Abstract
Purpose
To describe the retinal clinical features of a group of Mexican patients with Stargardt disease carrying the uncommon p.Ala1773Val founder mutation in ABCA4.
Methods
Ten patients carrying the p.Ala1773Val mutation, nine of them homozygously, were included. Visual function studies included best-corrected visual acuity, electroretinography, Goldmann kinetic visual fields, and full-field electroretinography (ERG). In addition, imaging studies, such as optical coherence tomography (OCT), short-wave autofluorescence imaging, and quantitative analyses of hypofluorescence, were performed in each patient.
Results
Best-corrected visual acuities ranged from 20/200 to 4/200. The median age of the patients at diagnosis was 23.3 years. The majority of the patients had photophobia and nyctalopia, and were classified as Fishman stage 4 (widespread choriocapillaris atrophy, resorption of flecks, and greatly reduced ERG amplitudes). An atypical retinal pigmentation pattern was observed in the patients, and the majority showed cone-rod dystrophy on full-field ERG. In vivo retinal microstructure assessment with OCT demonstrated central retinal thinning, variable loss of photoreceptors, and three different patterns of structural retinal degeneration. Two dissimilar patterns of abnormal autofluorescence were observed. No apparent age-related differences in the pattern of retinal degeneration were observed.
Conclusions
The results indicate that this particular mutation in ABCA4 is associated with a severe retinal phenotype and thus, could be classified as null. Careful phenotyping of patients carrying specific mutations in ABCA4 is essential to enhance our understanding of disease expression linked to particular mutations and the resulting genotype–phenotype correlations.
Introduction
Stargardt disease (STGD1; OMIM 248200), also known as ABCA4 retinopathy, is an autosomal recessive retinal dystrophy regarded as the leading cause of inherited macular dystrophy [1,2]. The condition has an estimated incidence of 1 in 10,000 individuals [3] and is typically associated with wide variations in age of onset and phenotype severity [4-6]. Patients with STGD1 generally acknowledge bilateral, gradual decline in vision between the ages of 6 and 20 years, with visual loss in the range of 20/30 to 20/200 or worse. A bilateral central scotoma and a red-green color perception defect may also occur in advanced stages of the disease [7,8]. Retinal fundus findings are heterogeneous ranging from a beaten-bronze appearance to atrophy, often presenting with characteristic yellowish-white round or pisciform macular flecks at the level of the RPE [9]. In most patients with STGD1, fluorescein angiography (FA) shows the characteristic dark choroid sign, which is caused by the blockage of normal choriocapillaris fluorescence with lipofuscin [7].
STGD1 arises from biallelic mutations in ABCA4, a gene encoding a retina-specific ATP-binding cassette (ABC) transporter protein that has a role in the retinoid cycling between photoreceptors and the RPE [10]. The absence of a functional ABCA4 protein causes lipofuscin accumulation in the photoreceptor outer segment and the RPE, which subsequently results in photoreceptor apoptosis [2,11]. It has also been demonstrated that ABCA4-deficient cones simultaneously generate more A2E bisretinoid (a major lipofuscin component) than rods and that primary cone toxicity may contribute to macular vision loss in addition to cone death secondary to RPE atrophy [12].
STGD1 is one of the most mutationally heterogeneous retinal dystrophies as more than 1,000 ABCA4 pathogenic variants distributed along the entire gene have been described to date in affected individuals [13-15]. The estimated carrier frequency for an ABCA4 defective allele in the general population ranges from 1/10 to 1/20 [16,17], explaining the high proportion of compound heterozygous mutations detected in patients with STGD1. Given this substantial number of different mutant alleles in populations, the existence of more than one ABCA4 disease-causing mutation should be considered even in consanguineous affected families [18].
However, several instances of STGD1 founder mutations have been identified in particular ethnic groups [19-22]. The identification of recurrent or founder mutations in a population is of great importance because testing for one or a few prevalent mutations is more efficient and low-cost than testing for many rare variants, and because it allows presymptomatic diagnosis and the application of carrier-detection programs. In addition, careful phenotyping of patients who are homozygous for specific mutations in ABCA4 is essential to enhance our understanding of disease expression linked to particular mutations and the resulting genotype–phenotype correlations [23].
Recently, a p.Ala1773Val missense mutation in ABCA4 was identified in numerous individuals with STGD1 originating from the same geographic region in Mexico, thus suggesting a founder mutation effect [24]. The aim of the present study was to characterize the clinical, functional, and optical coherence tomography (OCT) retinal imaging phenotypes associated with this uncommon mutation in ABCA4.
Methods
Patients
The study protocol was approved by the Institutional Review Board, and the procedures followed the tenets of the Declaration of Helsinki. A total of ten patients with molecularly diagnosed STGD1 were included in the study. Of them, nine were homozygous, and one was heterozygous for the p.Ala1773Val mutation in ABCA4. The clinical features of five patients were previously reported in a limited fashion by our group [24]. The patients belonged to six apparently unrelated Mexican families arising from the central region of the country, and aside from their retinal disease, they were healthy individuals.
