Abstract
The nematode Caenorhabditis elegans produces tens, if not hundreds, of different ascarosides as pheromones to communicate with other members of its species. Overlapping mixtures of these pheromones affect the development of the worm and a variety of different behaviors. The ascarosides represent a unique tool for dissecting the neural circuitry that controls behavior and that connects to important signaling pathways, such as the insulin and TGFβ pathways, that lie at the nexus of development, metabolism, and lifespan in C. elegans. However, the exact physiological roles of many of the ascarosides are unclear, especially since many of these pheromones likely have multiple functions depending on their concentrations, the presence of other pheromones, and a variety of other factors. Determining these physiological roles will be facilitated by top-down approaches to characterize the pheromone receptors and their function, as well as bottom-up approaches to characterize the pheromone biosynthetic enzymes and their regulation.
1. Introduction
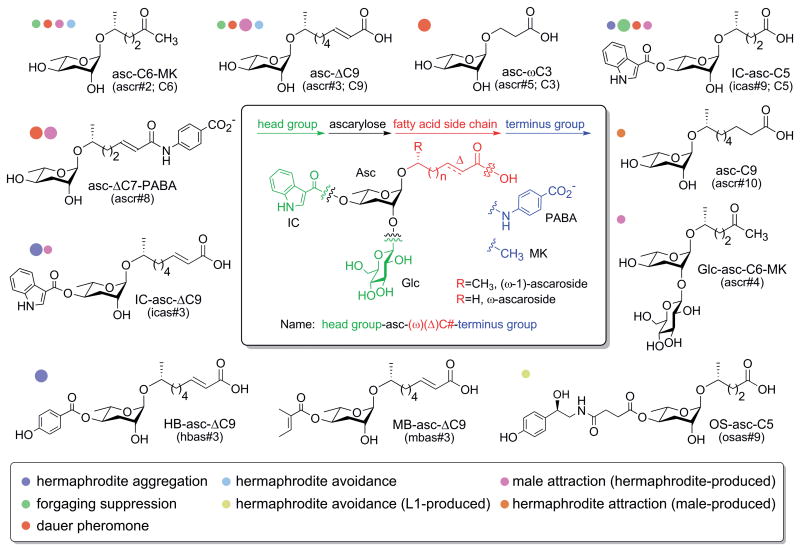
C. elegans survives in nature on rotting plant material, where it competes for food (bacteria) with other C. elegans and other nematodes. Using a class of pheromones called the ascarosides, C. elegans senses the larval stage, sex, metabolic state, and population density of conspecifics and adapts its behavior and development accordingly in order to improve its competitive advantage.1-7 The ascarosides are derivatives of the 3,6-dideoxysugar ascarylose that have different fatty acid-derived side chains of various lengths that are attached to the ascarylose sugar at their penultimate (ω-1) or terminal (ω) carbon (Fig. 1). The ascarosides can also have a variety of modifications on the sugar and the side chain. When food resources are plentiful, C. elegans uses the ascarosides to optimize its feeding strategy. Certain ascarosides, such as those modified with indole carbonyl (IC) group, stimulate social feeding where the nematodes aggregate on food (Fig. 1, purple dots).8 As population density increases, but food is still available, another subset of ascarosides influence foraging behavior by suppressing exploration to seek new food sources and promoting dwelling (Fig. 1, green dots).9, 10 When population density overwhelms available food resources, another subset of ascarosides, collectively known as the dauer pheromone, suppress neuroendocrine signaling by the insulin and TGFβ pathways, and thereby induce development of the stress-resistant dauer larval stage, which is specialized for dispersal to a new food source (Fig. 1, red dots).1-3, 5, 11 Certain ascarosides also induce avoidance, possibly enabling C. elegans to seek more favorable conditions (Fig. 1, light blue and yellow dots).7, 12 C. elegans also uses the ascarosides to help it find a mate. While most C. elegans are hermaphrodites, some are males, and both hermaphrodites and males secrete specific ascarosides to attract each other (Fig. 1, pink and orange dots).4, 7 In its natural environment, C. elegans also encounters other nematode species, many of which have been shown to use ascarosides in chemical communication.13, 14 In fact, ascaroside signaling appears to be broadly conserved in both free-living and parasitic nematodes species, and thus, investigations of ascaroside signaling in C. elegans have important implications for nematode communication in general.14-20
Figure 1.
