Abstract
Substantial evidence supports the view that inflammatory processes contribute to brain alterations in HIV infection. Mechanisms recently proposed to underlie neuropathology in Alcohol Use Disorder (AUD) include elevations in peripheral cytokines that sensitize the brain to the damaging effects of alcohol. This study included 4 groups: healthy controls, individuals with AUD (abstinent from alcohol at examination), those infected with HIV, and those comorbid for HIV and AUD. The aim was to determine whether inflammatory cytokines are elevated in AUD as they are in HIV infection. Cytokines showing group differences included interferon gamma-induced protein 10 (IP-10) and tumor necrosis factor α (TNFα). Follow-up t-tests revealed that TNFα and IP-10 were higher in AUD than controls but only in AUD patients who were seropositive for Hepatitis C virus (HCV). Specificity of TNFα and IP-10 elevations to HCV infection status was provided by correlations between cytokine levels and HCV viral load and indices of liver integrity including albumin/globulin ratio, fibrosis scores, and AST/platelet count ratio. Because TNFα levels were mediated by HCV infection, this study provides no evidence for elevations in peripheral cytokines in "uncomplicated", abstinent alcoholics, independent of liver disease or HCV infection. Nonetheless, these results corroborate evidence for elevations in IP-10 and TNFα in HIV and for IP-10 levels in HIV+HCV co-infection.
Introduction
Patients with chronic HIV infection typically show elevations in plasma cytokine levels [1–4]. HIV infection of the central nervous system (CNS) appears to begin with the transmigration of peripheral HIV-infected cells (e.g., monocytes or macrophages) across the blood brain barrier [5–12] and consequent infection of microglia [13–18]. Activation of macrophages and microglia and the release of chemokines, cytokines, and neurotoxins [19] promote further HIV replication [20], trafficking of macrophages into the CNS [21], glial activation [22], altered neuronal signaling and repair processes [23–26], and ultimately, compromised neuronal integrity [27–31]. Select studies in HIV patients have reported correlations between elevated plasma cytokine concentrations and cognitive deficits [32–35]. Elevations in plasma Tumor Necrosis Factor α (TNFα) [32, 36, 37] and Interferon γ-induced Protein 10 (IP-10) [33, 38–40] are frequently reported in the HIV literature and are considered biomarkers of HIV viral load (Table 1provides an acronym key).
Table 1. Acronym key.
| AIC | Akaike Information Criterion |
| AGR | Albumin / Globulin Ratio |
| AUD | Alcohol Use Disorder |
| APRI | AST/Platelet count Ratio Index |
| CSF | Cerebrospinal Fluid |
| EGF | Epidermal Growth Factor |
| EtOH | Ethanol |
| FGF | Fibroblast Growth Factor |
| Fib-4 | Fibrosis score |
| Flt3 | Fms-related tyrosine kinase 3 ligand |
| GCSF | Granulocyte Colony-Stimulating Factor |
| GMCSF | Granulocyte Macrophage Colony-Stimulating Factor |
| GRO | Growth Regulated Oncogene |
| HCV | Hepatitis C Virus |
| HIV | Human Immunodeficiency Virus |
| IP-10 | IFN-γ-induced protein 10 |
| IFN | Interferon |
| IL | Interleukin |
| MIP | Macrophage Inflammatory Protein |
| MDC | Macrophage-Derived Chemokine |
| MFI | Mean Fluorescence Intensity |
| MAPK | Mitogen-Activated Protein Kinase |
| MCP | Monocyte Chemoattractant Protein |
| NFκβ | Nuclear Factor kappa beta |
| PDGF | Platelet-Derived Growth Factor |
| RANTES | Regulated on Activation, Normal T cell Expressed and Secreted |
| SES | Socio-economic Status |
| CD40L | soluble CD40 ligand |
| SCID | Structured Clinical Interview for DSM-IV |
| TLR-4 | Toll-like Receptor 4 |
| TGF | Transforming Growth Factor |
| TNF | Tumor Necrosis Factor |
| VEGF | Vascular Endothelial Growth Factor |
| VACS | Veterans Aging Cohort Study Index |
Mechanisms of neuroimmune signaling in the pathogenesis of Alcohol Use Disorder (AUD) and associated brain atrophy have been proposed based primarily on animal studies [41–46]. In mice and rats, ethanol (EtOH) has been shown to activate Toll-like receptor 4 (TLR-4)[47–49], but see [50], which activates signaling molecules (e.g., members of the P38 mitogen-activated protein kinase (MAPK) family) and downstream transcription factors such as nuclear factor kappa beta (NFκβ) [51–55], to increase production of proinflammatory cytokines [56] and oxidative stress [57]. EtOH exposure in rodents has been shown to activate microglia [56, 58, 59] and upregulate proinflammatory cytokine mRNA and protein levels (e.g., monocyte chemotactic protein-1 [MCP-1]/chemokine ligand-2 [CCL2], TNFα, and interleukin (IL)-1β [IL-1β]) in several brain regions [60, 61], including frontal cortex [62, 63], cortical mantle [64, 65], hippocampus [66–69], cerebellum [49], and amygdala [70–72]. Additional support for the involvement of neuroimmune signaling in the pathogenesis AUD includes evidence for a proinflammatory environment underlying myelin disruption in EtOH-exposed mice [47]; alcohol-preferring P rats exhibiting innately elevated MCP-1 levels in the amygdala [73]; and reductions in MCP-1 in the amygdala (via silencing RNA) associated with reduced binge drinking in the P rat [74–79].
In humans, gene expression studies evaluating postmortem brain tissue from AUD relative to healthy controls showed a strong representation of immune- and inflammation- related genes in the AUD brain [80, 81]. A number of studies have evaluated whether polymorphisms in innate immune genes (e.g., NFκβ, TNFβ) contribute to the genetic risk for alcoholism, with equivocal results [82–85] but see [86–90]. These findings were elaborated by an influential paper showing in AUD relative to control human brain tissue higher MCP-1 protein levels in the ventral tegmental area (VTA), substantia nigra, hippocampus, and amygdala, and altered microglial morphology in the cingulate cortex, VTA, and midbrain [48, 91, 92]]. In vivo, withdrawal from alcohol has been associated with higher cerebrospinal fluid (CSF) levels of MCP-1 in alcoholics relative to healthy controls [93]. Stimulation of macrophages and mononuclear cells isolated from human subjects with AUD results in augmented proinflammatory cytokine production compared to cells from healthy controls [94, 95]. Peripheral (plasma/serum) cytokines reported as elevated in AUD include IL-1β [96], IL6 [97, 98], IP-10, and MCP-1 [99–106]]. Higher than control levels of TNFα have frequently been reported [107, 108] but see [109] and associated with AUD severity [97, 110, 111] and alcohol craving at early abstinence [98].
The considerable comorbidity of HIV infection and alcoholism [112–118] negatively impacts multiple biological systems, but particularly affects the progression of liver disease [119–122], which has emerged as a major cause of morbidity and mortality among HIV-infected patients [123]. In rodent models, EtOH exposure to HIV-infected animals resulted in greater elevations in MIP-2 [124] or MCP-1 [125] than HIV infection alone. In macaque models, muscle TNFα mRNA expression was markedly increased above baseline levels at 10 months post-infection in simian immunodeficiency (SIV) + EtOH-exposed animals [126]; IFNα levels were higher in the spleen of EtOH-exposed relative to vehicle exposed SIV-infected monkeys [127]. In humans, peripheral IL-6 levels were high in HIV-infected patients with alcohol problems [128, 129].
To evaluate whether peripheral cytokines are elevated in AUD relative to the HIV phenotype, this study compared 4 groups of human participants: those with AUD or HIV, those with HIV+AUD, and those without either condition (i.e., healthy controls). Based on the extant literature, we hypothesized that 1) HIV infection would be associated with elevated levels of IP-10 and TNFα; 2) an AUD diagnosis would be associated with elevated levels of TNFα; and 3) comorbidity for HIV+AUD would be associated with synergistic effects on elevating TNFα levels. Secondary analyses considered contributions to observed differences from disease-related factors, such as hematological indices of liver function.
Methods
Participants
This study was conducted in accordance with protocols approved by the Institutional Review Boards of Stanford University and SRI International. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki by the signing of consent documents in the presence of staff after staff ensured that each participant understood the information provided and appreciated the reasonably foreseeable consequences of a participating in the study. Study participants were healthy controls (26 women/28 men, 50.7±10.9 years), individuals with AUD (27 women/54 men, 51.1±8.8 years; currently sober as demonstrated by a negative Breathalyzer test given immediately following consent), those infected with HIV (16 women/28 men, 55.8±7.3 years), and those comorbid for HIV and AUD (16 women/28 men, 55.4±6.3 years).
AUD participants were recruited from local substance abuse treatment programs. HIV patients were referred from local outpatient or treatment centers, or recruited during presentations by project staff and by distribution of flyers at community events. Comparison participants were recruited from the local community by referrals and flyers. All participants were then screened using the Structured Clinical Interview for DSM-IV (SCID) [130], structured health questionnaires, and a semi-structured timeline follow-back interview to quantify lifetime alcohol consumption [131, 132]. Upon initial assessment, subjects were excluded if they had a significant history of medical (e.g., epilepsy, stroke, multiple sclerosis, uncontrolled diabetes, or loss of consciousness > 30 minutes), psychiatric (i.e., schizophrenia or bipolar I disorder), or neurological disorders (e.g., neurodegenerative disease) other than alcohol abuse or dependence in the AUD group. Other exclusionary criteria were recent (i.e., past 3 months) substance dependence other than alcohol in the AUD group or any DSM-IV Axis I disorder in the control group. Severity of depressive symptoms was assessed with the Beck Depression Inventory-II [133] in all groups.
Table 2presents demographic data for each of the 4 groups. The control and AUD groups were younger than the HIV and HIV+AUD groups (p = .0019). The 3 patient groups relative to the control group were less educated, had poorer socio-economic status (SES) [134] and global functioning (i.e., GAF) [135], scored lower on the Wechsler Test of Adult Reading (WTAR) [136] and the Dementia Rating Scale (DRS) [137], and had more depressive symptoms (as determined by the BDI-II) (all p≤.0001). The Veterans Aging Cohort Study (VACS) index, which predicts all-cause mortality, cause-specific mortality, and other outcomes in those living with HIV infection [138] was higher in the 2 HIV groups (HIV and HIV+AUD) than the control and AUD groups; the Karnofsky score, a standard to measure patients ability to perform ordinary tasks [139] was low in the HIV+AUD group relative to the 3 comparison groups.