Mutation screening of ABCA4
Patients P1, P2, P5, and P6 (Table 1) were previously demonstrated to carry the p.Ala1773Val mutation in both ABCA4 alleles; patient P9 was a heterozygous carrier of the mutation [24], and the second pathogenic allele could not be identified after sequencing the entire ABCA4 coding region (50 exons). Patients P3, P4, P7, P8, and P10 were novel cases with a clinical diagnosis of STGD1 and were genotyped for this study. The molecular methods for ABCA4 genetic screening included DNA isolation from peripheral leukocytes, PCR amplification of the ABCA4 gene, and direct nucleotide sequencing of the amplicons, following procedures reported elsewhere [24]. These five novel patients were also demonstrated to carry the c.5318C>T (p.Ala1773Val) homozygous mutation. Familial segregation analysis was performed in all of the patients demonstrating that the phenotype segregates exclusively with homozygous patients. Importantly, analysis of the intragenic single nucleotide polymorphism (SNP) haplotype linked to the ABCA4 p.A1773V substitution in DNA from all affected patients showed concordance for the same haplotype of SNPs, rs4847281, rs3112831, rs4147831, rs1801666, rs1801574, and c.6543C>T, further supporting a founder effect for this particular mutation in this population.
Table 1. Clinical data of STGD patients carrying the p.Ala1773Val mutation in ABCA4.
| Age/Sex* | Photophobia† | Nyctalopia† | VA‡ | RefractionΙΙ | Mutation | Evolution time (yr) | Stage# | |
|---|---|---|---|---|---|---|---|---|
| P1 |
32/F |
Y |
Y |
20/400 |
−1.75 |
Homozygous |
28 |
4 |
| P2 |
26/F |
Y |
Y |
20/200 |
−2.25 |
Homozygous |
11 |
4 |
| P3 |
27/F |
Y |
Y |
20/400 |
−2.25 |
Homozygous |
18 |
4 |
| P4 |
16/F |
Y |
N |
20/400–20/200 |
−2.00 |
Homozygous |
8 |
3 |
| P5 |
29/F |
Y |
N |
4/200–20/300 |
−4.00 |
Homozygous |
21 |
4 |
| P6 |
30/F |
Y |
Y |
20/300–20/200 |
−3.25 |
Homozygous |
23 |
4 |
| P7 |
32/F |
N |
Y |
20/300 |
−2.50 |
Homozygous |
19 |
4 |
| P8 |
9/M |
N |
Y |
20/200 |
−0.50 |
Homozygous |
2 |
2 |
| P9 |
19/M |
Y |
Y |
20/300 |
−2.00 |
Heterozygous |
12 |
3 |
| P10 | 13/F | Y | Y | 20/200 | −2.50 | Homozygous | 5 | 3 |
Age at diagnosis / Sex of the patient; † Photophobia/Nyctalopia; yes (Y) / NO (N); ‡ Best corrected visual acuity at diagnosis visit; similar in the two eyes; otherwise, specified individually as RE-LE; ΙΙSpherical equivalent at diagnosis visit; average of both eyes.; # Fishman classification (Mild: 1-2) (Severe: 3-4).; Fishman et al., 1999 [4].
Visual function studies
Patients underwent a complete eye examination, including best-corrected visual acuity, Goldman kinetic visual fields, and electroretinography (ERG). Full-field ERG (Metrovision ERG system, Perenchies, France) was performed in all the patients using the International Society for Clinical Electrophysiology of Vision (ISCEV) standard stimuli [25]. A contact lens electrode (Jet ERG) was used in all the studies. Dim blue flashes after 20 min of dark adaptation were used to elicit pure rod responses. Flicker amplitudes were used as the main measurement of the cone responses. Classification of cone-rod dystrophy or rod-cone dystrophy in all patients with ABCA4 retinopathy was performed by comparing the percentage of amplitude reduction between the rod and cone responses. The percentage of reduction in the photoreceptor response was calculated according to the average of the normal values.
Imaging studies
Retinal cross-sections were obtained with OCT (Spectralis; Heidelberg Engineering GmbH, Heidelberg, Germany). A 9-mm line scan along the horizontal meridian crossing the fovea and using the eye-tracking feature (ART) was performed in all patients. Eccentricity of the outer nuclear layer (ONL) and the ellipsoid zone (EZ) line were determined by manual segmentation by using the Spectralis built-in measurement software. The ONL was defined as the area between the scleral side of the outer plexiform layer (OPL) and the vitreal side of the external limiting membrane (ELM). The EZ line was defined as the second hyperreflective band in the outer retina [26]. En face short-wave autofluorescence (SW-AF) imaging was used to estimate RPE health. SW-AF (488 nm) was performed in the high-speed mode where the 30° x 30° field was sampled on a 768 × 768 pixel image. Quantitative analyses of hypofluorescent central areas were manually measured by delineation of areas of decreased autofluorescence. The optic nerve head and blood vessels were used as a reference for hypofluorescence, and the peripheral healthy retina was used as a reference for normal autofluorescence. A proposed classification of abnormal autofluorescence patterns in Stargardt disease was used for the description of this cohort of patients [27]. Briefly, type 1 was defined as a localized low AF signal at the fovea surrounded by a homogeneous background with or without perifoveal loci of a high or low signal, type 2 as a localized low AF signal at the macula surrounded by a heterogeneous background and widespread foci of a high or low AF signal extending anterior to the vascular arcades, and type 3 as multiple areas of a low AF signal at the posterior pole with a heterogeneous background with or without foci of a high or low signal.