Chemical structures of some key ascaroside pheromones. Colored dots next to the ascaroside structures indicate whether a particular ascaroside has been shown to have a particular activity. The size of the dot gives an indication of the relative strength of that activity compared to other ascarosides with that activity. Many of the ascarosides shown have not been tested for all activities listed so this figure does not give a complete activity profile many of the ascarosides. All of the ascarosides share a modular structure that is diagrammed in the central box. The ascarosides can be named based on this modular structure, and this structure-based name is listed below each ascaroside. The ascarosides can also be named using a name based primarily on the order of discovery (ascr#, icas#, mbas#, hbas#, osas#) or a name based on an earlier nomenclature (C6, C9, C3, or C5, indicating only the number of carbons in the side chain). For the structure-based name, the simplest ascarosides have an ascarylose sugar attached to a saturated fatty acid side chain of a particular length (C#) at its penultimate (ω-1) carbon. Deviations from that simple structure are indicated as follows: head group-asc-(ω)(Δ)C#-terminus group. ω indicates attachment at the terminal (ω), instead of the penultimate (ω-1) carbon. Δ indicates a double bond at the α-β position. Head groups include indole-3-carbonyl (IC), 4-hydroxybenzoyl (HB), 2-(E)-methyl-2-butenoyl (MB), and octopamine succinyl (OS) at the 4′-position and glucosyl (Glc) at the 2′-position. Terminus groups include a methyl ketone (MK) and para-aminobenzoic acid (PABA) instead of a carboxylic acid. The figure in the central box is adapted from ref. 26.
Although many ascarosides have been linked to a particular biological activity, others have not, and very little is known regarding the role of ascarosides in the context of the complex mixtures in which they function. Those ascarosides that were discovered through activity-guided fractionation (such as the dauer pheromone1-3 and hermaphrodite-produced sex pheromone4) have assigned biological roles, but many of the ascarosides that were discovered through metabolomic-based approaches do not yet have assigned biological roles.21 Furthermore, even those ascarosides with an assigned biological role may potentially have many additional as-yet-unknown activities. Complicating studies of these biological roles is the fact that the activities of the ascarosides are concentration-dependent. While ascarosides that affect behavior often work at lower concentrations (femtomolar to picomolar4, 6-8), those that affect dauer work at much higher concentrations (low to mid-nanomolar1-3, 5). Furthermore, the ascarosides work as mixtures of pheromones, and for the most part, the individual roles of the ascarosides in the mixtures are not fully defined. For example, the dauer pheromone has at least five ascaroside components that work synergistically together, and it is unclear what the distinct purpose of the individual components is.
This viewpoint will highlight recent studies that help to further define the specific roles of the ascaroside pheromones. We will first focus on studies that are determining the receptor targets of individual ascarosides and how this knowledge is helping to determine the downstream signaling pathways influenced by specific ascarosides and the role of particular ascarosides in providing a competitive advantage. We will then focus on studies that are determining when and under which conditions specific ascarosides are made and how this information is helping to further refine the individual roles of ascaroside pheromones.
