Abstract
Human cardiomyocytes (CMs) do not proliferate in culture and are difficult to obtain for practical reasons. As such, our understanding of the mechanisms that underlie the physiological and pathophysiological development of the human heart is mostly extrapolated from studies of the mouse and other animal models or heterologus expression of defective gene product(s) in non-human cells. Although these studies provided numerous important insights, much of the exact behavior in human cells remains unexplored given that significant species differences exist. With the derivation of human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSCs) from patients with underlying heart disease, a source of human CMs for disease modeling, cardiotoxicity screening and drug discovery is now available. In this review, we focus our discussion on the use of hESC/ iPSC-derived cardiac cells and tissues for studying various heart rhythm disorders and the associated pro-arrhythmogenic properties in relation to advancements in electrophysiology and tissue engineering.
Introduction
In 2007, human induced pluripotent stem cells (hiPSCs) exhibiting pluripotency and self-renewal characteristics of human embryonic stem cells (hESCs) were successfully derived from dermal fibroblasts through transgenic expression of a combination of pluripotency transcription factors [1,2]. The similarities and differences between hESCs and hiPSCs are extensively described elsewhere (e.g. other reviews in this thematic issue). Recently, many patient-specific hiPSC lines with monogenetic cardiac defects have been generated as prime candidates for drug testing and as alternative models for studying cardiac diseases in human cardiomyocytes (CMs) due to scarcity of their source and non-regenerative nature. This review discusses the generation of hiPSC-derived CMs (hiPSC-CMs) into tissue constructs for studying cardiac electrophysiology.
Directed cardiac differentiation and ventricular specification
The efficiency of directed cardiac differentiation of hESC/ iPSCs has significantly improved in the last decade [3–10]. It remains to be determined if a limit to the CM yield exists because the development and survival of CMs may be dependent on the presence of other cell types [11]. Currently, directed cardiac differentiation still yields a heterogeneous mixture of nodal, atrial and ventricular subtypes. A few labs have shown that ventricular CM subtype specification from hESCs can be augmented by neuregulin-1 [12], noggin in conjunction with retinoic acid receptor inhibition [13] or Dkk1 inhibitor IWR-1 (Ioannis Karakikes and Roger Hajjar, unpublished data).
There are several points to consider when evaluating the efficiency of cardiac differentiation. First, the number of contracting clusters is not an accurate assessment of the CM yield because the actual number of CM in a beating cluster varies significantly [14]. Further, not all derived CMs spontaneously contract. Second, the post-differentiation time point chosen for assessing the CM yield differs among publications. Because CMs remain proliferative past day 60 post-differentiation [15], the yield may include those that originate from proliferation not differentiation. Finally, adherent versus suspension culture of the CM clusters can also affect the yield. For instance, more proliferative fibroblasts in the adherent beating clusters can quickly migrate and divide into the unoccupied culture space and decrease the CM percentage relative to the total cell count. Therefore, the percent yield can be difficult to compare among the publications. A more accurate method would be to quantify the number of CMs per initial pluripotent cell.
Electrophysiology of human pluripotent stem cell-derived CMs: is classification based on AP parameters objective?
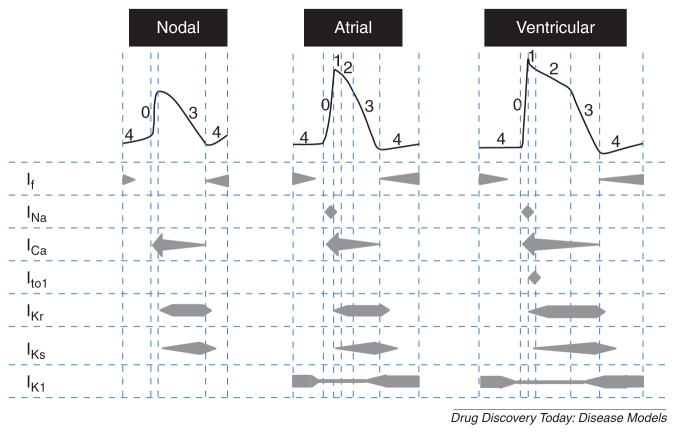
Excitation–contraction coupling in CMs is initiated by an action potential (AP) dictated by a combination of depolarizing and repolarizing ionic currents. hESC/hiPSC-CMs are no exception, with AP profiles that are reminiscent of those of human nodal, atrial or ventricular CMs harvested from the heart, prompting their classification into cellular subtypes based on their AP profile (Fig. 1) [15–17]. The depolarizing currents reported are: a funny current (If) that contributes to the generation of spontaneous APs, tetrodotoxin-sensitive Na+ current (INa) and an L-type Ca2+ current (ICa). The opposing repolarizing currents include: a rapid component of the delayed rectifying K+ current (IKr) and a slow component of the delayed rectifying K+ current (IKs). Recently, a more detailed analysis of hiPSC-CMs has also detected the transiently outward K+ current (Ito) and the membrane stabilizing inwardly rectifying K+ current (IK1) [17]. Of note, AP parameters of heterogeneous derived-CM populations are not discrete but in continuum; the ranges for classifying the different chamber-specific types are often arbitrary and differ among laboratories. As such, classification based on AP parameters alone is not entirely accurate. A more systematic and objective approach involving two or more parameters needs to be developed.
