Abstract
Objectives
The tumor suppressor gene SMAD4 (DPC4) is genetically inactivated in approximately half of pancreatic ductal adenocarcinomas (PDA). We examined whether Smad4 tumor status was associated with outcomes following adjuvant chemoradiation (CRT) for resected PDA.
Methods
Patients treated with adjuvant CRT were identified (N = 145). Smad4 status was determined by immunolabeling and graded as intact or lost. Kaplan-Meier method and multivariable competing risk analyses were performed.
Results
On multivariate competing risk analysis, Smad4 loss was associated with increased risk of local recurrence (LR), (hazard ratio [HR], 2.37; 95% confidence interval [CI], 1.10–5.11; P = 0.027), distant failure (DF), (HR, 1.71; 95% CI, 1.03–2.83; P = 0.037) and synchronous LR and DF at first recurrence (14.9 % vs. 5.3%, P = 0.07) compared to Smad4 intact cancers. Smad4 loss was not associated with median overall survival (22 vs. 22 months; P = 0.63) or disease-free survival (lost [13.6] vs. intact [13.5] months, P = 0.79).
Conclusion
Following PDA resection and adjuvant CRT, Smad4 loss correlated with higher risk of LR and DF, but not with survival. Smad4 loss may help predict which surgical patients are at higher risk for failure after definitive management and may benefit from intensified adjuvant therapy.
Keywords: SMAD4, DPC4, Pancreatic ductal adenocarcinoma, chemoradiation therapy, survival outcomes, adjuvant therapy
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDA) is now the third leading cause of cancer death in the United States with a 5-year overall survival rate of only 6%.1 Surgery, radiation, and chemotherapy are available therapies, but they rarely lead to a cure.2 Upfront surgery is the standard of care for resectable PDA,3 and typically, those who undergo upfront surgical resection receive adjuvant chemotherapy with or without concurrent radiation.4–6 Despite surgical resection, 70% of patients succumb to metastatic or locally recurrent disease and only 20–25% of surgically resected patients survive beyond five years.7–10
Although there are known prognostic factors (comorbidities, tumor grade, margin and nodal positivity),11 it is unclear why some patients develop recurrences and others do not. Identification of tumor-specific biomarker predictors for local and distant recurrence could guide the administration of specific therapies; however, no such biomarkers are consistently used for PDA.12 A potential prognostic biomarker for PDA is the tumor suppressor gene SMAD4 (DPC4).13,14 SMAD4 (DPC4) encodes a protein (Smad4) that functions as a central mediator of the transforming growth factor-beta (TGF-β) signaling pathway and regulates cellular processes including proliferation, differentiation, apoptosis, and migration. SMAD4 is inactivated in approximately 55% of pancreatic cancers.15–17 Previous studies have suggested that Smad4 protein expression or genetic mutation/deletion status of the SMAD4 gene influences overall survival following pancreatic cancer resection18,19 and correlates with patterns of recurrence in patients with metastatic disease. In an autopsy series of PDA, tumors with intact Smad4 tended to have patterns of predominantly localized disease, whereas loss of Smad4 more correlated with widely disseminated metastatic disease.15 Because this autopsy series included mostly patients with advanced disease and only 29% underwent surgical resection, it remains unclear if Smad4 status influences patterns of failure in patients who have undergone resection and adjuvant chemoradiation therapy (CRT). Herein, we sought to evaluate whether Smad4 expression predicts for patterns of failure and survival following PDA resection and adjuvant CRT.
MATERIAL AND METHODS
Patients
After Institutional Review Board approval, hospital charts were retrospectively reviewed to obtain patient follow-up information. Only patients who received adjuvant therapy at Johns Hopkins Hospital or Robert Wood Johnson University Hospital were included in the study. A total 145 patients who received adjuvant CRT at Johns Hopkins Hospital (N = 132, 91%) or Robert Wood Johnson University Hospital (N = 13, 9%) between 1994 and 2009 were included. Follow-up images were re-reviewed by two radiologists blinded to Smad4 status to ensure consistency of interpretation. A single pathologist (CID) blinded to patient outcomes, determined Smad4 status by immunolabeling of the resected carcinoma and graded it as either intact or lost. As a quality control measure of labeling quality, for ten patients, (five Smad4 intact and five Smad4 lost), immunolabeling was repeated on sections cut from a different paraffin block of the same carcinoma. In all cases, the pattern of immunolabeling was identical. As a quality control measure of labeling interpretation, immunolabeled sections of 40 patients were reviewed at a later time point by a second pathologist (MC) with complete concordance in all cases.
