Abstract
ALC1 (amplified in liver cancer 1), an SNF2 superfamily chromatin-remodeling factor also known as CHD1L (chromodomain helicase/ATPase DNA binding protein 1-like), is implicated in base-excision repair, where PARP (Poly(ADP-ribose) polymerase) mediated Poly(ADP-ribose) signaling facilitates the recruitment of this protein to damage sites. We here demonstrate the critical role played by ALC1 in the regulation of replication-fork progression in cleaved template strands. To analyze the role played by ALC1 as well as its functional relationship with PARP1, we generated ALC1-/-, PARP1-/-, and ALC1-/-/PARP1-/- cells from chicken DT40 cells. We then exposed these cells to camptothecin (CPT), a topoisomerase I poison that generates single-strand breaks and causes the collapse of replication forks. The ALC1-/- and PARP1-/- cells exhibited both higher sensitivity to CPT and an increased number of chromosome aberrations, compared with wild-type cells. Moreover, phenotypes were very similar across all three mutants, indicating that the role played by ALC1 in CPT tolerance is dependent upon the PARP pathway. Remarkably, inactivation of ALC1 resulted in a failure to slow replication-fork progression after CPT exposure, indicating that ALC1 regulates replication-fork progression at DNA-damage sites. We disrupted ATPase activity by inserting the E165Q mutation into the ALC1 gene, and found that the resulting ALC1-/E165Q cells displayed a CPT sensitivity indistinguishable from that of the null-mutant cells. This observation suggests that ALC1 contributes to cellular tolerance to CPT, possibly as a chromatin remodeler. This idea is supported by the fact that CPT exposure induced chromatin relaxation in the vicinity of newly synthesized DNA in wild-type but not in ALC1-/- cells. This implies a previously unappreciated role for ALC1 in DNA replication, in which ALC1 may regulate replication-fork slowing at CPT-induced DNA-damage sites.
Introduction
ALC1 is a member of the SNF2 superfamily of ATPases, which can function as chromatin-remodeling enzymes [1–3]. ALC1 possesses a macrodomain that recognizes Poly(ADP-ribose) (PAR) in vitro and in vivo [4, 5]. PAR is generated by Poly(ADP-ribose) polymerase (PARP), which is activated by single-strand DNA breaks (SSBs) and gaps [6, 7]. SSBs occur during base-excision repair (BER) [8], which removes base damage, including oxidation and alkylation of bases [8, 9]. Formation of PAR at chromatin proteins in the vicinity of SSBs facilitates the recruitment of ALC1 and other BER factors to damaged-base sites [4, 10]. PARP1 stimulates the chromatin-repositioning enzyme activity of ALC1 [11, 12]. Collaboration between ALC1 and PARP is also implicated in the nucleotide-excision repair of ultraviolet light (UV)-induced DNA damage [13]. It remains unclear whether ALC1 plays a role in DNA replication.
Camptothecins (CPTs) have emerged as a promising chemotherapeutic agent for cancer [14, 15]. These drugs target topoisomerase I (TOPI), which mediates DNA nicking, rotation, and resealing associated with DNA relaxation during replication [14, 15]. CPT interferes with TOPI after DNA nicking, resulting in a covalently attached TOPI-DNA cleavage complex (TOPI-cc) at the 3’ SSB end. DNA double-strand breaks (DSBs) are created by the collision of replication forks with TOPI-cc [16–19], which explains why rapidly cycling cells exhibit a strong sensitivity to CPT [14, 15]. PARP1 is able to interact and play role with Tyrosyl-DNA phosphodiesterase 1 (TDP1), which eliminates TOP1 from TOPI-cc [20]. Although this decreases the number of collisions between TOPI-ccs and replication forks, PARP1 decreases the rate of replication-fork progression upon exposure of cells to CPT [21, 22]. Thus, PARP1 may decrease the progression of replication forks independently of the activation of TDP1 by PARP, which would further reduce the number of collisions between TOP1-ccs and replication forks. Thus, PARP1 contributes to cellular tolerance to CPT via two independent mechanisms: the activation of TDP1 and the regulation of replication-fork progression following DNA damage. It is unclear whether ALC1 collaborates with PARP1 in the latter mechanism.
Using a PARP1-/- clone generated from the chicken DT40 B cell line is advantageous for analyzing the PARP pathway, as PARP1-/- DT40 cells are equivalent to PARP1-/-/PARP2-/- mammalian cells due to the absence of the PARP2 gene in the chicken genome [23]. In this study, we explore the role played by ALC1 as well as its functional relationship with the PARylation pathway. ALC1-/- cells were more sensitive to CPT than were wild-type cells, while loss of ALC1 had no detectable impact on the sensitivity of PARP1-/- cells to CPT. This data establishes an epistatic relationship between ALC1 and PARP1 in cellular tolerance to CPT. Moreover, loss of ALC1 resulted in the failure of safe replication-fork arrest. In summary, our data suggest that ALC1 and PARP1 collaborate to mediate safe replication-fork arrest at CPT-induced TopI-cc sites. This study unveils the previously unappreciated role played by ALC1 in regulating replication-fork progression to prevent the collapse of replication forks at TopI-cc sites.
Materials and methods
DT40 cell culture, ALC1 targeting construct, and ALC1-ATPase dead knock-in construct
The DT40 cell line was obtained from the Takeda laboratory (Kyoto University) [24]. DT40 cells were cultured in RPMI-1640 medium supplemented with 50 μM β-mercaptoethanol, penicillin, streptomycin, 10% fetal calf serum, and 1% chicken serum (Gibco) at 39.5°C. Methods used to generate the ALC1-targeting construct and the ALC1-ATPase dead knock-in construct were as described previously [25].
Generation of ALC1-/- and ALC1-/-/PARP1-/- mutant cells
We sequentially transfected ALC1-bsr and ALC1-hisD with targeting constructs to obtain ALC1-/- cells from wild-type DT40 cells as described previously [25]. We similarly generated ALC1-/-/PARP1-/- mutants from previously established PARP1-/- DT40 cells [26].