Results
Clinical characteristics of patients with ABCA4 retinopathy due to the p.Ala1773Val mutation
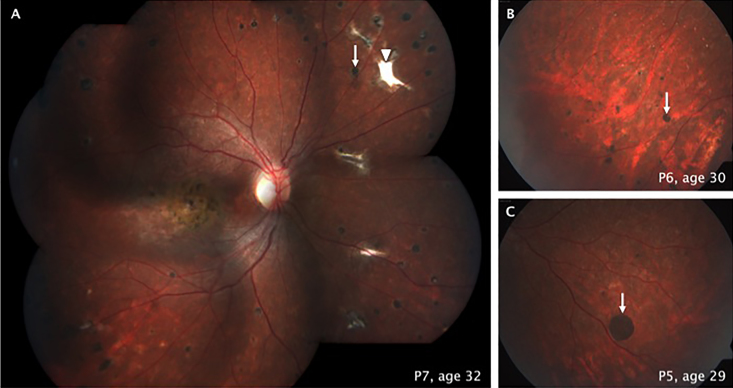
A summary of the clinical findings in this cohort is presented in Table 1. Four familial cases were included: Patients P1 and P2, P3 and P4, and P5 and P6 were pairs of sisters; patients P7 and P8 were mother and child. The remaining two patients (P9 and P10) were sporadic cases. Patients’ age ranged from 9 to 32 years (median: 26.5 years) when first diagnosed. The average reported age of central visual symptoms was 8.6 years. All patients denied a history of smoking or use of vitamin A or its derivatives. Time of evolution from onset of symptoms to present clinical evaluation was, on average, 14.7 years. Best-corrected visual acuities ranged from 20/200 to 4/200 in the eye with better vision at the first examination (Table 1). A myopic refractive error was identified in all the patients (spherical equivalent range: −4.00 to −0.50; Table 1). The majority of patients (eight out of ten) referred photophobia and nyctalopia during the first decade of life. On the ophthalmoscopic examination, only one patient (P8) showed flecks within and outside the vascular arcades, and this was the youngest patient in the cohort at the time of the examination. The disease severity classification proposed by Fishman et al. [7] was used for all patients. Accordingly, the majority of the patients (P1–P3 and P5–P7) were classified as stage 4; this stage is characterized by widespread choriocapillaris atrophy, resorption of flecks, and greatly reduced ERG amplitudes. As shown in Table 1, there seemed to be a tendency for correlation between the age of clinical examination and severity of the disease. An important fundoscopic finding in six affected individuals (P1–P3 and P5–P7) was the presence of pigmented lesions with well-defined borders located mainly in the mid- and far-peripheral retina (Figure 1).
Figure 1.
Fundoscopic findings in patients with the p.Ala1773Val mutation in ABCA4. Pigmented, round, well-demarcated sub-retinal lesions on the mid-peripheral retina in three patients (white arrows in A, B, and C). White, well-defined, non-pigmented, sub-retinal lesions shown in patient P7 (arrowhead in A).
Visual function in ABCA4 retinopathy due to the p.Ala1773Val mutation
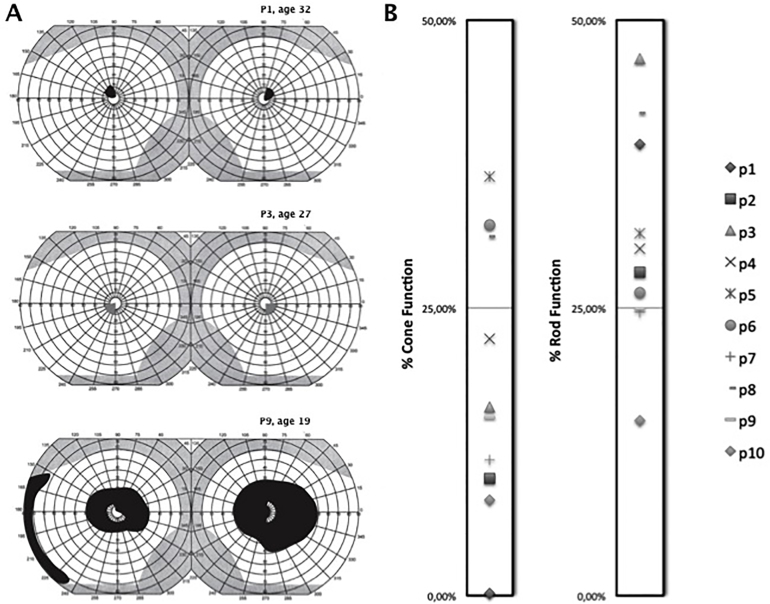
Three distinct kinetic perimetry patterns were found in all patients included in the study. The most common pattern of visual field abnormality, present in seven patients (P1, P2, P5–P8, and P10), was characterized by an absolute scotoma involving the central 5 degrees of vision with normal extent of the peripheral visual field (Figure 2A, top). In contrast, patient siblings P3 and P4 showed a relative central scotoma with a normal peripheral visual field at age 27 and 16 years, respectively (Figure 2A, middle). The last pattern, observed in patient P9, was characterized by a reduction in the peripheral visual field associated with a midperipheral absolute scotoma involving the central 5 degrees of vision (Figure 2A, bottom). All patients had full-field ERG recorded, and all had detectable signals. Two siblings, patients P5 and P6, showed more peripheral cone than rod function. The rest of the patients included in the cohort showed cone-rod dystrophy on full-field ERG (Figure 2B).
Figure 2.
Visual function in patients with the p.Ala1773Val mutation in ABCA4. A: Three different kinetic perimetry patterns in patients with ABCA4 retinopathy. Absolute central scotoma involving the central 10 degrees of vision (top, black shadow). Relative central scotoma involving the central vision (middle, gray shadow). Absolute central scotoma associated with reduction of the peripheral visual field (bottom, black shadow) B:Measured electroretinography (ERG) recordings shown as the percentage of cone and rod function compared with normative data in ten patients with the p.Ala1773Val mutation in ABCA4.
