Abstract
IMPORTANCE
The current recommendation is to perform re-resection for select patients with incidentally discovered gallbladder cancer. The optimal time interval for re-resection for both patient selection and long-term survival is not known.
OBJECTIVE
To assess the association of time interval from the initial cholecystectomy to reoperation with overall survival.
DESIGN, SETTING, AND PARTICIPANTS
This cohort study was conducted from January 1, 2000, to December 31, 2014 at 10 US academic institutions. A total of 207 patients with incidentally discovered gallbladder cancer who underwent reoperation and had available data on the date of their initial cholecystectomy were included.
EXPOSURES
Time interval from the initial cholecystectomy to reoperation: group A: less than 4 weeks; group B: 4 to 8 weeks; and group C: greater than 8 weeks.
MAIN OUTCOMES AND MEASURES
Primary outcome was overall survival.
RESULTS
Of 449 patients with gallbladder cancer, 207 cases (46%) were discovered incidentally and underwent reoperation at 3 different time intervals from the date of the original cholecystectomy: group A: less than 4 weeks (25 patients, 12%); B: 4 to 8 weeks (91 patients, 44%); C: more than 8 weeks (91 patients, 44%). The mean (SD) ages of patients in groups A, B, and C were 65 (9), 64 (11), and 66 (12) years, respectively. All groups were similar for baseline demographics, extent of resection, presence of residual disease, T stage, resection margin status, lymph node involvement, and postoperative complications. Patients who underwent reoperation between 4 and 8 weeks had the longest median overall survival (group B: 40.4 months) compared with those who underwent early (group A: 17.4 months) or late (group C: 22.4 months) reoperation (log-rank P = .03). Group A and C time intervals (vs group B), presence of residual disease, an R2 resection, advanced T stage, and lymph node involvement were associated with decreased overall survival on univariable Cox regression. Only group A (hazard ratio, 2.63; 95%CI, 1.25–5.54) and group C (hazard ratio, 2.07; 95%CI, 1.17–3.66) time intervals (vs group B), R2 resection (hazard ratio, 2.69; 95%CI, 1.27–5.69), and advanced T stage (hazard ratio, 1.85; 95%CI, 1.11–3.08) persisted on multivariable Cox regression analysis.
CONCLUSIONS AND RELEVANCE
The optimal time interval for re-resection for incidentally discovered gallbladder cancer appears to be between 4 and 8 weeks after the initial cholecystectomy.
The sixth most common gastrointestinal cancer, gallbladder carcinoma is a rare disease with a poor overall prognosis.1,2 Resection is the only potentially curative treatment option, yet reported survival rates following surgery vary greatly, from10% to 100% at 5 years, depending on tumor biology, stage of disease, and extent of resection.2–4 Approximately 50% to 70% of gallbladder cancers are found incidentally during or after an elective cholecystectomy for presumed benign disease, which represents 0.7% of all cholecystectomy specimens.5–7
Current management guidelines for incidental gallbladder cancer (IGBC) recommend re-resection for T1b, T2, and T3 lesions, unless contraindicated by advanced disease or poor performance status.8 However, to our knowledge, there are few data on the timing of re-resection, which can vary between 1 day and more than 2 years following the initial cholecystectomy.9 In the benign setting, most surgeons generally elect to reoperate either within the first 7 to 10 days—before the inflammatory processes have peaked—or after approximately 4 to 6 weeks, when these processes have begun to subside. In malignancy, tumor biology, in addition to technical considerations, plays an important role in defining the optimal timing of reoperation.
In several other cancers, such as esophageal and rectal cancers, the timing of definitive surgery following the initial treatment has been studied in detail, yet has primarily focused on the timing of surgery following neoadjuvant radiation.10,11 In IGBC, to our knowledge, no study has examined the effect of the timing of reoperation after the initial cholecystectomy on outcomes. The purpose of this study was to assess the association of time interval from the initial cholecystectomy to reoperation with overall survival (OS).
Key Points.
Question
Is there an association between time interval from the initial cholecystectomy to reoperation and overall survival?
