Abstract
We previously showed that, in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease (PD), vaccination with bacillus Calmette-Guerin (BCG) prior to MPTP exposure limited the loss of striatal dopamine (DA) and dopamine transporter (DAT) and prevented the activation of nigral microglia. Here, we conducted BCG dose studies and investigated the mechanisms underlying BCG vaccination’s neuroprotective effects in this model. We found that a dose of 1 × 106 cfu BCG led to higher levels of striatal DA and DAT ligand binding (28% and 42%, respectively) in BCG-vaccinated vs. unvaccinated MPTP-treated mice, but without a significant increase in substantia nigra tyrosine hydroxylase-staining neurons. Previous studies showed that BCG can induce regulatory T cells (Tregs) and that Tregs are neuroprotective in models of neurodegenerative diseases. However, MPTP is lymphotoxic, so it was unclear whether Tregs were maintained after MPTP treatment and whether a relationship existed between Tregs and the preservation of striatal DA system integrity. We found that, 21 days post-MPTP treatment, Treg levels in mice that had received BCG prior to MPTP were threefold greater than those in MPTP-only-treated mice and elevated above those in saline-only-treated mice, suggesting that the persistent BCG infection continually promoted Treg responses. Notably, the magnitude of the Treg response correlated positively with both striatal DA levels and DAT ligand binding. Therefore, BCG vaccine-mediated neuroprotection is associated with Treg levels in this mouse model. Our results suggest that BCG-induced Tregs could provide a new adjunctive therapeutic approach to ameliorating pathology associated with PD and other neurodegenerative diseases.
Keywords: Parkinson’s disease, MPTP, immune response, T cells
During the progression of Parkinson’s disease (PD), oxidative stress and other insults promote the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc; Dauer and Przedborski, 2003; Hirsch and Hunot, 2009; Qian et al., 2010). Several treatments can temporarily ameliorate the symptoms of PD, but none can slow the degeneration of dopaminergic neurons. Therefore, the discovery of new therapies that can preserve dopaminergic neurons or their functional capacity would be of great significance for PD disease management.
Numerous studies have shown that vaccination with CNS antigens can induce autoreactive T-cell responses that home to sites of injury in the CNS and inhibit neuronal degeneration in different models of neurological diseases and injury (for review see Schwartz and Cohen, 2000; Nevo et al., 2003). In the course of these studies, we and other investigators have noted that vaccination with complete Freund’s adjuvant (CFA) alone can have a neuroprotective effect (Benner et al., 2004; Jones et al., 2004; Kurkowska-Jastrzebska et al., 2005; Armentero et al., 2006; Yong et al., 2011). The major immunogenic component in CFA is heat-killed Mycobacterium tuberculosis. Bacille Calmette-Guérin (BCG) is an attenuated live bovine tuberculosis bacillus that is closely related to M. tuberculosis and is used in live vaccines against tuberculosis in humans. We therefore tested whether BCG vaccination was neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model. We found that mice vaccinated with BCG prior to MPTP treatment had higher levels of (DA) and dopamine transporter (DAT) ligand binding in the striatum than unvaccinated MPTP-treated mice and that BCG vaccination blocked the MPTP-induced increase in activated microglia in the substantia nigra (Yong et al., 2011). Because BCG has a well-established safety profile in humans, it is an excellent candidate for potential use as adjunctive therapy in those newly diagnosed with PD. We therefore wanted to learn more about the potential impact of BCG dose on the preservation of dopaminergic function in the MPTP model and the mechanisms by which BCG vaccination confers its neuroprotective effect in this model.
Mycobacterium tuberculosis (TB) infection and BCG vaccination have long been known to induce strong proinflammatory T-cell responses. More recently, both M. tuberculosis and BCG have been shown also to induce anti-inflammatory T cell responses, particularly Tregs, which limit inflammatory responses and tissue damage during persistent infection (Guyot-Revol et al., 2006; Ribeiro-Rodrigues et al., 2006; Scott-Browne et al., 2007; Jaron et al., 2008; Li and Shen, 2009; Fedatto et al., 2012). Tregs can suppress the activation and cytokine production of macrophages and microglial cells and have been associated with neuroprotection in some models of neurodegenerative disease (Reynolds et al., 2007, 2010; Walsh and Kipnis, 2011). Additionally, Tregs appear to have beneficial effects in amyotrophic lateral sclerosis (ALS) patients (Beers et al., 2011; Henkel et al., 2013). However, because MPTP is lymphotoxic (Benner et al., 2004), previous studies of Treg mediated-neuroprotection in the MPTP mouse model have utilized Tregs that were adoptively transferred from mice that had not received MPTP (Reynolds et al., 2007, 2010; Beers et al., 2011). It was therefore unclear whether Tregs would be maintained in BCG-vaccinated mice after MPTP treatment and whether Treg levels would be associated with BCG vaccination’s neuroprotective effects. Alternatively, it was possible that BCG’s beneficial effects could be due to the induced immune responses altering DA metabolism or inhibiting the conversion of MPTP to its neurotoxic metabolite MPP+.
The present study examines the impact in MPTP-treated mice of BCG vaccination dose on striatal DA levels and DAT ligand binding as well as on the number of tyrosine hydroxylase-positive (TH+) cells in the SNpc. We also assessed BCG treatment’s effect on the levels of DA and serotonin (5-HT) and their metabolites in striatum as well as on the conversion of MPTP to MPP+. Finally, we examined BCG vaccination’s effect on the number of splenic CD4+Foxp3+ Tregs in MPTP-treated mice and whether Treg levels were associated with DA and DAT ligand binding levels in individual mice. The results suggest that BCG-induced Tregs could provide a new therapeutic approach to ameliorate pathology associated with PD.
MATERIALS AND METHODS
Mice
Male C57Bl/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and were housed in a specific-pathogen-free facility. All studies were approved by the UCLA Chancellor’s Animal Research Committee.
Vaccination With BCG Prior to MPTP Treatment and Tissue Sampling
Freeze-dried BCG (TheraCys; Sanofi Pasteur, Swift-water, PA) made from the Connaught BCG strain was reconstituted with the accompanying diluent, which contains 0.85% w/v sodium chloride, 0.025% w/v polysorbate 80, 0.06% w/v sodium dihydrogen phosphate, and 0.25% w/v disodium hydrogen phosphate. Mice (8–10 weeks in age) were randomized and vaccinated intraperitoneally (i.p.) with saline (control) or 1 or 6 × 106 colony-forming units (cfu) BCG. Ten days after saline or BCG vaccination, mice were injected i.p., with 18 mg/kg MPTP-HCl for 5 consecutive days as previously described (Meredith et al., 2008a,b; Yong et al., 2011). The mice were killed by decapitation 21 days after the last MPTP treatment (36 days after BCG vaccination). Their brains were removed, placed in a mouse brain mold on ice, and sectioned into 1-mm blocks beginning from the anterior pole of cortex and proceeding caudally for ~4 mm. Left and right striatal tissues were dissected out from three consecutive blocks on an ice-cold stainless-steel plate to provide two samples for analysis, which were immediately frozen in dry ice and stored at −80°C. The entire midbrain containing the substantia nigra was postfixed in 4% paraformaldehyde for 24 hr at 4°C and then transferred into 30% sucrose/PBS. Spleens were also removed immediately following sacrifice, and mononuclear cells were isolated for FACS analysis.
