Abstract
Normal aging is associated with low-grade neuroinflammation that results from age-related priming of microglial cells. Further, aging alters the response to several anti-inflammatory factors, including interleukin (IL)-4 and IL-13. One intervention that has been shown to modulate microglia activation in the aged brain, both basally and following an immune challenge, is exercise. However, whether engaging in exercise can improve responsiveness to anti-inflammatory cytokines is presently unknown. The current study evaluated whether prior exercise training increases sensitivity to anti-inflammatory cytokines that promote the M2 (alternative) microglia phenotype in adult (5-month-old) and aged (23-month-old) C57BL/6J mice. After 8 weeks of exercise or control housing, mice received bilateral hippocampal injections of an IL-4/IL-13 cocktail or vehicle. Twenty-four hours later hippocampal samples were collected and analyzed for expression of genes associated with the M1 (inflammatory) and M2 microglia phenotypes. Results show that IL-4/IL-13 administration increased expression of the M2-associated genes Fizz1, Ym1, Arginase-1 (Arg1), SOCS1, IL-1ra, and CD206. In response to IL-4/IL-13 administration, aged mice showed increased hippocampal expression of the M2-related genes Arg1, SOCS1, Ym1, and CD206 relative to adult mice. Aged mice also showed increased expression of IL-1β relative to adults, which was unaffected by wheel running or IL-4/IL-13. Wheel running was found to have modest effects on expression of Ym1 and Fizz1 in aged and adult mice. Collectively, our findings indicate that aged mice show a differential response to anti-inflammatory cytokines relative to adult mice and that exercise has limited effects on modulating this response.
Keywords: M2, anti-inflammatory, wheel running, IL-1ra, microglia, cytokine
INTRODUCTION
Microglia are the resident immune cells of the central nervous system. In their ramified resting state these cells constantly scan the microenvironment and upon detecting a change, they rapidly activate (Kettenmann et al., 2011). The form of this activation is dependent on the stimulus encountered. Detection of any pathological changes or inflammatory molecules induces microglia to express the classic inflammatory form of activation, referred to as the M1 phenotype (Kreutzberg, 1996). M1 microglia increase levels of the activation markers CD86, major histocompatibility complex II and CD11b, proliferate, and release a host of proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α (Kettenmann et al., 2011). Induction of the M1 phenotype provides a rapid and non-specific immune response in order to clear an invading pathogen by triggering inflammation. In contrast, microglia are also capable of expressing an alternative or M2 phenotype. This activation state is neuroprotective, characterized by the release of anti-inflammatory molecules including IL-4, IL-13, and IL-10 as well as neurotrophic factors and is thought to promote healing through the resolution of inflammation (Mosser, 2003, Ponomarev et al., 2007, Pepe et al., 2014). Additionally, the M2 phenotype increases levels of arginase-1 (Arg1) which contributes to wound healing and matrix deposition, chitinase-like 3 (Ym1), found in inflammatory zone 1 (Fizz1) which promotes deposition of the extracellular matrix, and CD206 a mannose receptor (Cherry et al., 2014). Prior work has shown that microglia can be shifted to this neuroprotective phenotype through exposure to IL-4 and/or IL-13 (Butovsky et al., 2005, Lee et al., 2013). M2 microglia have been further broken down into the functional sub-phenotypes M2a, which deals with repair/regeneration, M2b, which is immunoregulatory, and M2c, which is associated with acquired-deactivation (Chhor et al., 2013). These M2 categories were originally described in peripheral macrophages, but microglia show similar forms of activation (Mosser, 2003, Mosser and Edwards, 2008). M2a and M2c phenotypes are known to reduce M1 inflammatory cytokines while increasing the anti-inflammatory cytokines IL-10 and IL-4 (Roszer, 2015). Clearly, cells expressing the M2 phenotype mediate the resolution of inflammation and allow an organism to recover from an insult.
As the brain ages, microglia become primed towards the inflammatory M1 state (Sierra et al., 2007). These age-related changes translate to an increase in basal levels of inflammatory cytokines as well as a prolonged neuroinflammatory and behavioral response following an immune challenge (Godbout et al., 2005, Sierra et al., 2007, Dilger and Johnson, 2008). An attenuated response to regulatory factors that limit microglial cell activation likely contributes to the development of low-grade chronic inflammation within the aged brain. (Fenn et al., 2012, Lee et al., 2013, Norden and Godbout, 2013). For instance, aged animals show reduced expression of CD200, which is released by neurons and reduces microglial cell activation (Frank et al., 2006). Additionally, following exposure to the bacterial endotoxin lipopolysaccharide (LPS), microglia from aged mice exhibit prolonged down-regulation of the fractalakine receptor. Activation of the fractalakine receptor helps maintain microglia in a resting state as well as attenuate inflammation during recovery from an immune challenge (Wynne et al., 2010, Norden and Godbout, 2013). Further, Fenn et al. (2012) report that exposing M1 activated microglia from adult mice to IL-4 induced the M2 anti-inflammatory phenotype as evidenced by increased levels of Arg1, IL-10, suppressor of cytokine signaling (SOCS)-1, and SOCS3. However, M1 microglia from aged mice were unresponsive to IL-4 exposure and maintained a classically activated phenotype. In addition, aged mice failed to show an increase in the surface expression of IL-4 receptor-alpha following an immune challenge (Fenn et al., 2012), indicating that age-related deficits in the IL-4 and IL-13 signaling pathways likely contribute to aberrant microglia activation. Lee et al. (2013) administered an IL-4/IL-13 cocktail without prior cell activation and found that three days post treatment aged mice had lower expression of Fizz1 and failed to induce Arg1, Ym1, and insulin-like growth factor (IGF)-1 compared to adult and middle-aged mice, providing further evidence that induction of the M2 response following stimulation with IL-4/IL-13 is diminished in the aged.
One possible intervention for attenuating the age-related dysfunction of microglia is exercise. In aged animals exercise has been shown to down-regulate microglia activation, attenuate LPS-induced IL-1β production, decrease microglia proliferation, and increase the proportion of microglia that co-label with IGF-1 and brain derived neurotrophic factor (BDNF) (Nichol et al., 2008, Barrientos et al., 2011, Kohman et al., 2012, Littlefield et al., 2015). However, reductions in LPS-induced cytokine expression are not consistently seen. For example, prior work found that voluntary wheel running did not attenuate LPS-induced reduction in BDNF or increases in TNF-α, IL-1β, IL-6, and IL-10 in aged mice (Martin et al., 2013, Martin et al., 2014). In the absence of an immune challenge, exercise has been shown to increase levels of anti-inflammatory cytokines such as IL-10 as well as neurotrophic factors such as BDNF in the brain of young mice (de Almeida et al., 2013). Collectively, the evidence indicates that exercise may modify microglia activation in the aged brain, potentially attenuating the age-related priming toward the classic inflammatory phenotype. Whether exercise is capable of modulating how microglia in the aged brain respond to M2-inducing signals is currently unknown.
