Abstract
This study investigated the relationship between measures of Long-Term Average Spectrum (LTAS) for speakers with Parkinson’s disease (PD) and Multiple Sclerosis (MS) and scaled estimates of perceived speech severity. Perceived severity was operationally defined as listeners’ overall impression of voice, resonance, articulatory precision, and prosody without regard to intelligibility. Healthy control talkers were also studied. Speakers were audio recorded while reading Harvard Sentences and the Grandfather Passage. Using TF32 (Milenkovic, 2005), the LTAS was computed for sentences. Coefficients of the first four moments were used to characterize energy across the speech spectrum. Supplemental acoustic measures of articulatory rate, vocal intensity, and fundamental frequency also were obtained. Three speech-language pathologists scaled speech severity for the reading passages. Results indicated no group differences in acoustic measures. The absolute magnitude of correlations between LTAS moment coefficients and perceptual estimates of scaled severity within and across speaker groups ranged from .16 to .53, with the strongest correlations for the PD group. These results suggest that the LTAS may prove useful in conjunction with perceptual judgments to document speech spectral changes related to treatment or disease progression. Findings further suggest that different acoustic models of severity are likely needed for dysarthria secondary to PD and dysarthria secondary to MS.
Keywords: dysarthria, speech severity, Long term average spectrum
INTRODUCTION
The Long-Term Average Spectrum (LTAS) is a fast Fourier transform-generated power spectrum of the frequencies comprising a speech sample. Thus, the LTAS is a composite signal representing the spectrum of the glottal source as well as the spectrum or resonant characteristics of the vocal tract. Although the relationship of the LTAS to the phonatory mechanism is not entirely transparent, the LTAS holds promise as an acoustic index of voice quality, at least when segmental production is reasonably intact (e.g., Cannito, Buder, & Chorna, 2005; Hartmann & von Cramon, 1984; Tanner, Roy, Ash, & Buder, 2005). For example, relatively weak harmonic energy in the higher frequencies of the speech spectrum and a corresponding increase in spectral tilt are characteristic of breathy or hypo-functional signals (Dromey, 2003; Hillenbrand & Houde, 1996). In contrast, excessive vocal fold impact and turbulent noise, both of which have been noted in functional dysphonia, are associated with relatively greater energy in the higher frequencies of the speech spectrum (Tanner, Roy, Ash, & Buder, 2005).
In one of the few studies to perform LTAS measures of dysarthria, Dromey (2003) reported that moment coefficients of the LTAS distinguished connected speech samples for healthy controls and speakers with Parkinson’s disease (PD). Moment coefficients are statistical constructs that reflect the central tendency and shape of a distribution. Thus, moment coefficients of the LTAS reflect the central tendency and shape of the speech spectrum computed over a designated frequency range. Dromey (2003) found that the LTAS for the Rainbow Passage produced by speakers with PD was characterized by an overall lower mean, as indicated by lower first moment coefficients, and greater spectral tilt, as indicated by higher third moment coefficients, compared to healthy controls [for related data in PD see Neel (2009)]. This finding of reduced energy in the higher speech frequencies is consistent with breathy voice or hypophonia often observed in dysarthria secondary to PD (Duffy, 2005). Although acoustic measures were not strongly correlated with perceptual judgments of speech, Dromey (2003) suggested that the LTAS may hold promise as an objective index of dysarthria severity. This suggestion is an attractive possibility given the relative ease in computing the LTAS. Moreover, a relationship between the LTAS and perceptual judgments of speech for both normal talkers and individuals with laryngeal pathology has been demonstrated in several studies (e.g., Hazan & Markham, 2004; Krause & Braida, 2004; Tanner et al., 2005).
The present study sought to further evaluate the suggestion that the LTAS may prove useful as an acoustic index of dysarthria severity. Specifically, this study investigated the relationship between scaled estimates of perceived speech severity and characteristics of the LTAS for a group of speakers with PD and a group of speakers with Multiple Sclerosis (MS). Healthy controls were included for comparison purposes. Supplemental acoustic measures of articulatory rate, vocal intensity, and fundamental frequency also were obtained.
