Abstract
STAT3 is a master regulator of the immune responses. Here we show that M. tuberculosis-infected stat3fl/fl lysm cre mice, defective for STAT3 in myeloid cells, contained lower bacterial load in lungs and spleens, reduced granuloma extension but higher levels of pulmonary neutrophils. STAT3-deficient macrophages showed no improved control of intracellular mycobacterial growth. Instead, protection associated to elevated ability of stat3fl/fl lysm cre antigen-presenting cells (APCs) to release IL-6 and IL-23 and to stimulate IL-17 secretion by mycobacteria-specific T cells. The increased IL-17 secretion accounted for the improved control of infection since neutralization of IL-17 receptor A in stat3fl/fl lysm cre mice hampered bacterial control. APCs lacking SOCS3, which inhibits STAT3 activation via several cytokine receptors, were poor inducers of priming and of the IL-17 production by mycobacteria-specific T cells. In agreement, socs3fl/fl cd11c cre mice deficient of SOCS3 in DCs showed increased susceptibility to M. tuberculosis infection. While STAT3 in APCs hampered IL-17 responses, STAT3 in mycobacteria-specific T cells was critical for IL-17 secretion, while SOCS3 in T cells impeded IL-17 secretion. Altogether, STAT3 signalling in myeloid cells is deleterious in the control of infection with M. tuberculosis.
Author summary
We studied the role of STAT3, a major regulator of immunity, in the control of the infection with M. tuberculosis. Stat3fl/fl lysm cre mice, deficient in STAT3 in myeloid cells, showed lower bacterial levels in organs and reduced extension of lung granulomas after infection with M. tuberculosis. STAT3-deficient APCs stimulated with innate receptor agonists released high levels of IL-6 and IL-23, and promoted IL-17 production by mycobacteria-specific CD4+ T cells. Increased IL-17 levels accounted for the increased resistance to M. tuberculosis of the STAT3-deficient mice. Instead, stat3fl/fl lysm cre macrophages showed no improved control of mycobacterial growth. SOCS3 is a negative regulator of STAT3 activation. The ability of socs3fl/fl lysm cre APCs to secrete IL-6 and IL-23 and to stimulate IL-17 production by antigen-specific T cells was reduced. In agreement, mice lacking SOCS3 in DCs showed increased susceptibility to M. tuberculosis infection. Different to a role in myeloid cells, STAT3 expression by mycobacteria-specific T cells was required for IL-17 secretion while SOCS3 in T cells hampered IL-17 production. Therefore, despite STAT3 expression in T cells is required for Th17 differentiation, STAT3 in APCs hampers secretion of Th17 promoting cytokines and the secretion of IL-17 by mycobacteria-specific T cells and reduces the resistance of mice to infection with M. tuberculosis.
Introduction
Tuberculosis (TB), caused by infection with Mycobacterium tuberculosis, remains a leading public health problem worldwide. TB causes 9 million new cases and 1.5 million deaths each year [1]. However, host factors determining the outcome of infection are not completely understood.
A host counters mycobacterial infections primarily via TH1 immune responses that involve cellular effector mechanisms such as macrophage activation [2, 3]. IL-12 secreted by APCs is crucial for the differentiation and maintenance of IFN-γ-secreting antigen-specific TH1 cells [4, 5] and both IL-12 and IFN-γ mediate mycobacterial control in mice and man [6–9].
The transcription factor STAT3 is a central regulator of immunity, mediating inflammatory but also anti-inflammatory responses [10, 11]. The functions of STAT3 are pleiotropic. STAT3 is activated by phosphorylation in response to cytokines of the IFN-receptor family (such as IL-10) and by some members of the IL-2 receptor family that uses the common γ chain receptor or after stimulation of several receptor tyrosine kinases (EGF, CSF-1, and PDGF). Additionally, STAT3 is activated by the common signal transducing molecule gp130 utilized by the IL-6 receptor family [12], and in response to G-CSF and leptin as their receptors are homologous to gp130.
STAT3 is critical for defense against bacterial and fungal infections. Low IL-17 secreting T-cell proportions were reported in patients bearing STAT3 mutations. These patients were prone to chronic candidiasis and staphylococcal diseases [13]. Chronic candidiasis is frequently present in patients deficient in IL-17 receptor A [14]. STAT3 deficient patients may also display impaired immunity against chronic viral infections [15, 16].
In mice, knockout of STAT3 is lethal, so in vivo studies on STAT3 functions have been performed using conditional knock out mice. Stat3fl/fl lysm cre mice, deficient in STAT3 in myeloid cells, display enhanced susceptibility to endotoxic shock and develop chronic enterocolitis with age [17]. The phenotype of these animals is similar to IL-10-/- mice, including increased expression of TNF and other inflammatory cytokines, since IL-10 suppresses induction of TNF-α via STAT3 [18]. Recently, STAT3 was shown to favour intracellular growth of M. tuberculosis in human macrophages [19]. Moreover, the presence of pSTAT3+ monocytes associated with the progression of the disease in M. tuberculosis infected non-human primates [20].
We have previously analysed the role of SOCS3, a molecule that inhibits STAT3 activation after triggering of several cytokine and growth factor receptors, and found that mice devoid in SOCS3 in myeloid or lymphoid cells showed increased susceptibility to M. tuberculosis [21].
The role of STAT3 during infection with M. tuberculosis in vivo is still unknown. We here examine the role of STAT3 in M. tuberculosis by using stat3fl/fl lysm cre mice. We highlight that STAT3 expression in APCs inhibits TH17 associated responses resulting in an increased susceptibility to infection with M. tuberculosis.
Results
Stat3fl/fl lysm cre mice are resistant to infection with M. tuberculosis
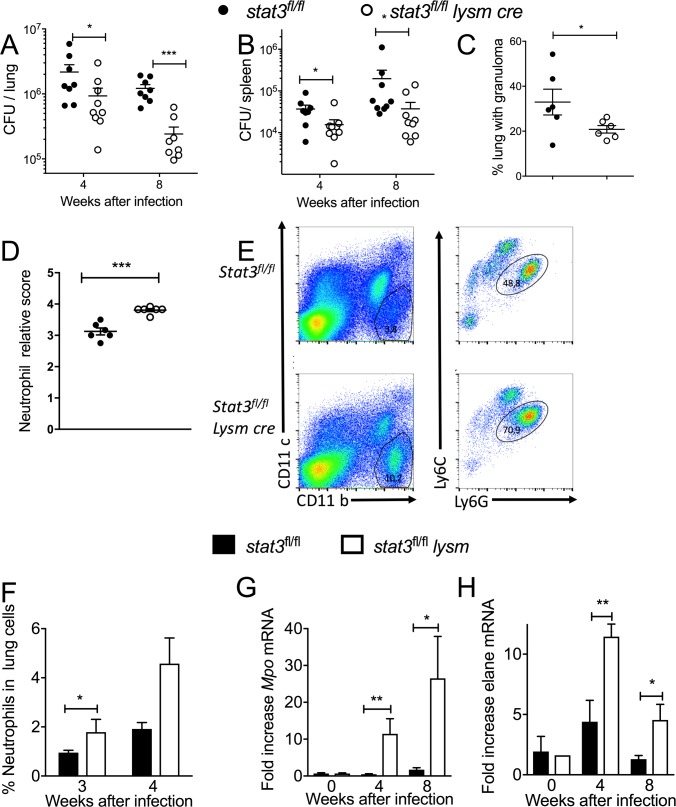
First, the role of STAT3 expression in myeloid cells in the control of infection with M. tuberculosis was examined using stat3fl/fl lysm cre mice. Lungs and spleens from stat3fl/fl lysm cre mice after 4 and 8 weeks of infection showed significantly lower M. tuberculosis burden than stat3fl/fl littermates (Fig 1A and 1B). A smaller area of the lung parenchyma of stat3fl/fl lysm cre mice was occupied by granulomas when compared to control lungs 4 but not at 8 weeks after infection (Fig 1C).
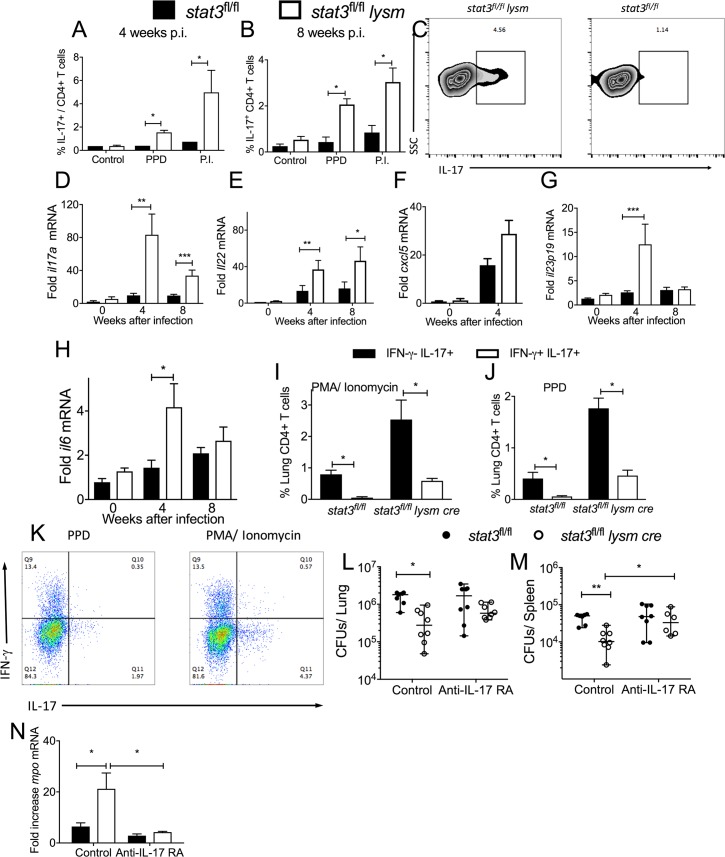
Fig 1. Stat3fl/fl lysm cre mice are resistant to infection with M. tuberculosis.
Stat3fl/fl lysm cre and stat3fl/fl littermate controls were sacrificed at indicated time points after aerosol infection with M. tuberculosis and colony forming units (CFU) per lung (A) and spleen (B) were assessed. The CFU per organ of individual mice and the median per group at the indicated time points after infection are depicted. For each time point 8–10 control and 8–10 mutant mice were infected simultaneously. We performed separated experiments for 4 and 8 weeks post infection. Only one representative of the two experiments for 4 as well as 8 weeks post infection is depicted. Differences in CFU are significant (*p<0.05, **p<0.01, ***p<0.001 Mann Whitney U test). Histopathological scoring of hematoxylin-eosin stained paraffin lung sections from stat3fl/fl lysm cre and stat3fl/fl mice measured 4 and 8 weeks after infection with M. tuberculosis. The mean ± SEM % lung area with granulomas (C) and the relative neutrophil density score (D) are depicted. Differences are significant (*p<0.05, ***p<0.001 Student t test). A representative dot plot (E) and the frequency (F) of CD11b+CD11c-Ly6Cdim Ly6G+ neutrophils in lungs stat3fl/fl lysm cre and stat3fl/fl mice 3 and 4 weeks after infection with M. tuberculosis are shown. Differences between 4 mice at each time point are significant (*p<0.05 Student’s t test). Total RNA was extracted from the lungs of stat3fl/fl lysm cre and stat3fl/fl mice at the indicated time points after infection with M. tuberculosis. The relative concentration of neutrophil elastase (elane) (G) and myeloperoxidase (mpo) transcripts (H) in relation to hprt mRNA levels in the same sample was determined by real time PCR. The mean fold induction of these transcripts ± SEM is depicted. Differences with control mice are significant (*p<0.05, **p<0.01 Student t-test).
