Abstract
Alzheimer’s disease (AD) is the most common form of dementia that affects more than 5 million Americans. It is the only disease among the 10 causes of death that cannot be slowed or cured, thus raising the need for identification of early preclinical markers that could be the focus of preventative efforts. Although evidence is escalating that abnormalities in olfactory structure and function precede AD development and early cognitive impairments by one or more decades, the importance of olfaction is largely overlooked in AD, and such testing is not routinely performed in neurology clinics. Nevertheless, research using the olfactory model, has begun to advance our understanding of the preclinical pathophysiology of AD. Notably, an interesting series of studies is beginning to illuminate the relationship between Apolipoprotein E (ApoE) ε4 polymorphism and olfactory dysfunction and late-onset Alzheimer’s disease. In this article, we reviewed present research on the significance of ApoE and olfaction to AD, summarized current studies on the associations and mechanisms of ApoE and olfactory dysfunction, and highlighted important gaps for future work to further advance the translational application of the olfactory paradigm to early, preclinical diagnosis and treatment of AD.
Keywords: Apolipoprotein E, Alzheimer’s disease, Olfactory bulb, Olfactory cortex, Olfactory function, Odor evoked response potentials
Introduction
ApoE4 is a risk factor for AD
Numerous genetic studies have revealed that inheritance of ApoE4 allele increases the risk and rate of progression of late-onset Alzheimer disease, LOAD [1–3]. The human APOE gene exists as three polymorphic alleles—ε2, ε3 and ε4—which have population frequencies of 8.4%, 77.9% and 13.7%, respectively [4]. However, the frequency of the ε4 allele is dramatically increased to ~40% in patients with AD (4). Additionally, 65-80% of AD patients have at least one ApoE4 allele. Inheritance of a single ApoE ε4 variant increases a person’s risk of developing AD by a factor of three in men and four in women, and having two copies of the ε4 allele increases risk by up to 15-fold compared to persons without the ε4 variant [4,5]. Furthermore, ApoE4 inheritance decreases the age of onset of AD [1,6–8].
Apolipoprotein E (ApoE) mediates lipid transport from one tissue or cell type to another [9,10], thus participates in the regulation of lipid homeostasis. In peripheral tissues, ApoE is mainly produced by the liver and macrophages, and mediates cholesterol metabolism in an isoform-dependent manner. ApoE4 is associated with hypercholesterolemia, which is a risk factor for atherosclerosis, coronary heart disease and stroke [9,10]. In the CNS, ApoE is mainly produced by astrocytes, but also can be expressed by oligodendrocytes, and ependymal layer cells [11,12]. Cholesterol is transported to neurons by ApoE via ApoE receptors, which are members of the low-density lipoprotein receptor (LDLR) family [13]. Increasing evidence suggests that under diverse pathophysiological conditions, CNS neurons also express ApoE, although at lower levels than astrocytes [14–17]. The cellular origin of ApoE appears to influence its effects on AD pathology [18,19].
Neurofibrillary tangles and amyloid plaques, two neuropathological hallmarks of AD, are increased in brain samples from ApoE4 carriers as compared to non-ApoE4 carriers [3,7]. Both plaques and tangles appear earlier in ApoE4 carriers as compared to non-carriers of ApoE4. In addition, AD patients with ApoE4 genotype showed widespread degeneration of neurons in areas of the brain related to learning and memory, compared to non-ApoE4 patients [20]. Although various hypotheses have been proposed to explain the relationship between ApoE4 and AD, the mechanism by which ApoE4 leads to AD in humans, if at all, is still unclear. A comprehensive study of the pathophysiological effect of ApoE4 on AD progression is hampered by ethical limitations to sampling of brain tissues in living persons as they progress through preclinical to clinical stages of the disease. The early involvement of the more accessible olfactory pathways by AD biology, therefore offers some hope that the olfactory paradigm can be applied to investigative efforts aimed at elucidating preclinical pathophysiology of disease progression in people with genetic risk of AD. This review examines the scientific premise in support of the validity of the olfactory paradigm for investigation of early and progressive AD in ApoE4 carriers. To provide a template for interpreting data on ApoE-related changes in olfaction in AD, we first provide an overview of basic neurobiology of olfaction and a brief description of methods used for assessment of olfaction. This is followed by the review of extant data that examined whether the olfactory system reliably reflects the effect of apoE variations on AD vulnerability.
Neurobiology of olfaction
The olfactory system enables us to perceive smell from the environment, and flavors from food. Loss of olfaction is linked to many neurodegenerative diseases, including Parkinson’s disease (PD), fronto-temporal dementia (FTD) and AD [21,22]. The olfactory system begins with the olfactory mucosa, a pseudostratified columnar epithelium in the posterior region of the nasal cavity, from which odor information is projected into the olfactory bulb (OB) at the base of the brain (Figure 1). In all mammals, nasal airflow carries odorants into contact with olfactory receptors (ORs) located on the cilia of olfactory receptor neurons (ORNs) in the nasal olfactory mucosa. ORNs express only one OR type [23], and ORNs expressing the same OR innervate up to two glomeruli per OB [24]. Odorant binding with an OR triggers a G-coupled protein–mediated intracellular signaling cascade, ultimately producing an action potential [25,26]. The ORs possess unique chemosensory tuning properties [27,28], that provide a first step at which the olfactory system can sort the limitless number of odorants it may encounter. Action potentials in the ORNs transmit odorant information into discrete zones in the OB whose activation is dictated by nasal airflow [29–31]. These zones in the OB form a “spatial map” of odorant information [32–35] and are modulated by local glomerular layer neurons. This type of organization is believed to be important for the most basic aspects of olfactory perception, including odor perception and discrimination [36–38]. The OB is a six-layer structure in which the sequential stages of odor information processing take place (Figure 1). The axons of ORNs form the olfactory nerve layer of the OB [36–38]. Secondary olfactory neurons, called mitral cells (MCs) located in the mitral cell layer of OB, and tufted cells (TCs) located in the external plexiform layer, all innervate OB glomeruli. Another major cell population in the OB are the granule cells, which are axon-less interneurons organized in patchy aggregated rows in the most central cell layer of the OB (interneuron). The apical dendrites of granule cells form synapsis with MCs and TCs. Granule cells also receive centrifugal input from some secondary olfactory structures and display broader odor-tuning characteristics than the upstream MCs and TCs [39]. Granule cells are mostly GABAergic and glutamatergic. In many mammalian species granule cells are constantly renewed by neurogenesis during adulthood [40–43]. The activity of MCs, TCs, and interneurons in the OB is subject to neuromodulation [44]. The OB receives dense noradrenergic projections from the locus ceruleus, cholinergic input from the horizontal limb of diagonal band of Broca, and serotoninergic afferents from the medial and dorsal raphe nuclei. Axons from MC and TC converge to form the lateral olfactory tract, whose distal projections innervate a variety of secondary olfactory structures, including the anterior olfactory nucleus (AON), piriform cortex, olfactory tubercle, the lateral entorhinal cortex, and para-amygdaloid complex (Figure 2). These secondary olfactory structures are regarded as the primary olfactory cortex. Cells in the AON cells are highly responsive to odor stimulation [45] and they are believed to aid in intra-nostril odor localization [46]. The piriform cortex is well-known for its role in modifying the processing of odors based upon experience and learning [47–51]. Olfactory tubercle neurons are reliably activated by tasks that assess odor valence, motivated behaviors, and acquisition of rewards, suggesting important roles of the olfactory tubercle in guiding hedonic and valence-dependent responses to odors [52,53]. Neurons within these secondary olfactory structures project into tertiary olfactory structures, which include the orbitofrontal cortex, the insular cortex, and the dorsal hippocampus [54]. Of particular relevance to AD, the entorhinal cortex innervates the hippocampus via the perforant pathway [55]. Additionally, thalamic regions receive olfactory information from several of the secondary olfactory structures, including the AON, piriform cortex, and olfactory tubercle. Olfactory information is also transmitted to the hypothalamus via the amygdaloid complex [56]. These foregoing discussions suggest that odor experience is intertwined with motivated behavior, emotion and cognition through overlap neural circuitry.
Figure 1.
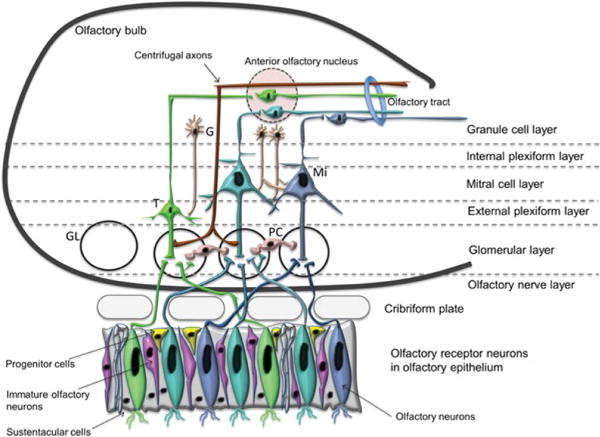
Schematic diagram of the olfactory neuroepithelium (OE) and the olfactory bulb (OB). The OE consists of cells at different stages of differentiation, including the proliferating progenitor cells (yellow color), the postmitotic immature olfactory neurons (pink color) and the olfactory sensory, OSN (also known as olfactory receptor neurons, ORN). Axons from the OSN pierce through the cribriform plate at the base of the skull to enter the OB, where they form the olfactory nerve layer. The OB, above the OE shows the laminar organization, the major cell types and the basic neuronal circuits. Interneurons shown are the granule cells (across different layers) and the periglomerular cells in the glomerular layer (GL). Efferent neurons of the olfactory bulb are tuffed and mitral cells.
Note: G: Granule cell; M: Mitral cell; T: Tufted cell; PC: Periglomerular cells
Figure 2.
