Practical Implications
Life-threatening attacks of spontaneous hypothermia can be a rare nonmotor manifestation of Parkinson disease and may respond to levodopa therapy.
An 88-year-old woman with history of atrial fibrillation, hypertension, and hypothyroidism was hospitalized for repeated episodes of hypothermia over 4 years. Initial rare episodes of mild hypothermia and confusion in winter months progressed to more frequent and severe episodes. The patient was hospitalized at least 3 times at other facilities before presenting to our hospital with recurrent daily episodes. During these episodes, her body temperature dropped spontaneously, followed by confusion, dysarthria, bradycardia, hypercarbic respiratory failure, and hypoglycemia.
Prior outpatient neurologic evaluation between attacks disclosed hypophonia, mild ataxia, positive Romberg sign, and diminished deep tendon reflexes. Extensive studies including endocrinologic workup, neuroimaging, CSF, paraneoplastic panel, and autoimmune workup were normal. The patient was diagnosed with spontaneous periodic hypothermia (SPH) and treated with low-dose cyproheptadine.
After initial improvement with warming during hospitalization, the patient developed recurrent severe hypothermia, bradycardia, hypotension, hypoglycemia, and hypercapnia refractory to active rewarming, and progressed to somnolence requiring intubation. Chest X-ray and white blood cell counts were normal. She was found to have a urinary tract infection, which was treated with antibiotics. EEG showed encephalopathy and brain MRI was negative. Workup for anterior pituitary, thyroid, and adrenal cortical functions was normal at baseline and during hypothermia events.
Trials of medication including cyproheptadine, methylprednisolone, carbamazepine, theophylline, modafinil, and pyridostigmine failed to improve the patient’s condition. She steadily deteriorated to stupor with occasional and inconsistent responsiveness. After a discussion of goals of care with her family, comfort measures only were instituted.
At this point, the patient’s 24-hour urinary catecholamine study returned, showing low levels of dopamine and norepinephrine, suggestive of central sympathetic failure. A trial of carbidopa/levodopa 50/500 mg was administered through her feeding tube. Within hours, she awoke and became interactive. Hypothermia episodes resolved and did not recur but she continued to have respiratory difficulty from heart failure.
After 3 doses of 25/250 mg of carbidopa/levodopa, the patient developed dyskinesia, which was possibly from a hyperdopaminergic state. There was also worsening of atrial fibrillation, heart failure, delirium, and agitation, which necessitated discontinuation of carbidopa/levodopa. Thereafter, she again became somnolent over a day. Her temperature did not fall but her respiratory difficulties persisted. Palliative measures were reinstituted after discussion with family and the patient died immediately after withdrawal of respiratory support.
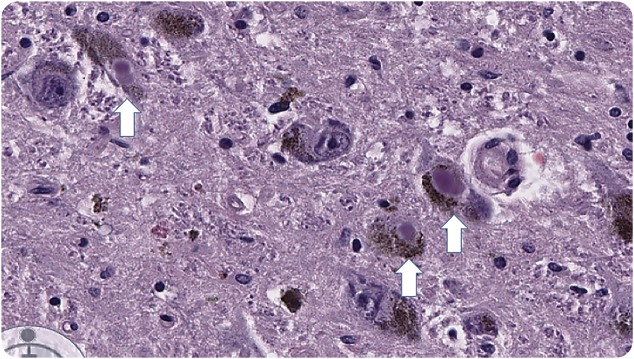
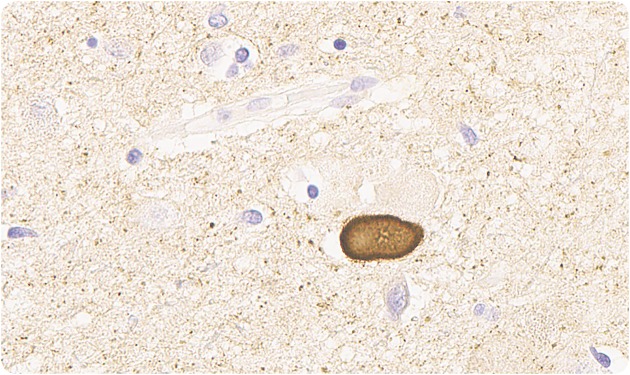
Macroscopic sections of cerebral hemispheres, brainstem, spinal cord, and substantia nigra were normal but microscopic sections showed moderate loss of neurons in the substantia nigra bilaterally with presence of Lewy bodies (figure 1). Lewy bodies were also seen in hypothalamus (figure 2), locus ceruleus, and intermediolateral spinal cord. None of the hypothalamic nuclei or neuronal groups in the spinal cord was destroyed. Cortex showed no pathologic changes with normal glial tissue but scattered Lewy bodies were seen. The pathologic diagnosis was Parkinson disease (PD), as the findings were not compatible with other diagnoses like multiple system atrophy or Lewy body disease, where there is more widespread involvement.