The density of granulocytes in the lung parenchyma was determined either by H&E staining of sections (Fig 1D) or by labelling of CD11b+CD11c-Ly6CdimLy6G+ neutrophils (Fig 1E and 1F) in lung suspensions from stat3fl/fl lysm cre and stat3fl/fl mice 3 and 4 weeks after M. tuberculosis infection. The neutrophil density (Fig 1D–1F) and the levels of neutrophil myeloperoxidase (mpo) and elastase (elane) mRNAs (Fig 1G and 1H) were also higher in lungs from stat3fl/fl lysm cre at mice 4 and 8 but not at 14 weeks after infection with M. tuberculosis- compared to controls (Fig 1D–1H and S1A–S1C Fig).
Stat3-deficient and control BMM show similar control of the growth of intracellular M. tuberculosis
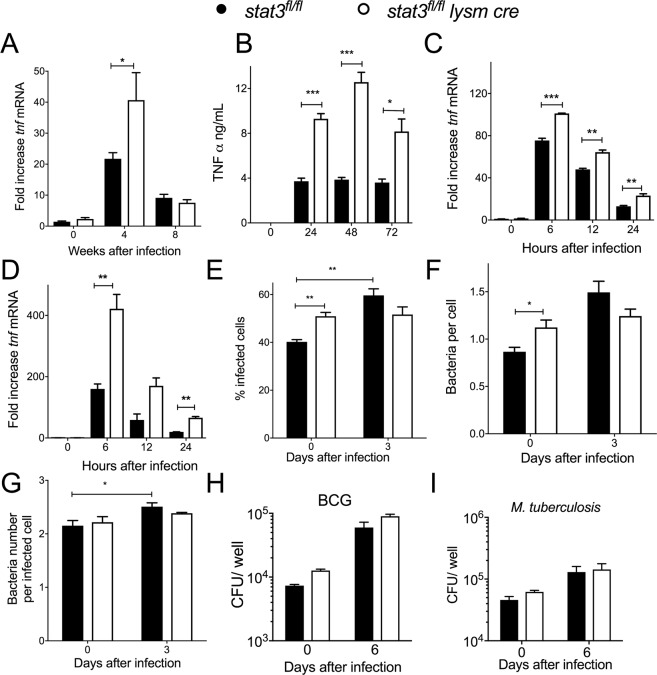
Activated STAT3 hampers TNF expression [22, 23]. Lungs from stat3fl/fl lysm cre mice infected with M. tuberculosis (Fig 2A) as well as BMM infected with M. tuberculosis or BCG (Fig 2B–2D) showed higher TNF protein and mRNA levels than controls. Since TNF has been shown to mediate M. tuberculosis control in macrophages [24], we speculated that stat3fl/fl lysm cre macrophages could display a better control of intracellular mycobacteria.
Fig 2. Stat3 deficient and control BMM show similar control of the intracellular growth of M. tuberculosis.
The levels of tnf mRNA in the lungs from stat3fl/fl lysm cre and stat3fl/fl mice at the indicated time points after infection with M. tuberculosis (A), and in stat3fl/fl lysm cre and stat3fl/fl BMM incubated with M. tuberculosis (C) or BCG (D) were determined by real time PCR. The mean fold increase of mRNA level ± SEM in 8 mice per group (A) or in triplicate independent cultures per condition compared to non-infected cultures (C, D) of one of two independent experiments is depicted (*p<0.05, **p<0.01, ***p<0.001 Student t test). The mean concentration of TNF in the supernatants of stat3fl/fl lysm cre and stat3fl/fl BMM at different times after M. tuberculosis infection were measured by ELISA (B). The mean % of infected BMM (E), the bacteria number per BMM (F) and the bacteria per infected BMM (G) ± SEM at 0 and 3 days after infection of stat3fl/fl lysm cre and stat3fl/fl BMM were determined after staining with auramin-rhodamin T for mycobacterial and DAPI for nuclei (E-G). One out of 2 independent experiments performed is depicted. Bacterial CFU were determined in stat3fl/fl lysm cre and stat3fl/fl BMM after infection with BCG (H) or M. tuberculosis (I) at a MOI of 5:1. The mean CFU ± SEM from triplicate cell cultures is shown. Three independent experiments for each panel were performed.
A higher frequency of stat3fl/fl lysm cre BMMs were infected when measured 4 h after co-incubation with the M. tuberculosis, although the number of bacteria per infected cell was similar (Fig 2E–2G). Three days after infection M. tuberculosis infected mutant and WT BMM showed similar numbers of infected cells and bacteria per total or infected cell (Fig 2E–2G). Stat3fl/fl lysm cre BMM showed no improved control of M. tuberculosis or BCG growth in vitro 6 days after infection as measured by CFU in lysates (Fig 2H and 2I). Altogether, we observed no indication of an improved bacterial growth control or reduced bacterial uptake in stat3fl/fl lysm cre BMM.
Role of STAT3 and SOCS3 in APCs in the regulation of T cell priming
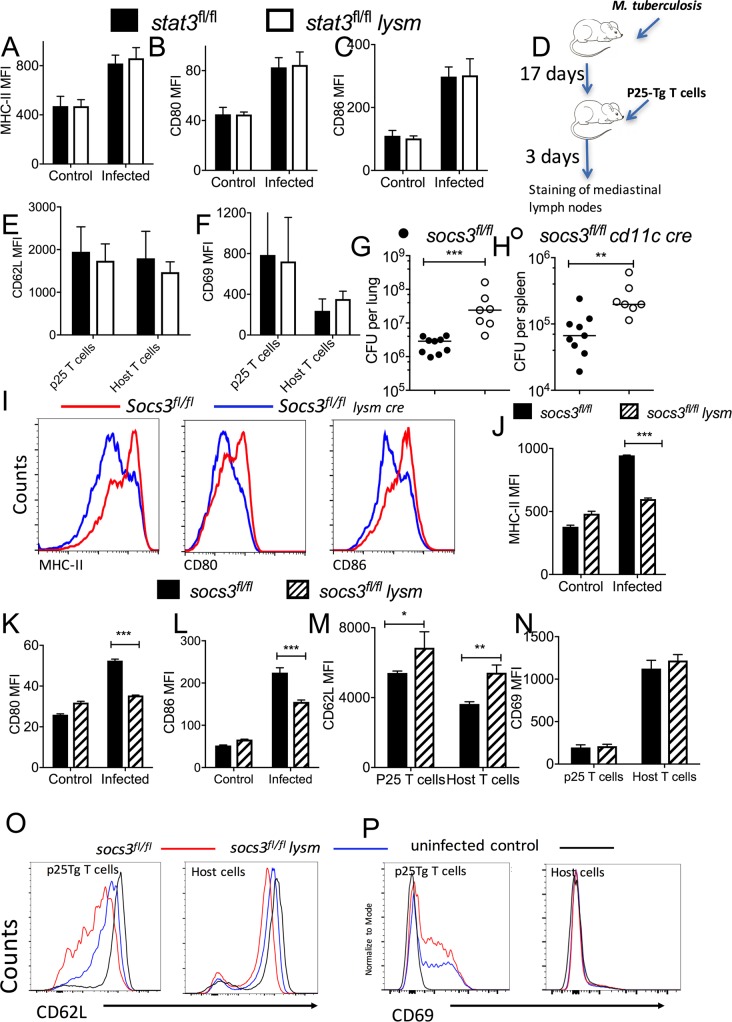
Several cytokines controlled by STAT3 are potent regulators of the expression of co-stimulatory molecules on APCs. Therefore, we studied whether STAT3 played a role in regulation of T cell priming. As expected, the density of co-stimulatory molecules CD80 and CD86 as well as MHC-II levels increased on BMDCs after mycobacterial stimulation. The expression of MHCII, CD80 and CD86 in either control or stat3fl/fl lysm cre BMDCs before or after mycobacterial stimulation was similar (Fig 3A–3C). To investigate if the expression of STAT3 by myeloid cells could modulate T cell priming during infection with M. tuberculosis T cell receptor transgenic T cells specific for the immunodominant mycobacterial Ag85B240-254 peptide (p25-tg) cells were inoculated i.v. into stat3fl/fl lysm cre or stat3fl/fl mice 17 days after infection with M. tuberculosis (Fig 3D). Three days after transfer, the expression of CD69 (which increases after T cell receptor triggering) and CD62L (the L-selectin ligand that hampers T cells to traffic to the periphery) was measured on p25-tg T cells and host T cells from the mediastinal lymph nodes (MLN). The expression of CD69 was increased and the expression of CD62L was reduced in p25-tg T cells from MLN of infected mice when compared to uninfected control mice. Similar levels of the CD69 and CD62L were expressed by p25-tg or host T cells from stat3/fl lysm cre or stat3fl/fl infected mice (Fig 3E and 3F).
Fig 3. Role of STAT3 and SOCS3 in APCs in the regulation of T cell priming.
The median fluorescent intensity (MFI) of MHCII, CD80 and CD86 on CD11c+ stat3fl/fl lysm cre or stat3fl/fl (A-C) BMDCs was determined by FACS analysis 24 h after infection with BCG or non-infected controls. The mean MFI ± SEM are depicted. Experimental scheme of M. tuberculosis infection followed 17 days after by transfer of 3. 106 p25-tg naïve T cells. Mice were sacrificed 3 days later (D). The mean MFI of CD62L (E) and CD69 (F) ± SEM on MLN CD4+ p25-tg or recipient T cells from either stat3fl/fl lysm cre or stat3fl/fl M. tuberculosis-infected mice. Socs3fl/fl cd11c cre and socs3fl/fl littermate controls were sacrificed 4 weeks after aerosol infection with M. tuberculosis and colony forming units (CFU) per lung (G) and spleen (H) were assessed. The CFU per organ of individual mice and the median per group at the indicated time points after infection are depicted. Differences in CFU are significant (**p<0.01, ***p<0.001 Mann Whitney U test). Representative FACS histograms and the mean MFI ± SEM of CD80, CD86 and MHC-II on socs3fl/fl lysm cre and socs3fl/fl BMDCs infected or not with BCG for 24 hs (I-L). Differences with socs3fl/fl BMDCs (n = 4 cultures per group) are significant (*p<0.05, ***p<0.001 Student t test). Representative FACS histograms and mean MFI levels of CD62L (M, O) and CD69 (N, P) ± SEM on p25-tg and host CD4+ MLN T cells before or 21 days after infection with M. tuberculosis. Differences with socs3fl/fl BMDCs are significant (*p<0.05 and **p<0.01 Student t test).