Table 2. Demographic characteristics of the 4 study groups: Mean ± SD / frequency count.
| Control (n = 54) | AUD (n = 81) | HIV (n = 44) | HIV + AUD (n = 44) | p-value* | |
|---|---|---|---|---|---|
| N (men/women) | 28/26 | 54/27 | 28/16 | 28/16 | 0.4684 |
| Age (years) | 50.7±10.9 | 51.1±8.8 | 55.8±7.3 | 55.4±6.3 | 0.0019 |
| Education (years) | 16.1±2.4 | 12.9±2.4 | 13.8±2.3 | 13.1±2.1 | < .0001 |
| Handedness (Right/Left/Ambidexterous) | 48/3/3 | 70/9/2 | 40/3/1 | 39/5/0 | 0.4885 |
| Body Mass Index | 26.9±5.0 | 28.2±5.0 | 26.2±4.8 | 27.1±4.5 | 0.2075 |
| Socioeconomic Statusa | 25.8±11.7 | 45.5±15.0 | 38.6±14.7 | 44.0±12.9 | < .0001 |
| WTAR IQ | 110.6±14.6 | 94.0±18.6 | 94.9±17.8 | 87.0±17.5 | < .0001 |
| Dementia Rating Scale | 139.5±3.2 | 134.8±5.6 | 137.4±4.3 | 134.5±4.4 | < .0001 |
| Global Assessment of Functioning | 84.9±7.0 | 70.6±11.2 | 73.9±10.6 | 68.7±10.5 | < .0001 |
| AUD onset age | - | 24.9±9.1 | - | 23.9±10.4 | 0.5640 |
| Lifetime Alcohol Consumption | 32.6±40.1 | 1424.2±1079.8 | 72.3±73.9 | 1147.6±1023.5 | < .0001 |
| Days since last Drink | 44.1±117.1 | 96.1±96.3 | 78.6±141.2 | 75.3±161.3 | 0.2229 |
| AUDIT scoresb | 2.4±2.5 | 16.4±11.2 | 2.2±2.5 | 9.9±10.3 | < .0001 |
| History of ER Detoxificationsc | - | 13/68 | - | 4/40 | 0.225 |
| Withdrawal Scoresd | - | 3.4±2.6 | - | 1.9±2.4 | 0.0015 |
| Beck Depression Inventory-II | 1.5±2.1 | 9.5±8.6 | 8.7±7.3 | 10.9±8.5 | < .0001 |
| Karnofsky score | 100.0±0 | 99.7±2.4 | 99.8±1.5 | 98.5±4.2 | 0.0366 |
| VACS Index | 14.56±10.84 | 17.67±12.53 | 33.44±17.49 | 29.24±14.40 | < .0001 |
| HIV onset age (years) | - | - | 35.9±10.0 | 33.6±7.3 | 0.2256 |
| HIV duration (days) | - | - | 7336.3±2785.1 | 8034.8±2421.0 | 0.2211 |
| CD4 cell count (100/mm3) | - | - | 669.9±265.3 | 675.7±335.4 | 0.2235 |
| CD4 cell count nadir (100/mm3) | - | - | 240.2±194.8 | 199.9±184.1 | 0.3867 |
| Viral Load (log copies/mL) | - | - | 1.7±0.9 | 1.9±1.1 | 0.3254 |
| AIDS-defining event (yes/no)e | - | - | 16/28 | 26/18 | < .0001 |
| HAART (yes/no) | - | - | 40/4 | 40/4 | 0.9449 |
| Efavirinz, including Atripla (yes/no) | - | - | 9/35 | 10/34 | 0.7956 |
| Hepatitis C Virus (positive/negative) | - | 16/65 | 13/31 | 21/23 | < .0001 |
| Treatment for HCV infectionf | - | 4/16 | 4/13 | 5/21 | 0.8984 |
| Smoker (never/past/current) | 51/1/2 | 16/23/42 | 25/7/12 | 13/10/21 | < .0001 |
| Self-Defined Ethnicity (Caucasian/AA)g | 44/10 | 40/41 | 26/18 | 14/30 | < .0001 |
*4-group comparisons: ANOVA used on continuous variables (e.g., age); χ2 used on nominal variables (e.g., handedness)
alower score = higher status
bAUDIT = Alcohol Use Disorders Identification Test
cSelf report of visit to emergency room for alcohol-related problems.
dSum of 8 possible withdrawal signs (autonomic signs, tremor, insomnia, nausea, agitation, anxiety, seizures, hallucinations)
eincluding AIDS-defining illness or CD4 prior nadir <200cells/μl
fSelf report of HCV treatment
gAA = African American
Sample collection and processing
Whole blood samples (n = 294), collected in lavender EDTA tubes between March 2013 and October 2016, were centrifuged (500 rcf at room temperature for 10min). Plasma was transferred to 1.5mL conical tubes, centrifuged at 13,000 rcf at room temperature for another 10min, and the resulting supernatant was transferred to 1.5mL conical tubes for storage at −80° C until analysis by the Human Immune Monitoring Center. Additional blood samples were collected and analyzed by Quest Diagnostics for complete blood count with differential, comprehensive metabolic panel, HIV and hepatitis C (HCV) screening, and RNA quantification when relevant (i.e., for HIV or HCV seropositive results). Quest laboratory results were missing for 11 control, 3 AUD, 1 HIV, and 3 HIV+AUD participants.
Immunological assays
The Human Immune Monitoring Center (http://iti.stanford.edu/himc/), which continually benchmarks processes to minimize technical variability (Maecker et al., 2005), performed immunological assays. Human 41-plex kits (HCYTOMAG-60K, 7 kits, each able to run 42 samples) were purchased from EMD Millipore and used according to the manufacturer’s recommendations with modifications as described. Briefly, samples were mixed with antibody-linked magnetic beads on a 96-well plate and incubated overnight incubation at 4°C with shaking. Cold and room temperature incubation steps were performed on an orbital shaker at 500–600 rpm. Plates were washed twice with wash buffer in a Biotek ELx405 washer. Following one hour incubation at room temperature with biotinylated detection antibody, streptavidin fluorochrome (i.e., streptavidin-PE) was added for 30 minutes with shaking. Plates were washed as above and PBS added to wells for reading in the Luminex 200 Instrument with a lower bound of 50–100 beads per sample per cytokine. Each sample was measured in duplicate. Custom assay control beads by Radix Biosolutions were added to all wells.
The 41 cytokines included in each kit belong to 4 families: hematopoietin (interleukin (IL)-1α, IL-1β, IL-1RA, IL2, IL3, IL4, IL5, IL6, IL7, IL9, IL10, IL12-p40, IL12-p70, IL13, IL15, IL17, soluble CD40 ligand (CD40L), Fms-related tyrosine kinase 3 ligand (Flt3 ligand), granulocyte colony-stimulating factor (GCSF), granulocyte macrophage CSF (GMCSF)), chemokines (epidermal growth factor (EGF), eotaxin (CCL11), fibroblast growth factor (FGF)-2, fractalkine, RANTES (regulated on activation, normal T cell expressed and secreted/CCL5), growth regulated oncogene (GRO/CXCl1), IL8, Interferon-γ-induced protein 10 (IP-10/CXCL10), monocyte chemoattractant protein 1 (MCP-1/CCL2), MCP-3 (CCL7), macrophage-derived chemokine (MDC/CCL22), macrophage inflammatory protein (MIP)-1α, MIP-1β, transforming growth factor (TGF)-α, vascular endothelial growth factor (VEGF)), growth factors (platelet-derived growth factor (PDGF)-AA, PDGF-BB, Tumor Necrosis Factor α (TNF-α), TNF-β), and interferons (IFN-α2, IFN-γ).
Liver status Assessments
We used standard laboratory results from Quest blood assays to calculate 2 noninvasive indices of liver fibrosis. The Fibrosis index (FIB-4: based on age, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet count) [140] and the AST/platelet count ratio (APRI) score both have high predictive accuracy for diagnoses of HCV e.g., [141, 142].
Statistical analysis
Of 294 samples, 6 individuals (1 AUD man, 2 HIV men, 1 HIV women, 1 HIV+AUD man, 1 HIV+AUD women) were excluded (e.g., low IQ, abnormal brain scan, diseases such as epilepsy or Progressive Multifocal Leukoencephalopathy). Longitudinal follow-up samples from individual subjects were also removed, yielding a total of 223 unique, single-visit samples (control n = 54, AUD n = 81, HIV n = 44, HIV+AUD n = 44). Based on a previous publication evaluating cytokine levels in AUD patients [98], and G*Power 3.1, we calculated an effect size of 3.8. Using this effect size with an alpha error probability of 0.5 and our control (n = 54) + AUD (n = 81) sample sizes, the current study was found to have a power of 1.
Based on the recommendation of the HIMC, the average of 2 readings for mean fluorescence intensity (MFI) for each analyte was used because these values have less variance than pg/mL measures (presented in S1 Table). In addition, corrected (studentized-residual) MFI values, based on results of an Akaike information criterion (AIC) model including kit number (nominal: 1–7), age (continuous), sex (nominal: M/F), socio-economic status (SES, continuous), and ethnicity (nominal: White/Black) were considered (S2 Table).
Diagnoses effects were evaluated using analysis of variance (ANOVA). Two-group comparisons used t-tests. Correlations were evaluated using Spearman’s ρ. Multiple regressions were used when relevant.
Results
4-group differences in cytokine levels
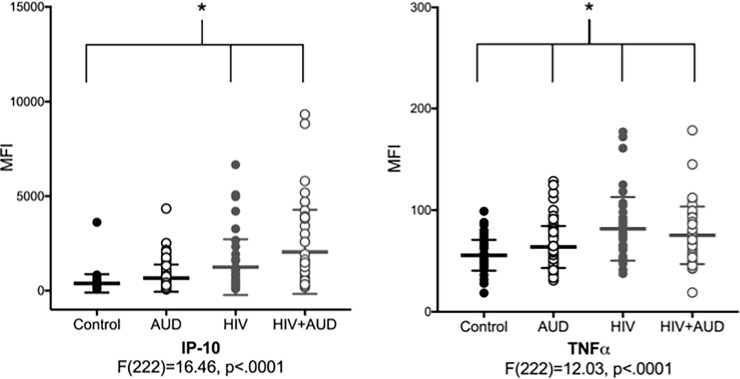
Results of separate 4-group ANOVAs for each of the 41 analytes are presented in Table 3. Post-hoc tests indicated that the most common results were lower levels of cytokines (i.e., IL-1α, IL-1β, IL2, IL3, IL9, IL12P40, and IL13) in the HIV and HIV+AUD groups relative to the control group. Cytokines that were higher in the 2 HIV groups (i.e., HIV and HIV+AUD) relative to the control group included IP-10 and MCP-1. TNFα was high in the 3 patient groups relative to the control group (Fig 1). IP-10 and TNFα results were similar when studentized-residual values were considered (S2 Table).
Table 3. Cytokine levels* in the 4 study groups: Mean ± SD and ANOVA results.