Age-related changes in immune function appear to alter the response to M2-inducing stimuli. Exercise has been shown to attenuate certain aspects of the age-related priming of microglia towards the M1 phenotype. Whether exercise alters the ability of aged subjects to express the M2 phenotype is presently unknown. The objective of the current study was to determine whether prior exercise increases microglia responsiveness to anti-inflammatory cytokines in aged animals. Specifically, we determined whether exercise in the form of voluntary wheel running alters hippocampal expression of M2 (i.e., Arg1, Ym1, Fizz1, IL-1 receptor antagonist [IL-1ra], transforming growth factor-β [TGF-β], CD206, and SOCS1) and M1 (i.e., IL-1β) associated genes in adult and aged mice following infusion of the anti-inflammatory cytokines IL-4 and IL-13.
EXPERIMENTAL PROCEDURES
Experimental subjects
Subjects were 31 adult (5-month-old) and 28 aged (23-month-old) C57BL/6J male mice. Aged mice were purchased from the National Institute on Aging rodent colony maintained by Charles River and adult mice were bred in-house from breeding stock purchased from The Jackson Laboratory (Bar Harbor, Maine). Mice were individually housed under a reverse light/dark cycle. Throughout the experiment mice were given free access to food and water. Experimental procedures and animal care were in accordance with the Guide for the Care and Use of Laboratory Animals and an approved protocol reviewed by the Institutional Animal Care and Use Committee at the University of North Carolina Wilmington.
Experimental design
Half of the adult and aged mice were semi-randomly assigned to the exercise condition and were individually housed in polypropylene cages (36 cm L × 20 cm W × 14 cm H) containing a running wheel (23 cm diameter; Respironics, Bend, OR). Mice had 24-hour access to the running wheel. The individual wheel cages were connected to a computer running the Vital View software (Respironics, Bend, OR) that collected the number of wheel rotations per minute. The remaining adult and aged mice were assigned to the control condition and were housed individually (29 cm L × 19 cm W × 13 cm H) without a running wheel. Following eight weeks of exercise or control housing, all mice received bilateral hippocampal injections of either an M2 promoting cytokine cocktail (containing IL-4 and IL-13) or vehicle (0.2M phosphate buffered saline (PBS)), procedure described below. Within an age group mice were assigned to receive the cytokine cocktail or PBS injection based on their body weight. For mice in the exercise condition, the total distance ran the week prior to treatment was also taken into consideration when assigning mice to the cytokine cocktail or PBS treatment group. These assignment parameters ensured that within an age group there were no differences in body weight or exercise levels between the treatment conditions. In total, each of the eight treatment conditions contained 7–8 mice per group.
Intra-hippocampal infusion procedure
In preparation all mice were given a subcutaneous (s.c.) injection of the analgesic, buprenorphine (0.05 mg/kg), 15 minutes prior to being anesthetized. Mice were placed in a small chamber and anesthetized using isoflurane (Allivet, St. Hialeah, FL) at 2.5–3% in air at 2.5 liters/minute, both of which were delivered through a vaporizer into the chamber. Once fully anesthetized the head was shaved, the mice were placed in the stereotax, and the eyes were coated with Vaseline to prevent corneal drying throughout the surgery. During the surgery, isoflurane was continuously delivered via a nose cone and levels were dropped to 1.5% and air was delivered at 1.5 liters/min. An incision was made to expose the skull and bregma was located for each individual animal. Bilateral hippocampal infusions were made −2.10 mm anteroposterior (Y), ± 1.25 mm lateral (X), −1.80 mm dorsal/ventral (Z) to bregma. A guarded 26-gauge needle was used to drill through the skull in order to allow passage of the infusion needle into the hippocampus. A 5.0 µl Hamilton syringe (Hamilton, Reno, NV) controlled by a Quintessential Stereotaxic Injector (Stoelting, Wood Dale, Illinois) was used to inject the cocktail of M2 promoting cytokines containing IL-4 (400 ng) and IL-13 (120 ng) in a total volume of 4 µl (2 µl per side) or an equivalent volume of vehicle (0.2M PBS) into the hippocampus. The vehicle or cytokine cocktail were infused at a rate of 0.5µl/min. The syringe was left in place for 5 minutes after the infusion was complete. Vetbond tissue adhesive was then used to close the incision. Bupivacaine at a dose of 2.5 mg/kg was given as a s.c. injection near the incision site. In order to replace fluids all mice received an intraperitoneal injection of 0.9% sterile saline (700 cc) before being placed in a recovery cage on top of a heating pad. Mice were monitored every 15 minutes for the first hour after surgery and then once an hour for the next 3 hours. To minimize discomfort, all mice received a second injection of buprenorphine (0.05 mg/kg s.c.) 8–12 hours after surgery. Individuals performing the infusion procedure were blinded to the animals housing condition (i.e., exercise or control) and age, though adult and aged mice are often visually distinct.
Tissue collection
Mice were sacrificed 24 hours after the vehicle or M2 cocktail infusion via transcardial perfusion with 0.9% RNase-free saline. Hippocampus samples within 1mm of the infusion sites were dissected on ice using a brain block and immediately placed in RNAlater solution (Qiagen, Valencia, CA) and kept at −20°C.
