Practical Implications
Hyperintensity in the corticomedullary junction on diffusion-weighted imaging is helpful in distinguishing neuronal intranuclear inclusion disease in patients with leukoencephalopathy.
Neuronal intranuclear inclusion disease (NIID) is a neurodegenerative disease characterized by eosinophilic hyaline intranuclear inclusions in cells in the central, peripheral, and autonomic nervous system and visceral organs. Although clinical diagnosis was difficult until recently, an increasing number of adult cases of leukoencephalopathy have been diagnosed antemortem with NIID based on the characteristic hyperintensity in the corticomedullary junction on diffusion-weighted imaging (DWI) and confirmatory skin biopsy.1 While adult-onset NIID is characterized by slowly progressive dementia, it may also present with acute symptoms including stroke-like episodes and epileptic seizures.1,2 However, pathophysiology of acute episodes remains largely unclear. Here we report a case of NIID with distinct neurologic deficits. Whereas chronic hypoperfusion on SPECT was reported in NIID,1 we observed unexpected perfusion abnormalities using arterial spin labeling (ASL), which is a noninvasive, repeatable perfusion MRI method using magnetically labeled arterial blood water protons as endogenous tracer particles.3
Case report
A 60-year-old retired surveyor was unable to work efficiently in subsequent reemployments and quit shortly after joining; thus he visited our department. The patient had a history of acute dysarthria when he was 59 and recovered completely afterwards. He had no history of migraine and was born to parents of consanguineous marriage. Neurologic examination demonstrated memorization and word recall impairment, saccadic pursuit eye movements, mild weakness of the left lower extremity, reduced tendon reflexes, Babinski sign on the left, snout and palmomental reflexes, and decreased vibratory sense. Brain MRI showed diffuse, bilateral white matter lesions; hyperintensity was evident in the white matter adjacent to the cortex on DWI, which remained stable afterwards (figure 1, A and B). He subsequently experienced 2 major episodes.
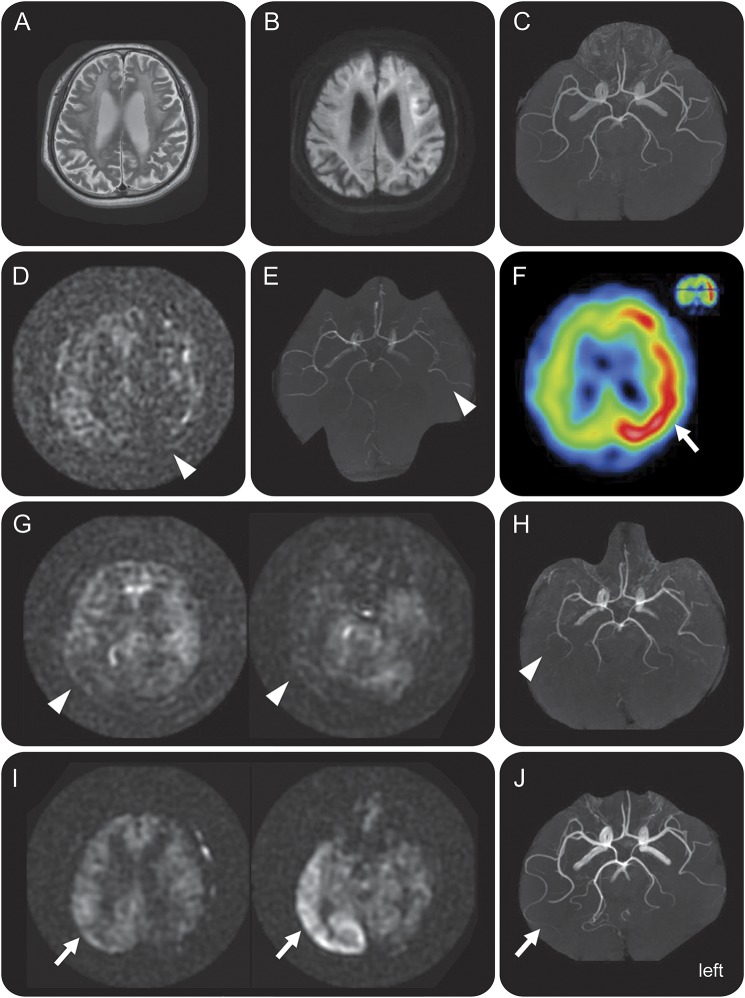
Figure 1. Dynamic perfusion changes during attacks in neuronal intranuclear inclusion disease.