| cytokine | Control (n = 54) | AUD (n = 81) | HIV (n = 44) | HIV + AUD (n = 44) | ANOVA | |
|---|---|---|---|---|---|---|
| F Ratio | p value | |||||
| CD40L | 68.85±51.01 | 139.95±267.02 | 204.74±923.29 | 62.30±44.57 | 1.09 | 0.35 |
| EGF | 28.56±30.31 | 25.64±23.01 | 76.67±320.78 | 17.09±8.44 | 1.61 | 0.19 |
| EOTAXIN | 81.38±55.35 | 92.74±79.23 | 127.24±101.47 | 103.28±81.39 | 2.93 | 0.03 |
| FGFB | 23.87±24.36 | 21.61±11.25 | 19.11±15.45 | 18.18±19.99 | 1.04 | 0.38 |
| FLT3L | 32.34±47.42 | 29.66±16.39 | 47.22±105.38 | 26.79±13.04 | 1.34 | 0.26 |
| Fractaline | 17.73±10.84 | 21.06±16.90 | 18.37±13.37 | 15.44±5.68 | 1.87 | 0.14 |
| GCSF | 32.36±21.34 | 26.46±10.43 | 24.62±10.85 | 28.58±22.05 | 2.15 | 0.09 |
| GMCSF | 26.44±10.65 | 37.19±100.92 | 22.31±5.71 | 23.81±9.84 | 0.79 | 0.50 |
| GRO | 336.12±464.17 | 664.88±873.39 | 423.09±708.46 | 415.28±530.30 | 2.89 | 0.04 |
| IFNA2 | 20.17±12.31 | 26.06±36.94 | 18.66±8.02 | 18.69±6.45 | 1.48 | 0.22 |
| IFNG | 79.88±75.19 | 42.45±37.29 | 49.30±78.31 | 45.07±51.70 | 4.75 | 0.003 |
| IL10 | 36.72±38.59 | 30.58±17.56 | 28.84±25.20 | 25.60±7.89 | 1.78 | 0.15 |
| IL12P40 | 30.46±26.63 | 27.12±15.56 | 21.90±7.35 | 20.24±6.36 | 3.98 | 0.009 |
| IL12P70 | 22.63±19.55 | 21.65±14.96 | 16.90±7.65 | 17.98±18.19 | 1.58 | 0.20 |
| IL13 | 29.54±47.57 | 21.34±25.20 | 15.82±9.78 | 14.42±5.22 | 2.92 | 0.04 |
| IL15 | 32.78±24.27 | 32.06±16.33 | 27.15±7.48 | 27.48±13.31 | 1.60 | 0.19 |
| IL17 | 61.01±88.69 | 39.74±39.58 | 42.72±69.16 | 30.39±27.26 | 2.37 | 0.07 |
| IL1A | 32.25±25.17 | 28.14±13.64 | 24.22±6.97 | 23.10±5.71 | 3.62 | 0.01 |
| IL1B | 24.13±22.38 | 22.29±13.74 | 16.20±5.52 | 15.22±4.50 | 4.96 | 0.002 |
| IL1RA | 27.25±18.66 | 27.10±26.55 | 31.43±48.26 | 25.05±15.42 | 0.38 | 0.77 |
| IL2 | 25.08±23.45 | 21.77±14.57 | 17.02±7.05 | 16.68±7.58 | 3.51 | 0.02 |
| IL3 | 24.25±15.13 | 22.02±11.52 | 19.05±4.46 | 19.43±3.79 | 2.66 | 0.05 |
| IL4 | 37.12±33.04 | 29.38±15.09 | 26.43±12.61 | 26.24±14.71 | 3.13 | 0.03 |
| IL5 | 20.78±34.52 | 16.04±12.18 | 18.74±35.47 | 13.25±6.94 | 0.89 | 0.45 |
| IL6 | 36.29±36.56 | 26.90±18.42 | 30.22±40.19 | 22.64±21.15 | 1.98 | 0.12 |
| IL7 | 23.69±14.89 | 23.03±11.32 | 20.03±7.59 | 20.86±11.64 | 1.11 | 0.34 |
| IL8 | 150.14±146.85 | 124.39±100.51 | 146.60±141.51 | 147.70±137.15 | 0.60 | 0.62 |
| IL9 | 28.88±26.92 | 26.03±21.88 | 18.52±5.90 | 17.75±5.32 | 4.25 | 0.006 |
| IP10 | 386.13±486.47 | 665.69±719.76 | 1250.73±1478.36 | 2057.29±2224.48 | 16.46 | < .0001 |
| MCP1 | 774.71±545.61 | 909.48±468.12 | 1112.89±876.39 | 1153.47±747.67 | 1.00 | 0.01 |
| MCP3 | 37.76±65.63 | 25.46±45.38 | 17.65±13.32 | 19.57±23.56 | 2.12 | 0.10 |
| MDC | 776.05±414.41 | 861.33±452.91 | 835.62±467.82 | 900.73±471.60 | 0.69 | 0.56 |
| MIP1A | 61.01±47.40 | 53.42±112.77 | 47.52±29.03 | 259.68±1243.43 | 1.61 | 0.19 |
| MIP1B | 52.44±62.30 | 41.26±16.26 | 41.08±33.53 | 90.75±346.86 | 1.07 | 0.36 |
| PDGFAA | 2727.60±1991.09 | 3826.70±3065.67 | 3029.29±3467.43 | 2861.59±1902.72 | 2.24 | 0.08 |
| PDGFBB | 414.39±533.71 | 636.60±637.51 | 594.13±1598.30 | 447.69±414.45 | 0.93 | 0.42 |
| RANTES | 8942.89±4267.98 | 8775.41±4176.13 | 9362.59±3455.16 | 9652.91±3444.59 | 0.57 | 0.64 |
| TGFA | 22.27±19.39 | 26.54±23.24 | 41.36±137.76 | 25.21±42.41 | 0.77 | 0.51 |
| TNFA | 55.66±15.13 | 63.78±20.58 | 81.68±31.27 | 75.26±28.28 | 12.03 | < .0001 |
| TNFB | 32.90±46.55 | 26.25±33.23 | 29.02±76.68 | 17.93±11.88 | 0.90 | 0.44 |
| VEGF | 29.74±32.42 | 26.19±16.79 | 32.13±78.11 | 22.09±28.51 | 0.51 | 0.67 |
*average of 2 mean fluorescence intensity (MFI) values per analyte
Fig 1.
Scatter plots of the a) the chemokine Interferon γ-induced Protein 10 (IP-10) and the b) cytokine Tumor Necrosis Factor α (TNFα) in the 4 groups (Control: black closed circles; AUD: black open circles; HIV: gray closed circles; HIV+AUD: gray open circles). * indicates significance at p = .001.
2-group differences in cytokine levels
For direct evaluation of single diagnoses effects on peripheral cytokine levels, additional statistics used t-tests to compare control and individual patient groups (Table 4). Table 4 also includes remaining comparisons (e.g., AUD vs. HIV; AUD vs. HIV+AUD; HIV vs. HIV+AUD). An AUD diagnosis, relative to healthy controls, was associated with higher levels of CD40L, GRO, PDGFAA, PDFGBB, IP-10, and TNFα and lower levels of IFN-γ and MIP-1α. This pattern of cytokines associated with an AUD diagnosis was significantly different from that presenting in HIV infection. In HIV relative to healthy controls, EGF, MCP-1, IP-10, and TNFα levels were high and GCSF, GMCSF, IL-1α, IL-1β, IL2, IL3, IL4, IL9, IL12p40, IL13, MCP-3, and TNFβ were low. The results relative to controls in the comorbid HIV+AUD group were very similar to those in the HIV only group: MCP-1, IP-10, and TNFα levels were high and EGF, FGFB, IFN-y, IL-1α, IL-1β, IL2, IL3, IL4, IL6, IL9, IL10, IL12p40, IL13, IL17, and TNFβ were low. In comparing HIV relative to HIV+AUD, only IP-10 was significantly different between groups, and was higher in HIV+AUD relative to HIV only. The only cytokines that were affected in all 3 (individual) patient groups relative to controls were IP-10 and TNFα. Results of 2-group comparisons were circumscribed when studentized-residual values were evaluated: relative to healthy controls, only TNFα levels were high in AUD and only IP-10 and TNFα levels were high in HIV or HIV+AUD (S3 Table).
Table 4. Two-group t-test* comparisons of cytokine levels.
| cytokine | Con. vs. AUD | Con. vs. HIV | Con. vs. HIV+AUD | AUD vs. HIV | AUD vs. HIV+AUD | HIV vs. HIV+AUD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t Ratio | p value | t Ratio | p value | t Ratio | p value | t Ratio | p value | t Ratio | p value | t Ratio | p value | |
| CD40L | 2.33 | 0.02 | n.s. | n.s. | n.s. | -2.55 | 0.01 | n.s. | ||||
| EGF | n.s. | 2.69 | 0.009 | -2.66 | 0.01 | n.s. | -2.99 | 0.003 | n.s. | |||
| EOTAXIN | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| FGFB | n.s. | n.s. | -2.25 | 0.03 | n.s. | n.s. | n.s. | |||||
| FLT3L | n.s. | n.s. | n.s | n.s. | n.s. | n.s. | ||||||
| Fractaline | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| GCSF | n.s. | -2.32 | 0.02 | n.s. | n.s. | n.s. | n.s. | |||||
| GMCSF | n.s. | -2.45 | 0.02 | n.s. | n.s. | n.s. | n.s. | |||||
| GRO | 2.84 | 0.005 | n.s. | n.s. | n.s. | -1.99 | 0.05 | n.s. | ||||
| IFNA2 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| IFNG | -3.39 | 0.001 | n.s. | -2.71 | 0.008 | n.s. | n.s. | n.s. | ||||
| IL1A | n.s. | -2.24 | 0.03 | -2.59 | 0.01 | -2.13 | 0.04 | -2.89 | 0.005 | n.s. | ||
| IL1B | n.s. | -2.51 | 0.01 | -2.86 | 0.006 | -3.50 | 0.0007 | -4.23 | < .0001 | n.s. | ||
| IL1RA | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| IL2 | n.s. | -2.40 | 0.02 | -2.48 | 0.02 | -2.45 | 0.02 | -2.57 | 0.01 | n.s. | ||
| IL3 | n.s. | -2.40 | 0.02 | -2.25 | 0.03 | -2.06 | 0.04 | n.s. | n.s. | |||
| IL4 | n.s. | -2.19 | 0.03 | -2.17 | 0.03 | n.s. | n.s. | n.s. | ||||
| IL5 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| IL6 | n.s. | n.s. | -2.31 | 0.02 | n.s. | n.s. | n.s. | |||||
| IL7 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| IL8 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| IL9 | n.s. | -2.75 | 0.008 | -2.97 | 0.004 | -2.90 | 0.005 | -3.23 | 0.002 | n.s. | ||
| IL10 | n.s. | n.s. | -2.07 | 0.04 | n.s | -2.18 | 0.03 | n.s. | ||||
| IL12P40 | n.s. | -2.26 | 0.03 | -2.73 | 0.008 | -2.54 | 0.01 | -3.48 | 0.0007 | n.s. | ||
| IL12P70 | n.s. | n.s. | n.s | -2.35 | 0.02 | n.s. | n.s. | |||||
| IL13 | n.s. | -2.07 | 0.04 | -2.32 | 0.02 | n.s | -2.38 | 0.02 | n.s. | |||
| IL15 | n.s. | n.s. | n.s | -2.23 | 0.02 | n.s. | n.s. | |||||
| IL17 | n.s. | n.s. | -2.40 | 0.02 | n.s | n.s. | n.s. | |||||
| IP10 | 2.69 | 0.008 | 3.72 | 0.0005 | 4.89 | < .0001 | 2.47 | 0.02 | 4.04 | 0.0002 | 2.00 | 0.05 |
| MCP1 | n.s. | 2.23 | 0.03 | 2.81 | 0.006 | n.s. | n.s. | n.s. | ||||
| MCP3 | n.s. | -2.20 | 0.03 | n.s. | n.s. | n.s. | n.s. | |||||
| MDC | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| MIP1A | -2.90 | 0.005 | n.s. | n.s. | n.s. | n.s. | n.s. | |||||
| MIP1B | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| PDGFAA | 2.53 | 0.01 | n.s. | n.s. | n.s. | -2.17 | 0.03 | n.s. | ||||
| PDGFBB | 2.19 | 0.03 | n.s. | n.s. | n.s. | -2.00 | 0.05 | n.s. | ||||
| RANTES | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| TGFA | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| TNFA | 2.64 | 0.009 | 5.06 | < .0001 | 4.14 | < .0001 | 3.42 | 0.0006 | 2.37 | 0.02 | n.s. | |
| TNFB | n.s. | -2.40 | 0.02 | -2.27 | 0.03 | n.s. | -2.03 | 0.05 | n.s. | |||
| VEGF | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
*directionality of change goes with second group listed in each comparison (e.g., CD40L is elevated in the AUD relative to the Control group)
Cytokine correlations
The functional significance of changes to peripheral cytokine levels was evaluated by exploring relationships with other blood markers; AUD-related variables (e.g., AUD onset age, lifetime alcohol consumption, days since last drink, scores on the AUD Identification Test [AUDIT], history of emergency room detoxifications/treatments, withdrawal scores); HIV-related variables (e.g., Karnofsky score, VACs Index, HIV onset age, HIV duration, CD4 cell count, CD4 cell count nadir, viral, AIDS-defining events); and general demographic variables such as body mass index (BMI) and smoking status.
