Practical Implications
Consider testing for Powassan virus immunoglobulin M/immunoglobulin G in the differential diagnosis of postencephalitic parkinsonism.
Postencephalitic parkinsonism has been described following infection with influenza A and Flaviviridae family viruses such as Japanese B encephalitis, St Louis encephalitis, and West Nile virus.1 Some of these viruses are neurotropic, and a few preferentially target the substantia nigra.2,3 We report a novel case of Powassan virus (POWV) causing postencephalitic parkinsonism with evidence of decreased presynaptic uptake of dopamine in the striatum.
Case Report
A 35-year-old woman from Wisconsin, with no recent travel history, presented with headache, vomiting, confusion, hypertension (blood pressure 240/140 mm Hg), and fever (temperature of 38.4°C). Within 24 hours, she developed a rightward gaze deviation and poor respiratory function requiring ventilation over 1.5 weeks. On withdrawal of sedation, she was minimally responsive. Two weeks later, she had reduced short-term memory, wide-based ataxic gait, and left abducens palsy. MRI brain without gadolinium showed marked T2 signal white matter hyperintensities in the cerebral hemispheres and posterior fossa bilaterally (figure 1). CSF analysis revealed an elevated opening pressure (65 cm H2O), elevated protein (76 mg/dL), normal glucose (47 mg/dL; blood glucose 87 mg/dL), and monocytic pleocytosis (343 total nucleated cells, 98% monocytes and 2% neutrophils). Initial extensive microbiologic evaluation including blood and CSF cultures were negative. Based on the patient's geographic location, POWV testing was performed. In the CSF, POWV immunoglobulin M (IgM) antibody was positive with a very high positive-to-negative (P/N) ratio of 23.7, with a neutralizing antibody titer of more than 32, confirming POWV as the cause of encephalitis. After 6 months of rehabilitation, the patient achieved an improved functional baseline (able to cook, converse, walk independently, and write her name). Ten months later (i.e., 16 months after her initial encephalitis), she developed new progressive gait festination and imbalance requiring an assistive device to ambulate, micrographia, hypomimia, abnormal posturing of the right upper limb, and bilateral reduced arm swing. She presented to our clinic 2 years after her initial encephalitic presentation. On examination, she was apathetic with hypometric vertical saccades, generalized bradykinesia, increased tone in the left lower limb, and diffuse hyperreflexia with absent Babinski responses. She had gait festination with en bloc turning, decreased arm swing, and loss of postural reflexes on retropulsion testing. A repeat MRI brain showed resolution of previous white matter changes but persistence of periventricular leukoaraiosis (figure 2A). Dopamine transporter SPECT imaging showed markedly reduced uptake in bilateral putamen and left greater than right caudate (figure 2B). Based on comparison with age-matched controls, the quantitated z scores were lower in the left putamen (−0.9 for right, −1.7 for left) and left caudate (0.1 for right, −1.8 for left) (MIM Software Inc., Cleveland, OH). Repeat CSF testing showed absence of POWV RNA, an elevated POWV IgM antibody P/N ratio of 5.4, and a neutralizing antibody titer of 4. Other CSF studies were normal: protein 34 mg/dL, glucose 84 mg/dL, total nucleated cells <1, normal immunoglobulin G index and synthesis rate, 4 oligoclonal bands (normal 0–4). She had partial improvement in gait with 900 mg/d of levodopa.
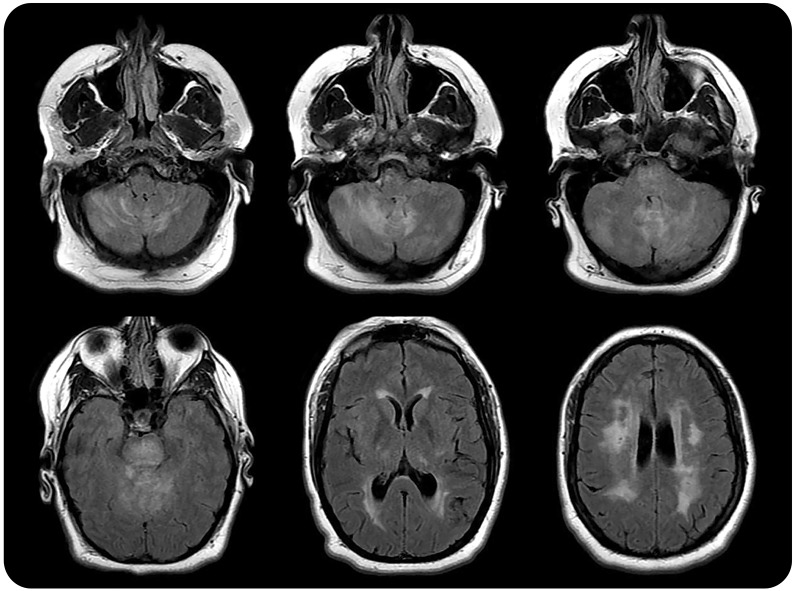
Figure 1. MRI brain (axial fluid-attenuated inversion recovery) in 2013.
MRI shows substantial T2 hyperintensities in the posterior fossa and periventricular subcortical white matter. The basal ganglia appear normal.