(A–C) The baseline study. T2-weighted imaging demonstrates diffuse, bilateral white matter lesion (A). Diffusion-weighted imaging (DWI) shows hyperintensity in the white matter adjacent to the cerebral cortex (B) with increased apparent diffusion coefficient. Magnetic resonance angiography (MRA) shows normal findings (C). (D–F) Studies during the first major episode. On admission, arterial spin labeling (ASL) and MRA indicate ipsilateral hypoperfusion (D, E; arrowheads). N-isopropyl-p-[123I]iodoamphetamine (123I-IMP) SPECT on the following day shows remarkable hyperperfusion in the corresponding regions (F; arrow). (G–J) Studies during the second major episode. On admission, ASL and MRA show hypoperfusion in the right posterior regions (G, H; arrowheads). Images the following day demonstrate marked hyperperfusion in the corresponding regions (I, J; arrows). Together, hypoperfusion and subsequent hyperperfusion was reproduced in the 2 episodes in the bilateral hemispheres.
One and a half years after the first visit, at the age of 62 years, the patient was unable to change his clothes one morning and felt disoriented at home. He was admitted to our hospital and presented with total aphasia and right hemiparesis. ASL3 revealed left hemisphere hypoperfusion (figure 1D); magnetic resonance angiography (MRA) demonstrated decreased flow, but no focal stenosis or occlusion in the left middle cerebral artery (MCA) (figure 1E). He developed drowsiness and complete paralysis of the right extremities within 2 hours of arrival. EEG showed suppressed activity but no epileptic discharge in the left hemisphere. Because an unobserved seizure followed by Todd paralysis was initially suspected, he received IV phenytoin followed by carbamazepine. His consciousness level improved the following day. Follow-up EEG was normal, but N-isopropyl-p-[123I]iodoamphetamine (123I-IMP) SPECT showed hyperperfusion in the left hemisphere (figure 1F). NIID was subsequently suspected based on the DWI findings (as the DWI characteristics of NIID became widely known among Japanese neuroradiologists then); abdominal skin biopsy later confirmed the diagnosis of NIID (figure 2). FMR1 gene testing showed no CGG premutations, thus excluding fragile X-associated tremor/ataxia syndrome, which may clinicopathologically resemble NIID. Polyneuropathy, which is common in NIID, was evident from diffuse, homogeneous delay in conduction velocity. After discharge, the patient experienced 3 mild episodes (right hemiparesis with aphasia or loss of consciousness) within 2 months during the outpatient follow-up. However, he had no major episodes for 1 year and 4 months after replacing carbamazepine with 1,000 mg/day levetiracetam.
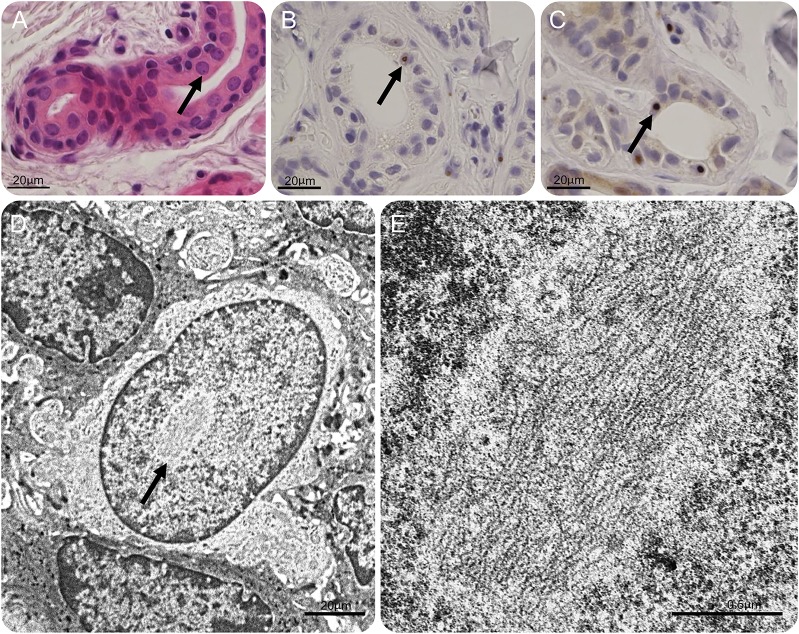
Figure 2. Skin biopsy.
(A) Hematoxylin & eosin staining and (B) immunohistochemistry with anti-ubiquitin and (C) p62 antibody of eccrine sweat gland. Eosinophilic, ubiquitin-immunoreactive, and p62-immunoreactive intranuclear inclusions are observed (arrows). Scale bars 20 μm. (D–E) Electron microscopic findings of an intranuclear inclusion without limiting membrane in the epithelial squamous cell at low (D, arrow) and high (E) magnification. Scale bars 2 μm (D), 0.5 μm (E).