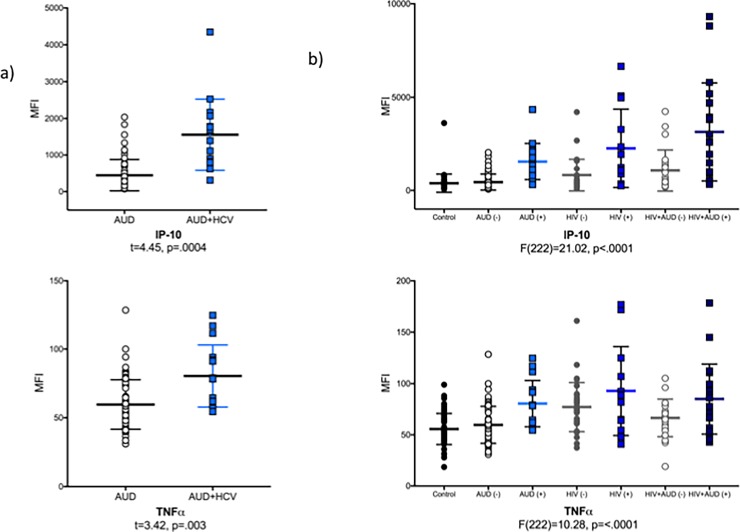
In the AUD group only, IP-10 (p = .0004) and TNFα (p = .003) levels were higher in AUD HCV-seropositive relative to AUD HCV-seronegative participants (Fig 2A). In addition, IP-10 levels correlated with depressive symptoms (i.e., total BDI-II score: ρ = .26, p = .03), alkaline phosphatase (AP: ρ = .28, p = .01), AST (ρ = .54, p < .0001), ALT (ρ = .42, p = .0002), and gamma-glutamyltransferase (GGT: ρ = .45, p < .0001). Similarly, TNFα levels in the AUD group only correlated with AP (ρ = .23, p = .04), AST (ρ = .31, p = .0006), ALT (ρ = .32, p = .005), and GGT (ρ = .35, p = .002). Of all the relationships evaluated between remaining cytokines affected by an AUD diagnosis and other blood markers, AUD-related variables, or general demographic variables, the only other significant correlation was between higher withdrawal scores and lower levels of MIP-1α (ρ = -.28, p = .01).
Fig 2.
Scatter plots of Interferon γ-induced Protein 10 (IP-10) and Tumor Necrosis Factor α (TNFα) in a) the AUD group by HCV status (i.e., AUD without HCV: open black circles; AUD+HCV: blue squares) and b) all 4 study groups by HCV status (Control: black closed circles; AUD (-): AUD without HCV, black open circles; AUD (+): AUD +HCV, blue squares; HIV (-): HIV without HCV, gray closed circles; HIV (+): HIV+HCV: dark blue squares; HIV+AUD (-): HIV+AUD without HCV, gray open circles; HIV+AUD (+): HIV+AUD+HCV, midnight squares).
In the HIV group only, IP-10 (p = .03) levels were also higher in HIV+HCV co-infected relative to mono-infected HIV seropositive individuals; MCP-1 (ρ = .46, p = .003), IP-10 (ρ = .42, p = .008), and TNFα (ρ = .42, p = .008) levels positively correlated with the VACS index; and GCSF levels were lower with longer HIV duration (ρ = -.31, p = .04). No other relationships emerged in the HIV group between affected cytokines and relevant variables.
In the HIV+AUD group, IP-10 (p = .002) and TNFα (p = .04) levels were higher in the HIV+AUD group with HCV relative to the group without HCV. Furthermore, in the HIV+AUD group alone, lower Karnofsky scores were associated with lower levels of IFN- γ and higher levels of IP-10 and TNFα. No other relationships emerged in the HIV+AUD group between affected cytokines and relevant variables.
An AIC to predict IP-10 levels across the 3 patient groups including all associated variables (i.e., HCV status, BDI score, AP, AST, ALT, GGT, VACS index, and Karnofsky score) highlighted GGT levels, Karnofsky score, VACS index, and HCV status. A multiple regression including these 4 variables was significant (F(143) = 19.52, p < .0001), explained 36% of the variance in IP-10 levels, and was driven by the HCV status (p < .0001). Indeed, HCV status alone explained 26% of the variance in IP-10 levels. For TNFα, a similar AIC (excluding BDI scores) highlighted AST levels, VACS index, and HCV status. A multiple regression including these 3 variables was significant (F(148) = 18.10, p < .0001), explained 27% of the variance in TNFα levels, and was driven by the VACS index (p < .0001).
Relevance of HCV infection
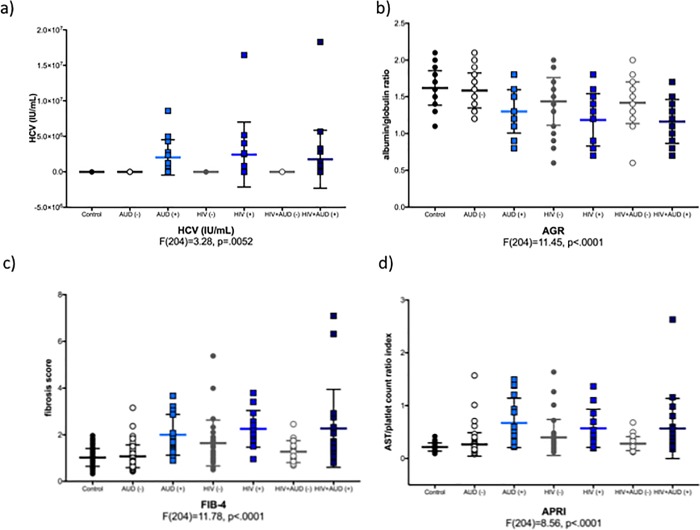
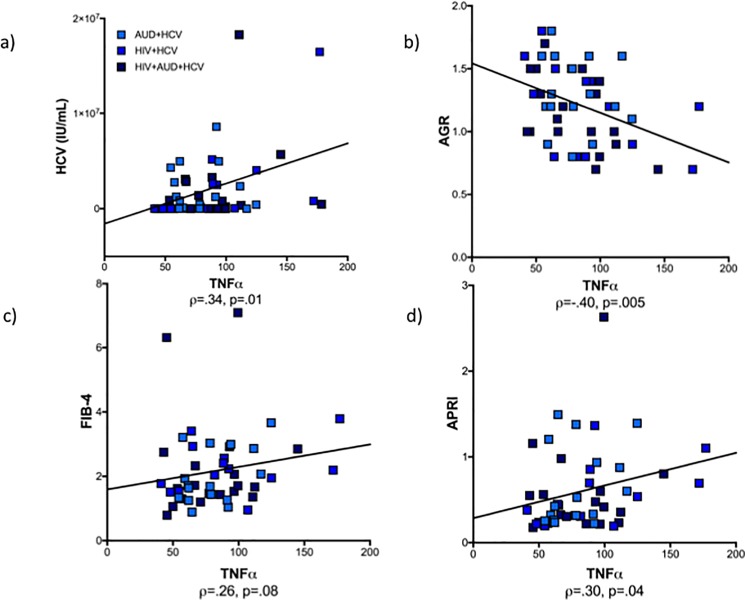
To pursue the potential effect of HCV on group differences, the initial 4 groups were subdivided by HCV status into 7 groups (control, and each of the 3 patient groups (AUD, HIV, HIV+AUD) with and without HCV). The patient subgroups infected with HCV had elevated IP-10 (F(222) = 21.02, p < .0001) and TNFα (F(222) = 10.28, p = < .0001) levels (Fig 2B). Thus, HCV-related measures were also evaluated for their effects on IP-10 and TNFα. Fig 3A demonstrates the presence of HCV viral load (International Units/mL) in patient subgroups with HCV. Additional 7-group ANOVAs demonstrated that the albumin/globulin ratio (AGR: F(204) = 11.45, p < .0001; Fig 3B) was low and FIB-4 (F(204) = 11.78, p < .0001; Fig 3C) and APRI (F(204) = 8.56, p < .0001; Fig 3D) scores were high in the HCV-infected subgroups. These indices of liver compromise (HCV viral load ρ = .34, p = .01; AGR ρ = -.40, p = .005; FIB-4 ρ = .26, p = .08; APRI ρ = .30, p = .04) correlated with TNFα levels in the HCV-seropositive patient subgroups (Fig 4). Correlations were similar for IP-10 (HCV viral load ρ = .43, p = .002; AGR ρ = -.26, p = .08; FIB-4 ρ = .21, p = .16; APRI ρ = .26, p = .08). Levels of IP-10 and TNFα were not related to self-report of treatment for HCV.
Fig 3.
Scatter plots of a) HCV viral load, b) albumin/globulin ratio (AGR), c) fibrosis score (FIB-4), and d) AST/platelet count ratio index (APRI) in the 4 study groups by HCV status (see legend to Fig 2 for details).
Fig 4.
Correlations in the HCV-seropositive patient subgroups between TNFα levels and a) HCV viral load, b) albumin/globulin ratio (AGR), c) fibrosis score (FIB-4), and d) AST/platelet count ratio index (APRI).
Discussion
The hypothesized role of the innate and adaptive immune systems in mood, psychiatric, and neurodegenerative disorders has gained significant support in the literature e.g., [143–145]. The aim of the current study was to determine whether uncomplicated alcoholism, that is, AUD in the absence of diagnosable medical concomitants, is associated with peripheral cytokine levels, in the context of similarly measured analytes in HIV, a disorder with a clearly demonstrated inflammatory component. Our results show that elevations in peripheral cytokines are associated not with an AUD diagnosis, but were associated with co-occurring HCV infection in abstinent drinkers.
A number of additional findings support the necessity of HCV infection to increase proinflammatory cytokine levels in AUD and HAART-controlled HIV subjects. When the HIV groups were similarly sub-categorized based on HCV status, the subgroups co-infected with HCV showed marked elevations in IP-10 and TNFα. Furthermore, across the HCV-infected individuals from the 3 patient groups, HCV viral load correlated with IP-10 and TNFα levels.
To provide further evidence that liver status affects cytokine levels in this population, we found that the albumin/globulin ratio (AGR) discriminated individuals with HCV relative to those without HCV. This comports with the literature demonstrating that low serum albumin levels can be used to predict HCV infection [146] and that albumin levels may be an important mortality risk factor for those co-infected with HIV and HCV [147]. In the HCV-infected patient subgroups included in this study, lower AGR correlated with higher IP-10 and TNFα levels.
We additionally calculated two descriptive, noninvasive indices of liver fibrosis [140]. FIB-4 scores (<1.45 absent; 1.45–3.25 intermediate fibrosis; >3.25 advanced fibrosis) have been used to predict and stage liver fibrosis in HCV and other forms of liver disease [128, 141, 142]. Our HCV patient subgroups had FIB-4 scores ranging from 2.00–2.27, indicating the presence of intermediate stage liver fibrosis. FIB-4 scores correlated weakly with IP-10 and TNFα levels in the subgroups with HCV infection.
In a meta-analysis of 40 studies, investigators concluded that an AST/platelet count ratio index (APRI) score greater than 1.0 had a sensitivity of 76% and specificity of 72% for predicting cirrhosis [148]: low APRI scores (<0.5) have negative predictive value to rule out cirrhosis; high APRI scores (> 1.5) have positive predictive value to diagnose cirrhosis. The APRI estimate has been used as alternative to frequent liver biopsies in HCV to detect and stage fibrosis e.g., [149–152]. The HCV subgroups included in this study had midrange APRI scores (0.56–0.67) and thus, cirrhosis cannot be ruled out. APRI scores also correlated with IP-10 and TNFα levels in subgroups with HCV infection.
The current finding of elevated TNFα in AUD + HCV is consistent with reports of hospitalized alcoholics showing correlations between high TNFα levels and liver dysfunction [89, 101, 107]. Alcoholic hepatitis is known to be associated with upregulation of serum cytokines [153, 154] and alcohol-related liver cirrhosis has been specifically associated with high TNFα levels [155], which have been used to predict mortality in alcoholic liver disease [156]. Our study contrasts with those reporting effects of “uncomplicated” AUD on increasing proinflammatory cytokine levels in notable ways: in the previously published studies, AUD subjects were currently actively drinking or hospitalized for drinking at the time of blood draw; and liver integrity, including presence of HCV, was not described e.g., [96–99, 108].
Our findings also comport with the HIV+HCV literature that has demonstrated a particular sensitivity of IP-10 levels to co-infection [157–159] and relationships between IP-10 levels and biomarkers of liver disease [160–162]. As has previously been suggested, however, alcoholism does not appear to have an effect on cytokine responses in HIV+HCV comorbidity [163].