qRT-PCR
RNA was extracted from hippocampal samples using the RNeasy Mini kit (Qiagen, Valencia, CA). The purity of extracted RNA was assessed by a Gen5 Epoch spectrophotometer (BioTek Instruments, Highland Park, VT); all samples exceeded a purity (260/280) of 1.95. The High-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) was used to convert the extracted RNA into cDNA, which was run in a thermal cycler using the following protocol: 10 min at 25°C, 120 min at 37°C, and 5 min at 85°C. cDNA samples were stored at −20° C before conducting real-time polymerase chain reaction (RT-PCR) to assess relative changes in the specific mRNA transcripts. Hippocampal samples were analyzed for expression of the target genes IL-1β (Mm00434228_m1), Fizz1 (Mm00445109_m1), mannose receptor (CD206; Mm00485148_m1), IL-1ra (Mm00446186_m1), SOCS1 (Mm0078255_s1), Ym1 (Mm00657889_mH), Arg1 (Mm00475988_m1), and TGF-β (Mm01178820_m1). For TGF-β, there was insufficient RNA remaining in several samples, therefore levels of TGF-β was assessed using fewer samples, with treatment conditions ranging from 4–8 mice per group. Expression levels of the target genes were normalized with the endogenous control gene β-actin (Mm00607939_s1). There were no significant differences in β-actin expression across groups. All samples were run in triplicate for the target genes and β–actin by an individual blinded to the treatment groups. Samples were run in 384-well plates, with each well containing a 10 µl reaction that consisted of cDNA (80 ng), master mix, and probe/primer. TaqMan™ probe and primer sets were used to determine relative levels of the target and control gene(s) (Applied Biosystems, Foster City, CA). Samples were run in an Applied Biosystems Viia7 PCR instrument (Applied Biosystems, Foster City, CA) with the following cycling conditions: 2 min at 50°C, 10 min at 95°, and 40 cycles of 15 sec at 95°C and 1 min at 60°C. Data analysis for RT-PCR (DART) was used to determine whether differences in amplification efficiency existed between treatment conditions as well as between individual samples within a condition (Peirson et al., 2003). Given that small changes in amplification efficiency can have sizable effects on gene expression, samples that showed significant variation in amplification efficiency were removed for a given gene. All treatment conditions were confirmed to have similar amplification efficiencies. Data were then analyzed with the 2−ΔΔCT method to determine relative changes in gene expression compared to the adult control vehicle-treatment mice.
Statistical analyses
Body weight data and wheel running data (distance ran) were analyzed by repeated measures ANOVA with age as the between-subject factor and day or week as the within-subject factor. All data had equal variance across treatment groups. Normality was determined by the Shapiro-Wilk test. When needed, log or exponential transformations were employed to achieve normality. Gene expression data were analyzed by three-way ANOVA with age (adult or aged), exercise condition (exercise or control), and infusion treatment (IL-4/IL-13 cocktail or vehicle) as the between-subject variables. If the overall F for an interaction was significant, Fisher’s least significant difference was used as the post hoc test to identify which groups were significantly different. A p<0.05 was considered statistically significant.
RESULTS
Wheel running data
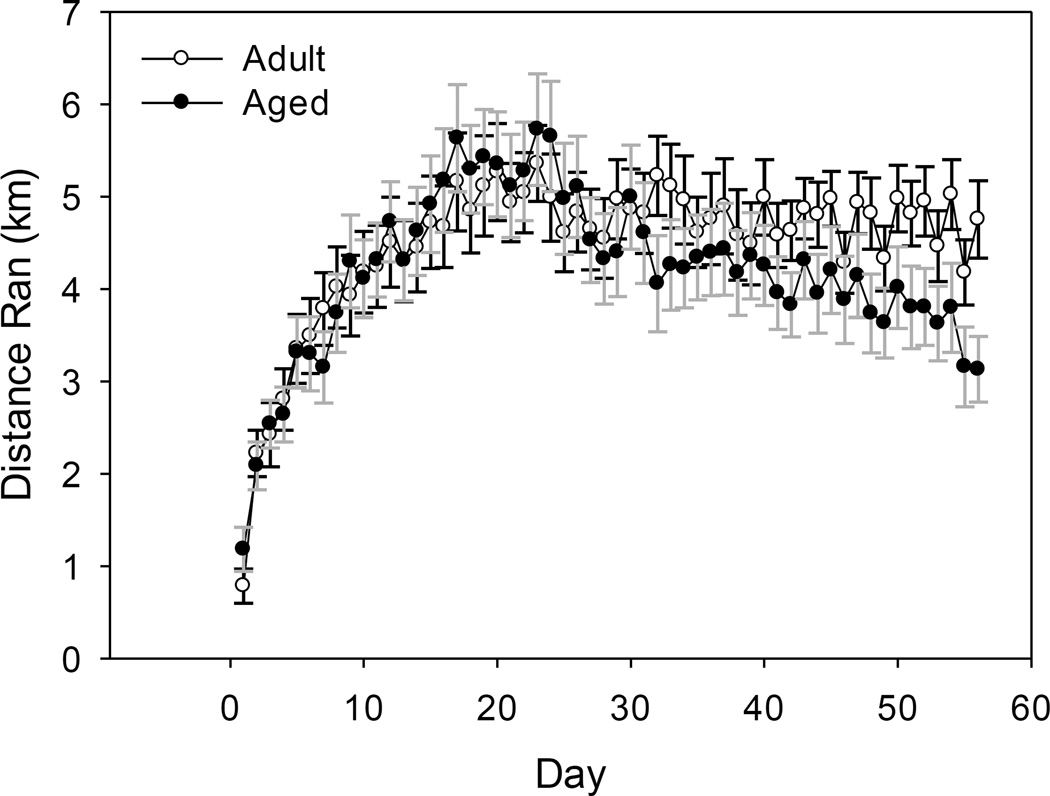
A significant age by day interaction showed that on select days during the 8 weeks of exercise the adult mice ran a longer distance than the aged mice (F(55, 1540)=2.04, p<0.001, see Figure 1). Overall the adult mice ran an average of 4.48 km/day and the aged mice ran an average of 4.18 km/day.
Figure 1.
Average distance (km) run per day by adult and aged mice over eight weeks of running wheel access. A significant age by day interaction showed that adult mice ran a farther distance than aged mice on select days (p<0.001). Lines represent means ± standard error of the mean (SEM).