Adjuvant therapy included continuous infusion or oral (capecitabine) based 5-fluorouracil (88%) or gemcitabine (12%) concurrent with radiotherapy. Following radiation therapy, most patients (59%) received maintenance 5-FU or gemcitabine therapy for an additional 2 to 6 months. Radiation fields were designed according to RTOG 9704 with 3D-conformal radiation (74%) or intensity-modulated radiation therapy (IMRT) (26%). The median total radiation dose was 50.4 Gy.
Routine follow-up occurred at 4-month intervals for the first 2 years, at 6-month intervals for years 3–5, and then annually thereafter. Disease recurrence was classified as: 1) local recurrence in the pancreatic resection bed and mesentery; 2) regional recurrence in the soft tissues or lymph nodes beyond the pancreatic bed; 3) distant recurrence with hepatic, pulmonary or other metastases in distant organs or the peritoneum.
Statistical Analysis
Initial analyses of baseline characteristics of patients were conducted using Chi-square tests of significance and their corresponding p-values were evaluated. Continuous variables were assessed for normality using the Shapiro-Wilk test. Normally distributed variables were compared using the Student’s t-test. Non-normally distributed variables were compared using the Wilcoxon rank sum test. Unadjusted Kaplan-Meier curves for product limit survival estimates and disease free survival (DFS) were computed for subjects. Curves were stratified on Smad4 status and compared using the log rank test. Time was defined as from surgical resection to the event of interest (death or last known follow up for overall survival; death, recurrence, or last known follow up for DFS). Survival analysis was carried out with Cox Proportional Hazards models. Variables significant on univariate analysis were included in the multivariate models. Fine and Gray competing risks regression analyses were conducted for local and distant failure. Cumulative incidence function curves for local and distant failure were computed for subjects. All analyses were conducted using SAS v9.3 (SAS Institute, Cary, NC) and the statistical level of significance was <0.05.
RESULTS
Patient Demographics
Patient demographics stratified by Smad4 status are outlined in Table 1. Smad4 loss was identified in 61% (N = 88) of the carcinomas. With regard to known prognostic factors, such as lymph node ratio (total number of positive lymph nodes/number of resected lymph nodes) and margin status there were no significant differences between the Smad4 intact versus Smad4 lost tumors (all P >0.05; Table 1).
TABLE 1.
Comparison of Smad4 Lost vs. Intact Tumors
| Characteristic | All (%) | Smad4 Lost, n (%) N = 88 |
Smad4 Intact, n (%) N = 57 |
P |
|---|---|---|---|---|
| Age, mean (SD), y | 62 (8.3) | 63 (11.2) | 0.54 | |
| Lymph node ratio* | 0.20 | 0.14 | 0.29 | |
| Tumor size, cm* | 3.0 | 3.0 | 0.95 | |
| Sex, male | 81 (55.6) | 45 (51.1) | 36 (63.2) | 0.15 |
| Grade 3/4 | 55 (38.5) | 32 (36.4) | 23 (40.4) | 0.71 |
| Positive surg margin | 57 (39.3) | 33 (37.5) | 24 (42.1) | 0.58 |
| 5-FU based CRT | 127 (88.8) | 78 (88.6) | 49 (86.0) | 0.38 |
| White | 126 (86.9) | 74 (84.1) | 52 (91.2) | 0.21 |
Median values reported
SD indicates standard deviation; surg, surgical; 5-FU- 5 Fluorouracil
Patterns of Failure
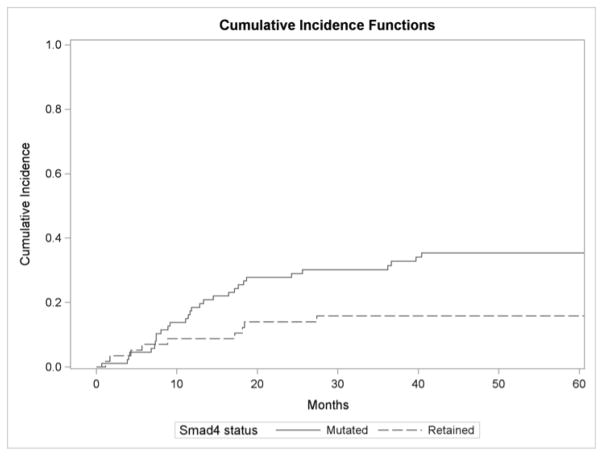
One-and two-year local failure rates in tumors whose cancers lost Smad4 expression were 19% and 29% respectively compared to 8% and 13% for in Smad4 intact cancers (P = 0.015) (Figure 1A). Cause specific hazard ratio (HR) demonstrated Smad4 loss was associated with increased risk of local failure (HR, 2.42; 95% CI, 1.15–5.15; P = 0.021). On multivariate competing risk analysis, Smad4 loss was associated with increased risk of local failure (HR, 2.37, 95% CI, 1.10–5.11; P = 0.027) (Table 2). Increasing lymph node ratio was also associated with local failure in this model (HR = 3.36; 95% CI, 2.02–5.58; P < 0.0001).