3. Receptors
G protein-coupled receptors (GPCRs) in nematodes are a large family of genes that have undergone recent expansion in several Caenorhabditis species, including C. elegans, where there are over 1,000 members.22 A few chemosensory receptors have been identified as the targets of specific ascarosides. This pioneering work represents the first steps in piecing together how the ascarosides influence nematode development and behavior and suggests that the field is poised for significant advancement. The chemoreceptors in C. elegans are fast-evolving and under selective pressure, such that even in different C. elegans strains they may not function in exactly the same way. Efforts to identify the chemosensory receptors of specific ascarosides have taken advantage of this fact by identifying strains that do and do not respond to a specific ascaroside and then zeroing in on the genomic regions responsible using quantitative trait locus mapping. Greene et al. discovered that most of the dauer pheromone ascarosides suppress exploratory foraging at low nanomolar concentrations in the standard C. elegans strain (N2).9, 10 However, one of the dauer pheromone components, IC-asc-C5 (icas#9) was not able to suppress exploratory foraging in certain wild strains (such as MY14). Recombinant inbred lines (RILs) were generated between MY14 and a strain that was responsive to IC-asc-C5 (icas#9), and the RILs were tested for sensitivity of IC-asc-C5 (icas#9), thereby enabling the identification of a genomic region (roam-1) that included srx-43 and srx-44 as candidate receptors. Strains such as MY14 were shown to be resistant to the effects of IC-asc-C5 (icas#9) on foraging behavior, in part because they express srx-43 at lower levels in the ASI neuron and in part because they change the expression pattern of srx-44.9, 10 The srx-43 and srx-44 channels regulate the insulin and TGFβ signaling pathways, which had previously been shown to affect foraging behavior.9, 10
Interestingly, Greene et al. were able to show that the effect of IC-asc-C5 (icas#9) on foraging behavior in responsive strains improves fitness, but only under certain conditions.9, 10 N2 and a strain containing the roam-1 genomic region from MY14 were allowed to grow together on a simple lawn for several days allowing food to become depleted, then the abundance of the two strains was measured and a small portion of the population was used to repeat the process. Under these conditions, N2 had the competitive advantage. However, when the experiment was run on a patchy lawn, the strain containing the roam-1 region, and the srx-43 gene specifically, had the competitive advantage, presumably because the strain was more apt to forage for the less available food. Thus, these results explain why different C. elegans strains maintain under balancing selection different alleles of the roam-1 region that are either responsive or non-responsive to IC-asc-C5 (icas#9).
Identification of some of the GPCRs that control dauer formation has begun to shed light on the mechanism by which the different components of the dauer pheromone contribute to the dauer decision. Long-term growth of C. elegans at a high population density in nutrient-rich conditions has provided dauer pheromone-resistant mutants. Generation of RILs from these resistant mutants has enabled the identification of the GPCRs SRG-36 and SRG-37 in the ASI neuron as targets of the dauer pheromone component asc-ωC3 (ascr#5).23 Several C. elegans and C. briggsae strains cultured under high density conditions were examined, and all of them had developed resistance to the dauer pheromone through mutation of srg-36 and srg-37. Thus, it is likely that loss of these receptors is a reproducible genetic change that enables nematodes to outcompete conspecifics. C. elegans will enter dauer at high population densities, even when cultured under nutrient rich conditions, and presumably those C. elegans that do not enter dauer under those conditions will eventually be able to take over the population.
The dauer pheromones asc-C6-MK (ascr#2) and asc-ΔC9 (ascr#3) target a different set of GPCRs. A genetic screen was used to identify SRBC-64 and -66 in the ASK chemosensory neuron as targets of the two dauer pheromones.24 A biochemical approach was used to identify DAF-37 and -38 in the ASI chemosensory neuron as additional targets of asc-C6-MK (ascr#2).25 Not surprisingly, the location of GPCR expression is key to their biological function. For example, asc-C6-MK (ascr#2) can either induce dauer by targeting the DAF-37 GPCR in the ASI neuron or can induce hermaphrodite-hermaphrodite repulsion by targeting the same receptor in the ASK neuron.25 Identification of these receptors will enable the downstream signaling pathways to be delineated and will reveal how certain dauer pheromone ascarosides work synergistically with others to induce dauer. Given the large number of ascarosides and their multiple activities, it is likely that many ascaroside receptors remain to be discovered.