A limitation of the current study was the absence of a non-AUD, HCV seropositive control group. It is our intention to include this comparison group in future studies. Further absent is a comparison group of recently detoxified alcoholics, who might be more likely to exhibit abnormal levels of cytokines cf., [96–99, 108].
In conclusion, this study reports elevations in TNFα in AUD individuals abstinent at examination that occurred only in the presence of HCV infection and suggests that changes in TNFα levels in AUD are dependent on derangement of liver function and not on alcohol-related variables. This finding encourages a careful characterization of alcoholics in human studies, including documentation of comorbid infections that can affect peripherally circulating levels of cytokines and chemokines.
Supporting information
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors would like to thank the staff at the Human Immune Monitoring Center, Stanford University. In particular, we would like to thank Yael Rosenberg-Hasson for her assistance in setting up and performing the assays.
Data Availability
The data have been publicly deposited at Dryad (http://datadryad.org/review?doi=doi:10.5061/dryad.rj61p).
Funding Statement
This study was supported with grant funding from the National Institute of Alcohol Abuse and Alcoholism (NIAAA) including U01 AA017347, U01 AA013521, K05 AA017168, R01 AA005965, R37 AA010723. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lahdevirta J, Maury CP, Teppo AM, Repo H. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1988;85(3):289–91. Epub 1988/09/01. . [DOI] [PubMed] [Google Scholar]
- 2.von Sydow M, Sonnerborg A, Gaines H, Strannegard O. Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res Hum Retroviruses. 1991;7(4):375–80. Epub 1991/04/01. doi: 10.1089/aid.1991.7.375 . [DOI] [PubMed] [Google Scholar]
- 3.Zangerle R, Gallati H, Sarcletti M, Wachter H, Fuchs D. Tumor necrosis factor alpha and soluble tumor necrosis factor receptors in individuals with human immunodeficiency virus infection. Immunol Lett. 1994;41(2–3):229–34. Epub 1994/07/01. . [DOI] [PubMed] [Google Scholar]
- 4.Keating SM, Golub ET, Nowicki M, Young M, Anastos K, Crystal H, et al. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS. 2011;25(15):1823–32. Epub 2011/05/17. doi: 10.1097/QAD.0b013e3283489d1f ; PubMed Central PMCID: PMC3314300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haase AT. The AIDS lentivirus connection. Microb Pathog. 1986;1(1):1–4. Epub 1986/02/01. 0882-4010(86)90026-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 6.Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28 Epub 2006/05/23. 1742-4690-3-28 [pii] doi: 10.1186/1742-4690-3-28 ; PubMed Central PMCID: PMC1513597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peluso R, Haase A, Stowring L, Edwards M, Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 1985;147(1):231–6. Epub 1985/11/01. . [DOI] [PubMed] [Google Scholar]
- 8.Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(18):7089–93. Epub 1986/09/01. ; PubMed Central PMCID: PMC386658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vazeux R, Brousse N, Jarry A, Henin D, Marche C, Vedrenne C, et al. AIDS subacute encephalitis. Identification of HIV-infected cells. The American journal of pathology. 1987;126(3):403–10. Epub 1987/03/01. ; PubMed Central PMCID: PMC1899654. [PMC free article] [PubMed] [Google Scholar]
- 10.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–94. Epub 2001/04/20. doi: 10.1038/35073667 35073667 [pii]. . [DOI] [PubMed] [Google Scholar]
- 11.Grovit-Ferbas K, Harris-White ME. Thinking about HIV: the intersection of virus, neuroinflammation and cognitive dysfunction. Immunol Res. 2010;48(1–3):40–58. Epub 2010/08/21. doi: 10.1007/s12026-010-8166-x . [DOI] [PubMed] [Google Scholar]
- 12.Rezai-Zadeh K, Gate D, Town T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J Neuroimmune Pharmacol. 2009;4(4):462–75. Epub 2009/08/12. doi: 10.1007/s11481-009-9166-2 ; PubMed Central PMCID: PMC2773117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS . Nature reviews. 2005;5(1):69–81. Epub 2005/01/05. nri1527 [pii] doi: 10.1038/nri1527 . [DOI] [PubMed] [Google Scholar]
- 14.Masliah E, DeTeresa RM, Mallory ME, Hansen LA. Changes in pathological findings at autopsy in AIDS cases for the last 15 years. AIDS. 2000;14(1):69–74. Epub 2000/03/14. . [DOI] [PubMed] [Google Scholar]
- 15.Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, et al. The neuropathogenesis of the AIDS dementia complex. AIDS. 1997;11 Suppl A:S35–45. Epub 1997/01/01. . [PubMed] [Google Scholar]
- 16.Cosenza MA, Zhao ML, Si Q, Lee SC. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain pathology (Zurich, Switzerland). 2002;12(4):442–55. Epub 2002/11/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer-Smith T, Croul S, Adeniyi A, Rybicka K, Morgello S, Khalili K, et al. Macrophage/microglial accumulation and proliferating cell nuclear antigen expression in the central nervous system in human immunodeficiency virus encephalopathy. The American journal of pathology. 2004;164(6):2089–99. Epub 2004/05/27. S0002-9440(10)63767-4 [pii] doi: 10.1016/S0002-9440(10)63767-4 ; PubMed Central PMCID: PMC1615769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111(2):194–213. Epub 2005/05/12. S0168-1702(05)00123-1 [pii] doi: 10.1016/j.virusres.2005.04.009 . [DOI] [PubMed] [Google Scholar]
- 19.Catalfamo M, Le Saout C, Lane HC. The role of cytokines in the pathogenesis and treatment of HIV infection. Cytokine & growth factor reviews. 2012;23(4–5):207–14. doi: 10.1016/j.cytogfr.2012.05.007 ; PubMed Central PMCID: PMC3726258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane BR, King SR, Bock PJ, Strieter RM, Coffey MJ, Markovitz DM. The C-X-C chemokine IP-10 stimulates HIV-1 replication. Virology. 2003;307(1):122–34. Epub 2003/04/02. S0042682202000454 [pii]. . [DOI] [PubMed] [Google Scholar]
- 21.Weiss JM, Nath A, Major EO, Berman JW. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J Immunol. 1999;163(5):2953–9. Epub 1999/08/24. ji_v163n5p2953 [pii]. . [PubMed] [Google Scholar]
- 22.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12 Suppl 1:878–92. Epub 2005/04/16. 4401623 [pii] doi: 10.1038/sj.cdd.4401623 . [DOI] [PubMed] [Google Scholar]
- 23.Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Annals of neurology. 1998;44(5):831–5. Epub 1998/11/18. doi: 10.1002/ana.410440521 . [DOI] [PubMed] [Google Scholar]
- 24.Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, et al. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis. 2001;184(8):1015–21. Epub 2001/09/28. JID010447 [pii] doi: 10.1086/323478 . [DOI] [PubMed] [Google Scholar]
- 25.Andersson J, Fehniger TE, Patterson BK, Pottage J, Agnoli M, Jones P, et al. Early reduction of immune activation in lymphoid tissue following highly active HIV therapy. AIDS. 1998;12(11):F123–9. Epub 1998/08/26. . [DOI] [PubMed] [Google Scholar]
- 26.Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63(11):2084–90. Epub 2004/12/15. 63/11/2084 [pii]. . [DOI] [PubMed] [Google Scholar]
- 27.Pulliam L, Herndier BG, Tang NM, McGrath MS. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. The Journal of clinical investigation. 1991;87(2):503–12. Epub 1991/02/01. doi: 10.1172/JCI115024 ; PubMed Central PMCID: PMC296337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gendelman HE, Baldwin T, Baca-Regen L, Swindells S, Loomis L, Skurkovich S. Regulation of HIV1 replication by interferon alpha: from laboratory bench to bedside. Res Immunol. 1994;145(8–9):679–84; discussion 84–5. Epub 1994/10/01. . [DOI] [PubMed] [Google Scholar]
- 29.Langford D, Masliah E. Crosstalk between components of the blood brain barrier and cells of the CNS in microglial activation in AIDS. Brain pathology (Zurich, Switzerland). 2001;11(3):306–12. Epub 2001/06/21. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. Journal of the neurological sciences. 2002;202(1–2):13–23. Epub 2002/09/11. S0022510X02002071 [pii]. . [DOI] [PubMed] [Google Scholar]
- 31.Wiley CA. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain pathology (Zurich, Switzerland). 2003;13(3):415; author reply -6. Epub 2003/08/30. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen KK, Pedersen M, Gaardbo JC, Ronit A, Hartling HJ, Bruunsgaard H, et al. Persisting inflammation and chronic immune activation but intact cognitive function in HIV-infected patients after long-term treatment with combination antiretroviral therapy. Journal of acquired immune deficiency syndromes. 2013;63(3):272–9. doi: 10.1097/QAI.0b013e318289bced . [DOI] [PubMed] [Google Scholar]
- 33.Cohen RA, de la Monte S, Gongvatana A, Ombao H, Gonzalez B, Devlin KN, et al. Plasma cytokine concentrations associated with HIV/hepatitis C coinfection are related to attention, executive and psychomotor functioning. Journal of neuroimmunology. 2011;233(1–2):204–10. doi: 10.1016/j.jneuroim.2010.11.006 ; PubMed Central PMCID: PMC3074016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Correia S, Cohen R, Gongvatana A, Ross S, Olchowski J, Devlin K, et al. Relationship of plasma cytokines and clinical biomarkers to memory performance in HIV. Journal of neuroimmunology. 2013;265(1–2):117–23. doi: 10.1016/j.jneuroim.2013.09.005 ; PubMed Central PMCID: PMC4422333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan LA, Zheng J, Brester M, Bohac D, Hahn F, Anderson J, et al. Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis. 2001;184(6):699–706. doi: 10.1086/323036 . [DOI] [PubMed] [Google Scholar]
- 36.Vaidya SA, Korner C, Sirignano MN, Amero M, Bazner S, Rychert J, et al. Tumor necrosis factor alpha is associated with viral control and early disease progression in patients with HIV type 1 infection. J Infect Dis. 2014;210(7):1042–6. doi: 10.1093/infdis/jiu206 ; PubMed Central PMCID: PMC4215080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tandon R, Chew GM, Byron MM, Borrow P, Niki T, Hirashima M, et al. Galectin-9 is rapidly released during acute HIV-1 infection and remains sustained at high levels despite viral suppression even in elite controllers. AIDS Res Hum Retroviruses. 2014;30(7):654–64. doi: 10.1089/AID.2014.0004 ; PubMed Central PMCID: PMC4077009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pastor L, Casellas A, Ruperez M, Carrillo J, Maculuve S, Jairoce C, et al. Interferon-gamma-Inducible Protein 10 (IP-10) as a Screening Tool to Optimize Human Immunodeficiency Virus RNA Monitoring in Resource-Limited Settings. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2017;65(10):1670–5. doi: 10.1093/cid/cix600 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiao Y, Zhang T, Wang R, Zhang H, Huang X, Yin J, et al. Plasma IP-10 is associated with rapid disease progression in early HIV-1 infection. Viral immunology. 2012;25(4):333–7. doi: 10.1089/vim.2012.0011 . [DOI] [PubMed] [Google Scholar]