OCT structural analysis of the retina in ABCA4 retinopathydue to the p.Ala1773Val mutation
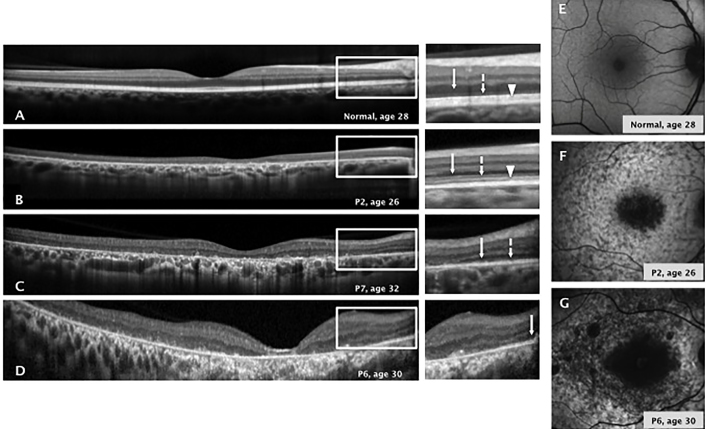
By OCT imaging, a healthy central retina is characterized by preserved lamination with normal ONL thickness and hyperreflective external bands (Figure 3A). In the study patients, in vivo microstructure analysis with OCT showed central retinal thinning and loss of photoreceptors with variable degrees of extension. None of the analyzed patients had preserved photoreceptors in the foveal region. Three different patterns of structural retinal degeneration were observed in the patients. The first pattern of central retinal degeneration was characterized by a detectable ONL, ELM, and EZ line within the central retina (Figure 3B) and was documented in patients P1–P4 and P8. The second pattern, observed in patients P5, P7, P9, and P10, was characterized by a detectable ONL and ELM within the central retina (Figure 3C); the third pattern was characterized by only the ONL detectable within the central retina and was observed in patient P6 (Figure 3D). RPE integrity was determined with en face SW-AF imaging. Normal SW-AF is characterized by a central area of decreased autofluorescence signal surrounded by a peak of signal at 10 degrees eccentric from the foveal center (Figure 3E). Two patterns of abnormal autofluorescence were found in the patient cohort. Patients P1–P5, P8, and P10 showed a localized central area of decreased autofluorescence surrounded by a heterogeneous background of high or low autofluorescence areas extending anterior to the vascular arcades (Figure 3F). In contrast, patients P6, P7, and P9 demonstrated multiple areas of decreased autofluorescence signals surrounded by a heterogeneous background of low and high autofluorescence areas (Figure 3G). Quantitation of the areas of decreased autofluorescence signals showed variable degrees of extension (mean ± standard deviation (SD) 7.25 ± 3.14 mm2). All patients included in this study showed preservation of autofluorescence signals in the peripapillary retina as has been described by others as a common autofluorescence imaging finding associated with ABCA4 retinopathy[28].
Figure 3.
Retinal structure in patients with the p.Ala1773Val mutation in ABCA4. A–D: Cross-sectional optical coherence tomography (OCT) images along the horizontal meridian through the fovea in a healthy subject compared with three patients with Stargardt disease (STGD1; P2, P6, and P7) with different patterns of central retinal degeneration. A:Retina from a 28-year-old healthy subject with normal central lamination. Magnified OCT images from the extrafoveolar regions delineated by white rectangles on the images illustrate the anatomy of the inner and outer retina. B:Retina from a 26-year-old patient with central retinal degeneration where the ONL, ELM, and EZ line are detectable along the central OCT scan. C: Retina from a 32-year-old patient with central retinal degeneration and a detectable ONL and ELM along the central OCT scan. D: Retina from a 30-year-old patient with central retinal degeneration and only the ONL detectable along the central OCT scan. The white arrow points to the outer nuclear layer. The dashed line with the white arrow points to the ELM. The white arrowhead points to the EZ line. ONL; outer nuclear layer. ELM; external limiting membrane. EZ; ellipsoid zone. E–G: Short-wave autofluorescence imaging results of a retina from a healthy subject compared with two representative patterns of abnormal autofluorescence imaging in two patients with STGD1 (P2 and P6) with ABCA4 retinopathy. E: SW-AF of a retina from a healthy 28-year-old male shows a central lower autofluorescence signal that increases eccentrically. The blood vessels and optic nerve appear dark. F:Retina from a 26-year-old patient with STGD1 shows a central hypoautofluorescence surrounded by a heterogeneous signal extending anterior to the vascular arcades. G:Retina from a 30-year-old patient with STGD1 shows a central hypoautofluorescence signal surrounded by patches of decreased fluorescence.
Discussion
ABCA4, the STGD1 gene, is one of the most mutationally heterogeneous retinal dystrophy genes. Remarkably, mutations in this gene have also been associated with non-STGD retinal phenotypes, such as autosomal recessive cone-rod dystrophies with retina-wide degeneration [29-31] or with early-onset severe dystrophy, sometimes mimicking retinitis pigmentosa [32-34].
As a result of the tremendous allelic heterogeneity in STGD1, most mutations in ABCA4 are private or occur in a minority of patients with STGD1. Nevertheless, several instances of recurrent mutations due to a founder mutation effect have been described, including p.C1490Y, p.R1129L, c.4254-15del23, and c.4539+2001G>A [19-22]. The identification of recurrent mutations in specific populations is important for directing ABCA4 molecular screening in patients from a particular ethnic group. In a recent report, Chacón-Camacho et al. [24] performed ABCA4 molecular analysis in a cohort of 31 unrelated patients with STGD1 from Mexico and identified the missense p.Ala1773Val pathogenic variant in eight out of a total of 46 disease-associated alleles (17.5%). Interestingly, all patients carrying this particular mutation originated from the same region of the country, and haplotype analyses of intragenic SNPs in four patients supported a common origin for this mutation [24].
