Abstract
Aim
To assess total retinal blood flow (TRBF) in diabetic retinopathy (DR) using multiplane en face Doppler optical coherence tomography (OCT).
Methods
A 70 kHz spectral-domain OCT system scanned a 2×2 mm area centred at the optic disc of the eyes with DR and healthy participants. The multiplane en face Doppler OCT algorithm generated a three-dimensional volumetric data set consisting of 195 en face planes. The TRBF was calculated from the maximum flow values of each branching retinal vein at an optimised en face plane. DR severity was graded according to the international clinical classification system. The generalised linear model method was used to compare flow values between DR groups and the control group.
Results
A total of 71 eyes from 71 participants were included. Ten eyes were excluded due to poor image quality. The within-visit repeatability of scans was 4.1% (coefficient of variation). There was no significant difference in the TRBF between the healthy (46.7±10.2 μL/min) and mild/moderate non-proliferative DR (44.9±12.6 μL/min) groups. The TRBF in severe non-proliferative DR (39.1±12.6 μL/min) and proliferative DR (28.9±8.85 μL/min) groups were significantly lower (p=0.04 and p<0.0001, respectively) than that of the healthy group. TRBF was correlated with DR disease severity (p<0.0001, linear trend test).
Conclusion
The novel multiplane en face Doppler OCT method provided reliable measurements of TRBF in DR eyes. This may be a useful tool in understanding the pathophysiology of DR.
INTRODUCTION
Retinal haemodynamics is an important part of the pathophysiology of diabetic retinopathy (DR). Capillary dropout and retinal perfusion changes are thought to play a role in the development of micro-vascular lesions of DR, diabetic macular oedema and neovascularisation.1,2 Multiple techniques such as blue field entoptic phenomenon,3 laser Doppler velocimetry,4–6 laser Doppler flowmetry,7 fluorescein angiography8,9 and colour Doppler10 have been used for retinal blood flow quantification in diabetics. These studies have reported some contradictory results, describing both increased and decreased retinal blood flow in DR.11
Recently, several groups have demonstrated the potential of Doppler optical coherence tomography (OCT) for assessment of retinal blood flow including in the diabetic eye.12–14 Accuracy of these techniques depends on an exact calculation of Doppler angle which can introduce significant measurement noise.15 Integration of the Doppler phase shift in the vessel region on the en face plane eliminates the need to calculate the Doppler angle.15–19 However, determining the flow of the central retinal vessels at the optic nerve head with this approach requires high-speed OCT (200 kHz or higher) because the axial velocities in these vessels are very high and their Doppler shift would exceed the phase wrapping limit.
In order to adopt this approach on clinically available spectral-domain OCT systems, we recently developed a ‘multiplane’ scheme to find suitable branches of the central retinal veins and the optimal en face planes to perform flow integration calculations. These planes are chosen so that the Doppler shifts do not exceed phase wrapping limit.15 This image-processing algorithm does not require angle determination, complex alignment or deep tissue penetration of the images. In this paper, we present the application of the more reliable multiplane en face Doppler OCT for evaluation of total retinal blood flow (TRBF) in DR.15
MATERIALS AND METHODS
Study population
Participants with diabetes with DR and age-matched healthy volunteers were recruited from the Casey Eye Institute at Oregon Health & Science University (OHSU). This cross-sectional study adhered to the Declaration of Helsinki in the treatment of human participants and the Institutional Review Board at OHSU approved the protocol. The nature of the study was explained to each participant and an informed consent was obtained.
Healthy volunteers without a history of diabetes were included in the control group. Participants with DR of varying severity were recruited based on clinical diagnosis from the Retina Service. Exclusion criteria for study eyes were presence of another ocular disease, inability to fixate, visual acuity worse than 20/200, significant media opacity and history of major ocular surgery. History of panretinal photocoagulation treatment (PRP) or anti-vascular endothelial growth factor (VEGF) injections were recorded from the electronic health records of the participants.