HPLC-EC Analysis of DA Content
Striatal samples from individual mice were homogenized in 0.5 ml ice-cold 0.2 M HClO4 containing 0.15% (w/v) Na2S2O5 and 0.05% (w/v) Na2EDTA and centrifuged at 10,000g for 15 min at 4°C. The supernatants were collected and filtered through a 0.2-µm polytetrafluoroethylene filter. A 20-µl aliquot of the filtrate was analyzed for DA and 5-HT and their metabolites, 3-O-methyldopamine (3-O-Me dopamine), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and 5-hydroxyindoleacetic acid (5-HIAA), by HPLC-EC in a blinded manner and normalized to striatal protein as described previously (O’Neil et al., 2006; Yong et al., 2011).
WIN Binding Assays
The DAT ligand binding levels in individual striatal samples were determined by [3H]WIN 35,428 (WIN; 87.0 Ci/mmol; Perkin Elmer Life Sciences, Boston, MA) binding assay, as described previously (O’Neil et al., 2006; Yong et al., 2011). Briefly, one portion of striatal sample was homogenized in binding buffer (20 mM sodium phosphate buffer, pH 7.4, 0.32 M sucrose) and centrifuged at 100,000g for 30 min, resuspended and respun. The pelleted proteins were resuspended in binding buffer, and, after quantification of protein concentrations, individual samples were incubated in triplicate with 5 nM [3H]WIN 35,428 at 0–4°C for 90 min (30 µM cocaine was used for nonspecifics), collected on filter paper, and analyzed by scintillation counting on a beta counter.
Histology and Stereological Analysis of TH+ Cells in the Substantia Nigra Pars Compacta
Methods for immunostaining and quantitation of TH+ neuron cell bodies in the SNpc followed similar protocols that we (Harvey et al., 2000) and others have previously described (Yoles and Schwartz, 1998; McCormack et al., 2002). For an initial analysis that might have revealed positive trends for a BCG neuroprotective effect, we selected subsets of MPTP- and BCG/MPTP-exposed midbrains (n = 4 per group) within their cohorts, based on their lowest and highest striatal DA levels, respectively.
Coronal sections (40 µm) of midbrain were obtained, and every tenth serial section was stained in 0.5% cresyl violet for anatomical determination of the SNpc region. Every fourth section in the region of SNpc was then stained for immunohistochemistry. The sections were first rinsed in PBS, and then endogenous peroxidases were blocked by incubation in 10% methanol/3% H2O2/PBS. Free-floating sections were preincubated at room temperature for 1 hr in 0.1 M PBST-azide (0.3% Triton X-100-PBST, 0.1% sodium azide) with 3% normal rabbit serum (Jackson Immunoresearch Laboratories, West Grove, PA) and then overnight in 0.1 M PBST-azide with 1.5% normal rabbit serum and the primary antibody, polyclonal sheep anti-TH (1:1,000; PelFreez, Rogers, AR). On the following day, sections were washed in PBS and then incubated at room temperature for 90 min in 0.1 M PBST with the secondary antibody, biotinylated rabbit anti-sheep IgG (1:200; Vector, Burlingame, CA) and 1.5% normal rabbit serum. After PBS rinses, sections were incubated at room temperature for 90 min in PBS/avidin-biotin complex (ABC method; 1:200; Vector) and visualized after 5–10 min of incubation in 0.02% 3,3′-diaminobenzidine tetrachloride (Sigma, St. Louis, MO) and 0.0015% H2O2 in 50 mM Tris buffer, pH 7.5, counterstaining with 0.06% pyronin Y for 20 min. For control sections, removal of the primary antibody step was followed by all remaining steps in the immunostaining protocol.
For stereological analysis of the TH+ neurons in the SNpc, coronal sections were selected that anatomically corresponded to the midbrain region between coordinates bregma −2.46 to −4.04 (Paxinos and Franklin, 2003). The number of TH+ neurons in the entire SNpc was determined using unbiased stereological methods (West et al., 1991; West, 2002) with StereoInvestigator software (MicroBrightField, Colchester, VT) and an Olympus BX-60 microscope equipped with a motorized stage (Ludl Electronic Products, Hawthorne, NY). Briefly, the SNpc was viewed and delineated on each section at ×4; a scan grid of 250 × 250 µm was placed onto its contour to define the counting area. A 100 × 100 µm counting frame was randomly placed on the counting area, which was then systematically sampled. The counting of TH+ cell bodies was performed with a ×40 objective. After sampling of the SNpc midbrain region (10–12 sections), the optical fractionator formula was used to estimate the total number of TH+ neurons in the analyzed brain volume. This protocol allowed for coefficient of error (CE) for the estimated cell number of <0.09 (usually 0.04–0.07).
Quantification of Levels of MPP+ in Mouse Brain
Animals (n = 4 per group) received either saline or 6 × 106 cfu BCG, i.p., and after 10 days were injected with 18 mg/kg MPTP-HCl, i.p. At 90 min post-MPTP, all animals were euthanized. This time point was previously shown to have maximal levels of MPP+ after MPTP injection. Formation of MPP+ was quantified by HPLC according to a published protocol (Jackson-Lewis and Przedborski, 2007).
Briefly, the brain was extracted and placed in ice-cold saline for 1 min. The brain was cut in a chilled brain matrix into 1-mm-thick blocks, from which striatum was dissected. Striatal tissue was weighed (wet weight) and frozen in dry ice until analyzed. The tissue was sonicated in 1:10 w/v 5% trifluoroacetic acid with 20 µg/ml 4-phenylpyridine (4PP) as an internal standard. The suspension was centrifuged for 15 min at 14,000 rpm, and the supernatant was filtered through a 0.2-µm Teflon syringe filter and analyzed by HPLC: 100 µl loop, column Luna 5 µm C18(2) 150 × 4.6 mm column (Phenomenex, Torrance, CA), UV detection at 295 nm, mobile phase 50 mM potassium phosphate buffer, pH 3.1/acetonitrile (9:1, v/v), flow rate 1.4 ml/min. MPP+ and 4PP were identified and quantified based on retention times corresponding with MPP+ and 4PP standards.
Treg Assay
At the time of sacrifice, splenic mononuclear cells were isolated and 1 × 106 cells/tube were blocked (in duplicate) with anti-CD16/32 on ice for 20 min and stained with fluorescein isothiocyanate (FITC)-anti-CD4 or isotype control (BD PharMigen, San Diego, CA) for 25 min. Subsequently, the cells were fixed and permeabilized according to the manufacturer’s instructions (Biolegend, San Diego, CA). After being washed, the cells were stained with PE-anti-Foxp3 or isotype control (Biolegend) and analyzed on a FACScan analyzer in CellQuest software (BD Biosciences, San Jose, CA). The cells were first gated on living cells, and the frequency of CD4+Foxp3+ and CD4+ T cells was analyzed. Finally, the number of splenic CD4+Foxp3+ Tregs in individual mice was calculated as the percentage of Tregs × total number of mononuclear cells.