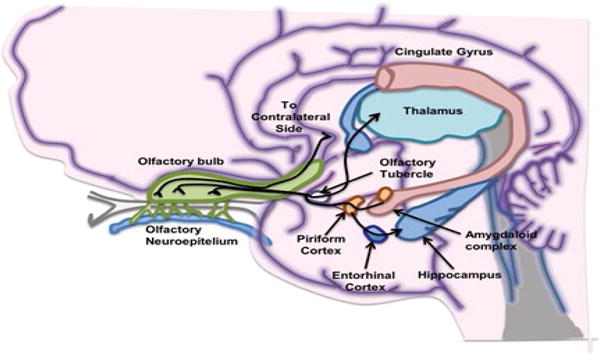
Simplified diagram of brain regions involved in the processing of olfactory information. The lateral olfactory tract project odor information into the primary olfactory cortex, which include the anterior olfactory nucleus (not shown), piriform cortex, olfactory tubercle, amygdaloid complex, and entorhinal cortex. From these primary olfactory cortical regions, odor information is projected to the thalamus, orbitofrontal cortex, insula cortex and hippocampus.
Psychophysical Tasks of Olfaction
Generally, psychophysical tasks of olfaction are based upon the presentation of odors to a test subject, followed by examination of the subject’s responses to questions about some characteristics of the odorants presented. Olfactory psychophysical tasks most commonly measured in studies of olfaction in aging include odor identification, odor memory, odor discrimination and odor threshold sensitivity tasks [57–59]. Other methods which have been employed for clinical assessment of olfaction include surveys of subjective rating of olfactory ability and quality of life [60] and assessments of odor familiarity [61] and hedonics [62].
Odor identification tasks
During odor identification tests, odorants are presented sequentially to test subjects at suprathreshold concentrations, and subjects are required to identify each odor from a list of descriptors. This forced-choice procedure is aimed at minimizing subjects’ response bias. Odor identification tests are considered the most advanced type of test depiction of higher-order cortical functions among other psychophysical tests [63–65]. However, a major problem of odor identification test is that it correlates with the verbal abilities of the subject and has a strong cultural precondition, as not all odors are known equally well in various cultural groups. The most widely used identification tests include the 40-odorant University of Pennsylvania Smell Identification Test (UPSIT) [59] and the Sniffin’ Sticks Identification Test [58]. However, several other tools are available, such as the 3-item Quick Smell Identification Test, Q-SIT [66]; the 12-item Brief Smell Identification Test, B-SIT [67]; the Smell Diskettes Olfaction Test [68]; the San Diego Odor Identification Test, (SDOIT), which consists of 8 common household odorants presented in opaque jars and includes a picture board to assist odor identification [20]; the Brief (Cross-Cultural) Smell Identification Test [67]; and the Scandinavian Odor Identification Test [69]. Additionally, odor identification can be assessed using flow-dilution olfactometers, such as the T&T Olfactometer [70].
Odor discrimination tasks
In odor discrimination procedures, different odorants are presented to subjects at suprathreshold concentrations, and subjects have to determine which of the odorants smell different [71]. This task examines the ability to distinguish between odors, not to recognize or identify them. In clinical applications discrimination tests are used in combination with identification and threshold tests. The Sniffin’ Sticks Test (SST) discrimination task involves a triple-forced-choice procedure. Per triplet, two distracter pens encompass identical smells, while the respective third pen (the clue) contains a different odor. The number of correctly identified clues represents the discrimination score [71].
Odor threshold tasks
The principle behind the threshold tests is that a subject is repeatedly exposed to ascending and descending concentrations of the same odorant and is required to identify the least detectable concentration for the index odor (usually n-butanol or phenyl-ethyl-alcohol) [72]. The widespread use of the latter odorant in odor threshold applications is based on the premise that it more selectively activates olfactory receptors than the trigeminal receptors in the nose [72]. This task is normally associated with the peripheral part of the olfactory pathway [73], and can be measured by means of the T&T olfactometer [70], the Threshold Test of the Sniffin’ Sticks Extended Test [71], and the Smell Threshold Test (STT) [74].
Odor memory tasks
Perhaps due to their relative complexity, odor memory tasks were introduced more recently, compared to threshold and identification tasks. Most clinical applications of odor memory use a recognition task carried out in two stages: the acquisition and the recognition stages. During the acquisition stage, the subject is asked to smell a small set of common household odors at intervals of 30 seconds to control for odor adaptation. This is followed by the recognition stage whereby the subject would be required to recognize the previously presented odors among distractor odors [75]. The total number of correctly recognized, percent of odors in the acquisition stage that was correctly identified, and recognition errors are outcome variables of odor memory tests commonly used in clinical studies [75,76].
Neuroimaging and Physiological Measures of Olfaction
Neuroimaging procedures, including structural and functional magnetic resonance imaging, positron emission tests (PET), single-photon emission computed tomography (SPECT), and electroencephalography (EEG), have been used in neuroscience research to characterize the neurobiology of olfactory processing. The imaging modalities provide good spatial localizations of regions relevant to olfaction, while the EEG applications reveal the sequence of neuronal activations with high temporal resolution [77]. However, due to its relative simplicity, neurobiological investigations of the relationship between ApoE and olfaction have almost exclusively employed EEG methods.
To characterize EEG patterns of olfactory perception, researchers either place electrodes intranasally to acquire odor-induced electrophysiological activity locally in the olfactory epithelium (i.e., electro-olfactography, EOG), or acquire cortical activations during odor exposure, through placement of electrodes on the scalp (i.e., odor event-related potentials, OERPs). These methods provide more objective measures of olfactory function, independent of patients’ response bias. Compared to EOG, OERPs are more commonly assessed in clinical populations, and their absence is often a strong indicator of olfactory loss [78]. Odor event-related potentials (OERPs) result from the sequential activation of different brain areas, beginning from olfactory bulbs and tracts and involving the orbitofrontal and insular cortices, along with rostrum-medial regions of the temporal lobe [79]. In most OERP designs, three scalp electrodes placed along the midline – frontal (Fz), central (Cz), and parietal (Pz) – regions allow for examination of relative activations of the cortical fronto-centro-parietal regions by olfactory stimuli [80]. Odor event-related potentials consist of a series of positive and negative voltage deflections, which are related to a set of underlying components. Most components are referred to by a letter (N/P) indicating polarity (negative/positive), followed by a number indicating either the latency in milliseconds or the component’s ordinal position in the waveform. For instance, a negative-going peak that often occurs about 100 milliseconds after a stimulus is presented is often called the N100 or N1. Typically, N1 is followed by a positive peak, known as the P200 or P2. The specified latencies for OERP components are often quite variable. For example, the P300 (P3) component may exhibit a peak anywhere between 250 ms – 700 ms [81]. These OERP parameters are important variables in studies investigating the influence of ApoE polymorphisms in olfactory functions and in AD.
ApoE4 association with olfactory dysfunction in AD
An overview of results from studies investigating the association between apoE polymorphisms and olfactory functions is depicted in Table 1. As shown, deficits in odor fluency, odor identification, odor recognition memory, and odor threshold sensitivity have all been associated with inheritance of the ApoE4 genotype in several studies [20,82–86]. These impairments in olfactory psychophysics are observed early in the course of AD, even before the onset of clinical dementia [20,86]. Non-demented carriers of ApoE4 polymorphism showed significant decline in olfactory processing as compared to individuals without ApoE4 allele [87,88]. Importantly, patients with increased brain atrophy have greater olfactory impairment [89], indicating that olfactory function is further diminished as AD progresses. In family studies, siblings of AD probands had lower olfactory identification scores compared to siblings of control probands [90]. Moreover, within families, siblings with ApoE4 allele showed greater deficits in odor identification tests than siblings without ApoE4 allele. This finding of cosegregation between ApoE4 and olfactory identification deficits suggests that odor identification deficits may reflect early disease expression in individuals at increased risk for developing the disease [90]. Data from longitudinal studies provide additional supporting evidence of the association between ApoE4 inheritance and poor scores in olfactory tests, but more importantly highlights the predictive effect of baseline ApoE4 status on progression of olfactory loss over a 4-year period [83]. A recent study suggests that domains of high-order olfactory functioning, like odor identification and remote memory measured by odor familiarity ratings, may be more impaired in AD E4/4 homozygotes compared to E3/4 heterozygotes and E3/3 homozygotes [91]. These deficits give insight into how the presence of two E4 alleles may differentially affect the progression of AD. Down Syndrome involves trisomy of chromosome 21, where the gene for amyloid precursor protein (APP) is located. Down Syndrome (DS) represents premature aging disorder and autopsy studies show that by age 40; the brains of almost all individuals with DS have significant levels of plaques and tangles, which are definitive neuropathological features of AD. These consistent brain changes notwithstanding, development of AD is not uniform in DS [92]. Interestingly, individuals with Down Syndrome (DS) who are carriers of ApoE4 allele, exhibit significantly greater deficits in odor identification than those who are negative for the allele [93]. It implies therefore, that olfactory tests and ApoE4 genotyping may contribute to improved prediction of AD risks in DS populations.
Table 1.