A masked, certified grader graded the retinopathy severity based on seven-field fundus photographs and assigned the level of severity.20 The eyes were divided into four groups: healthy, mild-to-moderate non-proliferative diabetic retinopathy (NPDR), severe NPDR and proliferative diabetic retinopathy (PDR). One eye of each participant was included. If the grading between the two eyes differed, the eye with less severe retinopathy was included. If both eyes were graded in the same severity group, one eye was randomly selected and included. Mean arterial pressure (MAP) was calculated as diastolic pressure plus one-third of the difference between systolic and diastolic pressures. Mean ocular perfusion pressure (MOPP) was calculated as two-thirds of MAP minus intraocular pressure.21
Doppler OCT
A commercial spectral-domain OCT system (RTVue-XR, Optovue, Fremont, California, USA) was used in this study. This instrument performs 70 000 A-scans per second with a light source centred at an 840 nm wavelength. It has a 2.3 mm scan depth with a 5 μm full-width-half-maximum depth resolution in tissue. The A-scan time interval was 14.3 μs and the phase wrapping velocity limit of RTVue-XR is 11.1 m/s.
The TRBF scan pattern consists of five consecutive volumes acquired in approximately 3 seconds. The scan covered a 2×2 mm area centred at the optic disc. Each volume contained 80 B-scans each consisting of 500 A-scans. The scan protocol included three sequentially repeated TRBF scans. A total of 15 volumes were acquired for each eye.
A customised software was used to calculate the Doppler phase shift using the phase-resolved technique and split-spectrum method. The split-spectrum method22,23 divided the full-spectrum fringe into several different bands. Averaging of the Doppler phase shift in these bands improves the signal-to-noise ratio in the Doppler OCT image.15
Total retinal blood flow measurement
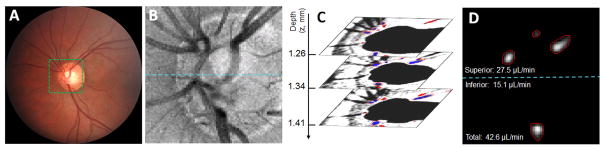
The software also calculates the TRBF (μL/min) using the multiple plane en face Doppler OCT technique.15 Based on the three-dimensional volumetric Doppler data, vessel boundaries were detected and vessels were then classified as either vein or artery based on the direction of the Doppler shift. Blood flow was integrated in each vein branch in each en face plane separately. For each vein, an optimised plane that yielded the maximum flow value was selected. The TRBF was then summed from all vein branches (figure 1A–C). A grader-set line divided the flow into superior and inferior hemispheres (figure 1D, dashed line) to assist in keeping track of the vasculature.
Figure 1.
Images from a healthy participant. (A) The volume covers the 2×2 mm area around the optic disc. (B) En face optical coherence tomography reflectance image. (C) Doppler image in three en face planes selected from 195 en face planes in the volumetric data. Red colour means flow into the disk and blue colour means flow out of the disk. The red lines indicated the detected boundaries of veins. (D) The projection of all vein branches. Dashed line is the middle line of disc in order to classify veins as superior and inferior.
Then the flow measurements were corrected for magnification variation due to axial length differences of the eye by applying a correction factor of the axial length squared divided by the square of the standard axial length of 24 mm to the calculated TRBF. To compensate for pulsatility during the cardiac cycle, the blood flow measurements were averaged from all valid volumes from the three scans, each consisting of five volumes over 3 seconds. While it is possible that diastole can be a part of these volumes, the volumes would certainly include systole. Invalid volumes were excluded when motion artefacts interfered with vein identification or flow calculation. An eye was included if at least 8 of the 15 acquired volumes were considered reliable. Following this step, a manual grader validated and corrected the vein decision by referencing corresponding fundus photos.24
Statistical analysis
TRBF mean and population SD were calculated for the healthy, mild/moderate NPDR, severe NPDR and PDR groups. The generalised linear model (GLM) method25 was used to examine if a significant difference exists between groups. Trend test with GLM method was used to determine whether there is a linear trend for TRBF across disease severity groups. The non-parametric Mann-Whitney U test was used to compare TRBF in treatment-naïve anti-VEGF and PRP groups. Coefficient of variation (CV) was used to determine within-visit repeatability of all combined groups. Analysis of variance (ANOVA) test was used to assess the statistical difference between controls and diabetic patients in terms of age and the number gradable scans. Correlation between TRBF and age, MOPP, body mass index (BMI), LogMar visual acuity and MAP was measured using Spearman’s rank correlation coefficient.
