Abstract
Objectives
Venovenous extracorporeal membrane oxygenation has been used to provide cardiopulmonary support in critically ill infants and children. Recently, dual-lumen venovenous extracorporeal membrane oxygenation has gained popularity in the pediatric population. Herein, we report our institutional experience using a bicaval dual-lumen catheter for pediatric venovenous extracorporeal membrane oxygenation support, which has been our unified approach for venovenous extracorporeal membrane oxygenation since 2009.
Design
This study is a retrospective review.
Setting
The setting is a tertiary children’s hospital in a major metropolitan area.
Patients
Between 2009 and 2011, 11 patients were cannulated using a dual-lumen bicaval venous catheter. Patient demographics, cannulation details, circuit complications, complications of catheter use, and patient outcomes were collected from a retrospective chart review.
Interventions
None.
Measurements and Main Results
Eleven of the patients were cannulated for venovenous extracorporeal membrane oxygenation using the dual-lumen bicaval cannula. The median age at the time of venovenous cannulation was 1.9 years (range, 0.14–17.1), and the median weight was 10.2 kg (range, 3–84). Three patients (27%) required conversion to venoarterial extracorporeal membrane oxygenation. The median duration of extracorporeal membrane oxygenation support was 10 days (2–38 days). Fifty-five percent of patients suffered from a bleeding complication (disseminated intravascular coagulation, pulmonary hemorrhage, or intraventricular hemorrhage), and 45% had a circuit complication. Adequate flow rates were achieved in all patients. The overall hospital mortality in the series was 55%. There were no cannula-related complications.
Conclusions
This review presents the first single-institution experience with the dual-lumen Avalon cannula in pediatric patients. Preliminary results indicate that the catheter can be safely placed and has an acceptable complication profile; however, continued study within larger trials is necessary to fully ascertain the clinical profile of this catheter.
Keywords: Avalon, cannulation, extracorporeal membrane oxygenation, pediatrics, venoarterial, venovenous
Extracorporeal membrane oxygenation (ECMO) support of cardiopulmonary failure in the pediatric patient was first reported by Bartlett et al (1) in 1976. The initial description of ECMO cannulation was via a venoarterial (VA) approach, with ligation of the distal carotid artery and internal jugular vein. However, one of the arguments against VA-ECMO has been its association with neuromotor dysfunction related to ligation of the carotid artery; in one trial, 50% of surviving patients had a neurologic disability at 4 years (2). Furthermore, a review of the Extracorporeal Life Support Organization (ELSO) database by Guner et al (3) demonstrated a higher frequency of seizures (11% vs 7%) and neurologic complications (16% vs 12%) after VA cannulation compared to venovenous (VV) cannulation in congenital diaphragm hernia patients on ECMO. Most recently, another ELSO study has found that patients undergoing VA cannulation have a higher likelihood of central nervous system injury when compared to VV cannulation (4).
In infants with hypoxemic respiratory failure without significant cardiac dysfunction, the use of VV-ECMO began to gain popularity in the early 1990s, with the number of cases rising from less than 1% to 23% of all ECMO cannulations over a 10-year period (3). VV-ECMO has the advantage of preserving the carotid artery, with the additional benefit of enhanced oxygenation of the pulmonary and coronary vasculature (3). Initially, cannulation of both the internal jugular and femoral veins was necessary for VV-ECMO; however, in the mid-1990s, the development of dual-lumen cannulas obviated the need for two-vessel cannulation in newborns and young infants. Recently, a bicaval, dual-lumen catheter (Avalon LLC, Rancho Dominguez, CA) has been developed that simultaneously removes deoxygenated blood from both the superior vena cava (SVC) and inferior vena cava (IVC) while infusing oxygenated blood through a central channel into the right ventricle. The cannula can be used to support patients of all ages, from the newborn period to adulthood. Bermudez et al (5) described their initial experience with this bicaval dual-lumen catheter in adults and reported no catheter-related deaths and minimal catheter-related complications. Further refinements in insertion technique have also led to continual improvement in patient outcomes and a broader use of this catheter in the adult population (6).
Although there have been reports of the use of the dual-lumen bicaval catheter in adults and neonates, there have been none on its use in the pediatric ECMO population (5–7). Herein, we report our single-center experience of patient outcomes using the bicaval dual-lumen Avalon cannula for VV-ECMO.
MATERIALS AND METHODS
Patients and Methods
Texas Children’s Hospital is an ELSO Center of Excellence, which provides ECMO to newborns and children with cardiopulmonary disease. Pediatric ECMO (beyond the neonatal period) in children without cardiac disease is carried out in the PICU. This current study is a retrospective chart review of pediatric patients who received ECMO in the PICU between 2009 and 2011 with the Avalon dual-lumen cannula. The review was approved by the Institutional Review Board of Baylor College of Medicine (H-27591).
