Abstract
Hepatocytes are highly differentiated epithelial cells that lose their phenotype and function when removed from the in vivo environment. Given the importance of hepatic cultures for drug toxicity, bioartificial liver assist devices and basic biology studies, considerable efforts have been focused on maintenance of hepatic function in vitro. The methods used to date include co-cultivation of hepatocytes with stromal cells, organizing these cells into spheroids and imbedding them into bioactive gels. Our team has recently demonstrated that primary rat hepatocytes confined to microfluidic channels in the absence of convection maintained epithelial phenotype through upregulation of endogenous signals including hepatocyte growth factor (HGF). The objective of the present study was to transition from microfluidic devices, which are somewhat specialized and challenging to use and towards low volume multiwell plates ubiquitous in the biology laboratories. Using a combination of 3D printing and micromolding we have constructed inserts that could be placed into standard 12-well plates and could be used to create low volume culture conditions under where primary hepatocytes maintained differentiated phenotype. This phenotype enhancement was confirmed by assays including albumin synthesis and expression. Importantly we confirmed upregulation of HGF inside the low volume culture plates and demonstrated that inhibition of HGF signaling degraded hepatic phenotype in our cell culture platform. Overall, this study outlines a new cell culture system that leverages low volume effects of microfluidic channels in a multiwell plate format. Beyond hepatocytes, such system may be of use in maintenance of other difficult-to-culture cells including stem cells and primary cancer cells.
Introduction
The liver is the primary organ responsible for drug metabolism. It is therefore essential to ascertain liver metabolism and toxicity in the process of developing new drugs 1, 2. There has been considerable interest over the years in establishing hepatocyte cultures liver surrogates. These efforts have been confounded somewhat by the fact that primary hepatocytes rapidly de-differentiate in vitro, losing epithelial phenotype and hepatic function. As a way to rescue hepatic phenotype, the liver biology community has introduced various culture formats attempting to mimic in vivo liver microenvironment, from hepatocyte spheroids to collagen gel sandwiches to co-cultures with stromal cells 3-11.
Our lab has recently pointed to a less studied aspect of liver microenvironment that may be important to integrate with in vitro cultures. Confining hepatocytes to small volume microfluidic cultures under conditions of minimal flow (<0.8μl/h) dramatically enhanced hepatic phenotype 12. This enhancement was shown to be the function of local volume or cell-to-volume ratio and was in part driven by the accumulation of endogenous hepato-inductive signals including HGF 12. It is worth noting that effects of low volume cultures are not limited to hepatocytes and have been observed by us recently for stem cells as well as cancer cells 13, 14. However, microfluidic channels used in these previous reports leave something to be desired in terms of simplicity of use, throughput and scale-up.
In the present study, a combination of 3D printing and micromolding was employed to fabricate polymer inserts with microstructured surfaces. These devices functioned in a manner similar to transwell inserts and were compatible with standard tissue culture plates. In addition to fabricating inserts, we characterized phenotype of hepatocytes, demonstrating that these cells remained epithelial and functional. Furthermore, similar to our microfluidic cultures, hepatocytes in low volume culture plates upregulated expression of HGF and were negatively impacted when HGF signaling was inhibited. The insert technology described in this study moves us closer to developing a biologist-friendly culture plates for harnessing endogenous signals from hepatocytes and other difficult-to-maintain cells.
Materials and Methods
Fabrication of multi-well plate inserts
Utilizing 3D printing technology and basic soft-lithography technique, inserts were fabricated in three steps.
Step I (3D printing the support structure)
The 3D printed support structure (Fig. S1) was designed in Autodesk Fusion 360 and printed at the TEAM facility (UC Davis) using PolyJet printing technology (Object260 Connex3). MED610, a biocompatible and transparent PolyJet photopolymer, was used for all tasks involving 3D printing.
Step II (Fabricating the master mold)
A master mold used to embed the PDMS layer into the 3D printed support structure was fabricated in two steps (Fig. S1b). First, following manufacture instructions (Microchame Corp), 75 μm thick features were micropatterned on a silicon wafer (University Wafer) by exposure of SU-8 2050 (Microchem Corp) to UV light through a photomask (CAD/Art Services). Following SU-8 pattering, a 3D printed retainer (MED610) was irreversibly bonded on the silicon wafer using super glue (Loctite) while encircling the SU-8 pattern as shown in Fig. S1b. This retainer was essential for ensuring correct placement of support structure on the SU8 pattern in step III.