RESULTS
Fifty eyes from 50 participants with diabetes and 21 eyes from 21 healthy participants were included. No statistically significant difference was observed between healthy control subjects and patients with diabetes (p=0.86, ANOVA). Ten eyes were excluded by the grader because they did not meet the minimum required 8/15 gradable volumes due to motion artefacts in those volumes interfering with vein identification: four from the PDR group, one from the severe NPDR, one from the mild/moderate NPDR and four from the healthy group. There was no significant difference in the number of gradable (included) volumes between groups (p>0.1, ANOVA). The within-visit repeatability of scans was 4.1% (CV).
The characteristics of participants and eyes from each study group are represented in table 1. Participants with PRP received treatment at an average of 17 months prior to scan (range 1–65 months). Participants with anti-VEGF received monthly injections starting an average of 21 months prior to the scan (range 2–46 months). The average time between the last injection and the scan was 1.4±1.4 months (range 1–6 months). In the healthy group, there was no significant correlation between TRBF and any of the following: age, MOPP, BMI or MAP. There was significant correlation between disease severity and LogMar visual acuity (Spearman’s r=0.439, p<0.001).
Table 1.
Characteristics of participants and eyes included
| Non-proliferative | ||||
|---|---|---|---|---|
| Healthy | Mild/Moderate | Severe | Proliferative | |
| Participants | 21 | 17 | 15 | 18 |
| Age | 56.4±12.2* | 59.5±8.8* | 59.1±11.6* | 49.7±13.6* |
| Gender | ||||
| Male | 5 | 7 | 8 | 10 |
| Female | 16 | 10 | 7 | 8 |
| Height, m | 1.64±0.09 | 1.69±0.10 | 1.68±0.12 | 1.69±0.10 |
| Weight, kg | 80.6±27.3 | 97.6±22.9 | 89.6±15.7 | 96.0±27.6 |
| Body mass index, kg/m2 | 29.4±8.2 | 33.9±6.1 | 31.8±5.5 | 34.0±11.4 |
| Diabetes present, % | 0.0 | 100.0 | 100.0 | 100.0 |
| Positive history of hypertension, % | 0.0 | 64.7 | 86.7 | 50.0 |
| Systolic blood pressure, mm Hg | 123.1±18.9 | 128.8±16.0 | 135.0±24.0 | 126.4±23.6 |
| Diastolic blood pressure, mm Hg | 76.5±12.2 | 69.3±13.7 | 70.7±15.0 | 73.2±11.4 |
| Mean arterial pressure, mm Hg | 92.0±13.8 | 89.1±13.0 | 92.2±15.8 | 90.9±13.5 |
| Eyes | 21 | 17 | 15 | 18 |
| LogMar | -0.03±0.05 | 0.08±0.14 | 0.19±0.19 | 0.11±0.12 |
| MOPP, mm Hg | 46.4±8.1 | 44.3±12.9 | 48.3±10.3 | 45.4±9.9 |
| ETDRS score | 89±3.6 | 81.0±7.8 | 75.3±9.2 | 79.2±7.0 |
| Intraocular pressure | 15.0±2.4 | 12.4±2.4 | 13.1±2.9 | 15.2±4.2 |
| Axial length, mm | 23.9±1.0 | 23.7±1.0 | 23.7±1.4 | 23.4±1.1 |
| Positive history of treatment | ||||
| Panretinal photocoagulation | N/A | 0 | 1 | 9 |
| Anti-VEGF | N/A | 2 | 8 | 6 |
| Treatment naïve | N/A | 15 | 6 | 7 |
p=0.86, analysis of variance.
Values are equal to n, mean±SD or percentage.
MOPP, mean ocular perfusion pressure; VEGF, vascular endothelial growth factor; N/A: not applicable.
