Abstract
Background
Cortical electrical stimulation of motor cortex in combination with rehabilitative training (CS/RT) has been shown to enhance motor recovery in animal models of focal cortical stroke yet in clinical trials the effects are much less robust. The variability of stroke location in human patient populations that include both cortical and subcortical brain regions may contribute to the failure to find consistent effects clinically.
Objective
This study sought to determine whether infarct location influences the enhanced motor recovery previously observed in response to CS/RT. The efficacy of CS/RT to promote improvements in motor function was examined in two different rat models of stroke that varied the amount and location of cortical and subcortical damage.
Methods
Ischemic infarctions were induced by injecting the vasoconstricting peptide endothelin-1 either 1) onto the middle cerebral artery (MCAo) producing damage to frontal cortex and lateral striatum or 2) into a subcortical region producing damage to the posterior thalamus and internal capsule (SCII). Daily CS/RT or RT alone was then given for twenty days during which time performance on a skilled reaching task was assessed.
Results
Animals with MCAo infarctions exhibited enhanced improvements on a skilled reaching task in response to CS/RT relative to RT alone. No such enhancement was observed in animals with SCII infarctions across the twenty days of treatment.
Conclusions
The efficacy of cortical stimulation for enhancing motor recovery after stroke may depend in part on the extent and location of the ischemic infarct.
Keywords: internal capsule, cortical stimulation, skilled reaching behavior, rodent models of stroke, motor cortex
Introduction
The combination of epidural electrical stimulation of motor cortex and motor rehabilitation (CS/RT) has been demonstrated to enhance motor recovery in preclinical animal studies of focal cortical ischemia1–7. This improved motor function is accompanied by increases in synapse density2 and enhanced synaptic responses7 in perilesional cortex. In addition, CS/RT also results in an expansion and reorganization of ICMS-derived forelimb movement representations in ipsilesional motor cortex beyond that observed with rehabilitative training alone4–6. Despite the robust effects in animal studies, the efficacy of CS/RT for ameliorating post stroke motor impairments in clinical studies has proven to be inconsistent. A recent large scale clinical trial failed to demonstrate any significant improvements in motor function in chronic stroke patients four weeks post CS/RT treatment8. In this same study, a positive treatment response was, however, observed in a subset of patients from which movements could be elicited in response to epidural electrical stimulation. The efficacy of cortical stimulation as a stroke treatment may thus depend on the functional integrity of residual neural tissue including the corticospinal tract9.
The nature and persistence of functional deficits after stroke can vary due to vast differences in infarct size and location10. Preclinical animal studies of stroke have predominantly used experimental models that primarily induce cortical damage11–14. Clinically however, damage often includes or may even be restricted to subcortical structures10–17. Indeed, enduring motor impairments can manifest as a result of damage to subcortical strokes including descending motor tracts18. Damage within subcortical white matter can result in cortical deafferentation causing dysfunction within widespread cortical areas outside of the infarction19–20. Small infarctions within the internal capsule, for example, result in severe motor impairments and poor motor recovery21–23.
Lesion location-dependent differences in the amount of motor recovery and cortical reorganization raise the possibility that the efficacy of any given treatment may depend on the specific pattern of brain damage in individual patients. The success of CS/RT for enhancing motor recovery in animal studies may be due to the fact that the stroke models used have targeted cortical rather than subcortical areas. In the present study we tested the efficacy of CS/RT to promote improvements in motor function in two different rat models of stroke producing two different patterns of brain injury.
Methods
Animals
Forty adult male Long-Evans hooded rats (350–420 g) were pair housed (2 animals/cage) in standard laboratory cages on a 12:12 hour light-dark cycle in the University of Florida’s Communicore Research Building vivarium. All experimentation occurred during the light cycle. Rats were given Lab Diet 5001 (PMI Feeds, St. Louis, MO), water ad libitum, and were cared for in accordance with the National Institutes Health Guide for the Care and Use of Laboratory Animals and with the approval of the University of Florida’s Institutional Animal Care and Use Committee.
Reach Training
The single pellet reaching task was used as previously described4–5, 24. Animals were trained to retrieve food pellets (45 mg; Bioserv) with their preferred limb for approximately two weeks (20 min/day). A successful reach was scored when the animal grasped the food pellet and brought it to their mouth without dropping it. The percentage of successful reaches [(# successful retrievals/the total # of reaches) x 100], i.e., reaching accuracy, was then calculated. All training sessions were video-recorded. Animals were sorted by pre-lesion performance to create groups with comparable baseline levels of reaching accuracy.