Measurement of sensitivity to genotoxic agents
Camptothecin (TopoGEN, Inc.), olaparib (AZD-2281, Astrazeneca), etoposide (VP16, Funakoshi), ICRF-193 (Funakoshi) and cis-diamminedichloroplatinum(II) (cisplatin, Nippon Kayaku) were used for the sensitivity assay, as described previously [27–30]. 104 cells were plated in duplicate onto 24-well cluster plates containing 1m of complete medium supplemented with the above-mentioned reagents and further incubated for 48 h. 3 × 105 cells were exposed to UV or irradiated by a 137Cs γ-ray source. 104 cells were then plated in duplicate onto 24-well cluster plates containing 1m of complete medium and culture for 48 h. 100 μl of incubated cell were transferred to 96-well plates and measured the amount of ATP using CellTiter-Glo (Promega), according to the manufacturer's instructions. Luminescence was measured by Fluoroskan Ascent FL (Thermo Fisher Scientific Inc, Whaltham, MA)
Chromosomal aberration analysis
DT40 clones were treated with 0.06% colcemid (Gibco BRL) for 2.5 h to arrest cells in the M-phase. Cells were pelleted by centrifugation, resuspended in 1 ml of 75 mM KCl for 15 min at room temperature, and fixed in 5 ml of a freshly prepared 3:1 mixture of methanol and acetic acid (Carnoy’s solution). The pelleted cells were then resuspended in 5 ml of Carnoy’s solution, dropped onto clean glass slides and air-dried. The slides were stained with a 5% HARLECO Giemsa stain solution (Nacalai Tesque) for 10 min, rinsed with water and acetone, and dried. All chromosomes in each mitotic cell were scored at 1000 × magnification.
DNA fiber assay
The DNA fiber assay was performed as previously described [27, 28], with a slight modification of the labeling method for the replicated tract. Cells were sequentially labeled for 15 min with 25 μM CldU and for 15 min with 250 μM IdU. Fiber length was measured using Image J (https://imagej.nih.gov/ij/), and the CldU/IdU ratio was calculated. Measurements were recorded from areas of the slides with untangled DNA fibers to prevent the possibility of recording labeled patches from tangled bundles of fibers.
Micrococcal nuclease digestion assay
5 ×107 DT40 cells were pulse-labeled with 20 μM BrdU for 10 min. Cells were then harvested and washed and resuspended in medium either with CPT (20 μM) or without CPT and further cultured for 15 min. Partial digestion of chromatin DNA with Micrococcal nuclease (MNase) was performed as described previously [31–33], with slight modifications. Briefly, the above-mentioned BrdU-labeled cells were suspended in 0.5 ml of lysis buffer (18% Ficoll 400, 10 mM KH2PO4, 10 mM K2HPO4, 1 mM MgCl2, 0.25 mM EGTA, 0.25 mM EDTA, and 1 mM Pefabloc SC [Roche, Mannheim, Germany]). After centrifugation at 14,000 rpm for 30 min at 4°C, the crude chromatin fraction was resuspended in 0.3 ml of buffer A (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 5 mM KCl, and 1 mM EDTA) containing a proteinase inhibitor cocktail (Complete, Roche). After addition of CaCl2 (5 mM final concentration), 0.1 ml aliquots of crude chromatin suspension were digested with several different amounts of MNase (0, 5, 10, and 20 U/ml) at 37°C for 5 min. The reaction was terminated by adding 25 mM EDTA, and DNA was purified. DNA samples were resolved in 2% agarose gel electrophoresis followed by membrane transfer and immunodetection using anti-BrdU antibody (Roche).
Results and discussion
ALC1-/- cells are moderately sensitive to camptothecin
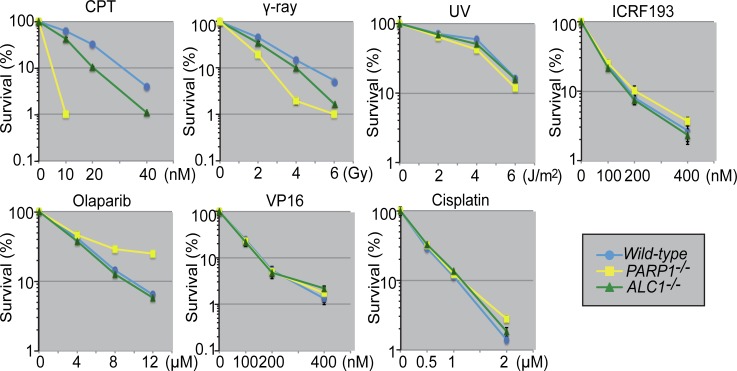
To determine in which DNA-repair pathways ALC1 plays a role, we generated ALC1-/- cells from the chicken DT40 cell line and measured cell survival after exposure to a wide variety of DNA-damaging agents. ALC1-/- and wild-type cells exhibited an indistinguishable sensitivity to cisplatin, UV, VP16, ICRF-193, and olaparib, but the ALC1-/- cells were moderately more sensitive to CPT than wild-type cells and showed four-fold reduction of viability in comparison to wild-type cells (Fig 1, at 40 nM CPT, wild-type and ALC1-/- cells showed 4% and 1% of survived cells, respectively). PARP1-/- cells were critically more sensitive to CPT than wild-type and ALC1-/- cells and showed 60-fold reduction of viability in comparison to wild-type cells (Fig 1, at 10 nM CPT, wild-type and PARP1-/- cells showed 60% and 1% of survived cells, respectively). These data indicate that ALC1 is involved in the cellular tolerance to CPT. Given the role ALC1 plays in PARP-mediated DNA repair [4, 11–13, 25, 34], we suggest that the two repair factors may work along a common pathway.
Fig 1. Role played by ALC1 in cell tolerance to CPT.