Findings
In this multi-institutional cohort study of 207 patients who underwent reoperation for incidental gallbladder cancer, reoperation between 4 and 8 weeks after the initial cholecystectomy was associated with improved median overall survival (40.8 months) compared with reoperation less than 4 weeks (17.4 months) and greater than 8 weeks (22.4 months).
Meaning
Reoperation between 4 and 8 weeks after the initial cholecystectomy appears to be the optimal time interval for re-resection in incidental gallbladder cancer.
Methods
Study Population
The US Extrahepatic Biliary Malignancy Consortium is a collaboration of 10 high-volume academic institutions: Emory University, Johns Hopkins University, New York University, The Ohio State University, Stanford University, University of Louisville, University of Wisconsin, Vanderbilt University, Wake Forest University, and Washington University in St Louis. All patients with IGBC who underwent reoperation from January 1, 2000, to December 31, 2014, were assessed. Only patients with IGBC who had information regarding the dates of their initial cholecystectomy and reoperation were included. Cases in which the diagnosis of IGBC was made intraoperatively and the definitive resection was performed under the same anesthesia were excluded.
Pertinent baseline demographic, preoperative, intraoperative, pathologic, and postoperative data were recorded. Pathology review was performed by experienced gastrointestinal pathologists at each institution, and staging was assigned as per American Joint Committee on Cancer 7th edition guidelines.12 Data regarding neoadjuvant and adjuvant therapy, disease recurrence, and survival were additionally collected. Survival information was verified with the Social Security Death Index when necessary. Institutional review board approval was obtained at each institution prior to data collection. Because this study involved only retrospective medical record review and posed minimal risk to patients, a waiver of consent was obtained at each institution.
Descriptive and comparative analyses were performed on the entire cohort. Thirty-day postoperative deaths (n = 4)were excluded for all survival analyses. Overall survival was calculated from the date of reoperation to the date of death or last follow-up. To account for potential length-time bias between groups, OS was also calculated from the date of the initial cholecystectomy to the date of death or last follow-up.
Time Interval Groups
The time interval from the date of the initial cholecystectomy to the date of reoperation was calculated for all patients. Patients were then separated into 3 groups according to their time interval to reoperation: group A (less than 4 weeks), B (4 to 8 weeks), and C (more than 8 weeks). The primary objective was to assess the difference in OS between groups to identify the optimal timing for reoperation and re-resection in patients with IGBC.
Statistical Analysis
All statistical analysis was performed using SPSS version 22.0 software (IBM Inc). The t test or 1-way analysis of variance was used to compare continuous variables, and χ2 analysis was used for categorical variables where indicated. Kaplan-Meier survival plots were calculated for OS. Univariable and multivariable Cox regression analyses were performed to assess the effect of time interval group on OS in the context of other clinically relevant clinicopathologic features. Statistical significance for each end point was predefined as 2-tailed P < .05.
Results
Of 449 patients with gallbladder cancer, 266 cases (59%) were discovered incidentally. The date of the initial cholecystectomy was not available for 33 patients, and in 26 patients, the definitive resection was performed at the time of incidental discovery, leaving 207 (46%) for inclusion in analysis. Among the entire cohort, the median time to reoperation was 7.4 weeks (interquartile range, 5.0–10.7). Twenty-five patients (12%) underwent reoperation less than 4 weeks (group A), 91 (44%) between 4 weeks and 8 weeks (group B), and 91 (44%) greater than 8 weeks (group C) after their initial cholecystectomy. Comparative analyses of clinicopathologic factors across groups are shown in Table 1. There was no difference in baseline demographics or underlying comorbidities between groups.
Table 1.