Hippocampus RNA M1 Marker: IL-1β
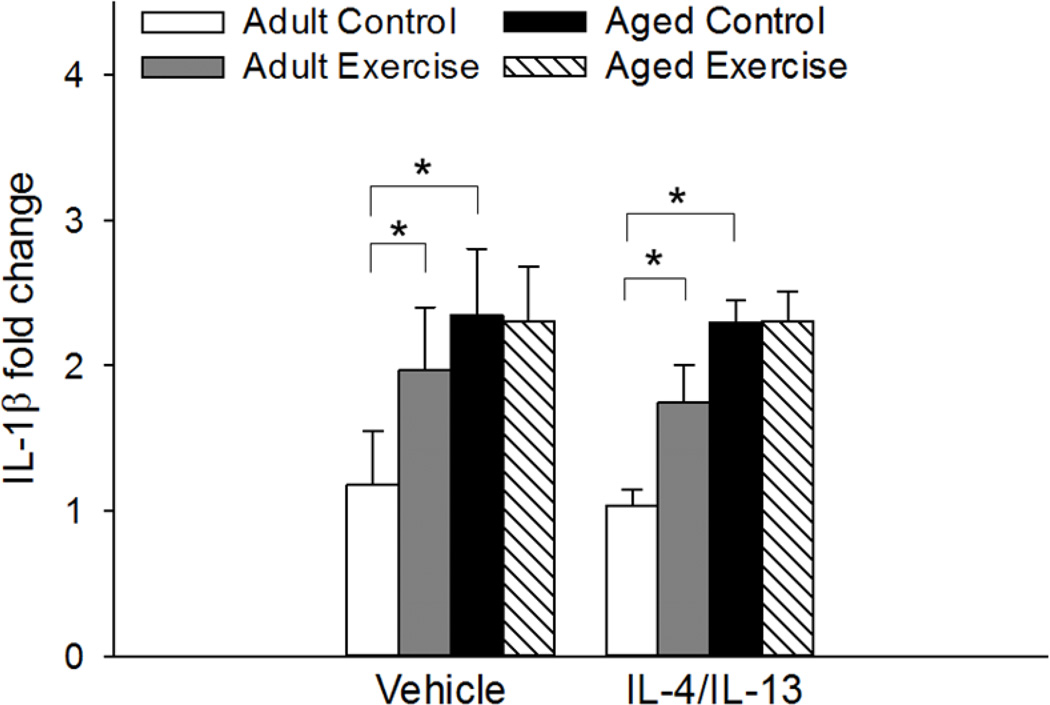
A significant main effect of age as well as an age by exercise condition interaction for hippocampal expression of IL-1β (F(1, 51)=15.787, p<0.001; F(1, 51)=4.41, p<0.05, respectively, see Figure 2) showed that aged control mice, regardless of their treatment condition, had higher levels of IL-1β compared to adult control mice (p<0.001). Exercise increased levels of IL-1β in adult, but not aged, mice (p<0.01). IL-4/IL-13 administration had no effect on IL-1β expression, as vehicle- and IL-4/IL-13-treated mice did not differ.
Figure 2.
Hippocampal expression of IL-1β was significantly increased in aged control mice relative to the adult control mice regardless of their treatment condition (p<0.001). Exercise increased expression of IL-1β in adult mice (p<0.01). IL-4/IL-13 treatment did not influence expression. Fold change is expressed relative to adult vehicle-treated control mice. * Indicates a significant difference between groups. Bars represent means ± SEM.
Hippocampus RNA M2 Markers: Fizz1, Ym1, Arg1, CD206, IL-1ra, SOCS1, and TGF-β
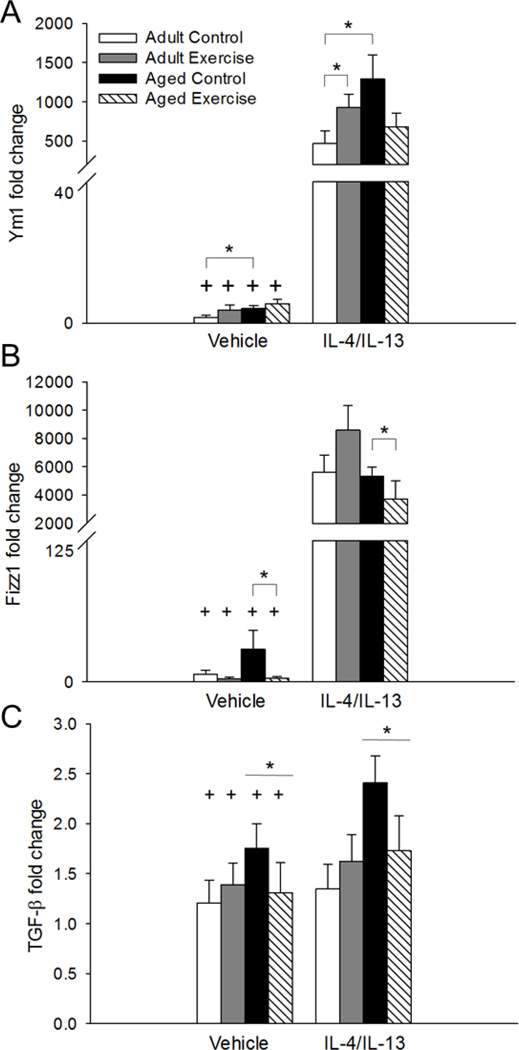
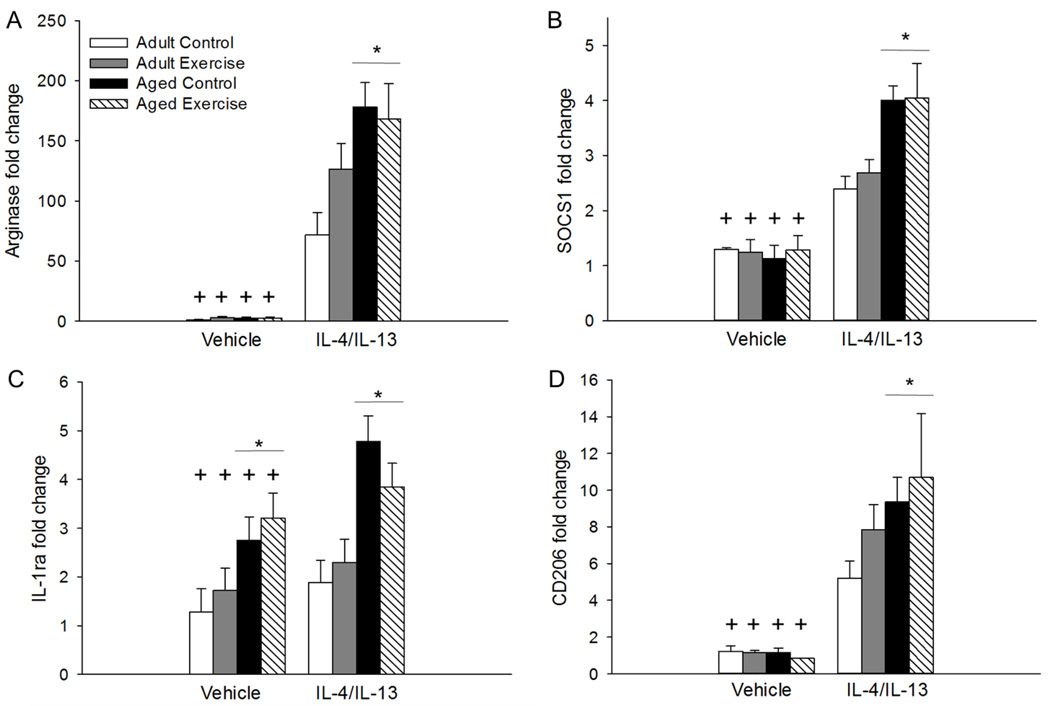
Administration of IL-4/IL-13 increased expression of all M2 genes relative to vehicle-treated mice, as shown by significant main effects of treatment for hippocampal expression of Ym1 (F(1, 51)=721.69, p<0.001, see Figure 3A), Fizz1 (F(1, 51)=711.75, p<0.001, see Figure 3B), TGF-β (F(1, 43)=7.52, p<0.005, see Figure 3C), Arg1 (F(1, 51)=414.596, p<0.001, see Figure 4A), SOCS1 (F(1, 47)=136.70, p<0.001, see Figure 4B), IL-1ra (F(1, 51)=7.34, p<0.01, see Figure 4C), and mannose receptor (CD206; F(1, 51)=205.46, p<0.001, see Figure 4D).