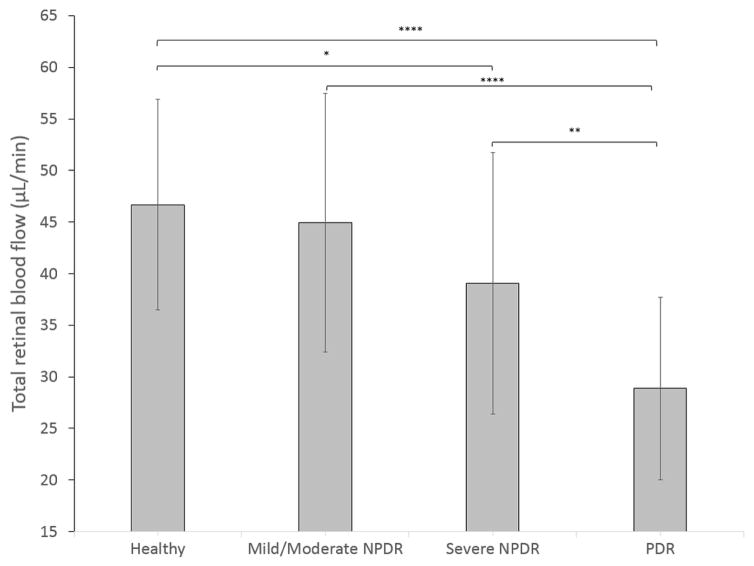
The TRBF (mean±SD) for each group and the statistical difference between them are shown in table 2. A linear trend test with GLM demonstrates that TRBF was linearly correlated with increasing DR disease severity (p<0.0001). TRBF in healthy eyes was higher than the severe NPDR and PDR group. Furthermore, mild/moderate NPDR and severe NPDR had higher TRBF than PDR (figure 2).
Table 2.
TRBF of each group and matrix comparing TRBF between each group
| p Value | ||||
|---|---|---|---|---|
| TRBF (mean±SD, μL/min) | Mild/moderate NPDR | Severe NPDR | PDR | |
| Healthy | 46.7±10.2 | 0.6 | 0.04* | <0.0001* |
| Mild/moderate NPDR | 44.9±12.6 | 0.14 | <0.0001* | |
| Severe NPDR | 39.1±12.6 | 0.01* | ||
| PDR | 28.9±8.8 | |||
p Values are based on generalised linear model.
Difference in TRBF is statistically significant.
NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; TRBF, total retinal blood flow.
Figure 2.
Column chart of total retinal blood flow of control group and diabetic retinopathy severity groups. NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. *p<0.05, **p≤0.01, ****p≤0.0001, based on generalised linear model.
The effect of anti-VEGF treatment on TRBF in DR eyes was examined. There were 28 treatment-naïve eyes (15 with mild/moderate NPDR, 6 with severe NPDR and 7 with PDR) and 16 anti-VEGF-treated eyes (two with mild/moderate NPDR, eight with severe NPDR and six with PDR) without history of PRP. The mean TRBF of treatment-naïve and anti-VEGF groups was 39.5±13.6 μL/min and 37.0±10.8 μL/min, respectively (p=0.36, Mann-Whitney U test). The effect of PRP on TRBF was also examined within the severe NPDR and PDR groups. Seven subjects were identified to have previously undergone PRP without history of anti-VEGF treatment. No significant difference in TRBF was observed between PRP (31.1±16.5 μL/min) and treatment-naïve (32.0±5.7 μL/min) groups (p=0.44, Mann-Whitney U test).
DISCUSSION
In this paper, we demonstrated that multiplane en face Doppler OCT using a commercially available spectral-domain OCT system can provide repeatable TRBF measurements in healthy eyes and eyes with DR. The within-visit repeatability from this study (4.1% CV) was better than previous reports on TRBF measurement of DR using Doppler OCT (10.2%,26 7.5%–9.2%).27,28 The observed TRBF values were in the same range as the previously reported values from Doppler OCT and laser Doppler studies measuring total retinal blood flow.14,15,29–33 We did not find a relationship between age and TRBF.