Clinicopathologic Features of Patients With Incidental Gallbladder Cancer by Time Interval Group
| Variable | No. (%) | P Value | ||
|---|---|---|---|---|
| Group A (<4 wk) | Group B (4–8 wk) | Group C (>8 wk) | ||
| Totala | 25 (12) | 91 (44) | 91 (44) | |
| Time to reoperation, median (range), wk | 2.9 (0.4–3.9) | 5.9 (4.1–8.0) | 11.4 (8.1–179.6) | |
| Age, mean (SD), y | 65 (9) | 64 (11) | 66 (12) | .75 |
| Male | 10 (40) | 34 (37) | 33 (36) | .94 |
| BMI, mean (SD) | 28.7 (6.5) | 29.0 (6.9) | 30.3 (7.0) | .40 |
| Race/ethnicity | ||||
| White | 21 (88) | 67 (77) | 68 (76) | .81 |
| African American | 0 | 11 (13) | 12 (13) | |
| Latino | 2 (8) | 5 (6) | 6 (7) | |
| Asian | 1 (4) | 2 (2) | 2 (2) | |
| Other | 0 | 2 (2) | 2 (2) | |
| ASA class | ||||
| 1 | 0 | 1 (2) | 1 (2) | .22 |
| 2 | 13 (62) | 19 (29) | 25 (37) | |
| 3 | 8 (38) | 44 (67) | 39 (57) | |
| 4 | 0 | 2 (3) | 3 (4) | |
| Comorbiditiesb | ||||
| 0 | 4 (17) | 32 (37) | 25 (28) | .16 |
| 1 | 15 (65) | 34 (39) | 37 (42) | |
| ≥2 | 4 (17) | 21 (24) | 26 (30) | |
| Clinical jaundice | 2 (8) | 9 (11) | 4 (5) | .34 |
| Location of original cholecystectomy | ||||
| Participating institution | 6 (24) | 8 (9) | 9 (10) | .09 |
| Locoregional residual disease | 14 (56) | 42 (47) | 42 (48) | .71 |
| Distant disease | 2 (8) | 18 (20) | 16 (18) | .38 |
| Resection | ||||
| Attempted | 22 (88) | 79 (87) | 77 (85) | .87 |
| Completed | 22 (88) | 74 (81) | 72 (79) | .60 |
| Extent of resection | ||||
| Radical cholecystectomy + portal LN | 21 (96) | 66 (87) | 69 (93) | .29 |
| Major hepatectomy | 1 (5) | 10 (13) | 5 (7) | |
| Operative approach | ||||
| Open | 23 (100) | 84 (97) | 85 (97) | .67 |
| Laparoscopic | 0 | 3 (3) | 3 (3) | |
| Common bile duct resection | 9 (41) | 29 (37) | 23 (30) | .54 |
| EBL, mean (SD), mL | 428 (318) | 294 (292) | 352 (396) | .26 |
| Final margin status | .10 | |||
| R0 | 19 (76) | 72 (79) | 69 (76) | |
| R1 | 3 (12) | 1 (1) | 3 (3) | |
| R2 | 3 (12) | 18 (20) | 19 (21) | |
| Tumor size, mean (SD), mm | 38.9 (18.1) | 28.4 (25.4) | 30.2 (19.9) | .31 |
| AJCC T stage | ||||
| T1a/b | 1 (5) | 5 (6) | 10 (12) | .11 |
| T2 | 11 (50) | 50 (63) | 35 (43) | |
| T3/4 | 10 (46) | 24 (30) | 36 (44) | |
| Grade | ||||
| Well/moderate | 13 (62) | 51 (71) | 56 (76) | .45 |
| Poor/undifferentiated | 8 (38) | 21 (29) | 18 (24) | |
| Lymphovascular invasion | 5 (46) | 20 (50) | 17 (41) | .69 |
| Perineural invasion | 8 (73) | 19 (46) | 25 (58) | .25 |
| LN positive | 9 (39) | 31 (47) | 30 (40) | .63 |
| Total LN retrieved, mean (SD) | 5.8 (5.5) | 5.2 (5.4) | 4.7 (4.9) | .63 |
| Major complication | 3 (13) | 8 (9) | 16 (18) | .24 |
| Neoadjuvant chemotherapy | 0 | 0 | 7 (8) | .01 |
| Adjuvant chemotherapy | 8 (44) | 41 (54) | 40 (52) | .77 |
Abbreviations: AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiology; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); EBL, estimated blood loss; LN, lymph node.
Total number varies depending on availability of data for each variable.
Includes hypertension, diabetes, prior cardiac event, and end-stage renal disease.