Indicated cells were incubated in medium containing the following DNA-damaging agents at 39.5°C for 48 h: CPT (camptothecin, a topoisomerase 1 poison), γ-ray, UV, ICRF-193 (a catalytic topoisomerase 2 inhibitor), olaparib (a PARP inhibitor), VP16 (a topoisomerase 2 poison), and cisplatin (cis-diamminedichloroplatinum[II]). Then, cell viability was assessed by ATP assay as described in materials and methods. Dosage is displayed on the x-axis on a linear scale, while cell-survival percentage is displayed on the y-axis on a logarithmic scale. Error bars represent standard deviations from three independent experiments.
ALC1 and PARP1 have an epistatic relationship in cellular tolerance to CPT
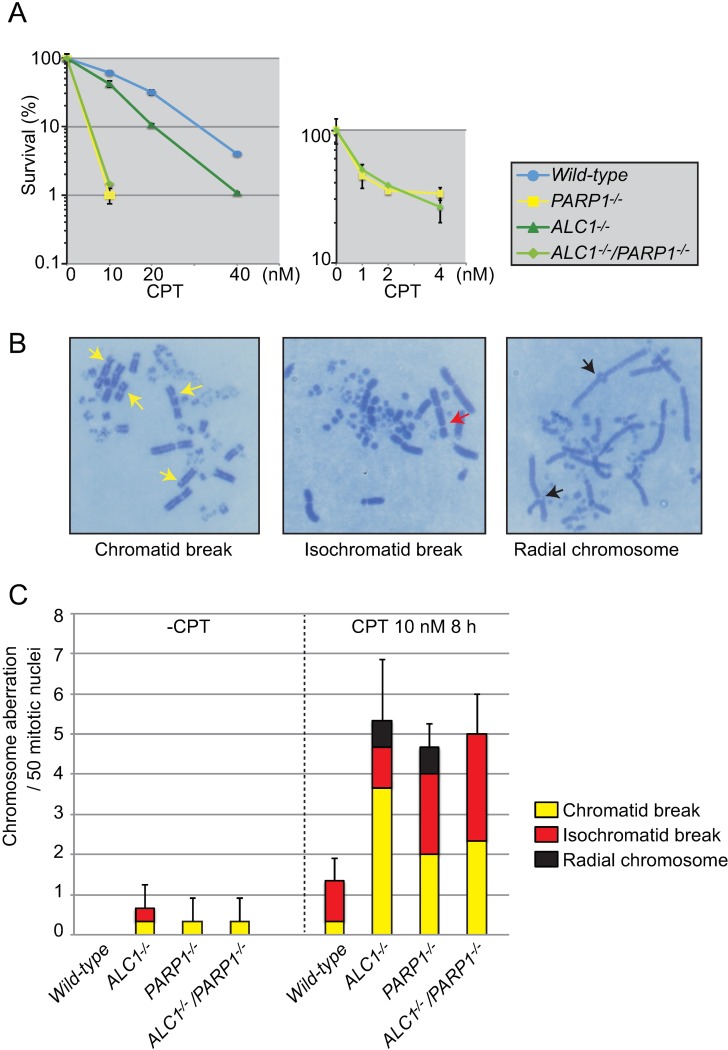
We next compared ALC1-/-, PARP1-/-, and ALC1-/-/PARP1-/- cells for sensitivity to CPT (Fig 2A). The ALC1-/- cells were less sensitive than the PARP1-/- cells, with the PARP1-/- and ALC1-/-/PARP1-/- cells exhibiting a similar sensitivity (Fig 2A). We therefore conclude that ALC1 and PARP1 collaborate in contributing to cellular tolerance to CPT. ALC1-/-, PARP1-/-, and ALC1-/-/PARP1-/- cells all showed an increase in the number of chromosome breaks after exposure to CPT, with the PARP1-/- and ALC1-/-/PARP1-/- cells exhibiting a very similar number of chromosome aberrations (Fig 2B). Note that loss of PARP1 in ALC1-/- cells changed the type of chromosome aberrations and resulted in an increase of isochromatid breaks (i.e., breaks at the same site of both sister chromatids.) This type of break is likely caused by defective resolution of recombination intermediates [30], and thus implying a possible involvement of PARP1 in the resolution of recombination intermediates.
Fig 2. Epistatic relationship between ALC1-/- and PARP1-/- in cellular tolerance to CPT.
(A) Cells with indicated genotypes were assessed for CPT sensitivity as in Fig 1. Dose is displayed on the x-axis, while the cell-survival percentage is displayed on y-axis. Error bars represent standard deviations from three independent experiments. The data for wild-type, ALC1-/- and PARP1-/- were same with the data in Fig 1. (B) Representative images showing DT40 chromosomes. Chromosomes from wild-type DT40 cells treated with mitomycinC were analyzed as described in Materials and Methods. The yellow, red and black arrows indicate chromatid breaks, isochromatid breaks and radial chromosomes, respectively. (C) Number of chromatid breaks, isochromatid breaks, and radial chromosomes in 50 mitotic cells. Chicken DT40 cells were exposed to CPT (10 nM) for 8 h with colcemid added 2.5 h before harvest to accumulate mitotic cells. Error bars represent standard deviations from three independent experiments.