The collected clinical data included patient demographics, such as patient gender, age at the time of cannulation, and diagnosis. Other patient and ECMO flow variables which were recorded included ECMO pump flow (cc/kg/min), duration of ECMO support, clinical complications, and survival to discharge. Statistical analysis was performed using SPSS version 19 (SPSS Corporation, Chicago, IL). Patient variables are expressed as median with the absolute range in parentheses.
Catheter Design
The Avalon bicaval dual-lumen cannula has three ports: proximal, middle, and distal. Deoxygenated blood is removed from the SVC/right atrium via the proximal port while the distal port removes blood from the IVC. Once oxygenated in the ECMO circuit, blood is infused through the middle port and directed toward the tricuspid valve into the right ventricle. Another aspect of the catheter design includes wire-reinforcement of the cannula to prevent bending and mechanical flow obstruction after catheter insertion. Catheter sizes range from 13F to 31F in our study group.
ECMO Cannulation Technique
The decision to initiate ECMO was made by the pediatric critical care team and ECMO surgeon, according to institutional indications for ECMO support. The criteria for ECMO initiation consisted of one or more of the following: persistent acidosis, hypoxia, hypercarbia, and hemodynamic instability in the setting of an underlying reversible pathologic disease process.
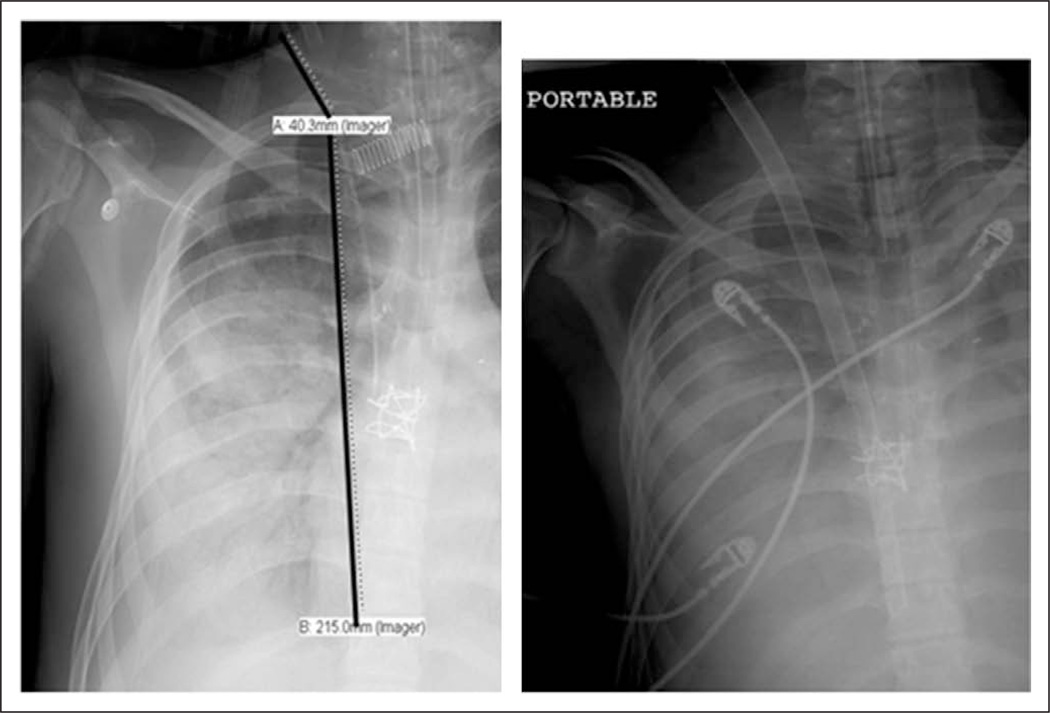
Eleven patients were cannulated for VV-ECMO using the bicaval dual-lumen cannula. Seven catheters were placed percutaneously and four via an open cannulation procedure. The open technique was reserved for younger patients (less than 1 yr old or less than 5 kg). Prior to cannula placement, the target distance from skin to cannula tip was measured on a chest radiograph, from the anticipated insertion site in the neck to the level of the suprahepatic IVC and right atrial junction (Fig. 1). Prior to placement, a portable ultrasound (Sonosite S Series, Sonosite, WA) was used to measure the diameter of the internal jugular vein in order to assist with appropriate cannula sizing.
Figure 1.
Pre- and postcannulation chest radiograph with 31F Avalon cannula. The chest radiograph on the left demonstrates a precannulation measurement of catheter length from the approximate level of insertion to the atriocaval junction. The right chest radiograph shows the cannula in situ at a depth of 25 cm.
The open cannulation technique has been previously described by Lazar et al (7). In brief, an incision was made one finger breadth above the clavicle, and the carotid artery and internal jugular vein were isolated and exposed. The vein was opened and the cannula was advanced to the appropriate length. For percutaneous placement, all cannulas were placed using a standard Seldinger technique with serial dilations to enlarge the subcutaneous tract. All catheter positions were confirmed by postplacement chest radiograph. Bedside echocardiography was used if there was any concern about the correct positioning of the catheter when visualized on postplacement chest radiograph, significant recirculation at the time of placement, or inability to achieve anticipated flow rates.