Patients in group A tended to be more likely to have undergone the initial cholecystectomy at their respective participating institution (24%), while patients in groups B and C tended to have undergone the initial cholecystectomy at outside hospitals (91%and 90%, respectively), although this was not statistically significant (P = .09). A similar proportion of patients in each group had locoregional residual or distant disease at the time of reoperation and underwent completed resections. There was no difference in the extent of the resection performed, with most patients undergoing the recommended partial hepatectomy (segments IVb and V) with portal lymph node dissection in all groups (96%, 87%, and 93%, respectively; P = .29). There was no difference between groups in margin status, T stage distribution, histologic grade, lymphovascular invasion, perineural invasion, or the presence of positive lymph nodes.
There was no difference in the incidence of major postoperative complications between groups (P = .24). Seven patients (8%) in group C received neoadjuvant therapy compared with zero patients in groups A and B (P = .01). A similar proportion of patients received adjuvant therapy in all groups.
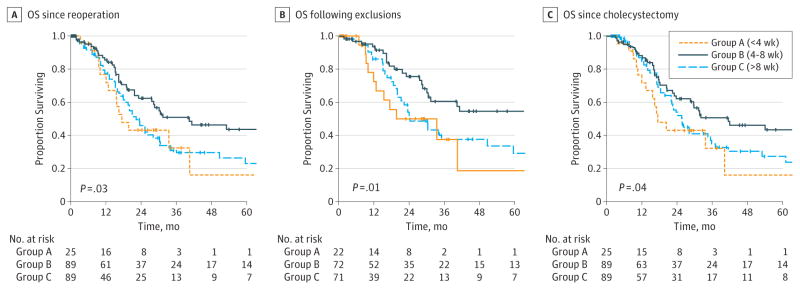
Median follow-up was 13.9 months (interquartile range, 2.7–37.5). Median OS for the entire cohortwas27.6months (95% CI, 21.4–33.8). Reoperation between 4 and 8 weeks (group B) was associated with improved OS (40.4 months; 95%CI, 16.4–64.4) compared with reoperation less than 4weeks (group A; 17.4 months; 95%CI, 11.1–23.7) or greater than 8weeks (group C; 22.4 months; 95% CI, 18.2–26.6) following the initial cholecystectomy (P = .03; Figure, A). Group B was still associated with improved OS compared with groups A and C when excludingR2 resections (110.3 months vs 33.5and24.3 months, respectively; P = .01; Figure, B). When calculating survival from the date of the initial cholecystectomy, group B was similarly associated with improved OS compared with groups A and C (41.5 months vs 17.4 and 25.9 months, respectively; P = .04; Figure, C).
Figure. Rates of Overall Survival (OS).
A, Overall survival from date of reoperation for all patients. Group B was associated with improved OS (40.4 months, n = 89) compared with groups A (17.4 months, n = 25) and C (22.4 months, n = 89) (P = .03). B, Overall survival from date of reoperation, excluding aborted procedures and R2 resections. Group B was associated with improved OS (110.3 months, n = 72) compared with groups A (33.5 months, n = 22) and C (24.3 months, n = 71) (P = .01). C, Overall survival from date of initial cholecystectomy for all patients. Group B was associated with improved OS (40.4 months, n = 89) compared with groups A (17.4 months, n = 25) and C (23.6 months, n = 91) (P = .04).
Univariable and multivariable Cox regression analyses for OS calculated from the date of reoperation are shown in Table 2. Time interval group (A and C vs B), advanced T stage (T3/4 vs T2), margin positivity, the presence of residual disease at reoperation, and lymph node positivity were all associated with worse survival on univariable analysis. Only time interval group, R2 resection, and advanced T stage were associated with worse survival on multivariable analysis. On multivariable Cox regression analysis calculating OS from the date of the initial cholecystectomy, group A (hazard ratio, 2.82; 95% CI, 1.33–5.97; P = .007) and group C (hazard ratio, 1.89; 95% CI, 1.07–3.33; P = .03) were still associated with worse survival compared with group B, as were advanced T stage andR2resection.
Table 2.