Step III (Molding culture chamber and transport channels in PDMS)
The 3D printed support structure (Fig. S1a) was coupled with the master mold fabricated in Step II (see Fig. S1b). Polydimethylsiloxane (PDMS; Dow Corning) prepolymer was mixed at 10 to 1 ratio of the base to the curing agent and was dispensed onto the SU-8 pattern through the hollow opening of the 3D printed support using a 1mL syringe (BD). PDMS was then degassed for 30 min and baked at 70°C for 80 min. The assembled insert, comprised of the 3D printed support structure and the PDMS layer, was then gently removed from the master mold. To ensure connectivity of the transport channels and the media reservoirs, any remaining PDMS overhang at the bottom of the insert was removed using a No. 11 scalpel. Inserts were rendered hydrophilic by treatment with oxygen plasma and then were placed in PBS to maintain hydrophilicity. Prior to seeding cells, inserts were sterilized using 70% ethanol and UV exposure in a tissue culture hood for 30 min, and then were washed twice with PBS and once with culture media.
When fabricating inserts with 1 mm tall cell culture chamber, steps I and III were identical to those described previously. A step II was modified in the following manner. First a 75 μm thick SU-8 master mold was fabricated and then the height of the cell culture chamber was adjusted by bonding a 1 mm thick, 8 mm diameter 3D printed disc over the top of the SU-8 pattern using super glue (Fig. S1b).
Fabrication of cell seeding inserts
A reservoir for seeding cells was designed in AutoCAD (Autodesk Fusion 360) and 3D printed at the TEAM facility (UC Davis) utilizing a biocompatible PolyJet photopolymer (MED610). A thin layer of PDMS membrane was incorporated to the bottom section of the reservoir by PDMS partial curing technique. First, mixture of 10:1 ratio of the base to curing agent was poured onto a clean silicon wafer and degassed for 30 min, followed by 20 mins baking at 70°C. The 3D printed reservoir was then placed on top of the partially cured PDMS layer and baked for additional 1 h. Using scalpel (Thermo Scientific), the reservoir was removed from the silicon wafer, with a thin PDMS layer reversibly bounded to the 3D printed support. Cell seeding reservoirs were sterilized with 70% ethanol and exposure to UV for 30 min prior to cell culture.
Cultivation of primary hepatocytes in low volume inserts
Isolation and purification of primary hepatocytes from adult female Lewis rats (Charles River Laboratories) was carried out as described previously in the literature15. On average, 100 to 120 million primary hepatocytes were isolated with above 90% viability. Hepatocytes were cultured in DMEM (Dublecco's Modified Eagle Medium; Gibco), supplemented with 1% (v/v) penicillin-streptomycin (Invitrogen), 10% FBS (Invitrogen), 0.5 U/mL Insulin (Novolin N), 20 ng/mL EGF (Epidermal Growth Factor; Invitrogen), 7 ng/mL Glucagon (Sigma) and 7.5μg/mL hydrocortisone sodium succinate (Prizer).
Seeding of hepatocytes proceeded as follows. Prior to culturing primary hepatocytes, tissue culture plates were coated with 0.1 mg/mL of collagen type I (Life Technology) dissolved in 20mM acetic acid for 1 h at 37 °C. Wells were then washed three times with PBS (Invitrogen) and once with culture media prior to cell culture. The seeding reservoirs were placed into wells of a 12-well plate. These reservoirs provided a way to localize hepatocytes to an 8 mm diameter region in the center of each well. Hepatocytes were resuspended in culture media at concentration of 2.5 × 105 cells/mL, dispensed into cell seeding reservoir and incubated for 1.5 h at 37 °C. Multi-well plates were gently shaken every 30 mins to enhance uniformity of cell seeding. Unattached cells were removed by one-time wash with culture media followed by addition of 1mL of fresh culture media in each well. This protocol typically resulted in attachment of ∼30,000-40,000 cells within an 8 mm diameter region. Following 24 h of culture, low volume inserts were placed into wells so as to register cell culture chambers with 8 mm diameter islands of hepatocytes created in the wells after the seeding step (Fig. 3). The culture media was changed every 48 h for the remainder of the experiment. In the case of HGF inhibition study, 5 μM c-met inhibitor (SU11274, Sigma) was added to the culture media following a day after cell seeding. All animal experiments were performed with the approval of the IACUC (Institutional Animal Care and Use Committee) and in accordance with the Ethical Guidelines for Animal Experimentation of UC Davis (University of California, Davis).
Figure 3. Seeding cells and setting up low volume culture conditions.