ApoE olfaction studies.
| Study | Sample (n) | Age (years) | Measure | Findings | Other notes |
|---|---|---|---|---|---|
| Wang et al. [85] | E4 + (25) E4 − (33) |
E4+ =70.70 ± 7.36; E4− =71.67 ± 6.28 | Cross-cultural Smell Identification Test (CC-SIT) | CC-SIT=9.11 ± 1.37 in E4− and CC-SIT=7.34 ± 1.43 in E4+; p<0.01 | ↓ smell identification in ApoE4 group |
| Kowalewski and Murphy [98] | E4 + (10) E4 − (10) |
69.3 (4.2) | Olfactory-visual semantic congruency task to investigate cross-modal odor identification disturbances | Significant differences in OERPs between E4+, and E4−. Significant main effect for congruency (F (1,16)=27.08, p <0.001). Significant 4-way interaction between LR, DV, congruency and ApoE4 (F (1,16)=9.41, p=0.007). E4+ had a larger difference in amplitude between congruous and incongruous odor-image pairs at right ventral electrode sites than E4−, t (9)=3.97, p=0.003. ApoE E4 carriers had a significantly smaller ERP amplitude difference than non-carriers (F (1,17)=5.12, p=0.037). |
A scalp topography of ApoE4 carriers was consistent with morphological and hypometabolic abnormalities found in PET, fMRI and MRI studies. OERPs reflected hemispheric asymmetries in E4 carriers that were line with a compensatory mechanism. OERPs in an odor/visual congruency task differentiated ApoE4+ and ApoE4−. |
| Corby et al. [97] | Young and middle-aged subjects with E4 polymorphisms: E4 + (20) E4 − (20) |
E4 +: Young=23.9+2.8 Middle age=50.6+2.4. E4 −: Young 22.2+3.0 Middle age=49.9+3.2. |
Chemosensory tests with the butanol odor threshold; SDOIT; OERPs. | Significant effects of ApoE status for P3 latency collapsed across age (F(1,36)= 21.91, p<0.001, η2=0.38), with ApoE E4− participants demonstrating shorter latencies than ApoE E4+ participants. In the young group olfactory P3 latency was the most significant predictor (χ2=7.69, p b 0.01) resulting in overall classification rate of 75% (Sensitivity=80%, Specificity=70%). In the middle age group olfactory P3 latency was also the most significant predictor (χ2=12.54, p=0.001) resulting in overall classification rate of 80% (Sensitivity=80%, Specificity=80%). |
OERP is sensitive to very subtle changes in the brain associated with the ApoE E4 allele, even at much younger ages than previously demonstrated. Additionally, the OERP is more sensitive to these changes than traditional tests of olfactory functioning. |
| Handley et al. [90] | Sibling group (24): E+ (10); E − (14) Control group (47) E+ (33); E − (14) |
Sibling group mean age (range): 74.08 (59 – 88). Control group: 73.17 (61 – 87) |
Odor identification performance test | Lower odor identification scores for the sibling group 4.17 ± 2.20 compared to control group 5.60 ± 2.22, F(1, 67) 10.42, p<0.01. No difference in odor identification accuracy between E4+ 5.17 ± 2.44, and E4− groups 5.09 ± 2.44), p=ns. The group × ApoE E4 status interaction was significant, F(1, 67) 6.10, p<0.05. The sibling E4+ group had the poorest mean odor identification scores, lower than control E4+ (t(22)= −4.12, p<0.01) and control E4− (t(44)= −2.33, p<0.05). |
Odor identification deficits may reflect early disease progression in individuals at increased risk for developing the disease. |
| Calhoun-Haney and Murphy [83] | Year 1 (baseline): E4+ (22) E− (28); Year 4 (follow-up): E4+ (22) E− (28) |
Year 1: E4 + =73; E4 − =71; Year 4: E4 + =77; E4 − =75. |
Butanol odor threshold test; SDOIT | At baseline there was no significant difference between performance on odor identification between the two allele groups, (F (1, 48)=2.9, p=0.09), although there was the trend toward poorer performance in the E4+ group. Allele status significantly affected performance on the odor identification measure at Year 4 follow-up, (F (1, 48)=20.0, p<0.0005), with performance significantly poorer in E4+ than in E4− individuals. E4+ individuals significantly declined in odor identification performance over time (F(1,21)=22.9, p<0.0005), but E4− individuals did not (F(1,27)=0.41, p=0.52). There was no main effect of time (F (1, 48)=0.39, p=0.54) or allele status (F (1,48)=1.7, p=0.20) on odor threshold. Detection of odor at higher dilution steps indicates better sensitivity. Neither the E4+ nor E4− group showed a significant decline in global cognitive performance over time. |
Study demonstrates that normal non-demented elderly adults who carry the E4+ allele and are thus at risk for AD, showed a significant decline over a 4-year time period in performance on odor identification but not on odor threshold, picture identification or the DRS. |
| Wetter and Murphy [94] | E4 + (10) E4 −(10) |
E4 + =75.7 ± 7.7; E4 − =75.3 ± 6.4 |
Amyl acetate odor threshold test; UPSIT; SDOIT; OERPs. | Significant delays for E4+ individuals at each OERP component at: N1 [F(1,18)=17.8, p=0.001)], P2 [F(1,18)=19.7, p=0.001], N2 [F(1,18)=22.4, p=0.001], and P3 [F(1,18)=16.1, p=0.001. Individuals with poorer ability to identify odors also showed increased olfactory P3 latencies, suggesting that the ability to identify odors is specifically associated with the speed of cognitive processing of olfactory stimuli. Psychophysical (UPSIT, and odor threshold) and cognitive (DRS and MMSE) measure revealed no significant effects of allele status in this sample size. |
ApoE4+ Individuals demonstrated delays in the processing of olfactory information compared to those who are E4−. OERP latency appears to be more sensitive for detecting olfactory deficits than the psychophysical measures utilized in this study. |
| Bacon et al. [86] | E+ (9); E− (6) | E+ =75.75 ± 5.01; E– =80.67 ± 5.39 |
Butanol odor threshold; MMSE; DRS | E4+ performed worse on DRS: t (12)= −2.18, p=0.05 and odor threshold. E4+ participants showed poorer odor sensitivity to butanol than E4−. No differences between groups on the MMSE |
First study documenting differences in performance on a test of olfaction in group of adults at risk for AD (E4+), more sensitive than MMSE. |
| Sundermann et al. [87] | MCI patients (converters and nonconverters to AD during 1–9 years follow up): Converters (39); Nonconverters (109); Controls (63) |
converters=73.2 ± 7.1; Nonconverters=64.9 ± 9.9 Controls=65.7 ± 9.3 |
UPSIT; Apolipoprotein E genotype: patients classified as E4+ or E4−. | UPSIT scores in: Converters=25.8 ± 8.4; Non-converters=33.2 ± 4.6; Controls=34.8 ± 4.2. % of E4 carriers: converters=34.3; non-converters=23.6; controls 22.4 |
Clinical sample of cognitively impaired, non-demented patients exhibiting memory complaints shows that ApoE and olfactory dysfunction can be combined for prediction of conversion rate to AD |
| Gilbert and Murphy [84] | Control group (38): E4+ (19); E4− (19) Probable AD (38): E4+ (24); E4− (14) Confirmed AD (38): E4+ (23); E4− (15) |
Control: E4+ =71.3 0± 2.24; E4− =71.44 ± 2.07; Probable AD: E4+ =74.15 ± 1.45; E4− =73.70 ± 1.93; Confirmed AD: E4+ =72.16 ± 1.31; E4− =75.90 ± 2.40 |
Odor threshold test; Recognition memory task developed by Murphy. | Significant effect of threshold for group F (2, 108)=6.13, p<0.01, but no significant effect for E4 status or an E4 status × group interaction, p>0.05. On the recognition memory task, E4− controls committed significantly fewer false positive errors than E4+ controls or AD patients. E4+ controls did not differ from AD patients in false positive errors. | The analysis of false positive errors in recognition memory for olfactory stimuli may be useful in identifying early deficits in cognition. |
| Gilbert and Murphy [84] | E4+ (21); E4− (21) |
E4+ =71.38 ± 1.88; E4−=71.45 ± 2.06 | Odor threshold test; Recognition Memory Task by Murphy. | No significant difference between the odor thresholds of the E4+ and E4− individuals (F(1, 40)=0:01; p=0.97). No differences between the mean number of hits committed by E4+ and E4− individuals on recognition memory tasks involving odors, faces, or symbols. E4+ individuals committed more false positive errors relative to E4− individuals for olfactory stimuli, but not for faces or symbols (F (1,40)=4,22; p=0:05*). No significant differences between E4+ and E4− individuals on recognition memory tasks involving odors F(1,40)=0.01; p=0.44; faces F(1,40)=2.20; p=0.15; or symbols F(1, 40)=1.98; p=0.28 |
The remote memory for olfactory and visual stimuli was not impaired in non-demented E4+ individuals compared to E4 positive controls. These data suggest that the areas of the brain involved in retrieval of remote memories are not significantly affected in non-demented individuals genetically at risk for AD. |
| Kjelvik et al. [89] | AD and Controls (% carriers of E4 alleles): Patients n=18; E4+ (73.3%) Controls n=30; E4+ (20.8%) |
Patients=74.6 ± 6.3 Controls=67.4 ± 7.6; |
B-SIT; SSIT; SSDT | Patients performed significantly worse than healthy controls on the two odor identification tests (B-SIT and SSIT), but not on the odor discrimination test SSDT. B-SIT: controls – 9.6 ± 2.0, patients – 6.6 ± 2.6 **p<0.005; SSIT: controls - 12.7 ± 2.4, patients - 9.4 ± 3.0 **p<0.005; The B-SIT score did not differentiate patients with aMCI from those with AD at baseline (p>0.4), but differentiated those patients persisting with aMCI from those who had progressed to AD 6–18 months later (mean 9.9 months, t-test; p=0.037). SSIT scores did not differentiate patients converting to AD. |
Competence in olfactory identification was also associated with the volume of several brain structures, particularly hippocampus, more than scores on memory tasks in aMCI and AD. Olfactory tests distinguished patients with aMCI and early AD from healthy control individuals, and suggested that patients with greater olfactory impairment have increased brain atrophy. |
| Corby et al. [97] | Young and middleaged subjects genotyped for ApoE Young E+ (10); E+ Middle E+ (10) Young E− (10); Middle E− (10) |
ApoE+: Young=22.2 ± 2.0; Middle=49.9 ± 3.2; ApoE−: Young=23.9 ± 2.8; Middle=50.6 ± 2.4 |
Butanol odor threshold; SDOIT; OERPs | DRS scores, butanol thresholds, and odor ID scores did not differ between age groups, or ApoE groups, and there were no interaction effects (p>0.05). Young participants produced significantly larger P3 amplitudes than middle age participants (F(1,36) = 7.96, p-0.01, η2 = 0.18). No significant effects of ApoE status on visual or olfactory amplitude (p>0.05), and ApoE status did not interact with age (p>0.05). Significant effects of ApoE status for P3 latency collapsed across age (F(1,36)=21.91, p=0.001, η2=0.38), with ApoE ε4- participants demonstrating shorter latencies than ApoE E4+ participants; Significant olfactory P3 latency in young subjects with E4+ participants producing longer P3 latencies than E4− participants (p=0.05, η2=0.31). For middle age participants analysis demonstrated longer P3 latencies (p=0.01, η2=0.47) for E4+ participants compared to E4− participants. |
In the young group olfactory P3 latency was the most significant predictor (χ2=7.69, p=0.01) resulting in overall classification rate of 75% (Sensitivity=80%, Specificity=70%). In the middle age group olfactory P3 latency was also the most significant predictor (χ2=12.54, p=0.001) resulting in overall classification rate of 80% (Sensitivity=80%, Specificity=80%). Study demonstrates that OERP seems to be sensitive to very subtle changes in the brain associated with the ApoE4 allele, even at much younger ages than previously shown. The OERP appears to be more sensitive to these changes than traditional tests of olfactory functioning. |