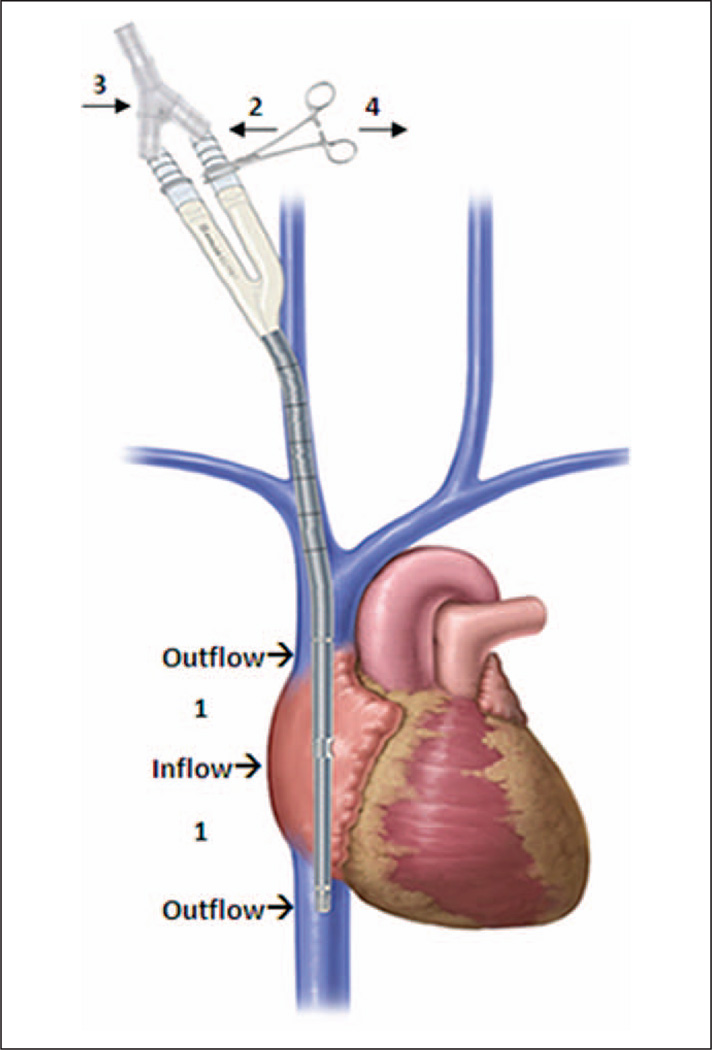
When a patient required conversion from VV-ECMO to VA-ECMO, the carotid artery was isolated in a standard fashion using a cut-down technique. Once the carotid artery was cannulated, the infusion (arterial) limb of the dual-lumen catheter was clamped to enable continued use of the dual-lumen cannula for venous drainage. The carotid cannula was then unclamped allowing for restoration of ECMO support and stabilization of the patient. To prevent stasis in the clamped infusion (arterial) limb of the Avalon catheter, a Y-connector was placed between both catheter limbs and connected to the venous return side on the ECMO circuit. This allowed the dual-lumen bicaval cannula to be converted into a venous drainage catheter while not requiring replacement with a standard single lumen cannula (Fig. 2).
Figure 2.
Conversion of venovenous (VV) to venoarterial (VA) cannulation with a Y-connector tubing. Steps for conversion of VV to VA cannulation: 1) Continue with VV bidirectional flow while performing open cutdown and cannulation of carotid artery. 2) Once the carotid artery cannula is in place, clamp the inflow port on the VV cannula and allow inflow from the carotid artery cannula. 3) Attach Y-connector tubing to the VV dual-lumen catheter. 4) Unclamp the previous inflow port so that both ports are now connected to the extracorporeal membrane oxygenation circuit venous return.