Univariable and Multivariable Cox Regression Analyses for Overall Survival From Date of Reoperation
| Variable | Analysis, HR (95% CI) | |||
|---|---|---|---|---|
| Univariable | P Value | Multivariable | P Value | |
| Time interval | ||||
| Group A (0–4 wk) | 1.94 (1.06–3.56) | .03 | 2.63 (1.25–5.54) | .01a |
| Group B (4.1–8 wk) | 1 [Reference] | 1 [Reference] | NA | |
| Group C (>8 wk) | 1.68 (1.08–2.59) | .02 | 2.07 (1.17–3.66) | .01a |
| Clinical jaundice | 1.69 (0.85–3.38) | .14 | NA | NA |
| Extent of resection | ||||
| Radical cholecystectomy + portal LN | 1 [Reference] | NA | NA | |
| Major hepatectomy | 1.35 (0.67–2.73) | .40 | NA | NA |
| Residual disease at reoperation | 3.10 (2.01–4.76) | <.001 | 1.51 (0.90–2.54) | .12 |
| Final margin status | ||||
| R0 | 1 [Reference] | 1 [Reference] | NA | |
| R1 | 2.73 (0.98–7.59) | .05 | 1.19 (0.34–4.18) | .79 |
| R2 | 4.33 (2.77–6.77) | <.001 | 2.69 (1.27–5.69) | .009a |
| AJCC T stage | ||||
| T1a/b | 0.16 (0.02–1.18) | .07 | 0.28 (0.04–2.08) | .21 |
| T2 | 1 [Reference] | 1 [Reference] | NA | |
| T3/4 | 2.16 (1.39–3.36) | .001 | 1.85 (1.11–3.08) | .02a |
| Grade | ||||
| Well/moderate | 1 [Reference] | NA | ||
| Poor/undifferentiated | 1.40 (0.87–2.26) | .16 | NA | NA |
| LN positive | 1.72 (1.07–2.76) | .03 | 1.56 (0.94–2.60) | .09 |
| Adjuvant chemotherapy | 0.99 (0.62–1.59) | .98 | NA | NA |
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; LN, lymph node; NA, not applicable.
P<.05.
Discussion
Gallbladder cancer is a rare and aggressive malignancy with a poor prognosis. Resection is the only potentially curative treatment option, and the timing of resection has been shown to be an important factor in determining outcomes—gallbladder cancer diagnosed in patients incidentally, which accounts for most cases, has better survival rates than gallbladder cancer diagnosed in patients only after the signs and symptoms of malignancy become apparent.7 Once IGBC is discovered, re-resection is the recommended treatment strategy for patients with T1b, T2, and T3 tumors.8 The choice of timing for reoperation is largely dictated by the waxing and waning of the inflammatory process to minimize complications and maximize patient safety. However, just as the timing of diagnosis of gallbladder cancer can translate to survival, so too may the timing of re-resection be an important, and heretofore under appreciated, determinant of outcomes in patients with IGBC. Indeed, the optimal timing of re-resection in IGBC that balances both technical considerations and tumor biology is currently not known.
In the current study, 207 patients underwent reoperation for IGBC. Baseline demographics, clinicopathologic characteristics, and outcomes of the entire cohort were similar to those in previous studies on IGBC.5–7,9,13,14 Overall, the median time to reoperation was 7.4 weeks (interquartile range, 5.0–10.7). This is in line with the general global practice patterns for this disease.7,9,13–15 Twenty-five patients (12%) underwent reoperation within 4 weeks (group A), 91 (44%) between 4 weeks and 8 weeks (group B), and 93 patients (44%) beyond 8 weeks (group C) after the initial cholecystectomy.
The groups were very similar with regard to baseline demographics and clinicopathologic characteristics. There were no differences in the presence of locoregional residual or distant disease at the time of reoperation, the percentage of aborted procedures and R2 resections, or the incidence of major complications between groups. Important prognostic factors other than margin status, such as T stage, grade, lymphovascular invasion, perineural invasion, and lymph node status were also similar between groups.