Infarction
Ischemic damage was induced by injecting the vasoactive peptide endothelin-1 either onto the proximal branches of the middle cerebral artery along the lateral aspect of the frontal cortex or into the subcortical territory within and surrounding the internal capsule as previously described11–12, 25. The hemisphere contralateral to each animal’s preferred reaching paw was targeted for injury. Animals were anesthetized with ketamine hydrochloride (70 mg/kg; i.p.) and xylazine (5 mg/kg; i.p.) with supplemental isofluorane (0.15%) and ketamine (20 mg/kg; i.p.). Under sterile conditions, a midline incision was made and burr holes created over the injections sites. Endothelin-1 (0.2 μg/μL American Peptide, Sunnyvale) was delivered by the Nanolitre injection system (World Precision Instruments, Sarasota, Fl) using a SYS-Micro 4 Controller (World Precision Instruments, Sarasota, Fl). Stereotaxic coordinates of the injection site for MCAo were: anteroposterior, +0.9 mm; mediolateral, −5.2mm; and dorsoventral, −8.6 mm with respect to bregma25 whereas for SCII were: anteroposterior, −3.0 mm; mediolateral, −3.0 mm; and dorsoventral, −7.0 mm with respect to bregma11–12. Doses of endothelin-1 were 3 μl for MCAo (240 pMol dissolved in 0.9% sterile saline) and1 μl for capsular injury (80 pMol dissolved in 0.9% sterile saline).
Cortical Electrode Implantation
Directly following MCAo or SCII (32 animals total), nine-pin electrode carriages (ABS Plug, Ginder Scientific Inc., Ottawa, Canada) were implanted over motor cortex in the injured hemisphere4. The surface electrode was placed over the exposed cortex between 1 mm posterior to 5 mm anterior to bregma and 0.5 mm to 5.5 mm lateral to midline. A return lead was fixed to the skull posterior to lambda and the craniotomy filled with gel foam. Both the electrode and gel foam were covered in non-exothermic PolyWave dental acrylic and cured with brief ultraviolet light. The electrode was fixed to skull screws with standard dental acrylic and the animals were given warm ringers solution (4 cc; s.c.) and metacam (0.10 mg/kg; s.c.).
Movement Thresholds
Three days after implantation, all animals had their individual cortical stimulation movement thresholds (MT’s) determined and then began motor rehabilitation. MT’s were assessed on post lesion training days 1, 10 and 19. MT’s were defined for each animal as the minimum current to cause an involuntary motor response. The animals were placed into a transparent cylinder and observed while 3 second trains of 1 millisecond 100 Hz monopolar cathodal pulses were given. Current was gradually increased by 5% increments until a movement of the contralateral forelimb could be clearly detected. Cortical stimulation movement thresholds for all animals were identified by one investigator who was blind to the treatment groups. Care was taken during cortical stimulation movement threshold testing as preliminary studies found that intensities of continuous CS set at greater than 50% threshold resulted in undesired evoked movements during CS/RT.
Cortical Electrical Stimulation And Rehabilitative Training
CS/RT or RT (20 minutes/day) were initiated 3 days after surgery and continued for 20 days. Cortical stimulation during rehabilitation training was then delivered at 50% of each animal’s CS movement threshold. Rehabilitative training was performed using the single pellet reaching task and all sessions were video-recorded for analysis of reaching accuracy and reach attempts. Animals receiving CS/RT were stimulated via the Vertis Stimulation System during these sessions. The cortical electrode was connected to a remote stimulator controlled by a personal computer. CS/RT was delivered as continuous monopolar cathodal stimulation with a frequency of 100 Hz and a current intensity dictated by each animal’s movement threshold. Each pulse was biphasic, charged balanced and asymmetric consisting of a square phase lasting 100± 10 microseconds and a decaying exponential phase lasting ~9900± 10 microseconds. Half of the animals that received MCAo were given RT alone (MCAo-RT; n=8) while the other half received the combination of CS/RT (MCAo-CS/RT; n=8). Half of the animals that received SCII were given RT alone (SCII-RT; n=8) while the other half received CS/RT (SCII-CS/RT; n=8). A group of animals with no injury were included as healthy controls (n=8) and were trained on the same task on all days.
Histology and Lesion Verification
Following the rehabilitation animals were given pentobarbital and then transcardially perfused with 0.1 M sodium phosphate buffer followed by 4% paraformaldehyde solution in 0.1 M sodium phosphate buffer. Serial 50 μm coronal sections were taken using a microtome. Ten sections spaced 600 μm apart and spanning approximately 2.7 mm anterior and 3.3 mm posterior to bregma were sampled for lesion verification. The sampled sections were stained with Toluidine blue and digitally scanned (Epson Perfection V500 Photo Scanner, Long Beach, CA). The area of spared tissue was traced using Image J software26 and cortical volumes were estimated with Cavalieri’s unbiased estimator method27:
Where “yi” is the cross sectional area of the “ith” section through the morphometric region, “d” is the distance between sections (600 μm) and “n” is the total number of sections (12). “ymax” is maximum value for the area of one section and “t” is the section thickness (50 μm) and their product is subtracted as a correction. Estimated volumes were analyzed as the percent affected to unaffected side (volume lesioned hemisphere/volume non lesioned hemisphere*100) to account for individual differences. Each group contained 8 animals.