METHODS
Participants
A total of 39 speakers were selected for study including 10 speakers with PD, 14 speakers with MS, and 15 healthy controls. Selected speaker characteristics are summarized in Table 1. Participants with medical diagnoses were recruited through patient support groups and newsletters for PD or MS in the western New York area. All speakers were native speakers of American English, had achieved at least a high school diploma, and had visual acuity or corrected acuity adequate for reading printed materials. Hearing aid use was an exclusion criterion. Participants with MS and PD were taking a variety of symptomatic medications, but no participant had undergone neurosurgical treatment for MS or PD. All speakers scored at least 26/30 on the Standardized Mini-Mental State (Molloy, 1999), with the exception of one male speaker with MS who received a score of 25/30.
TABLE 1.
Selected Speaker Characteristics are Summarized. Values in Parentheses are Standard Deviations.
| Number of Males | Number of Females | Mean Number of Years Post Diagnosis (SD) | Mean Age (SD) | Mean Percent Correct Sentence Intelligibility (SD) | |
|---|---|---|---|---|---|
| Control | 7 | 8 | N/A | 56 (14.2) | 94 (2.32) |
| MS | 4 | 10 | 11 (7.7) | 50 (11.6) | 92 (2.61) |
| PD | 5 | 5 | 9 (10.8) | 68 (8.2) | 90 (2.25) |
Sentence Intelligibility Test scores (Yorkston & Beukelman, 1996) in Table 1 reflect overall percent correct scores obtained for 10 inexperienced listeners. Intelligibility testing was undertaken to provide a baseline measure of overall dysarthria severity. Listeners who scored the sentence intelligibility test were recruited from the student population at the University at Buffalo, reported minimal experience with speech-language or hearing disorders, had no formal training in communication disorders or linguistics, and passed a pure tone hearing screening at 20 dB HL for octave frequencies between 250 to 8000 Hz. Sentences were presented to listeners via headphones in a double-walled audiometric booth, and listeners typed their response onto a computer. Listeners performed the task without knowledge of speaker identity or neurological status.
Table 1 suggests broadly similar intelligibility for all speaker groups. Indeed, average sentence intelligibility scores for normal and disordered speaker groups differ by only 2%–4%. This magnitude of difference likely is not clinically meaningful (see Hustad & Cahill, 2003 for similar conclusions concerning intelligibility in dysarthria secondary to cerebral palsy). Segmental integrity has long been considered to be important to intelligibility (Weismer, 2008). The fact that sentence intelligibility was similarly high across speaker groups suggests that segmental integrity was reasonably preserved for the current speakers with PD and MS. As such, any differences in LTAS measures for normal and disordered speakers likely cannot be attributed to differences in segmental integrity [see Dromey (2003) for related discussion of this issue in PD].
Finally, three speech-language pathologists (SLP) provided scaled estimates of speech severity. Each SLP had approximately 10 years of clinical experience in the management of neurogenic communication disorders. Procedures and materials for the scaling task are described in the following section.
Procedures and Instrumentation
Audio Recording
As part of a larger project, participants were audio recorded as they read 25 Harvard Sentences (IEEE, 1969) and the Grandfather Passage (Duffy, 2005). Online audio-recording of speech samples took place in a sound-treated room. The acoustic signal was transduced using an AKG C410 head-mounted microphone positioned 10 cm and 45–50 degrees from the left oral angle. The acoustic signal was preamplified, low pass-filtered at 9.8 kHz and digitized directly to computer hard disk at a sampling rate of 22 kHz using TF32 (Milenkovic, 2005). For each participant, a 1000 Hz calibration tone was recorded for use in calculating absolute sound pressure level (SPL) from the acoustic signal (see Tjaden & Wilding, 2004 for a complete description of these procedures). Speakers with PD were recorded approximately 1 hour prior to taking anti-Parkinsonian medications. Cyclical medication effects have not been documented in MS. Thus, participants with MS were recorded at a variety of times during the day.
Perceptual Estimates of Speech Severity
Speech-language pathologists (SLPs) scaled speech severity for the Grandfather Passage using a Visual Analog Scale (VAS) adapted from Cannito and colleagues (1997). A random ordering of all passage readings was created such that SLPs performed severity judgments without knowledge of speaker identity or neurological diagnosis. Passage readings were presented via headphones in a double-walled audiometric booth, and SLPs indicated their ratings on the VAS using a computer program. The VAS consisted of a 150-mm vertical line that appeared on the computer monitor with endpoints labeled “Severely Impaired” and “No Impairment”. SLPs placed a computer mouse pointer anywhere between the two endpoints to indicate a severity judgment. SLPs were instructed to judge severity on the basis of voice, resonance, articulatory precision, and prosody, without regard to intelligibility. Computer software converted the position of the mouse pointer to a scale value ranging from 0 to 1.0. Passage readings were presented at the same intensity level at which they were produced naturally by the speakers. The mean of the three SLP ratings for a given passage reading was calculated for use in data analysis.