In this work, we sought to characterize the retinal phenotype associated with the p.Ala1773Val mutation in ABCA4. A total of ten individuals (among them, nine were homozygous) carrying this pathogenic variant were subjected to clinical examination, as well as imaging and functional retinal studies.
Phenotype characterization showed individuals with STGD1 carrying the p.Ala1773Val mutation exhibited fundus areas of depigmentation and hyperpigmentation within the vascular arcades. The pattern of pigmentation of these lesions does not correspond to the typical scattered fine pigmentation of bone spicules that has been associated with cases of ABCA4 retinopathy. Fundoscopic findings of the mid- and peripheral retina in patients with ABCA4 retinopathy with wide retinal degeneration have been described as areas of hypopigmentation associated with scattered pigment clumping [35-37]. To the best of our knowledge, the pigmented well-demarcated lesions in the mid- and peripheral retina observed in 50% of the patients in this study have not been described in any form of ABCA4 retinopathy. Whether this clinical finding is related to the effect of this specific mutation is not known.
Autofluorescence imaging is a useful non-invasive tool for diagnosis and follow-up of ABCA4 retinopathy[27,38]. Abnormal autofluorescence is present in earlier stages of the disease [39], and we identified a variable extension of abnormal autofluorescence in all patients with the p.Ala1773Val mutation. The ring of preserved peripapillary autofluorescence that has been described as a common finding in patients with ABCA4 retinopathy [28] was also observed in all patients in this cohort. In this respect, recent studies have shown that nullizygosity for ABCA4 is associated with rapid progression shown by enlargement of central atrophy on fundus autofluorescence (FAF) while most patients harboring intermediate and null-like mutations display FAF abnormalities extending beyond the vascular arcades [40,41]. This latter pattern was observed in most (seven out of ten) of the patients in this cohort.
Variability of clinical expression has been documented in several cohorts of patients with ABCA4 retinopathy, and the residual functionality of ABCA4 is the proposed mechanism for this variable expression [42]. Approximately 2–5% of the mutations in ABCA4 result in a phenotype compatible with retinitis pigmentosa [43]. It has been proposed that severe mutations in both ABCA4 alleles are responsible for this phenotype. In our series, two cases had a retinitis pigmentosa pattern on ERG. These data indicate that additional factors, genetic or environmental, could modulate the final retinal phenotype associated with an identical mutation in the ABCA4 gene.
The p.Ala1773Val mutation has been identified in a few patients with sporadic STGD1 from other ethnic groups [23,37,44-49]. However, to date, homozygosity for this variant has not been demonstrated in non-Mexican patients with STGD1. Altogether, these data indicate that the p.Ala1773Val variant may have a higher allele frequency in Mexican patients with STGD1, an assumption supported by the fact that 11 out of 14 carriers of this variant included in the ExAC database are of Latino origin. Of note, the majority of patients with STGD1 heterozygous for the p.Ala1773Val allele and a different second pathogenic allele had late onset of disease (mean age of 26 years) and relatively well-preserved vision [23,37,45,49]. The relatively large cohort of patients homozygous for the p.Ala1773Val mutation included in this study enabled us to study the phenotypic effect of this specific variant and to categorize it in terms of severity. The early age of onset and extensive atrophy observed on FAF at an early age and early retina-wide involvement are similar to the phenotype of patients harboring null ABCA4 variants [40]. Thus, we hypothesize that p.Ala1773Val confers little to no ABCA4 function and could be categorized as a null-like mutation.
In this study, five new patients with STGD1 carrying the p.Ala1773Val mutation were included. Remarkably, all these patients originated from the same geographic region as the other patients carrying this variant we had reported previously [24]. The SNP haplotype analysis data confirmed our previous suggestion of a founder mutation effect for this particular mutation in patients with STGD1 from the central region of Mexico. These observations have prompted us to direct the genetic screening of STGD1 by searching first for the p.Ala1773Val mutation in ABCA4 in patients arising from this region. This approach has allowed us to diagnose additional patients with STGD1 from the same region carrying this pathogenic variant. (data not shown).
In conclusion, we described the retinal phenotype associated with a founder mutation in ABCA4 in a subgroup of patients with STGD1 from Mexico. Population genetic studies for known mutations to determine allele frequencies and their regional distributions could lead to more precise recommendations for genetic testing and could also help to define a better genotype–phenotype correlation in the group of ABCA4-linked retinal dystrophies. With the advent of novel treatment options, such as gene therapy, cell-based therapy, and optogenetics as potential effective treatments for ABCA4 retinopathy, comprehensive understanding of the disease phenotype represents a fundamental prerequisite for planning possible clinical trials in patients with STGD1.
Acknowledgments
This research was supported in part by the Foundation Fighting Blindness Grant CD-GE-0816–0711-OICV to the Institute of Ophthalmology “Conde de Valenciana” Foundation for the study of Mexican patients with inherited retinal degenerations. Partial Funding was also provided by CONACYT grant 234413. The authors do not have a proprietary interest.