ALC1 induces chromatin relaxation around TOPI-cc
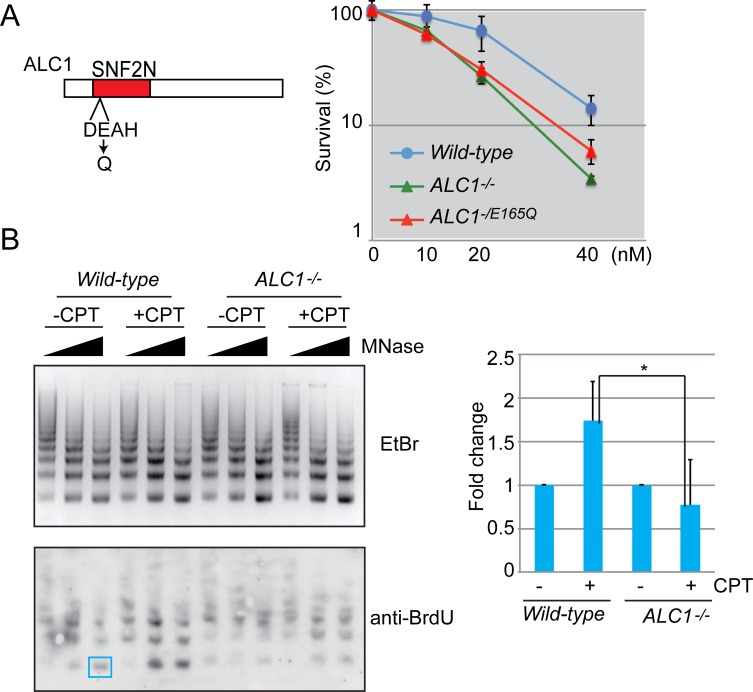
To determine ALC1’s function as a chromatin-remodeler, we measured cellular sensitivity to CPT in ALC1-/E165Q mutant cells, in which the ATPase activity of ALC1 is inactivated by mutating the essential E165 to Q (the same mutation used in previous biochemical studies [4, 11]) (Fig 3A). ALC1-/E165Q and ALC1-/- cells showed a virtually identical CPT sensitivity (Fig 3A), indicating that the chromatin-remodeling activity of ALC1 is required for cellular tolerance to CPT. This requirement suggests that ALC1 might induce chromatin relaxation around TOPI-cc and thereby participate in the CPT-tolerance mechanism. To examine this hypothesis, we analyzed the degree of chromatin condensation around replication forks stalled at TOPI-cc using an MNase digestion assay. We labeled newly replicated DNA with BrdU for 10 min, treated it with CPT for 15 min, then detected it with an anti-BrdU antibody (Fig 3B). The partially digested product corresponding to mononucleosomes (146 bp DNA, blue box in Fig 3B) was quantified. Following treatment with 20 μM CPT, MNase sensitivity was significantly increased and mononucleosomes were more efficiently digested in wild-type cells (Fig 3B), suggesting a change of chromatin state into a more open configuration. In marked contrast, MNase sensitivity in ALC1-/- cells was slightly reduced by the CPT treatment, suggesting that chromatin relaxation around the TOPI-cc site cannot be maintained in the absence of ALC1. We thus conclude that CPT induces chromatin decondensation and that ALC1 might be required for this process. This conclusion is consistent with the recently demonstrated pivotal role played by ALC1 in chromatin remodeling at DNA damages induced by laser microirradiation [34].
Fig 3. ALC1 induces chromatin relaxation around TOPI-cc.
(A) (Left) Schematic representation of the ALC1 domain. Glu-165 on ALC1 was altered by Gln to inhibit the ATPase activity of ALC1. (Right) The ALC1-ATPase dead mutant (ALC1-/E165Q) and the ALC1-/- cells showed a similar sensitivity to CPT. Error bars represent standard deviations from three independent experiments. (B) Nucleosome assembly in close proximity to TOPI-cc. 5 ×107 wild-type and ALC1-/- cells were pulse-labeled with BrdU for 10 min followed by treatment with CPT (20 μM) for 15 min. Nuclei were prepared and treated with 5, 10, and 20 units/ml MNase for 5 min. DNA was resolved in 2% agarose gel and stained with ethidium bromide (EtBr) (upper panel), transferred onto a membrane, and detected with an anti-BrdU antibody (lower panel). Blue box indicates quantified band corresponding to mononucleosome. P-value was calculated by Student’s t-test (*P<0.05).
Distinct contribution of the ALC1-PARP1 and RNF8 pathways to CPT tolerance
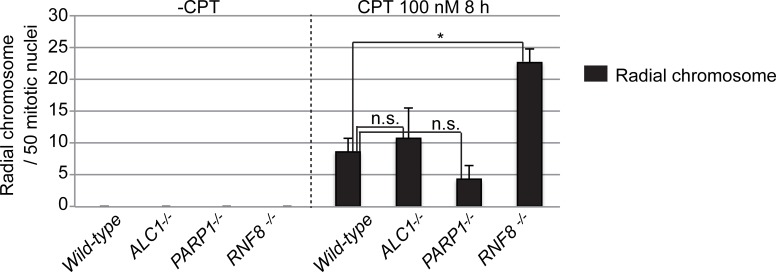
RNF8-/- cells showed a higher sensitivity to CPT than did wild-type cells [35], a phenotypic trait that is similar to that of ALC1-/- and PARP1-/-. We demonstrated that RNF8 suppressed toxic non-homologous end-joining (NHEJ), as evidenced by the increased number of radial chromosomes in the RNF8-/- cells [35]. This phenomenon occurs mainly as a consequence of aberrant NHEJ, as the number of radial chromosome events in NHEJ-deficient KU70-/- cells is several times lower than in wild-type cells [35, 36]. To examine whether the collaborative ALC1-PARP pathway also contributes to CPT tolerance by suppressing toxic NHEJ, we measured the number of radial chromosomes. Strikingly, there was no increase in radial chromosomes in either ALC1-/- or PARP1-/-, whereas the number of radial chromosome in RNF8-/- was two times higher than in wild-type cells (Fig 4). We thus conclude that the ALC1-PARP pathway contributes to CPT tolerance by some other mechanism(s) than the suppression of toxic NHEJ. This conclusion is consistent with our previous study [26, 35], which showed that RNF8 and RAD18 collaboratively suppress toxic NHEJ following CPT damage [35], whilePARP1 contributes to cellular tolerance to CPT independently of RAD18 [26].
Fig 4. The ALC1-PARP1 axis is not required for the suppression of toxic NHEJ following CPT exposure.
Number of radial chromosomes in 50 mitotic cells. DT40 cells were exposed to CPT (100 nM) for 8 h with colcemid added 2.5 h before harvest to accumulate mitotic cells. Error bars represent standard deviations from three independent experiments. P-value was calculated by a Student’s t-test (*P<0.05); n.s. = not significant.
