Abstract
SB-3CT is a potent and selective inhibitor of matrix metalloproteinase (MMP)-2 and -9, which has shown efficacy in an animal model of severe traumatic brain injury (TBI). However, SB-3CT is poorly water-soluble and is metabolized primarily to p-hydroxy SB-3CT (2), a more potent inhibitor than SB-3CT. We synthesized the O-phosphate prodrug (3) of compound 2 to enhance its water solubility by more than 2000-fold. The prodrug 3 was a poor MMP inhibitor, but readily hydrolyzed to the active 2 in human blood. Pharmacokinetics and brain distribution studies in mice showed that 2 crossed the blood-brain barrier (BBB) and achieved therapeutic concentrations in the brain. The prodrug 3/compound 2 was evaluated in a mouse model of severe TBI and found to significantly decrease the brain lesion volume and improve neurological outcomes. MMP-9 inhibition by a water-soluble thiirane inhibitor is a promising therapy for treatment of TBI.
Keywords: MMP-9, prodrug, brain distribution, traumatic brain injury
Graphical abstract
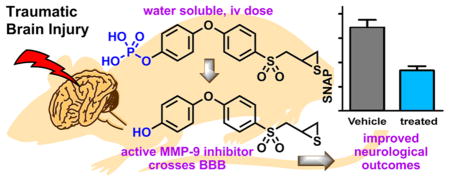
Traumatic brain injury (TBI) affects 1.7 million individuals in the United States every year, resulting in 52 000 annual deaths.1 TBIs range from mild to severe and result in various degrees of neurological deficits caused by primary and secondary injuries. Primary injury is the damage that occurs from the initial head trauma, which triggers a cascade of biochemical events that generate reactive-oxygen species and proinflammatory cytokines, resulting in inflammation, edema, blood-brain barrier (BBB) breakdown, oxidative stress, neurotoxicity, mitochondrial dysfunction, and cell death. This is referred to as secondary injury, which develops over the following few days after the primary injury. The time between the primary and secondary injuries provides a window of opportunity for therapeutic intervention to prevent further damage and improve prognosis. Insofar as matrix metalloproteinase (MMP)-9 plays an important role in the pathogenesis of TBI during this critical phase, it serves as a potential target of intervention in the disease.2,3
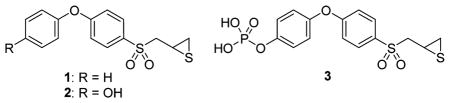
SB-3CT (compound 1) inhibits MMP-9 with an inhibition constant Ki of 400 ± 15 nM.4 The mode of inhibition of SB-3CT involves an active-site promoted ring opening of the thiirane to generate the corresponding thiolate, which then serves as a tight-binding inhibitor in the picomolar range.5 As indicated, this reaction occurs only at the active site, and once the enzyme is inhibited, the reversal of inhibition occurs slowly. In fact, the residence time, the time that the inhibitor remains complexed with the target, is 13.4 min,4 which is longer than that of the tissue inhibitor of matrix metalloproteinase (TIMP)-1 or -2 at 7.9 and 6.7 min, respectively.6 That is to say, the synthetic inhibitor SB-3CT is more potent at regulating MMP-9 activity than the natural TIMPs, which have evolved for the purpose. SB-3CT is metabolized primarily by oxidation of the terminal phenyl to p-hydroxy SB-3CT (compound 2), a more potent inhibitor of MMP-9 (Ki of 160 ± 80 nM)7 than the parent SB-3CT. In addition, SB-3CT is poorly water-soluble (2.3 μg/mL).8 In this report, we employed a prodrug strategy to increase water solubility of 2 and show that the prodrug (compound 3) is hydrolyzed to 2, which then crosses the BBB. We also found that administration of prodrug 3 and 2 decreases the lesion volume and improves neurological outcomes in a mouse model of severe TBI.
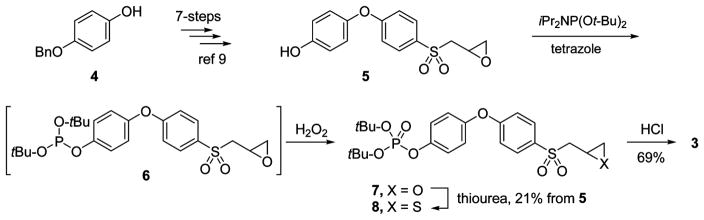
For the synthesis of prodrug 3, compound 5 was used as a key intermediate. Compound 5 was prepared from 4-benzyloxyphenol 4 in quantity in seven synthetic steps by the method developed by our laboratory (Scheme 1).9
Scheme 1.
Synthesis of Prodrug 3
Compound 5 was treated with di-tert-butyl diisopropylphosphoramidite in the presence of tetrazole to yield phosphite 6,
which was oxidized in situ to phosphate 7 by treatment with hydrogen peroxide. The oxirane in 7 was converted to the corresponding thiirane 8 by treatment with thiourea. The tert-butyl groups in 8 were removed by acid treatment to yield prodrug 3.