Figure 1. Neurons in the substantia nigra containing Lewy bodies (hematoxylin & eosin stain, ×225).
Figure 2. Lewy bodies in the hypothalamus (α-synuclein immunostain, ×350).
Hypothermic attacks in PD are rare.1,2 Our patient had been diagnosed with SPH, a rare syndrome occurring in early and midlife.3 Although the classic condition associated with SPH is Shapiro syndrome (corpus callosum agenesis, hyperhidrosis, and hypothermia from hyperactive parasympathetic output and hypoactive sympathetic output4), idiopathic cases also have been reported, suggesting a neurodegenerative process. Hypothermia in SPH is presumed to result from a failure in the neuroendocrine hypothalamic temperature set point. Several neurotransmitters are involved in temperature regulation in a complex pattern.5
There is increasing evidence of the role of dopamine in the central control of temperature. Dopamine lowers core temperature though a biphasic response to stress and other stimuli.6 Animal studies showed that stress combined with apomorphine (a dopamine agonist) produced hyperthermia not seen with apomorphine alone, suggesting that the effect of dopamine on the preoptic anterior hypothalamus varies with the thermal experience of the organism.7 The intricate interplay of dopamine with other hormones in temperature regulation has not been well-elucidated.
Autonomic dysfunction is a well-recognized nonmotor feature of PD. Temperature dysregulation leading to hyperthermia is more common than hypothermia in patients with PD. Our patient's age, functional decline over several years, hypophonia, and gait disturbance raised the suspicion for a neurodegenerative process. In retrospect, she had nonmotor manifestations of PD with postural instability and autonomic dysfunction. Hypothalamic dysfunction from synucleinopathy leading to temperature dysregulation may explain the SPH episodes. Autonomic dysfunction in other neurodegenerative conditions including multiple system atrophy and primary autonomic failure8 may also have similar underlying etiology. The dramatic improvement in sensorium and temperature stabilization following initiation of levodopa indicates a clear role of dopamine in temperature regulation in our patient’s case.
AUTHOR CONTRIBUTIONS
V. Renga and J.L. Bernat were equally involved in writing the manuscript. W.F. Hickey prepared and interpreted the pathology slides and wrote the description of the pathology findings.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
V. Renga and W.F. Hickey report no disclosures. J.L. Bernat serves on the editorial boards of Neurology® Clinical Practice, Neurocritical Care, Neurology Today®, The Physician's Index for Ethics and Medicine, and Multiple Sclerosis and Related Diseases; receives publishing royalties for Ethical and Legal Issues in Neurology (Elsevier, 2013), Ethical Issues in Neurology, 3rd ed. (Lippincott Williams & Wilkins, 2008), and Palliative Care in Neurology (Oxford University Press, 2004); and receives research support from NIH. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Li BY, Lou M. Severe hypothermia in Parkinson's disease. CNS Neurosci Ther 2012;18:864–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hama K, Miwa H, Kondo T. Life-threatening hypothermia in Parkinson's disease. Mov Disord 2009;24:945–946. [DOI] [PubMed] [Google Scholar]
- 3.Kloos RT. Spontaneous periodic hypothermia. Medicine 1995;74:268–280. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro WR, Williams GH, Plum F. Spontaneous recurrent hypothermia accompanying agenesis of the corpus callosum. Brain 1969;92:423–436. [DOI] [PubMed] [Google Scholar]
- 5.Lipton JM, Clark WG. Neurotransmitters in temperature control. Annu Rev Physiol 1986;48:613–623. [DOI] [PubMed] [Google Scholar]
- 6.Cox B, Tha S. The role of dopamine and noradrenaline in temperature control of normal and reserpine-pretreated mice. J Pharm Pharmacol 1975;27:242–247. [DOI] [PubMed] [Google Scholar]
- 7.Snow AE, Horita A. The stress-dependent nature of apomorphine hyperthermia. Brain Res 1976;117:163–168. [DOI] [PubMed] [Google Scholar]
- 8.Idiaquez J, Kaufmann H, Soza M, Necochea C. Pure autonomic failure: Bradbury Eggleston syndrome: case report [in Spanish]. Rev Med Chil 2005;133:215–218. [DOI] [PubMed] [Google Scholar]