ECMO Equipment and Postcannulation ECMO Management
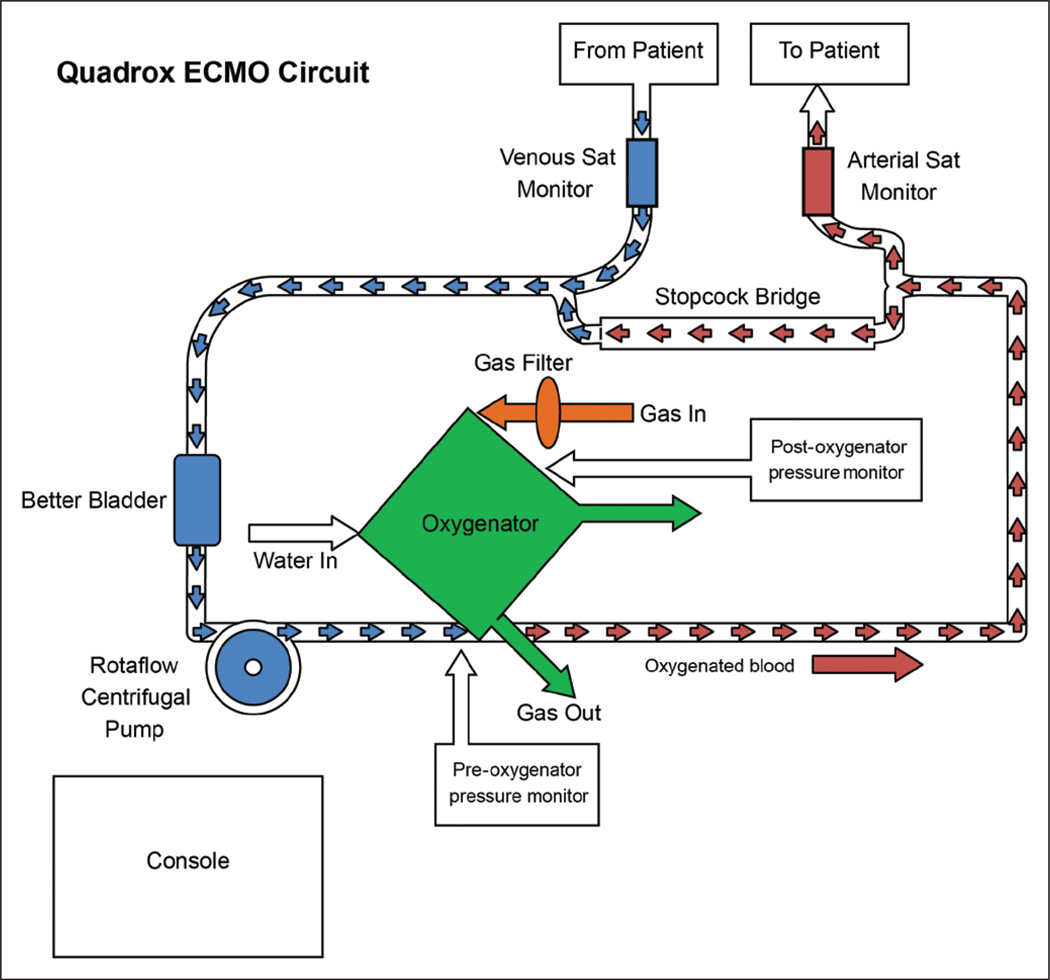
Patients were placed on ECMO machines that use a Maquet Bioline-coated circuit, which has a combined albumin/heparin matrix. The oxygenator is the Maquet Quadrox ID Bioline-coated oxygenator, which is made of polymethylpentene and works by gradient diffusion to remove carbon dioxide and deliver oxygen (Fig. 3). The manufacturing company specifies gas transfer rates of up to 180 cc/min for oxygen and 140 mm Hg/min for carbon dioxide. The circuit flow pump is a Maquet Rotaflow pump (Maquet Cardiovascular, Wayne, NJ).
Figure 3.
Schematic of extracorporeal membrane oxygenation (ECMO) system used in the pediatric ICU.
Once ECMO has been initiated, the anticoagulation protocol is driven by the titration of heparin based on coagulation panels drawn every 6 to 8 hours. The ECMO coagulation panel at the Texas Children’s Hospital consists of the following measured variables: activated clotting time (ACT) prothrombin time, partial thromboplastin time (PTT), PTT with heparinase, fibrinogen, antifactor Xa assay, D-dimer, and functional antithrombin. The platelet count was measured separately but at the same time. Target ACT levels are between 200 and 220 seconds, whereas the goal antifactor Xa assay level is between 0.3 and 0.7 U/mL. The desired platelet count is kept greater than 100^103/UL and fibrinogen is kept greater than 150 mg/dL. The functional antithrombin levels are maintained at levels greater than 80%. Ventilation management is based upon a lung-protective strategy to keep the desired tidal volumes at 3–5 mL/kg while maintaining lung recruitment with additional positive-end expiratory pressure to keep an oxygen saturation level above 80%. Paralytic medications are only used during cannula insertion, or manipulation, or when oxygenation is inadequate despite maximum support on ECMO. Combinations of fentanyl and midazolam are used for sedation, and sedation is held daily for neurologic examinations and ventilator trials.