As expected of a prodrug, 3 was a poor MMP inhibitor (Table 1). It inhibited MMP-2, MMP-9, and MMP-14 as a slow-binding inhibitor at mid to high micromolar levels. Prodrug 3 in the presence of human blood was quantitatively hydrolyzed to the active 2 with a half-life of 43.2 ± 2.1 min. The aqueous solubility of prodrug 3 was >2000-fold higher than that of compound 2 (52 200 μg/mL for 3 compared to 22.2 μg/mL for 2). Prodrug 3 was stable in rat liver S9, with a half-life of >60 min. Compound 2 and prodrug 3 are not toxic; hemolysis of red blood cells is not observed up to concentrations of 160 μM, and in the XTT cytotoxicity assay compound 2 and prodrug 3 have IC50 values of 114 ± 1 and 140 ± 1 μM, respectively.
Table 1.
Effects of Prodrug 3 on MMP Kinetic Parameters
| MMP | 105 kon (μM−1 s−1) | 103 koff (s−1) | Ki (μM) |
|---|---|---|---|
| MMP-2 | 8.5 ± 1.1 | 1.0 ± 0.2 | 11.7 ± 2.7 |
| MMP-9cat | 4.8 ± 0.6 | 1.7 ± 0.2 | 35.8 ± 6.7 |
| MMP-14cat | 3.9 ± 0.1 | 1.3 ± 0.1 | 32.6 ± 1.3 |
| MMP-1cat | 17% inhibition at 500 μM | ||
| MMP-7 | 43% inhibition at 500 μM | ||
| MMP-3cat | linear competitive | 142.1 ± 11.0 | |
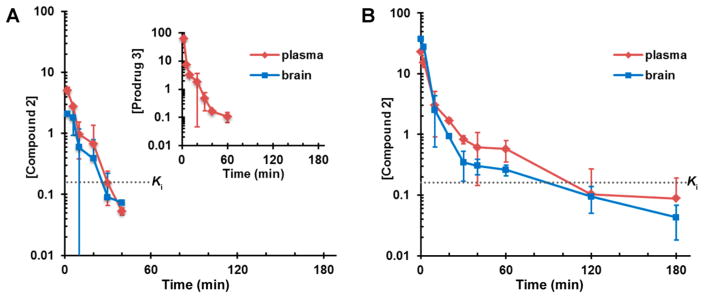
We next investigated the pharmacokinetics (PK) and brain distribution properties of prodrug 3 and compared these parameters to those of compound 2. Prodrug 3 was not detected in brain (Table 2). Systemic exposure, as measured by the area under the curve (AUC), was 8-fold higher for 3 than for its metabolite 2, indicating that the major species in circulation was the prodrug 3. However, metabolite 2 crossed the BBB, with a brain to plasma AUC ratio of 0.53. Levels of 2 in the brain were above Ki for MMP-9 for 20 min (Figure 1). Clearance of the prodrug 3 was moderate at 51 mL/min/kg. The volume of distribution of 3 was 0.185 L/kg, lower than total body water of 0.6 L/kg, indicating that 3 remained mostly in the extracellular fluid compartment and did not distribute to tissues, consistent with prodrug 3 being readily hydrolyzed in blood. After an intravenous (iv) dose of 2, brain AUC was higher than plasma AUC, with a brain to plasma AUC ratio of 1.2, indicating that 2 crossed the BBB avidly. Concentrations of 2 were above the Ki for MMP-9 for 60 min. Clearance of 2 was high at 105 mL/min/kg, greater than hepatic blood flow. Compound 2 distributed to tissues, with a volume of distribution of 0.850 L/kg.
Table 2.
Pharmacokinetic Parameters of Prodrug 3 and Compound 2 after a Single iv Dose to Male Mice
| 3 after Dose of 3 | 2 after Dose of 3 | 2 after Dose of 2 | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| parameter | brain | plasma | brain | plasma | brain | plasma |
| AUC0-lasta | NQb | 377 | 26 | 49 | 232 | 189 |
| AUC0-∞a | NQb | 380 | 27 | 49 | 235 | 195 |
| t1/2α (min) | 1.9 | 3.1 | 3.0 | 3.0 | 3.4 | |
| t1/2β (min) | 34 | 8.3 | 6.5 | 49 | 45 | |
| CL (mL/min/kg) | 51 | 105 | ||||
| Vd (L/kg) | 0.185 | 0.850 | ||||
| brainauc/plasmaauc | NCc | 0.53 | 1.2 | |||
AUC in pmol·min/mg for brain and in μM·min for plasma.
NQ = nonquantifiable.
NC = not calculated.
Figure 1.
Brain and plasma concentration–time curves of (A) single 7.8 mg/kg iv dose of prodrug 3 to mice and (B) single 6.3 mg/kg iv dose of compound 2 to mice. Doses of compounds 2 and 3 were equimolar. Concentrations in pmol/mg tissue for brain and in μM for plasma. Dashed lines indicate the Ki value of compound 2 for MMP-9.