Role played by ALC1 in the regulation of replication forks at CPT-induced lesions
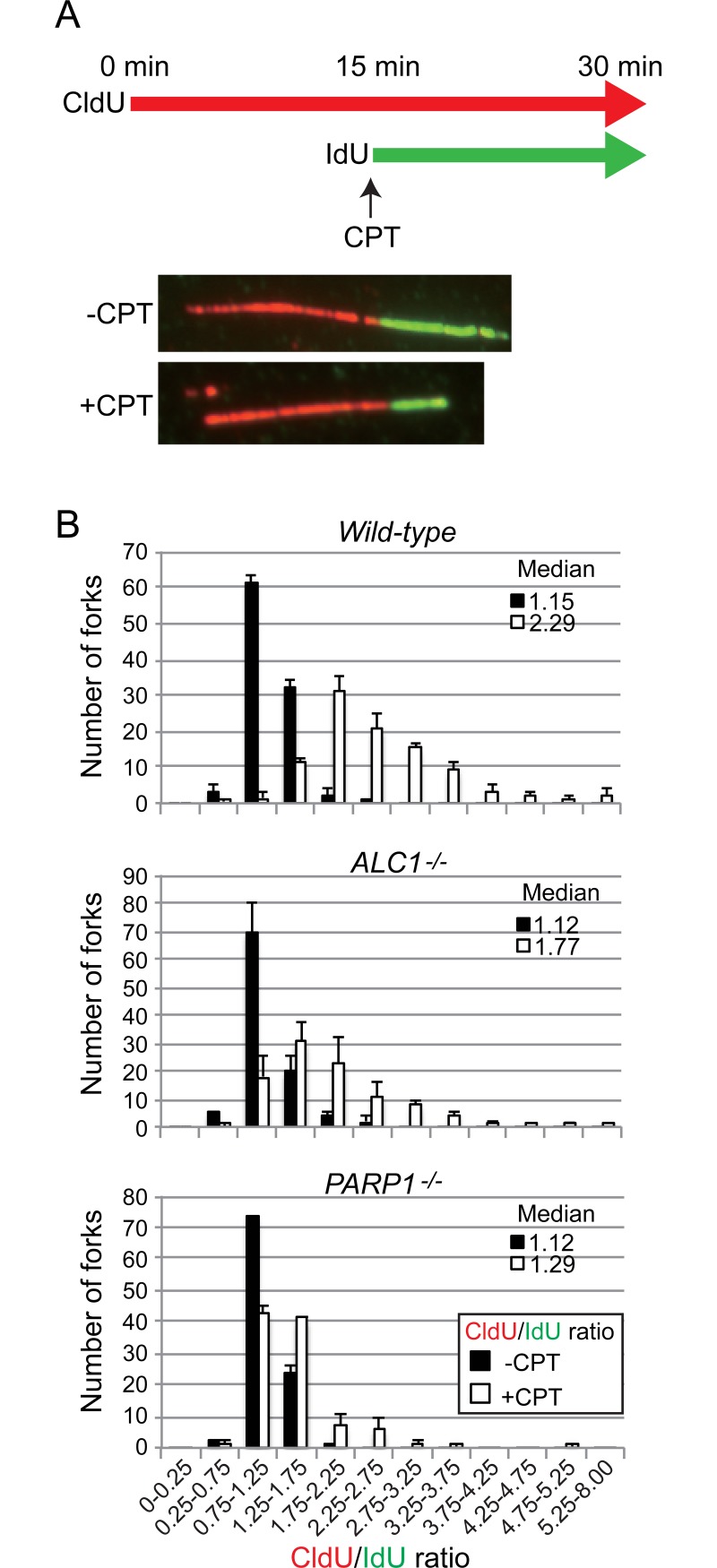
Since CPT induces replication-fork slowing [21, 22, 37], we next investigated what role ALC1 might play in the regulation of replication forks. We measured the length of the replicated tract before (CldU) and after (IdU) CPT treatment in ALC1-/-, PARP1-/-, and wild-type cells, then compared the CldU/IdU ratios (Fig 5). Consistent with our previous study, replication tracts in the wild-type cells were significantly shortened following CPT exposure, with a median CldU/IdU ratio of 2.29± 0.15 (Fig 5). In ALC1-/- cells, replication-fork slowing was partially impaired, with a median CldU/IdU ratio of 1.77± 0.08 (Fig 5). PARP1-/- cells showed an even more pronounced defect in replication-fork slowing (median CldU/IdU ratio of 1.29± 0.01). These results indicate that ALC1 is involved in the regulation of replication-fork slowing. The critical role played by the PARP pathway in safe fork arrest [21, 22] and the data showing an epistatic relationship between ALC1 and PARP1 in CPT tolerance combine to suggest that ALC1 may collaborate with the PARP pathway in the regulation of replication forks at TopI-cc sites.
Fig 5. Role played by the ALC1-PARP1 pathway in safe fork-slowing following CPT exposure.
(A) Representative images showing stained DNA fibers. DT40 cells were labeled sequentially with CldU and IdU with or without CPT treatment after CldU labeling. (B) Distribution of CldU/IdU ratios for replication forks in cells exposed to CPT. Indicated cells were incubated in medium containing CldU (25 μM) for 15 min, then incubated in medium containing IdU (250 μM) with CPT (10 μM) or without CPT for 15 min. The CldU/IdU ratios are shown on the x-axis. The number of fibers in each section is shown on the y-axis. 100 forks from each cell line were analyzed. Error bars represent standard deviations from three independent analyses.
In this study, we uncovered a previously unappreciated function of ALC1 in the regulation of DNA replication, wherein ALC1 slows replication-fork progression at TOPI-cc under the PARP pathway and thereby prevents replication-fork collapse. This conclusion is supported by a DNA-fiber assay demonstrating that ALC1 slows the progression of replication forks after CPT treatment (Fig 5). These data unveil the role played by ALC1 in the regulation of replication-fork progression following DNA damage. ALC1 contributes to cellular tolerance to DNA damage through multiple mechanisms: promotion of base-excision-repair pathways and prevention of DNA replication-fork collapse following DNA damage to template strands [4, 11–13, 38].