FIGURE 1.
Figure 1A: Cumulative incidence function for local failure by Smad4 status.
One-and two-year local failure rates in patients whose cancers lost Smad4 were 19% and 29% respectively compared to 8% and 13% for patients with Smad4 intact cancers (P = 0.015).
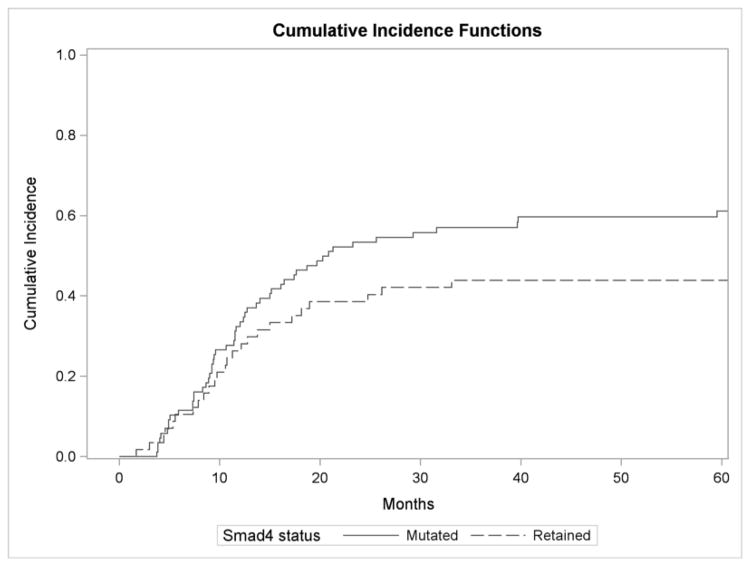
Figure 1B: Cumulative incidence function for distant failure by Smad4 status.
One- and two-year distant metastasis rates in patients with cancers with Smad4 loss were 34% and 53% respectively compared to 25% and 40% in Smad4 intact cases (P = 0.116).
TABLE 2.
Multivariable Competing Risk Analysis
| Characteristic | HR (95 % CI) | P |
|---|---|---|
| Local failure | ||
| Smad4 loss tumor* | 2.37 (1.10–5.11) | 0.027 |
| Lymph node ratio | 3.36 (2.02–5.58) | <0.0001 |
| Positive surgical margins | 0.99 (0.48–2.08) | 0.99 |
| Tumor size, cm | 1.10 (0.82–1.47) | 0.52 |
| Distant failure | ||
| Smad4 loss tumor* | 1.71 (1.03–2.83) | 0.037 |
| Lymph node ratio | 0.65 (0.41–1.04) | 0.07 |
| Positive surgical margins | 1.94 (1.20–3.12) | 0.0065 |
| Tumor size, cm | 1.28 (1.04–1.58) | 0.02 |
| Histologic grade 3/4 | 1.14 (0.71–1.84) | 0.59 |
By immunolabeling
One- and two-year distant metastasis rates in tumors with Smad4 loss were 34% and 53% respectively compared to 25% and 40% in Smad4 intact cases (P = 0.116) (Figure 1B). The most common sites of distant failure at five years included liver (N = 27; 50.9% [Smad4 lost] vs. N = 12; 44.4% [Smad4 intact]), followed by lung (N = 12; 22.6% [Smad4 lost] vs. N = 12; 44.4% [Smad4 intact]), and peritoneal failure (N = 12; 22.6% [Smad4 lost] vs. N = 2; 7.4% [Smad4 intact]). We did not observe a difference between Smad4 status when comparing the proportions of patients manifesting distant recurrence at the three specific sites listed above (P = 0.15). On univariate competing risk analysis, Smad4 loss trended towards significance for higher rates of distant failure (HR, 1.45; 95% CI, 0.91–2.31; P = 0.11). On multivariable competing risk analysis (Table 2), Smad4 loss was associated with a significantly increased risk of distant failure (HR, 1.71; 95% CI, 1.03–2.83; P = 0.037). In this model, increasing tumor size (HR, 1.28; 95% CI, 1.04–1.58; P = 0.02) and positive margins (HR, 1.94; 95% CI, 1.20–3.12; P = 0.0065) were also significantly associated with higher risk of distant metastasis.