As we showed that compound 2 crosses the BBB and can achieve therapeutic concentrations in the brain, we next investigated whether the compound was efficacious in a mouse model of severe TBI. Following craniotomy, we used the electromagnetic impactor to deliver a 2.5 mm depth precise controlled cortical impact (CCI) in the left parietotemporal cortex. As we had shown previously that MMP-9 is upregulated for 7 days after TBI,2 we administered multiple doses of the compound. However, since multiple iv injections are technically challenging to administer to mice, we dosed an initial iv bolus injection of prodrug 3 at 30 min after CCI, followed by subcutaneous (sc) injections of compound 2 at 1 h post CCI, with additional sc injections of 2 once a day for a total of 3 or 7 days of treatment. We administered the first dose iv for immediate absorption, and subsequent doses were given sc to achieve sustained concentrations. As prodrug 3 is hydrolyzed to compound 2 in blood, compound 2 was administered sc. The 7.8 mg/kg iv dose of prodrug 3 was chosen, since the PK study indicated that this dose level generated brain concentrations of 2 above the Ki for MMP-9. The 25 mg/kg sc dose of 2 was selected, as this is the dose level evaluated in TBI mice with SB-3CT,2 and we had shown that this dose achieved therapeutic brain concentrations.10
The brains of all animals showed severe contusion injuries, with substantial damage in the cortex and underlying hippocampus. Histopathological differences were assessed by staining with cresyl violet, followed by analysis using the stereology technique. Lesion area was plotted versus Bregma position and the data were fit to a second-degree polynomial (Figure 2A). Results showed a difference between vehicle-treated and prodrug 3/compound 2-treated mice. A significant reduction was observed in cortical lesion volume from 32 ± 3 mm3 in vehicle-treated mice compared to 23 ± 1 mm3 in mice treated with prodrug 3/compound 2 (Figure 2B) in both dosing regimens (3- and 7-day treatment). The reduction in brain damage was approximately 26% after drug treatment. The effect of prodrug 3/compound 2 on lesion volume was more effective than treatment with SB-3CT (14% reduction in brain damage after drug treatment).2 However, a direct comparison to SB-3CT cannot be made, as the routes of administration and dose regimens were different.
Figure 2.
Quantification of cortical lesion volumes by cresyl violet staining at 7 days after TBI. (A) Stereological plot of lesion areas from the cresyl violet stained brain sections of TBI mice treated with vehicle and prodrug 3/compound 2 for either 3 or 7 days. Each point represents the lesion area in one cresyl violet-stained brain section. Data are plotted based on the rostro-caudal axis of the brain coordinate to Bregma. Data were fit to a second-degree polynomial. The results indicate a difference in lesion areas between the vehicle-treated and prodrug 3/compound 2-treated mice, n = 6 in each group. (B) Quantification of cortical lesion volume, n = 6 for vehicle, n = 6 for 3-day treatment, and n = 7 for 7-day treatment, *p < 0.05, **p < 0.01. Data are expressed as mean ± SEM.
The effect of prodrug 3/compound 2 on neurobehavior after TBI was evaluated. Motor functions were assessed by beam-walking on an 80 cm length by 1.25 cm width beam on days 3 and 7. The number of mice falling, the average distance traveled prior to falling (Figure 3A), and the number of foot faults (Figure 3B) were determined. On day 3, there were no animals falling in the sham group, while 83% and 72% fell in the vehicle-treated and prodrug 3/compound 2-treated groups, respectively; these differences were statistically significant (p < 0.001 by χ2 test). On day 7, 42% and 33% fell in the vehicle and 3-day treatment, respectively, while there were no animals falling in the sham and 7-day treatment with prodrug 3/compound 2; these differences were statistically significant (p = 0.017). Significant improvement in the 7-day treatment was observed compared to the 3-day treatment (p = 0.034). There was a trend in reduction in the average distance traveled on day 3 among the TBI mice treated with prodrug 3/compound 2 compared to sham; however, no statistical differences were observed on day 7 (Figure 3A). The numbers of foot faults were significantly increased in the vehicle and 3-day treatment groups compared to sham, but not in the 7-day treatment group. There was a trend in foot fault reduction in the 7-day treatment animals compared to vehicle-treated mice, but it was not statistically significant (Figure 3B). The Simple Neuroassessment of Asymmetric Impairment (SNAP) score is a battery of eight tests to examine neurological deficits on vision, proprioception, posture, and motor strength.11 The SNAP score was performed before surgery and on days 3 and 7 post CCI; the lower the SNAP score, the better the outcome. Vehicle-treated mice had a SNAP score of 6.9 ± 0.6 (n = 12), compared to 4.6 ± 0.8 (n = 6) and 3.3 ± 0.4 (n = 12) for the prodrug 3/compound 2-treated animals for 3- or 7-day treatments, respectively. These differences in SNAP scores were statistically significant (Figure 3C). Thus, treatment with prodrug 3/compound 2 improved neurological outcomes.
Figure 3.

Effect of prodrug 3/compound 2 on sensorimotor functions. (A, B) Assessment of motor functions by beam-walking. (A) Quantification of the average distance traveled prior to falling on days 3 and 7. (B) Number of foot faults on day 7 in TBI mice after 3- or 7-day treatment compared to the sham animals and TBI mice treated with vehicle. (C) Assessment of sensorimotor functions by SNAP scores. n = 9 for sham, n = 12 for vehicle, n = 6 for 3-day treatment, and n = 12 for 7-day treatment. One-way ANOVA by Kruskal–Wallis test, p < 0.0001 for four groups; Dunn’s multiple comparison test: *p < 0.05 and ***p < 0.001 compared to sham; #p < 0.05 compared to vehicle; data are expressed as mean ± SEM for (B) and (C).