Figure 1.
Schematic action potential profiles of human pluripotent stem cell-derived cardiomyocyte subtypes. The currents involved in each phase of action potential for the nodal, atrial and ventricular cells are presented below the representative action potential for each cellular subtype. If: funny current responsible for pacemaking permeable to Na+ and K+, INa: Na+ current, ICa: Ca2+ current, Ito: transiently outward K+ current, IKr: rapid delayed rectifying K+ current, IKs: slow delayed rectifying K+ current, IK1: inwardly rectifying K+ current.
The electrophysiology of hESC/hiPSC-CMs changes, especially early on, during the in vitro differentiation process. Contractile hESC-CMs develop more mature electrophysiological properties with time, similar to maturation of fetal and neonatal CMs into adult cells [18]. hiPSC-CMs that are phenotypically comparable to hESC-CMs are likely to mature similarly. It is also important to keep in mind that the method for inducing cardiac differentiation and the culture environment can affect the electrophysiological phenotype. Repeated replating has been shown to alter the hESC-CM phenotype [19]. In fact, hESC-CMs differentiated in the embryoid body form exhibit a more hyperpolarized maximum diastolic potential (MDP) and shorter APD than the ones differentiated using the END2 co-culture method [20]. While the presence of non-CMs can enhance the maturation of hESC-CMs [11], other investigators have found superior electrophysiological functions in highly purified hiPSC-derived CMs [17]. The effects of non-CMs on hESC/hiPSC-CM phenotypic maturation may be dependent upon factors such as the differentiation or culture methods and the specific cell line used. Our data show that systematic electrical conditioning leads to an AP phenotype that is more mature than the unconditioned counterpart (Deborah Lieu and Ronald Li, unpublished data). Elucidation of these issues clearly requires further investigation.
Effects of tissue constructs on CM electrophysiology: 2D versus 3D
Much about hESC/hiPSC-CMs has been learned using traditional cell culture protocols and materials. However, culturing in a dish constrains cells to grow on a 2D surface, which is contrary to the 3D environment in vivo. Cellular morphology and function can change significantly as cells attempt to adapt to the environment. In 3D culture, CMs are not directed to exhibit surface polarity. For instance, protein distribution patterns can vary between 2D and 3D culture substrates attributed to the existence of a free cellular surface in the 2D geometry that does not allow the formation of cell–cell or cell–matrix junctions [21]. This raises the question whether the CM behavior observed on 2D culture surface is reflective of the actual behavior in vivo. To mimic the physiological environment, tissue engineering labs have devised different variations of tissue constructs that incorporate CMs into a 3D culture environment for studying CM function. Most cardiac constructs are in the form of a cardiac strip or patch [22–27]. In these configurations, the CMs exhibit many characteristics of natural myocardium, such as the formation of mechanical and electrical junctions and the alignment of myofilaments in a preferential axis [28]. Hence, engineered tissue configuration may facilitate hESC/hiPSC-CM maturation by providing more physiological and adult-like environmental cues.
One defining morphological feature of CMs in vivo is their anisotropic organization such that cells have a preferential axis of alignment [29,30]. Previously, we have shown this can be recreated by culturing hESC-CMs on microgroove substrates that induce cellular alignment parallel to the direction of the grooves by contact guidance. These cells showed morphological anisotropy but more importantly electrical anisotropy, with faster conduction velocity along the axis of alignment [31,32]. Furthermore, we found that increase in cellular alignment as shown by increase in anisotropic ratio of hESC-CMs reduces the spatial dispersion of AP propagation through the cell syncytium, a mechanism that can sustain reentrant arrhythmia, relative to randomly oriented cells, thereby, decreasing the susceptibility to arrhythmogenic stimuli (Jiaxian Wang and Ronald Li, unpublished data). Alternatively, alignment of hESC/hiPSC-CMs can also be achieved through application of mechanical loading. By culturing hESC-CMs in a 3D muscle strand anchored at the ends and subjected to either passive static or cyclic stretch, these CMs aligned themselves in the direction of the stretch, and the mechanically conditioned hESC-CMs showed signs of hypertrophy and formed cellular electrical couplings similar to mature CMs [22,33]. Schaaf et al. found electrophysiology variability in single hESC-CMs cultured in the human engineered heart tissue (hEHT), identifying two different behaviors: short and long ventricular CM-like APD, with the latter supporting phenotypic maturation [33], but their upstroke velocities are slow with depolarized MDP relative to hESC-CMs in embryoid bodies. Despite evidence of hEHT culture improvement of contractile and morphological maturity, these cells remain electrophysiologically immature.