| Landis et al. [77] | 60–66 age group (E4%): n=1121 (29.98); 72–78 age group: n=691 (29.67); 81–87: n=339 (22.44); 90+ age group: n=129 (18.52) | 60–66 age group: 63.06 ± 2.90; 72–78 age group=75.14 ± 2.99; 81–87 age group=83.92 ± 2.38; 90+ age group=91.95 ± 2.40 |
Olfactory memory comprised of episodic odor recognition memory and odor identification, based on the Sniffin’ Sticks test battery. Other measures: verbal and visual episodic and semantic memory |
E4+ had an effect on olfactory memory (standardized estimate=0.08, p<0.01), such that the presence of an E4 allele was associated with poorer olfactory memory. No interaction effects between APOE and age were observed with regard to olfactory memory and verbal or visual episodic memory (p>0.10). |
This study found that olfactory memory is more sensitive to effects of age and APOE genotype compared to episodic and semantic memory. |
| Murphy et al. [20] | Nondemented older persons E4+ (7); E4− (20) | E4+ =74.00 ± 5.34; E4− =79.43 ± 7.74 | Butanol odor threshold; SDOIT | SDOIT scores were significantly lower in E4+ versus E4 group (p=0.006). Odor threshold task did not reach significance between groups. | Study shows the dissociation between odor threshold and odor identification very early in the disease process, even before clinical signs of dementia. |
| Longitudinal study of episodic memory decline Decline group n=110: E4+ (45) and E4− (65). No decline group n=977: E4+ (279) and E4− (698) |
Decline group=65.6 ± 9.9. No decline group = 68.9 ± 10.8 |
SOIT | Odor identification scores were lower in participants with episodic memory decline (main effect, F(1,1083)=7.480, p=0.006). Odor identification scores were lower in participants with E4 (main effect, F(1,1083)=4.467, p=0.035). |
Olfaction was significantly impaired in participants with both E4 and an ongoing episodic memory decline. | |
| Longitudinal study of global cognitive decline E4+ (143); E4− (353) |
73.3 ± 7.1 | A version of SOIT | An odor identification deficit, in combination with older age and ApoE4 predicted decline in global cognitive function. | olfactory deficit can dissociate between benign and non-benign global cognitive development in nondemented, very old E4− carriers, who are at high risk of developing dementia. However, further longitudinal assessment are needed to resolve whether a combination of olfactory deficit, E4, and high age predicts clinical dementia over a more extended time frame. | |
| Population based longitudinal study E4+ (372); E4− (864) |
E4+ =61.5 ± 11.3; E4− =60.7 ± 10.8 | A version SOIT | Odor identification performance decreased most strongly in older participants with ApoE4; ApoE4 × age interaction was significant (p=0.033). Although several demographic, cognitive, and health variables affected odor identification, this results suggest that there was a unique effect of the ApoE4 × age interaction on odor identification. Predictor variables accounted for 21.3% of the explanatory variance in odor identification performance. |
The effect of ApoE4 on odor identification in the 75–80 age range is not driven by individuals that receive a dementia diagnosis within a 5-year period after olfactory assessment. The present results suggest that the ApoE gene plays a significant role for the integrity of the olfactory system in non-demented, elderly individuals. | |
| Young E4+ (11); Young E4− (13). Old E4+ (14) Old E4− (13) |
Young E4+ =22.1 ± 1.8; Young E4− =23.1 ± 2.7. Old E4+ =70.8 ± 5.0; Old E4− =71.6 ± 4.0 |
Odor threshold, olfactory ERP stimulus presentation, recording and analysis |
Latencies were longer in older adults than in young adults, F(1, 49)=106.54, p<0.001. Young adults who were positive for the ApoE E4 allele also showed shorter latencies than older adults with the ApoE E4 allele, F(1, 23)=236.05, p<0.001; only the ApoE E4 positive older adults maintained significant correlations between both measurements of adiposity and P3 latency at Pz, i.e., BMI [r(14)=0.629, p<0.05], waist circumference [r(14)=0.625, p<0.05] |
Study shows a positive linear relationship between adiposity and prolonged olfactory latencies in older adults. When analyzed separately, this relationship remained significant only in older adults who were positive for the ApoE4 allele. There were no significant differences in amplitude between groups. |
|
| E4+ (15); E4− (23) | E4+ =58.0 ± 6.3; E4− =58.0 ± 11.1 | B-SIT test | B-SIT score (M±SD) in E4+ vs. E4− =8.9 ± 1.9 and 10.1 ± 1.2, p<0.05 | Higher occurrence of olfactory dysfunction among Irish individuals at genetic risk of dementia. | |
| Sliger et al. [93] | Down Syndrome (DS) (34): E4+ (12); E4− (22); Control n 34 |
DS=31.2 ± 1.59; Control=31.3 ± 1.60 |
SDOIT | Participants with DS possessing at least one E4 allele performed significantly poorer on the odor identification test compared to those without the E4 allele. The mean number of odors identified in the E4+ group was 4.4, compared to 5.7 in the E4− group, F (1, 33)=4.51, p=0.04. | Individuals with DS carrying the ApoE4 allele, exhibit significantly greater deficits in odor identification than those who are negative for the allele. |
| Morgan and Murphy [99] | Young E4+ (10); Middle age E+ (10); Old E4+ (10). Young E4− (10); Middle age E4− (10); Old E4− (10). |
Young E4+ =23.1 ± 2.3; Middle age E4+ =50.2 ± 4.5; Old E4+ = 70.2 ± 2.9 Young E4− =22.6 ± 2.0); Middle age E4− =50.7 ± 1.7; Old E4− =71.2 ± 3.6. |
Odor Threshold test; SDOIT; OERPs | SDOIT test revealed no significant main effects or interaction effects involving ApoE status (p>0.05). Odor ERP task revealed a main effect of ApoE status collapsed across age groups (F(1,54)=4.54, p<0.05) η2=0.08). Varying patterns of brain activation were observed over the post-stimulus epoch for E4− versus E4+ individuals on topographical maps. Individuals with the E4 allele demonstrated different ERP peak latencies during identification of olfactory but not visual stimuli. |
Olfactory ERPs detected functional decline in individuals at risk for Alzheimer’s disease at much earlier ages than previously observed, suggesting the potential role of ERPs for pre-clinical detection of AD at very early stages. |
| Sundermann et al. [87] | Non-demented Older Females: E4+ on Hormonal Therapy, HT (n=8); E4+ No HT (n=11); E4− on HT (n=8); E4− No HT (n=24); AD Female Group: E4+ on HT (n=12); E4+ No HT (n = 35); E4− on HT (n=6; E4− No HT (n=24). |
Non-demented Older Females: E4+ on HT =70.41 ± 4.22; E4+ No HT =69.70 ± 3.60; E4− on HT =73.18 ± 4.22; E4− No HT =73.77 ± 2.43; AD Females: E4+ on HT =72.94 ± 2.61; E4+ No HT 76.48 ± 1.53; E4− on HT =72.97 ± 3.69; E4− No HT 75.61 ± 1.89. |
Butanol odor threshold | HT had no effect on olfactory sensitivity in female AD patients regardless of E4 genotype. Within the non-demented no HT group, E4− females had a significantly better threshold score than E4+ females. No significant differences existed in odor threshold scores between the E4+ and E4− females in the HT comparison group. | HT may exert neuroprotective effects on brain areas affected by AD. HT is protective against loss of odor sensitivity function in E4 positive individuals in preclinical stages of AD. |
| Murphy et al. [82] | 20 Non-demented Old Adults: E4+ (10); E4− (10) |
E4+ =75.1 ± 8.3; E4− =71 ± 6.1 |
Odor Threshold Test; SDOIT; OERP; EEG | No significant difference in odor threshold scores between ApoE4+ and E4− participants. P3 latency was significantly delayed in ApoE E4+ individuals, F(1,18)=9.6, p<0.05). The results support significant differences between E4+ and E4− individuals in P3 latency and in intra-class correlations of activity. ApoE E4+ individuals demonstrated significantly longer P3 latency than E4+ individuals and differential activity for all response types. Differential activity in ApoE E4+and E4− individuals, demonstrated by the intra-class correlation coefficients, is consistent with a compensatory hypothesis, which posits that E4+ individuals expend greater effort in cognitive processing and therefore require greater activation of neural tissue during retrieval attempts. |
Study suggest that cross-modal ERP studies of recognition memory in ApoE4+and E4− individuals are a useful measure for indexing functional brain integrity, for understanding the neurocognitive changes associated with the ApoE4 allele, and for discriminating between brain response in E4+ and E4− individuals. |
| Oleson and Murphy [91] |
Older adults with Probable AD Experiment 1 (n=51): E3/3 (n=17); E3/4 (n=17); E4/4 (n=17). Experiment 2 and 3: (n=69): E3/3 (n=23); E3/4 (n=23); E4/4 (n=23). |
Experiment 1: E3/3=74.38 ± 6.91; E3/4=74.71 ± 6.84; E4/4=74.88 ± 6.49 Experiment 2 and 3: E3/3=73.78 ± 7.08; E3/4=73.17 ± 6.89; E4/4=73.52 ± 6.59 |
Exp. 1: SDOIT Exp. 2: Remote Odor Memory Task Exp. 2, 3: Odor Threshold. |
Exp. 1: The effect of ApoE status across groups collapsing over tasks was marginal [F(2,46)=2.927, p=0.064]. The E4/4 homozygotes showed impaired performance in odor identification (M=13.79% correct) relative to E3/4 individuals (M=39.89% correct) and E3/3 individuals (M=42.19% correct)- p<0.05. No significant differences in visual task performance. No significant differences in performance between E3/4 and E3/3 individuals for visual memory tasks. Exp 2: When collapsing across both tasks, there was a main effect of ApoE status [F(2,64)=6.267, p=0.003]. E4/4 participants reported lower familiarity ratings compared to E3/3 participants, but not E3/4 participants. Exp. 3: The E4/4 patients showed poorer odor detection threshold in the left nostril compared to E3/4 patients [F(1,44)=5.650, p=0.022]. |
Percent correct odor identification scores for AD patients were noticeably lower than scores for the E3/4 group in the current study, suggesting that combining individuals with different levels of E4 allele status in a sample of AD individuals may show muted effects of AD on olfactory function. |
Note: AD Alzheimer’s disease, aMCI amnestic mild cognitive impairment, APOE apolipoprotein E, BOLD blood-oxygen-level dependent, B-SIT brief smell identification test, CC-SIT cross-cultural smell identification test, CDR clinical dementia rating, DRS2 dementia rating scale 2, DS Down syndrome, E4 + APOE with presence of at least one E4 allele, E4 - APOE without any E4 allele, EEG electroencephalography, fMRI functional magnetic resonance imaging, HC healthy control, HT hormone therapy, MMSE mini-mental status examination, MRI magnetic resonance imaging, OERPs olfactory and visual event-related potentials, POC primary olfactory cortex, PET positron emission tomography, SDOIT San Diego odor identification test, SOIT Scandinavian odor identification test, SSDT Sniffin sticks discrimination test, SSIT Sniffin sticks identification test, UPSIT University of Pennsylvania smell identification test
Olfactory event-related potentials (OERPs) have also demonstrated high sensitivity to subtle changes in olfactory functioning, and to AD and ApoE status [94–96]. A growing number of clinical studies have shown that brain changes associated with ApoE4 allele are captured much earlier in age through OERP recordings than through psychophysical tests of olfaction, thereby suggesting the potential utility of OERP for identification of preclinical stages of AD [82,97–99].