Data Analysis
Skilled reaching performance was assessed by measuring both the percentage change in both number of reaching attempts and reaching accuracy across the twenty days or training. For each animal, the percentage was calculated by subtracting the average of the first five rehabilitation days from the average of the final five rehabilitation days then normalizing to pre-injury values [Days 16 to 20 – Days 1 to 5)/Pre-injury X 100]. Motor performance and cortical stimulation movement thresholds were analyzed by One-Way Analysis of Variance (ANOVA) for percent changes or 2-Way repeated measures ANOVA and planned mean comparisons across treatment condition within each lesion model were conducted using Fisher’s PLSD. Spared tissue estimates were analyzed using One-Way ANOVA to assess differences across treatment conditions and planned mean comparisons across treatment condition within each lesion model were conducted using Fisher’s PLSD. Data are presented as mean ± SE and significance was set at p<0.05.
Results
Reaching Attempts
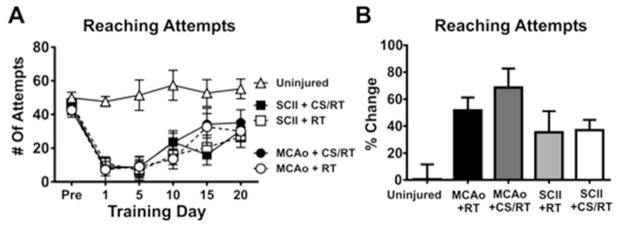
A Two-Way repeated measured ANOVA for training days 1, 5, 10, 15 and 20 for the number of each attempts revealed a significant Condition x Training Day interaction [F(20,175)= 2.019, p=0.0085] as well as a significant effect of Condition [F(4,35)= 12.62, p<0.0001] and Training Day [F(5,175)= 23.38, p<0.0001] (Figure 1A). Planned mean comparisons were used to assess differences between CS/RT and RT within each lesion model. No significant differences between MCAo+RT and MCAo+CS/RT conditions on number of reach attempts were detected on any of the training days: Pre-injury (p=0.76; FPLSD), Day 1 (p=0.94; FPLSD), Day 5 (p=0.99; FPLSD), Day 10 (p=0.25; FPLSD), Day 15 (p=0.86; FPLSD) or Day 20 (p=0.56; FPLSD). Animals given SCII+RT and SCII+CS/RT did not significantly differ in the number of reach attempts on any of the training days: Pre-injury (p=0.62; FPLSD), Day 1 (p=0.81; FPLSD), Day 5 (p=0.80; FPLSD), Day 10 (p=0.36; FPLSD), Day 15 (p=0.61; FPLSD) or Day 20 (p=0.66; FPLSD).
Figure 1. Number of reaching attempts during pre-injury and at 1, 5, 10, 15 and 20 days of rehabilitative training (RT) or cortical stimulation with rehabilitative training (CS/RT).
A. Histogram comparing the number of reach attempts during pre-injury and rehabilitative training. No significant differences were detected in the number of reach attempts between RT and CS/RT treatments for animals given either middle cerebral artery occlusion (MCAo) or subcortical capsular ischemic injury (SCII) during Pre-injury or rehabilitation days 1, 5, 10, 15 and 20. B. Histogram comparing percent change in the number of reach attempts during pre-injury and rehabilitative training. No significant differences were detected in the percent change in the number of reach attempts between RT and CS/RT treatments for animals given either MCAo or SCII.
For each animal, a percentage change in the number of reach attempts was calculated by subtracting the average of the first 5 rehabilitation days from the average of the final 5 rehabilitation days then normalizing to each pre-injury number of attempts [Days 16 to 20 – Days 1 to 5)/Pre-injury X 100]. A One-Way ANOVA for percentage change of reach attempts detected a significant overall difference between groups [F(4,35)= 4.46, p=0.0051]. Animals given MCAo+RT and MCAo+CS/RT did not differ in percentage change in the number of reach attempts (p=0.32; FPLSD). Similarly, animals given SCII+RT and SCII+CS/RT did not differ in percentage change in the number of reach attempts (p=0.93; FPLSD) (Figure 1B).
Reaching Accuracy
The animals’ percentage reach accuracy was analyzed in order to assess their ability to produce skilled forelimb movements during rehabilitative training.