At the age of 63 years, the patient developed headache upon waking up in the morning and felt disoriented at home; no convulsion was experienced. He was readmitted within a few hours. On examination, left hemispatial neglect, left-dominant visual inattention, and mild paresis of the left face and extremities was observed. MRI/MRA was repeated to evaluate potential stroke. ASL revealed hypoperfusion in the right posterior hemisphere; MRA showed decreased intensity in the distal part of the right MCA (figure 1, G–H). He subsequently presented with stupor and fever (CSF was unremarkable); they resolved over a few days, during which he received cooling, fluid replacement, and acetaminophen administration. Follow-up ASL on the following day demonstrated hyperperfusion in the right posterior area; MRA showed increased flow in the right MCA (figure 1, I–J). Furthermore, 123I-IMP SPECT performed 3 days later showed milder hyperperfusion in that region. Scores on Mini-Mental State Examination and Frontal Assessment Battery (25 and 11, respectively) were comparable to those during the first admission.
DISCUSSION
The episodes in the present case were distinct from strokes or epileptic seizures. Perfusion imaging showed initial hypoperfusion and subsequent hyperperfusion; the findings were in striking contrast with those in focal epilepsy, i.e., ictal hyperperfusion and postictal/interictal hypoperfusion.4 Rather, the perfusion changes in this patient were reminiscent of those in migraine with aura,5–7 although he had no migraine headaches throughout the course. In hemiplegic migraine aura, cerebral blood flow changes are biphasic, which begin with hypoperfusion and are followed by hyperperfusion.7 The present report demonstrates the ASL characteristics of certain acute neurologic deficits in NIID.
AUTHOR CONTRIBUTIONS
K. Fujita: design and conceptualization of the study, analysis and interpretation of the data, drafting and revising the manuscript. Y. Osaki: design and conceptualization of the study, analysis and interpretation of the data, drafting and revising the manuscript. R. Miyamoto: analysis and interpretation of the data, revising the manuscript. Y. Shimatani: analysis and interpretation of the data, revising the manuscript. T. Abe: analysis and interpretation of the data, revising the manuscript. H. Sumikura: analysis and interpretation of the data, revising the manuscript. S. Murayama: analysis and interpretation of the data, revising the manuscript. Y. Izumi: analysis and interpretation of the data, revising the manuscript. R. Kaji: analysis and interpretation of the data, revising the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
K. Fujita, Y. Osaki, R. Miyamoto, Y. Shimatani, T. Abe, and H. Sumikura report no disclosures. S. Murayama serves on the editorial board of Neuropathology and receives research support from the Japanese Governmental Bureau of Health, Labor and Welfare, National Center for Geriatrics and Gerontology, National Center of Neurology and Psychiatry, and the Japanese Governmental Bureau of Education, Technology and Sports. Y. Izumi reports no disclosures. R. Kaji serves as Section Editor for Frontiers in Neurology and Associate Editor for Neurology and Clinical Neuroscience; is author on a patent re: New Generation Botulinum Toxin Preparation (A2NTX); serves on the speakers' bureau for GlaxoSmithKline, Japan, Co. Ltd.; and receives research support from the Japanese Ministry of Health, Welfare, and Labor and the Japanese Ministry of Education, Culture and Science. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Sone J, Mori K, Inagaki T, et al. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain 2016;139:3170–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toyota T, Huang Z, Nohara S, et al. Neuronal intranuclear inclusion disease manifesting with new-onset epilepsy in the elderly. Neurol Clin Neurosci 2015;3:238–240. [Google Scholar]
- 3.Tada Y, Satomi J, Abe T, et al. Intra-arterial signal on arterial spin labeling perfusion MRI to identify the presence of acute middle cerebral artery occlusion. Cerebrovasc Dis 2014;38:191–196. [DOI] [PubMed] [Google Scholar]
- 4.Van Paesschen W. Ictal SPECT. Epilepsia 2004;45(suppl 4):35–40. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez del Rio M, Bakker D, Wu O, et al. Perfusion weighted imaging during migraine: spontaneous visual aura and headache. Cephalalgia 1999;19:701–707. [DOI] [PubMed] [Google Scholar]
- 6.Pollock JM, Deibler AR, Burdette JH, et al. Migraine associated cerebral hyperperfusion with arterial spin-labeled MR imaging. Am J Neuroradiol 2008;29:1494–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iizuka T, Tominaga N, Kaneko J, et al. Biphasic neurovascular changes in prolonged migraine aura in familial hemiplegic migraine type 2. J Neurol Neurosurg Psychiatry 2015;86:344–353. [DOI] [PubMed] [Google Scholar]