Figure 3.
IL-4/IL-13 treatment increased hippocampal expression of Ym1 (p<0.001; A), Fizz1 (p<0.001; B), and TGF-β (p<0.005; C) compared to vehicle-treated mice. Ym1 expression was higher in aged control mice compared to adult controls given either vehicle or IL-4/IL-13 treatment (p<0.05; A). However, there was no difference in Ym1 expression between the adult and aged mice in the exercise condition. In the IL-4/IL-13-treated adult mice, exercise increased Ym1 expression relative to adult controls (p<0.05; A). For Fizz1, exercise overall decreased expression in the aged mice (p<0.05; B). However, no differences in Fizz1 expression were detected between the adult and aged mice in the vehicle or IL-4/IL-13 treatment conditions. Aged mice overall showed increased expression of TGF-β compared to adult mice (p<0.05; C). Fold change is expressed relative to adult vehicle-treated control mice. + Indicates a significant difference from IL-4/IL-13-treated mice. * Indicates a significant difference between groups. Bars represent means ± SEM.
Figure 4.
IL-4/IL-13 treatment increased hippocampal expression of Arg1 (p<0.001; A), SOCS1 (p<0.001; B), IL-1ra (p<0.01; C), and CD206 (p<0.001; D) compared to vehicle-treated mice. Aged mice had higher expression of both Arg1 (p<0.001; A), SOCS1 (p<0.01; B), and CD206 (p<0.05; D) in response to IL-4/IL-13 treatment compared to adult mice regardless of their exercise condition. Aged mice, regardless of their treatment condition, showed higher expression of IL-1ra compared to adult vehicle-treated mice (p<0.01; C). Fold change is expressed relative to adult vehicle-treated control mice. + Indicates a significant difference from IL-4/IL-13-treated mice. * Indicates a significant difference between adult and aged mice regardless of their exercise condition. Bars represent means ± SEM.
For Ym1 there was a significant main effect of age and a three-way interaction between age, exercise, and infusion treatment (F(1, 51)=5.48, p<0.05; F(1, 51)=5.37, p<0.05, respectively, see Figure 3A). Findings showed that aged control mice in the vehicle- and IL-4/IL-13-treated groups had higher expression of Ym1 compared to adults in the corresponding treatment conditions (p<0.05). Further, adult IL-4/IL-13-treated exercise mice had higher Ym1 expression than adult IL-4/IL-13-treated control mice (p<0.05). Exercise and control aged IL-4/IL-13-treated mice did not differ (see Figure 3A). For Fizz1 there was a significant age by exercise condition interaction (F(1, 51)=4.62, p<0.05, see Figure 3B). Post hoc testing showed that Fizz1 expression was reduced in the aged exercise mice compared to aged control mice, when collapsed across the infusion treatment conditions (p<0.05). There were no differences between the adult and aged mice in either the IL-4/IL-13 or vehicle group. Further, there was no difference in Fizz1 expression between the adult exercise and control mice. Exercise had no effect on expression of Arg1, CD206, SOCS1, TGF-β, or IL-1ra.
For both Arg1 and SOCS1 there were significant main effects of age and significant age by infusion treatment interactions (F(1, 51)=6.76, p<0.01; F(1, 51)=8.34, p<0.005; F(1, 47)=4.35, p<0.05; F(1, 47)=11.65, p<0.001, respectively, see Figures 4A and 4B) that showed aged mice had higher expression of both Arg1 and SOCS1 in response to IL-4/IL-13 treatment as compared to adult mice regardless of their exercise condition (p<0.01). There was no difference in Arg1 or SOCS1 expression detected between the adult and aged mice in the vehicle-treated groups. There was a significant age by infusion treatment interaction for CD206 (F(1, 51)=4.32, p<0.05, see Figure 4D) that showed aged mice in the IL-4/IL-13 treatment group had higher expression of CD206 than adult mice (p<0.05). There was no difference in CD206 expression between the adult and aged mice in the vehicle treatment group. For Fizz1 there was a significant age by treatment interaction (F(1, 51)=4.40, p<0.05, see Figure 3B). Post hoc analysis showed that treatment with IL-4/IL-13 increased Fizz1 expression in both adult and aged mice (p<0.001). There was no difference in Fizz1 expression between the adult and aged mice in the IL-4/IL-13 or vehicle treatment groups.
For hippocampal IL-1ra expression there was a significant main effect of age (F(1, 48)=23.36, p<0.001, see Figure 4C). Overall aged mice, regardless of whether they received vehicle or IL-4/IL-13 had higher expression of IL-1ra relative to adult mice (p<0.01). A significant main effect of age for TGF-β expression (F(1,43)=6.80, p<0.05, see Figure 3C) showed that overall aged mice had higher expression of TGF-β regardless of their exercise or treatment condition. Table 1 provides a summary of the significant changes in gene expression related to age, treatment, and exercise.
Table 1.
The initial three columns summarize the age-related changes in expression of M1- and M2-associated genes within the vehicle and IL-4/IL-13 treatment groups. Subsequent columns contain statistical information for observed main effects and interactions. There were no significant main effects of exercise or treatment by exercise interactions detected and hence these were excluded from Table 1. Non-significant effects are notated by NS.