Based on data from the current study, it appears that reoperation between 4 and 8weeks (group B) is the optimal time interval for re-resection in patients with IGBC. Group Bhad significantly better survival than groups A and C on Kaplan-Meier, univariable Cox regression, and multivariable Cox regression analyses. Even when excluding patients with aborted procedures andR2resections, and calculating OS from the date of the initial cholecystectomy, group B patients still had better survival than those in both A and C. The possible reasons for this are many. First, reoperating earlier than 4 weeks may not allow for complete tumor evaluation and staging. Preliminary results based on frozen section analysis can be difficult to interpret and may be unreliable in the setting of acute inflammation. Furthermore, inflammation in the operative field can make visualization of important structures on cross-sectional imaging near impossible in the early postoperative period. Thus, it may take several weeks for adequate TNM and clinical staging to be completed, and rushing to the operating room may be doing so without all the information.
Second, reoperating outside the 4- to 8-weekwindowmay be suboptimal from a tumor biology standpoint. Reoperation too early (before4weeks)may not allow sufficient time for sub clinical disease, which was likely already present at the time of diagnosis, to be appreciated. Conversely, reoperation too late (after 8weeks)may allow too much time for disease dissemination. Although the percentage of patients with locoregional or distant disease at the time of reoperation was similar between groups Band C, this finding likely reflects selection bias and should be interpreted with caution—only patients who survived long enough, without evidence of locally advanced or distant disease preoperatively, underwent reoperation and were included in this study. Given this, one might expect patients in group C, who represent the “hearty survivors,” to have better survival than those in groups A and B; yet, group B patients still had better survival outcomes than group C patients, which may reflect more advanced subclinical disease in the latter group that might have been prevented had these patients been reoperated on sooner.
There are several limitations to this study. First, by including only patients who underwent reoperation, there is an inherent selection bias in this study, as previously discussed. However, this is not uncommon in studies examining the effect of surgery timing on patient outcomes, particularly in a tertiary care setting in which most patients are referred from outside facilities after diagnosis, as was the case in this study. Despite this bias, groups were still well matched for most baseline and clinicopathologic factors. Second, the retrospective nature of the study makes recurrence and disease-specific survival information difficult to capture. However, this study incorporates data from 10 high-volume, geographically dispersed academic institutions, which more closely represents the disease characteristics and general practice patterns of the United States and eliminates single-institution bias. In addition, given the aggressive nature of and poor prognosis associated with gallbladder cancer, OS is a good surrogate for disease-specific survival in most cases.
Conclusions
In conclusion, this is one of the largest series that examines patients who underwent reoperation for IGBC and, to our knowledge, the only study that assesses the effect of time from the initial cholecystectomy to reoperation on these patients’ survival. Between 4 and 8 weeks appears to be the optimal time interval for re-resection that balances both technical considerations and tumor biology in patients with IGBC.
Acknowledgments
Funding/Support: This study was supported in part by the Katz Foundation.
Role of the Funder/Sponsor: The Katz Foundation had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Disclaimer: Dr Pawlik is a Deputy Editor of JAMA Surgery but was not involved in the editorial review or the decision to accept the manuscript for publication.
Previous Presentations: This study was presented in part at the American Society of Clinical Oncology Gastrointestinal Cancer Symposium; January 22, 2016; San Francisco, CA; and at the Society of Surgical Oncology Annual Meeting; March 3, 2016; Boston, Massachusetts.
Author Contributions: Dr Maithel had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ethun, Pawlik, Poultsides, Fields, Jin, Weber, Schmidt, Beal, Hatzaras, Kooby, Maithel.
Acquisition, analysis, or interpretation of data: Ethun, Postlewait, Le, Pawlik, Buttner, Poultsides, Tran, Idrees, Isom, Jin, Weber, Salem, Martin, Scoggins, Shen, Mogal, Schmidt, Beal, Hatzaras, Shenoy, Maithel.
Drafting of the manuscript: Ethun, Le, Weber, Martin, Maithel.
Critical revision of the manuscript for important intellectual content: Ethun, Postlewait, Pawlik, Buttner, Poultsides, Tran, Idrees, Isom, Fields, Jin, Weber, Salem, Scoggins, Shen, Mogal, Schmidt, Beal, Hatzaras, Shenoy, Kooby, Maithel.
