Abstract
Glomerular sclerosis is characterized by mesangial cell proliferation and progressive extracellular matrix (ECM) accumulation. CCN3 belongs to the CCN family of matrix proteins; increasing evidence suggests that CCN3 is an endogenous negative regulator of the ECM and fibrosis. However, the exact role of CCN3 in the accumulation of ECM remains unknown. The aim of the present study was to investigate the effects of CCN3 on TGF-β1-induced production of ECM in human mesangial cells (HMCs) in vitro. Treatment with TGF-β1 (0.5–2.0 ng/mL) suppressed the mRNA and protein expression of CCN3 in HMCs in dose- and time-dependent manners. Furthermore, treatment with TGF-β1 significantly increased the expression of the two markers of renal fibrosis, fibronectin (FN) and type I collagen (COLI), in HMCs. Moreover, treatment with TGF-β1 significantly decreased the expression of metalloproteinase (MMP)-2 and MMP-9, and markedly increased the expression of tissue inhibitor of metalloproteinase (TIMP)-1 in HMCs. Pretreatment of HMCs with exogenous CCN3 (5–500 ng/mL) or overexpression of CCN3 significantly attenuated TGF-β1-induced changes in FN, COLI, MMP-2, MMP-9 and TIMP-1 in HMCs. These results suggest that CCN3 suppresses TGF-β1-induced accumulation of ECM in HMCs. CCN3 may have potential as a novel therapeutic target for alleviating glomerulosclerosis.
Keywords: renal fibrosis, glomerulosclerosis, CCN3, fibronectin, type I collagen, MMPs
Introduction
Glomerulosclerosis is considered to be the final and common pathway leading to progressive loss of renal function in various types of primary glomerulonephritis, diabetic nephropathy, and lupus nephritis1. The characteristics of glomerulosclerosis are mesangial cell proliferation and extracellular matrix (ECM) accumulation2, which lead to a decreased glomerular filtration rate and complete destruction and failure of the kidney3,4.
The ECM is a complex structure comprising structural and regulatory proteins that provide a supporting scaffold for cells5. Furthermore, ECM accumulation is the result of a balance between synthesis and degradation6. Under normal conditions, ECM compounds are continuously synthesized and degraded in a tightly regulated manner, because the integrity of glomerular matrices is strictly maintained throughout most of adult life7. However, loss of this coordinated regulation, leading to increased mesangial ECM deposition, occurs in response to aging-associated changes in glomeruli and during the development of glomerulosclerosis8. Mesangial cells are critical for the maintenance of normal glomerular structure and function, primarily through providing the correct mechanical tension and producing various cytokines, growth factors, and metalloproteinases, all of which are critical in regulating ECM turnover9.
Transforming growth factor-β (TGF-β) is an important mediator of renal fibrosis, which is characterized by the accumulation of ECM components in glomerulosclerosis and tubulointerstitial fibrosis3. Moreover, the balance between MMPs and TIMPs is one of the critical determinants controlling the integrity of the ECM10. Most MMPs are secreted from cells as zymogens, and their activity in the extracellular matrix is controlled by TIMPs11. MMP-2 and MMP-9 are two gelatinases, and although they share the ability to degrade both collagen and gelatin in basement membranes, their substrate specificity is not identical12. TIMP-1 preferentially interacts with MMP-9. Indeed, the expression of MMP-2 and MMP-9 is developmentally regulated by the TGF-β family13.
CCN3, also known as nephroblastoma overexpressed (NOV), was initially discovered in nephroblastoma14. On the basis of structural similarity, CCN3 belongs to the CCN family of matrix proteins, which also includes CCN1/CYR61, CCN2/CTGF, CCN4/WISP-1, CCN5/WISP-2 and CCN6/WISP-315. Recently, emerging evidence has indicated that CCN3 may act as an endogenous negative regulator of ECM and fibrosis16,17,18,19,20. We have previously demonstrated that urinary CCN3 mRNA in patients with chronic kidney disease is negatively correlated with glomerular matrix accumulation (r=−0.6146, P=0.0087)21. These observations suggest a potential protective role of CCN3 against renal fibrosis. However, the exact mechanism by which CCN3 alleviates renal fibrosis remains unknown. The goal of the present study was to investigate the role of CCN3 in regulating the production and degradation of the ECM.
Materials and methods
Cell culture
Human mesangial cells (HMCs) were purchased from ScienCell Research Laboratories (Carlsbad, CA). The cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (GIBCO, Carlsbad, CA, USA) and 1% penicillin/streptomycin solution (HyClone, Utah, USA). The cells were grown at 37 °C in 5% CO2. After reaching 70%–80% confluence, the cells were synchronized with serum-free culture medium for 24 h. Then, rhCCN3 was added at various doses for various periods of time, as indicated. The HMCs were treated with TGF-β1 for 1 h after CCN3 treatment. Then, the HMCs were collected for further study.
TGF-β1
TGF-β1 for pharmacokinetic and efficacy studies was purchased from R&D systems (Minneapolis, USA) and was diluted in 4 mmol/L HCl and 1 mg/mL BSA.
rhCCN3
rhCCN3 for pharmacokinetic and efficacy studies was purchased from PeproTech (NJ, USA). The rhCCN3 used in these studies was produced in Escherichia coli. The expressed protein was full-length CCN3 containing 331 amino acid residues (36.2 kDa) but lacking a signal peptide, and it was not glycosylated.
Plasmid construction and transfection
The plasmid encoding CCN3 was constructed by GenScript. HMCs were transfected with the pcDNA3.1(+)-CCN3 plasmid. Transfection was performed using LipofectamineTM 2000 reagent (Invitrogen, CA, USA), and the cells were incubated in serum-free RPMI-1640 medium for 24 h according to the manufacturer's instructions. For transfection, 90% confluent cells were plated in six-well plates with 4 μg of plasmid DNA and 10 μL of Lipofectamine 2000.
