Abstract
There is an unmet need to develop new agents or strategies against therapy resistant pancreatic cancer (PanCA). Recent studies from our laboratory showed that STAT3 negatively regulates NF-κB and that inhibition of this crosstalk using Nexrutine® (Nx) reduces transcriptional activity of COX-2. Inhibition of these molecular interactions impedes pancreatic cancer cell growth as well as reduces fibrosis in a preclinical animal model. Nx is an extract derived from the bark of Phellodendron amurense and has been utilized in traditional Chinese medicine as antidiarrheal, astringent, and anti-inflammatory agent for centuries. We hypothesized that “Nx-mediated inhibition of survival molecules like STAT3 and NF-κB in pancreatic cancer cells will improve the efficacy of the conventional chemotherapeutic agent, gemcitabine (GEM)”. Therefore, we explored the utility of Nx, one of its active constituents berberine and its derivatives, to enhance the effects of GEM. Using multiple human pancreatic cancer cells we found that combination treatment with Nx and GEM resulted in significant alterations of proteins in the STAT3/NF-κB signaling axis culminating in growth inhibition in a synergistic manner. Furthermore, GEM resistant cells were more sensitive to Nx treatment than their parental GEM-sensitive cells. Interestingly, although berberine, the Nx active component used, and its derivatives were biologically active in GEM sensitive cells they did not potentiate GEM activity when used in combination. Taken together, these results suggest that the natural extract, Nx, but not its active component, berberine, has the potential to improve GEM sensitivity, perhaps by down regulating STAT3/NF-κB signaling.
Introduction
Over the last several decades, numerous advances have been made in cancer therapy. However, a few cancers like pancreatic cancer (PanCA), still have a very poor prognosis for individuals diagnosed with the disease. In 2015 alone, it was estimated that over 48,000 individuals would be diagnosed with PanCA in the US [1]. Sadly, only around 7% of the people diagnosed will live beyond five years [1]. One of the leading causes for the poor survival rate of pancreatic cancer patients is drug resistance. Despite years of research, the best therapeutic option developed for PanCA has been and is still gemcitabine (GEM). GEM is a nucleoside analogue, that is metabolized into its active forms, difluorodeoxycytidine diphosphate (dFdCDP) and difluorodeoxycytidine triphosphate (dFdCTP) through a complex intracellular metabolic process [2]. These metabolites inhibit the production of deoxynucleotides through dFCDP, inhibition of ribonucleotide reductase and interfering with DNA transcription/replication by competing with and replacing deoxycytidine triphosphate (dCTP) in DNA [2]. Previous studies have demonstrated that resistance to GEM occurs via multiple processes. The main source of GEM resistance is from human equilibrative nucleoside transporter-1 (hENT1) deficiency, which causes decreased uptake of GEM into cells [3]. Additional resistance mechanisms include deoxycytidine kinase (dCK) deficiency, MUC4 expression, and ribonucleotide reductase (RR) overexpression [3,4]. Despite the recent development of new treatment regimens such as the GEM-erlotinib and nab-paclitaxel/GEM combinations as well as FOLFIRINOX, their use is limited to select groups of patients for multiple reasons including toxicity [5–7]. It is obvious that we must rethink our strategy if we are to develop novel treatments that are less toxic as well as more effective and universally usable.
The use of natural products has long been associated with traditional medicines to treat a range of ailments. However, the vast majority of these products lack scientific evidence to determine how and why they work or whether they may be useful in treating other diseases such as cancer. Nexrutine® (Nx) is a natural product derived from the bark of Phellodendron amurense, the cork tree [8]. Nx has been used in Traditional Chinese Medicine for thousands of years to treat inflammation, diarrhea, and rheumatism [8]. Studies from our group and others have demonstrated the beneficial use of Nx both in vitro and in vivo using various tumor models including prostate, pancreas, breast, liver, colon and melanoma [9–18]. Mechanistic investigations revealed that Nx inhibits a plethora of cellular processes, including cellular proliferation, autophagy, ROS production, and tumor cell invasion [12–15,18]. Remarkably, a clinical trial to assess the safety of Nx in prostate cancer patients showed that it is safe and well tolerated by prostate cancer patients [19]. Furthermore, the use of Nx as a neo-adjuvant to radiation therapy was associated with minimal side effects [19]. Although efficacy determination was not an endpoint of this trial, 81% of prostate cancer patients receiving Nx during neoadjuvant period showed reduction in PSA levels [19]. Encouraged by these studies, we examined whether Nx could be used to enhance the cytotoxic effects of GEM in a cell culture model. We show here that Nx as a whole extract, but not its individual components in combination with GEM may improve chemotherapeutic sensitivity perhaps by down regulation of STAT3/NF-κB signaling.
Materials and Methods
Reagents
Nexrutine® was provided by Next Pharmaceuticals, Inc. (Salinas, CA). Berberine, n-pentylbromide and 4-bromo benzyl bromide were purchased from Sigma (St. Louis, MO). Berberine and Nx were dissolved in 50% DMSO-50% nuclease free water solution at concentrations of 1 mg/mL and 5 mg/mL respectively. Gemcitabine (GEM) was obtained from Sigma and nuclease free water was used to make a 1 mM GEM master stock that was aliquoted and stored at −20°C until needed. All organic solvents used were of reagent grade quality and were used without further purification. Melting points were obtained with melting point apparatus. 1H NMR spectra were performed on a Varian Inova 400 MHz spectrometer (Varian, San Francisco, CA) in CDCl3/DMSO-d6 (as internal standard on a δ scale).
Synthesis of Ber 2-4 and Ber 2-8
The synthesis of Ber 2-4 and Ber 2-8 were achieved via intermediate, Tetrahydroberberine. The synthesis of Tetrahydroberberine was previously reported by Pingali et al. and has been obtained through a complete reduction of Berberine under refluxing conditions using equivalent amount of NaBH4 [20]. This resultant intermediate, Tetrahydroberberine, then undergoes a quaternization reaction with alkyl halides to achieve the corresponding quaternary ammonium salts: Ber 2-4 and Ber 2-8. Yang et al. have reported the method of synthesis earlier; however, Ber 2-4 and Ber 2-8 were not synthesized by them and have been reported here [21].