Statistical analysis: Ethun, Le, Tran, Weber, Maithel.
Administrative, technical, or material support: Ethun, Postlewait, Pawlik, Buttner, Poultsides, Idrees, Isom, Fields, Jin, Weber, Scoggins, Mogal, Beal, Maithel.
Study supervision: Pawlik, Poultsides, Martin, Schmidt, Hatzaras, Kooby, Maithel.
No additional contributions: Shen, Shenoy.
Other - Data Collection: Salem.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder: results of the French Surgical Association Survey. Ann Surg. 1994;219(3):275–280. doi: 10.1097/00000658-199403000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoist S, Panis Y, Fagniez PL French University Association for Surgical Research. Long-term results after curative resection for carcinoma of the gallbladder. Am J Surg. 1998;175(2):118–122. doi: 10.1016/s0002-9610(97)00269-9. [DOI] [PubMed] [Google Scholar]
- 4.Lendoire JC, Gil L, Duek F, et al. Relevance of residual disease after liver resection for incidental gallbladder cancer. HPB (Oxford) 2012;14(8):548–553. doi: 10.1111/j.1477-2574.2012.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi KS, Choi SB, Park P, Kim WB, Choi SY. Clinical characteristics of incidental or unsuspected gallbladder cancers diagnosed during or after cholecystectomy: a systematic review and meta-analysis. World J Gastroenterol. 2015;21(4):1315–1323. doi: 10.3748/wjg.v21.i4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuks D, Regimbeau JM, Le Treut YP, et al. Incidental gallbladder cancer by the AFC-GBC-2009 Study Group. World J Surg. 2011;35(8):1887–1897. doi: 10.1007/s00268-011-1134-3. [DOI] [PubMed] [Google Scholar]
- 7.Pawlik TM, Gleisner AL, Vigano L, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007;11(11):1478–1486. doi: 10.1007/s11605-007-0309-6. [DOI] [PubMed] [Google Scholar]
- 8.Aloia TA, Járufe N, Javle M, et al. Gallbladder cancer: expert consensus statement. HPB (Oxford) 2015;17(8):681–690. doi: 10.1111/hpb.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butte JM, Kingham TP, Gönen M, et al. Residual disease predicts outcomes after definitive resection for incidental gallbladder cancer. J Am Coll Surg. 2014;219(3):416–429. doi: 10.1016/j.jamcollsurg.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franko J, Voynov G, Goldman CD. Esophagectomy timing after neoadjuvant therapy for distal esophageal adenocarcinoma. Ann Thorac Surg. 2016;101(3):1123–1130. doi: 10.1016/j.athoracsur.2015.09.044. [DOI] [PubMed] [Google Scholar]
- 11.Huntington CR, Boselli D, Symanowski J, Hill JS, Crimaldi A, Salo JC. Optimal timing of surgical resection after radiation in locally advanced rectal adenocarcinoma: an analysis of the national cancer database. Ann Surg Oncol. 2016;23(3):877–887. doi: 10.1245/s10434-015-4927-z. [DOI] [PubMed] [Google Scholar]
- 12.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. Gallbladder; pp. 211–217. [Google Scholar]
- 13.Butte JM, Gönen M, Allen PJ, et al. The role of laparoscopic staging in patients with incidental gallbladder cancer. HPB (Oxford) 2011;13(7):463–472. doi: 10.1111/j.1477-2574.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butte JM, Waugh E, Meneses M, Parada H, De La Fuente HA. Incidental gallbladder cancer: analysis of surgical findings and survival. J Surg Oncol. 2010;102(6):620–625. doi: 10.1002/jso.21681. [DOI] [PubMed] [Google Scholar]
- 15.Isambert M, Leux C, Métairie S, Paineau J. Incidentally-discovered gallbladder cancer: when, why and which reoperation? J Visc Surg. 2011;148(2):e77–e84. doi: 10.1016/j.jviscsurg.2011.02.005. [DOI] [PubMed] [Google Scholar]