SOCS3 inhibits STAT3 activation by different cytokine receptors, e.g. those of the IL-6 receptor family [10]. In accordance with the results obtained with socs3fl/fl lysm cre mice [21], socs3fl/fl cd11c cre mice showed higher bacterial levels in lungs and spleens after infection with M. tuberculosis than control animals (Fig 3G and 3H). The cd11c cre transgene has been shown to be expressed in the majority of conventional and plasmacytoid DCs [25]
Mycobacteria-stimulated BMDC from socs3fl/fl lysm cre showed lower levels of MHCII, CD80 and CD86 than control cells (Fig 3I–3L). When T cell priming in vivo was studied by transfering p25-tg naïve T cells into M. tuberculosis infected animals, the density of CD62L on donor p25-tg T cells and on host MLN T cells was lower in WT mice as compared to socs3fl/fl lysm cre mice recipients (Fig 3M and 3O). The p25-tg T cells in the MLN of socs3fl/fl lysm cre mice also showed lower surface density of CD69 as compared to those from WT-infected mice (Fig 3N and 3P). However, the expression of CD69 in host MLN T cells from M. tuberculosis-infected WT and socs3fl/fl lysm cre mice was similar (Fig 3P).
Thus, deficiency of SOCS3 but not STAT3 in APCs regulates T cell priming during M. tuberculosis infection in vivo.
STAT3 in myeloid cells impairs IFN-γ secretion by mycobacteria-specific T cells
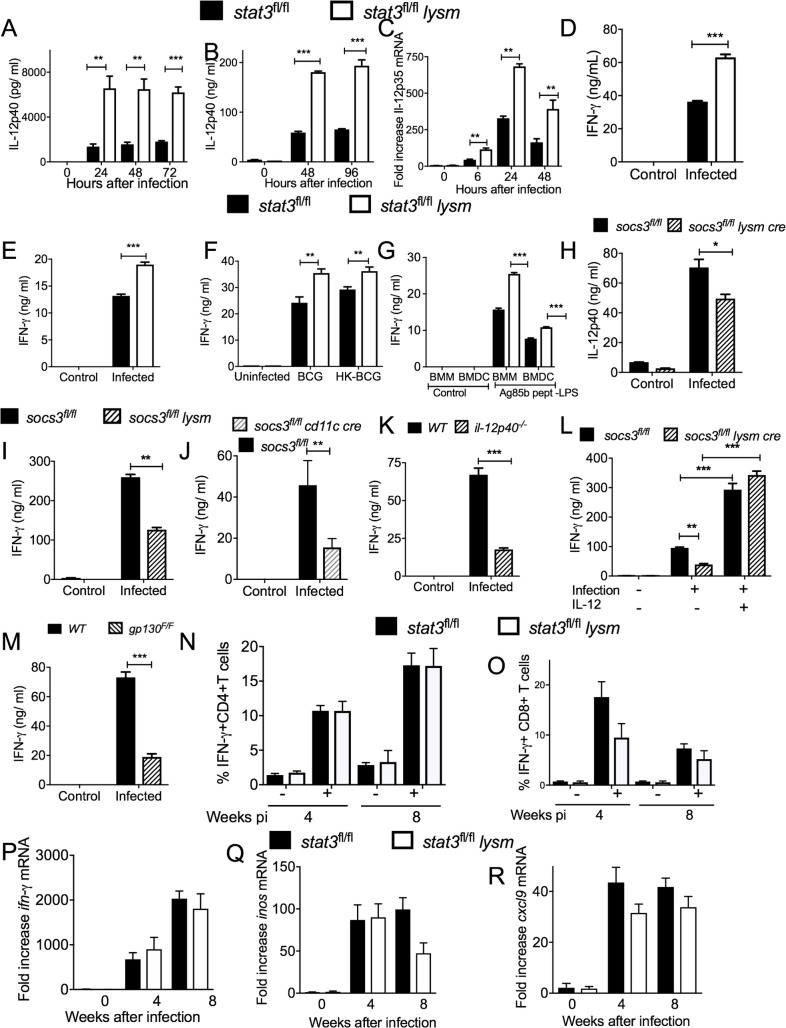
IFN-γ is required for protection against M. tuberculosis [2, 3]. STAT3 has been shown to inhibit the transcription of IL-12, a potent inducer of IFN-γ secretion by T cells [23]. We then analysed if the increased resistance to M. tuberculosis of stat3fl/fl lysm cre mice is associated with higher IFN-γ secretion by T cells. Higher levels of IL-12p40 (the α-chain of IL-12 and IL-23) in supernatants and il12p40 mRNA in cell lysates of BCG-infected stat3fl/fl lysm cre BMM or BMDC compared to controls were measured (Fig 4A and 4B). The il12p35 mRNA coding for the β-chain of the IL-12 heterodimer was also expressed in higher amounts by M. tuberculosis- or BCG-stimulated stat3fl/fl lysm cre BMDC compared to controls (Fig 4C).
Fig 4. STAT3 in myeloid cells impairs IFN-γ secretion by mycobacteria-specific T cells in vitro.
The concentration of IL-12 p40 in supernatants from mycobacteria-infected stat3fl/fl lysm cre and stat3fl/fl BMDCs (A) or BMM (B) at different times after incubation were determined by ELISA. The mean IL-12p40 ± SEM pg/ ml from triplicate cultures is depicted. Differences with stat3fl/fl BMDCs are significant (**p<0.01, ***p<0.001 Student t test). Total RNA was extracted from stat3fl/fl lysm cre and stat3fl/fl BMDC cultures 24 h after M. tuberculosis infection. The mean Il-12p35 mRNA levels ± SEM levels measured by real time PCR are depicted (C) (**p<0.01 Student t test). Stat3fl/fl lysm cre and stat3fl/fl BMDC were infected with either BCG (D), M. tuberculosis (E) or stimulated with heat killed BCG (F) or with LPS and peptide 25 of Ag85b (G) washed and incubated 6 h after with p25-tg CD4+ naïve T cells (at a ratio of 4:1 BMDC) (G). The concentration of IFN-γ in the culture supernatants was measured by ELISA 72h after co-incubation. The mean IFN-γ ± SEM from triplicate cultures is depicted (***p<0.001 Student t test). The concentration of IL-12p40 in supernatants from mycobacteria-infected socs3fl/fl lysm cre and socs3fl/fl BMDCs was determined by ELISA (H). The mean IL-12p40 ± SEM ng/ ml from triplicate cultures is depicted (**p<0.01 and ***p<0.001 Student t test). Socs3fl/fl lysm cre (I), socs3fl/fl cd11 cre (J) and socs3fl/fl BMDC were infected with BCG and incubated 6 h after with p25-tg T cells. The concentration of IFN-γ in the supernatants was measured by ELISA 72h after co-incubation. The mean IFN-γ ± SEM from triplicate cultures is depicted (**p<0.01 Student t test). Il12p40-/- (K), gp130F/F (M) and WT BMDC were infected with BCG and co-incubated with p25-tg T cells as described. Mycobacterial-infected socs3fl/fl lysm cre and socs3fl/fl. were cultured in presence of recombinant IL-12p70 or left untreated (K) and co-incubated with p25Tg-T cells (L). The mean IFN-γ ± SEM in supernatants from triplicate cultures was measured by ELISA (**p<0.01, ***p<0.001 Student t test). The frequency of IFN-γ-secreting cells in PPD-stimulated pulmonary T cells from stat3fl/fl lysm cre and stat3fl/fl mice 4 and 8 weeks after infection with M. tuberculosis was analysed by ICS (N, O). The mean frequency of IFN-γ-secreting within CD4+ cells (N) and CD8+ (O) ± SEM is displayed (n = 5 per group). The levels of ifng (P), inos (Q)and cxcl9 (R) mRNA in the lungs of stat3fl/fl lysm cre and stat3fl/fl mice before and at the indicated time points after aerosol infection with M. tuberculosis were determined by real time PCR.
Thus, whether STAT3-deficient APCs are better stimulators of IFN-γ secretion by mycobacteria-specific T cells than WT APCs was investigated. To test this hypothesis, p25-tg T cells were incubated with either BCG- or M. tuberculosis-infected stat3fl/fl lysm cre or stat3fl/fl BMDCs and the IFN-γ titers in the supernatants measured. IFN-γ levels in supernatants were elevated compared to those incubated with WT APCs (Fig 4D and 4E). Supernatants from cultures of p25-tg T cells incubated with heat-killed BCG-stimulated stat3fl/fl BMDC or BMM also contained higher levels of IFN-γ than those using control APCs (Fig 4F), indicating that infection is not required for such responses. In line with this, IFN-γ levels were higher in supernatants from p25-tg T cells co-incubated with stat3fl/fl lysm cre BMDC or BMM stimulated with oligopeptide p25 (amino acids 240–254) from Ag85b, a major immunodominant H2b epitope [26] recognized by the p25-tg T cells, in presence of LPS (Fig 4G).
Confirming previous results [21, 27], socs3fl/fl lysm cre BMDC showed diminished IL-12 secretion after mycobacterial stimulation (Fig 4H). Furthermore, IFN-γ secretion by p25-tg T-cells incubated with mycobacteria-infected socs3fl/fl lysm cre or socs3 fl/fl cd11 cre BMDCs was reduced (Fig 4I and 4J).
In line with these results, mycobacteria-infected il12p40-/- BMDCs showed reduced ability to trigger IFN-γ secretion by p25-tg T cells than controls (Fig 4K). Moreover, the addition of rec IL-12 restored the capacity of socs3fl/fl lysm cre BMDC to stimulate IFN-γ secretion by p25-tg T cells (Fig 4L).
Cells derived from gp130F/F mice, harbouring a mutation that ablates SOCS3 binding to the gp130, show exaggerated gp130-mediated STAT3 responses [28]. Mycobacteria-infected gp130F/F BMDCs also showed a reduced ability to stimulate IFN-γ secretion p25-tg T cells compared to WT cells (Fig 4M).
The frequency of IFN-γ-secreting mycobacteria-specific T cells in lung cell suspensions from stat3fl/fl lysm cre and stat3fl/fl mice 4 and 8 weeks after infection with M. tuberculosis was similar. The frequencies of lymphoid cell populations (S2A Fig) and of PPD- and PMA/ ionomycin -stimulated IFN-γ secreting CD4+ or CD8+ cells (Fig 4N and 4O and S2B and S2C Fig) from lungs stat3fl/fl lysm cre and stat3fl/fl mice 4 and 8 weeks were also similar. In addition, levels of ifng, and the IFN-γ-regulated inos and cxcl9 transcripts were increased in lungs after infection as compared to uninfected controls, but the titers of these transcripts in lungs from stat3fl/fl lysm cre and stat3fl/fl-infected mice were comparable (Fig 4P–4R).