MMP-9 is an important target of inhibition in the treatment of TBI. Ablation of MMP-9 resulted in less motor deficits in MMP-9 knockout animals than wild-type mice after CCI.12 The involvement of MMP-9 in the pathology of TBI has been confirmed by selective inhibition of MMP-9 with SB-3CT.2 Treatment with SB-3CT reduced brain lesion volumes, prevented neuronal loss and dendritic degeneration, and improved long-term neurological functions, including sensimotor function, as well as hippocampus-associated spatial learning and memory.2 In addition, SB-3CT treatment attenuated activation of microglia and astrocytes in the lesioned cortex.2 In a rat model of TBI, SB-3CT attenuated behavioral impairments and hippocampal loss.13 Significant elevation of MMP-9 has been observed in the cerebral spinal fluid of TBI patients.14 As compound 2 is a metabolite of SB-3CT and prodrug 3 is metabolized to 2, the underlying mechanism for the beneficial effects of prodrug 3/compound 2 may be similar to SB-3CT. These studies indicate that inhibition of MMP-9 is a promising therapy in the treatment of TBI. In order for an MMP-9 inhibitor to advance to clinical development, water solubility is necessary as the therapeutic must be given iv, the clinically relevant route of administration. SB-3CT, however, is poorly water-soluble. We took advantage of the fact that SB-3CT is metabolized to p-hydroxy-SB-3CT (2), a more potent inhibitor of MMP-9 than the parent SB-3CT. In order to increase water solubility, we made the phosphate prodrug 3, which hydrolyzed in human blood to the active 2. Compound 2 was able to cross the BBB and achieve therapeutic concentrations in the brain. The ability of compound 2 to cross the BBB is remarkable, as greater than 98% of small molecule drugs cannot distribute to the brain and represent the greatest challenge in the development of therapeutics for diseases of the central nervous system.15
We showed that treatment of TBI mice with prodrug 3/compound 2 reduces lesion volume to a level comparable to that with SB-3CT. These data indicate that MMP-9 inhibition by a water-soluble thiirane inhibitor is a promising therapeutic for the treatment of TBI.
METHODS
Compound 7
A mixture of compound 5 (0.6 g, 2.0 mmol)9 and tetrazole (0.2 g, 2.8 mmol) was stirred in THF (8 mL) in and ice-water bath, and di-tert-butyl diisopropylphosphoramidite (0.9 g, 3.4 mmol) was added. The resulting mixture was stirred for 1 h while temperature was gradually increased to room temperature. The mixture was cooled down in an ice-water bath and was treated with 30% H2O2 (0.9 mL). After 0.5 h, the mixture was diluted with sodium sulfite and extracted with ethyl acetate. The combined organic layer was dried with anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude material was purified by column chromatography on silica gel to give compound 7 (contaminated with phosphorylating agent). The analytical sample was further purified by column chromatography. 1H NMR (500 MHz, CDCl3) δ 1.49 (s, 18H), 2.45 (m, 1H), 2.79 (d, J = 2.6 Hz, 1H), 3.27 (s, 2H), 3.45 (m, 1H), 7.02 (d, J = 9.0 Hz, 2H), 7.04 (d, J = 9.0 Hz, 2H), 7.24 (d, J = 8.4 Hz, 2H), 7.85 (d, J = 8.8 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 29.99, 30.02, 46.0, 59.8, 84.0, 84.1, 117.4, 121.8 (d, J = 5.8 Hz), 130.7, 132.6, 148.7 (d, J = 7.4 Hz), 150.9, 163.2. 31P NMR (121 MHz, CDCl3) δ −14.14. HRMS (ESI) m/z: 499.1550 calcd for C23H32O8PS [M + H]+; 499.1556 obsd.
Compound 8
The crude compound 7 (~0.3 g, 0.6 mmol) in MeOH (5 mL) was mixed with thiourea (0.2 g, 3.0 mmol). After stirring for 18 h, solvent was removed under reduced pressure and the resultant solid was taken up in water and ethyl acetate. The organic layer was separated, dried with anhydrous MgSO4, filtered, and concentrated under reduced pressure. The crude material was purified by column chromatography on silica gel to give compound 8 (0.2 g, 21% from compound 5). 1H NMR (500 MHz, CDCl3) δ 1.49 (s, 18H), 2.12 (dd, J = 5.2, 1.6 Hz, 1H), 2.50 (dd, J = 6.2, 1.2 Hz, 1H), 3.02 (m, 1H), 3.15 (dd, J = 14.2, 7.8 Hz, 1H), 3.48 (dd, J = 14.4, 5.6 Hz, 2H), 7.01 (d, J = 9.0 Hz, 2H), 7.03 (d, J = 8.8 Hz, 2H), 7.24 (d, J = 8.4 Hz, 2H), 7.82 (d, J = 9.0 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 24.3, 26.2, 29.9, 30.0, 62.7, 84.0, 84.1, 117.5, 121.6, 121.8 (d, J = 5.8 Hz), 130.9, 132.0, 148.7 (d, J = 7.4 Hz), 150.9, 163.2. 31P NMR (121 MHz, CDCl3) δ −14.19. HRMS (ESI) m/z: 515.1321 calcd for C23H32O7PS2 [M + H]+; 515.1332 obsd.