Quantitative real-time PCR analysis
Total RNA was extracted using RNAiso Plus according to the manufacturer's directions (Takara, Dalian, China). RNA concentration and purity were confirmed with NanoDrop 2000 spectrophotometer (Thermo, MA, USA). Samples were used if they had a relative absorbance ratio of 260/280 nm between 1.8 and 2.0. cDNA was synthesized using a reverse transcription system kit (Takara, Liaoning, China). Real-time quantitative PCR was performed on an ABI PRISM 7300 real-time PCR System (Applied Biosystems, Foster City, CA, USA) with SYBR Green Real-time PCR Kit (Takara, Liaoning, China). The primer sequences are shown in Table 1. Relative mRNA values were normalized to those of β-actin and calculated using the comparative cycle threshold (ΔΔCt) method.
Table 1. Primer sequences for real-time RT-PCR.
| Gene | Strand | PCR primer sequence (5′–3′) | Accession number |
|---|---|---|---|
| FN | Sense | AAGCCCATAGCTGAGAAGTGTTTTG | NM_054034.2 |
| Anti-sense | GGATGTCCTTGTGTCCTGATCGT | ||
| COL1 | Sense | CGATGGATTCCAGTTCGAGTATG | NM_000088.3 |
| Anti-sense | TGTTCTTGCAGTGGTAGGTGATG3 | ||
| MMP-2 | Sense | TACAGGATCATTGGCTACACACC | NM_001127891.1 |
| Anti-sense | GGTCACATCGCTCCAGACT | ||
| MMP-9 | Sense | TGTACCGCTATGGTTACACTCG | NM_004994.2 |
| Anti-sense | GGCAGGGACAGTTGCTTCT | ||
| TIMP-1 | Sense | CTTCTGCAATTCCGACCTCGT | NM_003254.2 |
| Anti-sense | ACGCTGGTATAAGGTGGTCTG | ||
| β-Actin | Sense | TGACGTGGACATCCGCAAAG | NM_001101.3 |
| Anti-sense | CTGGAAGGTGGACAGCGAGG |
Western blot analysis
Total cellular protein was extracted by incubating the cells in lysis buffer according to the standard protocols. Protein concentrations were measured using a protein assay kit (KeyGEN, Jiangsu, China) according to the manufacturer's instructions. Equal amounts of protein from the samples were electrophoresed using 4%–20% SDS-polyacrylamide gel, transferred to PVDF membranes (Millipore, MA, USA) and blocked with 5% milk proteins. The blots were then incubated overnight at 4°C with primary antibodies against CCN3 (ab137677, Abcam, Cambridge, MA, USA), FN (ab2413, Abcam, Cambridge, MA, USA), collagen type I (ab34710, Abcam, Cambridge, MA, USA), MMP-2 (ab37150, Abcam, Cambridge, MA, USA), MMP-9 (#13667, CST, MA, USA) and TIMP-1 (sc-5538, Santa Cruz, CA, USA). The blots were washed and incubated with horseradish peroxidase-conjugated secondary antibodies as appropriate, and the signals were then detected using an advanced ECL system (Amersham Biosciences, Piscataway, NJ, USA). Relative protein levels were calculated by normalization to the level of β-actin protein.
Immunofluorescence staining
HMCs grown on coverslips were fixed with 4% paraformaldehyde for 15 min at 37 °C, permeabilized with 0.1% Triton X-100, and blocked with 10% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) at room temperature. The slides were then immunostained with primary antibodies against FN (ab2413, Abcam, Cambridge, MA, USA) and COLI (ab34710, Abcam, Cambridge, MA, USA) at 4°C overnight. After being washed with PBS, the cells were incubated with fluorescent secondary antibodies (goat anti-rabbit IgG H&L) (ab150077, Abcam, Cambridge, MA, USA) for 1 h at room temperature in the dark and rinsed with PBS. Images were captured using an Olympus FV 1000 Viewer.
Statistical analysis
The data are expressed as the mean±SD. Statistical significance was determined using one-way ANOVA for differences among multiple groups. A P value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 16.0.
Results
TGF-β1 down-regulates CCN3 expression in HMCs
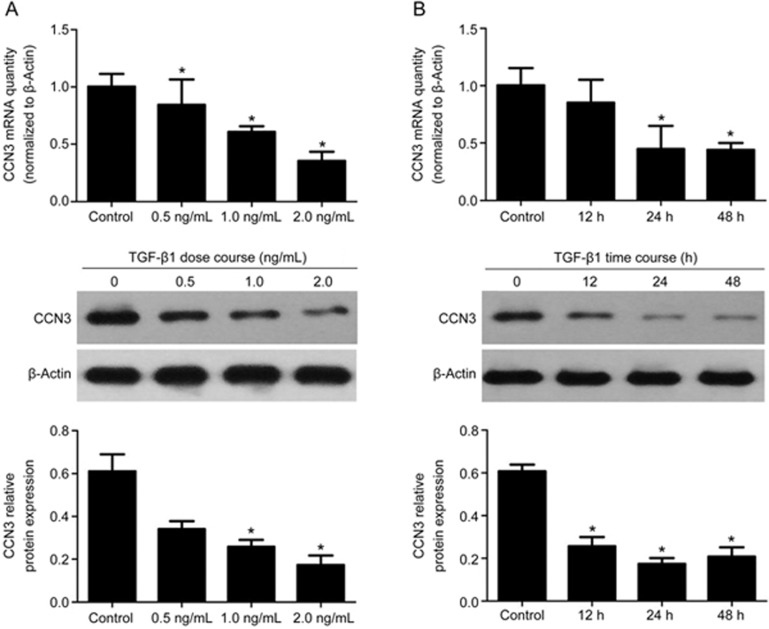
To evaluate the effect of TGF-β1 on CCN3 in HMCs, we assessed the expression levels of CCN3. On the basis of RT-PCR and Western blot analyses, the mRNA and protein levels of CCN3 decreased in response to TGF-β1 exposure in a dose- (0, 0.5, 1.0 and 2.0 ng/mL) and time-dependent manner (0, 12, 24 and 48 h) with the maximum effect at 2.0 ng/mL and 24 h (Figure 1, P<0.05), thus suggesting that TGF-β1 down-regulates CCN3 expression in HMCs.