RESULTS
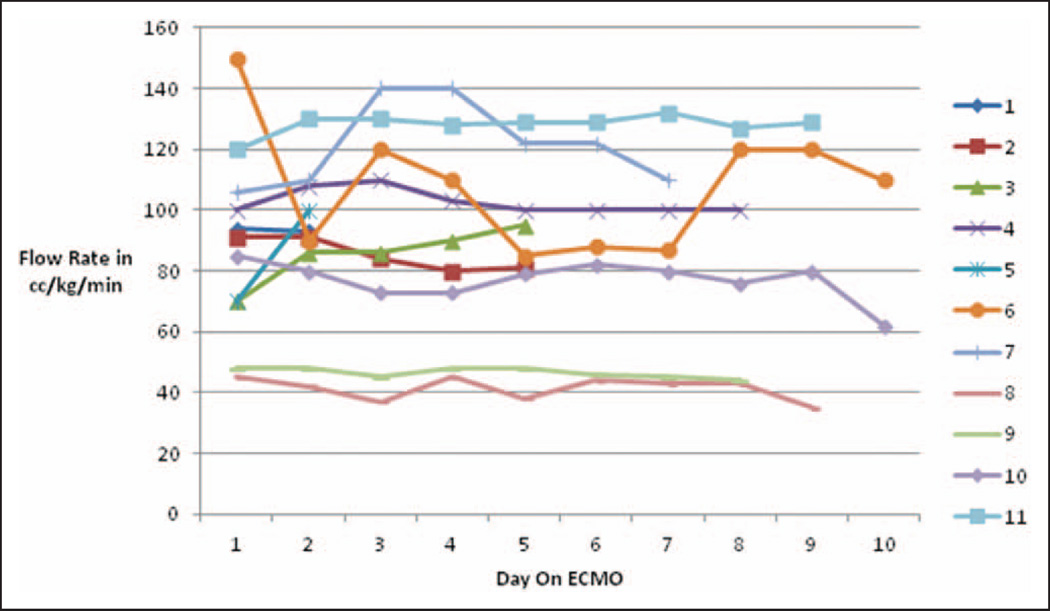
During this period of review, 17 PICU patients were placed on ECMO. Eleven of these patients had a dual-lumen VV-ECMO Avalon cannula placed, which included all of our patients placed on VV-ECMO. Six patients were placed on VA-ECMO due to a level of cardiac dysfunction deemed too severe for VV management. VV-ECMO patient demographics, diagnoses, and clinical data are presented in Table 1. The median age at the time of cannulation was 1.9 years (0.14–17.1), and the median weight was 10.2 kg (3–84). All VV-ECMO patients were acidotic prior to ECMO cannulation, with a median pH of 7.28 (range, 7.14–7.35), a median Paco2 of 70 mm Hg (range, 54–115), and a median Pao2 of 46 mm Hg (range, 25–97) (Table 2). ECMO flow rates in each patient, for the first 10 days of cannulation, are presented in Figure 4. Seven of 11 patients required vasopressor support during their ECMO course. The median duration of ECMO support was 10 days (range, 2–38).
Table 1.
Patient Background Data and Demographics
| Patient | Age (Yr) | Weight (kg) |
Indication for ECMO | Associated Comorbidities |
No. of Hospital Days Prior to ECMO |
Other Organ Failure at the Time of Cannulation |
|---|---|---|---|---|---|---|
| 1 | 1.7 | 10.2 | Sepsis | Spina bifida, hydrocephalus |
2 | Acute renal failure, pulmonary failure |
| 2 | 0.21 | 4.4 | H1N1 Influenza | Prematurity (32 wk) | 5 | Neurologic seizures |
| 3 | 5.8 | 21 | H1N1 Influenza | None | 7 | None |
| 4 | 0.41 | 3.4 | Bacterial pneumonia | Trisomy 21 | 148 | None |
| 5 | 6.68 | 17.8 | H1N1 Influenza | None | 0 | None |
| 6 | 1.91 | 9.7 | Bronchiolitis | None | 1 | None |
| 7 | 0.14 | 4.7 | RSV pneumonia | None | 8 | Acute renal failure |
| 8 | 15.6 | 65.1 | Adenovirus pneumonia | None | 2 | None |
| 9 | 14.5 | 84 | Acute respiratory distress syndrome after operative fixation of bilateral tibia/ fibula fracture |
None | 0 | None |
| 10 | 17.1 | 50 | Cytomegalovirus pneumonia | Cystic fibrosis | 8 | Acute renal failure |
| 11 | 0.21 | 3 | RSV pneumonia | Prematurity (28 wk) | 9 | None |
ECMO = extracorporeal membrane oxygenation, RSV = respiratory synctial virus.
Table 2.
Preextracorporeal Membrane Oxygenation Variables and Extracorporeal Membrane Oxygenation Physiology
| Patient | pH (Pre-ECMO) |
Po2 mL (Pre-ECMO) |
Pco2 mm Hg (Pre-ECMO) |
Flow Rate at 24 Hr (cc/min/kg) |
Vasopressors at Cannulation |
Duration Vasopressor Support (d) |
|---|---|---|---|---|---|---|
| 1 | 7.15 | 28 | 67 | 94 | Norepinephrine, dopamine, vasopressin |
2 |
| 2 | 7.28 | 54 | 91 | 93 | Dopamine | 3 |
| 3 | 7.24 | 50 | 115 | 86 | Norepinephrine, dopamine, epinephrine |
9 |
| 4 | 7.35 | 35 | 54 | 100 | None | NA |
| 5 | 7.14 | 25 | 70 | 101 | Dopamine, vasopressin, epinephrine |
2 |
| 6 | 7.30 | 45 | 66 | 124 | Vasopressin, milrinone | 4 |
| 7 | 7.28 | 49 | 67 | 106 | Dopamine, epinephrine | 2 |
| 8 | 7.31 | 53 | 70 | 44 | None | NA |
| 9 | 7.30 | 46 | 54 | 48 | None | NA |
| 10 | 7.19 | 46 | 101 | 80 | Dopamine | 9 |
| 11 | 7.30 | 97 | 106 | 120 | Dopamine | 3 |
ECMO = extracorporeal membrane oxygenation, NA = not applicable.