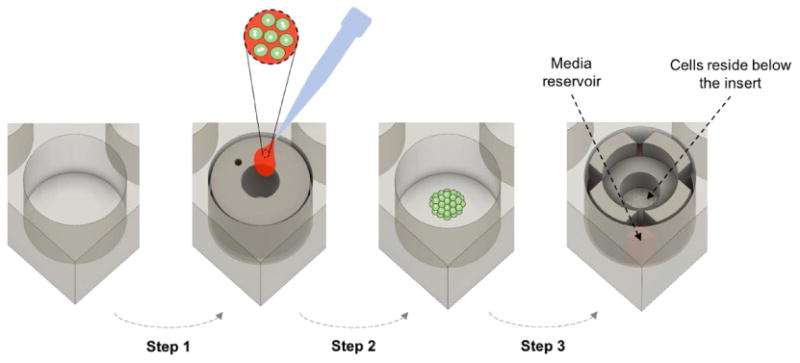
Schematic representation of main steps in utilizing the multiwell plate insert for cell culture. Step 1: seeding insert is placed in a well, coated with collagen and incubated with hepatocytes. Step 2: seeding insert is removed, well is washed and hepatocytes are incubated for 24 h. Step 3: culture insert is placed atop hepatocytes and low volume cultures commence.
Creating co-cultures of hepatocytes and mouse embryonic fibroblasts (MEFs)
The co-cultures were created by placing hepatocytes at the bottom of the well and MEFs on the roof of the insert. As shown in Fig. 6a, cell seeding reservoir was utilized to facilitate culturing MEFs on the PDMS roof of the insert. First, PDMS gasket of the reservoir and the PDMS roof of the insert were reversibly coupled during the cell seeding process (Fig.6a & S5a). Leakage was prevented through this bounding (Fig. S5a) and employing the reservoir restricted cell attachment to the PDMS ceiling of cell culture chamber at the center of the insert (8 mm dimeter) (Fig. 6a). Prior to culturing the growth-arrested MEFs (CF-1 mouse embryonic fibroblasts; MTI-GlobalStem), PDMS roof of the insert was first coated with collagen type-I (0.1 mg/mL) for 1.5 h at 37°C while employing the reservoir (Fig. 6a). MEFs were suspended in hepatocyte culture media at the concentration of 250,000 cells/mL and dispensed into seeding reservoir coupled with the insert followed by a 2 h incubation at 37°C. After removal of unattached cells by one time wash with fresh culture media, attached fibroblasts on the roof of the insert were incubated with hepatocyte culture media for 24 h in a 12 well plate (Fig. 6a). Using a new cell seeding reservoir, an 8 mm diameter island of hepatocytes was seeded in a collagen coated tissue culture well. As shown in Fig. 6a, following an overnight incubation at 37°C, insert with MEFs cultured on its ceiling was coupled with the well plate housing the 8 mm dimeter island of primary hepatocytes. 1 mL fresh hepatocyte culture media was distributed through the reservoirs and media was exchanged every 48 h. This physically segregated co-culture continued for one week while the cells resided in close proximity (Fig. 6b).
Figure 6. Creating co-cultures of hepatocytes and fibroblasts in low volume culture plates.

(a) 3D schematic demonstrating the process of culturing cells on the ceiling of the insert for the co-culture purposes. Step1: cell seeding reservoir is coupled with the insert and fibroblasts in suspension are dispensed into the reservoir. Step2: unattached cells are removed by one time wash with fresh culture media. Step3: insert with cultured fibroblasts on its roof is incubated with fresh culture media in a 12 well plate. Step4: 8 mm dimeter island of hepatocytes is seeded in a clean well plate. Step5: insert is coupled with the well housing the hepatocytes and fresh culture media is added. (b) 2D schematic illustrating position of fibroblasts and hepatocytes in the co-culture setting of multiwell plate insert. (c) Bright-field images of hepatocytes and fibroblasts in the co-culture setting on day 7. (d) RT-PCR analysis of albumin gene expression for hepatocytes cultured under mono and co-culture setting in the multi well plate insert on day 7. Data shown are mean ± standard deviation (n=2) for each sample type. *p<0.05. Scale bar=50μm.
ELISA
Albumin secretion rate was measured using commercially available ELISA assay (Bethyl Laboratories). Culture media was collected every 48 h and secretion rates were normalized by the cell number. ImageJ was used to estimate the number of cells for each sample after 7 days of culture.