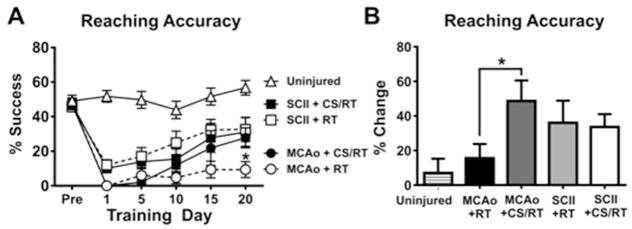
A Two-Way repeated measured ANOVA for training days 1, 5, 10, 15 and 20 on reaching accuracy revealed a significant Condition x Training Day interaction [F(20,175)= 4.18, p<0.0001] as well as a significant effect of Condition [F(4,35)= 18.29, p<0.0001] and Training Day [F(5,175)= 46.47, p<0.0001] (Figure 2A). Planned mean comparisons were used to assess differences between CS/RT and RT within each lesion model. Animals given MCAo+RT and MCAo+CS/RT did not significantly differ in reaching accuracy on the following training days: Pre-injury (p=0.91; FPLSD), Day 1 (p>0.99; FPLSD), Day 5 (p=0.58; FPLSD), Day 10 (p=0.31; FPLSD) or Day 15 (p=0.074; FPLSD). In contrast, the MCAo+CS/RT condition exhibited significantly greater reaching accuracy relative to the MCAo+RT condition on Day 20 (p=0.0090; FPLSD). Animals given SCII+RT and SCII+CS/RT did not significantly differ in reaching accuracy on any of the training days: Pre-injury (p=0.82; FPLSD), Day 1 (p=0.77; FPLSD), Day 5 (p=0.65; FPLSD), Day 10 (p=0.18; FPLSD), Day 15 (p=0.51; FPLSD) or Day 20 (p=0.83; FPLSD).
Figure 2. Percent successful reaching accuracy during pre-injury and at 1, 5, 10, 15 and 20 days of rehabilitative training (RT) or cortical stimulation with rehabilitative training (CS/RT).
A. Histogram comparing reaching accuracy during pre-injury and rehabilitative training for animals given either middle cerebral artery occlusion (MCAo) or subcortical capsular ischemic injury (SCII). No significant differences were detected in reaching accuracy between animals given SCII+RT and SCII+CS/RT. Animals given MCAo+CS/RT performed significantly greater reaching accuracy on rehabilitation day 20 (* p<0.05) whereas non-significant differences were detected between these groups at the other time points. B. Histogram comparing percent change in reaching accuracy during pre-injury and rehabilitative training. Animals given MCAo+CS/RT exhibited a significantly greater percent increase in reaching accuracy relative to animals given MCAo+RT (* p<0.05) whereas this difference was not observed in SCII animals.
A separate Two-Way repeated measures ANOVA for training days 1, 5, 10, 15 and 20 on reaching accuracy revealed a significant Lesion-Type x Training Day interaction [F(10,185)= 6.77, p<0.0001] as well as a significant effect of Lesion-Type [F(2,37)= 35.87, p<0.0001] and Training Day [F(5,185)= 29.14, p<0.0001]. Planned mean comparisons comparing MCAo and SCII models detected that animals given SCII exhibited significantly greater reaching accuracy relative to animals given MCAo on rehabilitation days: 1 (p=0.0264; FPLSD), 5 (p=0.022; FPLSD), 10 (p=0.017; FPLSD), 15 (p=0.0039; FPLSD) and 20 (p=0.0077; FPLSD).
For each animal, a percentage change in reaching accuracy was calculated by subtracting the average of the first 5 rehabilitation days from the average of the final 5 rehabilitation days then normalizing to each pre-injury accuracy [Days 16 to 20 – Days 1 to 5)/Pre-injury X 100]. A One-Way ANOVA for percentage improvement detected a significant overall main effect of Condition [F(4,35)= 2.83, p=0.039]. Planned mean comparisons comparing CS/RT and RT within each lesion model detected that the MCAo+CS/RT animals exhibited a significantly greater percentage change in reaching accuracy relative to animals given MCAo+RT (p=0.024; FPLSD). In contrast, no difference was detected between animals given SCII+RT and SCII+CS/RT (p=0.86; FPLSD) (Figure 2B).
These results demonstrate that whereas the combined therapy of CS/RT magnified motor improvements following MCAo, no CS treatment effect was detected in animals following SCII.
Cortical Stimulation Movement Thresholds
Cortical stimulation movement thresholds (MTs) were used to set stimulation intensity for each CS/RT animal and to provide insight into the integrity and plasticity of corticospinal pathways.