Figure 4.
Extracorporeal membrane oxygenation (ECMO) flow rates (cc/kg/min) in each patient for the first 10 days of cannulation.
Five patients required a circuit exchange, and the median time to the first exchange was 7 days (range, 2–22) (Table 3). All circuit changes were related to clot formation; in four of the patients, the circuits were able to be exchanged without complication. One patient had such an extensive clot in the ECMO cannula and circuit that an exchange was unable to be performed. The decision was made to decannulate the patient, who was transitioned to conventional medical support and successfully survived to discharge.
Table 3.
Extracorporeal Membrane Oxygenation Outcomes
| Patient | Duration of Extracorporeal Membrane Oxygenation (d)a |
Method of Insertion |
Access | Cannula Size |
Conversion to Venoarterial |
Circuit Exchange | Bleeding Complications | Survival | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | Percutaneous | IJ | 16F | No | No | Grade IV intraventricular hemorrhage | Dead | Brain herniation |
| 2 | 5 | Cutdown | IJ | 13F | No | No | DIC | Alive | |
| 3 | 23 | Percutaneous | IJ | 19F | Yes | Yes | Pulmonary hemorrhage requiring operation | Dead | Hypoxic respiratory failure |
| 4 | 7 | Cutdown | IJ | 13F | No | Yes | None | Dead | Hypoxic respiratory failure |
| 5 | 22 | Percutaneous | IJ | 16F | Yes | Yes | Pulmonary hemorrhage; ischemic stroke | Alive | |
| 6 | 19 | Percutaneous | IJ | 19F | No | No | DIC | Dead | Hypoxic respiratory failure |
| 7 | 38 | Cutdown | IJ | 13F | Yes | Yes | Coagulopathy | Dead | Multisystem organ failure |
| 8 | 9 | Percutaneous | IJ | 23F | No | No | None | Alive | |
| 9 | 8 | Percutaneous | IJ | 31F | No | No | None | Alive | |
| 10 | 22 | Percutaneous | IJ | 31F | No | No | None | Dead | Multisystem organ failure |
| 11 | 9 | Cutdown | IJ | 13F | No | Yes | None | Alive |
IJ = internal jugular, DIC = disseminated intravascular coagulation.
Including venoarterial run if conversion required.
Three patients required conversion from VV-ECMO to VA-ECMO after a median support time of 6 days. In all VV-ECMO to VA-ECMO conversions, the conversion to VA-ECMO was due to inadequate oxygenation and severe cardiac dysfunction. The first patient, 6 days postcannulation, developed a significant pulmonary hemorrhage requiring operative evacuation. Immediately following the procedure, the patient’s cardiac function acutely deteriorated and he was placed on maximum vasopressor support. At that time, he was converted to VA-ECMO. Another patient requiring transition to VA-ECMO developed persistent hypoxemia (Spo2 ~65%, Pao2 38 mm Hg) 24 hours after VV cannulation. The third patient was similarly converted to VA-ECMO for worsening oxygenation (Spo2 ~60%, Pao2 31 mm Hg) and cardiac dysfunction. The cardiac dysfunction was severe enough that it required a brief episode of cardiopulmonary resuscitation. There were no complications related to VV to VA conversion. Five of the 11 patients (45%) survived to hospital discharge, of which one had required conversion to VA-ECMO.
In regards to anticoagulation-related complications, one patient, with a ventriculoperitoneal shunt in place, developed a grade IV intraventricular hemorrhage with transtentorial herniation less than 24 hours after cannulation and was declared brain dead. Two patients developed pulmonary hemorrhages; one was managed with replacement of blood volume and correction of coagulopathy. The second patient developed a tension hemothorax, necessitating operative intervention for hematoma evacuation. Both patients had prior chest tubes placed for pneumothoraces before ECMO initiation. Three patients experienced significant clotting abnormalities; one patient required plasmapheresis, and two developed profound disseminated intravascular coagulopathy, with one necessitating decannulation and cessation of ECMO support (Table 3).
None of the patients experienced major catheter-related complications, including cardiac perforation or major vessel injury. Only one patient required operative repositioning of the cannula. The cannula needed to be retracted 4 cm as it had gradually migrated distally after 23 days of use; the adjustment resulted in increased venous return with improvement of the filling pressure from −110 to −95 mm Hg. No patients required insertion of an additional venous cannula for improved flow dynamics.