Mechanistic studies linking ApoE4 to olfactory function
Despite consistent evidence of the robust relationship between olfactory dysfunction, ApoE4 inheritance and AD, the mechanism underlying these relationships are not fully understood. However, murine models of ApoE are beginning to illuminate our understanding of the role of ApoE in olfactory structure and function. The results of mechanistic studies in mice and from in vitro biochemical assays are highlighted in Table 2. ApoE deficiency in apoE KO mice leads to deficits in several tasks of olfactory function, suggesting an important role of ApoE in the mice olfactory system [100,101]. Previous studies showed that ApoE is expressed at high levels by a variety of cell types in the olfactory epithelium (OE). In particular, high expression of ApoE in the basal cells and adjoining lamina propria of the OE suggests that ApoE may play a role in the differentiation, maturation and axonal growth of ORNs, perhaps by recycling lipids from degenerating ORN for use by growing axons [102]. Indeed, studies of ApoE KO mice provide support that ApoE plays an important role in olfactory nerve regeneration [103]. Another group of investigators examined the effects of ApoE isoforms on neuronal differentiation and neurite outgrowth in OE explant cultures [104]. They discovered that ApoE2 and apoE3 stimulate neurite outgrowth in OE cultures by interacting with the lipoprotein receptor, LRP. ApoE4, the isoform associated with AD, failed to promote neurite outgrowth, signifying a potential mechanism whereby ApoE4 may lead to olfactory dysfunction in AD patients [104]. Moreover, Nathan et al. [101] investigated the involvement of ApoE in propagating regeneration of OE cells by inducing OE lesions in ApoE and WT mice [101]. The results revealed that ApoE expression in the OE is highly regulated during the entire course of OE reconstitution post injury, and that ApoE deficiency in ApoE KO mice leads to delayed recovery of mature OMP+ cells in the reconstituting OE [105]. Glomerular Synaptophysin (Syn) density, measured by immunohistochemistry, was lower in KO mice at all time points following the lesion [105]. This lower concentration of whole bulb Syn paralleled the slower recovery of glomerular area in KO mice. In the absence of ApoE, synaptic recovery in whole bulb samples was significantly delayed compared to WT mice [106]. This study highlights the important role of ApoE in neuronal differentiation. It is noteworthy that ApoE has also been shown to modulate other molecular factors that are important for neurogenesis, including WNT2 and granulin [107–109]. Some studies have identified a close relationship between estrogen and apolipoprotein E (ApoE) in the central nervous system, and neuroprotective the role of hormone therapy (HT) in several neurological disorders. Estrogen and ApoE function synergistically to minimize the loss of mature sensory neurons and synapses in OB, and OE following ovariectomy [110–113].
Table 2.
ApoE mice studies.
| Author (year) | Animal model | Age (months) | Measure | Findings | Conclusion |
|---|---|---|---|---|---|
| Nathan et al. [101] | ApoE KO mice; WT C57BL/6 strain | 4 months | BFP test; OC test; OCTA test. | ApoE KO mice performed poorly in all three tests compared to WT mice, while they learned the tasks at a rate comparable to WT mice. Latency to find the buried pellet was significantly longer in ApoE KO mice than WT mice. ApoE KO mice did not differentiate the odorant and failed the avoidance test. | ApoE deficiency in ApoE KO mice leads to a deficit in olfactory function, suggesting an important role for ApoE in the olfactory system. |
| Nathan et al. [103] | ApoE KO mice; WT C57BL/6 strain | 2–3 months | OE Lesion; IHC of OB tissue (0, 3, 7, 21, 42, and 56 days post-lesion) | Slow OMP recovery in the OB in ApoE KO compared to WT mice. Recovery of glomerular area was similarly slower. GAP43 accumulation and restore in the OB were slower in KO mice. | Olfactory nerve regeneration is significantly slower in KO mice, suggesting ApoE participates in olfactory nerve regeneration. |
| MC Asey et al. [113] | ApoE KO mice; WT C57BL/6 strain | 2–4 months | Ovariectomy. Estradiol replacement. IHC of Olfactory tissues (5, 14, 28 and 49 days after OVX and pellet replacement). | GFAP concentrations were higher in the E2-deprived mice but did not increase in the E2-replaced group at 49 days. Syn and ApoE concentrations were significantly ↑ by 15% and 25%, respectively, in the E2-replaced compared to the vehicle-replaced group at 5 days, but by 14 days concentrations were equivalent. | Estradiol is able to suppress reactive gliosis. In addition, E2 replacement in OVX mice is associated with transiently higher levels of ApoE and Syn. |
| Nathan et al. [102] | ApoE KO mice; WT C57BL/6 strain | 4 months | IHC staining of OB and OE tissues fpr ApoE iimunoreactivity | The perikarya and processes of sustentacular (Sus) cells expressed ApoE-like immunoreactivity. The endothelial cells of blood vessels were intensely stained for ApoE in the lamina propria. Cells forming Bowman’s gland also immunostained for ApoE. The ApoE staining in the nerve fascicles was less intense, but was uniformly distributed throughout the core of the nerve bundles. Ensheathing glia, surrounding the nerve fascicles also stained heavily for ApoE. |
ApoE is expressed in the adult OE and lamina propria at strategic locations where it could facilitate the differentiation, maturation and axonal growth of the ORN, perhaps by recycling lipids from degenerating ORN for use by growing axons. |
| Cheng et al. [112] | ApoE KO mice; WT C57BL/6 strain | 2–4 months | Ovariectomy; Estradiol (E2) replacement; IHC staining of olfactory tissues. | Five days of E2 replacement significantly ↑ LRP expression in the hippocampus, OB and neocortex but not in cerebellum. In contrast, E2 treatment ↓ LRP expression in OB. |
Hormone therapy (HT) modification of both ApoE and LRP could have wide-spread effects on cellular function given LRP’s manifold signaling functions. |
| Nwosu et al. [105] | ApoE KO mice; WT C57BL/6 strain | 2–4 months | OE Lesion; IHC and IB staining of OB tissue (0, 3, 7, 21, 42, and 56 days post-nasal irrigation). | Sharp ↓ in concentrations of Syn in OB following injury in both WT and KO mice during the degenerative phase (3–7 days). Syn concentration in KO mice did not recover by day 56 whereas Syn density in WT was essentially restored to normal. IHC of glomerular Syn density revealed a lower density in KO mice at all-time points post lesion. Lower concentration of whole bulb Syn parallels the slower recovery of glomerular area in KO mice. |
In the absence of ApoE, synaptic recovery in whole bulb samples is substantially delayed compared to WT mice. |
| Nathan et al. [106] | ApoE KO mice; WT C57BL/6 strain | 2–4 months | OE Lesion; Tissue preparation (0, 3, 7, 21, 42, and 56 days post-treatment); IB; IHC | ApoE expression in the OE is highly regulated during the entire course of OE reconstitution post injury, and ApoE deficiency in ApoE KO mice leads to delayed recovery of mature OMP+ cells in the reconstituting OE. | ApoE production increases in the injured OE to facilitate maturation of olfactory sensory neurons. |
| Nathan et al. [106] | ApoE KO mice; WT C57BL/6 strain | 4 months | Ovariectomy; BrdU injections, Olfactory turbinates tissues; IHC | 3 days of estradiol replacement ↑ ApoE expression in the olfactory nerve and in the glomerular layer. Estradiol treatment also ↑cell proliferation, total cell numbers, number of mature neurons in the olfactory epithelium, and reactive astrocyte numbers in the OB in both WT and KO mice. Estradiol ↑ glomerular synaptophysin (Syn), but the magnitude of increase was potentiated by the presence of ApoE. |
ApoE may be required to elicit the complete effect of estradiol on Syn upregulation. |
| Nathan et al. [110] | ApoE KO mice; WT C57BL/6 strain | 2–4 months | Ovariectomy; BrdU injections, Olfactory tissues; IHC | Estradiol replacement ↑ ApoE staining in the olfactory nerve and glomerular layers. Estradiol ↑ astrocyte density and OE thickness regardless of the genotype. Estradiol treatment ↑ the number of mature neurons in the OE and glomerular synaptophysin in both genotypes, but the magnitude of increase was greater in the WT than in the KO mice. |
Estrogen and ApoE act synergistically to minimize the loss of mature sensory neurons and synapses following ovariectomy. |
| Hussain et al. [104] | ApoE KO mice; WT C57BL/6 J mice | post-natal pups (2 days old) | Olfactory explant epithelial culture; Immunocytochemistry; Measurement of neuronal numbers, halo size, and neurite outgrowth | The OE cultures derived from ApoE KO mice have significantly ↓ neurons with shorter neurite outgrowth than cultures from WT mice. Treatment with either purified human ApoE2 or with human ApoE3, but not ApoE4, significantly ↑ neurite outgrowth. The differential effects of human ApoE isoforms on neurite outgrowth were abolished by blocking the LRP with lactoferrin and RAP. | ApoE2 and ApoE3 stimulate neurite outgrowth in OE cultures by interacting with the LRP. ApoE4, the isoform associated with AD, failed to promote neurite outgrowth, suggesting a potential mechanism whereby apoE4 may lead to olfactory dysfunction in AD patients. |
| Peng et al. [100] | knock-in mice humanized to ApoE4 versus ApoE3 | 6 months; 12 months | Olfactory Perceptual Memory; in vivo resting and odor-evoked local field potentials (LPF) | Young ApoE4 compared to ApoE3 mice exhibited a behavioral olfactory deficit coinciding with hyperactive odor-evoked response magnitudes within the OB that were not observed in older ApoE4 mice; shift with aging in ApoE4−driven effects from OB to PCX; | Early ApoE4−driven olfactory memory impairments and OB network abnormalities may be a precursor to later network dysfunction in the PCX, a region that not only is targeted early in AD, but may be selectively vulnerable to ApoE4 genotype. |
Note: AD Alzheimer’s disease, BFP buried food pellet, BrdU bromodeoxyuridine, GAP 43 growth associated protein 43, GFAP Glial fibrillary acidic protein, IB immunoblotting, IHC immunohistochemistry, LRP low-density lipoprotein (LDL) receptor related protein, OB olfactory bulb, OC odor choice, OCTA odor cued taste avoidance, OE olfactory epithelium, OMP olfactory marker protein, ORN olfactory receptor neuron, OVX ovariectomized, PCX piriform cortex, RAP receptor-associated protein, Syn synaptophysin (a synaptic marker).