In diabetic eyes, there was a trend towards decreasing TRBF with increasing severity of the disease. There was, however, little difference in TRBF between control and the mild-to-moderate NPDR group. This could be explained by the fact that multiple factors influence the TRBF in DR. Capillary occlusion in DR is expected to increase resistance. Therefore, given the same perfusion pressure, the flow is expected to be decreased. However, a proposed mechanism of DR is an increased perfusion due to hypoxia and disordered autoregulation leading to relative hyper-perfusion.34–37 Previous studies of retinal blood flow in DR have supported both of these factors in pathophysiology. In the mild-to-moderate NPDR, it is possible that these opposing influences on TRBF cancel each other out, resulting in values similar to healthy eyes. This is in contrast to a report by Tayyari et al that TRBF of mild-to-moderate NPDR was significantly lower than control eyes using a different approach with Doppler OCT.38 This may be due to the fact that both studies have relatively few subjects and mild-to-moderate NPDR could represent a relatively wide range of disease, including ETDRS levels 20, 35 and 43. Other studies using laser Doppler, however, also found no significant decrease in flow in the early stages of DR, agreeing with our results.9,39
With more severe DR, this study showed that TRBF was significantly reduced compared with control or early NPDR, consistent with previous reports of TRBF in PDR using Doppler OCT.14,31 However, laser Doppler studies have reported both decreased and increased retinal blood flow in PDR.4,6,7,10 Again, this may be due to differences in the specific patient characteristics in the severe retinopathy group between studies. In our study, the majority of patients in the PDR group had received PRP. Previous studies have shown that eyes with PRP have lower retinal blood flow compared with untreated patients with PDR.40,41 However, in the current study, TRBF of eyes with PRP and untreated eyes were statistically equivalent within the more severe cohort of patients. This might be partially explained by the relatively small number of cases in each subgroup, as well as the cross-sectional design of this study. Moreover, the clinical response to PRP was found to be affected with different confounding factors, including the degree of glycaemic control,42 which was not assessed in the current study. Lee et al27 reported no significant difference in TRBF following PRP in a cohort of poorly controlled patients with PDR. Accordingly, the current study cannot provide conclusive results regarding the effect of PRP on retinal blood flow in patients with DR.
Anti-VEGF treatments for diabetic macular oedema have also been associated with decreased blood flow measured by laser Doppler.43 In our study, the TRBF of eyes that received anti-VEGF treatments compared with treatment naïve eyes were not significantly different. This, however, is a cross-sectional observation with relatively few eyes in each severity level. Furthermore, anti-VEGF treatments may influence the level of retinopathy observed,44 further confounding the analysis. For these reasons, it is difficult to draw conclusions about the effect of anti-VEGF injections on TRBF based on this study.
The diabetic groups were mixed of treated (anti-VEGF and PRP) and treatment-naïve patients, particularly the severe NPDR and PDR, for whom significant decrease of TRBF was observed. There is a possibility that the reduction in TRBF is caused by the treatment rather than the disease. However, in our study, no significant difference in TRBF was found between PRP and anti-VEGF groups and the treatment-naïve eyes in patients with DR. Thus, we believe the decreased TRBF is more likely to be caused by the disease itself, not the treatment.
These conflicting results reflect the complex relationship between TRBF, pathophysiology of DR and the effects of common treatments in DR. They also reflect the reality that DR is not a linear disease with degree of capillary damage resulting in a predictable clinical manifestation. In addition to non-perfusion, other factors such as haemodynamics of larger vessels, as well as genetic and cellular factors can influence disease progression, clinical manifestation and blood flow measurements. It is therefore unlikely that TRBF measurements solely could identify the full spectrum of retinopathy severity with reasonable sensitivity and specificity.
This study was limited by small number of participants and a cross-sectional design. Another potential limitation of the study is that we did not measure plasma glucose levels. Experimental models have shown that bolus hyperglycaemia can increase retinal blood flow.45 Although it is unclear if variations in glucose levels in patients with diabetes would have a similar effect, the glucose level may represent yet another confounder in the analysis of TRBF.