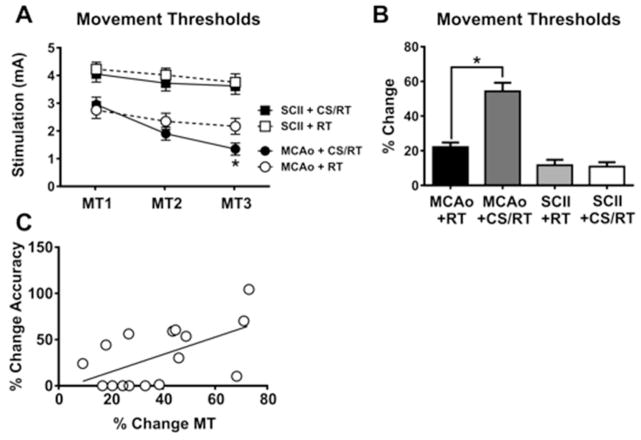
A Two-Way repeated measures ANOVA on movement thresholds during the three assessments detected significant effects of rehabilitation day [F(2,56)=130.80, p<0.0001], group [F(3,28)=13.10, p<0.0001], and the interaction [F(6,56)=17.50, p<0.0001] (Figure 3A). Planned mean comparisons were used to assess differences between CS/RT and RT within each lesion model. Animals given MCAo+RT were not significantly different on movement assessments relative to animals given MCAo+CS/RT on the first two assessments: MT1 (p=0.61; FPLSD) or MT2 (p=0.25; FPLSD). In contrast, the MCAo+CS/RT condition exhibited significantly lower movement thresholds relative to the MCAo+RT condition on the third assessment: MT3 (p=0.037; FPLSD). Animals given SCII+RT did not significantly differ in movement thresholds relative to animals given SCII+CS/RT during any of the assessments: MT1 (p=0.66; FPLSD), MT2 (p=0.45; FPLSD) or MT3 (p=0.73; FPLSD). Animals given SCII exhibited significantly greater movement thresholds relative to animals given MCAo on all three assessments: MT1 (SCII+RT versus MCAo+RT, p=0.00030 or SCII+CSRT versus MCAo+CS/RT, p=0.0055; FPLSD), MT2 (SCII+RT versus MCAo+RT, p<0.0001 or SCII+CSRT versus MCAo+CS/RT, p<0.0001; FPLSD) or MT3 (SCII+RT versus MCAo+RT, p<0.0001 or SCII+CSRT versus MCAo+CS/RT, p<0.0001; FPLSD).
Figure 3. Minimum thresholds to evoke involuntary movement with cortical stimulation (MTs).
Cortical stimulation movement thresholds (MT1–3) were assessed on rehabilitation days 1, 10 and 19, respectively. A. Histogram comparing movement thresholds for animals given either middle cerebral artery occlusion (MCAo) or subcortical capsular ischemic injury (SCII). No significant differences were detected in movement thresholds between animals given SCII+RT and SCII+CS/RT. Animals given MCAo+CS/RT exhibited significantly lower movement thresholds relative to animals given MCAo+RT on MT3 (* p<0.05) whereas non-significant differences were detected between these groups at the other time points. B. Histogram comparing percent change in movement thresholds between MT1 and MT3. Animals given MCAo+CS/RT exhibited a significantly greater percent reduction in movement thresholds relative to animals given MCAo+RT (* p<0.05) whereas this difference was not observed in SCII animals. C. For animals given MCAo, a significant positive correlation was detected between percent change in reaching accuracy and percent change in movement thresholds (r= 0.57; p<0.05).
The percentage change in movement thresholds was calculated by subtracting the threshold at the first assessment from the threshold at the final assessment and then normalized to the threshold at the first assessment [MT3 − MT1/MT1*100] (Figure 3B). A significant change in movement threshold was detected between groups [F(3,28)=35.14, p<0.0001]. Animals given MCAo+CS/RT exhibited a significantly greater percentage reduction in movement threshold in comparison to animals given MCAo+RT (p<0.0001; FPLSD). In contrast, animals given SCII+CS/RT exhibited no difference percentage change in movement threshold in comparison to animals given SCII+RT (p=0.87; FPLSD).
Separate correlations were used to test for relationships between the percentage change in movement threshold and the percentage change in reaching accuracy for either MCAo+CS/RT or SCII+CS/RT animals. A significant positive correlation was detected between percent change in movement threshold and percent change in reaching accuracy for animals given MCAo (r2= 0.33; p=0.021) (Figure 3C). No significant correlations between change in motor threshold and reaching accuracy were found in any of the other treatment conditions.
Spared Cortical Tissue
Spared cortical tissue was estimated by calculating the percent cortical volume from the injured hemisphere relative to the volume of the uninjured hemisphere (ipsilateral volume/contralateral volume X 100) (Figure 4A).
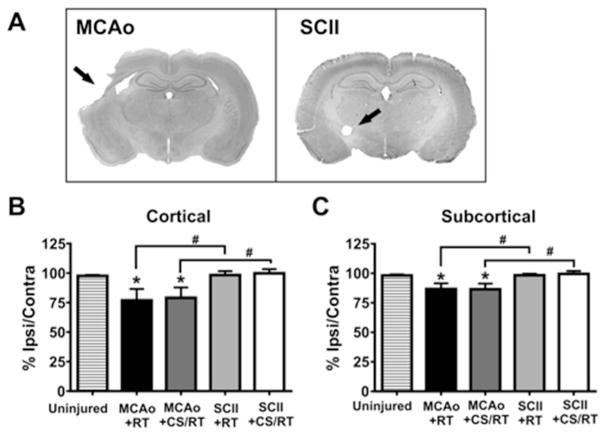
Figure 4. Spared tissue estimates following middle cerebral artery occlusion (MCAo) and subcortical capsular ischemic injury (SCII).