Statistical Analysis
All values are expressed as group mean ± SEM. Differences among groups were analyzed by one-way ANOVA with Bonferroni corrections for specific group comparisons. A t-test was used for analysis of TH+ cell counts in the SNpc; P < 0.05 was considered statistically significant.
RESULTS
Vaccination With a Lower Dose of BCG Still Preserves Striatal DA and DAT
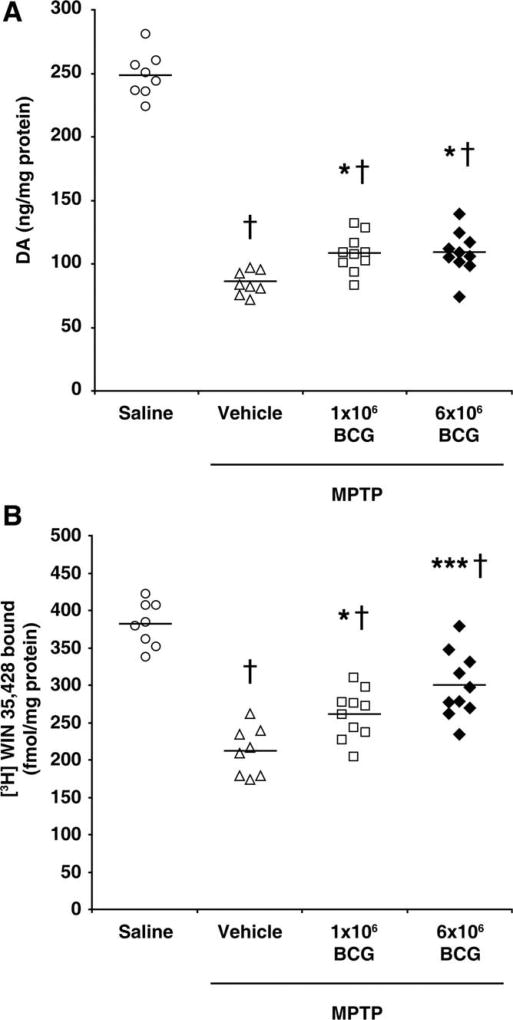
In our previous study, we vaccinated mice with BCG (20 × 106 cfu BCG) 10 days before MPTP treatment, because the acute toxicity of MPTP rapidly induces dopaminergic cell loss and activation of SNpc microglia, and it takes at least 10 days to mount vigorous immune responses to BCG in naïve mice (Yong et al., 2011). We observed that vaccination with BCG prior to MPTP treatment led to 16% higher levels of DA and 26% higher levels of DAT ligand binding in striatum when measured 21 days after the last MPTP treatment than in unvaccinated MPTP-treated controls (Yong et al., 2011). Here, we tested whether vaccination with lower doses of BCG relative to those used in our prior study could still provide neuroprotective effects. C57BL/6 mice were randomized into groups that received saline (alone), MPTP alone, or either 1 or 6 × 106 cfu BCG 10 days prior to MPTP treatment. Twenty-one days after the last MPTP treatment, we found that the levels of DA in the striatum of unvaccinated MPTP-treated mice were about 40% of those in the saline-treated mice (Fig. 1A). The levels of striatal DA in mice receiving either 1 or 6 × 106 BCG prior to MPTP treatment were about 28% higher than those in unvaccinated MPTP-treated mice (Fig. 1A), a statistically significant difference (P < 0.05).
Fig. 1.
BCG vaccination partially preserves DA and DAT-ligand binding levels in MPTP-treated mice. Mice were vaccinated with BCG (1 or 6 × 106 cfu) or received saline prior to MPTP treatment. A control group received only saline. Twenty-one days after the last dose of MPTP, we analyzed DA levels (A) and [3H]WIN-35,428 binding (B) in striatal homogenates from individual mice. Symbols represent individual mice in each group, and horizontal bars show mean for each group. N = 8–10 mice/group. *P < 0.05, ***P < 0.001 vs. unvaccinated MPTP-treated mice, †P < 0.001 relative to the saline-treated group, by one-way ANOVA.
Next, we determined the striatal DAT levels using a DAT ligand (WIN) binding assay. We found that the level of DAT ligand binding to striatal homogenates from mice treated with 1 or 6 × 106 cfu BCG was 23% and 42% higher, respectively, than that in striata from unvaccinated MPTP-treated mice (P < 0.05, and P < 0.01, respectively; Fig. 1B). These studies confirm our previous findings and suggest that BCG doses of 1–6 × 106 cfu BCG may have greater beneficial effects than the 20 × 106 cfu dose used in our initial study. In additional studies, we found that a lower dose of 1 × 105 cfu BCG was not neuroprotective (data not shown). Thus, a dose of 1– 6 × 106 cfu BCG appears to be optimal in our model.
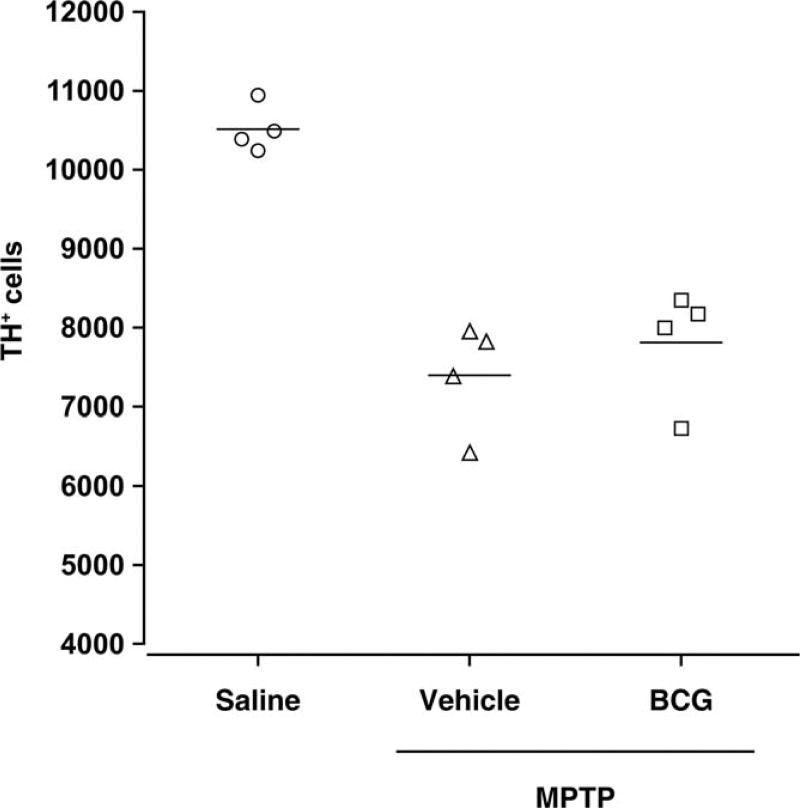
Stereological analysis revealed that the number of TH+ cells in the SNpc of BCG-vaccinated animals increased by an average of 5.6% compared with that in the mice injected with MPTP alone (7,814 ± 735 vs. 7,398 ± 692, respectively), although this difference was not statistically significant (Fig. 2; n = 5 mice/group). This finding is similar to that from our previous study (Yong et al., 2011) and suggests that BCG-induced immune responses primarily preserve the contents of DA and DAT in the striatum of mice.
Fig. 2.