SLPs judged a randomly selected 10% of passages a second time to estimate reliability. The Intraclass Correlation Coefficient (ICC) was used to assess reliability. The mean ICC Intraclass Correlation Coefficients for intrajudge and interjudge reliability were .97 and .96, respectively, suggesting high consistency of severity ratings both within and across judges.
Acoustic Analysis
Using TF32 (Milenkovic, 2005), a variety of acoustic measures were obtained for the Harvard sentences. Sentences were first segmented into speech runs, defined as a stretch of speech bounded by silent periods between words of at least 200 ms (Turner & Weismer, 1993). Acoustic measures were performed for each speech run or entire sentences in cases where no operationally defined pauses were present. Conventional acoustic criteria were used to identify run onsets and offsets, such as stop release bursts, frication, or voicing energy. For each run, the first four spectral moments (i.e., mean, standard deviation, skewness, and kurtosis) were used to characterize the central tendency and shape of the LTA spectrum from 0 to 11 kHz. The LTA was computed using a Hamming-window Fourier spectrum with the default pre-emphasis applied. Moment coefficients were averaged across speech runs for use in the data analysis. The first four moments of the LTAS were obtained for completeness. However, the first and third moments were of primary interest as the interpretation of these measures is most straightforward in so far as distributions with higher means (higher first moment) tend to be more negatively skewed (lower third moment) and vice versa.
Supplemental acoustic measures of articulatory rate, SPL, and fundamental frequency (f0) for Harvard Sentences were obtained to further characterize speaker characteristics (see also Dromey, 2003). Articulatory rate, in syllables per second (syll/sec), was determined by counting the number of syllables in each run and dividing by run duration. Articulatory rates were averaged across speech runs to yield a mean articulatory rate for each speaker. Average SPL for each speech run as well as the standard deviation (sd) of SPL were computed from the RMS trace to provide indices of mean vocal intensity and vocal intensity variation within a speech run, respectively. For this purpose, RMS voltages were exported to Excel and converted to dB SPL with reference to each talker’s calibration tone. Finally, f0 traces were generated to provide indices of average speaking f0 and f0 variation within speech runs. The f0 traces were visually inspected for computer-generated tracking errors and errors were hand-corrected on a pitch-period–by–pitch-period basis. Fundamental frequency (f0) traces were exported to Excel and converted to semitones. An average f0 and f0 sd were calculated for each speech run and then averaged across speech runs for use in the data analysis.
Data Analyses
One-way ANOVA was used to evaluate potential group differences in scaled estimates of speech severity as well as acoustic measures. Gender was used as a covariate in all tests. Average SPL was used as a covariate in all analyses for moment coefficients of the LTAS, owing to the known effects of vocal intensity on the LTAS (Nordenberg & Sundberg, 2004). Post-hoc testing was accomplished using the Tukey-Kramer adjustment for multiple comparisons. All tests were two-tailed and a nominal significance level of .05 was used. Correlation analysis was used to examine the relationship between moment coefficients of the LTAS and scaled severity. Standard diagnostic plots were used to assess model fit and the transformation of the dependent variable was done in some cases in order to meet statistical assumptions. All statistical tests were carried out using SAS version 9.1.3 (Cary, NC).