A. Representative nissl-stained coronal sections from animals given experimental MCAo or SCII. Black arrows are used to indicate regions of tissue damage. B. Histogram comparing percent spared cortical tissue in MCAo and SCII animals. Animals given MCAo exhibit significantly less spared cortical tissue relative to uninjured control animals (* p<0.05) or animals given SCII (# p<0.05). C. Histogram comparing percent spared subcortical tissue in MCAo and SCII animals. Animals given MCAo exhibit significantly less spared subcortical tissue relative to uninjured control animals (* p<0.05) or animals given SCII (# p<0.05).
A One-way ANOVA revealed a significant main effect of CONDITION on the percentage of spared cortical tissue [F(4,35)= 3.30; p=0.021] (Figure 4B). Planned mean comparisons were used to assess differences between CS/RT and RT within each lesion model. Animals given MCAo presented with significantly less spared cortical tissue in comparison to uninjured control animals (control versus MCAo+RT: p=0.025; control versus MCAo+CS/RT: p=0.043; FPLSD). The two groups of animals given MCAo did not differ from one another in the amount of spared cortical tissue (MCAo+RT versus MCAo+CS/RT: p=0.81). The two groups of animals given SCII did not differ from one another in the amount of spared cortical tissue (SCII+RT versus SCII+CS/RT: p=0.87; FPLSD). Animals given SCII did not significantly differ in the amount of spared cortical tissue in comparison to uninjured control animals (control versus SCII+RT: p=0.93; control versus SCII+CS/RT: p=0.81; FPLSD). The MCAo model exhbited significantly less spared cortical tissue relative to the SCII model (MCAo+RT versus SCII+RT: p=0.020 or MCAo+CS/RT versus SCII+CS/RT: p=0.025).
Spared Subcortical Tissue
A one-way ANOVA revealed a significant main effect of CONDITION on the percentage of spared subcortical tissue [F(4,35)= 4.046; p=0.0084] (Figure 4C). Planned mean comparisons were used to assess differences between CS/RT and RT within each lesion model. Animals given MCAo presented with significantly less spared subcortical tissue in comparison to uninjured control animals (control versus MCAo+RT: p=0.019; control versus MCAo+CS/RT: p=0.017; FPLSD). The two groups of animals given MCAo did not differ from one another in the amount of spared subcortical tissue (MCAo+RT versus MCAo+CS/RT: p=0.96; FPLSD). The two groups of animals given SCII did not differ from one another in the amount of spared subcortical tissue (SCII+RT: versus SCII+CS/RT: p=0.81; FPLSD). Animals given SCII did not significantly differ in the amount of spared subcortical tissue in comparison to uninjured control animals (control versus SCII+RT: p=0.98; control versus SCII+CS/RT: p=0.78; FPLSD). The MCAo model exhibited significantly less spared subcortical tissue relative to the SCII model (MCAo+RT versus SCII+RT: p=0.018 or MCAo+CS/RT versus SCII+CS/RT: p=0.0084; FPLSD).
Discussion
The present study tested the efficacy of CS/RT to enhance motor recovery after stroke in two different models of cerebral ischemia that produced two different patterns of brain injury. The MCAo model produced damage to the lateral frontal and parietal cortex as well as the dorsolateral striatum. The SCII model produced damage that was restricted to the lateral aspect of the posterior thalamus including the internal capsule. The results demonstrated that CS/RT differentially affected both post stroke motor performance and cortical physiology across these two injury models. The MCAo+CS/RT animals showed a significant increase in reaching accuracy as compared to the MCAo+RT animals. No such differences were observed between the SCII+CS/RT and SCII+RT animals. Further, animals in the MCAo+CS/RT condition showed significant reductions of motor thresholds as compared the MCAo+RT animals and these reductions correlated with improvements in reaching accuracy. Again, no such differences were found between the SCII+RT SCII+CS/RT animals. Together the results demonstrate that CS/RT treatment in animals with cortical infarctions augmented motor function as evidenced by the increase in reaching accuracy and altered cortical physiology as evidenced by the reduction in motor thresholds.