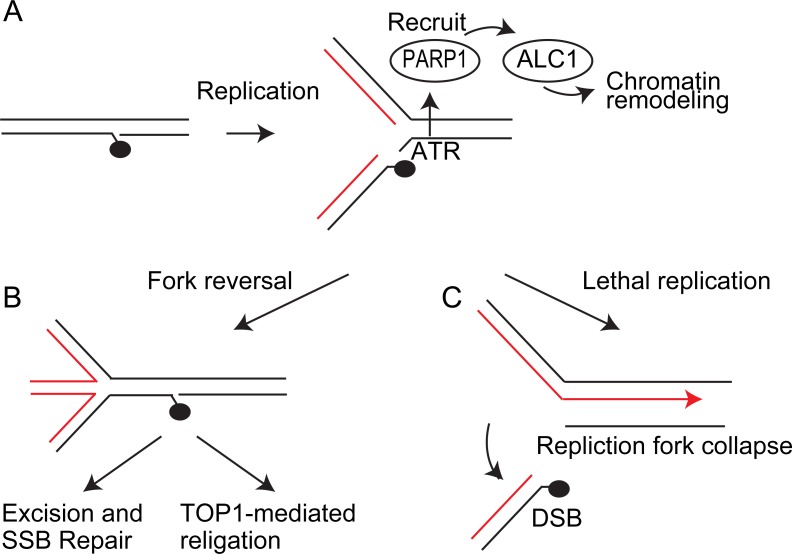
An important question is, how does ALC1 contribute to the regulation of replication forks? It is possible that, during fork-slowing, some aspect of the PARP-ALC1 axis is under the control of the S-phase-checkpoint pathway that comprises ATR-CHK1 [39]. TopI-cc interferes with DNA replication by avoiding origin firing and fork progression via the ATR-CHK1 signal pathway [37]. One possible scenario is that transient replication-fork arrest at TOPI-cc sites activates the ATR-CHK1 checkpoint (Fig 6A), which in turn activates the PARP-ALC1 axis, leading to fork reversal in which the newly synthesized strands are annealed to one another (Fig 6B). This hypothesis is supported by previous reports that visualize fork reversal at TOPI-cc and other DNA damage sites on template strands using an electronic microscope [21, 40]. Fork reversal might also require homologous recombination mechanisms, including the RAD51-XRCC3 complex [41]. As a result of fork reversal for the safe replication fork slowing, TOP1-cc at the 3’ SSB end might be efficiently excised by nucleases including TDP1 (Fig 6B). In addition, removal of CPT allows TOP1-mediated religation, [14, 15] (Fig 6B). Loss of fork reversal and following lethal replication may result in one-end DSB at fork leading to fork collapse (Fig 6C).
Fig 6. A model.
Schematic representation of the model showing the regulation of safe replication fork arrest at TopI-cc by PARP-ALC1 pathway. (A) Replication-fork arrest at TOP1-cc activates the ATR-CHK1 checkpoint, which in turn activates the PARP1. PARylation via PARP1 enzyme mediates recruitment of ALC1. ALC1 mediates chromatin remodeling and facilitates replication-fork reversal. (B) As a result of fork reversal, TOP1-cc is located at the 3’ end of SSB and efficiently excised and repaired. (C) Lethal replication results in one-end DSB and following fork collapse.
The ALC1 gene is frequently amplified and overexpressed in human hepatocellular carcinoma and numerous solid tumors. This overexpression is associated with lymph-node metastasis, tumor differentiation, and distant metastasis [42, 43], suggesting that the ALC1 gene plays a role in invasion and metastasis of cancer cells. In contrast to the hypoxic microenvironment of solid tumors, malignant cells are exposed to a high concentration of oxygen during hematogenous metastasis. One possible explanation is that the overexpression of ALC1 might increase cellular tolerance to oxidative stress during metastasis. Further analysis of ALC1’s role might clarify the role of ALC1 in cancer development.
Acknowledgments
We would like to thank the Radioisotope Research Center in Tokyo Metropolitan University for their support in the use of isotopes. Olaparib was supplied by Astrazeneca.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the JSPS KAKENHI Grant Number (JP16K12598, JP16H02957 and JP16H01314 to KH, and JP16H06306 to ST), the JSPS Core-to-Core Program (A) Advanced Research Networks (to ST), the Takeda Science Foundation and Yamada Science Foundation (to KH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Eisen JA, Sweder KS, Hanawalt PC. Evolution of the SNF2 family of proteins: subfamilies with distinct sequences and functions. Nucleic Acids Res. 1995;23(14):2715–23. Epub 1995/07/25. doi: 5a0167 [pii]. ; PubMed Central PMCID: PMC307096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flaus A, Martin DM, Barton GJ, Owen-Hughes T. Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res. 2006;34(10):2887–905. Epub 2006/06/02. doi: 34/10/2887 [pii] doi: 10.1093/nar/gkl295 ; PubMed Central PMCID: PMC1474054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorbalenya AE, Koonin EV, Donchenko AP, Blinov VM. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989;17(12):4713–30. Epub 1989/06/26. ; PubMed Central PMCID: PMC318027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahel D, Horejsi Z, Wiechens N, Polo SE, Garcia-Wilson E, Ahel I, et al. Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science. 2009;325(5945):1240–3. Epub 2009/08/08. doi: 1177321 [pii] doi: 10.1126/science.1177321 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karras GI, Kustatscher G, Buhecha HR, Allen MD, Pugieux C, Sait F, et al. The macro domain is an ADP-ribose binding module. EMBO J. 2005;24(11):1911–20. Epub 2005/05/20. doi: 7600664 [pii] doi: 10.1038/sj.emboj.7600664 ; PubMed Central PMCID: PMC1142602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croset A, Cordelieres FP, Berthault N, Buhler C, Sun JS, Quanz M, et al. Inhibition of DNA damage repair by artificial activation of PARP with siDNA. Nucleic Acids Res. 2013;41(15):7344–55. doi: 10.1093/nar/gkt522 ; PubMed Central PMCID: PMCPMC3753643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Silva I, Pelletier JD, Lagueux J, D'Amours D, Chaudhry MA, Weinfeld M, et al. Relative affinities of poly(ADP-ribose) polymerase and DNA-dependent protein kinase for DNA strand interruptions. Biochim Biophys Acta. 1999;1430(1):119–26. . [DOI] [PubMed] [Google Scholar]