9,10-dimethoxy-7-pentyl-5,6,7,8,13,13a-hexahydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium chloride (Ber 2-4): To a stirred suspension of Tetrahydroberberine (339 mg, 1 mmol,) and K2CO3 (410 mg, 3 mmol) in 15 mL of acetonitrile was added n-pentylbromide (0.62 mL, 5 mmol) drop wise. After the addition, the mixture was stirred for an additional 5 min and then was heated at 60°C for 12 hours with the condenser attached. After the reaction was complete, the reaction mixture was filtered to remove un-dissolved impurities and the excess solvent was removed by distillation. The residue thus obtained was washed with ether (2 × 10 mL) to remove unreacted halide. Then the yellow powdery substance obtained was recrystallized using 5 mL of MeOH: Acetone (1:1) in a reflux condenser to obtain pure product (267 mg, 65%). M.P.: 136–138°C. MS m/z 410 (M - Br)+. 1H NMR: δ 0.87 (t, J = 7.2 Hz, 3H), 1.18–1.28 (m, 2H), 1.71 (m, 1H), 1.87 (m, 1H), 3.05–3.14 (m, 3H), 3.38–3.40 m, 2H), 3.65–3.75 (m, 2H), 3.78–3.83 (m, 1H), 3.79 (s, 3H), 3.81 (s, 3H), 4.65 (d, J = 12.4 Hz, 1H), 4.74 (q, J = 5.6 Hz, 1H), 4.80 (d, J = 12.4 Hz, 1H), 6.03 (d, J = 8.0 Hz, 2H), 6.88 (s, 1H), 7.07 (s, 1H), 7.09 (d, J = 9.2 Hz, 1H), 7.12 (d, J = 8.8 Hz, 1H).
7-(4-bromobenzyl)-9,10-dimethoxy-5,6,7,8,13,13a-hexahydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium bromide (Ber 2-8): To a stirred suspension of Tetrahydroberberine (339 mg, 1 mmol,) and K2CO3 (410 mg, 3 mmol) in 15 mL of acetonitrile was added 4-bromo benzyl bromide (1.25 g, 0.005 mol, 5 Eq) drop wise. After the addition, the mixture was stirred for an additional 5 min and then was heated at 70°C for 12 hours with the condenser attached. After the reaction was complete, the reaction mixture was filtered to remove un-dissolved impurities and the excess solvent was removed by distillation. The residue thus obtained was washed with ether (2 × 10 mL) to remove unreacted halide. Then the yellow powdery substance obtained was recrystallized using 5 mL of MeOH: Acetone (1:1) in a reflux condenser to obtain pure product (295 mg, 58%). M.P.: 175–178°C. MS m/z 509 (M - Br)+. 1H NMR: δ 3.23 (q, J = 14.4 Hz, 1H), 3.38 (q, J = 12.4 Hz, 1H), 3.64–3.71 (m, 3H), 3.74 (s, 3H), 3.87 (s, 3H), 4.10–4.24 (m, 1H), 4.19 (m, 2H), 4.36 (d, J = 16.4 Hz, 1H), 4.48 (d, J = 16.4 Hz, 1H), 5.34 (q, J = 5.6 Hz, 1H), 6.08 (d, J = 5.6 Hz, 2H), 6.95 (s, 1H), 7.14 (s, 1H), 7.14–7.25 (m, 4H), 7.45–7.52 (m, 3H).
Cell Culture
Human pancreatic cancer cell lines BxPC-3, Capan-2, MIA PaCa-2, and PANC-1 were obtained from American Type Cell Culture (ATCC, Manassas, VA). The non-tumorigenic, HPNE cell line transformed with the KRASG12D mutation, HPNE-Ras, was generous gifts from Dr James W Freeman. GEM-resistant BxPC-3 (GRB) cells were generated in the Freeman laboratory and GEM-resistant Capan-2 (GRC) cells were generated in the Kumar laboratory [22]. Complete RPMI contained 10% fetal bovine serum (FBS, Atlanta Biologicals, Norcross, GA), 100 μg/mL amphotericin, and 100 μg/mL penicillin-streptomycin in RPMI medium. This was used to culture Capan-2 and BxPC-3. Complete RPMI supplemented with GEM was used to culture GRC and GRB cells. Media for MIA PaCa-2 cells contained 10% FBS, 5% horse serum (Sigma), and 100 μg/mL penicillin-streptomycin in DMEM medium containing high glucose. DMEM containing 10% FBS, 100 μg/mL amphotericin, and 100 μg/mL penicillin-streptomycin was used to culture PANC-1 cells. Culture media for HPNE and HPNE-Ras consisted of 5.5mM D-glucose, 10ng/mL recombinant EGF, 50 μg/mL gentamycin, and 5% FBS in HPNE base medium (25% M3 medium + 75% glucose free DMEM with 2mM L-glutamine). All cells were cultured in a humidified incubator at 37°C and 5% CO2.
Development of GEM Resistant Capan-2
To develop GEM resistant Capan-2 cells, 1 × 105 cells were seeded in a T25 flask and allowed to attach. Two days after seeding, cell media (complete RPMI) was replaced with fresh media containing 5 μM GEM. Cells were maintained in 5 μM GEM for four days before media was switched back to normal media without GEM as a majority of cells died. Two weeks after removal of GEM, the surviving cells had grown back to confluence and were again exposed to GEM. Cells were treated for 1 week with 5 μM GEM, followed by two weeks of culture in normal media without GEM. For the next treatment, cells were treated for 1 week with 7.5 μM GEM and then cultured two weeks without GEM. This pattern of GEM treatment followed by culture in normal media continued while treatment times were slowly increased and the GEM concentration was raised to 10 μM. Cells were cultured in 10 μM GEM for 4 months. Phenotypic changes of the resistant cells compared to their parental cell line include reduced proliferation and changed cell morphology.
Proliferation Assays
Cells were plated at a density of 4 × 103 cells per well in 96-well plates. 24h later, cells were treated with varying doses of Nx, berberine, a berberine derivative, gemcitabine, or a combination of gemcitabine with Nx, berberine or a berberine derivative. A modified MTT assay (CellTiter 96® Non-Radioactive Cell Proliferation Assay; Promega Corp., Madison, WI) was used to determine proliferation as previously described [13]. DMSO was used as the solvent control.