Figure 1.
Effects of TGF-β1 on CCN3 expression. (A) Human mesangial cells (HMCs) were incubated for 24 h with increasing concentrations of TGF-β1 (0, 0.5, 1.0 and 2.0 ng/mL). (B) HMCs were grown in 2 ng/mL TGF-β1-containing medium for 12–48 h. Total RNA and cell protein were harvested for qRT-PCR or Western blotting, respectively. The data are expressed as the mean±SD. Experiments were repeated four times. *P<0.05 vs control.
Exogenous CCN3 treatment attenuates TGF-β1-induced ECM accumulation in HMCs.
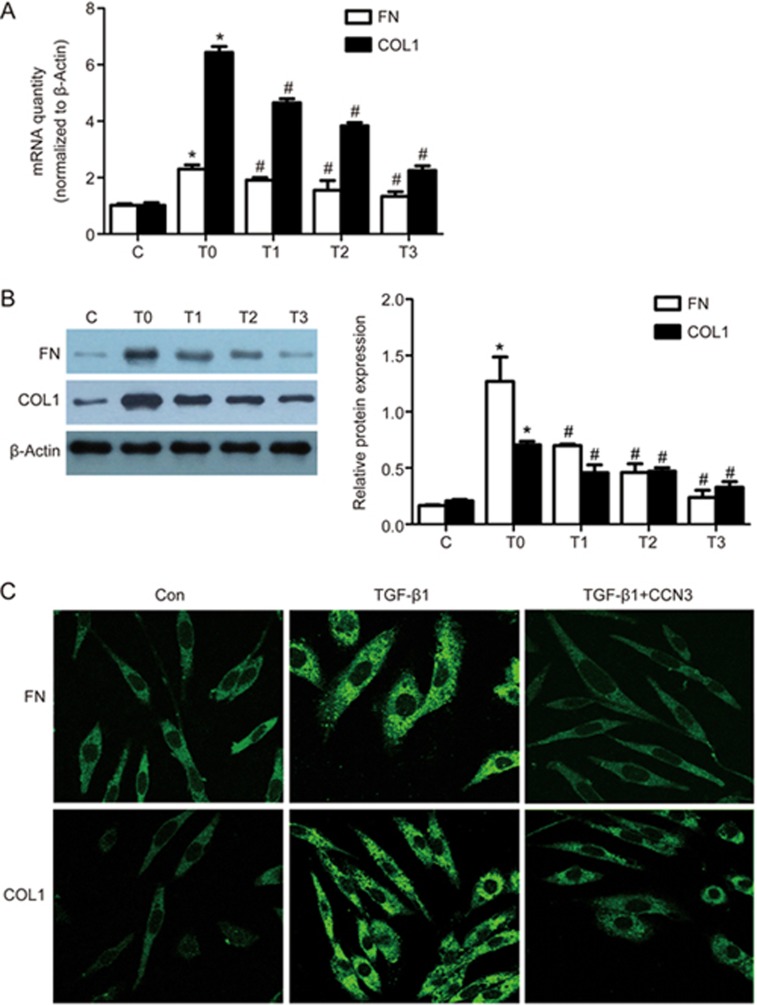
To explore the roles of CCN3 in TGF-β1-induced renal fibrosis and ECM accumulation, we determined the expression levels of fibronectin (FN) and type I collagen (COLI), which were used as markers of renal fibrosis. TGF-β1 treatment increased the mRNA and protein levels of both markers (P<0.05). However, a 1-h pretreatment of the cells with exogenous CCN3 down-regulated the expression of FN and COLI in a dose-dependent (0, 5, 50 and 500 ng/mL) manner, with the maximum effect at 500 ng/mL (P<0.05, Figure 2A, B), thus suggesting that exogenous CCN3 attenuates TGF-β1-induced ECM accumulation. We also examined the localization of FN and COLI in HMCs by using fluorescence microscopy. As shown in Figure 2C, the addition of CCN3 before TGF-β1 exposure prevented the up-regulation of FN and COLI.
Figure 2.
Effects of exogenous CCN3 on the expression of TGF-β1-induced fibronectin (FN) and type I collagen (COLI). HMCs were pretreated for 1 h with various doses of exogenous CCN3 and then incubated in RPMI-1640 medium containing TGF-β1 (2 ng/mL) for 24 h. RT-PCR and Western blotting were used to analyze the mRNA and protein levels of FN (A) and COLI (B). The data are expressed as the mean±SD. Experiments were repeated four times. C (control), T0 (TGF-β1, 2 ng/mL), T1 (TGF-β1, 2 ng/mL+CCN3, 5 ng/mL), T2 (TGF-β1, 2 ng/mL+CCN3, 50 ng/mL), T3 (TGF-β1, 2 ng/mL+CCN3, 500 ng/mL). *P<0.05 vs C. #P<0.05 vs T0. Representative immunofluorescence images showing FN (green) and COLI (green) labeling in HMCs (C). HMCs were treated without or with 2 ng/mL TGF-β1 or with 2 ng/mL TGF-β1 and 500 ng/mL CCN3 for 24 h in serum-free medium.