RESULTS
Perceptual Estimates of Speech Severity
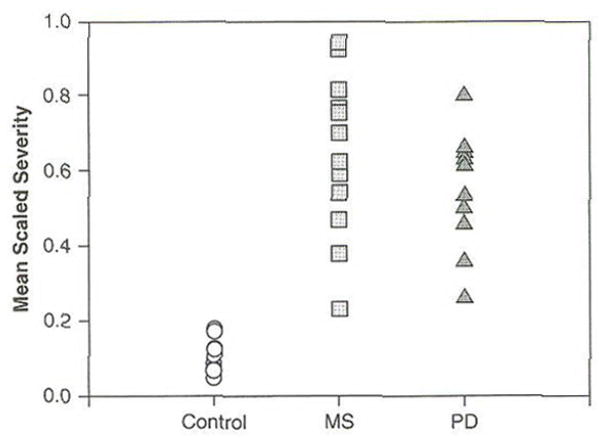
Figure 1 reports scaled estimates of severity for the Grandfather Passage. Larger-scale values indicate more severe impairment. Each symbol corresponds to the SLP’s mean rating for an individual speaker. Figure 1 suggests overlapping distributions for the MS and PD groups which are shifted toward higher or more severe ratings as compared to the Control group. ANOVA further indicated a main effect of group (p < .0001). Post-hoc testing revealed that scaled estimates of severity differed for the Control and MS groups as well as for the Control and PD groups (p < .03). Scaled estimates of severity did not differ for the MS and PD groups, however. Thus, despite similar sentence intelligibility (Table 1), speakers with MS and PD were perceived to have more severely impaired speech compared to controls.
Figure 1.
Mean Severity Ratings for each Speaker’s Reading of the Grandfather Passage. The Possible Range of Scale Values was 0 (No Impairment) to 1.0 (Severely Impaired)
Acoustic Measures
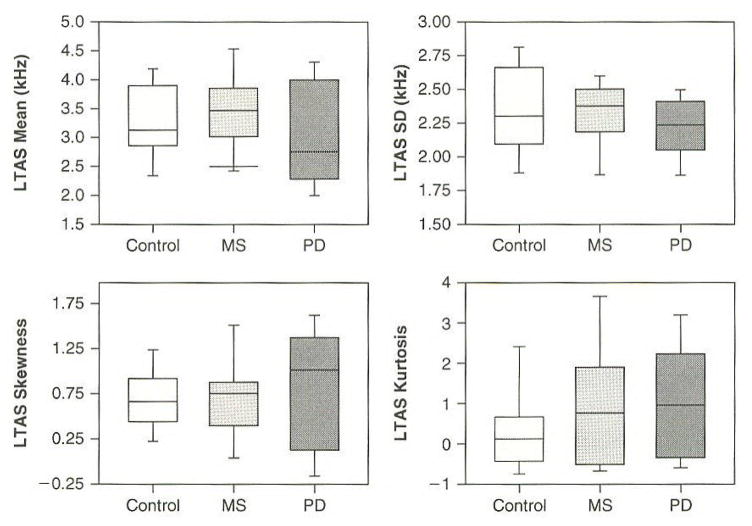
Table 2 summarizes descriptive statistics for the supplemental acoustic measures of articulatory rate, vocal intensity, and fundamental frequency. Statistical analyses indicated no group differences for any of the acoustic measures in Table 2. Figure 2 reports distributions of moment coefficients of the LTAS in the form of box and whiskers plots. This figure suggests substantial overlap of distributions for the three speaker groups. Statistical analyses indicated no group differences in moment coefficients of the LTAS.
TABLE 2.
Mean Group Values for Supplemental Acoustic Measures are Reported. Standard Deviations are Shown in Parentheses. Columns Labeled SPL SD and F0 SD Reflect Intensity and Fundamental Frequency Variability Over the Course of Speech Runs.
| Mean Artic Rate (syll/sec) | Mean SPL (dB) | SPL SD (dB) | Mean F0 (Semitone) | F0 SD (Semitone) | |
|---|---|---|---|---|---|
| Control | 3.77 (.46) | 73.1 (2.66) | 8.9 (.89) | 84.7 (4.78) | 3.3 (.69) |
| MS | 3.41 (.72) | 70.7 (2.56) | 8.6 (.99) | 85.9 (4.25) | 3.9 (1.38) |
| PD | 3.90 (.58) | 72.4 (2.72) | 8.6 (.93) | 84.8 (3.66) | 3.5 (1.14) |
Figure 2.