4. Biosynthesis
Defining when and under which conditions different ascarosides are produced by C. elegans will be critical to defining their biological role in development and behavior. Environmental conditions (food availability and temperature), as well as larval stage and sex, have been shown to affect which ascarosides are produced by C. elegans.6, 7, 26-28 It is unclear why the pheromones in C. elegans, such as the dauer pheromone and sex pheromone, consist of multiple ascaroside components. It has been speculated that for the dauer pheromone, in addition to population density, the different components of the dauer pheromone encode additional information about the status of conspecifics, such as their metabolic status, that helps to further inform the dauer decision. Increased food availability leads to increased production of many of the ascarosides, although to varying degrees.26, 28 In adults, shorter chain (ω-1)-ascarosides, such as the dauer pheromones asc-C6-MK (ascr#2) and IC-asc-C5 (icas#9), are not induced by food as much as other ascaroside pheromones.26 Perhaps, this shift in ascaroside production enables the worm to produce a less potent dauer pheromone under well fed conditions. On the other hand, in early larval stage worms, a short-chain ω-ascaroside, the potent dauer pheromone asc-ωC3 (ascr#5), is much more strongly induced by food than other ascarosides.26 It is unclear why a potent dauer pheromone component would be induced by food, since favorable conditions such as abundant food should counteract dauer formation. Clearly, this dauer pheromone is special in that it works synergistically with other dauer pheromone components to induce dauer,2 and it targets a unique set of GPCRs.23 One hypothesis for why C. elegans might produce more asc-ωC3 (ascr#5) on food is that it gauges the rate of population growth and food availability and realizes that while it has plenty of food now, its progeny will not, and thus, it produces a potent dauer pheromone to enable its progeny to enter dauer. Asc-ωC3 (ascr#5) also has unique behavioral effects from other dauer pheromone components that might in some way provide a survival benefit on food (Fig. 1).
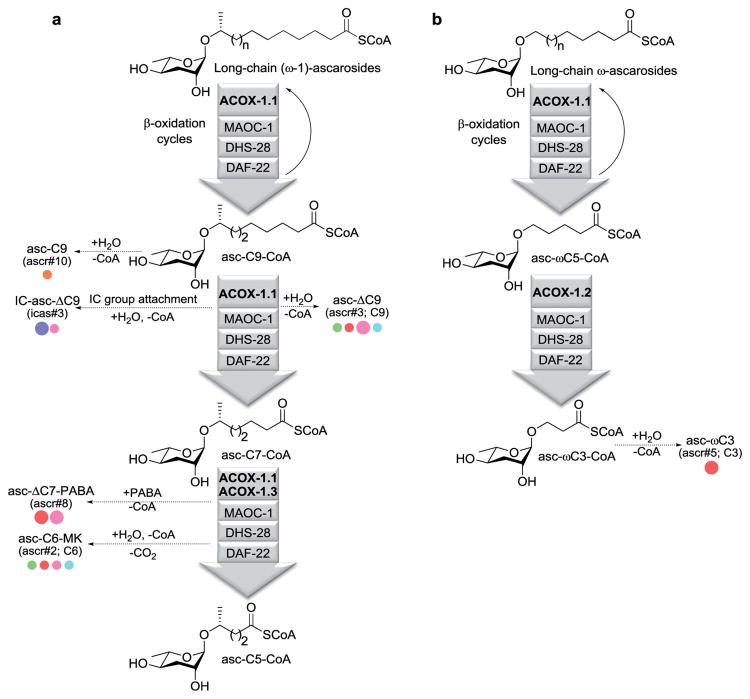
Although analysis of when and under which conditions different ascarosides are produced will help to define their functions, interpretation of this data is often challenging. It is difficult to change one variable in a C. elegans culture, such as food amount or temperature, without changing others. Identifying the biosynthetic enzymes that are important for the production of different ascarosides and determining their regulation will help to characterize the signaling pathways and conditions that induce pheromone production. C. elegans makes ascarosides with long side chains and then uses peroxisomal β-oxidation cycles to shorten those side chains by two carbons per cycle in order to produce the short-chain ascaroside pheromones.21, 26, 29-31 Separate β-oxidation pathways process the ω- and (ω-1)-ascarosides (Fig. 2a, b).21, 26 Acyl-CoA oxidases (ACOXs) catalyze the first step in each β-oxidation cycle, the installation of a double bond (Δ) α-β to the carbonyl, and have different substrate preferences. Thus far, three of the seven ACOXs in C. elegans have been implicated in ascaroside biosynthesis, and these enzymes represent a mechanism for C. elegans to regulate the production of specific ascarosides.26 Analysis of when and under what conditions these ACOX enzymes are expressed could potentially provide insight into the biological role of