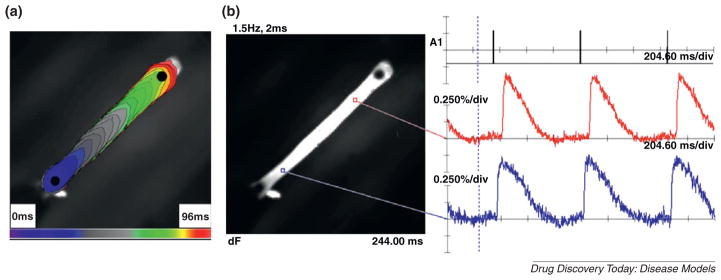
Although the electrophysiology of these constructs was not investigated, structural anisotropy can increase the diffusion rate in the direction of alignment and may facilitate organization of ion channels, which preferentially localize along the z-lines and intercalated discs in vivo [34,35]. Our group has recently created spontaneously beating hEHTs from hESC-CMs (Irene Turnbull and Kevin Costa, unpublished data), and preliminary studies using optical mapping with voltage-sensitive fluorescent dye revealed a longitudinal and unidirectional cable-like electrical conduction (Fig. 2), pacing length dependence of APD and loss of 1:1 capture at high pacing rates similar to natural human myocardium. Alteration in the sarcomere length by mechanical strain also plays an important role in the excitation–contraction coupling and the Ca2+-handling properties of CMs [36,37]. In addition, Costa et al. have shown that the presence of a free surface, creating a boundary constraint, can be a dominating factor over that of local tension and contact guidance in directing the cellular alignment [38].
Figure 2.
Electrophysiology of hEHT in tissue strip configuration constructed from hESC-CMs. (a) Isochrone map generated from optical mapping of point-stimulated hEHT conduction with electrode placed at bottom left. (b) Action potentials from two locations on the hEHT are shown.
To further improve upon the available tissue configurations, Costa et al. have engineered a cardiac tissue chamber that resembles a pumping mini-ventricle [39]. The spherical symmetry of this configuration is conducive to optical mapping of electrical conduction patterns and reentrant arrhythmias, and avoids artifacts from non-conductive boundary conditions created by the edges of traditional tissue patches or strips. It is also possible to assess the active contractile function, and the passive pressure–volume and stress–strain relationships.
Finally, CMs grown as part of a multicellular construct can establish intercellular electrical coupling through gap junctions, which is important for evaluating arrhythmogenesis. Automaticity and formation of early (EADs) and delayed (DADs) after-depolarizations in single CMs merely act as triggers or initiators of arrhythmias. Actual occurrence of arrhythmias depends on mechanisms that perpetuate the untimely electrical signal. In multicellular constructs, substrates for arrhythmia, such as cellular conduction velocity mismatch and the dispersion of repolarization dictated by electrical coupling, the cellular aspect ratio and organization may be observed and assessed.
Methods for functional assessment of hESC/iPSC-CM constructs at the single- and multi-cellular levels
The electrophysiology of hESC/hiPSC-CMs can be functionally assessed as an individual entity with the patch-clamp technique or as a syncytium of monolayer or tissue constructs. Traditional patch-clamp requires a technically skilled operator to form a Giga-ohm seal between the recording glass pipette electrode with the cell membrane before rupturing of the membrane with a burst of negative pressure or perforating agents to enable measurements of ionic currents in the voltage-clamp mode and membrane potential in the current-clamp mode. A crude method of measuring APs using a sharp electrode that impales a cluster of cells has also been used to measure the APs [15]. Automated patch-clamp instruments have been developed commercially for higher throughput assessment of ionic currents and are technically less challenging. The applicability of automated patch-clamp setup for measuring ionic currents of hiPSC-CMs has been demonstrated [17]. One disadvantage is the cell number on the order of millions in suspension required for each experiment. The large cell number of a homogenous population may not be feasible for low differentiation cell yield. It is also not clear at this point if progenitors and immature hESC/ hiPSC-CMs that are unable to retain their shape in suspension have altered ionic currents.
For tissue constructs mentioned above, information on the AP propagation may be assessed with microelectrode arrays (MEA) or optical mapping using voltage- or calcium-sensitive dyes to acquire information on the cellular conduction as a syncytium [40,41]. MEA requires plating of the cells on a substrate with embedded electrodes. Physical contact between the cells and the electrodes allows extracellular measurements of the cellular electrical activity. Unlike patch-clamp methods, the measurements are noninvasive and can be performed repeatedly over an extended period of time. Some drawbacks of this method are the limited number of spatial sampling points, no control over the locations of the sampling points and the high quality of the cell-electrode contacts needed. For greater spatial sampling and control of the sampling location, optical mapping of the tissue constructs is a better alternative. This is possible with the development of faster and more sensitive photodetectors for the acquisition of fast AP and Ca2+ transient responses [32,42,43]. This technique requires loading of the constructs with a voltage-sensitive or Ca2+-sensitive fluorescent dye that may have cytotoxic effects [44]. The dyes are also light-sensitive and can photobleach over extended exposure time, thus limiting the measurement time and the ability to reassess the constructs. From the raw MEA and optical mapping data, a conduction map can be generated giving information such as conduction velocity, direction of signal propagation and visualization of arrhythmia associated with abnormal beats or reentry.
