentage is significantly lower than the one observed in the CSBA (71% of users) (Ravaglia et al., 2008). While TCAs are no longer widely prescribed to treat depression in the elderly, our findings highlight the continued disproportionate use of this group of medications in managing non-mental health-related illnesses including chronic persistent pain and urinary incontinence. WHIMS also did not assess if women were receiving antidepressants alone or in combination with psychotherapeutic interventions, for treatment of LLD. Depressive symptoms that were assessed using the eight-item Burnam screen have excellent sensitivity and specificity to diagnose syndromal depression but have poor positive predictive value (Tuunainen et al., 2001). This screening instrument is not a replacement for the clinician-administered Structured Clinical Interview for DSM-IV disorders (SCID) and could have resulted in an inaccurate estimation of the percentage of women with syndromal depression. Even though we used depression score as a continuous covariate, it is still possible that our findings of antidepressant use-cognitive impairment relationship is due to residual confounding by depression and we are unable to fully unravel the specific effects of antidepressant use from that of depression. While the lack of antidepressant class differences (i.e. our findings that both SSRI and TCA antidepressant use are associated with incident cognitive impairment) may also be taken as evidence of residual confounding by depression, this concern is somewhat mitigated by the fact that we did not see an increased risk in the multiple/other antidepressant group (Table 3). In addition, the age of onset, number of prior depressive episodes, and the duration of current depressive symptoms are not known. It is also important to note that depressive symptoms and antidepressant use status were defined based on cross-sectional assessments at baseline. Therefore, the changes in these exposure variables during follow-up could have resulted in misclassification of true exposure prior to the event, though this might have resulted in a bias toward null. The operational definition of MCI has continued to evolve since WHIMS was initiated and the women classified as MCI could include individuals with both amnestic and non-amnestic MCI. Our findings are based on women who volunteered and were eligible for a hormone therapy trial, and may not generalize to other cohorts. Finally, the results reported here should be interpreted in the context of an observational study and are not a substitute for randomized double blind, clinical trials.
Our results should not discourage use of antidepressants, rather they further underline the need for the judicious use of these pharmacologic agents to treat LLD. Our study also highlights the complexities of managing depression in late-life using antidepressants. First, antidepressants have beneficial effects in acute and maintenance treatment of LLD (Rajji et al., 2008). However, suboptimal treatment is common in LLD and appropriate dosage, medication adherence, and adequate duration of treatment are essential to achieve remission. On the other hand, older adults using antidepressants are more likely to have other comorbid physical and psychiatric diseases, and be prescribed a wide variety of non-psychotropic and psychotropic medications that can cause synergistic side effects and have drug–drug interactions with antidepressants (Mark et al., 2011). Although at the outset, antidepressant drugs can be perceived as being detrimental to future cognitive health in postmenopausal women, our findings also point to the fact that different gradations of depressive symptom severity may be associated with variable risk of incident cognitive impairment. Since less than 50% of older depressed patients on antidepressant therapy achieve full remission (Alexopoulos, 2005; Rajji et al., 2008; Reynolds et al., 2011), it is critical to augment medications with non-pharmacologic interventions for adequate treatment of LLD. These strategies may also be crucial for preventing MCI and dementia in cognitively intact depressed older adults. Our findings further suggest the need for ongoing monitoring of cognitive function in the depressed elderly. Second, early-onset and late-onset depression may be associated with cognitive decline via different pathophysiological mechanisms. History of depression increases the risk of developing AD approximately twofold, supporting a causal factor hypothesis (Ownby et al., 2006). Younger age of onset and longer duration of untreated depression can result in excessive stress hormone exposure of the MTL structures leading to atrophy (Sapolsky, 2000). Interestingly, antidepressant-induced hippocampal neurogenesis is suggested as an essential component needed for the clinical benefits of antidepressants (Santarelli et al., 2003). In contrast, depressive symptoms may be the earliest non-cognitive manifestation of AD and may be a marker of incipient dementia, suggesting a reverse causality hypothesis (Alexopoulos, 2005; Steffens et al., 2006). Adequate antidepressant treatment of early-onset and mid-life depression, prevention of recurrent episodes, and shortening the duration of untreated depression may prevent future cognitive decline and development of dementia.