- 8.Gupte R, Liu Z, Kraus WL. PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes. Genes Dev. 2017;31(2):101–26. doi: 10.1101/gad.291518.116 ; PubMed Central PMCID: PMCPMC5322727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu J, Selby CP, Adar S, Adebali O, Sancar A. Molecular mechanisms and genomic maps of DNA excision repair in E.coli and humans. J Biol Chem. 2017. doi: 10.1074/jbc.R117.807453 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanzlikova H, Gittens W, Krejcikova K, Zeng Z, Caldecott KW. Overlapping roles for PARP1 and PARP2 in the recruitment of endogenous XRCC1 and PNKP into oxidized chromatin. Nucleic Acids Res. 2016. doi: 10.1093/nar/gkw1246 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottschalk AJ, Timinszky G, Kong SE, Jin J, Cai Y, Swanson SK, et al. Poly(ADP-ribosyl)ation directs recruitment and activation of an ATP-dependent chromatin remodeler. Proc Natl Acad Sci U S A. 2009;106(33):13770–4. Epub 2009/08/12. doi: 0906920106 [pii] doi: 10.1073/pnas.0906920106 ; PubMed Central PMCID: PMC2722505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottschalk AJ, Trivedi RD, Conaway JW, Conaway RC. Activation of the SNF2 family ATPase ALC1 by poly(ADP-ribose) in a stable ALC1.PARP1.nucleosome intermediate. J Biol Chem. 2012;287(52):43527–32. doi: 10.1074/jbc.M112.401141 ; PubMed Central PMCID: PMCPMC3527939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pines A, Vrouwe MG, Marteijn JA, Typas D, Luijsterburg MS, Cansoy M, et al. PARP1 promotes nucleotide excision repair through DDB2 stabilization and recruitment of ALC1. J Cell Biol. 2012;199(2):235–49. doi: 10.1083/jcb.201112132 ; PubMed Central PMCID: PMCPMC3471223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6(10):789–802. Epub 2006/09/23. doi: nrc1977 [pii] doi: 10.1038/nrc1977 . [DOI] [PubMed] [Google Scholar]
- 15.Pommier Y. DNA topoisomerase I inhibitors: chemistry, biology, and interfacial inhibition. Chem Rev. 2009;109(7):2894–902. doi: 10.1021/cr900097c ; PubMed Central PMCID: PMCPMC2707511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holm C, Covey JM, Kerrigan D, Pommier Y. Differential requirement of DNA replication for the cytotoxicity of DNA topoisomerase I and II inhibitors in Chinese hamster DC3F cells. Cancer Res. 1989;49(22):6365–8. . [PubMed] [Google Scholar]
- 17.Hsiang YH, Lihou MG, Liu LF. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989;49(18):5077–82. . [PubMed] [Google Scholar]
- 18.Strumberg D, Pilon AA, Smith M, Hickey R, Malkas L, Pommier Y. Conversion of topoisomerase I cleavage complexes on the leading strand of ribosomal DNA into 5'-phosphorylated DNA double-strand breaks by replication runoff. Mol Cell Biol. 2000;20(11):3977–87. ; PubMed Central PMCID: PMCPMC85758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takemura H, Rao VA, Sordet O, Furuta T, Miao ZH, Meng L, et al. Defective Mre11-dependent activation of Chk2 by ataxia telangiectasia mutated in colorectal carcinoma cells in response to replication-dependent DNA double strand breaks. J Biol Chem. 2006;281(41):30814–23. doi: 10.1074/jbc.M603747200 . [DOI] [PubMed] [Google Scholar]
- 20.Das BB, Huang SY, Murai J, Rehman I, Ame JC, Sengupta S, et al. PARP1-TDP1 coupling for the repair of topoisomerase I-induced DNA damage. Nucleic Acids Res. 2014;42(7):4435–49. doi: 10.1093/nar/gku088 ; PubMed Central PMCID: PMCPMC3985661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray Chaudhuri A, Hashimoto Y, Herrador R, Neelsen KJ, Fachinetti D, Bermejo R, et al. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat Struct Mol Biol. 2012;19(4):417–23. doi: 10.1038/nsmb.2258 . [DOI] [PubMed] [Google Scholar]
- 22.Sugimura K, Takebayashi S, Taguchi H, Takeda S, Okumura K. PARP-1 ensures regulation of replication fork progression by homologous recombination on damaged DNA. J Cell Biol. 2008;183(7):1203–12. doi: 10.1083/jcb.200806068 ; PubMed Central PMCID: PMCPMC2606964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ball LG, Xiao W. Molecular basis of ataxia telangiectasia and related diseases. Acta Pharmacol Sin. 2005;26(8):897–907. Epub 2005/07/26. doi: 10.1111/j.1745-7254.2005.00165.x . [DOI] [PubMed] [Google Scholar]
- 24.Buerstedde JM, Takeda S. Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell. 1991;67(1):179–88. . [DOI] [PubMed] [Google Scholar]
- 25.Tsuda M, Cho K, Ooka M, Shimizu N, Watanabe R, Yasui A, et al. ALC1/CHD1L, a chromatin-remodeling enzyme, is required for efficient base excision repair. PloS One. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saberi A, Hochegger H, Szuts D, Lan L, Yasui A, Sale JE, et al. RAD18 and poly(ADP-ribose) polymerase independently suppress the access of nonhomologous end joining to double-strand breaks and facilitate homologous recombination-mediated repair. Mol Cell Biol. 2007;27(7):2562–71. Epub 2007/01/24. doi: MCB.01243-06 [pii] doi: 10.1128/MCB.01243-06 ; PubMed Central PMCID: PMC1899888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirota K, Tsuda M, Mohiuddin, Tsurimoto T, Cohen IS, Livneh Z, et al. In vivo evidence for translesion synthesis by the replicative DNA polymerase delta. Nucleic Acids Res. 2016;44(15):7242–50. doi: 10.1093/nar/gkw439 ; PubMed Central PMCID: PMCPMC5009730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirota K, Yoshikiyo K, Guilbaud G, Tsurimoto T, Murai J, Tsuda M, et al. The POLD3 subunit of DNA polymerase delta can promote translesion synthesis independently of DNA polymerase zeta. Nucleic Acids Res. 2015;43(3):1671–83. doi: 10.1093/nar/gkv023 ; PubMed Central PMCID: PMCPMC4330384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ooka M, Kobayashi K, Abe T, Akiyama K, Hada M, Takeda S, et al. Determination of genotoxic potential by comparison of structurally related azo dyes using DNA repair-deficient DT40 mutant panels. Chemosphere. 2016;164:106–12. doi: 10.1016/j.chemosphere.2016.08.092 . [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto KN, Kobayashi S, Tsuda M, Kurumizaka H, Takata M, Kono K, et al. Involvement of SLX4 in interstrand cross-link repair is regulated by the Fanconi anemia pathway. Proc Natl Acad Sci U S A. 2011;108(16):6492–6. doi: 10.1073/pnas.1018487108 ; PubMed Central PMCID: PMC3080998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asada R, Takemata N, Hoffman CS, Ohta K, Hirota K. Antagonistic controls of chromatin and mRNA start site selection by Tup family corepressors and the CCAAT-binding factor. Mol Cell Biol. 2015;35(5):847–55. doi: 10.1128/MCB.00924-14 ; PubMed Central PMCID: PMCPMC4323495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirota K, Miyoshi T, Kugou K, Hoffman CS, Shibata T, Ohta K. Stepwise chromatin remodelling by a cascade of transcription initiation of non-coding RNAs. Nature. 2008;456(7218):130–4. Epub 2008/09/30. doi: nature07348 [pii] doi: 10.1038/nature07348 . [DOI] [PubMed] [Google Scholar]
- 33.Hirota K, Mizuno K, Shibata T, Ohta K. Distinct chromatin modulators regulate the formation of accessible and repressive chromatin at the fission yeast recombination hotspot ade6-M26. Mol Biol Cell. 2008;19(3):1162–73. Epub 2008/01/18. doi: E07-04-0377 [pii] doi: 10.1091/mbc.E07-04-0377 ; PubMed Central PMCID: PMC2262960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sellou H, Lebeaupin T, Chapuis C, Smith R, Hegele A, Singh HR, et al. The poly(ADP-ribose)-dependent chromatin remodeler Alc1 induces local chromatin relaxation upon DNA damage. Mol Biol Cell. 2016;27(24):3791–9. doi: 10.1091/mbc.E16-05-0269 ; PubMed Central PMCID: PMCPMC5170603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi S, Kasaishi Y, Nakada S, Takagi T, Era S, Motegi A, et al. Rad18 and Rnf8 facilitate homologous recombination by two distinct mechanisms, promoting Rad51 focus formation and suppressing the toxic effect of nonhomologous end joining. Oncogene. 2015;34(33):4403–11. doi: 10.1038/onc.2014.371 . [DOI] [PubMed] [Google Scholar]
- 36.Bunting SF, Callen E, Wong N, Chen HT, Polato F, Gunn A, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141(2):243–54. Epub 2010/04/07. doi: S0092-8674(10)00285-0 [pii] doi: 10.1016/j.cell.2010.03.012 ; PubMed Central PMCID: PMC2857570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seiler JA, Conti C, Syed A, Aladjem MI, Pommier Y. The intra-S-phase checkpoint affects both DNA replication initiation and elongation: single-cell and -DNA fiber analyses. Mol Cell Biol. 2007;27(16):5806–18. doi: 10.1128/MCB.02278-06 ; PubMed Central PMCID: PMCPMC1952133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuda M, Cho K, Ooka M, Shimizu N, Watanabe R, Yasui A, et al. ALC1/CHD1L, a chromatin-remodeling enzyme, is required for efficient base excision repair. PLoS One. 2017;12(11):e0188320 doi: 10.1371/journal.pone.0188320 ; PubMed Central PMCID: PMCPMC5693467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saldivar JC, Cortez D, Cimprich KA. The essential kinase ATR: ensuring faithful duplication of a challenging genome. Nat Rev Mol Cell Biol. 2017. doi: 10.1038/nrm.2017.67 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zellweger R, Dalcher D, Mutreja K, Berti M, Schmid JA, Herrador R, et al. Rad51-mediated replication fork reversal is a global response to genotoxic treatments in human cells. J Cell Biol. 2015;208(5):563–79. doi: 10.1083/jcb.201406099 ; PubMed Central PMCID: PMCPMC4347635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henry-Mowatt J, Jackson D, Masson JY, Johnson PA, Clements PM, Benson FE, et al. XRCC3 and Rad51 modulate replication fork progression on damaged vertebrate chromosomes. Mol Cell. 2003;11(4):1109–17. . [DOI] [PubMed] [Google Scholar]
- 42.Ma NF, Hu L, Fung JM, Xie D, Zheng BJ, Chen L, et al. Isolation and characterization of a novel oncogene, amplified in liver cancer 1, within a commonly amplified region at 1q21 in hepatocellular carcinoma. Hepatology. 2008;47(2):503–10. doi: 10.1002/hep.22072 . [DOI] [PubMed] [Google Scholar]
- 43.Mu QJ, Li HL, Yao Y, Liu SC, Yin CG, Ma XZ. Chromodomain Helicase/ATPase DNA-Binding Protein 1-Like Gene (CHD1L) Expression and Implications for Invasion and Metastasis of Breast Cancer. PLoS One. 2015;10(11):e0143030 doi: 10.1371/journal.pone.0143030 ; PubMed Central PMCID: PMCPMC4657932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.