Immunofluorescent staining of cells
Primary rat hepatocytes cultured in collagen coated wells and the mouse embryonic fibroblasts (MEFs) cultured on the PDMS membrane of the inserts were fixed separately. Prior to fixing, culture media was removed and each sample was washed once with PBS. Cells were then fixed and permeabilized simultaneously at room temperature for 15 mins by a 4% paraformaldehyde solution (Electron Microscopy Sciences) containing 0.2% Triton-X100 (Invitrogen) in PBS. Samples were then washed three times with PBS to remove fixate solution prior to incubation with the blocking solution (1% bovine serum albumin in PBS) for 1 h at room temperature. The excess of blocking solution was then removed by washing in PBS three times at room temperature. Subsequently, cells were incubated for 90 min at room temperature with the first antibody (Sheep anti-rat albumin; 1:100; Bethyl lab Inc.). After washing off primary antibody with PBS, the secondary antibody was introduced and incubated at room temperature for 1 h (Alexa-488 donkey anti-sheep IgG; 1:1000; Invitrogen). Lastly, wells were washed with PBS and stored in 4 °C prior to imaging. All antibodies were diluted in 1% BSA. Staining of cell nuclei proceeded according to the same permeabilization protocol, which was followed by incubation with DAPI (1:1000, Invitrogen). Stained cells were imaged using a laser scanning confocal microscopy (LSM700, Carl Zeiss, Jena, Germany).
Quantitative real-time PCR
To remove hepatocytes from tissue culture wells, cells were incubated with 0.05% trypsin-EDTA (Gibco) for 5 min at 37 °C. Total RNA was then harvested from the dissociated cells according to the manufacturer's instruction (Roche). cDNA was obtained through reverse transcription of total mRNA and RT-qPCR was carried out using universal SYBER Green master (Roche). Using the comparative threshold cycle method, relative expression levels for each gene was calculated with GAPDH (glyceraldehyde 3-phophate dehydrogenase) used as the housekeeping gene. Forward and reverse primers used for RT-qPCR are listed in Table S1.
COMSOL modeling of HGF accumulation, glucose level and oxygen tension
Numerical simulations were performed as previously described 12 using COMSOL Multiphysics 4.3 software (COMSOL Inc., Los Angeles, CA) to estimate the HGF concentration, glucose level and oxygen tension in the low volume multiwall plate and standard culture system. All utilized parameters, equations and assumptions were listed in our previous study 12 , with an exception of flow rate and cell density. For both culture system, we used the same cell seeding region (r = 4 mm) with same cell density (8.3 × 104 cells/cm2). Also, to simplify numerical simulation, we assumed zero flow in both platforms. Similarly to our microchambers12, the presence of slow flow in our system is explained as the consequence of surface tension differences between media reservoirs and high resistant of transport channels.
Statistical analysis
Minimum two biological duplicates were used for each condition. All error bars represent standard deviation (SD) for biological duplicates. Student t-test was used for statistical analysis. Differences were considered statistically significant at p<0.05.
Results
Design of low volume multiwell plates
The main objective of this study was to convert a microfluidic platform employed by us previously for culturing hepatocytes 12 into a multiwell plate. As shown in Fig. 1, we designed an insert capable of recapitulating a similar low volume cell culture platform within a conventional 12-well plate. Some of the design parameters that were found favorable for microfluidic cultures and were to be recapitulated in multiwell plates included the rate of accumulation of endogenous signals such as HGF (∼12-fold higher than standard plate) as well as oxygenation of cells (∼120 mmHg oxygen tension in low volume plates vs. 50 mmHg in standard plates) 12. Satisfying these design parameters required decreasing the height of the liquid head above the cells from ∼2.5 mm in the case of a standard 12-well plate to ∼75 μm for low volume cultures. This was achieved by molding microscale cell culture chambers and nutrient supply channels in PDMS – a material that is both amenable to precise fabrication by soft lithography and has excellent oxygen transport properties 16 (Fig. 2a). A microstructured PDMS membrane was embedded within a 3D printed support structure composed of MED610 polymer to achieve a snug fit into a well. As show in Fig. 2a, each insert contained a cell culture chamber with surface area of ∼50 mm2 and of volume of ∼4 μl, capable of supporting ∼40,000 cells. Nutrients were to be delivered by diffusion from four reservoirs via a network of transport channels (Fig. 2a-b).
Figure 1. Low volume multi-well plate for harnessing cell-secreted growth factors (GFs).

Top drawing shows the concept of low volume cell cultures where hepatocytes are cultured in shallow chambers and are confined to local volume of ∼4 μL. Bottom drawing shows placement of an insert into a 12-well plate.
Figure 2. Cell culture insert and cell seeding reservoir.