the ascaroside pheromones that they produce. Transcriptional regulation of ACOXs is correlated with the production of the ascarosides that they control. For example, ACOX-1.1 (previously known as ACOX-1) catalyzes the first step in the β-oxidation cycle that shortens a 9-carbon (ω-1)-ascaroside to a 7-carbon (ω-1)-ascaroside, and transcriptional downregulation of this ACOX enzyme in males is thought to contribute to the accumulation of a male-produced sex pheromone, asc-C9 (ascr#10) (Fig. 2a).6 ACOX-1.1 and ACOX-1.3 (previously known as ACOX-3) catalyze the first step in the β-oxidation cycle that shortens a 7-carbon (ω-1)-ascaroside to a 5-carbon (ω-1)-ascaroside to produce shorter chain (ω-1)-ascarosides, such as the dauer pheromone asc-C6-MK (ascr#2) (Fig. 2a).26 ACOX-1.3 expression has been shown to be suppressed by food, which could potentially contribute to the decreased production of shorter chain (ω-1)-ascarosides upon addition of food.26 Additionally, ACOX-1.2 (previously known as ACOX-2) catalyzes the first step in the β-oxidation cycle that shortens a 5-carbon ω-ascaroside to a 3-carbon ω-ascaroside to produce the dauer pheromone asc-ωC3 (ascr#5).26 ACOX-1.2 expression has been shown to be induced by food, which could potentially contribute the increased production of asc-ωC3 (ascr#5) upon addition of food.26 Other mechanisms besides transcription may regulate the ACOX enzymes. For example, many of the ACOX enzymes have been shown to bind ATP, which influences enzymatic activity by affecting the binding of an FAD cofactor.32 ATP could potentially affect the activity of certain ACOXs and thus link the mixture of ascarosides produced to metabolic activity in C. elegans, although this possibility remains to be tested. Investigation of additional factors that influence ACOX expression and activity could reveal additional information about the roles of the pheromones that they are responsible for producing.
Figure 2.
Biosynthesis of ascaroside pheromones. The short-chain ascaroside pheromones are biosynthesized through two parallel pathways, one shortening the side chains of long-chain (ω-1)-ascarosides (a) and one shortening the side chains of long-chain ω-ascarosides (b). β-oxidation cycles shorten the side chains by two carbons per cycle. ACOX enzymes, including ACOX-1.1, ACOX-1.2, and ACOX-1.3, install a double bond α-β to the carbonyl of the ascaroside intermediates. Likely biosynthetic pathways to some ascaroside pheromones have indicated if there is some evidence to support the pathways. The biological activities of these pheromones has been indicated by colored dots that can be interpreted using the key in Fig. 1.
In addition to the length of the fatty acid-derived side chain, the addition of various head groups to the 2′- and 4′-positions of the ascarylose sugar and terminus groups to the end of the side chain can have dramatic consequences for the biological activity of the ascarosides. For example, those ascarosides modified with the IC group on the 4′-position tend to induce aggregation, while those without this group tend to induce avoidance (Fig. 1).8 Although it is known that the IC group is biosynthesized from tryptophan and is added relatively late in the biosynthetic process, the enzymes involved in the biosynthesis and attachment are unknown.8, 21 Identification of these enzymes, as well as identification of conditions that induce production of the IC-ascarosides would help to define the biological role of these ascarosides and might help to explain how aggregation on IC-ascarosides provides a survival advantage.