Exogenous CCN3 treatment improves the TGF-β1-induced expression of MMPs while inhibiting TIMP production in HMCs.
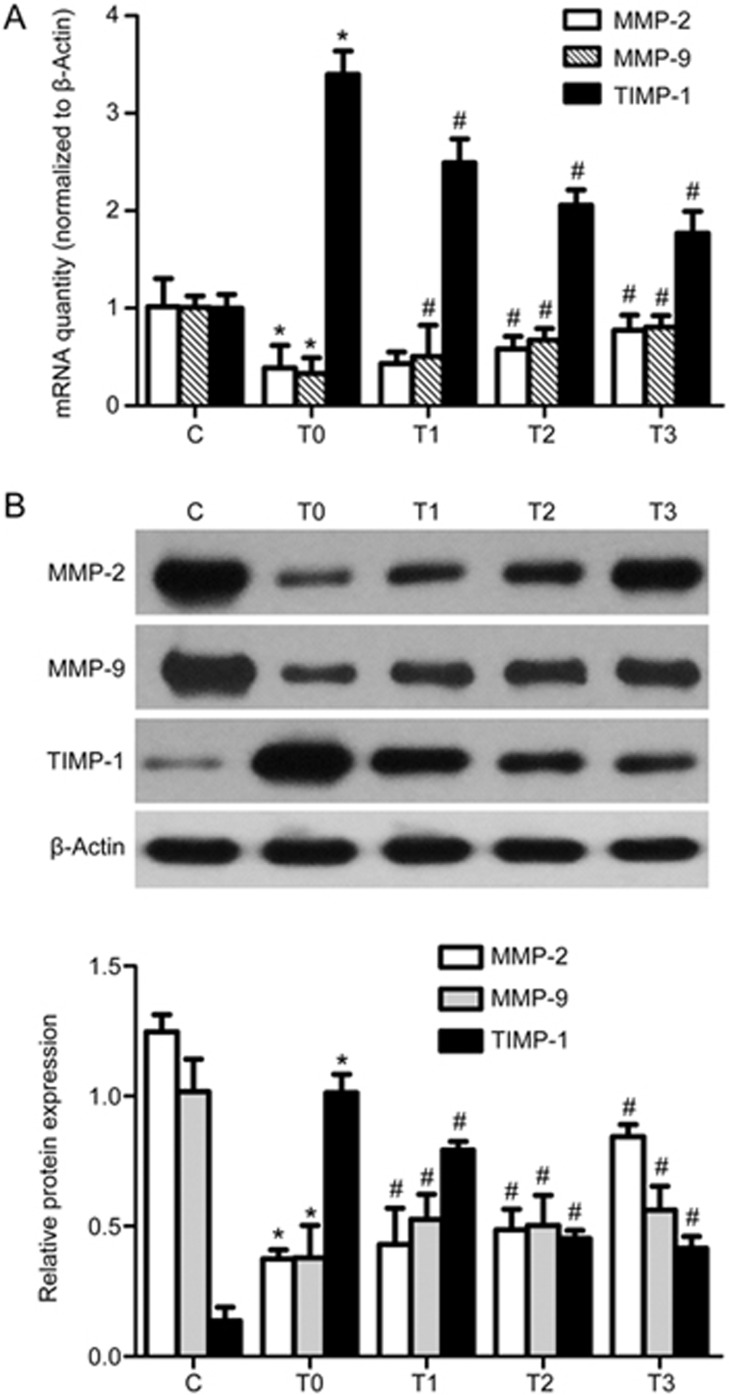
ECM accumulation is a net result of the balance between synthesis and degradation. Previous studies have demonstrated that MMPs and TIMPs play a role in the development of glomerular sclerosis and interstitial fibrosis. In particular, MMP-2 and MMP-9 have been studied extensively in kidneys and in renal transplantation models of acute allograft rejection and chronic allograft nephropathy. To further evaluate whether CCN3 promotes ECM degradation, we determined the expression levels of MMP-2, MMP-9 and TIMP-1. On the basis of RT-PCR and Western blot analyses of cell lysates, the mRNA and protein levels of MMP-2 and MMP-9 were up-regulated, and the level of TIMP-1 was significantly down-regulated by CCN3 pretreatment in a dose-dependent manner (0, 5, 50 and 500 ng/mL), with the maximum effect at 500 ng/mL (P<0.05) (Figure 3).
Figure 3.
Effects of exogenous CCN3 on the expression of TGF-β1-induced MMPs and TIMP. HMCs were pretreated for 1 h with various doses of exogenous CCN3 and then incubated in RPMI-1640 medium containing TGF-β1 (2 ng/mL) for 24 h. RT-PCR and western blotting were used to analyze the mRNA and protein levels of MMP-2, MMP-9 and TIMP-1 (A and B). The data are expressed as the mean±SD. Experiments were repeated four times. C (control), T0 (TGF-β1, 2 ng/mL), T1 (TGF-β1, 2 ng/mL+ CCN3, 5 ng/mL), T2 (TGF-β1, 2 ng/mL+CCN3, 50 ng/mL), T3 (TGF-β1, 2 ng/mL+CCN3, 500 ng/mL). *P<0.05 vs C. #P<0.05 vs T0.