| Vehicle | IL-4/IL-13 | Main effect: Treatment |
Main effect: Age |
Age × treatment |
Age × exercise |
Age × exercise × treatment |
|
|---|---|---|---|---|---|---|---|
| M1-associated gene | |||||||
| IL-1β | Control: Adult < Aged |
Control: Adult < Aged |
NS | p<0.001 | NS | p<0.05 | NS |
| Exercise: Adult = Aged |
Exercise: Adult = Aged |
||||||
| M2-associated genes | |||||||
| Arg1 | Adult = Aged | Adult < Aged | p<0.001 | p<0.01 | p<0.05 | NS | NS |
| SOCS1 | Adult = Aged | Adult < Aged | p<0.001 | p<0.005 | p<0.001 | NS | NS |
| CD206 | Adult = Aged | Adult < Aged | p<0.001 | NS | p<0.05 | NS | NS |
| IL-1ra | Adult < Aged | Adult < Aged | p<0.01 | p<0.001 | NS | NS | NS |
| Ym1 | Control: Adult < Aged |
Control: Adult < Aged |
p<0.001 | p<0.05 | NS | p<0.05 | p<0.05 |
| Exercise: Adult = Aged |
Exercise: Adult = Aged |
||||||
| Fizz1 | Adult = Aged | Adult = Aged | p<0.001 | NS | p<0.05 | p<0.05 | NS |
| TGF-β | Adult < Aged | Adult < Aged | p<0.005 | p<0.05 | NS | NS | NS |
P values are included for significant main effects and 2- and 3-way interactions. Equivalent expression levels between the adult and aged mice within an infusion treatment is notated by Adult = Aged. Increased expression of an individual gene in the aged compared to the adult mice within an infusion treatment is notated by Adult < Aged. When a difference was detected, groups were split into their exercise or control condition within an infusion treatment.
DISCUSSION
The current study determined whether voluntary wheel running altered the immune response to the anti-inflammatory cytokines IL-4 and IL-13 in adult and aged mice. Results demonstrate that IL-4/IL-13 increased hippocampal expression of several M2-associated genes in both adult and aged mice. However, the aged mice showed heightened expression of the M2-related genes Arg1, CD206, Ym1, and SOCS1 in response to IL-4/IL-13. Further, the current exercise protocol had minimal effects on the anti-inflammatory response, as expression of majority of the M2-assocaited genes were unaffected by exercise. Collectively, the data indicate that normal aging can dysregulate the immune response to anti-inflammatory cytokines and that exercise has a limited ability to modulate this response.
Age-related priming of microglia has been well established to produce a heightened and/or prolonged M1 response following an immune challenge (Dilger and Johnson, 2008). However, less is known about how aging affects the induction of an anti-inflammatory M2 response. The present data confirm that infusion of the anti-inflammatory cytokines IL-4 and IL-13 induces expression of the M2-associated genes, namely, Arg1, Fizz1, CD206, SOCS1, Ym1, TGF-β, and IL-1ra (Butovsky et al., 2005, Cecilio et al., 2011, Pepe et al., 2014). However, the animal’s age modulated this response, as aged mice showed increased hippocampal expression of Arg1, CD206, SOCS1, and Ym1 in response to IL-4/IL-13 administration relative to adults. These data are in agreement with prior work showing that macrophages from aged mice show increased Arg1 expression in response to IL-4 administration (Cecilio et al., 2011). Similarly, Kumar et al. (2013) report that twenty-four hours following a traumatic brain injury (TBI) aged mice showed increased expression of the M2a-associated genes Arg1, Ym1, and CD206 relative to adult mice. Though genes associated with the M2c acquired deactivation phenotype such as IL-4 receptor-β and SOCS3 were attenuated in the aged mice following TBI. In response to LPS, aged mice show increased central expression of both M1- and M2-associated genes when measured 8 or 24 hours after treatment (Henry et al., 2009, Fenn et al., 2012). One possibility is that the increased expression of the M2-associated genes in the aged mice results from an increase in TGF-β. Prior research has shown that exposing cultured microglia to TGF-β in combination with IL-4 potentiates expression of Arg1 and Ym1 relative to IL-4 alone (Zhou et al., 2012). Normal aging has been reported to increase TGF-β signaling relative to young adults (Doyle et al., 2010), an effect that was replicated in the current study. Potentially, the age-related increase in TGF-β signaling produced a greater induction of the M2-associated genes following IL-4/IL-13 exposure. Taken together the data indicate that age-related changes in immune activity can alter the response to anti-inflammatory as well as proinflammatory events.
In contrast to the present data, Lee et al., (2013) report that aged mice show attenuated expression of Ym1, Arg1, and IGF-1 relative to adult mice following central administration of IL-4 and IL-13. These seemingly contradictory results likely reflect differences in the sampling time, as the current study analyzed hippocampal samples 24 hours after treatment whereas Lee et al. (2013) collected tissue 3 days following treatment. Analysis of the time course of M2-associated genes in the frontal cortex demonstrates that expression of Fizz1 and Arg1 are higher 16 hours after IL-4 infusion and begin to decline by 48 hours. Further, in response to TBI hippocampal expression of the M2 genes Arg1, Fizz1, Ym1, and CD206 were elevated within twenty-four hours of injury (Ansari, 2015). Collectively these data may indicate that normal aging leads to an exaggerated M2 response, but that the duration of this response is likely blunted in the aged as research demonstrates increased expression early on and decreased expression at later time points. Additional work is needed to determine whether the temporal dynamics of the M2 response differ in adult and aged subjects.
Engaging in voluntary wheel running had minimal effects on the M2 response following IL-4/IL-13 administration, as exercise and control mice within an age group showed similar levels of Arg1, SOCS1, IL-1ra, TGF-β, and CD206. However, exercise modulated Ym1 expression in the adult and aged mice. Specifically, exercise increased expression of Ym1 in the adult mice in the IL-4/IL-13 treatment group. Moreover, aged control mice showed higher IL-4/IL-13-induced expression of Ym1 relative to adult controls, whereas adult and aged mice in the exercise condition did not differ indicating that exercise may mildly attenuate the increase in the aged mice. Additionally, exercise was found to reduce Fizz1 expression in the aged, but not adult, mice regardless of their treatment condition. Both Ym1 and Fizz1 have anti-inflammatory effects and are implicated in tissue repair, as they participate in restructuring the extracellular matrix (Colton, 2009). Ym1 levels are elevated in wounded tissue and decline as the tissue is repaired (Hung et al., 2002, Goren et al., 2014). Ym1 is proposed to facilitate extracellular matrix organization via interactions with heparin sulfate and maintaining levels of growth factors that promote repair (Hung et al., 2002, Colton, 2009). There are no prior data on the effects of exercise on Ym1 expression. However, research has shown that exercise attenuates Fizz1 expression in lung tissue following cigarette smoke inhalation (Ma et al., 2013). The authors suggest that exercise-induced changes in the IL-4 and IL-13 pathway may contribute to the reduction in Fizz1 expression in the lungs. Whether a similar mechanism occurs in the aged brain is presently unknown.