Distributions of Moment Coefficients of the LTAS are Reported in the Form of Box and Whiskers Plots
Relationship between LTAS and Scaled Severity
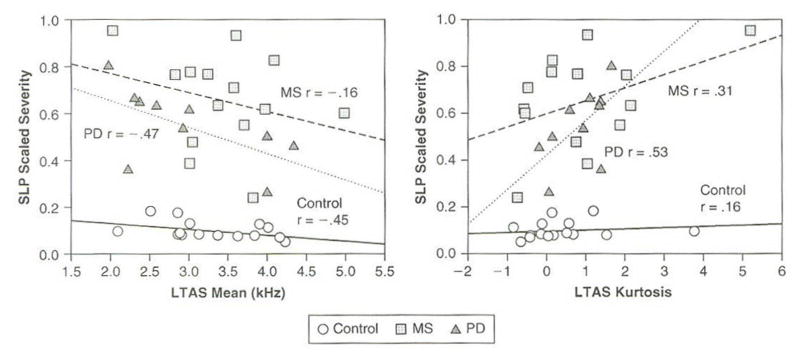
Table 3 reports the results of the partial correlation analysis relating LTAS moment coefficients and scaled estimates of severity, controlling for average SPL. Correlations are reported for the pooled group of 39 speakers as well as for individual speaker groups. The absolute magnitude of correlations ranged from .16 to .53. Scatterplots illustrating the relationship between selected moment coefficients of the LTAS and scaled severity are shown in Figure 3. These data were selected to illustrate the varied relationship between a given moment coefficient and scaled severity for the three speaker groups. Group affiliation in Figure 3 is indicated by symbol shape. Linear regression functions have been fit to the data to illustrate the direction and strength of the relationship between moment coefficients of the LTAS and scaled severity for each speaker group. Line type (i.e., dotted, dashed, solid) is used to indicate group affiliation, and r values from the partial correlation analysis reported in Table 3 are included for completeness.
TABLE 3.
Results of the Partial Correlation Analysis Relating Moment Coefficients of the LTAS and Scaled Estimates of Severity, Controlling for Average SPL. Correlations are Reported Separately for Each Speaker Group as well as for Pooled Speaker Groups (All Groups).
| LTAS Mean | LTAS SD | LTAS Skewness | LTAS Kurtosis | |
|---|---|---|---|---|
| Control | −.45 | −.38 | .23 | .16 |
| MS | −.16 | −.36 | .23 | .31 |
| PD | −.47 | −.39 | .45 | .53 |
| All Groups | −.24 | −.28 | .31 | .45 |
Figure 3.
Scatterplots Relating Select Moment Coefficients of the LTAS and Scaled Severity are Shown. Group Affiliation in Each Panel is Indicated by Symbol Shape. Partial Correlation Coefficients in the Form of R Values also are Shown in Each Plot. Simple Regression Lines have been Fit to Each Speaker Group’s Data to Illustrate the Relationship Between Acoustic and Perceptual Measures
There are several notable observations to be made regarding the data in Table 3 and Figure 3. First, the sign of correlations and hence the direction of the relationship between moment coefficients of the LTAS and perceived severity was consistent for all speaker groups. For example, for all three speaker groups, correlations for the first moment coefficient (Mean) of the LTAS and perceived severity had a negative sign, and correlations for the third moment coefficient (Skewness) and perceived severity had a positive sign. Thus, an overall lower mean (i.e., lower first moment of the LTAS) and greater spectral tilt (i.e., greater third moment of the LTAS)—both suggestive of less energy in the higher speech frequencies— were associated with higher or poorer estimates of severity for all speaker groups. It also should be noted that the strength of the relationship between the LTAS measures and perceived severity was most robust for the PD group (average absolute partial correlation coefficient r = .46), followed by control (absolute average correlation coefficient r = .30) and MS (average absolute correlation = .27) groups.
DISCUSSION
The suggestion that the LTAS might prove useful as an objective, instrumental index of dysarthria severity was originally advanced by Dromey (2003). In that study, the LTAS for PD talkers’ production of the Rainbow Passage was characterized by an overall lower mean and greater spectral tilt compared to a group of neurologically normal talkers. Measures of the LTAS were not strongly related to perceptual impressions of speech, but the fact that there were group differences in measures of the LTAS as well as perceptual impressions of voice for speakers with PD and healthy controls suggests that additional studies are warranted. The present study sought to extend this line of inquiry to additional speakers with PD as well as speakers with MS. MS provides an interesting comparison or foil for PD because the nature of the dysarthria tends to be distinct for these medical diagnoses, at least as characterized by the Mayo perceptual classification system for dysarthria (Duffy, 2005).