References
- 1.Noble KG, Carr RE. Stargardt’s disease and fundus flavimaculatus. Arch Ophthalmol. 1979;97:1281–5. doi: 10.1001/archopht.1979.01020020023005. [DOI] [PubMed] [Google Scholar]
- 2.Westerfeld C, Mukai S. Stargardt’s disease and the ABCR gene. Semin Ophthalmol. 2008;23:59–65. doi: 10.1080/08820530701745249. [DOI] [PubMed] [Google Scholar]
- 3.Blacharski PA. Fundus flavimaculatus. In: Newsome, D. A. (ed.): Retinal Dystrophies and Degenerations. New York: Raven Press 1988. Pp. 135–159. [Google Scholar]
- 4.Fishman GA, Stone EM, Grover S, Derlacki DJ, Haines HL, Hockey RR. Variation of clinical expression in patients with Stargardt dystrophy and sequence variations in the ABCR gene. Arch Ophthalmol. 1999;117:504–10. doi: 10.1001/archopht.117.4.504. [DOI] [PubMed] [Google Scholar]
- 5.Lois N, Holder GE, Bunce C, Fitzke FW, Bird AC. Phenotypic subtypes of Stargardt macular dystrophy-fundus flavimaculatus. Arch Ophthalmol. 2001;119:359–69. doi: 10.1001/archopht.119.3.359. [DOI] [PubMed] [Google Scholar]
- 6.Klevering BJ, Maugeri A, Wagner A, Go SL, Vink C, Cremers FP, Hoyng CB. Three families displaying the combination of Stargardt’s disease with cone-rod dystrophy or retinitis pigmentosa. Ophthalmology. 2004;111:546–53. doi: 10.1016/j.ophtha.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Fishman GA, Farber M, Patel BS, Derlacki DJ. Visual acuity loss in patients with Stargardt’s macular dystrophy. Ophthalmology. 1987;94:809–14. doi: 10.1016/s0161-6420(87)33533-x. [DOI] [PubMed] [Google Scholar]
- 8.Mäntyjärvi M, Tuppurainen K. Color vision in Stargardt’s disease. Int Ophthalmol. 1992;16:423–8. doi: 10.1007/BF00918432. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong JD, Meyer D, Xu S, Elfervig JL. Long-term follow-up of Stargardt’s disease and fundus flavimaculatus. Ophthalmology. 1998;105:448–57. doi: 10.1016/S0161-6420(98)93026-3. [DOI] [PubMed] [Google Scholar]
- 10.Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–46. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 11.Pollock NL, Callaghan R. The lipid translocase, ABCA4: seeing is believing. FEBS J. 2011;278:3204–14. doi: 10.1111/j.1742-4658.2011.08169.x. [DOI] [PubMed] [Google Scholar]
- 12.Conley SM, Cai X, Makkia R, Wu Y, Sparrow JR, Naash MI. Increased cone sensitivity to ABCA4 deficiency provides insight into macular vision loss in Stargardt’s dystrophy. Biochim Biophys Acta. 1822;2012l:1169–79. doi: 10.1016/j.bbadis.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014;133:1–9. doi: 10.1007/s00439-013-1358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelis SS, Bax NM, Zernant J, Allikmets R, Fritsche LG, den Dunnen JT, Ajmal M, Hoyng CB, Cremers FP. In Silico Functional Meta-Analysis of 5,962 ABCA4 Variants in 3,928 Retinal Dystrophy Cases. Hum Mutat. 2017;38:400–8. doi: 10.1002/humu.23165. [DOI] [PubMed] [Google Scholar]
- 15.Fokkema IF, Taschner PE, Schaafsma GC, Celli J, Laros JF, den Dunnen JT. LOVD v.2.0: the next generation in gene variant databases. Hum Mutat. 2011;32:557–63. doi: 10.1002/humu.21438. [DOI] [PubMed] [Google Scholar]
- 16.Jaakson K, Zernant J, Külm M, Hutchinson A, Tonisson N, Glavac D, Ravnik-Glavac M, Hawlina M, Meltzer MR, Caruso RC, Testa F, Maugeri A, Hoyng CB, Gouras P, Simonelli F, Lewis RA, Lupski JR, Cremers FP, Allikmets R. Genotyping microarray (gene chip) for the ABCR (ABCA4) gene. Hum Mutat. 2003;22:395–403. doi: 10.1002/humu.10263. [DOI] [PubMed] [Google Scholar]
- 17.Riveiro-Alvarez R, Aguirre-Lamban J, Lopez-Martinez MA, Trujillo-Tiebas MJ, Cantalapiedra D, Vallespin E, Avila-Fernandez A, Ramos C, Ayuso C. Frequency of ABCA4 mutations in 278 Spanish controls: an insight into the prevalence of autosomal recessive Stargardt disease. Br J Ophthalmol. 2009;93:1359–64. doi: 10.1136/bjo.2008.148155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ducroq D, Shalev S, Habib A, Munnich A, Kaplan J, Rozet JM. Three different ABCA4 mutations in the same large family with several consanguineous loops affected with autosomal recessive cone-rod dystrophy. Eur J Hum Genet. 2006;14:1269–73. doi: 10.1038/sj.ejhg.5201691. [DOI] [PubMed] [Google Scholar]
- 19.September AV, Vorster AA, Ramesar RS, Greenberg LJ. Mutation spectrum and founder chromosomes for the ABCA4 gene in South African patients with Stargardt disease. Invest Ophthalmol Vis Sci. 2004;45:1705–11. doi: 10.1167/iovs.03-1167. [DOI] [PubMed] [Google Scholar]
- 20.Valverde D, Riveiro-Alvarez R, Bernal S, Jaakson K, Baiget M, Navarro R, Ayuso C. Microarray-based mutation analysis of the ABCA4 gene in Spanish patients with Stargardt disease: evidence of a prevalent mutated allele. Mol Vis. 2006;12:902–8. [PubMed] [Google Scholar]