Pancreatic tumors with Smad4 loss were more likely to have synchronous local and distant failure at first recurrence following adjuvant therapy. At five years, the rate of synchronous local and distant failure was 14.9% in Smad4 mutant cancer compared to 5.3% in Smad4 intact cases (P = 0.07). Patients with synchronous local and distant failure as first recurrence had worse survival (16.1 months) compared to those with recurrence at a single site (20.4 months) or no recurrence (100.8 months; P < 0.001).
Overall Survival
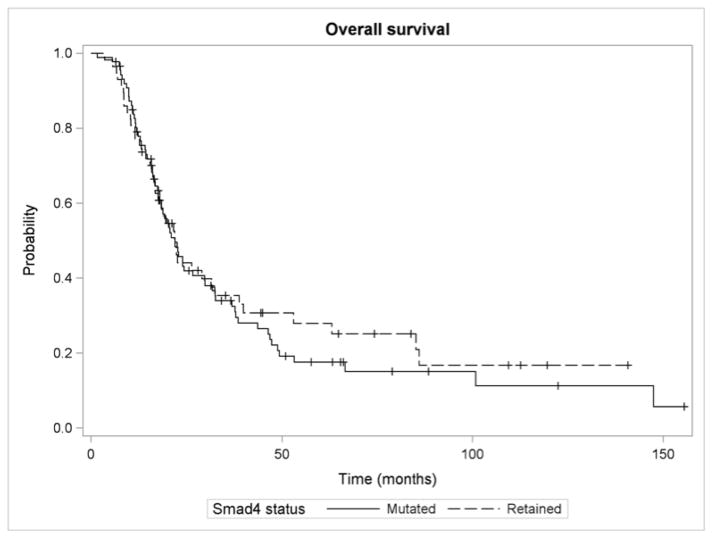
Median overall survival (OS) did not differ significantly in cancers with loss of Smad4 (22 months; 95% CI, 18.0–29.8) compared with intact Smad4 (22 months; 95% CI, 16.8–32.4; P = 0.626) (Figure 2). On univariate analysis, factors associated with decreased OS included pathologic grade 3/4 disease (HR, 1.58; 95% CI, 1.07–2.34; P = 0.022), margin positive resection (HR, 2.27; 95% CI, 1.53–3.38; P < 0.0001), increasing lymph node ratio (HR, 2.05; 95% CI, 1.47–2.87; P < 0.0001), and increasing tumor size (HR, 1.26; 95% CI, 1.09–1.47; P = 0.002).
FIGURE 2. Overall survival by Smad4 status.
Median overall survival did not differ significantly in patients with loss of Smad4 (22 months; 95% CI [18.0 – 29.8]) compared with intact Smad4 (22 months; 95% CI [16.8 – 32.4]; P = 0.626).
On multivariable analysis, margin positive resections (HR, 2.03; 95% CI, 1.33–3.08; P = 0.001), increasing lymph node ratio (HR, 1.46; 95% CI, 1.01–2.10; P = 0.04), and increasing tumor size (HR, 1.22; 95% CI, 1.01–1.44; P = 0.02) remained independent predictors for decreased OS. While controlling for other prognostic factors including margin status, node ratio, and tumor size, OS was not associated with Smad4 status (HR, 1.27; 95% CI, 0.84–1.91; P = 0.26) (Table 3).
TABLE 3.