Myeloid STAT3 hamper TH17 responses during M. tuberculosis infection
The neutrophil density and the levels of neutrophil transcripts were enhanced in the lungs of M. tuberculosis-infected stat3fl/fl lysm cre as compared to control mice (Fig 1D–1H). IL-17 has been shown to stimulate granulopoiesis via G-CSF production and to induce the expression of CXC chemokines involved in granulocyte recruitment [29]. Thus, we investigated whether the increased neutrophil levels in lungs from M. tuberculosis-infected stat3fl/fl lysm cre was associated with augmented TH17 responses. The frequency of IL-17-secreting, PPD-stimulated CD4+ T cells from lungs from stat3fl/fl lysm cre mice 4 and 8 weeks after infection with M. tuberculosis were elevated when compared to stat3fl/fl controls (Fig 5A–5C). Instead, the frequency of γδ T cells in lungs and the frequency of IL-17 secreting pulmonary γδ+ T cells from WT or stat3fl/fl lysm cre infected mice was similar (S3A–S3C Fig).
Fig 5. Myeloid cell expression of STAT3 hamper IL-17 secretion by T cells during M. tuberculosis infection.
The frequency of IL-17-secreting PPD and PMA and ionomycin (P.I) -stimulated CD4+ pulmonary T cells from stat3fl/fl lysm cre and stat3fl/fl mice 4 (A) and 8 (B) weeks after infection with M. tuberculosis was measured by FACS. A representative graph plot from PPD stimulated lungs at 4 w after infection (C) and the mean percentage of IL-17-secreting CD4+ cells ± SEM (A, B) are displayed (n = 6 per group, *p<0.05, **p<0.01 and ***p<0.001 Mann Whitney U test). The mean fold increase of il17a (D), il22 (E), cxcxl5 (F), il23p19 (G) and il6(H) mRNA ± SEM was measured by real time PCR in the total RNA from lungs of stat3fl/fl lysm cre and stat3fl/fl mice at different time points after M. tuberculosis infection (n = 8 per group **p<0.01 Student’s t test). The mean frequency of PPD (I) and PMA and ionomycin (J) -stimulated CD4+ pulmonary T cells from stat3fl/fl lysm cre and stat3fl/fl mice 8 weeks after infection with M. tuberculosis co-secreting or not IFN-γ was measured by FACS (n = 4 per group, *p<0.05, **p<0.01 and ***p<0.001 Mann Whitney U test). A representative graph plot from CD3+CD4+ gated PPD and P.I. stimulated IFN-γ and / or IL-17+ lung cells (K) is depicted. The frequency of both IL-17 and IFN-γ and only IL-17+ secreting cells from stat3fl/fllysm cre is higher as compared to stat3fl/fl controls; the frequency of IL-17+/IFN-γ- is higher than IL-17+/IFN-γ+ cells. This was determined after either PPD or PMA/ ionomycin stimulation (Two-way ANOVA *p<0.05 and *** p<0.0001). Stat3fl/fl lysm cre and stat3fl/fl littermate controls were treated i.p. 1 day before and once per week after aerosol infection with M. tuberculosis with 500 μg anti-IL-17RA M751or left untreated. Mice were sacrificed 4 weeks after the infection and colony forming units (CFU) per lung (L) and spleen (M) were assessed. The CFU per organ of individual mice and the median per group at the indicated time points after infection are depicted. Differences in CFU are significant (*p<0.05, **p<0.01 Mann Whitney U test). The mean fold increase of mpo mRNA ± SEM was measured by real time PCR in lysates from lungs of stat3fl/fl lysm cre and stat3fl/fl mice 4 weeks after infection with M. tuberculosis treated or not with anti-IL-17RA as described above (n≥ 4 per group, *p<0.05 Student’s t test) (N).
In addition, levels of il17a and il22, transcripts that code for TH17 cytokines were higher in lungs from stat3fl/fl lysm cre mice than in those from littermate controls when measured at 4 and 8 weeks after infection (Fig 5D and 5E). Higher levels of il17 mRNA were also observed in stat3fl/fllysm cre mice 14 weeks after infection with M. tuberculosis, while the increase of Il22 mRNA did not reach statistical significance (S4A and S4B Fig). CXCL5 is a neutrophil chemotactic protein stimulated by IL-17 [30]. The level of cxcl5 mRNA was increased in lungs from M. tuberculosis-infected stat3fl/fl lysm cre mice (Fig 5F).
Substantial in vivo data support the notion that IL-6 and IL-23 are required at different stages of TH17-cell differentiation [31, 32]. Levels of il6 and il23 mRNA were elevated in the lungs of M. tuberculosis-infected stat3fl/fl lysm cre mice when compared to levels in lungs from WT mice (Fig 5G and 5H).
TH17 cells show a high degree of developmental flexibility, and when exposed to IL-12 or IL-23, they can rapidly acquire effector functions that are normally associated with TH1 responses such as IFN-γ production [33]. These IFN-γ and IL-17 secreting cells were shown to be pathogenic in murine models of autoimmune diseases, and were also associated with murine colitis and human IBDs [34]. The majority of PPD or PMA/ ionomycin-stimulated IL-17 secreting CD4+ T cells in lungs from M. tuberculosis-infected stat3fl/fl lysm cre or stat3fl/fl mice were not IFN-γ co-producers (Fig 5I–5K). We next studied whether IL-17 played a role in the increased control of infection of stat3fl/fl lysm cre mice. For these experiments, mice were treated with neutralizing IL-17RA mab (M751) before and during infection with M. tuberculosis. Similar bacterial levels were found in lungs and spleens from stat3fl/fl lysm cre and stat3fl/fl animals treated with anti-IL17RA mAb. As expected, stat3fl/fl lysm cre mice from untreated mice showed reduced bacterial numbers in lungs and spleens than those from stat3fl/fl controls (Fig 5L and 5M).
The levels of mpo mRNA was measured in lungs from anti-IL-17RA to control for the IL17RA neutralization. As expected, levels of mpo mRNA were increased in lungs from M. tuberculosis infected stat3fl/fl lysm cre mice as compared to WT. In contrast, levels of mpo mRNA in lungs from infected or anti-IL17RA treated stat3fl/fl lysm cre and stat3fl/fl mice was similar. Lower titters of mpo mRNA were found in stat3fl/fl lysm cre infected mice treated with anti-IL-17RA compared to untreated infected controls, while mpo mRNA levels in anti-IL17RA treated or untreated infeceted stat3fl/fl mice were similar (Fig 5N).
Hence, increased M. tuberculosis control during infection of stat3fl/fl lysm cre mice is dependent on IL-17, but IL-17 neutralization did not increase the susceptibility to M. tuberculosis of mice with normal STAT3 function.
STAT3 expression in antigen presenting cells inhibit the generation of IL-17 secreting mycobacteria-specific T cells
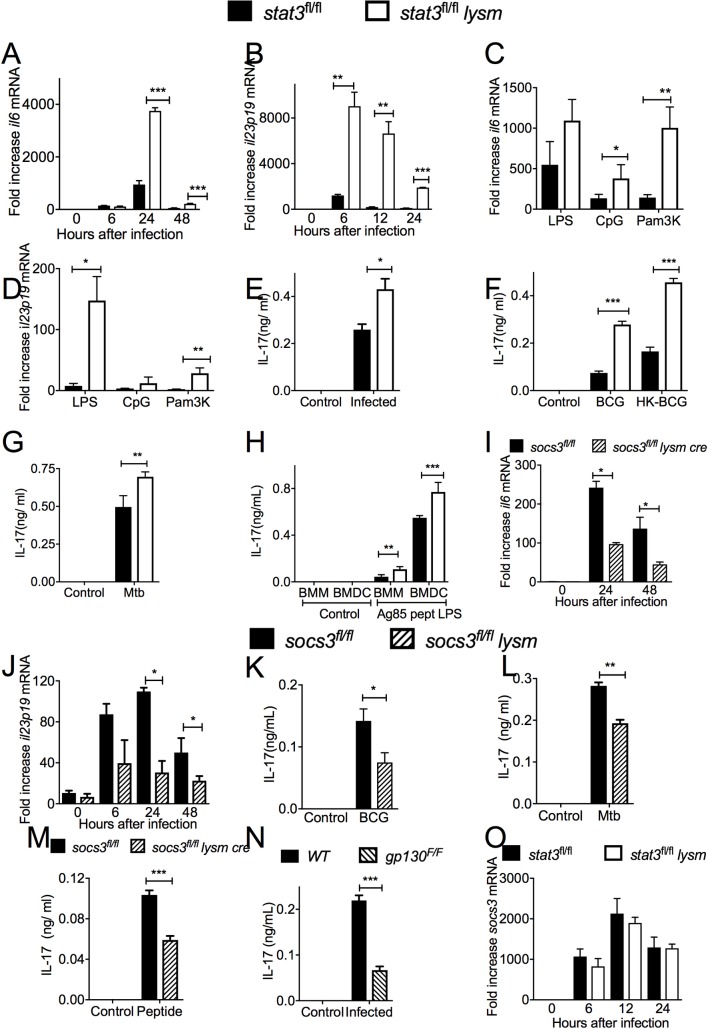
The role by which STAT3 in activated APCs regulates IL-17 secretion by specific T cells was then studied. The mRNA levels of Il6 and il23p19 were both increased in BMM and BMDCs co-incubated with mycobacteria in vitro (Fig 6A and 6B and S5A Fig). An increased accumulation of il6 and il23p19 mRNA was also observed after stimulation with the TLR agonists LPS, CpG or Pam3K of stat3fl/fl lysm cre as compared to stat3fl/fl BMM at 6 and 24 h after stimulation (Fig 6C and 6D and S5B Fig). This indicates that STAT3-mediated inhibition of the expression of IL-6 and IL-23 is not restricted to mycobacterial infection or stimulation with mycobacterial molecules. Supernatants from cultures of mycobacteria-infected stat3fl/f lysm cre BMM or BMDC co-incubated with naïve p25-tg T cells contained higher titers of IL-17 than those using stat3fl/fl controls (Fig 6E–6G). IL-17 levels were also higher in supernatants from p25-tg T cells co-incubated with stat3fl/fl lysm cre BMDC stimulated with either live or heat-killed BCG, M. tuberculosis or peptide 25 from Ag85b in presence of LPS (Fig 6F–6H).
Fig 6. STAT3 and SOCS3 regulate the secretion of IL-17 by antigen-specific T cells.