- 21.Beit-Ya’acov A, Mizrahi-Meissonnier L, Obolensky A, Landau C, Blumenfeld A, Rosenmann A, Banin E, Sharon D. Homozygosity for a novel ABCA4 founder splicing mutation is associated with progressive and severe Stargardt-like disease. Invest Ophthalmol Vis Sci. 2007;48:4308–14. doi: 10.1167/iovs.07-0244. [DOI] [PubMed] [Google Scholar]
- 22.Bauwens M, De Zaeytijd J, Weisschuh N, Kohl S, Meire F, Dahan K, Depasse F, De Jaegere S, De Ravel T, De Rademaeker M, Loeys B, Coppieters F, Leroy BP, De Baere E. An augmented ABCA4 screen targeting noncoding regions reveals a deep intronic founder variant in Belgian Stargardt patients. Hum Mutat. 2015;36:39–42. doi: 10.1002/humu.22716. [DOI] [PubMed] [Google Scholar]
- 23.Burke TR, Allikmets R, Smith RT, Gouras R, Tsang SH. Loss of peripapillary sparing in non-group I Stargardt disease. Exp Eye Res. 2010;91:592–600. doi: 10.1016/j.exer.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chacón-Camacho OF, Granillo-Alvarez M, Ayala-Ramirez R, Zenteno JC. ABCA4 mutational spectrum in Mexican patients with Stargardt disease: identification of 12 novel mutations and evidence of a founder effect for the common p.A1773V mutations. Exp Eye Res. 2013;109:77–82. doi: 10.1016/j.exer.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 25.McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 26.Staurenghi G, Sadda S, Chakravarthy U, Spaide RF, International Nomenclature for Optical Coherence Tomography (IN•OCT) Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN•OCT consensus. Ophthalmology. 2014;121:1572–8. doi: 10.1016/j.ophtha.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Fujinami K, Lois N, Mukherjee R, McBain VA, Tsunoda K, Tsubota K, Stone EM, Fitzke FW, Bunce C, Moore AT, Webster AR, Michaelides M. A longitudinal study of Stargardt disease: quantitative assessment of fundus autofluorescence, progression, and genotype correlations. Invest Ophthalmol Vis Sci. 2013;54:8181–9. doi: 10.1167/iovs.13-12104. [DOI] [PubMed] [Google Scholar]
- 28.Cideciyan AV, Swider M, Aleman TS, Sumaroka A, Schwartz SB, Roman MI, Milam AH, Bennett J, Stone EM. ABCA4-associated retinal degenerations spare structure and function of the human parapapillary retina. Invest Ophthalmol Vis Sci. 2005;46:4739–46. doi: 10.1167/iovs.05-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cremers FP, van de Pol DJ, van Driel M, den Hollander AI, van Haren FJ, Knoers NV, Tijmes N, Bergen AA, Rohrschneider K, Blankenagel A, Pinckers AJ, Deutman AF, Hoyng CB. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt’s disease gene ABCR. Hum Mol Genet. 1998;7:355–62. doi: 10.1093/hmg/7.3.355. [DOI] [PubMed] [Google Scholar]
- 30.Maugeri A, Klevering BJ, Rohrschneider K, Blankenagel A, Brunner HG, Deutman AF, Hoyng CB, Cremers FP. Mutations in the ABCA4 (ABCR) gene are the major cause of autosomal recessive cone-rod dystrophy. Am J Hum Genet. 2000;67:960–6. doi: 10.1086/303079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitiratschky VB, Grau T, Bernd A, Zrenner E, Jägle H, Renner AB, Keller U, Rudolph G, Jacobson SG, Cideciyan AV, Schaich S, Kohl S, Wissinger B. ABCA4 gene analysis in patients with autosomal recessive cone and cone rod dystrophies. Eur J Hum Genet. 2008;16:812–9. doi: 10.1038/ejhg.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-Mir A, Paloma E, Allikmets R, Ayuso C, del Rio T, Dean M, Vilageliu L, González-Duarte R, Balcells S. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet. 1998;18:11–2. doi: 10.1038/ng0198-11. [DOI] [PubMed] [Google Scholar]
- 33.Rozet JM, Gerber S, Ghazi I, Perrault I, Ducrop D, Souied E, Cabot A, Dufier JL, Munnich A, Kaplan J. Mutations of the retinal specific ATP binding transporter gene (ABCR) in a single family segregating both autosomal recessive retinitis pigmentosa RP19 and Stargardt disease: evidence of clinical heterogeneity at this locus. J Med Genet. 1999;36:447–51. [PMC free article] [PubMed] [Google Scholar]
- 34.Wiszniewski W, Zaremba CM, Yatsenko AN, Jamrich M, Wensel TG, Lewis RA, Lupski JR. ABCA4 mutations causing mislocalization are found frequently in patients with severe retinal dystrophies. Hum Mol Genet. 2005;14:2769–78. doi: 10.1093/hmg/ddi310. [DOI] [PubMed] [Google Scholar]
- 35.Fukui T, Yamamoto S, Nakano K, Tsujikawa M, Morimura H, Nishida K, Ohguro N, Fujikado T, Irifune M, Kuniyoshi K, Okada AA, Hirakata A, Miyake Y, Tano Y. ABCA4 gene mutations in Japanese patients with Stargardt disease and retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002;43:2819–24. [PubMed] [Google Scholar]