In summary, antidepressant use was associated with subsequent cognitive impairment in a large cohort of postmenopausal women. While we are unable to fully disentangle the specific effects of depression and antidepressant use in this study, different levels of depressive symptom severity were associated with varying trajectories with respect to the development of incident cognitive impairment. Moreover, while antidepressant use in women with remitted depression did not prevent subsequent cognitive decline, our results suggest a decreased risk of incident cognitive decline with optimal treatment of depression. Further research is essential to elucidate the role of antidepressant use in the depression–dementia relationship and to examine if newer generation antidepressants can prevent dementia in mid- and late-life depression subtypes with different levels of symptom severity.
Supplementary Material
Acknowledgments
The Women’s Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, and 32122. The active study drug and placebo were supplied by Wyeth-Ayerst Research Laboratories, Philadelphia, PA. The Women’s Health Initiative Memory Study was funded in part by Wyeth Pharmaceuticals as an ancillary study to the WHI. Wyeth Pharmaceuticals did not participate in the design and conduct of the studies, in the collection, analysis, and interpretation of the data, nor in the preparation, review, or approval of this paper.
Appendix: Short list of WHI investigators
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, MD) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Aleksandar Rajkovic; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Yvonne Michael; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael Simon.
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Footnotes
Conflict of interest
None.
Description of authors’ roles
J. S. Goveas, M. A. Espeland, J. M. Kotchen, A. Tummala, N. F. Woods, and S. Wassertheil-Smoller gave final approval to the paper. M. A. Espeland and P. Hogan were engaged in data analysis. J. S. Goveas worked on the initial draft of the paper and provided guidance with data analysis. N. L. Denburg, M. A. Espeland, J. S. Goveas, P. E. Hogan, J. M. Kotchen, J. E. Manson, W. J. Mysiw, J. Ockene, J. Smoller, A. Tummala, S. Wassertheil-Smoller, and N. F. Woods were involved in study design, data acquistion, analysis, and/or interpretation of data. N. L. Denburg, M. A. Espeland, J. S. Goveas, P. E. Hogan, J. M. Kotchen, J. E. Manson, W. J. Mysiw, J. Ockene, J. Smoller, A. Tummala, S. Wassertheil-Smoller, and N. F. Woods provided assistance and were involved in critical revision of the manuscript for important intellectual content.
References
- Aboukhatwa M, Dosanjh L, Luo Y. Antidepressants are a rational complementary therapy for the treatment of Alzheimer’s disease. Molecular Neurodegenertion. 2010;5:10. doi: 10.1186/1750-1326-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Alfonso H, Hankey GJ, Flicker L. Depression, antidepressant use and mortality in later life: the health in men study. PLoS One. 2010;5:e11266. doi: 10.1371/journal.pone.0011266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla RK, et al. Patterns of mild cognitive impairment after treatment of depression in the elderly. American Journal of Geriatric Psychiatry. 2009;17:308–316. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borhani NO, et al. Systolic Hypertension in the Elderly Program (SHEP). Part 1: rationale and design. Hypertension. 1991;17:II2–II15. doi: 10.1161/01.hyp.17.3_suppl.ii2. [DOI] [PubMed] [Google Scholar]
- Butters MA, et al. Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh Compound-B. Alzheimer Disease and Associated Disorders. 2008;22:261–268. doi: 10.1097/WAD.0b013e31816c92bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343:d4551. doi: 10.1136/bmj.d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culang ME, et al. Change in cognitive functioning following acute antidepressant treatment in late-life depression. American Journal of Geriatric Psychiatry. 2009;17:881–888. doi: 10.1097/jgp.0b013e3181b4bf4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goveas JS, Espeland MA, Woods NF, Wassertheil-Smoller S, Kotchen JM. Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly women: the Women’s Health Initiative Memory Study. Journal of the American Geriatric Society. 2011;59:57–66. doi: 10.1111/j.1532-5415.2010.03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney EM, Warden SJ, Bliziotes MM. Effects of selective serotonin reuptake inhibitors on bone health in adults: time for recommendations about screening, prevention and management? Bone. 2010;46:13–17. doi: 10.1016/j.bone.2009.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashioka S, Mcgeer PL, Monji A, Kanba S. Anti-inflammatory effects of antidepressants: possibilities for preventives against Alzheimer’s disease. Central Nervous System Agents in Medicinal Chemistry. 2009;9:12–19. doi: 10.2174/187152409787601897. [DOI] [PubMed] [Google Scholar]