Compound 3
Compound 8 (0.2 g, 0.4 mmol) in CH2Cl2/EtOAc (1:1, 8 mL) in an ice-water bath was mixed with 4N HCl in dioxane (0.4 mL, 1.6 mmol). The stirring was continued for 3 h, and the mixture was concentrated under reduced pressure. The resultant residue was triturated with diethyl ether, and the desired product was collected by filtration (0.1 g, 69%). Compound 3 was >95% pure as determined by UPLC. The UPLC conditions are given in the Liquid Chromatography/Mass Spectrometry subsection. 1H NMR (500 MHz, CD3OD) δ 2.13 (d, J = 4.8 Hz, 1H), 2.51 (d, J = 6.4 Hz, 1H), 3.04 (quin, J = 6.4 Hz, 1H), 3.42 (dd, J = 14.4, 6.4 Hz, 1H), 3.54 (dd, J = 14.4, 6.4 Hz, 1H), 7.12–7.15 (m, 4H), 7.30 (d, J = 8.0 Hz, 2H), 7.90 (d, J = 8.0 Hz, 2H). 13C NMR (126 MHz, CD3OD) δ 24.3, 27.1, 63.3, 118.7, 122.9, 123.4 (d, J = 4.1 Hz), 132.3, 133.7, 150.0, 150.1, 152.9 (d, J = 1.6 Hz), 164.6. 31P NMR (121 MHz, CD3OD) δ −3.95. HRMS (ESI) m/z: 400.9913 calcd for C15H14O7PS2 [M – H]−; 400.9941 obsd.
Compound 2
Compound 2 was synthesized as described previously.9
Enzyme Inhibition Studies
Human recombinant active MMP-2 and MMP-7 and catalytic domains of MMP-3 and MMP-14 were purchased from EMD Biosciences (La Jolla, CA). The catalytic domains of human recombinant MMP-1 and MMP-9 were from Enzo Life Sciences (Farmingdale, NY). Fluorogenic substrates MOCAcPLGLA2-pr(Dnp)AR-NH2 (used for MMP-2, -7, -9, -14) and MOCAcRPKPV-E(Nva)WRK(Dnp)-NH2 (for MMP-3) were purchased from Peptides International (Louisville, KY) while (Dnp)P(Cha)GC(Me)HAK-(NMa)NH2 (for MMP-1) was obtained from R&D Systems (Minneapolis, MN). The Km values used for the reaction of MMP-2, -9, and -14 with the fluorogenic substrate were 18.9 ± 1.0, 5.0 ± 0.1, and 5.6 ± 0.4 μM, respectively. The methodology for enzyme inhibition and assays was the same as described previously.16
Water Solubility Determination
A saturated solution of a compound 2 or 3 in water was prepared. The solution was centrifuged, and the supernatant was analyzed by ultraperformance liquid chromatography (UPLC) with UV detection at 245 nm. Calibration curves for each of the compounds were prepared using known concentrations of the compounds from which the concentration in the supernatant was determined. The UPLC conditions are given in the Liquid Chromatography/Mass Spectrometry subsection.
Ex Vivo Hydrolysis in Blood and Metabolic Stability in Rat Liver S9
The procedures for the ex vivo hydrolysis in human blood, and metabolic stability in rat S9 of prodrug 3, including the HPLC conditions for analysis, were the same as previously reported.17
Hemolysis
A 10 mL aliquot of horse whole blood (Innovative Research, Burlington, ON, Canada) was centrifuged at 7 000g for 5 min. The supernatant was removed, and 5 mL of 0.1MPBS was added to the red blood cell (RBC) pellet, followed by centrifugation. This step was repeated three times to afford washed RBCs, which were resuspended in 5 mL of 0.1MPBS. Stock solutions of compound 2 and prodrug 3 were prepared in DMSO. For the assays, a 50 μL aliquot of the each compound in DMSO was mixed with 750 μL of PBS and 200 μL of RBCs suspended in PBS to final concentrations of 5, 10, 20, 40, 80, and 160 μM, and incubated at 37 °C for 1 h. The positive control consisted of 50 μL of DMSO, 750 μL of 2% Triton X-100 in 0.1 M PBS, and 200 μL of RBCs. The negative control contained 50 μL of DMSO, 750 μL of PBS, and 200 μL of RBCs. All samples were prepared in duplicate. After incubation, the samples were centrifuged at 13 000g for 5 min, and the supernatant was collected and analyzed by UV spectroscopy at 541 nm using an Epoch microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT). Spectroscopic data were plotted in Microsoft Excel for calculation of IC50 values.
XTT Cytotoxicity Assay
This assay was performed in triplicate using HepG2 cells (ATCC HB-8065, American Type Culture Collection, Manassas, VA), as previously described.18 The IC50 values were calculated with GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA).