- 40.Ploquin MJ, Madec Y, Casrouge A, Huot N, Passaes C, Lecuroux C, et al. Elevated Basal Pre-infection CXCL10 in Plasma and in the Small Intestine after Infection Are Associated with More Rapid HIV/SIV Disease Onset. PLoS pathogens. 2016;12(8):e1005774 doi: 10.1371/journal.ppat.1005774 ; PubMed Central PMCID: PMC4980058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crews FT, Lawrimore CJ, Walter TJ, Coleman LG Jr. The role of neuroimmune signaling in alcoholism. Neuropharmacology. 2017. doi: 10.1016/j.neuropharm.2017.01.031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE. Minocycline reduces ethanol drinking. Brain, behavior, and immunity. 2011;25 Suppl 1:S165–9. doi: 10.1016/j.bbi.2011.03.002 ; PubMed Central PMCID: PMC3098317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30(24):8285–95. doi: 10.1523/JNEUROSCI.0976-10.2010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corrigan F, Wu Y, Tuke J, Coller JK, Rice KC, Diener KR, et al. Alcohol-induced sedation and synergistic interactions between alcohol and morphine: a key mechanistic role for Toll-like receptors and MyD88-dependent signaling. Brain, behavior, and immunity. 2015;45:245–52. doi: 10.1016/j.bbi.2014.12.019 ; PubMed Central PMCID: PMC4394865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behavioural brain research. 2005;165(1):110–25. doi: 10.1016/j.bbr.2005.06.026 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Timary P, Starkel P, Delzenne NM, Leclercq S. A role for the peripheral immune system in the development of alcohol use disorders? Neuropharmacology. 2017. doi: 10.1016/j.neuropharm.2017.04.013 . [DOI] [PubMed] [Google Scholar]
- 47.Alfonso-Loeches S, Pascual M, Gomez-Pinedo U, Pascual-Lucas M, Renau-Piqueras J, Guerri C. Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse. Glia. 2012;60(6):948–64. Epub 2012/03/21. doi: 10.1002/glia.22327 . [DOI] [PubMed] [Google Scholar]
- 48.Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biological psychiatry. 2013;73(7):602–12. doi: 10.1016/j.biopsych.2012.09.030 ; PubMed Central PMCID: PMC3602398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lippai D, Bala S, Csak T, Kurt-Jones EA, Szabo G. Chronic alcohol-induced microRNA-155 contributes to neuroinflammation in a TLR4-dependent manner in mice. PLoS One. 2013;8(8):e70945 doi: 10.1371/journal.pone.0070945 ; PubMed Central PMCID: PMC3739772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris RA, Bajo M, Bell RL, Blednov YA, Varodayan FP, Truitt JM, et al. Genetic and Pharmacologic Manipulation of TLR4 Has Minimal Impact on Ethanol Consumption in Rodents. J Neurosci. 2017;37(5):1139–55. doi: 10.1523/JNEUROSCI.2002-16.2016 ; PubMed Central PMCID: PMC5296793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blanco AM, Valles SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175(10):6893–9. Epub 2005/11/08. 175/10/6893 [pii]. . [DOI] [PubMed] [Google Scholar]
- 52.Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Coller JK, Rice KC, et al. Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. Br J Pharmacol. 2012;165(5):1319–29. Epub 2011/10/01. doi: 10.1111/j.1476-5381.2011.01572.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis RL, Syapin PJ. Ethanol increases nuclear factor-kappa B activity in human astroglial cells. Neuroscience letters. 2004;371(2–3):128–32. Epub 2004/11/03. S0304-3940(04)01062-6 [pii] doi: 10.1016/j.neulet.2004.08.051 . [DOI] [PubMed] [Google Scholar]
- 54.Zou J, Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-kappaB and proinflammatory cytokines. Alcoholism, clinical and experimental research. 2010;34(5):777–89. Epub 2010/03/06. doi: 10.1111/j.1530-0277.2010.01150.x . [DOI] [PubMed] [Google Scholar]
- 55.Zou JY, Crews FT. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS One. 2014;9(2):e87915 doi: 10.1371/journal.pone.0087915 ; PubMed Central PMCID: PMC3925099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. Journal of neuroinflammation. 2008;5:10 doi: 10.1186/1742-2094-5-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jaatinen P, Riikonen J, Riihioja P, Kajander O, Hervonen A. Interaction of aging and intermittent ethanol exposure on brain cytochrome c oxidase activity levels. Alcohol (Fayetteville, NY. 2003;29(2):91–100. Epub 2003/06/05. . [DOI] [PubMed] [Google Scholar]
- 58.Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, et al. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcoholism, clinical and experimental research. 2006;30(11):1938–49. doi: 10.1111/j.1530-0277.2006.00239.x . [DOI] [PubMed] [Google Scholar]
- 59.Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183(7):4733–44. Epub 2009/09/16. jimmunol.0803590 [pii] doi: 10.4049/jimmunol.0803590 . [DOI] [PubMed] [Google Scholar]
- 60.Baxter-Potter LN, Henricks AM, Berger AL, Bieniasz KV, Lugo JM, McLaughlin RJ. Alcohol vapor exposure differentially impacts mesocorticolimbic cytokine expression in a sex-, region-, and duration-specific manner. Neuroscience. 2017;346:238–46. doi: 10.1016/j.neuroscience.2017.01.015 . [DOI] [PubMed] [Google Scholar]
- 61.Knapp DJ, Harper KM, Whitman BA, Zimomra Z, Breese GR. Stress and Withdrawal from Chronic Ethanol Induce Selective Changes in Neuroimmune mRNAs in Differing Brain Sites. Brain sciences. 2016;6(3). doi: 10.3390/brainsci6030025 ; PubMed Central PMCID: PMC5039454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider R Jr., Bandiera S, Souza DG, Bellaver B, Caletti G, Quincozes-Santos A, et al. N-acetylcysteine Prevents Alcohol Related Neuroinflammation in Rats. Neurochemical research. 2017. doi: 10.1007/s11064-017-2218-8 . [DOI] [PubMed] [Google Scholar]
- 63.Pascual M, Montesinos J, Marcos M, Torres JL, Costa-Alba P, Garcia-Garcia F, et al. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addiction biology. 2016. doi: 10.1111/adb.12461 . [DOI] [PubMed] [Google Scholar]
- 64.Cui SQ, Wang Q, Zheng Y, Xiao B, Sun HW, Gu XL, et al. Puerarin protects against damage to spatial learning and memory ability in mice with chronic alcohol poisoning. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al. ]. 2015;48(6):515–22. doi: 10.1590/1414-431X20144250 ; PubMed Central PMCID: PMC4470310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harper KM, Knapp DJ, Breese GR. Withdrawal from Chronic Alcohol Induces a Unique CCL2 mRNA Increase in Adolescent But Not Adult Brain-Relationship to Blood Alcohol Levels and Seizures. Alcoholism, clinical and experimental research. 2015;39(12):2375–85. doi: 10.1111/acer.12898 ; PubMed Central PMCID: PMC4712108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain research. 2005;1034(1–2):11–24. doi: 10.1016/j.brainres.2004.11.014 . [DOI] [PubMed] [Google Scholar]
- 67.Qin L, Crews FT. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. Journal of neuroinflammation. 2012;9:130 doi: 10.1186/1742-2094-9-130 ; PubMed Central PMCID: PMC3412752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, de Witte P, et al. Neuro-inflammation induced in the hippocampus of 'binge drinking' rats may be mediated by elevated extracellular glutamate content. Journal of neurochemistry. 2009;111(5):1119–28. Epub 2009/09/22. JNC6389 [pii] doi: 10.1111/j.1471-4159.2009.06389.x . [DOI] [PubMed] [Google Scholar]
- 69.Marshall SA, Geil CR, Nixon K. Prior Binge Ethanol Exposure Potentiates the Microglial Response in a Model of Alcohol-Induced Neurodegeneration. Brain sciences. 2016;6(2). doi: 10.3390/brainsci6020016 ; PubMed Central PMCID: PMC4931493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freeman K, Brureau A, Vadigepalli R, Staehle MM, Brureau MM, Gonye GE, et al. Temporal changes in innate immune signals in a rat model of alcohol withdrawal in emotional and cardiorespiratory homeostatic nuclei. Journal of neuroinflammation. 2012;9:97 doi: 10.1186/1742-2094-9-97 ; PubMed Central PMCID: PMC3411448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bajo M, Varodayan FP, Madamba SG, Robert AJ, Casal LM, Oleata CS, et al. IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala. Front Pharmacol. 2015;6:49 doi: 10.3389/fphar.2015.00049 ; PubMed Central PMCID: PMC4365713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bajo M, Herman MA, Varodayan FP, Oleata CS, Madamba SG, Harris RA, et al. Role of the IL-1 receptor antagonist in ethanol-induced regulation of GABAergic transmission in the central amygdala. Brain, behavior, and immunity. 2015;45:189–97. doi: 10.1016/j.bbi.2014.11.011 ; PubMed Central PMCID: PMC4405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.June HL, Liu J, Warnock KT, Bell KA, Balan I, Bollino D, et al. CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology. 2015;40(6):1549–59. doi: 10.1038/npp.2015.4 ; PubMed Central PMCID: PMC4397415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL Jr., et al. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(11):4465–70. doi: 10.1073/pnas.1019020108 ; PubMed Central PMCID: PMC3060224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addiction biology. 2012;17(1):108–20. doi: 10.1111/j.1369-1600.2010.00284.x ; PubMed Central PMCID: PMC3117922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blednov YA, Benavidez JM, Black M, Harris RA. Inhibition of phosphodiesterase 4 reduces ethanol intake and preference in C57BL/6J mice. Front Neurosci. 2014;8:129 doi: 10.3389/fnins.2014.00129 ; PubMed Central PMCID: PMC4034339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mayfield J, Ferguson L, Harris RA. Neuroimmune signaling: a key component of alcohol abuse. Current opinion in neurobiology. 2013;23(4):513–20. doi: 10.1016/j.conb.2013.01.024 ; PubMed Central PMCID: PMC3694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karlsson C, Schank JR, Rehman F, Stojakovic A, Bjork K, Barbier E, et al. Proinflammatory signaling regulates voluntary alcohol intake and stress-induced consumption after exposure to social defeat stress in mice. Addiction biology. 2016. doi: 10.1111/adb.12416 . [DOI] [PubMed] [Google Scholar]
- 79.Valenta JP, Gonzales RA. Chronic Intracerebroventricular Infusion of Monocyte Chemoattractant Protein-1 Leads to a Persistent Increase in Sweetened Ethanol Consumption During Operant Self-Administration But Does Not Influence Sucrose Consumption in Long-Evans Rats. Alcoholism, clinical and experimental research. 2016;40(1):187–95. doi: 10.1111/acer.12928 ; PubMed Central PMCID: PMC4701601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu J, Lewohl JM, Harris RA, Iyer VR, Dodd PR, Randall PK, et al. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 2006;31(7):1574–82. Epub 2005/11/18. 1300947 [pii] doi: 10.1038/sj.npp.1300947 . [DOI] [PubMed] [Google Scholar]
- 81.Flatscher-Bader T, van der Brug M, Hwang JW, Gochee PA, Matsumoto I, Niwa S, et al. Alcohol-responsive genes in the frontal cortex and nucleus accumbens of human alcoholics. Journal of neurochemistry. 2005;93(2):359–70. Epub 2005/04/09. JNC3021 [pii] doi: 10.1111/j.1471-4159.2004.03021.x . [DOI] [PubMed] [Google Scholar]