Recreating human heart diseases in a dish from patient-specific hiPSC-CMs: shortcomings of existing animal and cellular models
Many cardiac diseases are hereditary. They have been categorized into contractile or electrophysiological dysfunction known as cardiomyopathies or channelopathies, respectively. Electrophysiology is largely affected by channelopathies or pathologies of ion channels (or their subunits) due to a gene mutation, affecting the activation or inactivation of the ion channels at the molecular level. This translates into an altered depolarizing or repolarizing ion current at the cellular level. Altered currents can generate abnormal pulse from increased automaticity or triggered activity by EADs or DADs. These pro-arrhythmogenic conditions are classified as long QT (LQT) syndrome, short QT (SQT) syndrome, Brugada syndrome (BS), cardiac conduction disease (CCD), sick sinus syndrome (SSS) and catecholaminergic polymorphic ventricular tachycardia (CPVT) [45]. These conditions are of great clinical interest because the subsequent development of arrhythmias or deviation in the normal cardiac rhythm can be fatal.
Although studies using murine models or heterologous expression of mutant ion channels in human embryonic kidney 293 cells or Xenopus oocytes have improved our understanding of the disease mechanisms, it does not provide definitive proof of the electrophysiological dysfunction in human CMs. First, mouse hearts have an intrinsic rate that is more than seven times the human rate, with significantly shorter cardiac APs. Therefore, mouse models cannot accurately recapitulate human electrophysiology due to the intrinsic differences in ion channel expression. Aside from the current magnitude differences, many human cardiac ion channels, such as IKr and IKs, are absent in mice [46]. Second, ion channel modulating subunits may be absent in the heterologously expressed system, therefore, not fully recapitulating the physiological condition of human CMs.
Patient-specific hiPSC-CMs are a great tool for studying the mechanisms of cardiac diseases and assessing the pharmacological effects in human cells, which eliminates possible non-specific effects produced by the mouse model from the transgenic expression of dominant-negative mutants or the animal species themselves [47]. They can be first probed for the corresponding genetic defects and then used for investigating the mechanism of the molecular dysfunction and the ramifications of these defects in human cells. Moreover, the diseased hiPSC-CMs also allows for pharmacological testing, not only at a general level for studying the disease but on a personal level for titrating the drug dosage for patients with distinct genetic backgrounds such as polymorphisms. All the hiPSC lines reported thus far, LQT1, LQT2 and LQT8 (aka Timothy Syndrome), are of channelopathies of a single mis-sense gene mutation, with the exception of one report on cardiomyopathic LEOPARD syndrome [48]. LQT is characterized by a prolonged QT interval in the EKG that reflects an increase in the depolarizing currents or a decrease in the repolarizing currents that prolongs the APD at the cellular level. Although LEOPARD syndrome is a cardiomyopathy, it does present abnormal EKG, but these diseased hiPSC-CMs were not assessed for their electrophysiology.
Currently, twelve LQT syndromes and other channelopathies attributed to different channel mutations have been documented, but few have been investigated using hiPSC-CMs (Table 1). It is important to note that not all the diseases may be modeled in a dish using these cells. To recapitulate the disease in vitro, the defective ion channels or proteins responsible for the symptoms must be present in hiPSC-CMs at the stage when they are interrogated; otherwise, the defects will not be symptomatic. To date, IK1 has not been detected by all labs studying hESC/hiPSC-CMs [49]. In rodent CMs, this current has been shown to progressively increase from embryonic day 12–18 [50]. It is possible that ion channels encoding IK1 may be expressed in more mature hiPSC-CMs. If not, a hiPSC model for LQT7 (Anderson–Tawil syndrome) with a mutation altering IK1 in 60% of the patients [51] may not be possible. Besides being hereditary, channelopathies can also be acquired. Previously, several FDA approved drugs have been recalled for their inhibitory effects on the human ether-à-go-go-related gene channels (hERGs), either by direct channel block or by reduced expression, resulting in acquired LQT2 that can lead to fatal torsade de pointes arrhythmia [52,53]. Therefore, hERG-expressing hESC/hiPSC-CMs [17,18] make superb candidates for pharmacological safety screening.
Table 1.