Stereological analysis of midbrain TH+ cell counts. The numbers of TH+ cells in the midbrain regions were determined using stereological analysis. Bars show mean for each group.
BCG Vaccination Does Not Alter Striatal Levels of DA and 5-HT or Their Metabolites or the Metabolism of MPTP
BCG vaccination induces inflammatory responses, and the associated cytokines may alter metabolism in some organs. Accordingly, we evaluated in striatum whether BCG vaccination itself leads to alterations in levels of DA and 5-HT and their metabolites or in the conversion of MPTP to its neurotoxic metabolite MPP+ that might have confounded interpretation of the neuroprotective effects of BCG vaccination.
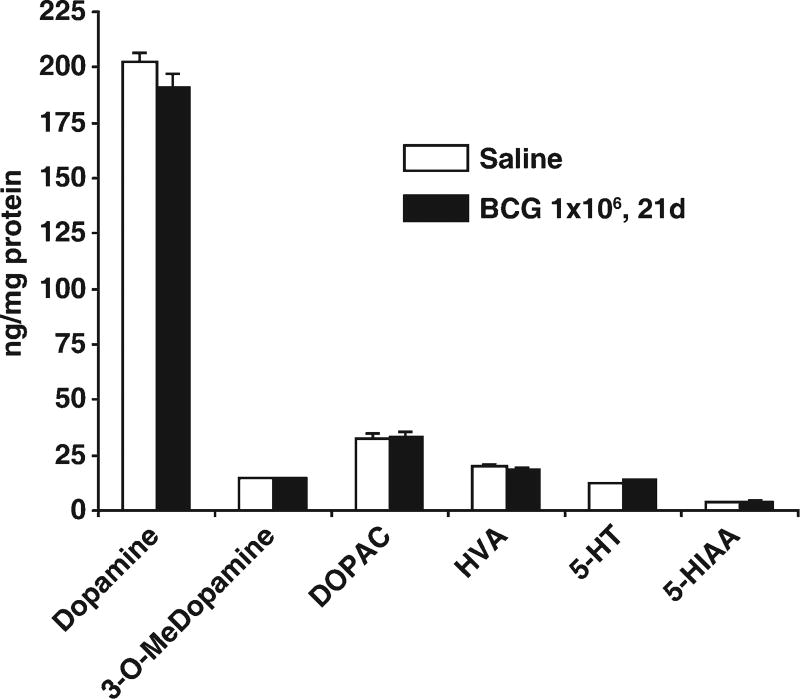
To determine potential effects of BCG vaccination on DA and 5-HT contents and their metabolites, 10-week-old mice were administered either saline or 1 × 106 cfu BCG, i.p. Twenty-one days later, the animals were sacrificed. HPLC-EC analysis of striatal homogenates showed that BCG-induced immune responses did not significantly change striatal levels of DA, 3-O-Me dopamine, DOPAC, HVA, 5-HT, or 5-HIAA (Fig. 3). Thus, at this time point, DA and 5-HT parameters in striatum were not altered by BCG vaccination.
Fig. 3.
BCG treatment does not change striatal levels of DA or 5-HT or their metabolites. Animals were sacrificed at 21 days following administration of either saline or 1 × 106 cfu BCG, i.p. (nine mice/group). Striatal levels of DA and 5-HT and their metabolites were analyzed by HPLC. There were no statistically significant differences between the two treatments for any measured parameter: DA, 3-O-methyldopamine (3-O-Me dopamine), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-HT, or 5-hydroxyindoleacetic acid (5-HIAA).
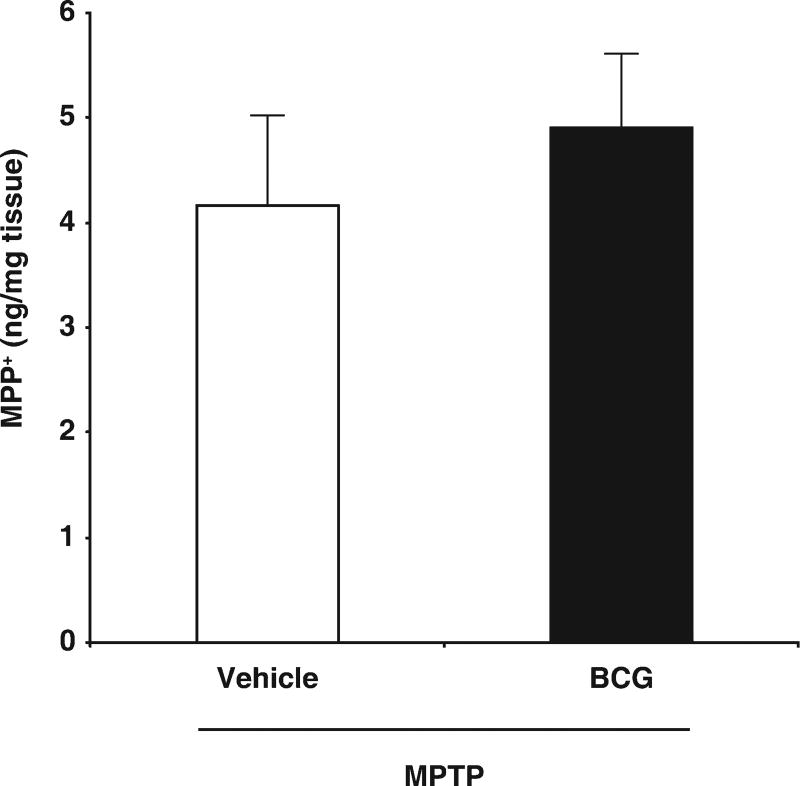
To determine whether BCG vaccination altered the metabolism of MPTP, mice were vaccinated with saline or 6 × 106 cfu BCG, and 10 days later they were injected with MPTP. After 90 min, we determined the levels of striatal MPP+ by HPLC. We found comparable levels of MPP+ in the striatum of both saline and BCG-vaccinated mice (Fig. 4). Thus, BCG’s neuroprotective effects are not due to metabolic alterations in the conversion of MPTP to MPP+.
Fig. 4.
BCG-induced immune responses do not alter the metabolism of MPTP. Mice were vaccinated with saline vehicle or BCG (6 × 106 cfu) and 10 days later were treated with MPTP. Ninety minutes later, the levels of striatal MPP+ in individual mice were determined by HPLC. N = 4 mice/group.
BCG Vaccination Increases the Number and Frequency of Splenic Tregs, Which Are Positively Correlated With Striatal DA and DAT Levels in MPTP-Treated Mice
Besides inducing inflammatory responses, BCG vaccination induces Tregs (Ribeiro-Rodrigues et al., 2006; Scott-Browne et al., 2007; Jaron et al., 2008). Consistent with those studies, we found that vaccination with BCG (1 × 106 cfu) led to an 85% increase in the percentage of CD4+Foxp3+ splenic Tregs in C57Bl/6 mice when measured 21 days after immunization (P < 0.001, data not shown). Previous studies have shown that Tregs can be neuroprotective in the MPTP mouse model, but, in these studies, Tregs were adoptively transferred from Copaxone-treated mice to mice that had been MPTP treated in order to avoid MPTP’s lymphotoxic effects on Copaxone-induced immune responses (Benner et al., 2004; Reynolds et al., 2007). Thus, it was unclear whether Treg responses would be sustained in BCG-vaccinated mice after MPTP treatment or whether Treg levels would be associated with neuroprotection in our model.