Gaps in current studies and future directions
Current diagnosis of Alzheimer’s disease (AD) is based on clinical examination, neuropsychological testing and brain imaging; however, a definite diagnosis can only be made by postmortem examination. Although brain imaging and cerebrospinal fluid biomarkers are applied in patients with mild or questionable symptoms to increase the level of diagnostic certainty, and peripheral bio-fluids are largely investigated, no definitive diagnostic tests are available yet. Biomarkers that reliably predict development of AD would greatly assist preventative and management treatments. This review demonstrates the potential relevance of olfactory system for both biomarker and pathophysiology studies of AD progression. However, many questions must still be answered. It is unclear how to identify and differentiate age-related olfactory changes and olfactory dysfunction caused by disease. Smell loss is also associated with other neurodegenerative disorders, like Parkinson’s disease. Olfactory testing would need to be used with other biomarkers, specific to each disease, or olfactory changes in each disease have to be better specified. While the role of ApoE in olfactory neurogenesis has been reasonably demonstrated in basic studies, it remains uncertain how ApoE4 promotes AD amyloid and tau pathology in the olfactory system, especially in OB tissue. There are numerous studies examining interactions between ApoE4 protein and Amyloid or Tau proteins in cortical and hippocampal tissues [114]. ApoE is thought to be involved in plaque formation, but how exactly ApoE is involved in pathogenesis of AD is not well understood. The hypothesis gaining widespread support is that ApoE is involved in deposition or clearance of Abeta by direct protein-to-protein interaction. When associated with lipid, ApoE4 bound preferentially to an intermediate aggregated form of Abeta and had higher avidity than did ApoE2 or ApoE3 [115]. The tenability of this hypothesis in olfactory tissues has not been studied. Moreover, mechanistic correlations between ApoE and olfaction in AD to date are performed in animal models, not in humans. There are substantial differences between olfactory systems in rodents and humans. Further research is necessary to clarify these uncertainties. Patient-derived olfactory neurons offer an excellent alternative tool to study correlations between ApoE4 and olfactory impairment. Moreover, these olfactory cells can be collected from people at high risk (e.g. ApoE4 carriers), particularly those who display progressive impairments in psychophysical and physiological tests of olfaction. With future accomplishment of these goals in mind, it is reasonable to anticipate that combination of psychophysical, neuroimaging, electrophysiological and molecular studies of olfactory tissues may hold promise for characterization preclinical stages of the disease in people at risk, such as the case of ApoE4 inheritance.
Conclusion
This review summarizes the research showing that ApoE4 is a significant player in olfactory impairment, an early AD symptom. Furthermore, we discussed the mechanistic studies that have been evaluated in ApoE KO mice. Our review stresses the importance of olfactory function as a biomarker of AD, and a potential useful test for prediction of AD development in those with genetic (i.e., ApoE4) risk. Olfactory tests should be incorporated in the assessment of populations at high risk for dementia, like ApoE4 alleles for early recognition of AD and successful intervention.
Acknowledgments
We are grateful to Ms. McLean and Dr. Rai at The Translational Neuroscience Laboratory of Howard University, for their assistance with the editorial changes made to this review article.
Disclaimer
Sources of Funding/Disclaimers This project has been funded in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. Funding support for olfactory testing, and for the enrollment and neuropsychological testing of the control subjects is through USPHS grant MH-091460 (PI, Nwulia). This publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Disclaimer: Dr. Kapetanovic contributed to this article as part of his official duties while working in the NIMH Intramural Office.
References
- 1.Corder EH, Saunder AM, Strittmatter WJ, Schmechel DE, Gaskell PC, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 2.Corder EH, Lannfelt L, Bogdanovic N, Fratiglioni L, Mori H. The role of APOE polymorphisms in late-onset dementias. Cell Mol Life Sci. 1998;54:928–934. doi: 10.1007/s000180050223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohm TG, Scharnagl H, Marz W, Bohl J. Apolipoprotein E isoforms and the development of low and high Braak stages of Alzheimer’s disease-related lesions. Acta Neuropathol. 1999;98:273–280. doi: 10.1007/s004010051080. [DOI] [PubMed] [Google Scholar]
- 4.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 5.Bertram L, Tanzi RE. The genetic epidemiology of neurodegenerative disease. J Clin Invest. 2005;115:1449–1457. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueki A, Kawano M, Namba Y, Kawakami M, Ikeda K. A high frequency of apolipoprotein E4 isoprotein in Japanese patients with late-onset nonfamilial Alzheimer’s disease. Neurosci Lett. 1993;163:166–168. doi: 10.1016/0304-3940(93)90373-s. [DOI] [PubMed] [Google Scholar]
- 7.Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saunders AM, Schmader K, Breitner JC, Benson MD, Brown WT, et al. Apolipoprotein E epsilon 4 allele distributions in late-onset Alzheimer’s disease and in other amyloid-forming diseases. Lancet. 1993;342:710–711. doi: 10.1016/0140-6736(93)91709-u. [DOI] [PubMed] [Google Scholar]
- 9.Lahoz CE, Schaefer J, Cupples LA, Wilson PW, Levy D, et al. Apolipoprotein E genotype and cardiovascular disease in the Framingham Heart Study. Atherosclerosis. 2001;154:529–537. doi: 10.1016/s0021-9150(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 10.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 11.Poirier J, Hess M, May PC, Finch CE. Astrocytic apolipoprotein E mRNA and GFAP mRNA in hippocampus after entorhinal cortex lesioning. Brain Res Mol Brain Res. 1991;11:97–106. doi: 10.1016/0169-328x(91)90111-a. [DOI] [PubMed] [Google Scholar]
- 12.Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76:1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu PT, Schmechel D, Qiu HL, Herbstreith M, Rothrock-Christian T, et al. Sialylated human apolipoprotein E (apoEs) is preferentially associated with neuron-enriched cultures from APOE transgenic mice. Neurobiol Dis. 1999;6:63–75. doi: 10.1006/nbdi.1998.0213. [DOI] [PubMed] [Google Scholar]
- 15.Beffert U, Poirier J. Apolipoprotein E, plaques, tangles and cholinergic dysfunction in Alzheimer’s disease. Ann N Y Acad Sci. 1996;777:166–174. doi: 10.1111/j.1749-6632.1996.tb34415.x. [DOI] [PubMed] [Google Scholar]
- 16.Metzger RE, LaDu MJ, Pan JB, Getz GS, Frail DE, et al. Neurons of the human frontal cortex display apolipoprotein E immunoreactivity: implications for Alzheimer’s disease. J Neuropathol Exp Neurol. 1996;55:372–380. doi: 10.1097/00005072-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Han SH, Einstein G, Weisgraber KH, Strittmatter WJ, Saunders AM, et al. Apolipoprotein E is localized to the cytoplasm of human cortical neurons: a light and electron microscopic study. J Neuropathol Exp Neurol. 1994;53:535–544. doi: 10.1097/00005072-199409000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y. Molecular and cellular mechanisms of apolipoprotein E4 neurotoxicity and potential therapeutic strategies. Curr Opin Drug Discov Devel. 2006;9:627–641. [PubMed] [Google Scholar]
- 19.Huang Y, Weisgraber KH, Mucke L, Mahley RW. Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer’s disease. J Mol Neurosci. 2004;23:189–204. doi: 10.1385/JMN:23:3:189. [DOI] [PubMed] [Google Scholar]
- 20.Murphy C, Bacon AW, Bondi MW, Salmon DP. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Ann N Y Acad Sci. 1998;855:744–750. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- 21.Doty RL. The olfactory system and its disorders. Semin Neurol. 2009;29:74–81. doi: 10.1055/s-0028-1124025. [DOI] [PubMed] [Google Scholar]
- 22.Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, et al. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. 1999;53:1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- 23.Chess A, Simon I, Cedar H, Axel R. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78:823–834. doi: 10.1016/s0092-8674(94)90562-2. [DOI] [PubMed] [Google Scholar]
- 24.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, et al. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 25.Kato A, Reisert J, Ihara S, Yoshikawa K, Touhara K. Evaluation of the role of g protein-coupled receptor kinase 3 in desensitization of mouse odorant receptors in a Mammalian cell line and in olfactory sensory neurons. Chem Senses. 2014;39:771–780. doi: 10.1093/chemse/bju050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshikawa K, Touhara K. Myr-Ric-8A enhances G(alpha15)-mediated Ca2+ response of vertebrate olfactory receptors. Chem Senses. 2009;34:15–23. doi: 10.1093/chemse/bjn047. [DOI] [PubMed] [Google Scholar]