Determination of transfection efficiency
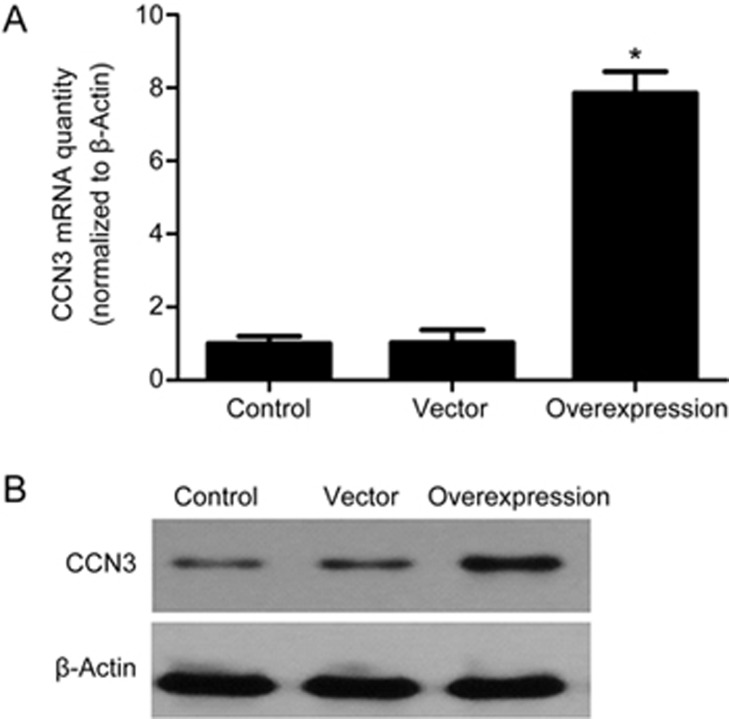
To test the efficiency of CCN3 transfection, RT-PCR and Western blot analyses were used to evaluate the expression of mRNA and protein. As shown in Figure 4, the HMCs in the control group expressed only low levels of CCN3. Similarly, transfection of the cells with pcDNA3.1(+) without CCN3 scarcely increased CCN3 expression. The expression of CCN3 significantly increased after transfection with pcDNA3.1(+)-CCN3. These results indicated that transfection with pcDNA3.1(+)-CCN3 effectively increases CCN3 expression in HMCs.
Figure 4.
Effects of CCN3 transfection (48 h) on the expression of CCN3 in HMCs were determined by RT-PCR (A) and Western blot analysis (B). The data are expressed as the mean±SD. Experiments were repeated four times. *P<0.05 vs control.
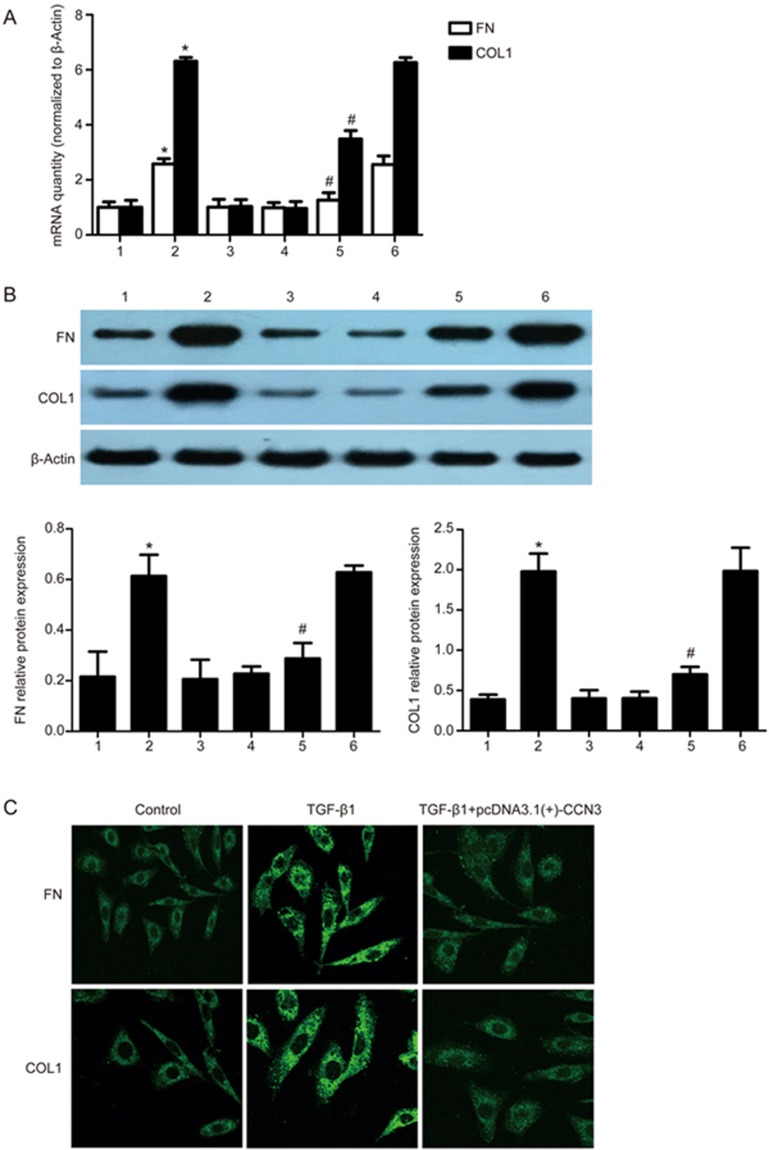
Overexpression of human CCN3 gene down-regulates FN and COLI in HMCs
To gain further insight into the effects of CCN3 on ECM formation, we performed further studies in which we exposed HMCs transfected with pcDNA3.1(+)-CCN3 to TGF-β1 (2 ng/mL). The protein and mRNA expression levels of FN and COLI were measured. The results showed that the protein and mRNA expression levels of FN and COLI were significantly lower in the TGF-β1+pcDNA3.1(+)-CCN3 group than in the TGF-β1 group and TGF-β1+pcDNA3.1(+)-blank vector group (P<0.05) (Figure 5A–D). We also examined the localization of FN and COLI in HMCs by using immunofluorescence microscopy as shown in Figure 5C.