Colony Formation Assays
Colony formation was carried out using the CytoSelect 96-well Cell Transformation Assay (Soft Agar Colony Formation) according to manufacturer’s directions (Cell Biolabs, San Diego, CA). Cells were cultured for 7 days with Gem (0.5 μM) and Nx (IC20) alone or in combination in the soft agar matrix. Colony formation was determined according to manufacturer’s directions using the SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA) using 485/520 nm filters. Nx IC20s were: Capan-2 = 50 μg/mL; BxPC-3 = 20 μg/mL; MIA PaCa-2 = 20 μg/mL; and HPNE-Ras = 20 μg/mL.
Apoptosis Assays
3 × 105 cells/well were seeded in six well plates and approximately 24h after plating, cells were treated for 24h with either media alone, 0.5 μM GEM, their Nx IC20 dose (Capan-2 = 50 μg/mL and BxPC-3 = 20 μg/mL), or a combination of 0.5 μM GEM + Nx IC20 dose. Apoptosis was assessed using the Annexin-V-FITC Apoptosis Detection Kit from BD Biosciences (San Diego, CA) as per the manufacture’s protocol.
Western Blotting
To assess STAT3/NF-κB signaling changes, whole cell extracts were prepared following treatment for 24h with either media alone, the IC20 dose of Nx for each cell line (Capan-2 = 50 μg/mL; BxPC-3 = 20 μg/mL; MIA PaCa-2 = 20 μg/mL; and HPNE-Ras = 20 μg/mL), 0.5 μM GEM, or a combination of both GEM and Nx. Cells were lysed in 2x SDS buffer containing fresh protease and phosphatase inhibitors. Lysates were fractionated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels. Proteins were transferred to nitrocellulose membranes as previously described [9]. Blots were blocked for 1 hour at room temperature in either 5% nonfat milk in 1x TBS containing 0.1% Tween 20 (TBST) or 5% BSA in TBST. Primary antibodies (purchased from Cell Signaling Technology, Danvers, MA and Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were used to probe for specific proteins for 2h at room temperature. This was followed by incubation with secondary antibody (HRP-conjugated from Sigma and Santa Cruz Biotechnology) for 1h. Blots were then developed in a Syngene G:Box Imaging System (Frederick, MD) using Western Lightning® Plus-ECL (Perkin Elmer, Waltham, MA) chemiluminescence. Band quantification was done as previously described [23].
Combination Index Analysis
Cell proliferation was carried out as described above. The combinatorial effect of Nx and GEM on proliferation inhibition was calculated by combination index (CI) using the Chou and Talalay method [24,25]. According to this calculation, CI value = 1 denotes an additive drug effect, while values < 1 indicate strong synergy between drugs and values > 1 indicate antagonism between drugs [25].
Statistical Analysis
All experiments were done in triplicate in at least three independent experiments and the data generated was then tested for significance by two-way ANOVA followed by student T-test. P-values < 0.05 were considered significant.
Results
Nx-GEM combination exhibits synergistic anti-cancer effects
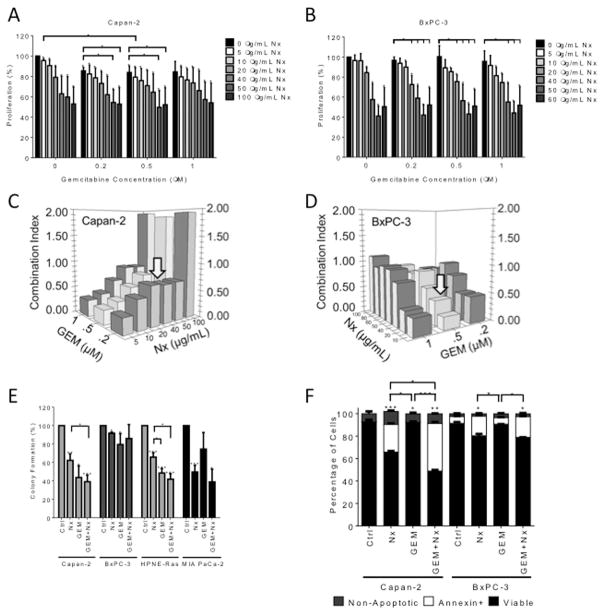
Previous work from our laboratory demonstrated the ability of Nx to inhibit cell growth as well as modulate inflammatory (STAT3/NF-κB) and autophagic signaling pathways in PanCA cells [12,13]. Given that these pathways contribute to therapeutic resistance, we assessed the effect of Nx in combination with the conventional therapeutic agent, gemcitabine (GEM). We first measured the effect of Nx and GEM on cell proliferation of human pancreatic cancer cell lines. Treatment of Capan-2 and BxPC-3 cells with both Nx and GEM reduced proliferation significantly (Fig. 1A and 1B). The observed anti-proliferative effects were analyzed using Isobologram analysis to determine whether these effects were synergistic or additive. A combination index (CI) value of 1 denotes additive; CI of <1 indicates synergistic and >1 indicates antagonistic interaction [25]. A CI of 0.5 was achieved using 50 and 20 μg/mL Nx (Capan-2 and BxPC-3 respectively) in combination with 0.5 μM GEM indicating a strong synergistic interaction (Figs. 1C and 1D). Based on these data, we chose 50 and 20 μg/mL Nx (Capan-2 and BxPC-3 respectively) in combination with 0.5 μM GEM for subsequent experiments.
Fig. 1. Nx and Gemcitabine combination treatment has a synergistic anti-cancer effect.
(A & B) Capan-2 and BxPC-3 were treated with different doses of Nx in the presence and absence of varying concentrations of GEM for 24hr. Cell proliferation was determined by MTT assay. Combination index values of Capan-2 (C) and BxPC-3 (D) cells were calculated with Calcusyn Software as a function of the level of anti-proliferative activity (Combination index = 1 denotes additivity; combination index > 1 is antagonism; and combination index < 1 is defined as synergy). (E) Capan-2, BxPC-3, HPNE-Ras and MIA PaCa-2 cells were treated with IC20 doses of Nx (Capan-2 = 50 μg/mL; BxPC-3 = 20 μg/mL; MIA PaCa-2 = 20 μg/mL; and HPNE-Ras = 20 μg/mL) in the presence and absence of GEM (0.5 μM) for 7 days. Colony formation was determined using the CytoSelect 96-well Cell Transformation Assay according to manufacturer’s directions (Cell Biolabs, San Diego, CA). (F) Percentages of apoptotic, non-apoptotic and viable cells were measured using Annexin V-FITC Apoptosis Detection Kit (BD Biosciences, CA) in Capan-2 and BxPC-3 cells treated for 24h with either solvent control, Nx alone (50 and 20 μg/ml respectively for Capan-2 and BxPC-3 cells), 0.5 μM GEM alone, or Nx-GEM combination (Nx 50 and 20 μg/ml respectively for Capan-2 and BxPC-3 cells + 0.5 μM GEM) as described in methods. P values: * <0.05; ** <0.001; *** <0.0005.