4. Conclusions
Ascaroside signaling in C. elegans represents a unique opportunity to map a collection of pheromones targeting development, metabolism, and lifespan, and diverse set of behaviors to the neural network controlling these processes. Determining how the receptor targets of the ascarosides interact with each other and with downstream signaling pathways will be a fundamental step forward in our understanding of the neural circuitry and will provide insights into how pheromones influence the insulin, TGFβ, and other important signaling pathways. The GPCRs targeted by the ascarosides are from several different chemoreceptor superfamilies, suggesting that GPCRs cannot be easily identified as ascaroside targets through some set of common structural features. However, newer techniques, such as quantitative trait locus mapping and genetic engineering via CRISPR-Cas, are helping to identify these receptors. The specificity of these receptors and when, where, and under which conditions they are expressed will help to define the biological roles of the ascaroside pheromones that they detect. Further clues to these biological roles will be provided by knowledge of the biosynthetic pathways that coordinated ascaroside production and how they are regulated. Relatively little is currently known about these biosynthetic pathways. Identification of the ascaroside biosynthetic enzymes is complicated by the fact that the genes involved are not clustered in the genome and are difficult to distinguish from those involved in primary metabolism. Thus, identification of these enzymes might benefit from more global approaches, such as RNAi-based screens. Finally, better analytical techniques for detecting small changes in ascaroside production levels under dynamic environmental conditions will help to determine the biological role of the ascarosides.
Acknowledgments
This work was supported by grants from the NIH (GM118775), NSF (Career - 1555050), Alfred P. Sloan Foundation, and the Research Corporation for Science Advancement.
Rebecca A. Butcher was raised in Fort Myers, FL, and received her A.B. in Chemistry at Harvard University working with Gregory Verdine. She earned her Ph.D. with Stuart Schreiber at Harvard as an NSF predoctoral fellow. She then pursued postdoctoral studies with Jon Clardy at Harvard Medical School, supported by an NIH NRSA postdoctoral fellowship and an NIH Pathway to Independence award. She began her independent career in 2010 in the Department of Chemistry at the University of Florida, where she is currently an assistant professor.
References
- 1.Butcher RA, Fujita M, Schroeder FC, Clardy J. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 2.Butcher RA, Ragains JR, Kim E, Clardy J. Proc Natl Acad Sci U S A. 2008;105:14288–14292. doi: 10.1073/pnas.0806676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butcher RA, Ragains JR, Clardy J. Org Lett. 2009;11:3100–3103. doi: 10.1021/ol901011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PE, Malik RU, Edison AS, Sternberg PW, Schroeder FC. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pungaliya C, Srinivasan J, Fox BW, Malik RU, Ludewig AH, Sternberg PW, Schroeder FC. Proc Natl Acad Sci U S A. 2009;106:7708–7713. doi: 10.1073/pnas.0811918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izrayelit Y, Srinivasan J, Campbell SL, Jo Y, von Reuss SH, Genoff MC, Sternberg PW, Schroeder FC. ACS Chem Biol. 2012;7:1321–1325. doi: 10.1021/cb300169c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artyukhin AB, Yim JJ, Srinivasan J, Izrayelit Y, Bose N, von Reuss SH, Jo Y, Jordan JM, Baugh LR, Cheong M, Sternberg PW, Avery L, Schroeder FC. J Biol Chem. 2013;288:18778–18783. doi: 10.1074/jbc.C113.477000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivasan J, von Reuss SH, Bose N, Zaslaver A, Mahanti P, Ho MC, O'Doherty OG, Edison AS, Sternberg PW, Schroeder FC. PLoS Biol. 2012;10:e1001237. doi: 10.1371/journal.pbio.1001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene JS, Brown M, Dobosiewicz M, Ishida IG, Macosko EZ, Zhang X, Butcher RA, Cline DJ, McGrath PT, Bargmann CI. Nature. 2016;539:254–258. doi: 10.1038/nature19848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene JS, Dobosiewicz M, Butcher RA, McGrath PT, Bargmann CI. Elife. 2016;5:e21454. doi: 10.7554/eLife.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fielenbach N, Antebi A. Genes Dev. 2008;22:2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong C, Dolke F, von Reuss SH. Org Biomol Chem. 2016;14:7217–7225. doi: 10.1039/c6ob01230b. [DOI] [PubMed] [Google Scholar]