Compound Formulations for PK and TBI Studies
Compounds 2 and 3 were formulated in 70% propylene glycol/30% water as a solution at a concentration of 3.1 and 3.9 mg/mL, respectively. The solutions were sterilized by filtration through an Acrodisc syringe filter (Pall Life Sciences, New York, NY; 0.2 μm, 13 mm diameter, PTFE membrane).
PK and Brain Distribution Studies
Mice (male C57Bl/6J, 6–8 weeks old, 21–26 g body weight, n = 3 per time point) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were fed Teklad 2019 Extruded Rodent Diet (Harlan, Madison, WI) and water ad libitum. Animals were housed in polycarbonate shoebox cages with 1/4″ corncob/Alpha-dri 1:1 bedding; the room was maintained at 72 ± 2 °F, with a 12 h light/dark cycle. All procedures were performed in accordance with the University of Notre Dame Institutional Animal Care and Use Committee.
Mice (n = 3 per time point) were administered a single dose consisting of 50 μL of compounds 2 or 3 (equivalent to 6.3 and 7.8 mg/kg respectively) by tail vein injection. Doses of compounds 2 and 3 were adjusted to be equimolar. Terminal blood samples (n = 3 per time point) were collected by cardiac puncture using heparin at 2, 6, 10, 20, 30, 40, 60, 120, and 180 min. Blood samples were centrifuged to obtain plasma. Transcardial perfusion with saline was performed before collection of brain samples. Brain samples were weighed, immediately flash-frozen in liquid nitrogen, and stored at −80 °C until analysis.
PK Sample Analysis
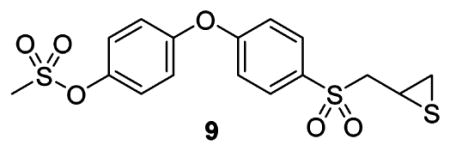
A 50 μL aliquot of plasma was mixed with 100 μL of internal standard 9 in ACN to a final concentration of 10 μM. The sample was centrifuged at 20 000g for 15 min. Brain samples were weighed and homogenized for 5 min in one volume equivalent of cold acetonitrile containing internal standard 9 using a bullet blender (Next Advance, Inc., Averill Park, NY) at 4 °C. The brain homogenates were centrifuged at 20 000g for 20 min at 4 °C. The plasma and brain supernatants were analyzed by reversed phase UPLC/electrospray ionization (ESI)-multiple-reaction monitoring (MRM) of the transitions 321 → 184 for 2, 401 → 79 for 3, and 399 → 184 for internal standard 9. Calibration curves of compounds 2 and 3 were prepared by fortification of blank mouse plasma and blank mouse brain with compounds 2 and 3 at concentrations ranging from 0.05 up to 20 μM. The methods were linear over the specified range with coefficients of correlation R2 of 0.993 and 0.998 for compound 2 in plasma and brain, respectively, and 0.999 and 0.986 for compound 3 in plasma and brain, respectively. Peak area ratios relative to the internal standard and linear regression parameters from the calibration curve standards were used for quantification. PK parameters were calculated as described previously.17
Liquid Chromatography/Mass Spectrometry
The LC-MS-MS instrument consisted of a Waters Acquity UPLC system (Waters Corporation, Milford, MA) equipped with a binary solvent manager, an autosampler, a column heater, and a photodiode array detector coupled with a Waters TQD tandem quadrupole detector (Milford, MA) monitored with MassLynx MS software. Mass spectrometry acquisition was performed in the negative ESI mode with MRM using the following parameters: capillary voltage = 2.8 kV, cone voltage = 25 V, extractor voltage = 3 V, RF lens voltage = 0.1 V, nitrogen as both desolvation gas (650 L/h) and cone gas (50 L/h flow rate), argon for collision gas (0.03 mL/min), source temperature = 150 °C, and desolvation temperature = 350 °C. Chromatographic separation was performed on an Acclaim RSLC 120 C18 column (2.2 μm, 2.1 mm i.d. × 100 mm, Dionex, Thermo Fisher Scientific Inc., Waltham, MA). The mobile phase consisted of elution at 0.5 mL/min with 100% A for 1 min, followed by a 0.1 min linear gradient to 60% A/40% B, 3 min linear gradient to 30% A/70% B, 1 min linear gradient to 100% B, 1 min with 100% B, 4 min linear gradient to 100% A, and then 100% A for 1 min (A = 10 mM dibutylammonium acetate in 95% water/5% ACN, B = 10 mM dibutylammonium acetate in 16% water/84% ACN).
TBI Mouse Model
Male C57Bl/6J mice (8–10 weeks old, 20–25 g) were obtained from the Jackson Laboratory, Bar Harbor, ME. TBI was inflicted using an electromagnetic impactor for controlled cortical impact (CCI) as described previously.2 A 5 mm diameter craniotomy in the left parietotemporal skull was performed. The CCI was performed using a velocity of 5.0 m/s, a dwell time of 100 ms, at a depth of 2.5 mm. For sham mice, only the craniotomy was performed. All efforts were made to minimize animal suffering. Animal procedures were performed under compliance with the University of Missouri Animal Care and Use Committee.