Summary of channelopathies and the responsible ion channels and currents. Channelopathies highlighted in black have been modeled by patient-specific hiPSC-derived cardiomyocytes. Ion channels highlighted in gray are those that have been detected in hiPSC-derived cardiomyocytes. LQT: long QT syndrome, SQT: short QT syndrome, BS: Brugada syndrome, SSS: sick sinus syndrome, CCD: cardiac conduction disease, CPVT: catecholaminergic polymorphic ventricular tachycardia
| gene (protein) |
Na+ channels | K+ channels | Ca2+channels | Other Channels | Non-channel Proteins | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCN1B (Navβ.1) |
SCN3B (Navβ.3) |
SCN4B (Navβ.4) |
SCN5a (Nav1.5) |
KCNE1 (MinK) |
KCNE2 (MiRP1) |
KCNE3 (MiRP2) |
KCNH2 (Kv11.1, hERG) |
KCNJ2 (Kir2.1) |
KCNQ1 (Kv7.1, KvLQT1) |
CACNA1c (Cav1.2) |
CACNB2b (Cavβ2b) |
HCN4 | RyR2 | ANK2 | AKAP9 | ANTA1 | CAV3 | CPD1L | CASQ2 | Ionic currents |
||
| Channelopathies | LQT1 | x | IKs | |||||||||||||||||||
| LQT2 | x | Ikr | ||||||||||||||||||||
| LQT3 | x | INa | ||||||||||||||||||||
| LQT4 | x | (INCX, INa-K-ATPase, among others) | ||||||||||||||||||||
| LQT5 | x | IKs | ||||||||||||||||||||
| LQT6 | x | Ikr | ||||||||||||||||||||
| LQT7 | x | IK1 | ||||||||||||||||||||
| LQT8 | x | ICa-L | ||||||||||||||||||||
| LQT9 | x | INa | ||||||||||||||||||||
| LQT10 | x | INa | ||||||||||||||||||||
| LQT11 | x | IKs | ||||||||||||||||||||
| LQT12 | x | INa | ||||||||||||||||||||
| SQT1 | x | Ikr | ||||||||||||||||||||
| SQT2 | x | IKs | ||||||||||||||||||||
| SQT3 | x | IK1 | ||||||||||||||||||||
| BS1 | x | INa | ||||||||||||||||||||
| BS2 | x | INa | ||||||||||||||||||||
| BS3 | x | ICa-L | ||||||||||||||||||||
| BS4 | x | ICa-L | ||||||||||||||||||||
| BS5 | x | INa | ||||||||||||||||||||
| BS6 | x | IKs | ||||||||||||||||||||
| BS7 | INa | |||||||||||||||||||||
| BS8 | x | If | ||||||||||||||||||||
| SSS1 | x | If | ||||||||||||||||||||
| SSS2 | x | INa | ||||||||||||||||||||
| CCD2 | x | INa | ||||||||||||||||||||
| CCD4 | x | INa | ||||||||||||||||||||
| CPVT1 | x | SRCa2+ release | ||||||||||||||||||||
| CPVT2 | x | SR Ca2+ release | ||||||||||||||||||||
Limitations of hiPSC-CMs as a disease model
AP characteristics depend on a balance between interacting ionic currents. Hence, the level of expression of endogenous ionic currents other than the defective ionic current of interest may promote the presence of triggered EADs or DADs. The electrophysiological immaturities of hiPSC-CMs may be a limiting factor for their ability to fully model some channelopathies. For example, IKs has been shown to be present in hiPSC-CMs, but in only ~30% of them [17] compared to ~50% in the heart [54], let alone their smaller current amplitudes even when expressed. However, the heterogeneous IKs presence and the current kinetics did not affect the presentation of the disease phenotype for LQT1-hiPSC-CMs carrying an IKs mutation [55], but it is not clear if the severity of the presenting symptoms is affected. Ion channels normally not present in the contractile CMs such as the hyperpolarization-activated cyclic nucleotide-modulated (HCN) channels, can contribute to automaticity creating a pro-arrhythmogenic condition by inducing membrane depolarization. This poses a problem for a suggested application of immature hiPSC-CMs for personalized treatment in titrating the drug dosage because the ion channel expression in the patient’s hiPSC-CMs may not truly reflect the channel expression in the patient’s heart, which can result in either underestimating the drug dosage that does not effectively shorten the APD enough to restore the normal QT interval or overestimating the drug dosage that creates a pathological condition of short QT interval that has also proved to be pro-arrhythmogenic [56]. An ionic profile that accurately recapitulates the adult CMs may be necessary for titrating the drug dosage to prevent arrhythmia. Other factors to consider when using patient-specific hiPSCs as a disease model include the effects of their epigenetic memory and possible mutagenesis that may arise from the reprogramming method and long-term culturing [57,58]. Future work needs to assess the accuracy of these patient-specific hiPSC-CMs in modeling the diseases.