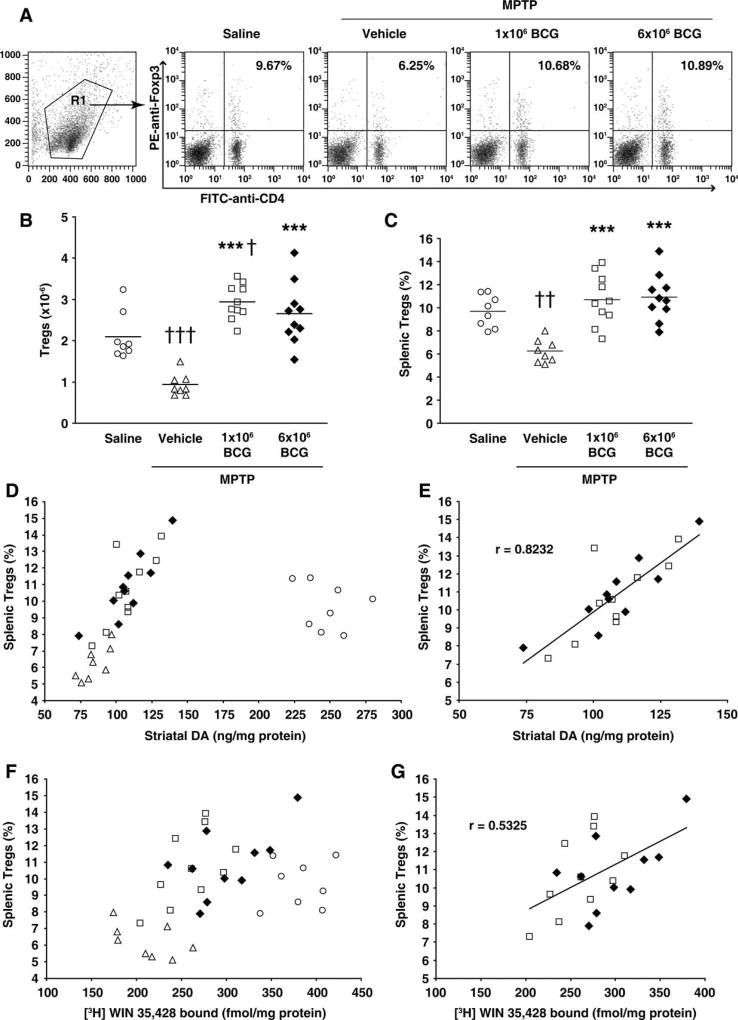
Mice received saline or BCG (1 or 6 × 106 cfu) prior to treatment with MPTP or saline, as described above. Twenty-one days after the last MPTP treatment, we determined the number of splenic CD4+Foxp3+ Tregs as well as the striatal DA and DAT levels in individual mice. Representative FACS analysis of CD4+Foxp3+ cells from each mouse group are shown in Figure 5A. We found that the number of Tregs was greatly reduced in unvaccinated mice that had received MPTP (Fig. 5B). BCG vaccination prior to MPTP treatment led to a greater number and percentage of Tregs compared with that in unvaccinated MPTP-treated mice (Fig. 5B,C). The number of splenic Tregs was about threefold higher in mice that had received 1 or 6 × 106 BCG prior to MPTP treatment than in unvaccinated MPTP-treated mice. Moreover, Treg numbers in BCG/MPTP-treated mice were significantly higher than those in control saline-treated mice that had not been exposed to MPTP (Fig. 5B). This is likely to be due to the persistent BCG infection, which continually promoted Treg responses, thereby increasing the total numbers of Tregs in the MPTP-treated BCG-vaccinated mice. Consequently, despite receiving lymphotoxic MPTP, Treg numbers were significantly higher in mice that had been BCG vaccinated than in control saline-treated mice 21 days post-MPTP treatment (Fig. 5B).
Fig. 5.
BCG vaccination induces Tregs; Treg response correlates positively with striatal DA and DAT levels in MPTP-treated mice. Mice were treated with 1 × 106 cfu BCG (open squares), 6 × 106 cfu BCG (black diamonds), or vehicle (triangles) and 10 days later were given a 5-day course of MPTP. Control mice received only saline (open circles). Twenty-one days later, the numbers and percentage of CD4+Foxp3+ splenic Tregs and striatal DA and DAT ligand binding levels in individual mice were measured. A: Representative charts from two cytometry analyses of splenic CD4+Foxp3+ cells from mice in each group. B,C: Quantitative analysis of splenic Treg number (B) and frequency (C) in individual mice of each group. Horizontal bars show the mean for each group. ***P < 0.001 relative to the vehicle/MPTP-treated group. †P < 0.05, ††P < 0.01, †††P < 0.001 relative to the saline-treated group. D: Splenic Treg frequency and striatal DA levels in individual mice from all mouse groups. E: Correlation analysis of splenic Treg frequency and striatal DA levels in individual mice that had received BCG prior to MPTP treatment. The two dosages of BCG resulted in magnitudes of effect on DA and DAT ligand-binding parameters that were not significantly different, so the Treg frequencies in all BCG-treated mice were pooled; a positive correlation of r = 0.82 was obtained for Treg frequencies and DA levels from individual animals. Striatal DA levels also correlated positively with the number of splenic Tregs in individual mice (r = 0.582; data not shown). F: Splenic Treg frequency and striatal DAT ligand-binding levels in individual mice from all groups. G: Correlation analysis of splenic Treg frequency and striatal DAT ligand binding levels in mice that had received BCG. Striatal DAT ligand binding also showed some association with the number of splenic Tregs in individual mice (r = 0.255; data not shown).
The frequency of Tregs and striatal levels of DA in individual mice from all mouse groups is shown in Figure 5D. Correlation analysis revealed that both the number and the percentage of splenic Tregs in BCG/MPTP-treated mice correlated positively with the levels of striatal DA (Fig. 5E). The frequency of Tregs and striatal DAT ligand binding in individual mice from all mouse groups is shown in Figure 5F. Similar positive correlations between the number and percentage of splenic Tregs and the amount of DAT ligand binding were also observed in the BCG/MPTP-treated mice (Fig. 5G). Thus, BCG vaccination increased Treg responses, and the magnitude of this response correlated positively with dopaminergic neuroprotection in individual MPTP-treated mice.
DISCUSSION
We began our studies by assessing whether BCG vaccination dose affects markers of dopaminergic function after MPTP treatment. We found that a dose of 1 or 6 × 106 cfu BCG leads to about 28% higher striatal DA levels and 23% and 42% higher DAT-ligand binding levels, respectively, compared with unvaccinated MPTP-treated mice. The results of this study confirm our prior finding that BCG vaccination can be neuroprotective in the MPTP mouse model (Yong et al., 2011) and indicate that relatively lower dosages of BCG may have greater beneficial effects. Although we observed a slightly greater number of TH+ cells in the SNpc of MPTP treated mice vaccinated with BCG than in unvaccinated MPTP-treated mice in both our previous and our current studies, these differences were not statistically significant, suggesting that BCG-induced immune factors act in the striatum primarily to preserve aspects of the striatal DA system integrity. Nevertheless, because current PD therapy is directed at DA replacement in the striatum, BCG vaccination could be a valuable adjunctive therapy for PD based on its neuroprotective effects. Moreover, because the MPTP model that we have utilized induces acute neurotoxicity, conceivably neuroprotective immune responses might be better able to limit dopaminergic cell loss in the more slowly progressing human PD.