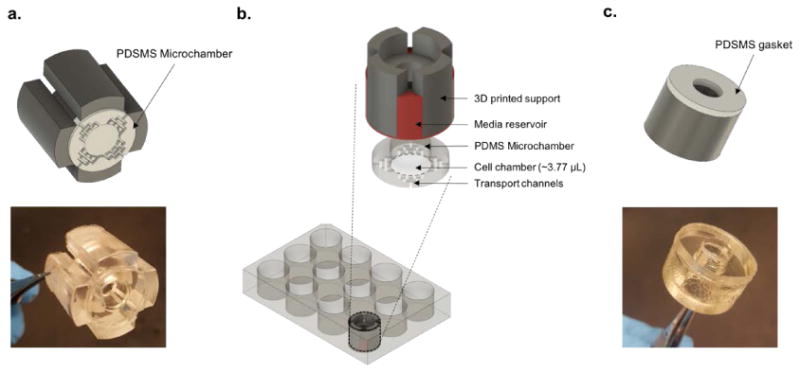
(a) 3D schematic and image of the multiwell plate insert displaying the PDMS based microchamber embedded at the bottom of the 3D printed support structure. (b) 3D schematic illustrating position of the multiwell plate insert with respect to cultured cells. Cells confined in a PDMS based microscale cell chamber at the center of the well. (c) Image of the cell seeding reservoir with the thin PDMS gasket embedded at the bottom of the hollow cylindrical reservoir.
Seeding cells into low volume multi-well plates
We wanted to seed cells selectively into the central region of a well to ensure that when an insert is placed into a well at a later time point it will be in contact with tissue culture plastic and will make an effective seal. In addition, given that paracrine and autocrine signals are amplified in our culture system we wanted to minimize damage to cells and proliferation of distress signals that may negatively impact quality of hepatocyte cultures. To achieve these goals, we designed and 3D printed a hollow cylindrical “cell seeding reservoir” with 8 mm inner diameter (Fig. 2c) using a biocompatible photopolymer (MED610). A thin flat PDMS gasket was embedded at the bottom of the reservoir to ensure a tight seal with the bottom of the well (Fig. 2c). As shown in Fig. 3, once reservoirs were in place, plates were incubated with collagen I and then with hepatocytes. Upon removal of seeding reservoirs, hepatocytes occupied 8 mm diameter region in the center of each well. Low volume inserts were then placed into wells to commence cell culture experiments.
Accumulation of endogenous signals and oxygen transport in low volume cultures
As discussed previously, inserts were designed to enhance local concentration of endogenous signals by shrinking the volume of cell cultures. The height of each insert was 75 μm resulting in confinement of ∼40 thousands of cells in ∼ 4 μL volume (∼ 10000 cells/μL). One should contrast this to a standard 12-well plate where cells reside in ∼2.5 mm of culture media at a 300 cells/μL. Therefore, cell-to-volume ratio in low volume culture plates was over 30 times higher when compared to standard culture plates. The differences in cell-to-volume ratio were expected to translate into much higher local concentrations of secreted GFs. In fact, COMSOL modeling of secretion and accumulation of HGF (shown in Fig. 4a) revealed that this morphogen accumulated rapidly within a low volume insert, reaching concentrations of 0.1 nM at 3 h and 0.8 nM at 24 h. In contrast, in a standard 12-well plate, HGF levels were predicted to reach 0.01 nM and 0.03 nM at 3h and 24 h respectively. Fig. 4a also highlights the fact that low volume inserts resulted in establishment and maintenance of gradients, with HGF concentration ranging from ∼ 0.8 nM in the cell culture chamber to <0.007 nM in the media reservoirs. Such gradients were not expected to be present in a well of standard multi-well plate.
Figure 4. Modeling of HGF and oxygen in low volume cultures.

(a) COMSOL modeling of HGF accumulation in low volume wells vs. standard plates over the course of 24 h. (b) Simulation for oxygen tension at the surface of hepatocytes cultured in standard plate vs. multi-well plate insert after 24 h.
We should note that global or average concentration of HGF was expected to be similar for both low volume and standard volume cultures as shown by modeling results in Fig. S2a-b. However, local concentration in the vicinity of cells was expected to be many fold higher in the case low volume plates.
Another important design criterion was to ensure sufficient delivery of oxygen to primary hepatocytes, which are known to have high oxygen consumption rates 17-19. Using previously reported oxygen consumption rates for hepatocytes we set up consumption-diffusion model in COMSOL to predict oxygen tension at the cell surface. This model demonstrates that hepatocytes in low volume culture plates were expected to be significantly better oxygenated compared to standard plates, with oxygen tension predicted to be 131 mmHg for former and 54 mmHg for latter cases (Fig. 4b).
In addition, we modeled glucose concentration at the cell surface during the 24h of culture period. As described in Fig. S3, the glucose in the low volume well was expected to be in the 3900 mg/ml range – somewhat lower compared to glucose in a standard culture plate (4491 mg/ml) but still within the range reported to be acceptable for hepatocytes 20, 21. Therefore, we expected nutrition transport in our platform to be sufficient for maintenance of such metabolic active cells as hepatocytes.