Proarrhythmic triggers at the single-cell level versus multi-cellular reentrant arrhythmias
The generation of ventricular fibrillation (VF), the most common cause for sudden death, requires both a cellular trigger (e.g. AP prolongation, EADs and DADs), as well as multicellular reentrant events (e.g. spiral wave reentry). Thus far, all the patient-specific hiPSCs-CMs reported have been dealing with the abnormal pulse formation or single-cell events contributing to arrhythmia. Multi-cellular sustained reentry as a prerequisite mechanism for arrhythmogenesis has not yet been dealt with using hiPSC-CMs. The observation of reentry is not possible at the single cell level, but becomes apparent when the cells are electrically coupled as a syncytium. Although cellular presence of EADs and DADs suggests probable occurrence of arrhythmia, it is only an indirect indication. In fact, formation of EADs generally becomes more apparent when it occurs in the ventricular mid-myocardium leading to a more prominent transmural dispersion of repolarization which is a substrate for reentry [53]. Therefore, the cellular electrophysiological disorder only initiates the condition for arrhythmia, but the actual occurrence depends on the existence of a condition that perpetuates the electrical propagation. While single cell patch-clamp revealed a significant difference in the length of repolarization period between healthy and LQT2-specific hiPSC-CMs, the differences observed atthe multi-cellular level usingMEA werediminished [59,60]. The authors hypothesized that cell-to-cell contacts in the multi-cellular form may have compensatory effects in dissipating abnormal repolarization. Evidence suggests that single cell assessment may be more sensitive for channelopathy detection, but the severity of the disease and the drug titration for treatment may best be evaluated as a multi-cellular construct. When using hESC/iPSC-CMs in a construct, it is important to be sure any arrhythmias observed are not an artifact of the conduction path that can be created by cells with heterogeneous electrophysiology. Cardiac differentiation from these human pluripotent stem cells yields non-CM cardiac cells such as fibroblasts and endothelial cells. Contaminates of non-excitable cells may create a conduction block or a sink for ionic currents that can alter the conduction propagation. Therefore, pure CMs may be necessary to generate reproducible and reliable results.
A CM enrichment strategy often used is the generation of cardiac-specific reporter lines but this is a laborious process [61,62]. Recently, purification of hESC/hiPSC-CMs through the labeling of surface markers [63–65] or mitochondria [66] have been reported, but the surface markers identified have not been shown to be completely restricted to CMs and the mitochondrial level requires at least 50 days post-differentiation to become distinctly higher than other cell types. We reported a method of identifying hESC/hiPSC-CMs using a non-genetic, label-free method based on the intrinsic nature of the myosin filament organization that generates a second harmonic signal [67]. This may yield a source of CMs that has not been manipulated or perturbed by labeling reagents.
The enriched hESC/hiPSC-CM population is still heterogeneous of nodal, atrial and ventricular subtypes with very different electrophysiological properties that can create a conduction mismatch in the tissue construct and promote arrhythmogenesis. It is often difficult to separate the cause of arrhythomogenesis, as Itzhaki et al. point out that the observed arrhythmia in the LQT-hiPSC-CMs cannot be completely confirmed to be attributed to the defective ion channel alone [68]. Factors such as CM automaticity (intrinsic of hESC/ hiPSC-CMs) and reentry (from multi-cellular organization and coupling) may contribute to the observed irregular electrophysiological phenotype. Therefore, a pure population of ventricular subtype may be more appropriate for some drug screening applications. Currently, a reliable method of identifying ventricular subtype is through the generation of reporter lines driven by the myosin light chain (MLC)-2v promoter [69,70]. Future work may improve hiPSC-CM disease modeling by isolating the correct CM subtype to model the corresponding disease, for instance, such that nodal and ventricular CMs are used for studying SSS and LQT, respectively.
Conclusions
With the derivation of patient-specific hiPSCs, human pluripotent stem cells now have new directions to be pursued. For the cardiac field alone, not only is generating a source of autologous cells possible, the research on inherited heart diseases, drug safety screening and cardiac drug testing in human CMs as well as personalized treatment strategy are also one step closer to reality. Although the challenges discussed above may prevent immature diseased hiPSC-CMs from presenting the exact responses of adult cells in vivo, the results from the several diseased-hiPSC linesgenerated as heart disease in a dish have certainly been encouraging. Advancements in the electrophysiology measuring instruments and culture conditions designed to mimic the in vivo environment will further accelerate our progress in understanding the mechanisms inducing abnormal electrophysiology in CMs.
Acknowledgments
This work was supported by grants from the NIH-R01 HL72857, the CC Wong Foundation Stem Cell Fund and the Research Grant Council (T13-706/11 and 103544).