Total RNA was extracted from stat3fl/fl lysm cre and stat3fl/fl BMDC cultures 24 h after M. tuberculosis infection. Il6 and il23p19 mRNA were measured by real time PCR and normalized to the hprt mRNA levels in the same samples. The mean and il6 (A) il-23p19 (B) mRNA fold increase ± SEM levels in triplicate independent cultures are depicted (*p<0.05, **p<0.01 and ***p<0.001 Student’s t test). The mean fold increase of il6 (C) and il23p19 (D) ± SEM were measured by real-time PCR in triplicate cultures of stat3fl/fl lysm cre and stat3fl/fl BMDCs 24 h after stimulation with either LPS, CpG or Pam3K (*p<0.05 and **p<0.01 Student’s t test). Stat3fl/fl lysm cre and stat3fl/fl BMDC (E-H) or BMM (H) were stimulated with either BCG (E), heat killed BCG (F), M. tuberculosis (G), or with LPS and the peptide 25 of Ag85b (H) and incubated 6 h after with p25-tg CD4+ naïve T cells (at a ratio of 4:1 BMDC). The concentration of IL-17 in the culture supernatants was measured by ELISA 72h after co-incubation. The mean IL-17 ng/ ml ± SEM from triplicate cultures is depicted (* p<0.05; **p<0.01 and ***p<0.001 Student’s t test). Total RNA was extracted from socs3fl/fl lysm cre and socs3fl/fl BMDC cultures at different times after infection with mycobacteria. The mean Il6 (I) and IL23p19 (J) mRNA levels ± SEM levels determined by real time PCR are depicted (**p<0.01 Student’s t test). The concentration of IL-17 was measured 72h supernatants of co cultures of p25-tg naïve T cells incubated with either BCG (K), M. tuberculosis (L) or pept25 and LPS (M)-stimulated socs3fl/fl lysm cre and socs3fl/fl BMDCs. The mean IL-17 ng/ ml ± SEM from triplicate independent cultures were determined by ELISA (*p<0.05; **p<0.01 and ***p<0.001 Student’s t test). IL-17 was measured in 72 h culture supernatants from control or BCG-infected gp130F/F BMDC and p25-tg T cells. The mean IL-17 ng/ ml ± SEM from triplicate cultures from infected or control BMDCs is shown in (N). Differences are significant at ***p<0.001 Student’s t test. The mean fold increase of socs3 transcript ± SEM in total RNA from stat3fl/fl lysm cre and stat3fl/fl BMDC at different time points after M. tuberculosis infection as compared to uninfected controls were measured by real time PCR are depicted (O).
Whether SOCS3 in mycobacteria-infected APCs also regulated IL-17 secretion by antigen-specific T cells was then measured. We found that mycobacteria-infected socs3fl/fl lysm cre BMM contained lower levels of il6 and il23p19 mRNA than their WT counterparts (Fig 6I and 6J). Moreover, p25-tg T cells incubated with socs3fl/fl lysm cre BMM stimulated either with BCG, M. tuberculosis or the cognate p25 peptide secreted lower levels of IL-17 than those stimulated by WT BMDCs (Fig 6K–6M). Similarly, supernatants from co-cultures of p25-tg T cells with mycobacteria-infected gp130F/F BMDC contained higher levels of IL-17 than those using wild type BMDCs (Fig 6N).
We then asked whether STAT3 deficiency regulated the levels of socs3 mRNA transcripts in mycobacteria-infected macrophages. M. tuberculosis-infected stat3fl/fl lysm cre and stat3fl/fl BMMs showed similar levels of socs3 mRNA (Fig 6O).
STAT3 and SOCS3 in T cells show a differential regulation of IL-17 and IFN-γ secretion
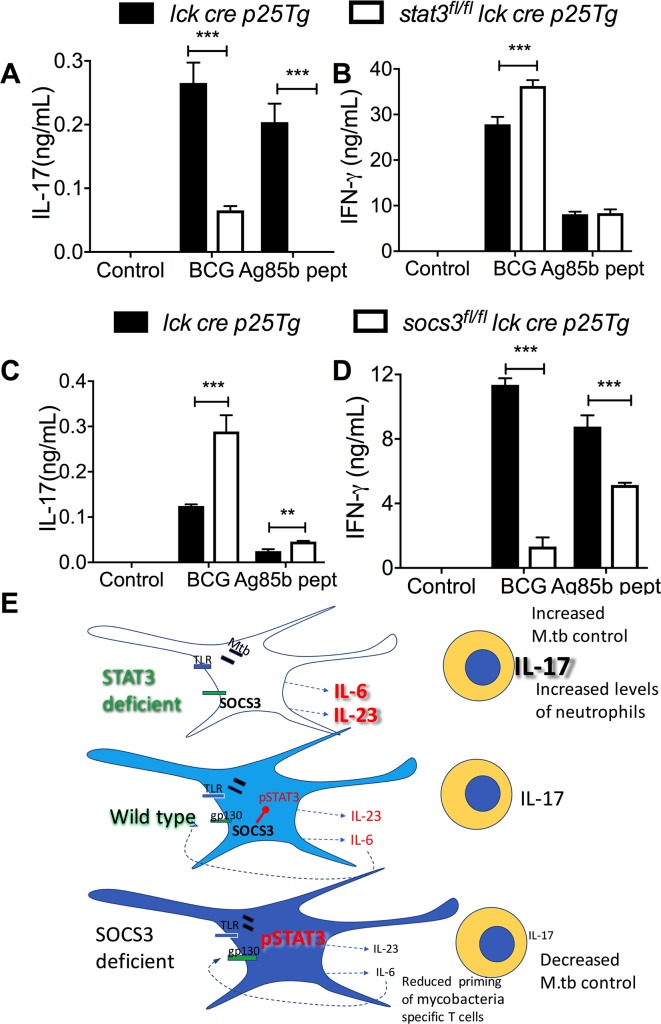
Different to the inhibitory role of STAT3 in myeloid cells we here showed, STAT3 expression in T cells has been indicated to be required for TH17 cell differentiation in vitro and in vivo [31]. SOCS3, via hyperactivation of STAT3, has been shown to increase IL-17 secretion [35]. In order to compare the role of STAT3 and SOCS3 in T cells and APCs in the regulation of cytokine secretion by T cells, stat3fl/fl lck cre p25-tg and socs3 fl/f lck cre p25-tg mice were generated. The culture supernatants of stat3fl/fl lck cre p25-tg T cells stimulated with BCG-infected or Ag85b peptide-pulsed BMDCs showed low or undetectable levels of IL-17 as compared to controls (lck cre p25-tg T cells) (Fig 7A). Instead IL-17 levels in supernatants from socs3fl/fl lck cre p25-tg T cells co-incubated with BCG or peptide loaded BMDCs were higher than controls (Fig 7C).
Fig 7. SOCS3 and STAT3 in antigen-specific T cells are important regulators of IL-17 and IFN-γ secretion.
Stat3fl/fl lck cre p25-tg T cells (A, B), socs3fl/fl lck cre p25-tg T cells (C, D) or control p25-tg T cells were incubated for 3 days with either BCG, peptide 25 and LPS-stimulated or untreated BMDCs. The mean concentration of IL-17 (A, C) and IFN-γ (B, D) ± SEM in supernatants from triplicate cultures is shown (**p<0.01 and ***p<0.001 Student’s t test). Graphical summary (E): Mice deficient in STAT3 in myeloid cells show increased resistance while socs3fl/fl lysm cre mice displaying augmented STAT3 activation had impaired resistance against infection with M. tuberculosis. The differential control of infection in excess or deficiency of STAT3 is not due to the intrinsic regulation of bacterial control by macrophages but rather to differences in the ability of DCs to regulate the differentiation of specific T-cells. Stat3fl/fl lysm cre APCs release higher levels of IL-6 and IL-23 after stimulation with mycobacterial or other TLR agonists, while secretion of these cytokines is reduced in socs3fl/fl lysm cre APCs. Stat3fl/fl lysm cre APCs show improved ability to trigger IL-17 release by mycobacteria-specific T cells while the opposite is observed when using socs3fl/fl lysm cre APCs. The IL-17 secretion by T cells is also controlled via gp130R signalling, indicating an autocrine or paracrine loop by IL-6 family cytokines. The increased resistance to M. tuberculosis infection of stat3fl/fl lysm cre mice was IL-17 dependent. Socs3fl/fl lysm cre (but not stat3fl/fl lysm cre) DCs improved capacity of mycobacteria-specific T cells priming in vivo.
The titters of IFN-γ in the supernatants of stat3 fl/fl p25-tg T cells incubated with BCG- (but not with Ag85b peptide-) stimulated BMDCs were higher than those incubated with control T cells (Fig 7B). IFN-γ levels in supernatants from mycobacteria or peptide pulsed BMDCs incubated with socs3fl/fl lck cre p25-tg were instead lower than those using control p25-tg T cells (Fig 7D).
Thus, STAT3 in APCs and T cells, has a dissimilar ability to regulate IL-17 secretion by ag-specific T cells, while SOCS3 in APCs and T cells promote T cell mediated IFN-γ secretion.
Discussion
We report here that stat3fl/f lysm cre mice show reduced M. tuberculosis load in lungs and spleens, indicating that STAT3 expression in myeloid cells is detrimental for the control of infection with M. tuberculosis. Despite reduced area of the lung occupied by granuloma the area with inflammation was not reduced and the numbers of infiltrating pulmonary neutrophils were elevated in stat3fl/fllysm cre mice. Neutrophil accumulation late during infection have been associated with susceptibility to M. tuberculosis, whereas early after infection neutrophils play a protective role and contribute to early priming of T cells in the draining lymph node [36–39].
Although mice lacking STAT3 expression in bone marrow progenitors display peripheral neutrophilia under resting conditions [40], the pathways involved in neutrophil mobilization and response to chemokines during inflammation have been shown to be STAT3 dependent [41, 42]. Thus, we consider it unlikely that the increase in pulmonary neutrophils observed during infection occurs as a direct consequence of STAT3 deficiency in these cells. Rather, increased levels of IL-17 and IL-22, cytokines that stimulate the expression of neutrophil recruiting chemokines [29], might contribute to the accumulation of granulocytes in lungs from M. tuberculosis-infected stat3fl/fl lysm cre mice. In agreement with this hypothesis, the frequency of IL-17 secreting mycobacteria-specific CD4+ T cells, but not of γδ+ T cells, were elevated in the lungs from stat3fl/fl lysm cre mice compared to controls.
STAT3 expression in APCs proved to be a major regulator of the expression of cytokines that control T cell differentiation. This was shown in STAT3- and in SOCS3-deficient APC, which display higher levels of activated STAT3 when stimulated with mycobacteria or other innate receptor agonists [21, 43]. Thus, while mycobacteria-infected STAT3 deficient APCs showed an improved ability to trigger IL-17 secretion by antigen-specific T cells, the opposite was observed using socs3fl/fl lysm cre or gp130F/F BMDCs or macrophages as APCs.
We observed an increased IFN-γ secretion by antigen-specific T cells incubated with STAT3-deficient, mycobacteria-infected APCs. However, IFN-γ responses were not increased in vivo. Whether this is due to the present of cytokines that might stimulate IL-17 responses while antagonizing TH1 cells (such as for example TGF-β) remains to be explored. Whereas in vitro cultures used provide a proper tool to gain mechanistic insights, the diversity of populations, the tissue localization and the balance between the host immune responses and mycobacteria in the chronic infection might account for differences observed between in vitro responses and the control of infection in mice.