What about accumulation of waste byproducts of cellular metabolism? While advantages of low volume cultures for harnessing endogenous signals have been highlighted, the buildup of toxic products of cellular metabolism may be of concern. Typical byproducts of hepatic metabolism (carbon dioxide, ammonia, urea) are small molecules with diffusion coefficients two orders of magnitude larger than that of proteins (see Table S2). Therefore, toxic byproducts are expected to diffuse much more rapidly than secreted proteins. Furthermore, silicone rubber comprising the roof of the low volume well is gas permeable and allows for rapid diffusion of gaseous byproducts such as carbon dioxide.
Function of hepatocytes cultured in low volume multi-well plates
Hepatocytes in low volume cultures had more pronounced epithelial phenotype (cobblestone morphology, prominent nuclei and cell borders) compared to cells in standard culture plates (Fig. 5a). Hepatocytes in low volume culture plates were also more functional, producing 10 times more albumin at day 9 and 60 times more at day 11 compared to cells in standard cultures (Fig. 5b). PCR analysis for albumin, CYP3A1 and E-cadherin also revealed that these epithelial/hepatic genes were expressed at much higher level in low volume cell cultures (Fig. 5c).
Figure 5. Characterizing phenotype of hepatocytes and exploring the role of endogenous HGF in low volume culture plates.

(a) Bright field images of primary hepatocytes on day 1 and 7 of culture in low volume and standard plates. (b) Albumin secretion for hepatocytes cultured in a multi-well plate in the presence or absence of low volume inserts. (c) RT-PCR analysis of hepatic and epithelial gene expression in hepatocytes cultured for 11 days under standard and low volume conditions. (d) RT-PCR analysis of HGF gene expression for hepatocytes cultured under standard and low volume conditions. (e) Albumin synthesis for hepatocytes maintained in low volume cultures in presence or absence of c-met (SU11274) and hepatocytes maintained in 1mm height multiwell plate insert. Secretion rates were normalized by cell numbers. All data shown are mean ± standard deviation (n=2) for each sample type. *p<0.05. Scale bar=100μm.
Gene expression of E-cadherin a cell adhesion molecules associated with epithelial cells, was upregulated ∼ 8-fold in low volume hepatocyte cultures whereas cytochrome P450 (CYP)3A1 – one of the enzymes responsible for liver metabolism – was upregulated ∼16-fold in these cultures (Fig. 5c). On the whole, molecular biology analysis of hepatocyte cultures revealed enhancement of hepatic/epithelial phenotype for cells maintained in small volume multiwell plates compared to the conventional 12-well plates.
The role of endogenous HGF in enhancing hepatic phenotype
HGF has been well-established as a potent hepato-inductive signal during liver development and regeneration 22, 23. An important finding of the present study was that low volume culture conditions elicited higher levels of HGF gene expression compared to standard cultures (Fig. 5d). This suggested that HGF may also be involved in the low volume-induced enhancement of hepatic phenotype observed with inserts.
To investigate the importance of HGF, hepatocytes were cultured in low volume plates in the presence of HGF receptor (c-met) inhibitor (SU11274) for 10 days. As seen from Fig. 5e, these cells secreted 3 times less albumin compared to cells cultured under low volume conditions in the absence of c-met inhibitor. It is interesting to note that the presence of c-met inhibitor did not drive albumin secretion down to the level of standard culture plates (see Fig. 5b for example). A plausible explanation for this may be that HGF is not the only morphogen affecting hepatic phenotype under low volume conditions. In fact, we have shown previously that insulin-like growth factor (IGF) and fibroblast growth factor (FGF)-7 are upregulated in microfluidic cultures of hepatocytes12.
In another experiment highlighting the connection between local volume and hepatic function, we fabricated inserts with 1 mm tall cell culture chambers (Fig. S1). This represented ∼13 fold dilution compared to 75 μm tall culture chambers typically utilized in our study. As seen from Fig. 5e, the effect of increasing the local volume was slightly more critical compared to the effects of c-met inhibition in terms of attenuating production of albumin. It should be noted that HGF gene expression for hepatocytes cultured in 75 μm inserts was higher compared to hepatocytes in 1 mm tall inserts (Fig. S4).