To validate the proliferation inhibitory capability of the Nx-GEM combination, we tested the anchorage-independent growth of Capan-2 and BxPC-3 cells using soft agar colony formation assay. These cells were treated with single agent alone or combination treatment for 7 days to allow cells to form colonies. Results are displayed as the percentage of colonies formed compared to the untreated control. Irrespective of cell type, single agent treatments (Nx or GEM) significantly reduced colony-forming ability compared to untreated controls (Fig. 1E). Combination treatment reduced the colony forming ability of cell lines expressing mutant KRAS (Capan-2, MIA PaCa-2 and HPNE-RAS), but not wild type KRAS (BxPC-3). The observed results suggest that the combination of Nx and GEM inhibits anchorage-independent growth of multiple human pancreatic cancer cell lines. Further, these effects appear to be more effective in cell lines with oncogenic Ras.
Given that the Nx-GEM combination inhibits growth of pancreatic cancer cells, we hypothesized that this combination induces apoptosis in the cells that undergo growth inhibition. Using Annexin V apoptosis assay, we assessed apoptotic activity in response to Nx-GEM combination treatment in Capan-2 and BxPC-3 cells. We did not observe Annexin positive cells in Capan-2 cells treated with GEM or media alone (Fig. 1F). Conversely, Nx treatment alone induced apoptosis in these cells (p value < 0.005). Moreover, Capan-2 cells treated with both Nx and GEM showed greater apoptosis induction compared to all other treatments (p values < 0.005). Similar to Capan-2, BxPC-3 cells displayed increased levels of apoptotic cells in Nx and not GEM alone treatments compared to the control (p value < 0.05). Further, combination of Nx plus GEM showed significantly increased apoptosis compared to GEM alone (p value < 0.005). However, there was not a significant difference between the Nx alone and Nx-GEM combination treatments in these cells. Taken together, these data demonstrate that Capan-2 cells expressing KRAS mutation were markedly more sensitive to the combination treatment than BxPC-3 cells expressing WT KRAS. Furthermore, combination of Nx plus GEM may inhibit growth of these cells synergistically possibly independent of apoptotic cell death.
Nx-GEM combination decreases STAT3 activity in cell lines with oncogenic KRAS
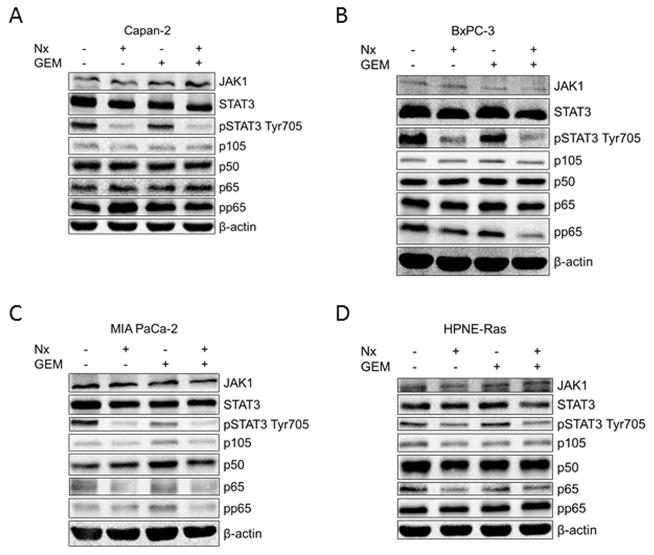
Since our published findings showed that Nx inhibits pancreatic cancer cell growth by inhibiting STAT3 and NF-κB signaling, we examined whether Nx plus GEM mediated growth inhibitory effects are due to changes in STAT3/NF-κB signaling molecule expression and activity. Whole cell extracts prepared from various treatment regimens were used in immunoblot analysis with antibodies against JAK1, STAT3, phosho-STAT3 (pSTAT3), p105, p50, p65, and phospho-p65 (pp65). Cumulative analyses of these data indicate that Nx alone or Nx-GEM combination, but not GEM alone significantly reduced levels of pSTAT3 in Capan-2, BxPC-3 and HPNE-Ras cells. Furthermore, we observed a significant decrease in the levels of pp65 in BxPC-3 cells in response to combination treatment. It is noteworthy to mention that GEM treatment induced p105, p50 and pp65 in MIA PaCa-2 cells (despite the low levels of pp65 in this cell line); while the combination treatment did not induce these proteins. We observed notable changes in JAK1 both in Nx and Nx plus GEM conditions in MIA PaCa-2 as well as in the GEM alone condition for BxPC-3 cells (Fig. 2). Quantification of the western blot signaling data from Fig. 2 is shown in supplemental figures S1A – S1D.
Fig. 2. Nx/GEM combination effect on the STAT3/NF-κB signaling axis.
Capan-2 (A), BxPC-3 (B), MIA PaCa-2 (C), and HPNE-Ras (D) cells were treated for 24h with either media alone, Nx (Capan-2 = 50 μg/mL; BxPC-3 = 20 μg/mL; MIA PaCa-2 = 20 μg/mL; and HPNE-Ras = 20 μg/mL), 0.5 μM GEM, or Nx-GEM combination (Capan-2 = 50 μg/mL; BxPC-3 = 20 μg/mL; MIA PaCa-2 = 20 μg/mL; and HPNE-Ras = 20 μg/mL + 0.5 μM GEM). Whole cell extracts were prepared following 24h treatment and assessed for changes to the STAT3/NF-κB signaling axis by Immunoblot analysis. A representative image from three independent experiments is shown.