Multivariate Cox Proportional Hazard Models
| Characteristic | HR (95 % CI) | P |
|---|---|---|
| Overall Survival | ||
| Smad4 loss tumor* | 1.27 (0.84–1.91) | 0.26 |
| Positive surgical margins | 2.03 (1.33–3.08) | 0.001 |
| Histologic grade ¾ | 1.42 (0.94–2.14) | 0.09 |
| Lymph node ratio | 1.46 (1.01–2.10) | 0.04 |
| 5-FU based CRT | 0.66 (0.34–1.27) | 0.21 |
| Tumor size (cm) | 1.22 (1.04–1.44) | 0.02 |
| Disease free survival | 0.47 | |
| Smad4 loss tumor* | 1.15 (0.79–1.68) | 0.007 |
| Positive surgical margins | 1.75 (1.17–2.61) | 0.01 |
| Histologic grade 3/4 | 1.61 (1.10–2.35) | 0.003 |
| Lymph node ratio | 2.98 (1.44–6.18) | 0.11 |
| 5-FU based CRT | 0.59 (0.30–1.13) | 0.01 |
By immunolabeling
5-FU indicates 5 Fluorouracil; CRT – chemoradiation therapy
Disease Free Survival
For DFS, the log-rank test did not demonstrate a significant difference based on Smad4 status (P = 0.79). Median DFS was 13.6 months (95% CI, 11.5–17.4) for Smad4 lost and 13.5 months (95% CI, 9.7–18.1) for Smad4 intact tumors. DFS at 1- and 2-years for Smad4 loss was 57% and 26% and for intact tumors was 53% and 30%. Similarly, there was no association between Smad4 status and DFS in multivariable Cox proportional hazards analysis (HR, 1.15; 95% CI, 0.79–1.68, P = 0.47) (Table 3).
DISCUSSION
This study aimed to determine whether Smad4 expression status of surgically resected PDA could predict for patterns of failure and survival following adjuvant CRT. To our knowledge, this is the largest study to evaluate whether Smad4 status influences patterns of failure after surgery and adjuvant therapy. After controlling for other known risk factors, Smad4 loss was significantly associated with both local recurrence and distant failure. Additionally, Smad4 loss was associated with higher rates of synchronous local and distant failure. We did not observe a statistically significant correlation between Smad4 status and OS or DFS in this patient population.
In oncology, the concept of “personalized medicine” has flourished, especially in breast, lung, and colorectal cancers, by selecting the optimal treatment regimen based on biomarker profiling of the tumor.20 There has been significant interest in improving prognostic capacity and the ability to forecast patterns of progression in patients with PDA to optimally individualize therapeutic regimens.21 Among the most promising of these markers for PDA is the tumor suppressor gene, SMAD4/DPC4.
Other studies have investigated the correlation between Smad4 status and OS, yet none have yielded a conclusive or consistent answer. Following PDA resection, Tascilar et al,22 Singh et al,23 and Oshima et al24 reported that Smad4 loss was associated with significantly shorter OS. In unresectable pancreas cancer, Kadera et al,25 similarly showed Smad4 to be associated with shorter OS; however, the majority (38 of 49; 77.6%) of patients did not receive any radiation therapy. Blackford et al. also found Smad4 gene inactivation to be associated with shorter survival rates in the setting of resected and unresectable PDA, many of whom were treated with a variety of chemotherapeutic agents and radiation therapy doses.19 In contrast, Hua et al26 and Khorana et al27 found no OS association with Smad4 status following surgical resection. Biankin et al. also found Smad4 to not be an independent predictor of OS in a population receiving a combination of surgical, radiation and chemotherapy options28 In our analysis, all patients underwent curative intent surgical resection and received adjuvant CRT. All patients in Tascilar et al, Singh et al, Hua et al, and Oshima et al underwent surgery, but no mention was made as to how many received adjuvant RT. Only 10% and 20% of patients in Biankin et al and Kadera et al received adjuvant radiation therapy, respectively.
Khorana et al. included the highest proportion of patients receiving adjuvant CRT (70%). However, the analysis included bile duct, duodenal, and ampullary tumors as well as other pancreatic tumor histologies (mucinous cystic adenocarcinoma and intraductal papillary mucinous neoplasms with a component of adenocarcinoma) and it demonstrated an improved survival in patients with loss of DPC4. In contrast, most studies have correlated Smad4 loss with poor survival in PDA29,30 and worsened recurrence-free survival.31,32 Boone et al. demonstrated that loss of SMAD4 was associated with distant metastases.17,33 Our study is unique in that it evaluated patterns of failure based on Smad4 status in a population who all received adjuvant therapy. Oshima et al is the only other study to examine this; however, the authors did not make mention of the type of adjuvant therapy received. Similar to our study, they found Smad4 loss significantly correlated with both locoregional and distant failure.