Creating hepatocyte-stromal cell co-cultures using inserts
As noted earlier in this paper, methods for enhancing function of hepatocytes in vitro include co-cultivation with stromal cells such as fibroblasts24, 25. To demonstrate flexibility of our technology, we wanted to implement hepatocyte-fibroblast co-cultures with the inserts being developed in this study. To achieve this, growth arrested mouse embryonic fibroblasts (MEFs) were seeded onto a roof of a PDMS insert and, upon placement of an insert into a well, became positioned a short distance away from hepatocytes (Fig. 6a-c & S5). As expected, the presence of MEFs further enhanced hepatic function as highlighted by elevated albumin gene expression level (Fig. 6d). These results suggest that a combination of heterotypic paracrine interactions and low volume cultures may represent a promising application of this technology.
Conclusions
Over the years, liver biology community has devised means to rescue hepatic phenotype and function by culturing these cells in collagen gel sandwiches, 3D spheroids and in co-cultures with stromal cells4, 6, 25, 26. Recently, interest in our laboratory has turned to spatial confinement as a design parameter that may have important implications on cell phenotype and function. Our studies were informed and stimulated by previous reports in the field suggesting that microfluidic channels may provide a different way of culturing cells where endogenous signals may be manipulated and possibly enhanced27-30. Employing microfluidic chambers our lab has previously confirmed that accumulating endogenous signals were the key drivers of phenotype enhancement for a variety of cell types from undifferentiated embryonic stem cells to highly differentiated hepatocytes12, 13.
In this paper, we wanted to adapt low volume culture conditions to a multi-well plate format in order to make this culture system more biologist-friendly. To achieve this, a combination of 3D printing and soft lithography was used to fabricate inserts that could be used to create low volume conditions for primary hepatocytes in standard a 12-well plate. The use of this newly developed low volume inserts resulted in significant improvement of hepatic phenotype and function compared to standard volume culture plates. Inhibition of HGF signaling in low volume cultures led to a decrease in albumin production, thereby establishing a connection between this endogenous morphogen and cell function. In this work we focused on HGF, a well-known hepato-inductive factor; however, accumulation of various endogenous factors could have resulted in our observed function enhancement as suggested in our previous study 12. In addition, we demonstrated that microfabricated inserts may be used to create hepatocyte-fibroblast co-cultures in multi-well plates and that such co-cultures further improve hepatic function. Moving forward, low volume multi-well plates described here may be used for harnessing endogenous signals from primary hepatocytes or other difficult-to-maintain cells. Such multi-well plates may decrease the reliance on expensive exogenous signals (e.g. recombinant growth factors) and may be amenable for scale-up or high-throughput screening experiments.
Supplementary Material
Acknowledgments
We thank Ali Rahimian for help with hepatocytes isolation and Tam Vu for help in confocal imaging. Financial support for this project was provided by NIH (DK107255) and NSF (1403561) grants awarded to AR and from the NSF graduate research fellowship (1650042) awarded to PG.
Footnotes
Conflicts of Interests: The authors declare no conflict of interest.
References
- 1.Ulrich RG, et al. Cultured hepatocytes as investigational models for hepatic toxicity: Practical applications in drug discovery and development. Toxicol Lett. 1995;82-3:107–115. doi: 10.1016/0378-4274(95)03547-8. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Danielsson A, Zern MA. Toxicity of hepatotoxins: new insights into mechanisms and therapy. Expert Opin Investig Drugs. 1999;8:585–607. doi: 10.1517/13543784.8.5.585. [DOI] [PubMed] [Google Scholar]
- 3.Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB J. 1996;10:1471–1484. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 5.Kim M, Lee JY, Jones CN, Revzin A, Tae G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials. 2010;31:3596–3603. doi: 10.1016/j.biomaterials.2010.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riccalton-Banks L, Liew C, Bhandari R, Fry J, Shakesheff K. Long-term culture of functional liver tissue: three-dimensional coculture of primary hepatocytes and stellate cells. Tissue Eng. 2003;9:401–410. doi: 10.1089/107632703322066589. [DOI] [PubMed] [Google Scholar]