- 82.Okvist A, Johansson S, Kuzmin A, Bazov I, Merino-Martinez R, Ponomarev I, et al. Neuroadaptations in human chronic alcoholics: dysregulation of the NF-kappaB system. PLoS One. 2007;2(9):e930 doi: 10.1371/journal.pone.0000930 ; PubMed Central PMCID: PMC1976556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kebir O, Gorsane MA, Blecha L, Krebs MO, Reynaud M, Benyamina A. Association of inflammation genes with alcohol dependence/abuse: a systematic review and a meta-analysis. European addiction research. 2011;17(3):146–53. doi: 10.1159/000324849 . [DOI] [PubMed] [Google Scholar]
- 84.Yamauchi M, Takamatsu M, Maezawa Y, Takagi M, Araki T, Satoh S, et al. Polymorphism of tumor necrosis factor-beta and alcohol dehydrogenase genes and alcoholic brain atrophy in Japanese patients. Alcoholism, clinical and experimental research. 2001;25(6 Suppl):7S–10S. . [DOI] [PubMed] [Google Scholar]
- 85.Pastor IJ, Laso FJ, Romero A, Gonzalez-Sarmiento R. -238 G>A polymorphism of tumor necrosis factor alpha gene (TNFA) is associated with alcoholic liver cirrhosis in alcoholic Spanish men. Alcoholism, clinical and experimental research. 2005;29(11):1928–31. . [DOI] [PubMed] [Google Scholar]
- 86.Augustynska B, Araszkiewicz A, Wozniak M, Grzybowski T, Skonieczna K, Wozniak A, et al. Assessment of the frequency of the transforming growth factor beta-1 sequence polymorphisms in patients with alcohol dependence syndrome. Acta Biochim Pol. 2015;62(1):63–7. . [DOI] [PubMed] [Google Scholar]
- 87.Auguet T, Vidal F, Broch M, Olona M, Aguilar C, Morancho B, et al. Polymorphisms in the interleukin-10 gene promoter and the risk of alcoholism and alcoholic liver disease in Caucasian Spaniard men. Alcohol (Fayetteville, NY. 2010;44(3):211–6. doi: 10.1016/j.alcohol.2010.02.007 . [DOI] [PubMed] [Google Scholar]
- 88.Serretti A, Liappas I, Mandelli L, Albani D, Forloni G, Malitas P, et al. Interleukin-1 alpha and beta, TNF-alpha and HTTLPR gene variants study on alcohol toxicity and detoxification outcome. Neuroscience letters. 2006;406(1–2):107–12. doi: 10.1016/j.neulet.2006.07.003 . [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez-Quintela A, Campos J, Loidi L, Quinteiro C, Perez LF, Gude F. Serum TNF-alpha levels in relation to alcohol consumption and common TNF gene polymorphisms. Alcohol (Fayetteville, NY. 2008;42(6):513–8. doi: 10.1016/j.alcohol.2008.04.008 . [DOI] [PubMed] [Google Scholar]
- 90.Marcos M, Gomez-Munuera M, Pastor I, Gonzalez-Sarmiento R, Laso FJ. Tumor necrosis factor polymorphisms and alcoholic liver disease: a HuGE review and meta-analysis. American journal of epidemiology. 2009;170(8):948–56. doi: 10.1093/aje/kwp236 . [DOI] [PubMed] [Google Scholar]
- 91.He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental neurology. 2008;210(2):349–58. doi: 10.1016/j.expneurol.2007.11.017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vetreno RP, Qin L, Crews FT. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiology of disease. 2013;59:52–62. doi: 10.1016/j.nbd.2013.07.002 ; PubMed Central PMCID: PMC3775891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Umhau JC, Schwandt M, Solomon MG, Yuan P, Nugent A, Zarate CA, et al. Cerebrospinal fluid monocyte chemoattractant protein-1 in alcoholics: support for a neuroinflammatory model of chronic alcoholism. Alcoholism, clinical and experimental research. 2014;38(5):1301–6. doi: 10.1111/acer.12367 . [DOI] [PubMed] [Google Scholar]
- 94.Gaydos J, McNally A, Guo R, Vandivier RW, Simonian PL, Burnham EL. Alcohol abuse and smoking alter inflammatory mediator production by pulmonary and systemic immune cells. American journal of physiology Lung cellular and molecular physiology. 2016;310(6):L507–18. doi: 10.1152/ajplung.00242.2015 ; PubMed Central PMCID: PMC4796259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoyt LR, Randall MJ, Ather JL, DePuccio DP, Landry CC, Qian X, et al. Mitochondrial ROS induced by chronic ethanol exposure promote hyper-activation of the NLRP3 inflammasome. Redox biology. 2017;12:883–96. doi: 10.1016/j.redox.2017.04.020 ; PubMed Central PMCID: PMC5413213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leclercq S, De Saeger C, Delzenne N, de Timary P, Starkel P. Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biological psychiatry. 2014;76(9):725–33. doi: 10.1016/j.biopsych.2014.02.003 . [DOI] [PubMed] [Google Scholar]
- 97.Heberlein A, Kaser M, Lichtinghagen R, Rhein M, Lenz B, Kornhuber J, et al. TNF-alpha and IL-6 serum levels: neurobiological markers of alcohol consumption in alcohol-dependent patients? Alcohol (Fayetteville, NY. 2014;48(7):671–6. doi: 10.1016/j.alcohol.2014.08.003 . [DOI] [PubMed] [Google Scholar]
- 98.Leclercq S, Cani PD, Neyrinck AM, Starkel P, Jamar F, Mikolajczak M, et al. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain, behavior, and immunity. 2012;26(6):911–8. doi: 10.1016/j.bbi.2012.04.001 . [DOI] [PubMed] [Google Scholar]
- 99.Manzardo AM, Poje AB, Penick EC, Butler MG. Multiplex Immunoassay of Plasma Cytokine Levels in Men with Alcoholism and the Relationship to Psychiatric Assessments. Int J Mol Sci. 2016;17(4):472 doi: 10.3390/ijms17040472 ; PubMed Central PMCID: PMC4848928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beech RD, Qu J, Leffert JJ, Lin A, Hong KA, Hansen J, et al. Altered expression of cytokine signaling pathway genes in peripheral blood cells of alcohol dependent subjects: preliminary findings. Alcoholism, clinical and experimental research. 2012;36(9):1487–96. doi: 10.1111/j.1530-0277.2012.01775.x ; PubMed Central PMCID: PMC3393821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gonzalez-Reimers E, Santolaria-Fernandez F, Medina-Garcia JA, Gonzalez-Perez JM, de la Vega-Prieto MJ, Medina-Vega L, et al. TH-1 and TH-2 cytokines in stable chronic alcoholics. Alcohol and alcoholism (Oxford, Oxfordshire). 2012;47(4):390–6. doi: 10.1093/alcalc/ags041 . [DOI] [PubMed] [Google Scholar]
- 102.Neupane SP, Lien L, Martinez P, Aukrust P, Ueland T, Mollnes TE, et al. High frequency and intensity of drinking may attenuate increased inflammatory cytokine levels of major depression in alcohol-use disorders. CNS Neurosci Ther. 2014;20(10):898–904. doi: 10.1111/cns.12303 ; PubMed Central PMCID: PMC4257130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laso FJ, Iglesias MC, Lopez A, Ciudad J, San Miguel JF, Orfao A. Increased interleukin-12 serum levels in chronic alcoholism. Journal of hepatology. 1998;28(5):771–7. . [DOI] [PubMed] [Google Scholar]
- 104.Gonzalez-Quintela A, Dominguez-Santalla MJ, Perez LF, Vidal C, Lojo S, Barrio E. Influence of acute alcohol intake and alcohol withdrawal on circulating levels of IL-6, IL-8, IL-10 and IL-12. Cytokine. 2000;12(9):1437–40. 0.1006/cyto.2000.0715. doi: 10.1006/cyto.2000.0715 . [DOI] [PubMed] [Google Scholar]
- 105.Garcia-Valdecasas-Campelo E, Gonzalez-Reimers E, Santolaria-Fernandez F, De La Vega-Prieto MJ, Milena-Abril A, Sanchez-Perez MJ, et al. Brain atrophy in alcoholics: relationship with alcohol intake; liver disease; nutritional status, and inflammation. Alcohol and alcoholism (Oxford, Oxfordshire). 2007;42(6):533–8. Epub 2007/09/15. agm065 [pii] doi: 10.1093/alcalc/agm065 . [DOI] [PubMed] [Google Scholar]
- 106.Maes M, Lin A, Bosmans E, Vandoolaeghe E, Bonaccorso S, Kenis G, et al. Serotonin-immune interactions in detoxified chronic alcoholic patients without apparent liver disease: activation of the inflammatory response system and lower plasma total tryptophan. Psychiatry research. 1998;78(3):151–61. . [DOI] [PubMed] [Google Scholar]
- 107.Gonzalez-Reimers E, Fernandez-Rodriguez CM, Santolaria-Fernandez F, de la Vega-Prieto MJ, Martin-Gonzalez C, Gomez-Rodriguez MA, et al. Interleukin-15 and other myokines in chronic alcoholics. Alcohol and alcoholism (Oxford, Oxfordshire). 2011;46(5):529–33. doi: 10.1093/alcalc/agr064 . [DOI] [PubMed] [Google Scholar]
- 108.Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain, behavior, and immunity. 2004;18(4):349–60. doi: 10.1016/j.bbi.2004.02.001 . [DOI] [PubMed] [Google Scholar]
- 109.Franchi S, Sacerdote P, Moretti S, Gerra G, Leccese V, Tallone MV, et al. The effects of alcoholism pharmacotherapy on immune responses in alcohol-dependent patients. International journal of immunopathology and pharmacology. 2010;23(3):847–55. doi: 10.1177/039463201002300320 . [DOI] [PubMed] [Google Scholar]
- 110.Neupane SP, Lien L, Ueland T, Mollnes TE, Aukrust P, Bramness JG. Serum brain-derived neurotrophic factor levels in relation to comorbid depression and cytokine levels in Nepalese men with alcohol-use disorders. Alcohol (Fayetteville, NY. 2015;49(5):471–8. doi: 10.1016/j.alcohol.2015.01.012 . [DOI] [PubMed] [Google Scholar]
- 111.Neupane SP, Lien L, Martinez P, Hestad K, Bramness JG. The relationship of alcohol use disorders and depressive symptoms to tryptophan metabolism: cross-sectional data from a Nepalese alcohol treatment sample. Alcoholism, clinical and experimental research. 2015;39(3):514–21. doi: 10.1111/acer.12651 ; PubMed Central PMCID: PMC4348238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bonacini M. Alcohol use among patients with HIV infection. Ann Hepatol. 2011;10(4):502–7. Epub 2011/09/14. 958681 [pii]. . [PubMed] [Google Scholar]
- 113.Miguez MJ, Shor-Posner G, Morales G, Rodriguez A, Burbano X. HIV treatment in drug abusers: impact of alcohol use. Addict Biol. 2003;8(1):33–7. doi: 10.1080/1355621031000069855 . [DOI] [PubMed] [Google Scholar]
- 114.Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16(2):83–8. doi: 10.1111/j.1525-1497.2001.00122.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Samet JH, Phillips SJ, Horton NJ, Traphagen ET, Freedberg KA. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Res Hum Retroviruses. 2004;20(2):151–5. doi: 10.1089/088922204773004860 [DOI] [PubMed] [Google Scholar]
- 116.Conigliaro J, Justice AC, Gordon AJ, Bryant K. Role of alcohol in determining human immunodeficiency virus (HIV)-relevant outcomes: A conceptual model to guide the implementation of evidence-based interventions into practice. Med Care. 2006;44(8 Suppl 2):S1–6. doi: 10.1097/01.mlr.0000223659.36369.cf . [DOI] [PubMed] [Google Scholar]
- 117.Samet JH, Walley AY, Bridden C. Illicit drugs, alcohol, and addiction in human immunodeficiency virus. Panminerva Med. 2007;49(2):67–77. . [PubMed] [Google Scholar]
- 118.Fuller BE, Loftis JM, Rodriguez VL, McQuesten MJ, Hauser P. Psychiatric and substance use disorders comorbidities in veterans with hepatitis C virus and HIV coinfection. Current opinion in psychiatry. 2009;22(4):401–8. doi: 10.1097/YCO.0b013e32832cadb9 . [DOI] [PubMed] [Google Scholar]
- 119.Tran BX, Nguyen LT, Do CD, Nguyen QL, Maher RM. Associations between alcohol use disorders and adherence to antiretroviral treatment and quality of life amongst people living with HIV/AIDS. BMC public health. 2014;14:27 doi: 10.1186/1471-2458-14-27 ; PubMed Central PMCID: PMC3893525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Braithwaite RS, Conigliaro J, Roberts MS, Shechter S, Schaefer A, McGinnis K, et al. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS care. 2007;19(4):459–66. doi: 10.1080/09540120601095734 ; PubMed Central PMCID: PMC3460376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petry NM. Alcohol use in HIV patients: what we don't know may hurt us. Int J STD AIDS. 1999;10(9):561–70. doi: 10.1258/0956462991914654 . [DOI] [PubMed] [Google Scholar]