- 27.Bhandawat V, Reisert J, Yau KW. Elementary response of olfactory receptor neurons to odorants. Science. 2005;308:1931–1934. doi: 10.1126/science.1109886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araneda RC, Kini AD, Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- 29.Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci. 2007;10:631–639. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- 30.Buonviso N, Amat C, Litaudon P. Respiratory modulation of olfactory neurons in the rodent brain. Chem Senses. 2006;31:145–154. doi: 10.1093/chemse/bjj010. [DOI] [PubMed] [Google Scholar]
- 31.Mozell MM. Olfactory Discrimination: Electrophysiological Spatiotemporal Basis. Science. 1964;143:1336–1337. doi: 10.1126/science.143.3612.1336. [DOI] [PubMed] [Google Scholar]
- 32.Spors H, Wachowiak M, Cohen LB, Friedrich RW. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci. 2006;26:1247–1259. doi: 10.1523/JNEUROSCI.3100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson BA, Farahbod H, Xu Z, Saber S, Leon M. Local and global chemotopic organization: general features of the glomerular representations of aliphatic odorants differing in carbon number. J Comp Neurol. 2004;480:234–249. doi: 10.1002/cne.20335. [DOI] [PubMed] [Google Scholar]
- 34.Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- 35.Sharp FR, Kauer JS, Shepherd GM. Local sites of activity-related glucose metabolism in rat olfactory bulb during olfactory stimulation. Brain Res. 1975;98:596–600. doi: 10.1016/0006-8993(75)90377-7. [DOI] [PubMed] [Google Scholar]
- 36.Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol. 2006;17:411–423. doi: 10.1016/j.semcdb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: a major excitatory element that coordinates glomerular activity. J Neurosci. 2004;24:6676–6685. doi: 10.1523/JNEUROSCI.1367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, et al. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. doi: 10.1038/nature02185. [DOI] [PubMed] [Google Scholar]
- 39.Tan J, Savigner A, Ma M, Luo M. Odor information processing by the olfactory bulb analyzed in gene-targeted mice. Neuron. 2010;65:912–926. doi: 10.1016/j.neuron.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brill MS, Ninkovic J, Winpenny E, Hodge RD, Ozen I, et al. Adult generation of glutamatergic olfactory bulb interneurons. Nat Neurosci. 2009;12:1524–1533. doi: 10.1038/nn.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 42.Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 43.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 44.Linster C, Fontanini A. Functional neuromodulation of chemosensation in vertebrates. Curr Opin Neurobiol. 2014;29:82–87. doi: 10.1016/j.conb.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kay RB, Meyer EA, Illig KR, Brunjes PC. Spatial distribution of neural activity in the anterior olfactory nucleus evoked by odor and electrical stimulation. J Comp Neurol. 2011;519:277–289. doi: 10.1002/cne.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kikuta S, Sato K, Kashiwadani H, Tsunoda K, Yamasoba T, et al. From the Cover: Neurons in the anterior olfactory nucleus pars externa detect right or left localization of odor sources. Proc Natl Acad Sci USA. 2010;107:12363–12368. doi: 10.1073/pnas.1003999107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson DA, Sullivan RM. Cortical processing of odor objects. Neuron. 2011;72:506–19. doi: 10.1016/j.neuron.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gottfried JA. Central mechanisms of odour object perception. Nat Rev Neurosci. 2010;11:628–641. doi: 10.1038/nrn2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barkai E, Saar D. Cellular correlates of olfactory learning in the rat piriform cortex. Rev Neurosci. 2001;12:111–120. doi: 10.1515/revneuro.2001.12.2.111. [DOI] [PubMed] [Google Scholar]
- 50.Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995a;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- 51.Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. II. Ensemble activity in orbitofrontal cortex. J Neurophysiol. 1995c;74:751–762. doi: 10.1152/jn.1995.74.2.751. [DOI] [PubMed] [Google Scholar]
- 52.Gadziola MA, Wesson DW. The Neural Representation of Goal-Directed Actions and Outcomes in the Ventral Striatum’s Olfactory Tubercle. J Neurosci. 2016;36:548–560. doi: 10.1523/JNEUROSCI.3328-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gadziola MA, Tylicki KA, Christian DL, Wesson DW. The olfactory tubercle encodes odor valence in behaving mice. J Neurosci. 2015;35:4515–4527. doi: 10.1523/JNEUROSCI.4750-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doty RL, Philip S, Reddy K, Kerr KL. Influences of antihypertensive and antihyperlipidemic drugs on the senses of taste and smell: a review. J Hypertens. 2003;21:1805–1813. doi: 10.1097/00004872-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kareken DA, Mosnik DM, Doty RL, Dzemidzic M, Hutchins GD. Functional anatomy of human odor sensation, discrimination, and identification in health and aging. Neuropsychology. 2003;17:482–495. doi: 10.1037/0894-4105.17.3.482. [DOI] [PubMed] [Google Scholar]
- 57.Lotsch J, Reichmann H, Hummel T. Different odor tests contribute differently to the evaluation of olfactory loss. Chem Senses. 2008;33:17–21. doi: 10.1093/chemse/bjm058. [DOI] [PubMed] [Google Scholar]
- 58.Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, et al. “Sniffin’ sticks”: screening of olfactory performance. Rhinology. 1996;34:222–226. [PubMed] [Google Scholar]
- 59.Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, et al. Smell identification ability: changes with age. Science. 1984;226:1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- 60.Frasnelli J, Hummel T. Olfactory dysfunction and daily life. Eur Arch Otorhinolaryngol. 2005;262:231–235. doi: 10.1007/s00405-004-0796-y. [DOI] [PubMed] [Google Scholar]
- 61.Plailly J, d’Amato T, Saoud M, Royet JP. Left temporo-limbic and orbital dysfunction in schizophrenia during odor familiarity and hedonicity judgments. Neuroimage. 2006;29:302–313. doi: 10.1016/j.neuroimage.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 62.Joussain P, Thevenet M, Rouby C, Bensafi M. Effect of aging on hedonic appreciation of pleasant and unpleasant odors. PLoS One. 2013;8:e61376. doi: 10.1371/journal.pone.0061376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki Y, Critchley HD, Rowe A, Howlin P, Murphy DG. Impaired olfactory identification in Asperger’s syndrome. J Neuropsychiatry Clin Neurosci. 2003;15:105–107. doi: 10.1176/jnp.15.1.105. [DOI] [PubMed] [Google Scholar]
- 64.Zatorre RJ, Jones-Gotman M. Human olfactory discrimination after unilateral frontal or temporal lobectomy. Brain. 1991;114(Pt 1A):71–84. [PubMed] [Google Scholar]
- 65.Tanabe T, Iino M, Takagi SF. Discrimination of odors in olfactory bulb, pyriform-amygdaloid areas, and orbitofrontal cortex of the monkey. J Neurophysiol. 1975;38:1284–1296. doi: 10.1152/jn.1975.38.5.1284. [DOI] [PubMed] [Google Scholar]
- 66.Jackman AH, Doty RL. Utility of a three-item smell identification test in detecting olfactory dysfunction. Laryngoscope. 2005;115:2209–2212. doi: 10.1097/01.mlg.0000183194.17484.bb. [DOI] [PubMed] [Google Scholar]
- 67.Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT) Laryngoscope. 1996;106:353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 68.Simmen D, Briner HR, Hess K. Screening of olfaction with smell diskettes. Laryngorhinootologie. 1999;78:125–130. doi: 10.1055/s-2007-996844. [DOI] [PubMed] [Google Scholar]
- 69.Nordin S, Bramerson A, Liden E, Bende M. The Scandinavian Odor-Identification Test: development, reliability, validity and normative data. Acta Otolaryngol. 1998;118:226–234. doi: 10.1080/00016489850154946. [DOI] [PubMed] [Google Scholar]
- 70.Toyota B, Kitamura T, Takagi SF. Olfactory Disorders–Olfactometry and Therapy. Igaku-Shoin; Tokyo: 1978. [Google Scholar]
- 71.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 72.Deems DA, Doty RL. Age-related changes in the phenyl ethyl alcohol odor detection threshold. Trans Pa Acad Ophthalmol Otolaryngol. 1987;39:646–650. [PubMed] [Google Scholar]
- 73.Guarneros M, Ortiz-Romo N, Alcaraz-Zubeldia M, Drucker-Colin R, Hudson R. Nonoccupational environmental exposure to manganese is linked to deficits in peripheral and central olfactory function. Chem Senses. 2013;38:783–791. doi: 10.1093/chemse/bjt045. [DOI] [PubMed] [Google Scholar]
- 74.Doty RL. The Smell Threshold Test™ administration manual. Sensonics Inc; Haddon Heights, NJ: 2000. [Google Scholar]
- 75.Sulmont C, Issanchou S, Köster EP. Selection of odorants for memory tests on the basis of familiarity, perceived complexity, pleasantness, similarity and identification. Chem Senses. 2002;27:307–317. doi: 10.1093/chemse/27.4.307. [DOI] [PubMed] [Google Scholar]
- 76.Lehrner JP, Brücke T, Dal-Bianco P, Gatterer G, Kryspin-Exner I. Olfactory functions in Parkinson’s disease and Alzheimer’s disease. Chem Senses. 1997;22:105–110. doi: 10.1093/chemse/22.1.105. [DOI] [PubMed] [Google Scholar]
- 77.Landis BN, Negoias S, Friedrich H. Chemosensory Event Related Potentials. Epileptologie. 2016;33:189–196. [Google Scholar]