GEM resistant pancreatic cancer cells with oncogenic KRAS are more sensitive to Nx than their parental cells
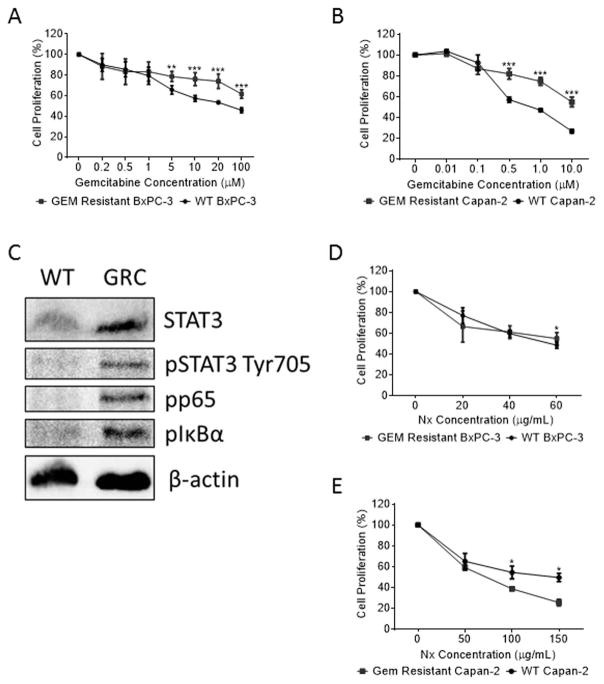
Due to the high preponderance of GEM resistance seen in patients undergoing chemotherapy and our findings that Nx enhanced the effect of GEM, we sought to determine the effects of Nx on GEM resistant cells. As can be seen in Figs. 3A and 3B, GRB and GRC cells bear a significantly higher level of tolerance to GEM as compared to their WT parental cell lines. Western blot analysis of GRC cells compared to WT Capan-2 cells showed higher levels of phosphorylated p65 and IκBα as well as both total and phosphorylated STAT3 (Fig. 3C). Collectively, these data suggest that the GRC cells have increased STAT3/NF-κB inflammatory signaling. Furthermore, Nx was able to inhibit cellular proliferation of GRB cells at levels equivalent to that of WT BxPC-3 cells (Fig. 3D). Intriguingly, Nx exhibited a much stronger effect on GRC cells than on the WT Capan-2 cells (Fig. 3E). The difference in Nx sensitivity between the two cell lines could possibly be related to their KRAS status as BxPC-3 cells express WT KRAS and Capan-2 express mutant KRASG12D. Though there are a number of possibilities for the development of GEM resistance in the GRB and GRC cell lines, our data suggests that STAT3/NFκB inflammatory signaling may be involved.
Fig. 3. Characterization of Gem Resistant cells.
GEM resistant BxPC-3 (A) and Capan-2 (B) cells were treated with indicated doses of gemcitabine (GEM) for 24h and proliferation was measured as described in methods. (C) Whole cell lysates prepared from Capan-2 WT and GEM resistant GRC cells were assessed for alterations in STAT3, pSTAT3, pp65, pIκBα and β–actin using Western blot analysis. (D & E) GEM resistant BxPC-3 and Capan-2 cells were treated with indicated doses of Nexrutine (Nx) for 24h and proliferation was measured as described in methods. Data is presented as average±SD from three independent experiments conducted in triplicate. P values: * <0.05; ** <0.001; *** <0.0005.
Berberine and its derivatives, Ber 2-4 & Ber 2-8, inhibit PanCA cell proliferation
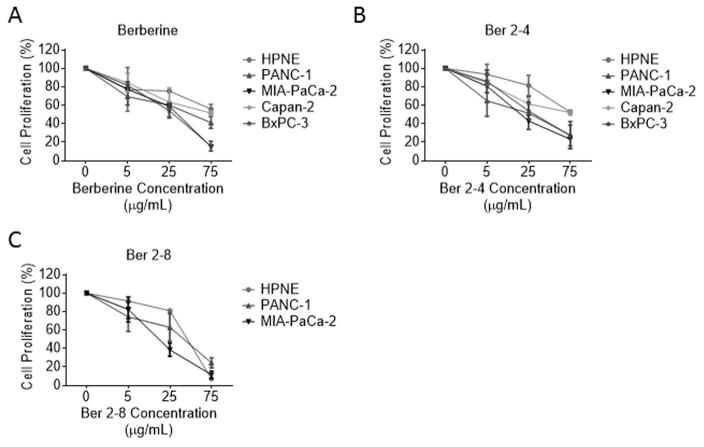
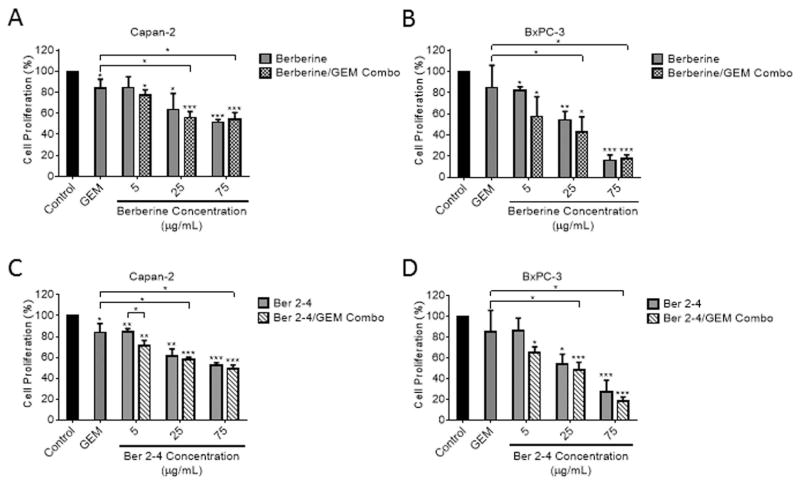
Previous biochemical fractionation studies showed berberine and palmatine as some of the major active components associated with the biological activity of Nx [26]. Here we tested, if berberine or some of its derivatives could recapitulate the Nx-induced combinatorial growth inhibitory effects [27–32]. Chemical structures of Ber and its derivatives are shown in Fig. S2. We observed significant dose-dependent inhibition of proliferation in all cell lines with MIA PaCa-2 and BxPC-3 cells being the most sensitive to Ber treatment compared to untreated cells (Fig. 4A). Furthermore, the berberine derivatives, Ber 2-4 and Ber 2-8, demonstrated strongly significant dose dependent inhibition of proliferation in PanCA cell lines. The most interesting finding between these two Ber derivatives and Ber however, was the notable difference in proliferation inhibition between pancreatic cancer cell lines and HPNE cells treated with Ber 2-4 (Fig. 4B). This difference is discernable at the 25 μg/mL and 75 μg/mL Ber 2-4 doses. At the median dose of Ber 2-4 tested (25 μg/mL), all cell lines displayed significantly higher proliferation inhibition than HPNE cells (p values < 0.05, Fig. 4B). Only Capan-2 cells at the highest Ber 2-4 dose (75 μg/mL) were not significantly less inhibited than HPNE cells (p values for other cell lines were < 0.00001). A summary of the Ber derivative structures along with their respective IC20 and IC50 doses for all cell lines is shown in Table 1. Based on the data presented in figures 1C and 1D, regarding Nx-mediated synergy when combined with 0.5 μM GEM, we tested whether Ber or Ber 2-4 would also have an additive effect when combined with GEM. As such, we treated Capan-2 and BxPC-3 cells with a range of Ber or Ber 2-4 doses in combination with 0.5 μM GEM for 24h. Our results show that although combination of Ber or Ber 2-4 with GEM inhibits proliferation, the observed effects were not statistically significant compared to monotherapy (GEM or individual compounds alone; Figs. 5 A–D). These data suggest that although Ber and Ber 2-4 are able to effectively inhibit PanCA cell proliferation, there is no additive effect to combining the compounds with 0.5 μM GEM. Taken together, these data suggest that Nx, as a whole extract, may be better than individual components in potentiating GEM activity in these cells.