References
- 1.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Mummery C, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 4.Yoon BS, et al. Enhanced differentiation of human embryonic stem cells into cardiomyocytes by combining hanging drop culture and 5-azacytidine treatment. Differentiation. 2006;74:149–159. doi: 10.1111/j.1432-0436.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara M, et al. Induction and enhancement of cardiac cell differentiation from mouse and human induced pluripotent stem cells with cyclosporin-A. PLoS One. 2011;6:e16734. doi: 10.1371/journal.pone.0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takei S, et al. Bone morphogenetic protein-4 promotes induction of cardiomyocytes from human embryonic stem cells in serum-based embryoid body development. Am J Physiol Heart Circ Physiol. 2009;296:H1793–H1803. doi: 10.1152/ajpheart.01288.2008. [DOI] [PubMed] [Google Scholar]
- 7.Laflamme MA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 9.Willems E, et al. Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ Res. 2011;109:360–364. doi: 10.1161/CIRCRESAHA.111.249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burridge PW, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6:e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim C, et al. Non-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiation. Stem Cells Dev. 2010;19:783–795. doi: 10.1089/scd.2009.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu WZ, et al. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010;107:776–786. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21:579–587. doi: 10.1038/cr.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohr JC, et al. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials. 2010;31:1885–1893. doi: 10.1016/j.biomaterials.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta A, et al. Pharmacological response of human cardiomyocytes derived from virus-free induced pluripotent stem cells. Cardiovasc Res. 2011;91:577–586. doi: 10.1093/cvr/cvr132. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, et al. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol. 2011;301:H2006–H2017. doi: 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sartiani L, et al. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25:1136–1144. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 19.Otsuji TG, et al. Progressive maturation in contracting cardiomyocytes derived from human embryonic stem cells: qualitative effects on electrophysiological responses to drugs. Stem Cell Res. 2010;4:201–213. doi: 10.1016/j.scr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Pekkanen-Mattila M, et al. Human embryonic stem cell-derived cardiomyocytes: demonstration of a portion of cardiac cells with fairly mature electrical phenotype. Exp Biol Med (Maywood) 2010;235:522–530. doi: 10.1258/ebm.2010.009345. [DOI] [PubMed] [Google Scholar]
- 21.Di Felice V, et al. HSP90 and eNOS partially co-localize and change cellular localization in relation to different ECM components in 2D and 3D cultures of adult rat cardiomyocytes. Biol Cell. 2007;99:689–699. doi: 10.1042/BC20070043. [DOI] [PubMed] [Google Scholar]
- 22.Tulloch NL, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bursac N, et al. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am J Physiol. 1999;277(Pt 2):H433–H444. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- 24.Radisic M, et al. Optical mapping of impulse propagation in engineered cardiac tissue. Tissue Eng Part A. 2009;15:851–860. doi: 10.1089/ten.tea.2008.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmermann WH, et al. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 26.Zhao YS, et al. Construction of a unidirectionally beating 3-dimensional cardiac muscle construct. J Heart Lung Transplant. 2005;24:1091–1097. doi: 10.1016/j.healun.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Caspi O, et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 28.Akins RE, et al. Cardiac organogenesis in vitro: reestablishment of three-dimensional tissue architecture by dissociated neonatal rat ventricular cells. Tissue Eng. 1999;5:103–118. doi: 10.1089/ten.1999.5.103. [DOI] [PubMed] [Google Scholar]
- 29.Streeter DD, Jr, et al. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res. 1969;24:339–347. doi: 10.1161/01.res.24.3.339. [DOI] [PubMed] [Google Scholar]
- 30.LeGrice IJ, et al. Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am J Physiol. 1995;269(Pt 2):H571–H582. doi: 10.1152/ajpheart.1995.269.2.H571. [DOI] [PubMed] [Google Scholar]
- 31.Luna JI, et al. Multi-scale biomimetic topography for the alignment of neonatal and embryonic stem cell-derived heart cells. Tissue Eng Part C Methods. 2011;17:579–588. doi: 10.1089/ten.TEC.2010.0410. [DOI] [PubMed] [Google Scholar]
- 32.Chen A, et al. Shrink-film configurable multiscale wrinkles for functional alignment of human embryonic stem cells and their cardiac derivatives. Adv Mater. 2011;23:5785–5791. doi: 10.1002/adma.201103463. [DOI] [PubMed] [Google Scholar]
- 33.Schaaf S, et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One. 2011;6:e26397. doi: 10.1371/journal.pone.0026397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He J, et al. Reduction in density of transverse tubules and L-type Ca(2+) channels in canine tachycardia-induced heart failure. Cardiovasc Res. 2001;49:298–307. doi: 10.1016/s0008-6363(00)00256-x. [DOI] [PubMed] [Google Scholar]
- 35.Maier SK, et al. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation. 2004;109:1421–1427. doi: 10.1161/01.CIR.0000121421.61896.24. [DOI] [PubMed] [Google Scholar]
- 36.Izu LT, et al. Eavesdropping on the social lives of Ca(2+) sparks. Biophys J. 2007;93:3408–3420. doi: 10.1529/biophysj.107.112466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibrahim M, et al. Prolonged mechanical unloading affects cardiomyocyte excitation-contraction coupling, transverse-tubule structure, and the cell surface. FASEB J. 2010;24:3321–3329. doi: 10.1096/fj.10-156638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costa KD, et al. Creating alignment and anisotropy in engineered heart tissue: role of boundary conditions in a model three-dimensional culture system. Tissue Eng. 2003;9:567–577. doi: 10.1089/107632703768247278. [DOI] [PubMed] [Google Scholar]