Figure 5.
Overexpression of human CCN3 down-regulates FN and COLI in HMCs. We performed gene overexpression experiments via transfection with pcDNA3.1(+)-CCN3. mRNA and protein expression of FN and COLI was assessed by qRT-PCR or Western blotting (A and B). The data are expressed as the mean±SD. Experiments were repeated four times. 1: control, 2: TGF-β1 (2 ng/mL), 3: pcDNA3.1(+)-CCN3, 4: pcDNA3.1(+)-blank vector, 5: TGF-β1+pcDNA3.1(+)-CCN3, 6: TGF-β1+pcDNA3.1(+)-blank vector. *P<0.05 vs 1, 3 or 4. #P<0.05 vs 2 or 6. Representative immunofluorescence images showing FN (green) and COLI (green) labeling in HMCs (C). The first and second group of HMCs were treated without or with 2 ng/mL TGF-β1 for 24 h in serum-free medium. The third group of HMCs that were transfected with pcDNA3.1(+)-CCN3 was treated with 2 ng/mL TGF-β1 for 24 h in serum-free medium.
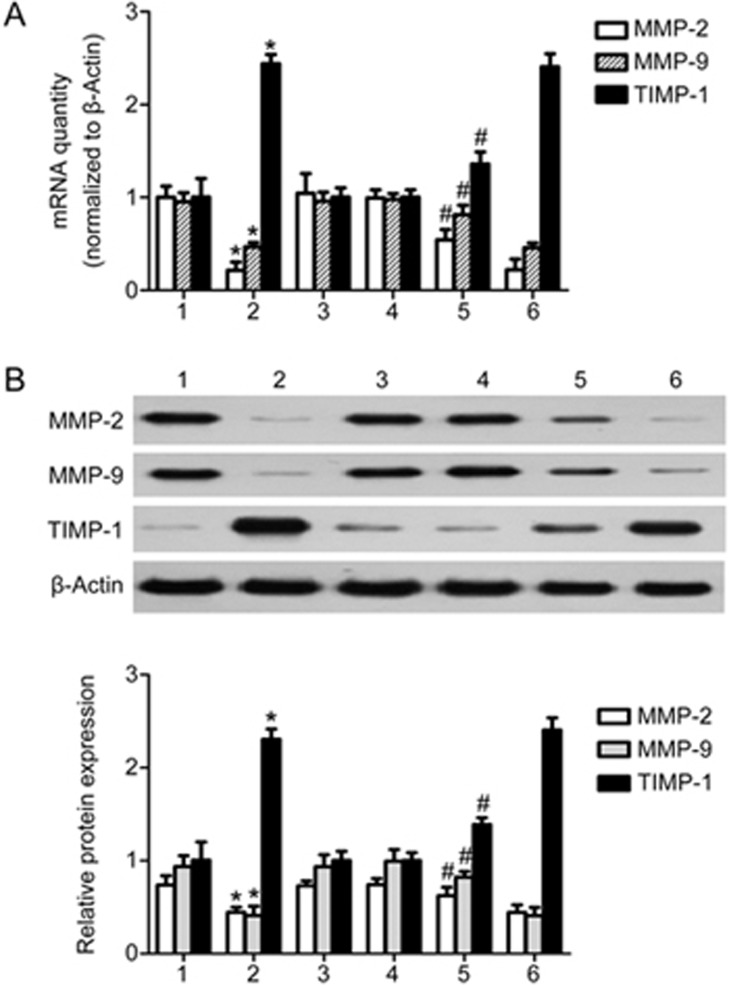
Overexpression of human CCN3 gene improves the TGF-β1-induced expression of MMPs while inhibiting the production of TIMP in HMCs
We also explored the influence of the overexpression of human CCN3 on the expression of MMP-2, MMP-9 and TIMP-1. As shown in Figure 6, the selective high degree of expression of CCN3 resulted in a significant increase in the expression of MMP-2 and MMP-9 at mRNA and protein levels in the TGF-β1+pcDNA3.1(+)-CCN3 group compared with the TGF-β1 group and TGF-β1+pcDNA3.1(+)-blank vector group. Furthermore, the expression of TIMP-1 was inhibited in the TGF-β1+ pcDNA3.1(+)-CCN3 group compared with the TGF-β1 group and TGF-β1+pcDNA3.1(+)-blank vector group (P<0.05).
Figure 6.
Overexpression of human CCN3 up-regulates expression of MMPs and down-regulates expression of TIMP in HMCs stimulated with TGF-β1. We performed gene overexpression experiments via transfection with pcDNA3.1(+)-CCN3. The mRNA and protein expression of MMP-2, MMP-9 and TIMP-1 was assessed by qRT-PCR or Western blotting (A, B). The data are expressed as the mean±SD. Experiments were repeated four times. 1: control, 2: TGF-β1 (2 ng/mL), 3: pcDNA3.1(+)-CCN3, 4: pcDNA(3.1+)-blank vector, 5: TGF-β1+pcDNA3.1(+)-CCN3, 6: TGF-β1+ pcDNA3.1(+)-blank vector. *P<0.05 vs 1, 3 or 4. #P<0.05 vs 2 or 6.
Discussion
In the present study, we provide the first demonstration that CCN3 not only inhibits ECM formation but also promotes TGF-β1-induced degradation of ECM in human mesangial cells. Our results provide evidence that CCN3 is a novel regulator of ECM formation, thus suggesting that CCN3 may play an antifibrotic role in the treatment of glomerulosclerosis.