Fig. 4. Derivatives of the Nx active component, berberine, are able to inhibit pancreatic cell proliferation.
Human pancreatic cell lines (HPNE, PANC-1, MIA-PaCa-2 Capan-2 and BxPC-3) were treated with different doses of Berberine (A), Berberine derivatives Ber 2-4 (B) and Ber 2-8 (C) for 24hr. Cell proliferation was determined by MTT assay essentially as descried for Fig 1. Data is presented as average±SD from three independent experiments conducted in triplicate.
Table 1.
| ||||
|---|---|---|---|---|
| Compound | R-group | Salt | Approx. IC20 | Approx. IC50 |
| Berberine | None | 5 μg/mL (HPNE) 2.5 μg/mL (Panc-1) 5 μg/mL (MIA PaCa-2) 5 μg/mL (Capan-2) 5 μg/mL (BxPC-3) |
75 μg/mL (HPNE) 50 μg/mL (Panc-1) 25 μg/mL (MIA PaCa-2) 75 μg/mL (Capan-2) 25 μg/mL (BxPC-3) |
|
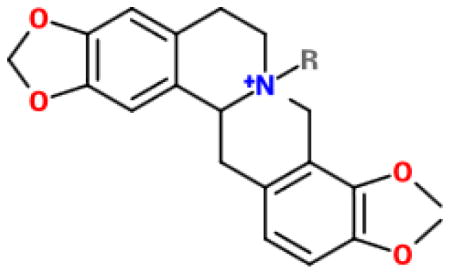
| Ber 2-4 |
|
Br- | 25 μg/mL (HPNE) 1 μg/mL (Panc-1) 10 μg/mL (MIA PaCa-2) 5 μg/mL (Capan-2) 5 μg/mL (BxPC-3) |
75 μg/mL (HPNE) 5 μg/mL (Panc-1) 30 μg/mL (MIA PaCa-2) 75 μg/mL (Capan-2) 25 μg/mL (BxPC-3) |
| Ber 2-8 |
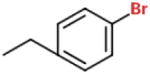
|
Br- | 25 μg/mL (HPNE) 5 μg/mL (Panc-1) 5 μg/mL (MIA PaCa-2) |
50 μg/mL (HPNE) 50 μg/mL (Panc-1) 20 μg/mL (MIA PaCa-2) |
Fig. 5. Effect of Ber and Ber 2-4 combination treatment with gemcitabine.
Capan-2 (A and C) and BxPC-3 cells (B and D) were treated with the indicated doses of Ber (A and B), Ber 2-4 (C and D) in the presence or absence of 0.5 μM GEM for 24. Following 24h incubation, cell proliferation was measured as described in method. Data is presented as average±SD from three independent experiments conducted in triplicate. P values were: * < 0.05, ** < 0.001, and *** < 0.0005.
Discussion
Despite recent advancements to PanCA treatment, the longevity of patients has been improved by only a matter of months, depending on the treatment regime used [5–7]. Even with these small advances, resistance to treatment remains an unfortunately common occurrence. For those patients receiving GEM alone, resistance can rapidly occur. Natural products such as curcumin and unsaturated vitamin E have been indicated as chemo-sensitizers, which act through inhibiting inflammation by downregulation of NF-κB and STAT3 activity [33–35]. We previously established the ability of Nx to inhibit STAT3/NF-κB signaling [13]. Our current study sought to address drug resistance by highlighting the potential of Nx as an adjuvant to GEM treatment. Based off our collective results from Figs. 1–3, we determined that Nx is able to enhance the cytotoxic effects of GEM and that this likely occurs due to the ability of Nx to inhibit inflammatory signaling via downregulation of STAT3 and NF-κB activity. Although GEM resistance is generally associated with alterations in hENT1, dCK, MUC4, and RR expression, it is well established that GEM can enhance the production of pro-inflammatory signaling molecules like NF-κB and various cytokines [3,4,36,37]. Furthermore, pancreatic cancer cells that have reduced levels of STAT3 display increased sensitivity to GEM, thereby linking STAT3 activity to GEM resistance [38]. Collectively, these studies indicate that inflammatory signaling leads to the induction of a milieu of pro-survival and anti-apoptotic processes, which reduce sensitivity to GEM. Further, NF-κB has been linked to hENT1 regulation and MUC4-mediated GEM resistance was linked to NF-κB activation of pro-survival and anti-apoptotic pathways [4,39]. In addition, analysis of patient samples suggests that GEM resistance may also occur through induction of NF-κB via the pro-inflammatory cytokines, TNF-α and IL-1β [36]. Furthermore, STAT3 has been indicated as an inducer of MUC4 expression leading to chemo resistance in a gastric cancer model [40,41]. The increased levels of STAT3 in our GEM resistant Capan-2 cell line suggests that a STAT3/MUC4 mediated resistance could be involved in pancreatic cancer as well. However, more detailed experiments such as determining MUC4 expression in response to IL-6/STAT3 stimulation and inhibition should be done to confirm this hypothesis. Collectively, these data indicate that inflammation could be targeted for treatment in conjunction with chemotherapy to develop more efficacious PanCA treatment strategies. Our data also indicates that GEM resistant pancreatic cancer cells bearing oncogenic KRAS mutation are more sensitive to Nx than their parent (GEM sensitive) cell line, while resistant cells with a WT KRAS did not show any modified effect compared to their parental line. The selectivity exhibited by Nx in targeting GEM resistant KRAS mutant cell lines as well as the synergistic activity between Nx and GEM on PanCA cell lines is very promising. Though a more detailed analysis of why the KRAS mutant GEM resistant cells were more sensitive to Nx needs to be conducted, our data suggests that the enhanced sensitivity may be due to increased levels of inflammatory signaling molecules like STAT3 in the resistant cell line.