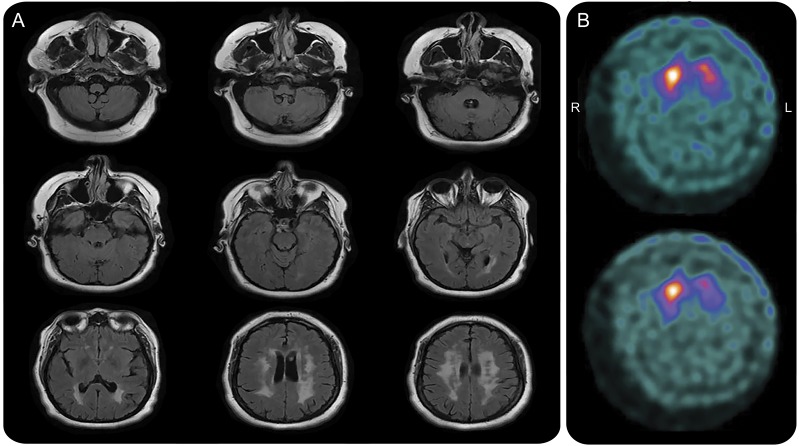
Figure 2. MRI and dopamine transporter (DaT) SPECT in 2015.
(A) MRI brain (axial fluid-attenuated inversion recovery) in 2015 shows resolution of T2 hyperintensities in the posterior fossa with persistence of periventricular subcortical white matter changes. There is mild generalized cerebral and cerebellar volume loss. (B) DaT SPECT in 2015 shows reduced uptake in the left greater than right putamen and caudate.
DISCUSSION
POWV is a rare positive-sense RNA virus that belongs to a group of viruses once referred to as tick-borne encephalitis viruses within the Flaviviridae family.4 It has been isolated from 4 species of North American ticks, most commonly reported in Canada, the northeast United States, and around the Great Lakes.5 The encephalitis associated with POWV is often severe, with respiratory distress, headache, vomiting, fever, cerebellar deficits, hemiplegia, and possible seizures.6 Laboratory diagnosis of POWV is usually accomplished with virus-specific IgM and neutralizing antibodies in serum or CSF. In our case of POWV, IgM is still detectable 2 years after acute infection.
The pathogenesis of postencephalitic parkinsonism is not entirely clear. In some cases, the virus preferentially targets the substantia nigra and ventral tegmental area prior to spreading to the rest of the CNS.3 Neuropathologic studies of patients with postinfluenza parkinsonism did not find evidence of α-synuclein or Lewy body pathology, but rather found gliosis, inflammation, and tau pathology.7 Other cases have found evidence of robust induction of cytokines and subsequent microglial activation, even in tissues not directly infected by the virus.1 POWV has demonstrated a high degree of neurotropism in animal models. There is pathologic evidence of inflammatory lymphocytic infiltration and tissue necrosis predominantly affecting the basal ganglia and rostral brainstem.6 We suggest that POWV may cause parkinsonism through nigrostriatal degeneration, similar to other viruses causing postencephalitic parkinsonism, based on decreased presynaptic dopamine uptake in the bilateral striatum on our patient's DaTscan. Our case adds POWV to the differential diagnosis for viruses causing postencephalitic parkinsonism.
AUTHOR CONTRIBUTIONS
S.O. Mittal: analysis of data, drafting and critical revision of manuscript. A. Hassan: data collection, data analysis, and critical revision of manuscript for intellectual content. J. Sanchez: data collection, data analysis, and critical revision of manuscript for intellectual content. C.E. Robertson: data collection, data analysis, and critical revision of manuscript for intellectual content.
ACKNOWLEDGMENT
The authors thank Dr. Gregory Wiseman for providing the interpretation and quantification of our patient's DaT-SPECT scan.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
S.O. Mittal reports no disclosures. A. Hassan serves on the editorial board of Parkinsonism and Related Disorders and serves as a consultant for Bioblast Pharma. J. Sanchez reports no disclosures. C.E. Robertson has served on a scientific advisory board for Amgen and receives publishing royalties from UpToDate (2012–2017). Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Jang H, Boltz DA, Webster RG, Smeyne RJ. Viral parkinsonism. Biochim Biophys Acta 2009;1792:714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerna F, Mehrad B, Luby JP, Burns D, Fleckenstein JL. St. Louis encephalitis and the substantia nigra: MR imaging evaluation. AJNR Am J Neuroradiol 1999;20:1281–1283. [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi M, Yamada T, Nakajima S, Nakajima K, Yamamoto T, Okada H. The substantia nigra is a major target for neurovirulent influenza A virus. J Exp Med 1995;181:2161–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grard G, Moureau G, Charrel RN, et al. Genetic characterization of tick-borne flaviviruses: new insights into evolution, pathogenetic determinants and taxonomy. Virology 2007;361:80–92. [DOI] [PubMed] [Google Scholar]
- 5.Birge J, Sonnesyn S. Powassan virus encephalitis, Minnesota, USA. Emerg Infect Dis 2012;18:1669–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gholam BI, Puksa S, Provias JP. Powassan encephalitis: a case report with neuropathology and literature review. CMAJ 1999;161:1419–1422. [PMC free article] [PubMed] [Google Scholar]
- 7.Jellinger KA. Absence of α-synuclein pathology in postencephalitic parkinsonism. Acta Neuropathol 2009;118:371–379. [DOI] [PubMed] [Google Scholar]