- 36.Fishman GA, Stone EM, Eliason DA, Taylor CM, Lindeman M, Derlacki DJ. ABCA4 gene sequence variations in patients with autosomal recessive cone-rod dystrophy. Arch Ophthalmol. 2003;121:851–5. doi: 10.1001/archopht.121.6.851. [DOI] [PubMed] [Google Scholar]
- 37.Burke TR, Duncker T, Woods RL, Greenberg JP, Zernant J, Tsang SH, Smith RT, Allikmets R, Sparrow JR, Delori FC. Quantitative fundus autofluorescence in recessive Stargardt disease. Invest Ophthalmol Vis Sci. 2014;55:2841–52. doi: 10.1167/iovs.13-13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cideciyan AV, Swider M, Schwartz SB, Stone EM, Jacobson SG. Predicting Progression of ABCA4-Associated Retinal Degenerations Based on Longitudinal Measurements of the Leading Disease Front. Invest Ophthalmol Vis Sci. 2015;56:5946–55. doi: 10.1167/iovs.15-17698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cideciyan AV, Swider M, Aleman TS, Tsybovsky Y, Schwartz SB, Windsor EA, Roman AJ, Sumaroka A, Steinberg JD, Jacobson SG, Stone EM, Palczewski K. ABCA4 disease progression and a proposed strategy for gene therapy. Hum Mol Genet. 2009;18:931–41. doi: 10.1093/hmg/ddn421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fakin A, Robson AG, Fujinami K, Moore AT, Michaelides M, Pei-Wen Chiang J. E Holder G, Webster AR. Phenotype and progression of retinal degeneration associated with nullizigosity of ABCA4. Invest Ophthalmol Vis Sci. 2016;57:4668–78. doi: 10.1167/iovs.16-19829. [DOI] [PubMed] [Google Scholar]
- 41.Fakin A, Robson AG, Chiang JP, Fujinami K, Moore AT, Michaelides M, Holder GE, Webster AR. The effect on retinal structure and function of 15 specific ABCA4 mutations: A detailed examination of 82 hemizygous patients. Invest Ophthalmol Vis Sci. 2016;57:5963–73. doi: 10.1167/iovs.16-20446. [DOI] [PubMed] [Google Scholar]
- 42.Cideciyan AV, Aleman TS, Swider M, Schwartz SB, Steinberg JD, Brucker AJ, Maguire AM, Bennett J, Stone EM, Jacobson SG. Mutations in ABCA4 result in accumulation of lipofuscin before slowing of the retinoid cycle: a reappraisal of the human disease sequence. Hum Mol Genet. 2004;13:525–34. doi: 10.1093/hmg/ddh048. [DOI] [PubMed] [Google Scholar]
- 43.Fahim AT, Daiger SP, Weleber RG. Nonsyndromic Retinitis Pigmentosa Overview. In: Pagon, R.A., Adam, M.P., Ardinger, H.H., Wallace, S.E., Amemiya, A, Bean, L.J.H., Bird, T.D., Ledbetter, N., Mefford H.C., Smith, R.J.H., Stephens, K., editors. GeneReviews® [Internet]. 2000 Aug 4 [Updated 2017 Jan 19]. Seattle (WA): University of Washington, Seattle; 1993–2017 [Google Scholar]
- 44.Stenirri S, Alaimo G, Manitto MP, Brancato R, Ferrari M, Cremonesi L. Are microarrays useful in the screening of ABCA4 mutations in Italian patients affected by macular degenerations? Clin Chem Lab Med. 2008;46:1250–5. doi: 10.1515/CCLM.2008.248. [DOI] [PubMed] [Google Scholar]
- 45.Nõupuu K, Lee W, Zernant J, Tsang SH, Allikmets R. Structural and genetic assessment of the ABCA4-associated optical gap phenotype. Invest Ophthalmol Vis Sci. 2014;55:7217–26. doi: 10.1167/iovs.14-14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zernant J, Xie YA, Ayuso C, Riveiro-Alvarez R, Lopez-Martinez MA, Simonelli F, Testa F, Gorin MB, Strom SP, Bertelsen M, Rosenberg T, Boone PM, Yuan B, Ayyagari R, Nagy PL, Tsang SH, Gouras P, Collison FT, Lupski JR, Fishman GA, Allikmets R. Analysis of the ABCA4 genomic locus in Stargardt disease. Hum Mol Genet. 2014;23:6797–806. doi: 10.1093/hmg/ddu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Ge X, Shi W, Huang P, Min Q, Li M, Yu X, Wu Y, Zhao G, Tong Y, Jin ZB, Qu J, Gu F. Molecular diagnosis of putative Stargardt disease by capture next generation sequencing. PLoS One. 2014;9:e95528. doi: 10.1371/journal.pone.0095528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boulanger-Scemama E, El Shamieh S, Démontant V, Condroyer C, Antonio A, Michiels C, Boyard F, Saraiva JP, Letexier M, Souied E, Mohand-Saïd S, Sahel JA, Zeitz C, Audo I. Next-generation sequencing applied to a large French cone and cone-rod dystrophy cohort: mutation spectrum and new genotype-phenotype correlation. Orphanet J Rare Dis. 2015;10:85. doi: 10.1186/s13023-015-0300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biswas P, Duncan JL, Maranhao B, Kozak I, Branham K, Gabriel L, Lin JH, Barteselli G, Navani M, Suk J, Parke M, Schlechter C, Weleber RG, Heckenlively JR, Dagnelie G, Lee P, Riazuddin SA, Ayyagari R. Genetic analysis of ten pedigrees with inherited retinal degeneration (IRD) by exome sequencing and phenotype-genotype association. Physiol Genomics. 2017;49:216–29. doi: 10.1152/physiolgenomics.00096.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]