An additional factor to consider is the potential for region specific variations in the microglia response. As evidence indicates that microglia vary in their activation profile depending on their location within the brain. For instance, Hart et al. (2012) report that microglia show subtle phenotypic differences in the aged brain depending on whether they reside in white matter or grey matter. Microglia in white matter tend to show greater age-related increases of several microglia activation markers compared to microglia in grey matter. Moreover, a recent report that employed a genome wide analysis of transcriptional changes in four regions of the adult brain confirmed that microglia phenotypes vary across the brain, as resting microglia in the cerebellum maintain a more reactive profile compared to resting microglia in the cerebral cortex and striatum. Whereas resting microglia in the hippocampus had a moderately reactive profile that fell between the phenotypes expressed by the cerebellar and cortical microglia (Grabert et al., 2016). These regional differences subsequently affect how aging impacts microglial cells. While microglia continue to show regional differences with aging, microglia within the hippocampus start to align with the microglia in cortical regions whereas microglia in the cerebellum continue to diverge. Further, microglia show regional differences in activation following LPS exposure, as the cerebellum and hippocampus show augmented expression of inflammatory-related genes relative to microglia in the cerebral cortex (Grabert et al., 2016). While aging and/or exposure to an immune challenge influence microglia activation in all areas of the brain the magnitude of these effects will vary by location. These regionally distinct microglia may have the potential to show unique reactions to interventions such as exercise.
In agreement with prior work (Sierra et al., 2007, Kohman et al., 2013), aged mice were shown to have higher expression levels of IL-1β, confirming that normal aging is associated with development of chronic low-grade neuroinflammation. In addition, we report that aged mice also show increased basal expression of IL-1ra relative to adults. Prior work has shown that serum levels of IL-1ra are elevated in older individuals (Catania et al., 1997, Ferrucci et al., 2005), but to the best of our knowledge the current data are the first to demonstrate an age-related increase in IL-1ra in the hippocampus. Administration of endogenous IL-1ra has been previously shown to normalize the prolonged behavioral deficits and inflammatory response following an immune challenge in aged animals (Abraham and Johnson, 2009, Frank et al., 2010), indicating that IL-1ra can attenuate the aberrant immune response in the aged. The elevated basal levels of IL-1ra in the aged may occur in reaction to the basal elevations of IL-1β, as IL-1β can initiate the release of IL-1ra along with several other molecules (Watkins et al., 1999). Though IL-1ra levels were elevated in the aged mice this did not reduce expression of IL-1β, as IL-1β levels were elevated basally in the aged mice. Further, expression of IL-1ra was significantly increased following IL-4/IL-13 infusion, but expression of IL-1β was unaltered by IL-4/IL-13 infusion. This inability of IL-1ra to suppress IL-1β expression likely reflects the fact that the physiological response to IL-1β requires binding of only a few IL-1 receptors and thus high levels of IL-1ra are needed to fully suppress IL-1β activity (Watkins et al., 1999). Findings indicate that normal aging may alter expression of anti-inflammatory molecules possibly in response to age-related changes in inflammatory molecules such as IL-1β.
In the vehicle-infused mice, exercise had minimal effects on expression of M1- and M2-associated genes. In the aged, exercise had no effect on basal levels of IL-1β or any of the anti-inflammatory M2 genes. Prior work reports that exercise reduces the age-related increase in IL-1β (Barrientos et al., 2011, Gibbons et al., 2014). However, other’s including the present study fail to replicate this effect (Martin et al., 2013, Martin et al., 2014). Potentially, the duration of exercise training may contribute to the divergent findings as studies using a shorter length of exercise training report attenuated IL-1β whereas those using longer training periods 2–3 months find no difference. Surprisingly, the young adults with access to a running wheel showed increased expression of IL-1β relative to control mice. Prior research has found that acute and chronic exercise can induce a transient increase in IL-1β within the brain (Carmichael et al., 2005, Inoue et al., 2015), potentially the increase in adult mice reflects an acute effect of exercise as they ran a farther distance than aged mice prior to tissue collection. Prior work has shown that exercise can increase efficiency of the immune response, as exercise rats showed higher levels of IL-1β within the hypothalamus and pituitary following an E. coli infection (Nickerson et al., 2005). This heightened response was associated with faster clearance of the E. coli bacteria, indicating faster recovery in the exercise rats. Potentially exercise may enhance aspects of the inflammatory response to aid in recovery. Further research is needed to disentangle how and under what conditions exercise stimulates inflammation in the adult brain.
In summary, the present data demonstrate that normal aging modulates the induction of an anti-inflammatory response, as aged mice showed heightened expression of several M2-associated genes following IL-4/IL-13 infusion. Additionally, the increase in the anti-inflammatory cytokines IL-1ra and TGF-β in the aged indicates that basal changes in immune activity are not limited to proinflammatory molecules. Lastly, results demonstrate that overall exercise had minimal effects on the induction of an M2 response, though exercise appeared to modulate expression of Ym1 and Fizz1. Ultimately, these data further our understanding of how normal aging dysregulates immune function, as aging influences induction of both the pro- and anti-inflammatory immune response.
Highlights.
Aging dysregulates the anti-inflammatory response to IL-4 and IL-13
Exercise has minimal effects on the anti-inflammatory response in the aged
Aging increases hippocampal expression of interleukin-1 receptor antagonist
Acknowledgments
This work was supported by the National Institute on Aging [R00AG040194]; and Alzheimer’s North Carolina Incorporated. Funding sources had no involvement in the experimental design or interpretation of the results.
ABBREVIATIONS
- IL
interleukin
- TNF
tumor necrosis factor
- Arg1
Arginase-1
- Ym1
chitinase-like 3
- Fizz1
found in inflammatory zone 1
- SOCS
suppressor of cytokine signaling
- LPS
lipopolysaccharide
- IGF
insulin-like growth factor
- BDNF
brain derived neurotrophic factor
- IL-1ra
IL-1 receptor antagonist
- PBS
phosphate buffered saline
- s.c.