DISCUSSION
In certain pediatric centers, ECMO has evolved into the standard of care for providing life-saving cardiopulmonary support for pediatric patients with reversible pathologies. Following the earliest report of VV-ECMO in three newborns with hypoxemic respiratory failure (8), the use of VV-ECMO has increased worldwide in newborns, older children, and adults with respiratory failure for a multitude of causes (3, 9). The most recent report in 2012 by Skinner et al (10) has validated the effectiveness of VV-ECMO even when risk adjusting for age, advanced respiratory support, and the use of vasopressor agents.
Historically, VV-ECMO has often required the use of two cannulae: one for venous drainage and the second for venous return. However, the advent of the dual-lumen ECMO cannula has obviated the need for two access sites. Although earlier dual-lumen cannulas had limited use due to lumen size, frequent recirculation, and mechanical catheter obstruction, the Avalon dual-lumen bicaval cannula has been designed to address each of these issues. Since its introduction, the cannula has been employed for VV-ECMO via a percutaneous or open technique. Bermudez et al (5) described their initial experience in eleven adult patients where the cannula was able to provide adequate oxygenation during ECMO support without any catheter-related complications or deaths (5). A recent report in 2011 by Javidfar et al (11) also described the successful use of the bicaval dual-lumen catheter in 27 adults, with an overall survival of 56% and one occurrence of SVC injury.
As with any new device, refinements in placement technique and use of image guidance have allowed for improved safety in the use of this device. We would agree with the previous studies and the manufacturer recommendation that the use of image guidance should be employed for safe placement of these cannulas (12, 13). One of the modifications in our placement technique is the measurement of the internal jugular vein diameter using bedside ultrasound prior to placement. This measurement allows the surgeon to accurately estimate the optimal cannula size that the vein will accommodate, thereby preventing any injury that may be caused by placing a cannula too large for the internal jugular vein. Furthermore, using a preplacement chest radiograph, the depth of insertion can be determined preoperatively based on the distance from the anticipated insertion site on the neck down to the level of the suprahepatic IVC/right atrium junction. This is especially helpful in situations where the patient’s bed is not fluoroscopycapable. A final safety measure is the use of real-time echocardiography during cannula placement to confirm that the catheter has been placed at the correct depth, to confirm that the oxygenated infusion port is pointed toward the tricuspid valve, to evaluate initial ECMO flow, and to observe that no vascular injury has occurred. Even though we do not feel it is necessary to use real-time echocardiography for each cannulation, whenever there is a question about correct positioning, we will use echocardiography to resolve the issue.
Our adverse outcome rate is similar to a recent longitudinal report of pediatric ECMO patients with data from the ELSO registry. Mortality in this series of over 3,000 patients ranged from 39% to 50% depending on the age of the patient group. The study found that mortality rates were higher in the VA-ECMO patients (49% vs 30%) and that mortality after conversion from VV to VA-ECMO was similar to the VA group (51%) (14). Although the mortality in our study is slightly higher than these reported numbers, comparisons are difficult given our small sample size. Other concerns with this cannula have revolved around the occurrence of major vascular injury during placement or postplacement, mainly in neonates and small infants. A recent article by Lazar et al (7) on the use of this cannula in a neonatal population did not report any cannula-related vascular or cardiac complications. Although we did not have any catheter-related complications or major vessel injury, serious events, including right ventricular rupture with tamponade, have been reported with use of the Avalon catheter (15). As these complications are rare, our small sample size is inadequate to detect the true rate of these potentially fatal outcomes and a larger multicenter trial or use of a national/international ECMO database may be the only avenue with which to answer this question.
Our high conversion rate is consistent with the reported VV-ECMO to VA-ECMO conversion rate in the literature (16). This specific cannula design allows for conversion to VA-ECMO without the need for replacement of the cannula. One important point for VA conversion is that the arterial limb of the cannula must have the Y-connector placed to allow for flow within that lumen. This prevents stasis and clot formation within this limb. This technique has been similarly described with success in the adult population, where the Avalon cannula was attached to a Y-connector and used as the venous drainage system for both cardiopulmonary bypass and VA-ECMO via the axillary artery (17). Another concern over conversion rates in dual-lumen VV-ECMO is due to the supposition that adequate flow rates cannot be achieved through an overall smaller cannula diameter; however, our patients were all able to establish adequate flow rates based on their weight (Fig. 1), and conversions were related to underlying severe physiologic disturbances.
CONCLUSIONS
This study presents the first institutional report of VV-ECMO using the Avalon dual-lumen bicaval cannula in a PICU population. In our institution, this cannula is the preferred method for VV-ECMO support in the pediatric population, and this series present some preliminary findings on the safety, flow, and complication profile for this catheter. As with any new technology, the continued study of this cannula is necessary to fully delineate its clinical profile and to allow for its safest use within the ECMO population.