- 14.Choe A, von Reuss SH, Kogan D, Gasser RB, Platzer EG, Schroeder FC, Sternberg PW. Curr Biol. 2012;22:772–780. doi: 10.1016/j.cub.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noguez JH, Conner ES, Zhou Y, Ciche TA, Ragains JR, Butcher RA. ACS Chem Biol. 2012;7:961–966. doi: 10.1021/cb300056q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choe A, Chuman T, von Reuss SH, Dossey AT, Yim JJ, Ajredini R, Kolawa AA, Kaplan F, Alborn HT, Teal PE, Schroeder FC, Sternberg PW, Edison AS. Proc Natl Acad Sci U S A. 2012;109:20949–20954. doi: 10.1073/pnas.1218302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bose N, Meyer JM, Yim JJ, Mayer MG, Markov GV, Ogawa A, Schroeder FC, Sommer RJ. Curr Biol. 2014;24:1536–1541. doi: 10.1016/j.cub.2014.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yim JJ, Bose N, Meyer JM, Sommer RJ, Schroeder FC. Org Lett. 2015;17:1648–1651. doi: 10.1021/acs.orglett.5b00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manosalva P, Manohar M, von Reuss SH, Chen S, Koch A, Kaplan F, Choe A, Micikas RJ, Wang X, Kogel KH, Sternberg PW, Williamson VM, Schroeder FC, Klessig DF. Nat Commun. 2015;6:7795. doi: 10.1038/ncomms8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao L, Zhang X, Chinta S, Lu M, Wei Y, Zhou J, Zhang W, Kong X, Liu Y, Zou Z, Butcher RA, Sun J. Nature Communications. 2016;7:e12341. doi: 10.1038/ncomms12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Reuss SH, Bose N, Srinivasan J, Yim JJ, Judkins JC, Sternberg PW, Schroeder FC. J Am Chem Soc. 2012;134:1817–1824. doi: 10.1021/ja210202y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bargmann CI. WormBook. 2006 doi: 10.1895/wormbook.1.123.1. www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 23.McGrath PT, Xu Y, Ailion M, Garrison JL, Butcher RA, Bargmann CI. Nature. 2011;477:321–325. doi: 10.1038/nature10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim K, Sato K, Shibuya M, Zeiger DM, Butcher RA, Ragains JR, Clardy J, Touhara K, Sengupta P. Science. 2009;326:994–998. doi: 10.1126/science.1176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park D, O'Doherty I, Somvanshi RK, Bethke A, Schroeder FC, Kumar U, Riddle DL. Proc Natl Acad Sci U S A. 2012;109:9917–9922. doi: 10.1073/pnas.1202216109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Feng L, Chinta S, Singh P, Wang Y, Nunnery JK, Butcher RA. Proc Natl Acad Sci U S A. 2015;112:3955–3960. doi: 10.1073/pnas.1423951112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan F, Srinivasan J, Mahanti P, Ajredini R, Durak O, Nimalendran R, Sternberg PW, Teal PE, Schroeder FC, Edison AS, Alborn HT. PLoS One. 2011;6:e17804. doi: 10.1371/journal.pone.0017804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joo HJ, Park S, Kim KY, Kim MY, Kim H, Park D, Paik YK. Biochem J. 2016;473:789–796. doi: 10.1042/BJ20150938. [DOI] [PubMed] [Google Scholar]
- 29.Butcher RA, Ragains JR, Li W, Ruvkun G, Clardy J, Mak HY. Proc Natl Acad Sci U S A. 2009;106:1875–1879. doi: 10.1073/pnas.0810338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joo HJ, Yim YH, Jeong PY, Jin YX, Lee JE, Kim H, Jeong SK, Chitwood DJ, Paik YK. Biochem J. 2009;422:61–71. doi: 10.1042/BJ20090513. [DOI] [PubMed] [Google Scholar]
- 31.Joo HJ, Kim KY, Yim YH, Jin YX, Kim H, Kim MY, Paik YK. J Biol Chem. 2010;285:29319–29325. doi: 10.1074/jbc.M110.122663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Li K, Jones RA, Bruner SD, Butcher RA. Proc Natl Acad Sci U S A. 2016;113:10055–10060. doi: 10.1073/pnas.1608262113. [DOI] [PMC free article] [PubMed] [Google Scholar]