Mice that received only BCG did not display alterations in their striatal levels of DA, 5-HT, or their metabolites. We also found equivalent levels of MPP+ in the striatum of vaccinated and unvaccinated mice that received MPTP, indicating that BCG vaccine’s neuroprotective effects were not due to changes in the metabolism of MPTP. Therefore, BCG-induced immune responses were likely to underlie this treatment’s neuroprotective effects. The immune response to BCG has been extensively studied in mice and humans (Teixeira et al., 1995; Kumar et al., 1999; Hussey et al., 2002; Wu et al., 2007; Ritz et al., 2008; Soares et al., 2008; Lalor et al., 2010). BCG is a potent inducer of Th1-type CD4+ T cells and activator of antigen-presenting cells. Additionally, BCG infection induces Tregs that limit inflammation and tissue damage during infection (Ribeiro-Rodrigues et al., 2006; Scott-Browne et al., 2007; Jaron et al., 2008). Previous studies have shown that Tregs control inflammation in models of autoimmune disease and that Copaxone-induced Tregs are neuroprotective in the MPTP mouse model, but, in those studies, Tregs had to be adoptively transferred from Copaxone-treated mice to MPTP-treated mice to avoid MPTP’s lymphotoxic effects (Benner et al., 2004; Reynolds et al., 2007). Therefore, in our model, it was unclear whether Tregs would persist in BCG-vaccinated mice after MPTP treatment and whether they would exert neuroprotective effects. We found that, 21 days after the last MPTP treatment, Treg levels were indeed quite reduced in mice that had received only MPTP, indicating that MPTP is toxic to Tregs. However, infection with BCG countered the negative effect of MPTP treatment such that the numbers of splenic Tregs in MPTP-treated mice were about threefold more than those in unvaccinated MPTP-treated mice and were even higher than those in control mice that received only saline. This is likely to be due to the persistent BCG infection, which continually promoted Treg responses, thereby increasing the total numbers of Tregs in the MPTP-treated BCG-vaccinated mice. Importantly, we observed a clear positive correlation between the magnitude of the BCG-induced Tregs response and the extent to which DA and DAT were preserved in individual mice. These findings suggest that Tregs mediate BCG’s neuroprotective effects. Thus, unlike the case in the Copaxone/MPTP model, MPTP treatment did not prevent BCG from inducing an effective neuroprotective response. This may be because after MPTP treatment the persistent BCG infection continued to induce Tregs.
Our previous study (Yong et al., 2011) showed that MPTP treatment increased the number of nigral microglia (Iba1+), as has been observed by others (see, e.g., Czlonkowska et al., 1996, 2002; Benner et al., 2004), which are thought to contribute to MPTP-induced nigrostriatal system damage. In contrast, mice that had been treated with BCG prior to MPTP treatment had numbers of nigral Iba1+ cells similar to those in saline-treated control mice. In addition, in BCG-treated mice, the nigra microglia had small cell bodies and long, ramified processes, indicating a resting state. Such microglia are thought to exert neurosupportive functions by their abilities to produce neurotrophins and eliminate excitotoxins. Several studies have shown that Tregs can inhibit the activation of microglia in the MPTP mouse PD model (Reynolds et al., 2007, 2009, 2010). Other studies with the MPTP model have pointed to CD4+ T cells as playing a key role in neurodegeneration (Brochard et al., 2009). Th17 cells recognizing nitrated α-synuclein exacerbate MPTP-induced neuronal cell loss but can be held in check by Tregs (Reynolds et al., 2007, 2010). Th17 cell development is also antagonized by interferon-γ (IFNγ; Harrington et al., 2005; Cruz et al., 2006). BCG is known to be a potent inducer of IFNγ-secreting Th1 responses and to induce Tregs (Teixeira et al., 1995; Kumar et al., 1999; Hussey et al., 2002; Wu et al., 2007; Ritz et al., 2008; Soares et al., 2008; Lalor et al., 2010; present study), both of which could inhibit Th17 cell expansion and function. In addition, the active BCG infection in the periphery may have diverted T cells, macrophages, and bone marrow-derived microglial precursors from responding to the CNS damage. Previous studies with the experimental autoimmune encephalomyelitis (EAE) model have shown that infection with BCG 6 weeks before the induction of EAE diverts activated myelinreactive CD4+ T cells from the CNS to granulomata in the spleen and liver (Sewell et al., 2003). Evidently, the peripheral inflammatory lesions nonspecifically attracted pathogenic T cells and blunted the development of EAE. Interestingly, in clinical trials, MS patients immunized with BCG had a 57% reduction of lesions as measured by MRI (Ristori et al., 1999; Paolillo et al., 2003). Thus, there is some clinical evidence that BCG treatment can suppress a neurodegenerative autoimmune response. Other possible neuroprotective mechanisms include the following. 1) BCG induced circulating neurosupportive cytokines/chemokines that might have a supportive effect on neurons in the area of injury and/or inhibit the activation and proliferation of resident microglia after MPTP treatment. 2) BCG-induced T-cell responses might be attracted to areas of damage in the CNS and locally release neurosupportive factors. Activated T cells can secrete BDNF, NGF, NT-3, and NT4/5 (Kerschensteiner et al., 1999a,b; Barouch et al., 2000; Hammarberg et al., 2000; Hohlfeld et al., 2000; Moalem et al., 2000), which may help to counteract factors that promote DA system degeneration, reduce the priming of surrounding neurons for secondary degeneration and promote neurorestoration. There may be other, as yet unknown neuroprotective mechanisms. Additional studies are needed to determine the contributions of these potential protective mechanisms to BCG vaccination’s neuroprotective effects.
There are several different BCG strains, each of which induces different types of immune responses in mice and humans and may confer a somewhat different degree of protection from tuberculosis (Fine et al., 1999; Davids et al., 2006; Wu et al., 2007; Ritz et al., 2008). In pilot studies, we tested the BCG Connaught strain (TheraCys) and the BCG Tice strain (Merck & Co.) side by side over a dose range and found that the BCG Connaught strain preserved striatal DA content significantly more than the Tice strain (data not shown). These differences in neuroprotection could be due to inducing different levels of Tregs, quantitative and qualitative differences in induced cytokine/chemokines, differences in the kinetics of their replication and biodistribution, differences in the ratio of living/dead BCG in the inocula, or other factors. The availability of different clinically applicable BCG strains that induce different types of immune responses provides an opportunity to dissect further the BCG-induced immune responses that are associated with neuroprotection in mice. Before attempting to translate BCG vaccination to human clinical trials with PD patients, it will be important to determine which BCG strains are the best candidates for therapy.