- 39.Lee EJ, et al. Engineered cardiac organoid chambers: toward a functional biological model ventricle. Tissue Eng Part A. 2008;14:215–225. doi: 10.1089/tea.2007.0351. [DOI] [PubMed] [Google Scholar]
- 40.Kehat I, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue T, et al. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- 42.Lieu DK, et al. Absence of transverse tubules contributes to nonuniform Ca2+ wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells Dev. 2009;18:1493–1500. doi: 10.1089/scd.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, et al. Facilitated maturation of Ca2+ handling properties of human embryonic stem cell-derived cardiomyocytes by calsequestrin expression. Am J Physiol Cell Physiol. 2009;297:C152–C159. doi: 10.1152/ajpcell.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaffer P, et al. Di-4-ANEPPS causes photodynamic damage to isolated cardiomyocytes. Pflugers Arch. 1994;426:548–551. doi: 10.1007/BF00378533. [DOI] [PubMed] [Google Scholar]
- 45.Bezzina CR. Genetics of cardiomyopathy and channelopathy. Heart Metab. 2008;41:5–10. [Google Scholar]
- 46.Davis RP, et al. Pluripotent stem cell models of cardiac disease and their implication for drug discovery and development. Trends Mol Med. 2011;17:475–484. doi: 10.1016/j.molmed.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Unternaehrer JJ, Daley GQ. Induced pluripotent stem cells for modelling human diseases. Philos Trans R Soc Lond B: Biol Sci. 2011;366:2274–2285. doi: 10.1098/rstb.2011.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carvajal-Vergara X, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465:808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Satin J, et al. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol. 2004;559(Pt 2):479–496. doi: 10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagashima M, et al. Alternation of inwardly rectifying background K+ channel during development of rat fetal cardiomyocytes. J Mol Cell Cardiol. 2001;33:533–543. doi: 10.1006/jmcc.2000.1327. [DOI] [PubMed] [Google Scholar]
- 51.Tristani-Firouzi M, Etheridge SP. Kir 2.1 channelopathies: the Andersen-Tawil syndrome. Pflugers Arch. 2010;460:289–294. doi: 10.1007/s00424-010-0820-6. [DOI] [PubMed] [Google Scholar]
- 52.Su X, et al. Microfluidic cell culture and its application in high-throughput drug screening: cardiotoxicity assay for hERG channels. J Biomol Screen. 2011;16:101–111. doi: 10.1177/1087057110386218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gussak I, et al. Electrical Diseases of the Heart: Genetics, Mechanisms, Treatment, Prevention. Springer; 2008. [Google Scholar]
- 54.Virag L, et al. The slow component of the delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovasc Res. 2001;49:790–797. doi: 10.1016/s0008-6363(00)00306-0. [DOI] [PubMed] [Google Scholar]
- 55.Moretti A, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 56.Patel C, Antzelevitch C. Pharmacological approach to the treatment of long and short QT syndromes. Pharmacol Ther. 2008;118:138–151. doi: 10.1016/j.pharmthera.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polo JM, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Narsinh K, et al. Derivation of human induced pluripotent stem cells for cardiovascular disease modeling. Circ Res. 2011;108:1146–1156. doi: 10.1161/CIRCRESAHA.111.240374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lahti AL, et al. Model for long QT syndrome type 2 using human iPS cells demonstrates arrhythmogenic characteristics in cell culture. Dis Model Mech. 2011;5:220–230. doi: 10.1242/dmm.008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsa E, et al. Drug evaluation in cardiomyocytes derived from human induced pluripotent stem cells carrying a long QT syndrome type 2 mutation. Eur Heart J. 2011;32:952–962. doi: 10.1093/eurheartj/ehr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kita-Matsuo H, et al. Lentiviral vectors and protocols for creation of stable hESC lines for fluorescent tracking and drug resistance selection of cardiomyocytes. PLoS One. 2009;4:e5046. doi: 10.1371/journal.pone.0005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elliott DA, et al. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods. 2011;8:1037–1040. doi: 10.1038/nmeth.1740. [DOI] [PubMed] [Google Scholar]
- 63.Dubois NC, et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29:1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Hoof D, et al. Identification of cell surface proteins for antibody-based selection of human embryonic stem cell-derived cardiomyocytes. J Proteome Res. 2010;9:1610–1618. doi: 10.1021/pr901138a. [DOI] [PubMed] [Google Scholar]
- 65.Uosaki H, et al. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS One. 2011;6:e23657. doi: 10.1371/journal.pone.0023657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hattori F, et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods. 2010;7:61–66. doi: 10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- 67.Awasthi S, et al. Label-free identification and characterization of human pluripotent stem cell-derived cardiomyocytes using second harmonic generation (SHG) microscopy. J Biophotonics. 2012;5:57–66. doi: 10.1002/jbio.201100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Itzhaki I, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 69.Fu JD, et al. Na+/Ca2+ exchanger is a determinant of excitation-contraction coupling in human embryonic stem cell-derived ventricular cardiomyocytes. Stem Cells Dev. 2010;19:773–782. doi: 10.1089/scd.2009.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huber I, et al. Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. FASEB J. 2007;21:2551–2563. doi: 10.1096/fj.05-5711com. [DOI] [PubMed] [Google Scholar]