The LTAS is a composite signal reflecting the spectrum of the glottal source as well as the resonant characteristics of the vocal tract. Differences in measures of the LTAS for speakers with dysarthria and neurologically normal talkers therefore could be attributed to phonatory characteristics, articulatory characteristics, or both. Dromey’s (2003) study did not undertake an evaluation of articulatory variables, thus making it difficult to determine the source of the reported group differences in LTAS measures. The current approach was to use sentence intelligibility as a global, indirect metric of articulatory integrity. All speaker groups in the present study received almost identical scores on the Yorkston and Beukelman (1996) Sentence Intelligibility Test (see Table 1). To the extent that this finding indicates relatively preserved segmental integrity for speakers with PD and MS, any differences in measures of the LTAS for normal and disordered speaker groups would most likely reflect phonation or voice quality characteristics rather than articulation characteristics. The fact that sentence intelligibility scores were similar for normal and disordered speaker groups, however, raises the question of whether speakers with PD and MS did indeed have a dysarthria. Figure 1, which reports scaled estimates of speech severity for individual speakers, provides good evidence that speakers with PD and MS had a perceptually obvious dysarthria. The data in Figure 1 are especially compelling given that reading passages for all talkers were pooled and then randomized prior to presentation to SLPs. SLPs also were blinded to speaker identity or neurological diagnosis when judging speech samples.
Although the PD and MS groups were perceived to be more severe than healthy controls, moment coefficients of the LTAS (Figure 2) did not differ across speaker groups nor did the supplemental acoustic measures of SPL, articulatory rate or f0 differ (Table 2). In Dromey’s (2003) study, speakers with PD did not differ from neurologically normal talkers on measures of SPL or fundamental frequency. Previous studies from our lab have also reported similar articulatory rates for PD, MS, and control groups (Tjaden & Wilding, 2004). Deficits in SPL, f0, and articulatory rate thus are not ubiquitous in dysarthria secondary to PD or MS, perhaps especially for individuals with sentence intelligibility scores approaching 90%—a score consistent with descriptions of mild dysarthria (Yorkston, Miller, & Strand, 2004). However, the failure to find differences in LTAS measures for speakers with dysarthria and healthy controls is at odds with Dromey’s (2003) study. As previously mentioned, that study did not undertake an evaluation of articulatory characteristics. As noted by Dromey (2003), it is possible that the group differences in the LTAS that he reported were indicative of articulatory differences, whereas the sentence intelligibility data collected in the present study suggest largely preserved articulatory integrity for disordered speaker groups. To the extent that the LTAS measures in the present study primarily reflect glottal source characteristics, the implication is that there were no sizeable group differences in voice quality. Differences in the choice of speech sample, acoustics software, analysis parameters for computing the LTAS, metrics for computing moment coefficients, and even heterogeneity of disordered speakers also may help to explain the different findings. Adoption of a common set of procedures for quantifying LTAS characteristics in dysarthria would facilitate future cross-study comparisons (for related discussion, see Tanner et al., 2005).
Finally, negative correlations between the LTAS mean and scaled severity as well as positive correlations between LTAS skewness and perceived severity suggest that reduced high-frequency energy of the LTAS, as has been noted for breathy signals of both normal and disordered speaker populations, was associated with poorer perceptual impressions of speech (Hillenbrand & Houde, 1996; Neel, 2009). Relatedly, the positive relationship between LTAS kurtosis and scaled severity is consistent with Dromey’s (2003) observation that speakers with PD, judged to sound more impaired than controls, exhibited higher kurtosis values than controls. Thus, although there were no group differences in measures of the LTAS, the direction of the relationship of these acoustic measures to perceived severity is consistent with other published studies. A modest correlation between LTAS measures and scaled severity, most notably for the PD group, also suggests that LTAS measures may prove useful in conjunction with perceptual judgments to document speech performance, possibly related to disease progression or treatment. The fact that the strength of the relationship between moment coefficients of the LTAS and scaled severity differed for PD and MS groups in the present study further highlights the importance of comparative group studies of dysarthria. These results also suggest that variables contributing to an acoustic model of dysarthria severity will likely need to be weighted differently for dysarthria secondary to PD and dysarthria secondary to MS.
Acknowledgments
This research was supported by NIH DC004689.