Billions of infants have been vaccinated with BCG to protect against tuberculosis (Colditz et al., 1994; Fine et al., 1999; Barreto et al., 2006). For those vaccinated with BCG in infancy, there is little reason to expect that the vaccination would be associated with reduced PD incidence decades later, because BCG-induced immune responses, as assessed by skin sensitivity to tuberculin, wane within a few months or, at most, a few years (Menzies, 2000). For those vaccinated as children, e.g., ages 5–7 years, the BCG-induced immune response to tuberculin can last considerably longer, even decades, although in the great majority it will become negative within a decade or two. Even after the vaccinee becomes tuberculin negative, some BCG-reactive memory responses may persist, but these memory responses are quiescent until re-exposure to BCG or tuberculosis. Because BCG vaccine-induced immune responses wane over a period of months or a few years, it may be beneficial for PD patients to receive periodic revaccination with BCG. However, BCG infection evokes many different types of immune responses, and it is often found that immune responses to a second exposure to an immunogen can be quite different from the initial response. Therefore, it is important to understand the nature of the immune responses to subsequent BCG vaccination and whether these favor neuroprotection or may be deleterious.
Our results suggest that, although BCG vaccination has no effect on the unperturbed CNS, the presence of a BCG infection leads to a better outcome after CNS insult, which appears to be associated with Treg levels. Notably, Gendelman and colleagues recently reported that Treg dysfunction was associated with pathobiology and disease severity in PD patients (Saunders et al., 2012). Likewise, two studies recently showed that Treg levels are inversely correlated with disease progression rates in ALS patients (Beers et al., 2011; Henkel et al., 2013). Collectively, these findings suggest that it will be important to study how to induce Tregs efficiently and safely in humans and whether human Tregs can be neuroprotective and, if so, how elevated Treg levels can be safely maintained long term in PD patients. Our findings may be relevant to designing new adjunctive therapies to ameliorate pathology associated not only with PD but also with other human neurodegenerative diseases.
Acknowledgments
We thank Marie-Francoise Chesselet for her advice and constructive review of the manuscript.
Contract grant sponsor: Michael J. Fox Foundation.
Footnotes
The authors have no conflicting financial interests.
References
- Armentero MT, Levandis G, Nappi G, Bazzini E, Blandini F. Peripheral inflammation and neuroprotection: systemic pretreatment with complete Freund’s adjuvant reduces 6-hydroxydopamine toxicity in a rodent model of Parkinson’s disease. Neurobiol Dis. 2006;24:492–505. doi: 10.1016/j.nbd.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Barouch R, Appel E, Kazimirsky G, Braun A, Renz H, Brodie C. Differential regulation of neurotrophin expression by mitogens and neurotransmitters in mouse lymphocytes. J Neuroimmunol. 2000;103:112–121. doi: 10.1016/s0165-5728(99)00233-7. [DOI] [PubMed] [Google Scholar]
- Barreto ML, Pereira SM, Ferreira AA. BCG vaccine: efficacy and indications for vaccination and revaccination. J Pediatr (Rio de Janeiro) 2006;82(Suppl 3):S45–S54. doi: 10.2223/JPED.1499. [DOI] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Zhao W, Wang J, Huang A, Wen S, Liao B, Appel SH. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011;134:1293–1314. doi: 10.1093/brain/awr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner EJ, Mosley RL, Destache CJ, Lewis TB, Jackson-Lewis V, Gorantla S, Nemachek C, Green SR, Przedborski S, Gendelman HE. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2004;101:9435–9440. doi: 10.1073/pnas.0400569101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- Cruz A, Khader SA, Torrado E, Fraga A, Pearl JE, Pedrosa J, Cooper AM, Castro AG. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Czlonkowski A. Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson’s disease mice model. Neurodegeneration. 1996;5:137–143. doi: 10.1006/neur.1996.0020. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Kurkowska-Jastrzebska I, Czlonkowski A, Peter D, Stefano GB. Immune processes in the pathogenesis of Parkinson’s disease—a potential role for microglia and nitric oxide. Med Sci Monit. 2002;8:RA165–RA177. [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Davids V, Hanekom WA, Mansoor N, Gamieldien H, Gelderbloem SJ, Hawkridge A, Hussey GD, Hughes EJ, Soler J, Murray RA, Ress SR, Kaplan G. The effect of bacille Calmette-Guerin vaccine strain and route of administration on induced immune responses in vaccinated infants. J Infect Dis. 2006;193:531–536. doi: 10.1086/499825. [DOI] [PubMed] [Google Scholar]
- Fedatto PF, Sergio CA, Paula MO, Gembre AF, Franco LH, Wowk PF, Ramos SG, Horn C, Marchal G, Turato WM, Silva CL, da Fonseca DM, Bonato VL. Protection conferred by heterologous vaccination against tuberculosis is dependent on the ratio of CD4+/CD4+ Foxp3+ cells. Immunology. 2012;137:239–248. doi: 10.1111/imm.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine PE, Careiro I, Milstien JB, Clements CJ. World Health Organization; 1999. Issues relating to the use of BCG in immunization programs. ( www.who.int/gpv-documents) [Google Scholar]
- Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173:803–810. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- Hammarberg H, Lidman O, Lundberg C, Eltayeb SY, Gielen AW, Muhallab S, Svenningsson A, Linda H, van Der Meide PH, Cullheim S, Olsson T, Piehl F. Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J Neurosci. 2000;20:5283–5291. doi: 10.1523/JNEUROSCI.20-14-05283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Harvey DC, Lacan G, Tanious SP, Melega WP. Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res. 2000;871:259–270. doi: 10.1016/s0006-8993(00)02439-2. [DOI] [PubMed] [Google Scholar]
- Henkel JS, Beers DR, Wen S, Rivera AL, Toennis KM, Appel JE, Zhao W, Moore DH, Powell SZ, Appel SH. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol Med. 2013;5:64–79. doi: 10.1002/emmm.201201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R, Kerschensteiner M, Stadelmann C, Lassmann H, Wekerle H. The neuroprotective effect of inflammation: implications for the therapy of multiple sclerosis. J Neuroimmunol. 2000;107:161–166. doi: 10.1016/s0165-5728(00)00233-2. [DOI] [PubMed] [Google Scholar]