The increased resistance to M. tuberculosis in stat3fl/fl lysm cre mice is mirrored by data showing that mice with SOCS3 deficiency in myeloid cells display reduced resistance to TB and toxoplasmosis [21, 44]. Here we show that socs3fl/fl cd11c cre mice. CD11c cre in these mice has been shown to be expressed in ca 90% of splenic DCs, compared with <10% of lymphocytes and <1% of myeloid cells such as granulocytes [25]. Thus, these animals in which SOCS3 is deleted in DCs but not in inflammatory macrophages and neutrophils [25] are also more susceptible to infection with M. tuberculosis. This supports that the major role of STAT3 and SOCS3 in myeloid cells in the control of infection with M. tuberculosis is not due to an altered ability of SOCS3 or STAT3-deficient macrophages to control the growth of the intracellular mycobacteria in vitro, as shown here and ref [21].
To our knowledge, this is the first report showing that STAT3 deficiency in myeloid cells promotes IL-17 secretion by antigen-specific T cells in vitro and in vivo. Such a role was related to the increased secretion of TH17 inducing IL-6 and IL-23 by STAT3-deficient APCs. The increased expression of IL-6 and IL-23 in stat3fl/fl lysm cre APCs was not restricted to the infection with attenuated or virulent mycobacteria since, it was observed after incubating mutant APCs with different TLR agonists or bacterial lysates, confirming previous data [45]. The opposite effect was observed using socs3fl/fl lysm cre BMM, which were poor inducers of IL-17 secretion by mycobacteria-specific T cells. In relation to this, DC that secrete IL-12p40 (required for T cell differentiation into Th17 or Th1) in the lymph nodes of mycobacteria infected mice are primarily uninfected [46].
Since IL-6 can be produced by various hematopoietic and non-hematopoietic cells, we suggest that APCs are relevant cellular sources of IL-6 for the differentiation of IL-17 secreting cells during infection. Moreover, our data using gp130F/F BMDCs indicate that DC-derived IL-6 acts in an autocrine/ paracrine manner on DCs to regulate their ability to stimulate IL-17 secretion by T cells. A role for gp130/ IL6/ STAT3 pathway in susceptibility to M. tuberculosis has been previously determined. The high susceptibility of gp130F/F mice to infection with M. tuberculosis was not observed in gp130F/Fil6-/- or gp130F/F stat3+/- mice [21].
Our observations on stat3fl/fl lysm cre mice are reminiscent of those seen in il10-/- or anti-IL-10R mAb treated mice that resulted in enhanced lung TH1 and TH17 responses after BCG vaccination [47]. Depletion of IL-10 resulted in elevated protection to M. tuberculosis in some studies but not others [47–50]. However different to our model, IL-10 might not only impair the functions of APCs but is also secreted by T cells and has a direct inhibitory effect on TH1 or TH17 cells [51].
We showed that the improved M. tuberculosis control in stat3fl/fl lysm cre is IL-17-mediated, since administration of neutralization anti-IL-17RA antibodies abrogated differences in bacterial burden between mutant and control mice. IL-17 might contribute to long term protection, control of infection after vaccination or control of hypervirulent strains of M. tuberculosis [52–55]. IL-17 has been suggested to induce of protective TH1 responses against mycobacterial infection [52, 56]. IL-17 has been also shown to mediate CXCL13 induction in the lung, a chemokine that contributes to the localization of pro-inflammatory cytokine-producing CXCR5+ T cells within lymphoid structures, promoting those macrophage activation and mycobacterial control [53, 57].
However, other studies have indicated that IL-17 is dispensable after primary infection with M. tuberculosis [58]. In line with the later observations, we observed similar M. tuberculosis load in lungs or spleens of WT mice treated or not with anti-IL-17RA.
Levels of MHCII and CD80 and CD86 were lower on socs3fl/fl lysm cre BMDCs after mycobacterial stimulation confirming previous findings showing reduced MHCII and co-stimulatory molecules after LPS stimulation of SOCS3-deficient BMDCs [59]. Furthermore, the activation of mycobacteria specific p25-tg T cells was also diminished in MLN from M. tuberculosis-infected socs3fl/fl lysm cre mice as compared to controls. Of importance, p25-tg T cell proliferation was not detectable in the MLN of mice infected with an Ag85b deficient strain of M. tuberculosis indicating the specificity of p25-tg T cell priming [60]. The Ag85b KO M. tuberculosis strain grew in the lungs and disseminated to the MLN at a rate equivalent to that of wild-type bacteria. Instead, stat3fl/fl lysm cre and control BMDCs expressed similar levels of MHC-II and co-stimulatory molecules after mycobacterial stimulation in vitro and similar levels of activated antigen-specific T cells in vivo. Different to these results, STAT3 deficient APCs have been shown increased MHCII levels after IL-6 stimulation [61].
Finally, the role of STAT3 in T cells in regulation of antigen-specific IFN-γ and IL-17 T cell responses was investigated. Contrary to the role of STAT3 in APCs, IL-17 secretion was hampered in mycobacteria-specific STAT3-deficient T cells. STAT3 is required for the responses to both IL-6, IL-21 and IL-23 and for the expression of RORγt by T cells [62]. Instead, SOCS3-deficient antigen-specific T cells secreted higher IL-17 levels as previously reported in other systems [35], while IFN-γ responses were inhibited. Thus, while the role of STAT3 in T cells in the control of M. tuberculosis remains to be studied, these results illustrate the pleiotropic effect of STAT3 in regulation of infection-induced immune responses in different cell types.
In summary, we here showed using SOCS3- and STAT3-deficient mice that STAT3 in myeloid cells is detrimental for the control of infection with M. tuberculosis. Surprisingly, this occurs via impairing secretion of IL-17 by antigen-specific T cells (Fig 7E).
Materials and methods
Ethics statement
The animals were housed and handled at the Dept. of Microbiology, Tumor and Cell Biology and the Astrid Fagreus Laboratory, Karolinska Institute, Stockholm, according to directives and guidelines of the Swedish Board of Agriculture, the Swedish Animal Protection Agency, and the Karolinska Institute (djurskyddslagen 1988:534; djurskyddsförordningen 1988:539; djurskyddsmyndigheten DFS 2004:4). The study was performed under approval of the Stockholm North Ethical Committee on Animal Experiments permit number N397/13 and N487/11. Animals were housed under specific pathogen-free conditions.
Mice
Mice containing loxP-flanked stat3 and socs3 alleles have been described before [43]. For a myeloid-specific deletion these were bred with transgenic lysm cre mice [63]. Socs3fl/fl mice were also bred with cd11c cre transgenic animals. Stat3fl/fl or socs3fl/fl littermates negative for cre expression were used as controls for all experiments. Gp130F/F mice with a homozygous substitution of tyrosine (Y)757 to phenylalanine (F) within the common IL-6 family receptor gp130 abrogating the SOCS3 binding site have been described before [64]. Transgenic T cell receptor p25-tg mice with a T-cell receptor specific for peptide 25 (aa 240–254) of mycobacterial Ag85B on H2b haplotype were used [65]. p25-tg rag2-/- mice expressing ECFP were generated by crossing p25-tg with rag1-/- mice [65] with ECFP mice on a rag2-/- background (kindly provided by Dr. Ronald Germain, NIAID, NIH). The ECFP expression co-localized with Vβ11 used by p25tg T cells [65]. Socs3fl/fl lck cre and stat3fl/fl lck cre mice deficient in SOCS3 and STAT3 in T cells were crossed with p25-tg mice to generate p25-tg socs3fl/fl lck cre and p25-tg stat3fl/fl lck cre mice. P25-tg lck cre mice were also obtained and used as controls.
Infection and infectivity assay
BCG Montreal and M. tuberculosis Harlingen were grown in Middlebrook 7H9 (Difco, Detroit, MI) supplemented with albumin, dextrose, catalase and, for BCG cultures, 50 μg/ ml hygromycin (Sigma, St. Louis, MO). Mice were infected with 250 M. tuberculosis Harlingen strain by aerosol using a nose-only exposure unit (In-tox Products, Uppsala, Sweden)[66].
Bacteria were quantified on Middlebrook 7H11 agar containing 10% enrichment of oleic acid, albumin, dextrose, catalase, 5 μg of amphotericin B per ml and 8 μg/ ml polymyxin B grown for 3 weeks at 37°C.
Generation of mouse bone marrow-derived macrophages
Bone marrow was extracted from tibia and femurs of mice and resuspended in DMEM containing glucose and supplemented with 10% FCS and 30% L929 cell-conditioned medium (as a source of macrophage-colony stimulating factor). Bone marrow cells were passed through a 70 μm cell strainer, plated and incubated for 6 days at 37°C, 5% CO2. Bone marrow-derived macrophage (BMM) cultures were then washed vigorously to remove non-adherent cells, trypsinized, counted and cultured for one day at 37°C in 24, 12 or 6 well plates. We have previously shown that these BMM are F4/80+, CD14+ and Mac-3+ [67].
Quantification of intracellular mycobacteria
In order to quantify intracellular M. tuberculosis uptake and growth, BMM cells were plated on glass slides at 2.105 cells per well in 24 well plates, incubated for 4 h with M. tuberculosis (MOI 2) and washed with PBS for 3 times to remove the extracellular bacteria before either fixation or replacing the medium. Three days after infection cells were washed with PBS, fixed with 2% PFA and stained with phalloidin to label F-actin (Life technologies, 1:100), DAPI (1:500) and auramine-rhodamine T to label mycobacteria (BD). Micrographs from infected macrophages (400X) were obtained and a total of at least 1000 BMM from 3 independent cultures and categorized as infected or uninfected. The intracellular M. tuberculosis were enumerated. BMM harboring 5 or more bacteria were considered as containing 5. In some cultures, mycobacterial CFU from BMM 6 days after infection were determined.
Generation of mouse bone marrow-derived dendritic cells
Mouse bone marrow-derived dendritic cells (BMDC) were differentiated as previously described [68]. Briefly, bone marrow was extracted from tibia and femurs and cell suspensions cultured in RPMI-1640 medium containing 10% FCS and 2 ng/ ml GM-CSF (Peprotech, Rocky Hill, NJ). Fresh medium and cytokine were replaced after 3 days. After six days of culture, loosely adherent cells were harvested and seeded in concentrations for infection. Harvested cells were further selected for CD11c expression with magnetic beads (Miltenyi Biotech) before seeding.
T cell priming in vitro
BMDC or BMM were stimulated with either live or heat killed BCG, M. tuberculosis or Ag85b peptide in presence of LPS for 6 h. Then, cells were washed and co-incubated with p25-tg CD4+ lymph node transgenic T cells from rag2-/- p25-Tg mice (at a ratio of 4:1 BMDC). The cultures were further incubated for 24–48 hs at 37C 5% CO2. At these time points the concentration of IFN-γ and IL-17 in the supernatants was measured by ELISA.