- 7.Seo SJ, Kim IY, Choi YJ, Akaike T, Cho CS. Enhanced liver functions of hepatocytes cocultured with NIH 3T3 in the alginate/galactosylated chitosan scaffold. Biomaterials. 2006;27:1487–1495. doi: 10.1016/j.biomaterials.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 9.Dunn JCY, Tompkins RG, Yarmush ML. Long-Term Invitro Function of Adult Hepatocytes in a Collagen Sandwich Configuration. Biotechnol Progr. 1991;7:237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 10.Clement B, et al. Long-term co-cultures of adult human hepatocytes with rat liver epithelial cells: modulation of albumin secretion and accumulation of extracellular material. Hepatology. 1984;4:373–380. doi: 10.1002/hep.1840040305. [DOI] [PubMed] [Google Scholar]
- 11.Ijima H, Matsushita T, Nakazawa K, Fujii Y, Funatsu K. Hepatocyte spheroids in polyurethane foams: Functional analysis and application for a hybrid artificial liver. Tissue Engineering. 1998;4:213–226. [Google Scholar]
- 12.Haque A, et al. Cell biology is different in small volumes: endogenous signals shape phenotype of primary hepatocytes cultured in microfluidic channels. Sci Rep. 2016;6:33980. doi: 10.1038/srep33980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guild J, et al. Embryonic Stem Cells Cultured in Microfluidic Chambers Take Control of Their Fate by Producing Endogenous Signals Including LIF. Stem Cells. 2016;34:1501–1512. doi: 10.1002/stem.2324. [DOI] [PubMed] [Google Scholar]
- 14.Patel D, et al. Microfluidic co-cultures with hydrogel-based ligand trap to study paracrine signals giving rise to cancer drug resistance. Lab Chip. 2015;15:4614–4624. doi: 10.1039/c5lc00948k. [DOI] [PubMed] [Google Scholar]
- 15.Dunn JCY, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 16.Kim MC, Lam RHW, Thorsen T, Asada HH. Mathematical analysis of oxygen transfer through polydimethylsiloxane membrane between double layers of cell culture channel and gas chamber in microfluidic oxygenator. Microfluid Nanofluid. 2013;15:285–296. [Google Scholar]
- 17.Allen JW, Bhatia SN. Formation of steady-state oxygen gradients in vitro: application to liver zonation. Biotechnol Bioeng. 2003;82:253–262. doi: 10.1002/bit.10569. [DOI] [PubMed] [Google Scholar]
- 18.Cho CH, et al. Oxygen uptake rates and liver-specific functions of hepatocyte and 3T3 fibroblast co-cultures. Biotechnol Bioeng. 2007;97:188–199. doi: 10.1002/bit.21225. [DOI] [PubMed] [Google Scholar]
- 19.Kidambi S, et al. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci U S A. 2009;106:15714–15719. doi: 10.1073/pnas.0906820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Na GH, Kim DG, Kim YH, Han JH, Jung ES. Effects of glucose concentration in the medium on rat hepatocyte culture. Ann Surg Treat Res. 2014;87:53–60. doi: 10.4174/astr.2014.87.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thule PM, Liu J, Phillips LS. Glucose regulated production of human insulin in rat hepatocytes. Gene Ther. 2000;7:205–214. doi: 10.1038/sj.gt.3301076. [DOI] [PubMed] [Google Scholar]
- 22.Jones CN, et al. Cultivating hepatocytes on printed arrays of HGF and BMP7 to characterize protective effects of these growth factors during in vitro alcohol injury. Biomaterials. 2010;31:5936–5944. doi: 10.1016/j.biomaterials.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel D, Haque A, Gao Y, Revzin A. Using reconfigurable microfluidics to study the role of HGF in autocrine and paracrine signaling of hepatocytes. Integr Biol (Camb) 2015;7:815–824. doi: 10.1039/c5ib00105f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.March S, et al. Micropatterned coculture of primary human hepatocytes and supportive cells for the study of hepatotropic pathogens. Nat Protoc. 2015;10:2027–2053. doi: 10.1038/nprot.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy G, et al. Long-term culture and expansion of primary human hepatocytes. Nat Biotechnol. 2015;33:1264–1271. doi: 10.1038/nbt.3377. [DOI] [PubMed] [Google Scholar]
- 26.Yarmush ML, et al. Hepatic tissue engineering. Development of critical technologies. Ann N Y Acad Sci. 1992;665:238–252. doi: 10.1111/j.1749-6632.1992.tb42588.x. [DOI] [PubMed] [Google Scholar]
- 27.Paguirigan AL, Beebe DJ. From the cellular perspective: exploring differences in the cellular baseline in macroscale and microfluidic cultures. Integrative Biology. 2009;1:182–195. doi: 10.1039/b814565b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H, Meyvantsson I, Shkel IA, Beebe DJ. Diffusion dependent cell behavior in microenvironments. Lab on a Chip. 2005;5:1089–1095. doi: 10.1039/b504403k. [DOI] [PubMed] [Google Scholar]
- 29.Przybyla L, Voldman J. Probing Embryonic Stem Cell Autocrine and Paracrine Signaling Using Microfluidics. Annual Review of Analytical Chemistry, Vol 5. 2012;5:293–315. doi: 10.1146/annurev-anchem-062011-143122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Przybyla LM, Voldman J. Attenuation of extrinsic signaling reveals the importance of matrix remodeling on maintenance of embryonic stem cell self-renewal. Proc Natl Acad Sci U S A. 2012;109:835–840. doi: 10.1073/pnas.1103100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