- 122.Soboka M, Tesfaye M, Feyissa GT, Hanlon C. Alcohol use disorders and associated factors among people living with HIV who are attending services in south west Ethiopia. BMC research notes. 2014;7:828 doi: 10.1186/1756-0500-7-828 ; PubMed Central PMCID: PMC4289332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barve S, Kapoor R, Moghe A, Ramirez JA, Eaton JW, Gobejishvili L, et al. Focus on the liver: alcohol use, highly active antiretroviral therapy, and liver disease in HIV-infected patients. Alcohol Res Health. 2010;33(3):229–36. ; PubMed Central PMCID: PMC3860514. [PMC free article] [PubMed] [Google Scholar]
- 124.Bautista AP. Chronic alcohol intoxication induces hepatic injury through enhanced macrophage inflammatory protein-2 production and intercellular adhesion molecule-1 expression in the liver. Hepatology (Baltimore, Md. 1997;25(2):335–42. Epub 1997/02/01. S0270913997000724 [pii] doi: 10.1002/hep.510250214 . [DOI] [PubMed] [Google Scholar]
- 125.Banerjee A, Abdelmegeed MA, Jang S, Song BJ. Increased Sensitivity to Binge Alcohol-Induced Gut Leakiness and Inflammatory Liver Disease in HIV Transgenic Rats. PLoS One. 2015;10(10):e0140498 doi: 10.1371/journal.pone.0140498 ; PubMed Central PMCID: PMC4618849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Molina PE, McNurlan M, Rathmacher J, Lang CH, Zambell KL, Purcell J, et al. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcoholism: Clinical and Experimental Research. 2006;30(12):2065–78. [DOI] [PubMed] [Google Scholar]
- 127.Marcondes MC, Watry D, Zandonatti M, Flynn C, Taffe MA, Fox H. Chronic alcohol consumption generates a vulnerable immune environment during early SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2008;32(9):1583–92. doi: 10.1111/j.1530-0277.2008.00730.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fuster D, Tsui JI, Cheng DM, Quinn EK, Armah KA, Nunes D, et al. Interleukin-6 is associated with noninvasive markers of liver fibrosis in HIV-infected patients with alcohol problems. AIDS Res Hum Retroviruses. 2013;29(8):1110–6. doi: 10.1089/AID.2012.0348 ; PubMed Central PMCID: PMC3715787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fuster D, Cheng DM, Quinn EK, Armah KA, Saitz R, Freiberg MS, et al. Inflammatory cytokines and mortality in a cohort of HIV-infected adults with alcohol problems. AIDS. 2014;28(7):1059–64. doi: 10.1097/QAD.0000000000000184 ; PubMed Central PMCID: PMC4105144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. New York, NY: Biometrics Research Department, New York State Psychiatric Institute, 1998. [Google Scholar]
- 131.Skinner HA. Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. Toronto, Canada: Addiction Research Foundation; 1982. [Google Scholar]
- 132.Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. Journal of studies on alcohol. 1982;43(11):1157–70. . [DOI] [PubMed] [Google Scholar]
- 133.Beck AT, S R.A., B G.K. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation, (1996) [Google Scholar]
- 134.Hollingshead A. Four-factor index of social status. New Haven, CT: Department of Sociology, Yale University, 1975. [Google Scholar]
- 135.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–71. [DOI] [PubMed] [Google Scholar]
- 136.Wechsler D. Wechsler Test of Adult Reading: WTAR. San Antonio, TX: Pearson Education, Inc, 2001. [Google Scholar]
- 137.Mattis S. Dementia rating scale (DRS) professional manual. Odessa, FL: Psychological Assessment Resources, Inc, 1998. [Google Scholar]
- 138.Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27(4):563–72. doi: 10.1097/QAD.0b013e32835b8c7f ; PubMed Central PMCID: PMC4283204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Karnofsky DA. The clinical evaluation of chemotherapeutic agents in cancer In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. p. 191–205. [Google Scholar]
- 140.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology (Baltimore, Md. 2006;43(6):1317–25. doi: 10.1002/hep.21178 . [DOI] [PubMed] [Google Scholar]
- 141.Thandassery RB, Al Kaabi S, Soofi ME, Mohiuddin SA, John AK, Al Mohannadi M, et al. Mean Platelet Volume, Red Cell Distribution Width to Platelet Count Ratio, Globulin Platelet Index, and 16 Other Indirect Noninvasive Fibrosis Scores: How Much Do Routine Blood Tests Tell About Liver Fibrosis in Chronic Hepatitis C? Journal of clinical gastroenterology. 2016;50(6):518–23. doi: 10.1097/MCG.0000000000000489 . [DOI] [PubMed] [Google Scholar]
- 142.Fouad SA, Esmat S, Omran D, Rashid L, Kobaisi MH. Noninvasive assessment of hepatic fibrosis in Egyptian patients with chronic hepatitis C virus infection. World J Gastroenterol. 2012;18(23):2988–94. doi: 10.3748/wjg.v18.i23.2988 ; PubMed Central PMCID: PMC3380327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Swardfager W, Rosenblat JD, Benlamri M, McIntyre RS. Mapping inflammation onto mood: Inflammatory mediators of anhedonia. Neuroscience and biobehavioral reviews. 2016;64:148–66. doi: 10.1016/j.neubiorev.2016.02.017 . [DOI] [PubMed] [Google Scholar]
- 144.Trepanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016;21(8):1009–26. doi: 10.1038/mp.2016.90 ; PubMed Central PMCID: PMC4960446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bagyinszky E, Giau VV, Shim K, Suk K, An SSA, Kim S. Role of inflammatory molecules in the Alzheimer's disease progression and diagnosis. Journal of the neurological sciences. 2017;376:242–54. doi: 10.1016/j.jns.2017.03.031 . [DOI] [PubMed] [Google Scholar]
- 146.Lee CH, Shin HP, Lee JI, Joo KR, Cha JM, Jeon JW, et al. Predicting factors of present hepatitis C virus infection among patients positive for the hepatitis C virus antibody. Clinical and molecular hepatology. 2013;19(4):376–81. doi: 10.3350/cmh.2013.19.4.376 ; PubMed Central PMCID: PMC3894437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Scherzer R, Heymsfield SB, Rimland D, Powderly WG, Tien PC, Bacchetti P, et al. Association of serum albumin and aspartate transaminase with 5-year all-cause mortality in HIV/hepatitis C virus coinfection and HIV monoinfection. AIDS. 2017;31(1):71–9. doi: 10.1097/QAD.0000000000001278 ; PubMed Central PMCID: PMC5127775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology (Baltimore, Md. 2011;53(3):726–36. doi: 10.1002/hep.24105 . [DOI] [PubMed] [Google Scholar]
- 149.Borsoi Viana MS, Takei K, Collarile Yamaguti DC, Guz B, Strauss E. Use of AST platelet ratio index (APRI Score) as an alternative to liver biopsy for treatment indication in chronic hepatitis C. Annals of hepatology. 2009;8(1):26–31. . [PubMed] [Google Scholar]
- 150.El Serafy MA, Kassem AM, Omar H, Mahfouz MS, El Said El Raziky M. APRI test and hyaluronic acid as non-invasive diagnostic tools for post HCV liver fibrosis: Systematic review and meta-analysis. Arab journal of gastroenterology: the official publication of the Pan-Arab Association of Gastroenterology. 2017;18(2):51–7. doi: 10.1016/j.ajg.2017.05.005 . [DOI] [PubMed] [Google Scholar]
- 151.Matta B, Lee TH, Patel K. Use of Non-invasive Testing to Stage Liver Fibrosis in Patients with HIV. Current HIV/AIDS reports. 2016;13(5):279–88. doi: 10.1007/s11904-016-0329-5 . [DOI] [PubMed] [Google Scholar]
- 152.Peters L, Rockstroh JK. Biomarkers of fibrosis and impaired liver function in chronic hepatitis C: how well do they predict clinical outcomes? Current opinion in HIV and AIDS. 2010;5(6):517–23. doi: 10.1097/COH.0b013e32833e3ee6 . [DOI] [PubMed] [Google Scholar]
- 153.Afford SC, Fisher NC, Neil DA, Fear J, Brun P, Hubscher SG, et al. Distinct patterns of chemokine expression are associated with leukocyte recruitment in alcoholic hepatitis and alcoholic cirrhosis. The Journal of pathology. 1998;186(1):82–9. Epub 1999/01/06. doi: 10.1002/(SICI)1096-9896(199809)186:1<82::AID-PATH151>3.0.CO;2-D . [DOI] [PubMed] [Google Scholar]
- 154.Hill DB, Marsano LS, McClain CJ. Increased plasma interleukin-8 concentrations in alcoholic hepatitis. Hepatology (Baltimore, Md. 1993;18(3):576–80. Epub 1993/09/01. S0270913993002149 [pii]. . [PubMed] [Google Scholar]
- 155.Daniluk J, Szuster-Ciesielska A, Drabko J, Kandefer-Szerszen M. Serum cytokine levels in alcohol-related liver cirrhosis. Alcohol (Fayetteville, NY. 2001;23(1):29–34. . [DOI] [PubMed] [Google Scholar]
- 156.Sheron N, Bird G, Goka J, Alexander G, Williams R. Elevated plasma interleukin-6 and increased severity and mortality in alcoholic hepatitis. Clin Exp Immunol. 1991;84(3):449–53. ; PubMed Central PMCID: PMC1535433. [PMC free article] [PubMed] [Google Scholar]
- 157.Zeremski M, Markatou M, Brown QB, Dorante G, Cunningham-Rundles S, Talal AH. Interferon gamma-inducible protein 10: a predictive marker of successful treatment response in hepatitis C virus/HIV-coinfected patients. Journal of acquired immune deficiency syndromes. 2007;45(3):262–8. doi: 10.1097/QAI.0b013e3180559219 . [DOI] [PubMed] [Google Scholar]
- 158.Qu J, Zhang Q, Li Y, Liu W, Chen L, Zhu Y, et al. The Tat protein of human immunodeficiency virus-1 enhances hepatitis C virus replication through interferon gamma-inducible protein-10. BMC immunology. 2012;13:15 doi: 10.1186/1471-2172-13-15 ; PubMed Central PMCID: PMC3350415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Roe B, Coughlan S, Hassan J, Grogan A, Farrell G, Norris S, et al. Elevated serum levels of interferon- gamma -inducible protein-10 in patients coinfected with hepatitis C virus and HIV. J Infect Dis. 2007;196(7):1053–7. doi: 10.1086/520935 . [DOI] [PubMed] [Google Scholar]
- 160.Reiberger T, Aberle JH, Kundi M, Kohrgruber N, Rieger A, Gangl A, et al. IP-10 correlates with hepatitis C viral load, hepatic inflammation and fibrosis and predicts hepatitis C virus relapse or non-response in HIV-HCV coinfection. Antivir Ther. 2008;13(8):969–76. . [PubMed] [Google Scholar]
- 161.Sultana C, Erscoiu SM, Grancea C, Ceausu E, Ruta S. Predictors of Chronic Hepatitis C Evolution in HIV Co-Infected Patients From Romania. Hepatitis monthly. 2013;13(2):e8611 doi: 10.5812/hepatmon.8611 ; PubMed Central PMCID: PMC3632003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hajarizadeh B, Lamoury FM, Feld JJ, Amin J, Keoshkerian E, Matthews GV, et al. Alanine aminotransferase, HCV RNA levels and pro-inflammatory and pro-fibrogenic cytokines/chemokines during acute hepatitis C virus infection. Virology journal. 2016;13:32 doi: 10.1186/s12985-016-0482-x ; PubMed Central PMCID: PMC4765111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Graham CS, Wells A, Edwards EM, Herren T, Tumilty S, Stuver SO, et al. Effect of exposure to injection drugs or alcohol on antigen-specific immune responses in HIV and hepatitis C virus coinfection. The Journal of Infectious Diseases. 2007;195(6):847–56. doi: 10.1086/511990 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
The data have been publicly deposited at Dryad (http://datadryad.org/review?doi=doi:10.5061/dryad.rj61p).