Real time PCR
Transcripts were quantified by real time PCR as previously described[66]. Hprt was used as a control gene to calculate the ΔCt values for individual samples. The relative amount of cytokine/ hprt transcripts was calculated using the 2-(ΔΔCt) method. These values were then used to calculate the relative expression of cytokine mRNA in uninfected and infected cells and tissues.
Flow cytometry and intracellular cytokine staining
Lungs were perfused with PBS through the heart before removal from mice. Lungs were mechanically minced into small pieces and digested with 3 mg/ ml Collagenase D and 30 μg/ ml DNase I for 1 h at 37°C, and single-cell suspensions prepared by filtering lung tissue through 70-μm nylon cell strainers. To further remove impurities cells were loaded in 40/ 70% Percoll gradient in PBS and centrifuged 30 min room temperature. The cells at the interphase were collected and washed. Single spleen cell suspensions were obtained by mechanical disruption, lysis of erythrocytes and straining over a 70-μm nylon mesh. Lung, lymph node and spleen cells were stained for CD3, CD4, CD8, γδ-TCR, CD62L, CD69, CD44, CD11b, CD11c, Ly6C and Ly6G (all eBioscience) and fixed before acquisition.
For determination of IFN-γ and IL-17-producing cells, lung cells were incubated with PPD or with 50 ng/ml phorbol myristate acetate (PMA) and 2 μg/ml ionomycin (Sigma) for 6 or 18 h at 37 oC. Brefeldin (10 μg/ ml) was added to the cultures the last 4 h of stimulation. Cells were then stained with cell population-specific antibodies, and live/ dead staining, fixed, permeabilized using leukocyte permeabilization reagent IntraPrep™ (Immunotech, Marseille, France) and further stained with anti-IL-17a or anti-IFN-γ (eBioscience).
Data were acquired in a CyAn™ ADP (Beckman Coulter) or an LSRII Flow cytometry and analyzed with FlowJo software (Tree star Inc., Ashland, OR).
Histopathological analysis
Formalin fixed left lungs of mice experimentally inoculated with M. tuberculosis were blocked on paraffin. From each lung sample 4 sections were obtained, one longitudinal along the long axis of the lobe and 3 across/transversal of the remaining piece of lung.
The blocks were processed and sections were stained with haematoxylin-eosin. All sections were interpreted by the same pathologist (D. G-W.) and scored semi-quantitatively, blinded to the variables of the experiment.
The following features were scored:
Lung area occupied with granulomas (% of the total area of the section)
Lung area free of lesions or area of healthy lung (% of the total area of the section)
Statistics
The Mann Whitney test for the bacterial CFU load in vivo and of the ICS analysis. For each experiment, 8–10 control and 8–10 mutant mice were infected. We performed separated experiments for 4 and 8 weeks post infection. One of two independent experiments showing similar results is shown.
The analysis of cytokine secretion or mRNA, histopathological scores and frequencies was done using the Student’s t test for unpaired samples. All in vitro experiments were performed at least twice. A two-way ANOVA was used to compare the differences in IL-17 secretion between genotypes, as well as between cells that co-secrete IFN-γ or not.
Supporting information
The frequency (A) and numbers (B) of CD11b+CD11c-Ly6Cdim Ly6G+ neutrophils in lungs stat3fl/fl lysm cre and stat3fl/fl mice at 14 weeks after infection with M. tuberculosis ± SEM are shown (n = 4 mice per group); representative dot plots of neutrophil staining in lungs are shown (C).
(TIFF)
The mean frequency of PMA/ ionomycin-stimulated IFN-γ secreting CD4+ and CD8+ lung T cells from stat3fl/fl lysm cre and stat3fl/fl mice at 4 (B) and 8 (C) weeks after infection with M. tuberculosis ± SEM was measured by FACS (n = 4 per group).
(TIFF)
The mean frequency of CD4+, CD8+ and γδ+ cells within lung CD3+ T cells from stat3fl/fl lysm cre and stat3fl/fl mice 8 weeks after infection with M. tuberculosis was measured by FACS (n = 4 per group) (A).
The mean frequency of PPD and PMA/ ionomycin-stimulated IL-17 secreting γδ+ pulmonary T cells from stat3fl/fl lysm cre and stat3fl/fl mice at 4 (B) and 8 (C) weeks after infection with M. tuberculosis was measured by FACS (n = 4 per group).
(TIFF)
The mean fold increase of il17a (A), il22 (B) mRNA ± SEM was measured by real time PCR in the total RNA from lungs of stat3fl/fl lysm cre and stat3fl/fl mice at 14 weeks after M. tuberculosis infection (n = 5 per group *p<0.05 Student’s t test).
(TIFF)
The mean fold increase of il6 (A) and il23p19 (B) ± SEM were measured by real-time PCR in triplicate cultures of stat3fl/fl lysm cre and stat3fl/fl BMDCs 6 h after stimulation with either LPS, CpG or Pam3K (*p<0.05 and ***p<0.001 Student t test).
(TIFF)
Acknowledgments
We thank suggestions and comments to our study from Dr Benedict Chambers and Dr Susanne Nylen. We acknowledge the excellent technical help from Berit Olsson, Helen Braxenholm, Torun Söderberg and Ida Fahlen. We thank Dr Antonio Rothfuchs for suggestions and facilitating access to p25-tg mice. The rat anti-mouse IL-17RA (M751) blocking Ab was kindly provided by Amgen.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Swedish Research Council grant VR/2016-19/2015-02296 (www.vr.se/inenglish), The Swedish Foundation for International Cooperation in Research and Higher Education (www.stint.se/en), The Swedish Heart and Lung Foundation 2015-17/20140641 (www.hjart-lungfonden.se), the Chinese Scholarship Council (www.en.csc.edu.cn/) and the Karolinska Institutet (www.ki.se). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. Global tuberculosis control. Surveillance, planning, financing. WHO report. 2006;www.who.int/tb/publications/global_report/2006/download_centre/en/index.html.
- 2.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178(6):2243–7. Epub 1993/12/01. ; PMCID: PMC2191280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249–54. Epub 1993/12/01. ; PMCID: PMC2191274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng CG, Jankovic D, Kullberg M, Cheever A, Scanga CA, Hieny S, et al. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J Immunol. 2005;174(7):4185–92. Epub 2005/03/22. . [DOI] [PubMed] [Google Scholar]
- 5.Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203(7):1805–15. Epub 2006/07/05. doi: 10.1084/jem.20052545 ; PMCID: PMC2118335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168(3):1322–7. Epub 2002/01/22. . [DOI] [PubMed] [Google Scholar]
- 7.Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18(6):347–61. Epub 2006/09/26. doi: 10.1016/j.smim.2006.07.010 . [DOI] [PubMed] [Google Scholar]
- 8.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158(3):670–89. Epub 1983/09/01. ; PMCID: PMC2187114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annual review of immunology. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851 . [DOI] [PubMed] [Google Scholar]
- 10.Carow B, Rottenberg ME. SOCS3, a Major Regulator of Infection and Inflammation. Frontiers in immunology. 2014;5:58 doi: 10.3389/fimmu.2014.00058 ; PMCID: PMC3928676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nature reviews Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver JS, Hunter CA. gp130 at the nexus of inflammation, autoimmunity, and cancer. Journal of leukocyte biology. 2010;88(6):1145–56. doi: 10.1189/jlb.0410217 ; PMCID: PMC2996896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. 2012;12(6):616–22. doi: 10.1097/ACI.0b013e328358cc0b ; PMCID: PMCPMC3538358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–8. doi: 10.1126/science.1200439 ; PMCID: PMCPMC3070042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kane A, Deenick EK, Ma CS, Cook MC, Uzel G, Tangye SG. STAT3 is a central regulator of lymphocyte differentiation and function. Current opinion in immunology. 2014;28C:49–57. doi: 10.1016/j.coi.2014.01.015 . [DOI] [PubMed] [Google Scholar]
- 16.Siegel AM, Heimall J, Freeman AF, Hsu AP, Brittain E, Brenchley JM, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35(5):806–18. doi: 10.1016/j.immuni.2011.09.016 ; PMCID: PMC3228524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10(1):39–49. . [DOI] [PubMed] [Google Scholar]
- 18.Riley JK, Takeda K, Akira S, Schreiber RD. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J Biol Chem. 1999;274(23):16513–21. . [DOI] [PubMed] [Google Scholar]
- 19.Queval CJ, Song OR, Deboosere N, Delorme V, Debrie AS, Iantomasi R, et al. STAT3 Represses Nitric Oxide Synthesis in Human Macrophages upon Mycobacterium tuberculosis Infection. Sci Rep. 2016;6:29297 doi: 10.1038/srep29297 ; PMCID: PMCPMC4935992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lastrucci C, Benard A, Balboa L, Pingris K, Souriant S, Poincloux R, et al. Tuberculosis is associated with expansion of a motile, permissive and immunomodulatory CD16(+) monocyte population via the IL-10/STAT3 axis. Cell Res. 2015;25(12):1333–51. doi: 10.1038/cr.2015.123 ; PMCID: PMCPMC4670988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carow B, Reuschl AK, Gavier-Widen D, Jenkins BJ, Ernst M, Yoshimura A, et al. Critical and independent role for SOCS3 in either myeloid or T cells in resistance to Mycobacterium tuberculosis. PLoS Pathog. 2013;9(7):e1003442 Epub 2013/07/16. doi: 10.1371/journal.ppat.1003442 ; PMCID: PMC3701707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bromberg J, Darnell JE Jr. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19(21):2468–73. doi: 10.1038/sj.onc.1203476 . [DOI] [PubMed] [Google Scholar]
- 23.Hoentjen F, Sartor RB, Ozaki M, Jobin C. STAT3 regulates NF-kappaB recruitment to the IL-12p40 promoter in dendritic cells. Blood. 2005;105(2):689–96. doi: 10.1182/blood-2004-04-1309 . [DOI] [PubMed] [Google Scholar]
- 24.Bekker LG, Freeman S, Murray PJ, Ryffel B, Kaplan G. TNF-alpha controls intracellular mycobacterial growth by both inducible nitric oxide synthase-dependent and inducible nitric oxide synthase-independent pathways. J Immunol. 2001;166(11):6728–34. . [DOI] [PubMed] [Google Scholar]
- 25.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204(7):1653–64. doi: 10.1084/jem.20062648 ; PMCID: PMCPMC2118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kariyone A, Tamura T, Kano H, Iwakura Y, Takeda K, Akira S, et al. Immunogenicity of Peptide-25 of Ag85B in Th1 development: role of IFN-gamma. Int Immunol. 2003;15(10):1183–94. . [DOI] [PubMed] [Google Scholar]
- 27.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4(6):551–6. doi: 10.1038/ni938 . [DOI] [PubMed] [Google Scholar]