Acknowledgments
There are no relevant sources of financial support or relationships with the manufacturing company, Avalon, to report with respect to the contents of this manuscript.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
results
- 1.Bartlett RH, Gazzaniga AB, Jefferies MR, et al. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. 1976;22:80–93. [PubMed] [Google Scholar]
- 2.Bennett CC, Johnson A, Field DJ, et al. UK Collaborative ECMO Trial Group. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation: Follow-up to age 4 years. Lancet. 2001;357:1094–1096. doi: 10.1016/S0140-6736(00)04310-5. [DOI] [PubMed] [Google Scholar]
- 3.Guner YS, Khemani RG, Qureshi FG, et al. Outcome analysis of neonates with congenital diaphragmatic hernia treated with venovenous vs venoarterial extracorporeal membrane oxygenation. J Pediatr Surg. 2009;44:1691–1701. doi: 10.1016/j.jpedsurg.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Rollins MD, Hubbard A, Zabrocki L, et al. Extracorporeal membrane oxygenation cannulation trends for pediatric respiratory failure and central nervous system injury. J Pediatr Surg. 2012;47:68–75. doi: 10.1016/j.jpedsurg.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Bermudez CA, Rocha RV, Sappington PL, et al. Initial experience with single cannulation for venovenous extracorporeal oxygenation in adults. Ann Thorac Surg. 2010;90:991–995. doi: 10.1016/j.athoracsur.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Trimlett RH, Cordingley JJ, Griffiths MJ, et al. A modified technique for insertion of dual lumen bicaval cannulae for venovenous extracorporeal membrane oxygenation. Intensive Care Med. 2011;37:1036–1037. doi: 10.1007/s00134-011-2213-5. [DOI] [PubMed] [Google Scholar]
- 7.Lazar DA, Cass DL, Olutoye OO, et al. Venovenous cannulation for extracorporeal membrane oxygenation using a bicaval dual-lumen catheter in neonates. J Pediatr Surg. 2012;47:430–434. doi: 10.1016/j.jpedsurg.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 8.Andrews AF, Klein MD, Toomasian JM, et al. Venovenous extracorporeal membrane oxygenation in neonates with respiratory failure. J Pediatr Surg. 1983;18:339–346. doi: 10.1016/s0022-3468(83)80178-x. [DOI] [PubMed] [Google Scholar]
- 9.Patel SA, DeMare JS, Truemper EJ, et al. Successful use of venovenous extracorporeal membrane oxygenation for complicated H1N1 pneumonia refractory to mechanical ventilation. J Extra Corpor Technol. 2011;43:70–74. [PMC free article] [PubMed] [Google Scholar]
- 10.Skinner SC, Iocono JA, Ballard HO, et al. Improved survival in venovenous vs venoarterial extracorporeal membrane oxygenation for pediatric noncardiac sepsis patients: A study of the Extracorporeal Life Support Organization registry. J Pediatr Surg. 2012;47:63–67. doi: 10.1016/j.jpedsurg.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Javidfar J, Brodie D, Wang D, et al. Use of bicaval dual-lumen catheter for adult venovenous extracorporeal membrane oxygenation. Ann Thorac Surg. 2011;91:1763–1768. doi: 10.1016/j.athoracsur.2011.03.002. discussion 1769. [DOI] [PubMed] [Google Scholar]
- 12.Kuenzler KA, Arthur LG, Burchard AE, et al. Intraoperative ultrasound reduces ECMO catheter malposition requiring surgical correction. J Pediatr Surg. 2002;37:691–694. doi: 10.1053/jpsu.2002.32254. [DOI] [PubMed] [Google Scholar]
- 13.Thomas TH, Price R, Ramaciotti C, et al. Echocardiography, not chest radiography, for evaluation of cannula placement during pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2009;10:56–59. doi: 10.1097/PCC.0b013e3181937409. [DOI] [PubMed] [Google Scholar]
- 14.Zabrocki LA, Brogan TV, Statler KD, et al. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. 2011;39:364–370. doi: 10.1097/CCM.0b013e3181fb7b35. [DOI] [PubMed] [Google Scholar]
- 15.Hirose H, Yamane K, Marhefka G, et al. Right ventricular rupture and tamponade caused by malposition of the Avalon cannula for venovenous extracorporeal membrane oxygenation. J Cardiothorac Surg. 2012;7:36. doi: 10.1186/1749-8090-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim K, Mazor RL, Rycus PT, et al. Use of venovenous extracorporeal life support in pediatric patients for cardiac indications: A review of the Extracorporeal Life Support Organization registry. Pediatr Crit Care Med. 2012;13:285–289. doi: 10.1097/PCC.0b013e31822f1586. [DOI] [PubMed] [Google Scholar]
- 17.Bacchetta M, Javidfar J, et al. Ease of conversion from venovenous extracorporeal membrane oxygenation to cardiopulmonary bypass and venoarterial extracorporeal membrane oxygenation with a bicaval dual lumen catheter. ASAIO J. 2011;57:283–285. doi: 10.1097/MAT.0b013e31821d3f35. [DOI] [PMC free article] [PubMed] [Google Scholar]