Contributor Information
Kris Tjaden, Department of Communicative Disorders & Sciences, University at Buffalo
Joan E. Sussman, Department of Communicative Disorders & Sciences, University at Buffalo
Grace Liu, Department of Communicative Disorders & Sciences, University at Buffalo
Greg Wilding, Department, of Biostatistics, University at Buffalo
References
- Cannito M, Buder E, Chorna L. Spectral amplitude measures of adductor spasmodic dysphonic speech. Journal of Voice. 2005;19:391–410. doi: 10.1016/j.jvoice.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Cannito M, Burch AR, Watts C, Rappold P, Hood SB, Sherrard K. Disfluency in spasmodic dysphonia: A multivariate analysis. Journal of Speech, Language, and Hearing Research. 1997;40:627–641. doi: 10.1044/jslhr.4003.627. [DOI] [PubMed] [Google Scholar]
- Dromey C. Spectral measures and perceptual ratings of hypokinetic dysarthria. Journal of Medical Speech-Language Pathology. 2003;11:85–94. [Google Scholar]
- Duffy JR. Motor speech disorders: Substrates, differential diagnosis, and management. 2. St. Louis, MO: Mosby; 2005. [Google Scholar]
- IEEE. IEEE transactions on audio and electroacoustics. 1969;17:227–246. [Google Scholar]
- Hartmann E, von Cramon D. Acoustic measurements of voice quality in central dysphonia. Journal of Communication Disorders. 1984;17:425–440. doi: 10.1016/0021-9924(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Hazan V, Markham D. Acoustic-phonetic correlates of talker intelligibility for adults and children. Journal of the Acoustic Society of America. 2004;116:3108–3118. doi: 10.1121/1.1806826. [DOI] [PubMed] [Google Scholar]
- Hillenbrand J, Houde RA. Acoustic correlates of breathy vocal quality: Dysphonic voices and continuous speech. Journal of Speech and Hearing Research. 1996;39:311–321. doi: 10.1044/jshr.3902.311. [DOI] [PubMed] [Google Scholar]
- Hustad KC, Cahill MA. Effects of presentation mode and repeated familiarization on intelligibility of dysarthric speech. American Journal of Speech-Language Pathology. 2003;12:1–11. doi: 10.1044/1058-0360(2003/066). [DOI] [PubMed] [Google Scholar]
- Krause JC, Braida LD. Acoustic properties of naturally produced clear speech at normal speaking rates. Journal of the Acoustical Society of America. 2004;115:362–378. doi: 10.1121/1.1635842. [DOI] [PubMed] [Google Scholar]
- Milenkovic P. TF32. [Computer program] University of Wisconsin-Madison; 2005. [Google Scholar]
- Molloy DW. Standardized Mini-Mental State Examination. Troy, NY: New Grange Press; 1999. [Google Scholar]
- Neel A. Effects of loud and amplified speech on sentence and word intelligibility in Parkinson disease. Journal of Speech, Language, and Hearing Research. 2009;52:1021–1033. doi: 10.1044/1092-4388(2008/08-0119). [DOI] [PubMed] [Google Scholar]
- Nordenberg M, Sundberg S. Effect on LTAS of vocal loudness variation. Logopedics, Phoniatrics and Vocology. 2004;29:183–191. doi: 10.1080/14015430410004689. [DOI] [PubMed] [Google Scholar]
- Tanner K, Roy N, Ash A, Buder EH. Spectral moments of the long-term average spectrum: Sensitive indices of voice change after therapy? Journal of Voice. 2005;19:211–222. doi: 10.1016/j.jvoice.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Tjaden K, Wilding GE. Rate and loudness manipulations in dysarthria: Acoustic and perceptual findings. Journal of Speech, Language, and Hearing Research. 2004;47:766–783. doi: 10.1044/1092-4388(2004/058). [DOI] [PubMed] [Google Scholar]
- Turner GS, Weismer G. Characteristics of speaking rate in the dysarthria associated with amyotrophic lateral sclerosis. Journal of Speech and Hearing Research. 1993;36:1134–1144. doi: 10.1044/jshr.3606.1134. [DOI] [PubMed] [Google Scholar]
- Yorkston KM, Beukelman DR. Sentence intelligibility test. Lincoln, NE: Tice Technologies; 1996. [Google Scholar]
- Yorkston KM, Miller RM, Strand EA. Management of speech and swallowing in degenerative disease. 2. Austin, TX: Pro-Ed; 2004. [Google Scholar]
- Weismer G. Speech intelligibility. In: Ball MJ, Perkins MR, Muller N, Howard S, editors. The handbook of clinical linguistics. Oxford: Blackwell Publishing; 2008. pp. 568–582. [Google Scholar]