Evaluation of Prodrug 3 and Compound 2 in Mouse TBI Model
Prodrug 3 was administered to TBI mice as a single iv injection at 7.8 mg/kg at 30 min after CCI. A second injection of compound 2 at 25 mg/kg was given sc 30 min later, followed by once a day sc dosing for the following three or 7 days. A vehicle group (treated with 70% propylene glycol/30% water) was included. The study was performed in a randomized-blinded manner.
Determination of Brain Lesion
Mice were sacrificed at 7 days after TBI, and the brains were collected for assessment of brain lesion volumes using the stereology technique as described previously.2 Brain volumes were quantified from the 40 μm thickness coronal sections (120–150 sections per brain) by cresyl violet staining. The digital photomicrographs were analyzed in a double-blinded manner using ImageScope software, as we previously described.19 The area of the lesioned cortex was measured and subtracted from that of the contralateral one. Sections were assigned a position along the rostro-caudal axis of the brain based on Bregma position. The lesion area was plotted versus the Bregma position, and the curves were fitted in MS Excel to a second-degree polynomial. Lesion volumes were calculated from the lesion areas multiplied by 200 μm, which is the distance between adjacent sections; the lesion volumes for each section were added to yield the lesion volume for each brain.
Beam-Walking
Beam-walking evaluates complex motor coordination. 20 A beam of 80 cm length by 1.25 cm width had an aversive stimulus (bright lamp) at one end for a mouse to start walking and a black box with an opening at the other end. Animals were trained the day before and the day of surgery to ambulate into the box to avoid the aversive stimulus. The beam-walk evaluation was performed at 3 and 7 days after TBI. The numbers of mice falling, the average distance traveled prior to falling, and the numbers of foot faults for each animal were quantified as parameters for motor function outcomes.
SNAP
Neurological functions in mice were evaluated using the SNAP score to assess vision, proprioception, motor strength, and posture. The eight tests include (1) interactions with the examiner for the level of alertness, (2) cage grasp was evaluated by grip strength, (3) visual placing was evaluated by vision, torso strength, forelimb coordination, proprioception, and tactile input, (4) pacing or circling was observed for the ambulation direction, (5) gait and limb posture, (6) head tilt, (7) visual field was examined by waving a 2 mm diameter fiber-tipped applicator on either side of the head, and (8) baton was evaluated by coordination and proprioception.11 Each of the eight tests has a scoring range of 0–5. The results from each of the eight tests were summed to derive the overall SNAP score. Briefly, a neurologically intact animal would be expected to have a SNAP score of “0”. SNAP scores were high when asymmetric deficits were apparent. SNAP allows for ambiguous results (a score of “1”) and test scores of 2–5 were assigned when asymmetric deficits were apparent. In addition, a range was allowed when the deficit appeared to span more than one definition for a test score, and ranges were then averaged to derive the test score (e.g., a range of 1–2 would be scored as 1.5 for that test).
Statistical Analysis
Data are expressed as mean values ± SEM. Data were analyzed for statistical significance by the unpaired one-tailed Student’s t test for any two-group comparisons, by one-way analysis of variance (ANOVA) by Kruskal–Wallis test with Dunn’s multiple comparison test for multiple groups, and numbers of mice falling were analyzed by the χ2 test using Prism 5.
Acknowledgments
Funding
This work was supported by a grant from the Indiana Department of Health Spinal Cord and Brain Injury Fund. M.G. is a Fellow of the Chemistry-Biochemistry-Biology Interface Program, supported by a training grant GM075762 from the National Institutes of Health.
Footnotes
Author Contributions
M.L. synthesized compounds 2, 3, and 9 and performed the PK experiments. Z.C., B.N.T., and J.C performed the mouse TBI studies. M.G. determined enzyme kinetics, blood and S9 stability. D.H. contributed to the synthesis of compound 3. B.B. contributed to the development of the bioanalytical method for prodrug 3. E.L. performed the hemolysis and XTT assays. M.A.S., V.A.S., and W.R.W. performed the in-life portion of the PK and brain distribution animal studies. Z.C., B.N.T., M.R.J., R.N., J.C., and Z.G. analyzed the TBI data. J.C. and Z.G. designed the TBI studies. M.C. conceived and designed the experiments for this project and analyzed the results. S.M., Z.G., and M.C. wrote the manuscript, with input from the authors.
Notes
The authors declare no competing financial interest.