Glomerulosclerosis, an end-point lesion common in various glomerular diseases, is characterized by mesangial cell proliferation and ECM accumulation. TGF-β1 and its downstream Smad signaling pathway play an essential role in the formation of ECM22. MMPs catalyze the turnover of ECM macromolecules such as interstitial and basement membrane collagen as well as accessory ECM proteins such as fibronectin23. Moreover, many studies have suggested that TIMPs are often up-regulated in diseased kidneys24. Our results suggested that these cytokines play an important role in glomerulosclerosis. Many researchers have been seeking new therapeutic approaches to treat glomerulosclerosis by targeting mesangial cells and ECM accumulation.
CCN3 was first discovered as an anti-fibrosis molecule that was shown to play a role in embryogenesis and growth modulation in cancer25. A large body of evidence has indicated that CCN3 is a negative regulator in the fibrotic pathway. For example, Roeyen et al26 have found that, in anti-Thy1.1 rats, CCN3 is an endogenous inhibitor of mesangial cell growth, on the basis of PDGF-induced mitogenesis. Furthermore, they have also identified pro-angiogenic and anti-proliferative effects (on mesangial cells) of CCN3 in experimental glomerulonephritis20. In the present study, we demonstrated that CCN3 treatment attenuates TGF-β1-induced ECM accumulation. We found that either the addition of CCN3 or CCN3 overexpression produced a marked down-regulation of FN and COLI induced by TGF-β1 in human mesangial cells. This finding is critically important, because TGF-β is a potent profibrotic element in diseases occurring in a variety of organ systems, including the kidney. However, the exact mechanistic role of CCN3 in the inhibition of ECM accumulation remains unclear.
As previously reported, emerging evidence suggests that collagen turnover in the kidney is tightly regulated by a balance between MMPs, particularly the two gelatinases MMP-2 and MMP-9, and their endogenous inhibitors, TIMPs. In addition, MMP-9 has been shown to cleave type I and III collagen, both in vitro and in vivo27. We therefore hypothesized that CCN3 may act through MMPs and TIMPs in promoting ECM degradation in human mesangial cells. Increasing evidence shows that CCN3 differentially modulates the expression of MMP-1, MMP-3, MMP-2, MMP-9, and MMP-13 with respect to its pro- and anti-motility effects in a cell type-dependent manner28,29,30,31. Moreover, in vivo studies have indicated that CCN3 attenuates inflammation through the regulation of MMP-2 and MMP-929. Together, the findings in these studies demonstrate the important role of CCN3 in regulating the expression of MMP-2 and MMP-9. Our results further support these earlier findings. We demonstrated that CCN3 up-regulates the expression of MMP-2 and MMP-9, both of which are inhibited by TGF-β1 in human mesangial cells. Simultaneously, the expression of TIMP-1 is down-regulated.
In conclusion, our findings demonstrate that CCN3 decreases the production of ECM and promotes TGF-β1-induced ECM degradation. CCN3 may serve as a novel therapeutic tool to alleviate glomerulosclerosis. However, the detailed mechanistic role of CCN3 in regulating ECM accumulation requires further investigation.
Author contribution
Hai-fei LIU, Hong LIU, and Long CHEN designed research; Hai-fei LIU, Hong LIU , and Bi-cheng LIU performed research; Hai-fei LIU, Lin-li LV, and Kun-ling MA contributed new analytical tools and reagents; Hai-fei LIU, Hong LIU , and Yi WEN analyzed data; Hai-fei LIU, Hong LIU, and Long CHEN wrote the paper.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No 81300596), the Fifth Phase High-level Personnel Training Project of Jiangsu Provincial and the Jiangsu Provincial Special Program of Medical Science (No BK20161437).
References
- Randles MJ, Woolf AS, Huang JL, Byron A, Humphries JD, Price KL, et al. Genetic background is a key determinant of glomerular extracellular matrix composition and organization. J Am Soc Nephrol 2015; 26: 3021–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornigold N, Craven RA, Keen JN, Johnson T, Banks RE, Mooney AF. Upregulation of Hic-5 in glomerulosclerosis and its regulation of mesangial cell apoptosis. Kidney Int 2010; 77: 329–38. [DOI] [PubMed] [Google Scholar]
- López-Hernández FJ, López-Novoa JM. Role of TGF-β in chronic kidney disease: an integration of tubular, glomerular and vascular effects. Cell Tissue Res 2012; 347: 141–54. [DOI] [PubMed] [Google Scholar]
- Marchal PO, Kavvadas P, Abed A, Kazazian C, Authier F, Koseki H, et al. Reduced NOV/CCN3 expression limits inflammation and interstitial renal fibrosis after obstructive nephropathy in mice. PLoS One 2015; 10: e0137876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon R, Byron A, Humphries JD, Randles MJ, Carisey A, Murphy S, et al. Global analysis reveals the complexity of the human glomerular extracellular matrix. J Am Soc Nephrol 2014; 25: 939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Zhou LL, Cheng Q, Jiang SN, Sheng J, Sun JD, et al. Effect of bradykinin on renal mesangial cell proliferation and extracellular matrix secretion. Genet Mol Res 2014; 13: 490–8. [DOI] [PubMed] [Google Scholar]