subcutaneous
- RT-PCR
real-time polymerase chain reaction
- TBI
traumatic brain injury
- TGF-β
transforming growth factor-β
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests: All authors declare that there are no conflicts of interest
REFERENCES
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain, behavior, and immunity. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA. Temporal profile of M1 and M2 responses in the hippocampus following early 24h of neurotrauma. Journal of the neurological sciences. 2015;357:41–49. doi: 10.1016/j.jns.2015.06.062. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Molecular and cellular neurosciences. 2005;29:381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Carmichael MD, Davis JM, Murphy EA, Brown AS, Carson JA, Mayer E, Ghaffar A. Recovery of running performance following muscle-damaging exercise: relationship to brain IL-1beta. Brain, behavior, and immunity. 2005;19:445–452. doi: 10.1016/j.bbi.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Catania A, Airaghi L, Motta P, Manfredi MG, Annoni G, Pettenati C, Brambilla F, Lipton JM. Cytokine antagonists in aged subjects and their relation with cellular immunity. The journals of gerontology Series A, Biological sciences and medical sciences. 1997;52:B93–B97. doi: 10.1093/gerona/52a.2.b93. [DOI] [PubMed] [Google Scholar]
- Cecilio CA, Costa EH, Simioni PU, Gabriel DL, Tamashiro WM. Aging alters the production of iNOS, arginase and cytokines in murine macrophages. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al] 2011;44:671–681. doi: 10.1590/s0100-879x2011007500067. [DOI] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O'Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. Journal of neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhor V, Le Charpentier T, Lebon S, Ore MV, Celador IL, Josserand J, Degos V, Jacotot E, Hagberg H, Savman K, Mallard C, Gressens P, Fleiss B. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain, behavior, and immunity. 2013;32:70–85. doi: 10.1016/j.bbi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida AA, Gomes da Silva S, Fernandes J, Peixinho-Pena LF, Scorza FA, Cavalheiro EA, Arida RM. Differential effects of exercise intensities in hippocampal BDNF, inflammatory cytokines and cell proliferation in rats during the postnatal brain development. Neuroscience letters. 2013;553:1–6. doi: 10.1016/j.neulet.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. Journal of leukocyte biology. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS. TGFbeta signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. Journal of neuroinflammation. 2010;7:62. doi: 10.1186/1742-2094-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain, behavior, and immunity. 2012;26:766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiology of aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain, behavior, and immunity. 2010;24:254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons TE, Pence BD, Petr G, Ossyra JM, Mach HC, Bhattacharya TK, Perez S, Martin SA, McCusker RH, Kelley KW, Rhodes JS, Johnson RW, Woods JA. Voluntary wheel running, but not a diet containing (−)-epigallocatechin-3-gallate and beta-alanine, improves learning, memory and hippocampal neurogenesis in aged mice. Behavioural brain research. 2014;272:131–140. doi: 10.1016/j.bbr.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Goren I, Pfeilschifter J, Frank S. Uptake of neutrophil-derived Ym1 protein distinguishes wound macrophages in the absence of interleukin-4 signaling in murine wound healing. The American journal of pathology. 2014;184:3249–3261. doi: 10.1016/j.ajpath.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nature neuroscience. 2016;19:504–516. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AD, Wyttenbach A, Perry VH, Teeling JL. Age related changes in microglial phenotype vary between CNS regions: grey versus white matter differences. Brain, behavior, and immunity. 2012;26:754–765. doi: 10.1016/j.bbi.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain, behavior, and immunity. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SI, Chang AC, Kato I, Chang NC. Transient expression of Ym1, a heparin-binding lectin, during developmental hematopoiesis and inflammation. Journal of leukocyte biology. 2002;72:72–82. [PubMed] [Google Scholar]
- Inoue K, Okamoto M, Shibato J, Lee MC, Matsui T, Rakwal R, Soya H. Long-Term Mild, rather than Intense, Exercise Enhances Adult Hippocampal Neurogenesis and Greatly Changes the Transcriptomic Profile of the Hippocampus. PloS one. 2015;10:e0128720. doi: 10.1371/journal.pone.0128720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiological reviews. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Bhattacharya TK, Kilby C, Bucko P, Rhodes JS. Effects of minocycline on spatial learning, hippocampal neurogenesis and microglia in aged and adult mice. Behavioural brain research. 2013;242:17–24. doi: 10.1016/j.bbr.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, DeYoung EK, Bhattacharya TK, Peterson LN, Rhodes JS. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain, behavior, and immunity. 2012;26:803–810. doi: 10.1016/j.bbi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends in neurosciences. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiology of aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Ruiz CR, Lebson L, Selenica ML, Rizer J, Hunt JB, Jr, Rojiani R, Reid P, Kammath S, Nash K, Dickey CA, Gordon M, Morgan D. Aging enhances classical activation but mitigates alternative activation in the central nervous system. Neurobiology of aging. 2013;34:1610–1620. doi: 10.1016/j.neurobiolaging.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AM, Setti SE, Priester C, Kohman RA. Voluntary exercise attenuates LPS-induced reductions in neurogenesis and increases microglia expression of a proneurogenic phenotype in aged mice. Journal of neuroinflammation. 2015;12:138. doi: 10.1186/s12974-015-0362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WL, Cai PC, Xiong XZ, Ye H. Exercise training attenuated chronic cigarette smoking-induced up-regulation of FIZZ1/RELMalpha in lung of rats. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 2013;33:22–26. doi: 10.1007/s11596-013-1065-3. [DOI] [PubMed] [Google Scholar]
- Martin SA, Dantzer R, Kelley KW, Woods JA. Voluntary wheel running does not affect lipopolysaccharide-induced depressive-like behavior in young adult and aged mice. Neuroimmunomodulation. 2014;21:52–63. doi: 10.1159/000356144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SA, Pence BD, Greene RM, Johnson SJ, Dantzer R, Kelley KW, Woods JA. Effects of voluntary wheel running on LPS-induced sickness behavior in aged mice. Brain, behavior, and immunity. 2013;29:113–123. doi: 10.1016/j.bbi.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. Journal of leukocyte biology. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. Journal of neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson M, Elphick GF, Campisi J, Greenwood BN, Fleshner M. Physical activity alters the brain Hsp72 and IL-1beta responses to peripheral E. coli challenge. American journal of physiology Regulatory, integrative and comparative physiology. 2005;289:R1665–R1674. doi: 10.1152/ajpregu.00601.2004. [DOI] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathology and applied neurobiology. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic acids research. 2003;31:e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe G, Calderazzi G, De Maglie M, Villa AM, Vegeto E. Heterogeneous induction of microglia M2a phenotype by central administration of interleukin-4. Journal of neuroinflammation. 2014;11:211. doi: 10.1186/s12974-014-0211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:10714–10721. doi: 10.1523/JNEUROSCI.1922-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators of inflammation. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hansen MK, Nguyen KT, Lee JE, Maier SF. Dynamic regulation of the proinflammatory cytokine, interleukin-1beta: molecular biology for non-molecular biologists. Life sciences. 1999;65:449–481. doi: 10.1016/s0024-3205(99)00095-8. [DOI] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain, behavior, and immunity. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Spittau B, Krieglstein K. TGFbeta signalling plays an important role in IL4-induced alternative activation of microglia. Journal of neuroinflammation. 2012;9:210. doi: 10.1186/1742-2094-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]