In addition to our investigation of Nx-GEM combination, we set out to identify if Ber (one of the active components in Nx) was the source of the Nx-mediated effects on pancreatic cells by examining Ber and some of its derivatives. In general, berberine inhibition of pancreatic cells correlates to other studies indicating Ber toxicity at higher levels and also suggests that Ber may be responsible for some of the Nx mediated effects on PanCA cells. Next to Ber, its derivatives (Ber 2-4 and Ber 2-8) showed potential as anti-cancer therapeutics (Fig. 4B & 4C). Moreover, Ber 2-4 exerted stronger anti-proliferative effects on cancer cell lines than on the “normal” cell line, HPNE. This difference in proliferative inhibition suggests that Ber 2-4 may be less toxic to normal pancreatic cells. The implications of this variance between the “normal” cell line and both cancer and tumor promoting cell lines are very promising. However, we were unable to establish synergy between Ber 2-4 and GEM. Our inability to detect synergy was likely due to our experimental design, as we did not fully assess the potential of Ber 2-4 like we did Nx. If we had tested Ber 2-4 the same way we tested Nx in Figs. 1 and 2 we would have been able to conclusively determine whether this Ber derivative is able to act synergistically with GEM. Current studies in our lab are seeking to address this topic.
The collective results of this study suggest that Nx carries significant potential for translation into the clinic. This greater potential is due to its ability to exert synergistic anti-cancer effects on PanCA cell lines when combined with GEM and its ability to inhibit the proliferation of GEM resistant cell lines. Though these results along with the previous clinical trial in prostate cancer patients provide strong support for the use of Nx in PanCA, our study was limited by the lack of in vivo data from animal models. Nevertheless, these results provide evidence for the need to conduct more in-depth investigations to understand the mechanism associated with GEM-resistance, evaluating combinatorial effects of Nx plus GEM including selectivity towards KRAS using mutant cell lines and preclinical animal models.
Supplementary Material
(A–D) Quantification of Western blot data discussed in Fig. 2 using the data from three sample sets for each cell line (P values: * < 0.05).
(A–E) Structures of berberine and derivatives. Compound structures were drawn using the eMolecules chemical structure drawing tool found on their website (https://www.emolecules.com/).
Acknowledgments
This work was supported in part by funds from 1R01AT007448, Veterans Affairs-Merit Award I01 BX 000766-01 and CPRIT RP 150166 (APK). We acknowledge support provided by CTRC at UT Health Science Center San Antonio (UTHSCSA) through the National Cancer Institute support grant #2P30 CA 054174-17 for use of Flow Cytometry core facility. We acknowledge support provided by the CTRC 40th Anniversary Distinguished Professor of Oncology Endowment to APK. This publication was supported in part by funding from the NIGMS-BUILD grant number 8UL1GM118967 and the RCMI grant number 2G12MD007595-06 from the National Institute on Minority Health and Health Disparities (AM) and Louisiana Cancer Research Consortium (FPS).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2006;17(Suppl 5):12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 3.Nakano Y, Tanno S, Koizumi K, et al. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96(3):457–463. doi: 10.1038/sj.bjc.6603559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skrypek N, Duchene B, Hebbar M, Leteurtre E, van Seuningen I, Jonckheere N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family. Oncogene. 2013;32(13):1714–1723. doi: 10.1038/onc.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 6.Moore M, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff D, Ervin T, Arena F, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. The New England journal of medicine. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain S, Patel D, Ghosh R, Kumar A. Extracting the Benefit of Nexrutine® for Cancer Prevention. Curr Pharmacol Rep. 2015;1(6):365–372. doi: 10.1007/s40495-015-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia G, Nicole A, Bhaskaran S, Gupta… A. Akt-and CREB-mediated prostate cancer cell proliferation inhibition by Nexrutine, a Phellodendron amurense extract. Neoplasia. 2006;8(6):523–533. doi: 10.1593/neo.05745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh R, Garcia G, Crosby K, et al. Regulation of Cox-2 by cyclic AMP response element binding protein in prostate cancer: potential role for nexrutine. Neoplasia (New York, NY) 2007;9(11):893–899. doi: 10.1593/neo.07502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh R, Graham H, Rivas P, et al. Phellodendron amurense bark extract prevents progression of prostate tumors in transgenic adenocarcinoma of mouse prostate: potential for prostate cancer management. Anticancer research. 2010;30(3):857–865. [PubMed] [Google Scholar]
- 12.Gong J, Muñoz AR, Chan D, Ghosh R, Kumar AP. Stat3 down regulates LC3 to inhibit autophagy and pancreatic cancer cell growth: Role of NexrutineR. Oncotarget. 2014;5(9):2529–2541. doi: 10.18632/oncotarget.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong J, Xie J, Bedolla R, et al. Combined Targeting of STAT3/NF-κB/COX-2/EP4 for Effective Management of Pancreatic Cancer. Clin Cancer Res. 2014;20(5):1259–1273. doi: 10.1158/1078-0432.CCR-13-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan G, Lanza-Jacoby S, Wang C. Nexrutine inhibits survival and induces G1 cell cycle arrest, which is associated with apoptosis or autophagy depending on the breast cancer cell line. Nutr Cancer. 2014;66(3):506–516. doi: 10.1080/01635581.2013.780627. [DOI] [PubMed] [Google Scholar]