- Irie F, Masaki KH, Petrovitch H, Abbott RD, Ross GW, Taaffe DR. Apolipoprotein E epsilon4 allele genotype and the effect of depressive symptoms on the risk of dementia in men: the Honolulu-Asia Aging Study. Archives of General Psychiatry. 2008;65:906–912. doi: 10.1001/archpsyc.65.8.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knegtering H, Eijck M, Huijsman A. Effects of antidepressants on cognitive functioning of elderly patients. A review. Drugs and Aging. 1994;5:192–199. doi: 10.2165/00002512-199405030-00005. [DOI] [PubMed] [Google Scholar]
- Lee JS, Potter GG, Wagner HR, Welsh-Bohmer KA, Steffens DC. Persistent mild cognitive impairment in geriatric depression. International Psychogeriatrics. 2007;19:125–135. doi: 10.1017/S1041610206003607. [DOI] [PubMed] [Google Scholar]
- Mark TL, Joish VN, Hay JW, Sheehan DV, Johnston SS, Cao Z. Antidepressant use in geriatric populations: the burden of side effects and interactions and their impact on adherence and costs. American Journal of Geriatric Psychiatry. 2011;19:211–221. doi: 10.1097/jgp.0b013e3181f1803d. [DOI] [PubMed] [Google Scholar]
- Mossello E, Boncinelli M, Caleri V, Cavallini MC, Palermo E, Di Bari M. Is antidepressant treatment associated with reduced cognitive decline in Alzheimer’s disease? Dementia and Geriatric Cognitive Disorders. 2008;25:372–379. doi: 10.1159/000121334. [DOI] [PubMed] [Google Scholar]
- Nelson RL, et al. Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Experimental Neurology. 2007;205:166–176. doi: 10.1016/j.expneurol.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of General Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajji TK, Mulsant BH, Lotrich FE, Lokker C, Reynolds CF., III Use of antidepressants in late-life depression. Drugs and Aging. 2008;25:841–853. doi: 10.2165/00002512-200825100-00003. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, et al. Prevalent depressive symptoms as a risk factor for conversion to mild cognitive impairment in an elderly Italian cohort. American Journal of Geriatric Psychiatry. 2008;16:834–843. doi: 10.1097/JGP.0b013e318181f9b1. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, III, et al. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Archives of General Psychiatry. 2011;68:51–60. doi: 10.1001/archgenpsychiatry.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sheline YI, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Archives of General Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker SA, et al. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Controlled Clinical Trials. 1998;19:604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Smoller JW, et al. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women’s Health Initiative study. Archives of Internal Medicine. 2009;169:2128–2139. doi: 10.1001/archinternmed.2009.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, et al. Hippocampal volume in geriatric depression. Biological Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- Steffens DC, et al. Methodology and preliminary results from the neurocognitive outcomes of depression in the elderly study. Journal of Geriatric Psychiatry and Neurology. 2004;17:202–211. doi: 10.1177/0891988704269819. [DOI] [PubMed] [Google Scholar]
- Steffens DC, et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Archives of General Psychiatry. 2006;63:130–138. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Chung H, Krishnan KR, Longstreth WT, Jr, Carlson M, Burke GL. Antidepressant treatment and worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2008;39:857–862. doi: 10.1161/STROKEAHA.107.498097. [DOI] [PubMed] [Google Scholar]
- Tuunainen A, Langer RD, Klauber MR, Kripke DF. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Research. 2001;103:261–270. doi: 10.1016/s0165-1781(01)00278-5. [DOI] [PubMed] [Google Scholar]
- Weintraub D, et al. Sertraline for the treatment of depression in Alzheimer disease: week-24 outcomes. American Journal of Geriatric Psychiatry. 2010;18:332–340. doi: 10.1097/JGP.0b013e3181cc0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolcott JC, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Archives of Internal Medicine. 2009;169:1952–1960. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- Wright RM, et al. Effect of central nervous system medication use on decline in cognition in community-dwelling older adults: findings from the Health, Aging And Body Composition Study. Journal of the American Geriatric Society. 2009;57:243–250. doi: 10.1111/j.1532-5415.2008.02127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.