There are several potential explanations for the differential effects of CS/RT in the experimental models. First, the two stroke models used here resulted in different overall lesion sizes. MCAo animals had larger cortical and subcortical lesions than both controls and SCII animals. Further, there was no significant difference in cortical or subcortical lesion size between SCII animals and controls. This pattern of results emerges as a result of the way in which the infarctions were created. The MCAo lesion method used here primarily resulted in damage to the mediolateral frontal and parietal cortices but also damaged the dorsal and lateral aspects of the anterior striatum. The SCII lesion method resulted in very focal lesions that were limited to the lateral aspect of the posterior thalamus including the internal capsule. The method used to measure lesion size in this study may not have been sensitive enough to detect the small infarctions in SCII animals. However, the SCII animals did show impairments on the reaching task and had significantly higher motor thresholds than the MCAo animals indicating that there was tissue damage. The larger infarctions in the MCAo animals were also associated with greater post-stroke motor impairments than the SCII animals. With daily CS/RT, the MCAo animals achieved a performance comparable to the SCII+RT and SCII+CS/RT animals. The reduced impairment levels may have masked any potential additional improvements in SCII animals resulting from the CS/RT. A second potential explanation may be related to lesion location. Cortical stimulation is presumed to enhance motor recovery by encouraging the recruitment/reorganization of residual cortical areas5. Damage to the internal capsule might hinder the effects of any such plasticity as the output of those cortical areas would be impaired. This is consistent with the observation that SCII animals had significantly higher motor thresholds than the MCAo throughout the twenty days of treatment.
In clinical studies, reductions in the magnitude or absences of motor evoked potentials in response to cortical transcranial magnetic stimulation within days of stroke are associated with poor motor recovery28–29. In the present study, animals with SCII required significantly greater stimulation currents to evoke movement than animals given MCAo. This suggests a greater disruption in the integrity of the descending motor tracts in SCII animals relative to the MCAo animals. The fact that movement thresholds were present in SCII animals suggests some sparing of internal capsule fibers. This partial sparing coupled with absence of cortical damage may have supported the observed improvements in reaching accuracy.
Motor rehabilitation following cortical ischemia results in motor improvements that are accompanied by an expansion and reemergence of movement representations in motor cortex that are specific to the training30 and are likely mediated by synaptic plasticity31–32. CS/RT is thought to augment these endogenous plasticity processes and is associated with greater reorganization of motor maps, increased density of synapses2 and increased synaptic responses7 than RT alone4–6. CS/RT may also have neuroprotective, angiogenic, anti-inflammatory or growth factor-releasing properties33 that also contribute to enhanced motor recovery. Subcortical infarctions that damage the corticospinal tract would then limit the impact of any treatment, such as CS/RT, intended to enhance plasticity within motor cortex. This is consistent with the results of the recent Everest trial where no differences in the overall percentage of patients that met the primary efficacy end point where found between CS/RT patients (32%) and controls (29%) four weeks post treatment8. Yet in the 13 patients that did exhibit movements in response to the cortical stimulation, 69% met the primary efficacy end point. The results highlight the importance of determining the specific pattern of brain damage resulting from stroke prior to prescribing adjuvant therapies such as cortical stimulation.
Conclusions
The present findings demonstrate that the efficacy of cortical stimulation for enhancing motor recovery after stroke may depend in part on the extent and location of the ischemic infarct. The results further highlight the importance of understanding the interaction between the infarct location and extent and the use of brain stimulation to treat functional impairments after stroke.
Acknowledgments
Supported by National Institutes of Health Grant U54 NSO48126 (J.A.K.).
Footnotes
Disclosures
No part of this work has been published.
The authors declare no competing financial interests.
References Cited
- 1.Adkins DL, Campos P, Quach D, Borromeo M, Schallert K, Jones TA. Epidural cortical stimulation enhances motor function after sensorimotor cortex infarcts in rats. Exp Neurol. 2006;200:356–370. doi: 10.1016/j.expneurol.2006.02.131. [DOI] [PubMed] [Google Scholar]
- 2.Adkins DL, Hsu JE, Jones TA. Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Exp Neurol. 2008;212:14–28. doi: 10.1016/j.expneurol.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25:780–788. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- 4.Boychuk JA, Adkins DL, Kleim JA. Distributed versus focal cortical stimulation to enhance motor function and motor map plasticity in a rodent model of ischemia. Neuro Neural Repair. 2011;25:88–97. doi: 10.1177/1545968310385126. [DOI] [PubMed] [Google Scholar]