- 15.Alam S, Yadav RS, Pal A, et al. Dietary administration of Nexrutine inhibits rat liver tumorigenesis and induces apoptotic cell death in human hepatocellular carcinoma cells. Toxicology Reports. 2015;2:1–11. doi: 10.1016/j.toxrep.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alam S, Pal A, Kumar R, Mir SS, Ansari KM. Nexrutine inhibits azoxymethane-induced colonic aberrant crypt formation in rat colon and induced apoptotic cell death in colon adenocarcinoma cells. Molecular Carcinogenesis. 2015 doi: 10.1002/mc.22368. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 17.Kumar R, Das M, Ansari K. Nexrutine(R) inhibits tumorigenesis in mouse skin and induces apoptotic cell death in human squamous carcinoma A431 and human melanoma A375 cells. Carcinogenesis. 2012;33(10):1909–1918. doi: 10.1093/carcin/bgs219. [DOI] [PubMed] [Google Scholar]
- 18.Hambright HG, Meng P, Kumar AP, Ghosh R. Inhibition of PI3K/AKT/mTOR axis disrupts oxidative stress-mediated survival of melanoma cells. Oncotarget. 2015;6(9):7195–7208. doi: 10.18632/oncotarget.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swanson GP, Jones WE, 3rd, Ha CS, Jenkins CA, Kumar AP, Basler J. Tolerance of Phellodendron amurense bark extract (Nexrutine(R)) in patients with human prostate cancer. Phytother Res. 2015;29(1):40–42. doi: 10.1002/ptr.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pingali S, Donahue JP, Payton-Stewart F. Tetrahydroberberine, a pharmacologically active naturally occurring alkaloid. Acta Crystallogr C Struct Chem. 2015;71(Pt 4):262–265. doi: 10.1107/S2053229615004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang P, Song DQ, Li YH, et al. Synthesis and structure-activity relationships of berberine analogues as a novel class of low-density-lipoprotein receptor up-regulators. Bioorg Med Chem Lett. 2008;18(16):4675–4677. doi: 10.1016/j.bmcl.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Bera A, VenkataSubbaRao K, Manoharan MS, Hill P, Freeman JW. A miRNA signature of chemoresistant mesenchymal phenotype identifies novel molecular targets associated with advanced pancreatic cancer. PloS one. 2014;9(9):e106343. doi: 10.1371/journal.pone.0106343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hambright HG, Batth IS, Xie J, Ghosh R, Kumar AP. Palmatine inhibits growth and invasion in prostate cancer cell: Potential role for rpS6/NFkappaB/FLIP. Molecular Carcinogenesis. 2014 doi: 10.1002/mc.22192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in enzyme regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 25.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 26.Muralimanoharan S, Kunnumakkara A, Shylesh B, et al. Butanol fraction containing berberine or related compound from nexrutine inhibits NFkappaB signaling and induces apoptosis in prostate cancer cells. The Prostate. 2009;69(5):494–504. doi: 10.1002/pros.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kai K, Cecilia AB, Henri JH. Mitochondria and NMDA Receptor-Dependent Toxicity of Berberine Sensitizes Neurons to Glutamate and Rotenone Injury. PLoS ONE. 2014 doi: 10.1371/journal.pone.0107129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kheir MM, Wang Y, Hua L, et al. Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem Toxicol. 2010;48(4):1105–1110. doi: 10.1016/j.fct.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Ma BL, Ma YM, Shi R, et al. Identification of the toxic constituents in Rhizoma Coptidis. J Ethnopharmacol. 2010;128(2):357–364. doi: 10.1016/j.jep.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 30.Margherita C, Simone F, Cristina F, et al. Berberine behind the thriller of marked symptomatic bradycardia. World Journal of Cardiology. 2013 doi: 10.4330/wjc.v5.i7.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin KS, Choi HS, Zhao TT, et al. Neurotoxic effects of berberine on long-term L-DOPA administration in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Arch Pharm Res. 2013;36(6):759–767. doi: 10.1007/s12272-013-0051-4. [DOI] [PubMed] [Google Scholar]
- 32.Yin J, Ye J, Jia W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharmaceutica Sinica B. 2012;2(4):327–334. [Google Scholar]
- 33.Kunnumakkara A, Sung B, Ravindran J, et al. {Gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer research. 2010;70(21):8695–8705. doi: 10.1158/0008-5472.CAN-10-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lev-Ari S, Vexler A, Starr A, et al. Curcumin Augments Gemcitabine Cytotoxic Effect on Pancreatic Adenocarcinoma Cell Lines. Cancer Investigation. 2007;25(6):411418. doi: 10.1080/07357900701359577. [DOI] [PubMed] [Google Scholar]
- 35.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. The AAPS journal. 2012;15(1):195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delitto D, Black BS, Sorenson HL, et al. The inflammatory milieu within the pancreatic cancer microenvironment correlates with clinicopathologic parameters, chemoresistance and survival. BMC Cancer. 2015;15:783. doi: 10.1186/s12885-015-1820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arlt A, Gehrz A, Müerköster S, et al. Role of NF-κB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22(21):3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 38.Venkatasubbarao K, Peterson L, Zhao S, et al. Inhibiting signal transducer and activator of transcription-3 increases response to gemcitabine and delays progression of pancreatic cancer. Molecular cancer. 2013;12(1):104. doi: 10.1186/1476-4598-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montero TD, Racordon D, Bravo L, Owen GI, Bronfman ML, Leisewitz AV. PPARα and PPARγ regulate the nucleoside transporter hENT1. Biochemical and Biophysical Research Communications. 2012;419(2):405–411. doi: 10.1016/j.bbrc.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 40.Mejías-Luque R, Peiró S, Vincent A, Van Seuningen I, de Bolós C. IL-6 induces MUC4 expression through gp130/STAT3 pathway in gastric cancer cell lines. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2008;1783(10):1728–1736. doi: 10.1016/j.bbamcr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 41.Li G, Zhao L, Li W, et al. Feedback activation of STAT3 mediates trastuzumab resistance via upregulation of MUC1 and MUC4 expression. Oncotarget. 2014;5(18):8317–8329. doi: 10.18632/oncotarget.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A–D) Quantification of Western blot data discussed in Fig. 2 using the data from three sample sets for each cell line (P values: * < 0.05).
(A–E) Structures of berberine and derivatives. Compound structures were drawn using the eMolecules chemical structure drawing tool found on their website (https://www.emolecules.com/).