- 28.Silver JS, Stumhofer JS, Passos S, Ernst M, Hunter CA. IL-6 mediates the susceptibility of glycoprotein 130 hypermorphs to Toxoplasma gondii. J Immunol. 2011;187(1):350–60. doi: 10.4049/jimmunol.1004144 ; PMCID: PMC3119722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194(4):519–27. ; PMCID: PMCPMC2193502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fogli LK, Sundrud MS, Goel S, Bajwa S, Jensen K, Derudder E, et al. T cell-derived IL-17 mediates epithelial changes in the airway and drives pulmonary neutrophilia. J Immunol. 2013;191(6):3100–11. doi: 10.4049/jimmunol.1301360 ; PMCID: PMCPMC3822005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–74. doi: 10.1038/ni1488 . [DOI] [PubMed] [Google Scholar]
- 32.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–33. doi: 10.1016/j.cell.2006.07.035 . [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Godec J, Ben-Aissa K, Cui K, Zhao K, Pucsek AB, et al. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-gamma-producing T helper 17 cells. Immunity. 2014;40(3):355–66. doi: 10.1016/j.immuni.2014.01.002 ; PMCID: PMCPMC3965587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Current opinion in immunology. 2011;23(6):702–6. doi: 10.1016/j.coi.2011.08.007 ; PMCID: PMCPMC3232281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103(21):8137–42. Epub 2006/05/16. doi: 10.1073/pnas.0600666103 ; PMCID: PMC1459629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eruslanov EB, Lyadova IV, Kondratieva TK, Majorov KB, Scheglov IV, Orlova MO, et al. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infection and immunity. 2005;73(3):1744–53. doi: 10.1128/IAI.73.3.1744-1753.2005 ; PMCID: PMC1064912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell host & microbe. 2012;11(1):81–90. doi: 10.1016/j.chom.2011.11.012 ; PMCID: PMC3266554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng CG, Kaviratne M, Rothfuchs AG, Cheever A, Hieny S, Young HA, et al. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J Immunol. 2006;177(10):7086–93. . [DOI] [PubMed] [Google Scholar]
- 39.Pedrosa J, Saunders BM, Appelberg R, Orme IM, Silva MT, Cooper AM. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infection and immunity. 2000;68(2):577–83. ; PMCID: PMCPMC97179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee CK, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K, et al. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17(1):63–72. . [DOI] [PubMed] [Google Scholar]
- 41.Panopoulos AD, Zhang L, Snow JW, Jones DM, Smith AM, El Kasmi KC, et al. STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood. 2006;108(12):3682–90. doi: 10.1182/blood-2006-02-003012 ; PMCID: PMCPMC1895456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen-Jackson H, Panopoulos AD, Zhang H, Li HS, Watowich SS. STAT3 controls the neutrophil migratory response to CXCR2 ligands by direct activation of G-CSF-induced CXCR2 expression and via modulation of CXCR2 signal transduction. Blood. 2010;115(16):3354–63. doi: 10.1182/blood-2009-08-240317 ; PMCID: PMCPMC2858484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4(6):551–6. Epub 2003/05/20. doi: 10.1038/ni938 . [DOI] [PubMed] [Google Scholar]
- 44.Whitmarsh RJ, Gray CM, Gregg B, Christian DA, May MJ, Murray PJ, et al. A critical role for SOCS3 in innate resistance to Toxoplasma gondii. Cell host & microbe. 2011;10(3):224–36. Epub 2011/09/20. doi: 10.1016/j.chom.2011.07.009 ; PMCID: PMC3176442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melillo JA, Song L, Bhagat G, Blazquez AB, Plumlee CR, Lee C, et al. Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. J Immunol. 2010;184(5):2638–45. doi: 10.4049/jimmunol.0902960 ; PMCID: PMCPMC3099405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothfuchs AG, Egen JG, Feng CG, Antonelli LR, Bafica A, Winter N, et al. In situ IL-12/23p40 production during mycobacterial infection is sustained by CD11bhigh dendritic cells localized in tissue sites distinct from those harboring bacilli. J Immunol. 2009;182(11):6915–25. Epub 2009/05/21. doi: 10.4049/jimmunol.0900074 ; PMCID: PMCPMC2786988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pitt JM, Stavropoulos E, Redford PS, Beebe AM, Bancroft GJ, Young DB, et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol. 2012;189(8):4079–87. doi: 10.4049/jimmunol.1201061 ; PMCID: PMC3467194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung YJ, Ryan L, LaCourse R, North RJ. Increased interleukin-10 expression is not responsible for failure of T helper 1 immunity to resolve airborne Mycobacterium tuberculosis infection in mice. Immunology. 2003;109(2):295–9. ; PMCID: PMC1782960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Redford PS, Boonstra A, Read S, Pitt J, Graham C, Stavropoulos E, et al. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. European journal of immunology. 2010;40(8):2200–10. doi: 10.1002/eji.201040433 ; PMCID: PMC3378704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roque S, Nobrega C, Appelberg R, Correia-Neves M. IL-10 underlies distinct susceptibility of BALB/c and C57BL/6 mice to Mycobacterium avium infection and influences efficacy of antibiotic therapy. J Immunol. 2007;178(12):8028–35. . [DOI] [PubMed] [Google Scholar]
- 51.Huber S, Gagliani N, Esplugues E, O'Connor W Jr., Huber FJ, Chaudhry A, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34(4):554–65. doi: 10.1016/j.immuni.2011.01.020 ; PMCID: PMCPMC3113617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–77. doi: 10.1038/ni1449 . [DOI] [PubMed] [Google Scholar]
- 53.Gopal R, Monin L, Slight S, Uche U, Blanchard E, Fallert Junecko BA, et al. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 2014;10(5):e1004099 doi: 10.1371/journal.ppat.1004099 ; PMCID: PMC4022785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wozniak TM, Saunders BM, Ryan AA, Britton WJ. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infection and immunity. 2010;78(10):4187–94. doi: 10.1128/IAI.01392-09 ; PMCID: PMC2950338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine & growth factor reviews. 2010;21(6):455–62. doi: 10.1016/j.cytogfr.2010.10.004 ; PMCID: PMC3032416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178(6):3786–96. . [DOI] [PubMed] [Google Scholar]
- 57.Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA, et al. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 2013;6(5):972–84. Epub 2013/01/10. doi: 10.1038/mi.2012.135 ; PMCID: PMCPMC3732523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175(2):788–95. . [DOI] [PubMed] [Google Scholar]
- 59.Matsumura Y, Kobayashi T, Ichiyama K, Yoshida R, Hashimoto M, Takimoto T, et al. Selective expansion of foxp3-positive regulatory T cells and immunosuppression by suppressors of cytokine signaling 3-deficient dendritic cells. J Immunol. 2007;179(4):2170–9. . [DOI] [PubMed] [Google Scholar]
- 60.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205(1):105–15. Epub 2007/12/26. doi: 10.1084/jem.20071367 ; PMCID: PMCPMC2234384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitamura H, Kamon H, Sawa S, Park SJ, Katunuma N, Ishihara K, et al. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23(5):491–502. doi: 10.1016/j.immuni.2005.09.010 . [DOI] [PubMed] [Google Scholar]
- 62.Chen Z, O'Shea JJ. Th17 cells: a new fate for differentiating helper T cells. Immunol Res. 2008;41(2):87–102. doi: 10.1007/s12026-007-8014-9 . [DOI] [PubMed] [Google Scholar]
- 63.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8(4):265–77. Epub 2000/01/06. . [DOI] [PubMed] [Google Scholar]
- 64.Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8(10):1089–97. Epub 2002/09/10. doi: 10.1038/nm763 . [DOI] [PubMed] [Google Scholar]
- 65.Tamura T, Ariga H, Kinashi T, Uehara S, Kikuchi T, Nakada M, et al. The role of antigenic peptide in CD4+ T helper phenotype development in a T cell receptor transgenic model. Int Immunol. 2004;16(12):1691–9. Epub 2004/10/13. doi: 10.1093/intimm/dxh170 . [DOI] [PubMed] [Google Scholar]
- 66.Carow B, Qun Ye X, Gavier-Widen D, Bhuju S, Oehlmann W, Singh M, et al. Silencing Suppressor of Cytokine Signaling-1 (SOCS1) in Macrophages Improves Mycobacterium tuberculosis Control in an Interferon-{gamma} (IFN-{gamma})-dependent Manner. J Biol Chem. 2011;286(30):26873–87. Epub 2011/05/31. doi: 10.1074/jbc.M111.238287 ; PMCID: PMC3143647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rothfuchs AG, Gigliotti D, Palmblad K, Andersson U, Wigzell H, Rottenberg ME. IFN-alpha beta-dependent, IFN-gamma secretion by bone marrow-derived macrophages controls an intracellular bacterial infection. J Immunol. 2001;167(11):6453–61. Epub 2001/11/21. . [DOI] [PubMed] [Google Scholar]
- 68.Yang T, Stark P, Janik K, Wigzell H, Rottenberg ME. SOCS-1 protects against Chlamydia pneumoniae-induced lethal inflammation but hampers effective bacterial clearance. J Immunol. 2008;180(6):4040–9. Epub 2008/03/07. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The frequency (A) and numbers (B) of CD11b+CD11c-Ly6Cdim Ly6G+ neutrophils in lungs stat3fl/fl lysm cre and stat3fl/fl mice at 14 weeks after infection with M. tuberculosis ± SEM are shown (n = 4 mice per group); representative dot plots of neutrophil staining in lungs are shown (C).
(TIFF)
The mean frequency of PMA/ ionomycin-stimulated IFN-γ secreting CD4+ and CD8+ lung T cells from stat3fl/fl lysm cre and stat3fl/fl mice at 4 (B) and 8 (C) weeks after infection with M. tuberculosis ± SEM was measured by FACS (n = 4 per group).
(TIFF)
The mean frequency of CD4+, CD8+ and γδ+ cells within lung CD3+ T cells from stat3fl/fl lysm cre and stat3fl/fl mice 8 weeks after infection with M. tuberculosis was measured by FACS (n = 4 per group) (A).
The mean frequency of PPD and PMA/ ionomycin-stimulated IL-17 secreting γδ+ pulmonary T cells from stat3fl/fl lysm cre and stat3fl/fl mice at 4 (B) and 8 (C) weeks after infection with M. tuberculosis was measured by FACS (n = 4 per group).
(TIFF)
The mean fold increase of il17a (A), il22 (B) mRNA ± SEM was measured by real time PCR in the total RNA from lungs of stat3fl/fl lysm cre and stat3fl/fl mice at 14 weeks after M. tuberculosis infection (n = 5 per group *p<0.05 Student’s t test).
(TIFF)
The mean fold increase of il6 (A) and il23p19 (B) ± SEM were measured by real-time PCR in triplicate cultures of stat3fl/fl lysm cre and stat3fl/fl BMDCs 6 h after stimulation with either LPS, CpG or Pam3K (*p<0.05 and ***p<0.001 Student t test).
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.