In our cohort, Smad4 loss significantly predicted for local failure and synchronous local and distant failure. These results are concerning and suggest that more intensified local radiation is indicated. Although our results need to be prospectively validated, they suggest that patients who are found to have Smad4 loss at surgery should be considered for more intensified radiation therapy and systemic therapy. Newer radiation technologies including image guided radiation therapy, IMRT, and stereotactic body radiotherapy (SBRT) allow for higher doses of radiation to be delivered to the tumor bed while sparing dose to adjacent normal structures including bowel and stomach. Several studies have demonstrated that IMRT and SBRT can be used to escalate the dose of radiation therapy to the tumor bed.34,35
Since Smad4 loss showed a trend towards greater risk of both local and synchronous local/distant recurrence at five years as the first mode of failure following adjuvant therapy among all patients (14.9 % vs. 5.3%), it may be beneficial to consider incorporating more biologically active systemic therapies in patients whose cancers show a loss of Smad4 expression.36 Since patients with Smad4 loss may also be at risk of developing synchronous local and distant failure, more intensive, combination chemotherapy, such as combined 5-fluorouracil, oxaliplatin, leucovorin, and irinotecan (FOLFIRINOX) should be considered and is currently being studied in the adjuvant setting.37
The main limitation of this study is its retrospective nature and the resulting inherent biases that may not be fully accounted for even through the use of multivariate analysis. Despite these limitations, patient demographics and tumor characteristics, as well as survival outcomes described herein are similar to other retrospective and prospective studies in the literature.38 Nevertheless, RTOG/NRG Oncology 1201, a Phase II Randomized Trial of High versus Standard Intensity Local or Systemic Therapy for Unresectable Pancreatic Cancer, which planned to stratify patients based on SMAD4 status unfortunately closed due to poor accrual, so additional information about the role of SMAD4 in localized disease, will not be available. Our study provides additional clarity about the utility of SMAD4 as a predictor of patterns of failure. We advocate for the continued incorporation of molecular-based approaches in forthcoming pancreatic cancer clinical trials.
CONCLUSION
Resected PDA tumors with Smad4 loss may be at greater risk for local recurrence, distant failure and synchronous local/distant recurrence after adjuvant therapy that includes chemotherapy and RT. These patients may benefit from more aggressive therapeutic regimens that maximize efforts to improve both loco-regional and systemic disease control. Future studies should prospectively evaluate whether Smad4 status is associated with disease outcomes and patterns of failure for patients undergoing adjuvant therapy for pancreatic cancer.
Acknowledgments
Support: Supported by the GI SPORE grant, NCI CA 6; supported by the Biorespository at the Rutgers Cancer Institute of New Jersey
Footnotes
Disclosure: The authors declare no conflict of interest or funding.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 6.Le Scodan R, Mornex F, Partensky C, et al. Histologic assessment of treatment effect of preoperative chemoradiation in patients presenting with resectable pancreatic adenocarcinoma. Cancer Radiother. 2011;15:97–105. doi: 10.1016/j.canrad.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Oettle H, Neuhaus P. Adjuvant therapy in pancreatic cancer: a critical appraisal. Drugs. 2007;67:2293–2310. doi: 10.2165/00003495-200767160-00001. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 9.Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981–990. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regine WF, Winter KA, Abrams R, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–1326. doi: 10.1245/s10434-011-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 12.Herman JM, Regine WF. Adjuvant pancreatic cancer therapy: no one should go it alone or be left behind. Int J Radiat Oncol Biol Phys. 2010;77:645–647. doi: 10.1016/j.ijrobp.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 13.Lowery MA, O’Reilly EM. Pancreatic cancer: the role of molecular markers in diagnosis and management. Clin Adv Hematol Oncol. 2011;9:900–908. [PubMed] [Google Scholar]