- 5.Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003;25:789–793. doi: 10.1179/016164103771953862. [DOI] [PubMed] [Google Scholar]
- 6.Plautz EJ, Barbay S, Frost SB, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25:801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- 7.Teskey GC, Flynn C, Goertzen CD, Monfils MH, Young NA. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res. 2003;25:794–800. doi: 10.1179/016164103771953871. [DOI] [PubMed] [Google Scholar]
- 8.Levy RM, Harvey RL, Kissela BM, et al. Epidural Electrical Stimulation for Stroke Rehabilitation Results of the Prospective, Multicenter, Randomized, Single-Blinded Everest Trial. Neuro Neural Repair. 2015 doi: 10.1177/1545968315575613. 1545968315575613. [DOI] [PubMed] [Google Scholar]
- 9.Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive Cortical Stimulation to Promote Recovery of Function After Stroke A Critical Appraisal. Stroke. 2009;40:1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saczynski JS, Sigurdsson S, Jonsdottir MK, et al. Cerebral infarcts and cognitive performance importance of location and number of infarcts. Stroke. 2009;40:677–682. doi: 10.1161/STROKEAHA.108.530212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frost SB, Barbay S, Mumert ML, Stowe AM, Nudo RJ. An animal model of capsular infarct: endothelin-1 injections in the rat. Behav Brain Res. 2006;169:206–211. doi: 10.1016/j.bbr.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Kim RG, Kim H-S, et al. Longitudinal changes in resting-state brain activity in a capsular infarct model. J Cereb Blood Flow Metab. 2015;35:11–19. doi: 10.1038/jcbfm.2014.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecrux C, McCabe C, Weir CJ, et al. Effects of magnesium treatment in a model of internal capsule lesion in spontaneously hypertensive rats. Stroke. 2008;39:448–454. doi: 10.1161/STROKEAHA.107.492934. [DOI] [PubMed] [Google Scholar]
- 14.Sozmen EG, Hinman JD, Carmichael ST. Models that matter: white matter stroke models. Neurotherapeutics. 2012;9:349–358. doi: 10.1007/s13311-012-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bamford J, Sandercock P, Dennis M, Warlow C, Burn J. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 16.Bryan RN, Cai J, Burke G, et al. Prevalence and anatomic characteristics of infarct-like lesions on MR images of middle-aged adults: the atherosclerosis risk in communities study. AJNR. 1999;20:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider AT, Kissela B, Woo D, et al. Ischemic stroke subtypes A population-based study of incidence rates among blacks and whites. Stroke. 2004;35:1552–1556. doi: 10.1161/01.STR.0000129335.28301.f5. [DOI] [PubMed] [Google Scholar]
- 18.Norrving B. Long-term prognosis after lacunar infarction. Lancet Neurol. 2003;2:238–245. doi: 10.1016/s1474-4422(03)00352-1. [DOI] [PubMed] [Google Scholar]
- 19.Kraemer M, Schormann T, Hagemann G, Qi B, Witte OW, Seitz RJ. Delayed Shrinkage of the Brain After Ischemic Stroke: Preliminary Observations With Voxel-Guided Morphometry. J Neuroimaging. 2004;14:265–272. doi: 10.1177/1051228404264950. [DOI] [PubMed] [Google Scholar]
- 20.Seitz RJ, Schlaug G, Kleinschmidt A, et al. Remote depressions of cerebral metabolism in hemiparetic stroke: topography and relation to motor and somatosensory functions. Hum Brain Mapp. 1994;1:81–100. [Google Scholar]
- 21.Lie C, Hirsch J, Rossmanith C, Hennerici M, Gass A. Clinicotopographical Correlation of Corticospinal Tract Stroke A Color-Coded Diffusion Tensor Imaging Study. Stroke. 2004;35:86–92. doi: 10.1161/01.STR.0000106912.09663.EB. [DOI] [PubMed] [Google Scholar]
- 22.Morecraft RJ, Herrick JL, Stilwell-Morecraft KS, et al. Localization of arm representation in the corona radiata and internal capsule in the non-human primate. Brain. 2002;125:176–198. doi: 10.1093/brain/awf011. [DOI] [PubMed] [Google Scholar]
- 23.Wenzelburger R, Kopper F, Frenzel A, et al. Hand coordination following capsular stroke. Brain. 2005;128:64–74. doi: 10.1093/brain/awh317. [DOI] [PubMed] [Google Scholar]
- 24.Whishaw IQ, Pellis SM, Gorny BP, Pellis VC. The impairments in reaching and the movements of compensation in rats with motor cortex lesions: an endpoint, videorecording, and movement notation analysis. Behav Brain Res. 1991;42:77–91. doi: 10.1016/s0166-4328(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 25.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with Image J. Biophotonics International. 2004;11:36–43. [Google Scholar]
- 27.Gundersen H, Jensen E. The efficiency of systematic sampling in stereology and its prediction*. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 28.Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2002;83:1629–1637. doi: 10.1053/apmr.2002.35473. [DOI] [PubMed] [Google Scholar]
- 29.Pennisi G, Rapisarda G, Bella R, Calabrese V, de Noordhout AM, Delwaide PJ. Absence of Response to Early Transcranial Magnetic Stimulation in Ischemic Stroke Patients Prognostic Value for Hand Motor Recovery. Stroke. 1999;30:2666–2670. doi: 10.1161/01.str.30.12.2666. [DOI] [PubMed] [Google Scholar]
- 30.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 31.Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 32.Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baba T, Kameda M, Yasuhara T, et al. Electrical stimulation of the cerebral cortex exerts antiapoptotic, angiogenic, and anti-inflammatory effects in ischemic stroke rats through phosphoinositide 3-kinase/Akt signaling pathway. Stroke. 2009;40:598–605. doi: 10.1161/STROKEAHA.109.563627. [DOI] [PubMed] [Google Scholar]