- 78.Miao X, Yang L, Gu H, Ren Y, Chen G, et al. Evaluation of post-traumatic anosmia with MRI and chemosensory ERPs. Eur Arch Otorhinolaryngol. 2015;272:1945–1953. doi: 10.1007/s00405-014-3278-x. [DOI] [PubMed] [Google Scholar]
- 79.Barresi M, Ciurleo R, Giacoppo S, Foti Cuzzola V, Celi D, et al. Evaluation of olfactory dysfunction in neurodegenerative diseases. J Neurol Sci. 2012;323:16–24. doi: 10.1016/j.jns.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 80.Rombaux P, Mouraux A, Bertrand B, Guerit JM, Hummel T. Assessment of olfactory and trigeminal function using chemosensory event-related potentials. Neurophysiol Clin. 2006;36:53–62. doi: 10.1016/j.neucli.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 81.Kappenman ES, Luck SJ. The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology. 2010;47:888–904. doi: 10.1111/j.1469-8986.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murphy C, Solomon ES, Haase L, Wang M, Morgan CD. Olfaction in aging and Alzheimer’s disease: event-related potentials to a cross-modal odor-recognition memory task discriminate ApoE epsilon4+ and ApoE epsilon 4- individuals. Ann N Y Acad Sci. 2009;1170:647–657. doi: 10.1111/j.1749-6632.2009.04486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Calhoun-Haney R, Murphy C. Apolipoprotein epsilon4 is associated with more rapid decline in odor identification than in odor threshold or Dementia Rating Scale scores. Brain Cogn. 2005;58:178–182. doi: 10.1016/j.bandc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 84.Gilber PE, Murphy C. The effect of the ApoE epsilon4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease, and healthy elderly controls. J Clin Exp Neuropsychol. 2004;26:779–794. doi: 10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- 85.Wang QS, Tian L, Huang YL, Qin S, He LQ, et al. Olfactory identification and apolipoprotein E epsilon 4 allele in mild cognitive impairment. Brain Res. 2002;951:77–81. doi: 10.1016/s0006-8993(02)03137-2. [DOI] [PubMed] [Google Scholar]
- 86.Bacon AW, Bondi MW, Salmon DP, Murphy C. Very early changes in olfactory functioning due to Alzheimer’s disease and the role of apolipoprotein E in olfaction. Ann N Y Acad Sci. 1998;855:723–731. doi: 10.1111/j.1749-6632.1998.tb10651.x. [DOI] [PubMed] [Google Scholar]
- 87.Sundermann EE, Gilbert PE, Murphy C. The effect of hormone therapy on olfactory sensitivity is dependent on apolipoprotein E genotype. Horm Behav. 2008;54:528–533. doi: 10.1016/j.yhbeh.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sundermann EE, Gilbert PE, Murphy C. Apolipoprotein E epsilon4 genotype and gender: effects on memory. Am J Geriatr Psychiatry. 2007;15:69–78. doi: 10.1097/JGP.0b013e318065415f. [DOI] [PubMed] [Google Scholar]
- 89.Kjelvik G, Saltvedt I, White LR, Stenumgard P, Sletvold O, et al. The brain structural and cognitive basis of odor identification deficits in mild cognitive impairment and Alzheimer’s disease. BMC Neurol. 2014;14:168. doi: 10.1186/s12883-014-0168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Handley OJ, Morrison CM, Miles C, Bayer AJ. ApoE gene and familial risk of Alzheimer’s disease as predictors of odour identification in older adults. Neurobiol Aging. 2006;27:1425–1430. doi: 10.1016/j.neurobiolaging.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 91.Oleson S, Murphy C. Olfactory Dysfunction in ApoE varepsilon4/4 Homozygotes with Alzheimer’s Disease. J Alzheimers Dis. 2015;46:791–803. doi: 10.3233/JAD-150089. [DOI] [PubMed] [Google Scholar]
- 92.Zigman WB, Devenny DA, Krinsky-McHale SJ, Jenkins ED, Urv TK, et al. Alzheimer’s Disease in Adults with Down Syndrome. Int Rev Res Ment Retard. 2008;36:103–145. doi: 10.1016/S0074-7750(08)00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sliger M, Lander T, Murphy C. Effects of the ApoE epsilon4 allele on olfactory function in Down syndrome. J Alzheimers Dis. 2004;6:397–402. doi: 10.3233/jad-2004-6407. [DOI] [PubMed] [Google Scholar]
- 94.Wetter S, Murphy C. Apolipoprotein E epsilon4 positive individuals demonstrate delayed olfactory event-related potentials. Neurobiol Aging. 2001;22:439–447. doi: 10.1016/s0197-4580(01)00215-9. [DOI] [PubMed] [Google Scholar]
- 95.Murphy C, Morgan CD, Geisler MW, Wetter S, Covington J, et al. Olfactory event-related potentials and aging: normative data. Int J Psychophysiol. 2000;36:133–145. doi: 10.1016/s0167-8760(99)00107-5. [DOI] [PubMed] [Google Scholar]
- 96.Covington JW, Geisler MW, Polich J, Murphy C. Normal aging and odor intensity effects on the olfactory event-related potential. Int J Psychophysiol. 1999;32:205–214. doi: 10.1016/s0167-8760(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 97.Corby K, Morgan CD, Murphy C. Abnormal event-related potentials in young and middle-aged adults with the ApoE epsilon4 allele. Int J Psychophysiol. 2012;83:276–281. doi: 10.1016/j.ijpsycho.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kowalewski J, Murphy C. Olfactory ERPs in an odor/visual congruency task differentiate ApoE epsilon4 carriers from non-carriers. Brain Res. 2012;1442:5–65. doi: 10.1016/j.brainres.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morgan CD, Murphy C. Individuals at risk for Alzheimer’s disease show differential patterns of ERP brain activation during odor identification. Behav Brain Funct. 2012;8:37. doi: 10.1186/1744-9081-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng KY, Matthews PM, Levy E, Wilson DA. Apolipoprotein E4 causes early olfactory network abnormalities and short-term olfactory memory impairments. Neuroscience. 2017;343:364–371. doi: 10.1016/j.neuroscience.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nathan BP, Yost J, Litherland MR, Struble RG, Switzer PV. Olfactory function in apoE knockout mice. Behav Brain Res. 2004;150:1–7. doi: 10.1016/S0166-4328(03)00219-5. [DOI] [PubMed] [Google Scholar]
- 102.Nathan BP, Nannapaneni S, Gairhe S, Nwosu I, Struble RG. The distribution of apolipoprotein E in mouse olfactory epithelium. Brain Res. 2007;1137:78–83. doi: 10.1016/j.brainres.2006.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nathan BP, Nisar R, Short J, Randall S, Grissom E, et al. Delayed olfactory nerve regeneration in ApoE-deficient mice. Brain Res. 2005;1041:87–94. doi: 10.1016/j.brainres.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 104.Hussain A, Luong M, Pooley A, Nathan BP. Isoform-specific effects of apoE on neurite outgrowth in olfactory epithelium culture. J Biomed Sci. 2013;20:49. doi: 10.1186/1423-0127-20-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nwosu I, Gairhe S, Struble RG, Nathan BP. Impact of apoE deficiency during synaptic remodeling in the mouse olfactory bulb. Neurosci Lett. 2008;441:282–285. doi: 10.1016/j.neulet.2008.05.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nathan BP, Gairhe S, Nwosu I, Clark S, Struble RG. Reconstitution of the olfactory epithelium following injury in apoE-deficient mice. Exp Neurol. 2010a;226:40–46. doi: 10.1016/j.expneurol.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiba S, Suzuki M, Yamanouchi K, Nishihara M. Involvement of granulin in estrogen-induced neurogenesis in the adult rat hippocampus. J Reprod Dev. 2007;53:297–307. doi: 10.1262/jrd.18108. [DOI] [PubMed] [Google Scholar]
- 108.Morris DC, Zhang ZG, Wang Y, Zhang RL, Gregg S, et al. Wnt expression in the adult rat subventricular zone after stroke. Neurosci Lett. 2007;418:170–174. doi: 10.1016/j.neulet.2007.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Caruso A, Motolese M, Iacovelli L, Caraci F, Copani A, et al. Inhibition of the canonical Wnt signaling pathway by apolipoprotein E4 in PC12 cells. J Neurochem. 2006;98:364–371. doi: 10.1111/j.1471-4159.2006.03867.x. [DOI] [PubMed] [Google Scholar]
- 110.Nathan BP, Tonsor M, Struble RG. Long-term effects of estradiol replacement in the olfactory system. Exp Neurol. 2012;237:1–7. doi: 10.1016/j.expneurol.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nathan BP, Tonsor M, Struble RG. Acute responses to estradiol replacement in the olfactory system of apoE-deficient and wild-type mice. Brain Res. 2010c;1343:66–74. doi: 10.1016/j.brainres.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cheng X, McAsey ME, Li M, Randall S, Cady C, et al. Estradiol replacement increases the low-density lipoprotein receptor related protein (LRP) in the mouse brain. Neurosci Lett. 2007;417:50–54. doi: 10.1016/j.neulet.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 113.McAsey ME, Cady C, Jackson LM, Li M, Randall S, et al. Time course of response to estradiol replacement in ovariectomized mice: brain apolipoprotein E and synaptophysin transiently increase and glial fibrillary acidic protein is suppressed. Exp Neurol. 2006;197:197–205. doi: 10.1016/j.expneurol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 114.Richey PL, Siedlak SL, Smith MA, Perry G. Apolipoprotein E interaction with the neurofibrillary tangles and senile plaques in Alzheimer disease: implications for disease pathogenesis. Biochem Biophys Res Commun. 1995;208:657–663. doi: 10.1006/bbrc.1995.1389. [DOI] [PubMed] [Google Scholar]
- 115.Carter DB. The interaction of amyloid-beta with ApoE. Subcell Biochem. 2005;38:255–272. doi: 10.1007/0-387-23226-5_13. [DOI] [PubMed] [Google Scholar]