- Hussey GD, Watkins ML, Goddard EA, Gottschalk S, Hughes EJ, Iloni K, Kibel MA, Ress SR. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology. 2002;105:314–324. doi: 10.1046/j.1365-2567.2002.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Jaron B, Maranghi E, Leclerc C, Majlessi L. Effect of attenuation of Treg during BCG immunization on anti-mycobacterial Th1 responses and protection against Mycobacterium tuberculosis. PLoS One. 2008;3:e2833. doi: 10.1371/journal.pone.0002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TB, Ankeny DP, Guan Z, McGaughy V, Fisher LC, Basso DM, Popovich PG. Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats. J Neurosci. 2004;24:3752–3761. doi: 10.1523/JNEUROSCI.0406-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999a;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999b;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Behera AK, Matsuse H, Lockey RF, Mohapatra SS. A recombinant BCG vaccine generates a Th1-like response and inhibits IgE synthesis in BALB/c mice. Immunology. 1999;97:515–521. doi: 10.1046/j.1365-2567.1999.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkowska-Jastrzebska I, Balkowiec-Iskra E, Joniec I, Litwin T, Czlonkowski A, Czlonkowska A. Immunization with myelin oligodendrocyte glycoprotein and complete Freund adjuvant partially protects dopaminergic neurons from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced damage in mouse model of Parkinson’s disease. Neuroscience. 2005;131:247–254. doi: 10.1016/j.neuroscience.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Lalor MK, Smith SG, Floyd S, Gorak-Stolinska P, Weir RE, Blitz R, Branson K, Fine PE, Dockrell HM. Complex cytokine profiles induced by BCG vaccination in UK infants. Vaccine. 2010;28:1635–1641. doi: 10.1016/j.vaccine.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Shen HH. Neonatal bacillus Calmette-Guerin vaccination inhibits de novo allergic inflammatory response in mice via alteration of CD4+CD25+ T-regulatory cells. Acta Pharmacol Sin. 2009;30:125–133. doi: 10.1038/aps.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- Menzies D. What does tuberculin reactivity after bacille Calmette-Guerin vaccination tell us? Clin Infect Dis. 2000;31(Suppl 3):S71–S74. doi: 10.1086/314075. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Sonsalla PK, Chesselet MF. Animal models of Parkinson’s disease progression. Acta Neuropathol. 2008a;115:385–398. doi: 10.1007/s00401-008-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S, Potashkin JA, Surmeier DJ. Modeling PD pathogenesis in mice: advantages of a chronic MPTP protocol. Parkinsonism Rel Disorders. 2008b;14(Suppl 2):S112–S115. doi: 10.1016/j.parkreldis.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moalem G, Gdalyahu A, Shani Y, Otten U, Lazarovici P, Cohen IR, Schwartz M. Production of neurotrophins by activated T cells: implications for neuroprotective autoimmunity. J Autoimmun. 2000;15:331–345. doi: 10.1006/jaut.2000.0441. [DOI] [PubMed] [Google Scholar]
- Nevo U, Kipnis J, Golding I, Shaked I, Neumann A, Akselrod S, Schwartz M. Autoimmunity as a special case of immunity: removing threats from within. Trends Mol Med. 2003;9:88–93. doi: 10.1016/s1471-4914(03)00024-8. [DOI] [PubMed] [Google Scholar]
- O’Neil ML, Kuczenski R, Segal DS, Cho AK, Lacan G, Melega WP. Escalating dose pretreatment induces pharmacodynamic and not pharmacokinetic tolerance to a subsequent high-dose methamphetamine binge. Synapse. 2006;60:465–473. doi: 10.1002/syn.20320. [DOI] [PubMed] [Google Scholar]
- Paolillo A, Buzzi MG, Giugni E, Sabatini U, Bastianello S, Pozzilli C, Salvetti M, Ristori G. The effect of bacille Calmette-Guerin on the evolution of new enhancing lesions to hypointense T1 lesions in relapsing remitting MS. J Neurol. 2003;250:247–248. doi: 10.1007/s00415-003-0967-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates, compact. 2. San Diego: Academic Press; 2003. [Google Scholar]
- Qian L, Flood PM, Hong JS. Neuroinflammation is a key player in Parkinson’s disease and a prime target for therapy. J Neural Transm. 2010;117:971–979. doi: 10.1007/s00702-010-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J Leukoc Biol. 2007;82:1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Mosley RL, Gendelman HE. Proteomic studies of nitrated alpha-synuclein microglia regulation by CD4+CD25+ T cells. J Proteome Res. 2009;8:3497–3511. doi: 10.1021/pr9001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J Immunol. 2010;184:2261–2271. doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Rodrigues R, Resende Co T, Rojas R, Toossi Z, Dietze R, Boom WH, Maciel E, Hirsch CS. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristori G, Buzzi MG, Sabatini U, Giugni E, Bastianello S, Viselli F, Buttinelli C, Ruggieri S, Colonnese C, Pozzilli C, Salvetti M. Use of bacille Calmette-Guerin (BCG) in multiple sclerosis. Neurology. 1999;53:1588–1589. doi: 10.1212/wnl.53.7.1588. [DOI] [PubMed] [Google Scholar]
- Ritz N, Hanekom WA, Robins-Browne R, Britton WJ, Curtis N. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol Rev. 2008;32:821–841. doi: 10.1111/j.1574-6976.2008.00118.x. [DOI] [PubMed] [Google Scholar]
- Saunders JA, Estes KA, Kosloski LM, Allen HE, Dempsey KM, Torres-Russotto DR, Meza JL, Santamaria PM, Bertoni JM, Murman DL, Ali HH, Standaert DG, Mosley RL, Gendelman HE. CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson’s disease. J Neuroimmune Pharmacol. 2012;7:927–938. doi: 10.1007/s11481-012-9402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Cohen IR. Autoimmunity can benefit self-maintenance. Immunol Today. 2000;21:265–268. doi: 10.1016/s0167-5699(00)01633-9. [DOI] [PubMed] [Google Scholar]
- Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell DL, Reinke EK, Co DO, Hogan LH, Fritz RB, Sandor M, Fabry Z. Infection with Mycobacterium bovis BCG diverts traffic of myelin oligodendroglial glycoprotein autoantigen-specific T cells away from the central nervous system and ameliorates experimental autoimmune encephalomyelitis. Clin Diagn Lab Immunol. 2003;10:564–572. doi: 10.1128/CDLI.10.4.564-572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares AP, Scriba TJ, Joseph S, Harbacheuski R, Murray RA, Gelderbloem SJ, Hawkridge A, Hussey GD, Maecker H, Kaplan G, Hanekom WA. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180:3569–3577. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira HC, Munk ME, Kaufmann SH. Frequencies of IFN gamma- and IL-4-producing cells during Mycobacterium bovis BCG infection in two genetically susceptible mouse strains: role of alpha/beta T cells and NK1.1 cells. Immunol Lett. 1995;46:15–19. doi: 10.1016/0165-2478(95)00009-t. [DOI] [PubMed] [Google Scholar]
- Walsh JT, Kipnis J. Regulatory T cells in CNS injury: the simple, the complex and the confused. Trends Mol Med. 2011;17:541–547. doi: 10.1016/j.molmed.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Design-based stereological methods for counting neurons. Prog Brain Res. 2002;135:43–51. doi: 10.1016/S0079-6123(02)35006-4. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wu B, Huang C, Garcia L, Ponce de Leon A, Osornio JS, Bobadilla-del-Valle M, Ferreira L, Canizales S, Small P, Kato-Maeda M, Krensky AM, Clayberger C. Unique gene expression profiles in infants vaccinated with different strains of Mycobacterium bovis bacille Calmette-Guerin. Infect Immun. 2007;75:3658–3664. doi: 10.1128/IAI.00244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoles E, Schwartz M. Degeneration of spared axons following partial white matter lesion: implications for optic nerve neuropathies. Exp Neurol. 1998;153:1–7. doi: 10.1006/exnr.1998.6811. [DOI] [PubMed] [Google Scholar]
- Yong J, Lacan G, Dang H, Hsieh T, Middleton B, Wasserfall C, Tian J, Melega WP, Kaufman DL. BCG vaccine-induced neuroprotection in a mouse model of Parkinson’s disease. PloS One. 2011;6:e16610. doi: 10.1371/journal.pone.0016610. [DOI] [PMC free article] [PubMed] [Google Scholar]