References
- 1.Faul M, Xu L, Wald M, Coronado V. Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention; Atlanta, GA: 2010. [Google Scholar]
- 2.Hadass O, Tomlinson BN, Gooyit M, Chen SY, Purdy JJ, Walker JM, Zhang CY, Giritharan AB, Purnell W, Robinson CR, Shin D, Schroeder VA, Suckow MA, Simonyi A, Sun GY, Mobashery S, Cui JK, Chang M, Gu ZZ. Selective Inhibition of Matrix Metalloproteinase-9 Attenuates Secondary Damage Resulting from Severe Traumatic Brain Injury. PLoS One. 2013;8:e76904. doi: 10.1371/journal.pone.0076904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higashida T, Kreipke CW, Rafols JA, Peng CY, Schafer S, Schafer P, Ding JY, Dornbos D, Li XH, Guthikonda M, Rossi NF, Ding YC. The role of hypoxia-inducible factor-la, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury Laboratory investigation. J Neurosurg. 2011;114:92–101. doi: 10.3171/2010.6.JNS10207. [DOI] [PubMed] [Google Scholar]
- 4.Brown S, Bernardo MM, Li ZH, Kotra LP, Tanaka Y, Fridman R, Mobashery S. Potent and selective mechanism-based inhibition of gelatinases. J Am Chem Soc. 2000;122:6799–6800. [Google Scholar]
- 5.Forbes C, Shi QC, Fisher JF, Lee M, Hesek D, Llarrull LI, Toth M, Gossing M, Fridman R, Mobashery S. Active Site Ring-Opening of a Thiirane Moiety and Picomolar Inhibition of Gelatinases. Chem Biol Drug Des. 2009;74:527–534. doi: 10.1111/j.1747-0285.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson MW, Gervasi DC, Mobashery S, Fridman R. Kinetic analysis of the binding of human matrix metalloproteinase-2 and –9 to tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2. J Biol Chem. 1997;272:29975–29983. doi: 10.1074/jbc.272.47.29975. [DOI] [PubMed] [Google Scholar]
- 7.Lee M, Villegas-Estrada A, Celenza G, Boggess B, Toth M, Kreitinger G, Forbes C, Fridman R, Mobashery S, Chang M. Metabolism of a highly selective gelatinase inhibitor generates active metabolite. Chem Biol Drug Des. 2007;70:371–382. doi: 10.1111/j.1747-0285.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 8.Testero SA, Lee M, Staran RT, Espahbodi M, Llarrull LI, Toth M, Mobashery S, Chang M. Sulfonate-Containing Thiiranes as Selective Gelatinase Inhibitors. ACS Med Chem Lett. 2011;2:177–181. doi: 10.1021/ml100254e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee M, Celenza G, Boggess B, Blase J, Shi Q, Toth M, Bernardo MM, Wolter WR, Suckow MA, Hesek D, Noll BC, Fridman R, Mobashery S, Chang M. A potent gelatinase inhibitor with anti-tumor-invasive activity and its metabolic disposition. Chem Biol Drug Des. 2009;73:189–202. doi: 10.1111/j.1747-0285.2008.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gooyit M, Suckow MA, Schroeder VA, Wolter WR, Mobashery S, Chang M. Selective gelatinase inhibitor neuroprotective agents cross the blood-brain barrier. ACS Chem Neurosci. 2012;3:730–736. doi: 10.1021/cn300062w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shelton SB, Pettigrew DB, Hermann AD, Zhou W, Sullivan PM, Crutcher KA, Strauss KI. A simple, efficient tool for assessment of mice after unilateral cortex injury. J Neurosci Methods. 2008;168:431–442. doi: 10.1016/j.jneumeth.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang XY, Jung JC, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia F, Yin YH, Gao GY, Wang Y, Cen L, Jiang JY. MMP-9 Inhibitor SB-3CT Attenuates Behavioral Impairments and Hippocampal Loss after Traumatic Brain Injury in Rat. J Neurotrauma. 2014;31:1225–1234. doi: 10.1089/neu.2013.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossetete M, Phelps J, Arko L, Yonas H, Rosenberg GA. Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery. 2009;65:702–708. doi: 10.1227/01.NEU.0000351768.11363.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikejiri M, Bernardo MM, Bonfil RD, Toth M, Chang ML, Fridman R, Mobashery S. Potent mechanism-based inhibitors for matrix metalloproteinases. J Biol Chem. 2005;280:33992–34002. doi: 10.1074/jbc.M504303200. [DOI] [PubMed] [Google Scholar]
- 17.Gooyit M, Lee M, Schroeder VA, Ikejiri M, Suckow MA, Mobashery S, Chang M. Selective Water-Soluble Gelatinase Inhibitor Prodrugs. J Med Chem. 2011;54:6676–6690. doi: 10.1021/jm200566e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Daniel PI, Peng Z, Pi H, Testero SA, Ding D, Spink E, Leemans E, Boudreau MA, Yamaguchi T, Schroeder VA, Wolter WR, Llarrull LI, Song W, Lastochkin E, Kumarasiri M, Antunes NT, Espahbodi M, Lichtenwalter K, Suckow MA, Vakulenko S, Mobashery S, Chang M. Discovery of a new class of non-beta-lactam inhibitors of penicillin-binding proteins with Gram-positive antibacterial activity. J Am Chem Soc. 2014;136:3664–3672. doi: 10.1021/ja500053x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z, Shin D, Chen S, Mikhail K, Hadass O, Tomlison BN, Korkin D, Shyu CR, Cui J, Anthony DC, Gu Z. Histological quantitation of brain injury using whole slide imaging: a pilot validation study in mice. PLoS One. 2014;9:e92133. doi: 10.1371/journal.pone.0092133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker JM, Fowler SW, Miller DK, Sun AY, Weisman GA, Wood WG, Sun GY, Simonyi A, Schachtman TR. Spatial learning and memory impairment and increased locomotion in a transgenic amyloid precursor protein mouse model of Alzheimer’s disease. Behav Brain Res. 2011;222:169–175. doi: 10.1016/j.bbr.2011.03.049. [DOI] [PubMed] [Google Scholar]