- Rupprecht HD, Schöcklmann HO, Sterzel RB. Cell-matrix interactions in the glomerular mesangium. Kidney Int 1996; 49: 1575–82. [DOI] [PubMed] [Google Scholar]
- Hu C, Sun L, Xiao L, Han Y, Fu X, Xiong X, et al. Insights into the mechanisms involved in the expression and regulation of extracellular matrix proteins in diabetic nephropathy. Curr Med Chem 2015; 22: 2858–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riser BL, Cortes P, Yee J. Modelling the effects of vascular stress in mesangial cells. Curr Opin Nephrol Hypertens 2000; 9: 43–7. [DOI] [PubMed] [Google Scholar]
- Li W, Saji S, Sato F, Noda M, Toi M. Potential clinical applications of matrix metalloproteinase inhibitors and their future prospects. Int J Biol Markers 2013; 28: 117–30. [DOI] [PubMed] [Google Scholar]
- Coronato S, Laguens G, Di GV. Role of metalloproteinases and their inhibitors in tumors. Medicina (B Aires) 2012; 72: 495–502. [PubMed] [Google Scholar]
- Kapoor C, Vaidya S, Wadhwan V, Kaur G, Pathak A. Seesaw of matrix metalloproteinases (MMPs). J Cancer Res Ther 2016; 12: 28–35. [DOI] [PubMed] [Google Scholar]
- Chung B, Hinek A, Keating S, Weksberg R, Shah V, Blaser S, et al. Overgrowth with increased proliferation of fibroblast and matrix metalloproteinase activity related to reduced TIMP1: a newly recognized syndrome. Am J Med Genet A 2012; 158A: 2373–81. [DOI] [PubMed] [Google Scholar]
- Martinerie C, Viegas-Pequignot E, Guenard I, Dutrillaux B, Nguyen VC, Bernheim A, et al. Physical mapping of human loci homologous to the chicken nov proto-oncogene. Oncogene 1992; 7: 2529–34. [PubMed] [Google Scholar]
- Brigstock DR. The CCN family: a new stimulus package. J Endocrinol 2003; 178: 169–75. [DOI] [PubMed] [Google Scholar]
- Leask A. Yin and Yang revisited: CCN3 as an anti-fibrotic therapeutic. J Cell Commun Signal 2015; 9: 97–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Hou Y, Ma S, Tao Y, Li J, Cao H, et al. Effects of CCN3 on fibroblast proliferation, apoptosis and extracellular matrix production. Int J Mol Med 2014; 33: 1607–12. [DOI] [PubMed] [Google Scholar]
- Riser BL, Najmabadi F, Garchow K, Barnes JL, Peterson DR, Sukowski EJ. Treatment with the matricellular protein CCN3 blocks and/or reverses fibrosis development in obesity with diabetic nephropathy. Am J Pathol 2014; 184: 2908–21. [DOI] [PubMed] [Google Scholar]
- Riser BL, Najmabadi F, Perbal B, Peterson DR, Rambow JA, Riser ML, et al. CCN3 (NOV) is a negative regulator of CCN2 (CTGF) and a novel endogenous inhibitor of the fibrotic pathway in an in vitro model of renal disease. Am J Pathol 2009; 174: 1725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roeyen CR, Boor P, Borkham-Kamphorst E, Rong S, Kunter U, Martin IV, et al. A novel, dual role of CCN3 in experimental glomerulonephritis: pro-angiogenic and antimesangioproliferative effects.t. Am J Pathol 2012; 180: 1979–90. [DOI] [PubMed] [Google Scholar]
- Chen L, Wu YG, Liu D, Lv LL, Zheng M, Ni HF, et al. Urinary mRNA expression of CCN2/CCN3 as a noninvasive marker for monitoring glomerular structure changes in nondiabetic chronic kidney disease. Biomarkers 2012; 17: 714–20. [DOI] [PubMed] [Google Scholar]
- Meng XM, Tang PM, Li J, Lan HY. TGF-β/Smad signaling in renal fibrosis. Front Physiol 2015; 6: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci 2006; 11: 1696–701. [DOI] [PubMed] [Google Scholar]
- Ahmed AK, Haylor JL, El NAM, Johnson TS. Localization of matrix metalloproteinases and their inhibitors in experimental progressive kidney scarring. Kidney Int 2007; 71: 755–63. [DOI] [PubMed] [Google Scholar]
- Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol 2001; 54: 57–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roeyen CR, Eitner F, Scholl T, Boor P, Kunter U, Planque N, et al. CCN3 is a novel endogenous PDGF-regulated inhibitor of glomerular cell proliferation. Kidney Int 2008; 73: 86–94. [DOI] [PubMed] [Google Scholar]
- Bigg HF, Rowan AD, Barker MD, Cawston TE. Activity of matrix metalloproteinase-9 against native collagen types I and III. FEBS J 2007; 274: 1246–55. [DOI] [PubMed] [Google Scholar]
- Fukunaga-Kalabis M, Martinez G, Telson SM, Liu ZJ, Balint K, Juhasz I, et al. Downregulation of CCN3 expression as a potential mechanism for melanoma progression. Oncogene 2008; 27: 2552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kular L, Rivat C, Lelongt B, Calmel C, Laurent M, Pohl M, et al. NOV/CCN3 attenuates inflammatory pain through regulation of matrix metalloproteinases-2 and -9. J Neuroinflammation 2012; 9: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CG, Chen CC, Leu SJ, Grzeszkiewicz TM, Lau LF. Integrin-dependent functions of the angiogenic inducer NOV (CCN3): implication in wound healing. J Biol Chem 2005; 280: 8229–37. [DOI] [PubMed] [Google Scholar]
- Tzeng HE, Chen JC, Tsai CH, Kuo CC, Hsu HC, Hwang WL, et al. CCN3 increases cell motility and MMP-13 expression in human chondrosarcoma through integrin-dependent pathway. J Cell Physiol 2011; 226: 3181–9. [DOI] [PubMed] [Google Scholar]