- 14.Garrido-Laguna I, Uson M, Rajeshkumar NV, et al. Tumor engraftment in nude mice and enrichment in stroma- related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin Cancer Res. 2011;17:5793–5800. doi: 10.1158/1078-0432.CCR-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biankin AV, Biankin SA, Kench JG, et al. Aberrant p16(INK4A) and DPC4/Smad4 expression in intraductal papillary mucinous tumours of the pancreas is associated with invasive ductal adenocarcinoma. Gut. 2002;50:861–868. doi: 10.1136/gut.50.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crane CH, Varadhachary GR, Yordy JS, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29:3037–3043. doi: 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 19.Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res. 2009;15:4674–4679. doi: 10.1158/1078-0432.CCR-09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao L, Fan X, Cheng N, et al. Shifting from population-wide to personalized cancer prognosis with microarrays. PLoS One. 2012;7:e29534. doi: 10.1371/journal.pone.0029534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger AC, Garcia M, Jr, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008;26:5918–5922. doi: 10.1200/JCO.2008.18.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tascilar M, Skinner HG, Rosty C, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115–4121. [PubMed] [Google Scholar]
- 23.Singh P, Srinivasan R, Wig JD. SMAD4 genetic alterations predict a worse prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2012;41:541–546. doi: 10.1097/MPA.0b013e318247d6af. [DOI] [PubMed] [Google Scholar]
- 24.Oshima M, Okano K, Muraki S, et al. Immunohistochemically detected expression of 3 major genes (CDKN2A/p16, TP53, and SMAD4/DPC4) strongly predicts survival in patients with resectable pancreatic cancer. Ann Surg. 2013;258:336–346. doi: 10.1097/SLA.0b013e3182827a65. [DOI] [PubMed] [Google Scholar]
- 25.Kadera BE, Sunjaya DB, Isacoff WH, et al. Locally advanced pancreatic cancer: association between prolonged preoperative treatment and lymph-node negativity and overall survival. JAMA Surg. 2014;149:145–153. doi: 10.1001/jamasurg.2013.2690. [DOI] [PubMed] [Google Scholar]
- 26.Hua Z, Zhang YC, Hu XM, et al. Loss of DPC4 expression and its correlation with clinicopathological parameters in pancreatic carcinoma. World J Gastroenterol. 2003;9:2764–2767. doi: 10.3748/wjg.v9.i12.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khorana AA, Hu YC, Ryan CK, et al. Vascular endothelial growth factor and DPC4 predict adjuvant therapy outcomes in resected pancreatic cancer. J Gastrointest Surg. 2005;9:903–911. doi: 10.1016/j.gassur.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Biankin AV, Morey AL, Lee CS, et al. DPC4/Smad4 expression and outcome in pancreatic ductal adenocarcinoma. J Clin Oncol. 2002;20:4531–4542. doi: 10.1200/JCO.2002.12.063. [DOI] [PubMed] [Google Scholar]
- 29.Jin J, Liao W, Yao W, et al. Aldo-keto Reductase Family 1 Member B 10 Mediates Liver Cancer Cell Proliferation through Sphingosine-1-Phosphate. Sci Rep. 2016;6:22746. doi: 10.1038/srep22746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shugang X, Hongfa Y, Jianpeng L, et al. Prognostic Value of SMAD4 in Pancreatic Cancer: A Meta-Analysis. Transl Oncol. 2016;9:1–7. doi: 10.1016/j.tranon.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herman JM, Fan KY, Wild AT, et al. Correlation of Smad4 status with outcomes in patients receiving erlotinib combined with adjuvant chemoradiation and chemotherapy after resection for pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2013;87:458–459. doi: 10.1016/j.ijrobp.2013.06.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada S, Fujii T, Shimoyama Y, et al. SMAD4 expression predicts local spread and treatment failure in resected pancreatic cancer. Pancreas. 2015;44:660–664. doi: 10.1097/MPA.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 33.Boone BA, Sabbaghian S, Zenati M, et al. Loss of SMAD4 staining in pre-operative cell blocks is associated with distant metastases following pancreaticoduodenectomy with venous resection for pancreatic cancer. J Surg Oncol. 2014;110:171–175. doi: 10.1002/jso.23606. [DOI] [PubMed] [Google Scholar]
- 34.Dholakia AS, Kumar R, Raman SP, et al. Mapping patterns of local recurrence after pancreaticoduodenectomy for pancreatic adenocarcinoma: a new approach to adjuvant radiation field design. Int J Radiat Oncol Biol Phys. 2013;87:1007–1015. doi: 10.1016/j.ijrobp.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herman JM, Fan KY, Wild AT, et al. Phase 2 study of erlotinib combined with adjuvant chemoradiation and chemotherapy in patients with resectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;86:678–685. doi: 10.1016/j.ijrobp.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang ML, Foo KF. Adjuvant chemoradiotherapy for high-risk pancreatic cancer. Singapore Med J. 2009;50:43–48. [PubMed] [Google Scholar]
- 37.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 38.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]