It is clear that retinal haemodynamics play an important pathogenic role in DR and studies to date may be inconclusive due to conflicting and non-repeatable results. A reliable method of measuring TRBF, such as one reported here, applied to a larger number of DR subjects and a strict control on potential confounding factors such as history of anti-VEGF treatment and PRP could help settle long-running controversies about the relationship between DR and alterations in TRBF. Paired with other biomarkers, such as total retinal non-perfusion area, TRBF could help elucidate the respective role of non-perfusion and disordered autoregulation in the development of DR. A longitudinal design of study could further clarify the role of retinal haemodynamics in DR.
Acknowledgments
Funding This work was supported by grant DP3 DK104397, R01 EY024544, P30 EY010572 from the National Institutes of Health (Bethesda, Maryland) and by unrestricted departmental funding from Research to Prevent Blindness (New York, New York).
Footnotes
Contributors Study conception and design were done by ADP, YJ, TSH, DH, OT, DJW and MP. OT and YJ did software development. Data processing was done by ADP, AMH and LL. Data collection and statistical analysis were done by ADP, AMH, LL, XZ and MP. Writing of the manuscript was done by ADP, TSH and AMH. Figures and tables preparation were done by AMH and ADP. Editing and reviewing the manuscript were done by TSH, AMH, YJ and DH. Also, YJ and TSH were guarantors. All authors accepted the final version of the manuscript.
Competing interests Oregon Health & Science University (OHSU), OT, DH and YJ have a significant financial interest in Optovue, a company that may have a commercial interest in the results of this research and technology. OT and DH have a significant financial interest in Carl Zeiss Meditec. These potential conflicts of interest have been reviewed and managed by OHSU.
Ethics approval Institutional Review Board at Oregon Health and Science University.
Provenance and peer review Not commissioned; externally peer reviewed.
No commercial use is permitted unless otherwise expressly granted.
References
- 1.Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye. 2009;23:1496–508. doi: 10.1038/eye.2009.108. [DOI] [PubMed] [Google Scholar]
- 2.Antonetti DA, Barber AJ, Bronson SK, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–11. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 3.Fallon TJ, Chowiencyzk P, Kohner EM. Measurement of retinal blood flow in diabetes by the blue-light entoptic phenomenon. Br J Ophthalmol. 1986;70:43–6. doi: 10.1136/bjo.70.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel V, Rassam S, Newsom R, et al. Retinal blood flow in diabetic retinopathy. BMJ. 1992;305:678–83. doi: 10.1136/bmj.305.6855.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagaoka T, Sato E, Takahashi A, et al. Impaired retinal circulation in patients with type diabetes mellitus: retinal laser Doppler velocimetry study. Invest Ophthalmol Vis Sci. 2010;51:6729–34. doi: 10.1167/iovs.10-5364. [DOI] [PubMed] [Google Scholar]
- 6.Grunwald JE, Riva CE, Sinclair SH, et al. Laser Doppler velocimetry study of retinal circulation in diabetes mellitus. Arch Ophthalmol. 1986;104:991–6. doi: 10.1001/archopht.1986.01050190049038. [DOI] [PubMed] [Google Scholar]
- 7.Cuypers MH, Kasanardjo JS, Polak BC. Retinal blood flow changes in diabetic retinopathy measured with the Heidelberg scanning laser Doppler flowmeter. Graefes Arch Clin Exp Ophthalmol. 2000;238:935–41. doi: 10.1007/s004170000207. [DOI] [PubMed] [Google Scholar]
- 8.Pemp B, Schmetterer L. Ocular blood flow in diabetes and age-related macular degeneration. Can J Ophthalmol. 2008;43:295–301. doi: 10.3129/i08-049. [DOI] [PubMed] [Google Scholar]
- 9.Blair NP, Feke GT, Morales-Stoppello J, et al. Prolongation of the retinal mean circulation time in diabetes. Arch Ophthalmol. 1982;100:764–8. doi: 10.1001/archopht.1982.01030030768009. [DOI] [PubMed] [Google Scholar]
- 10.Goebel W, Lieb WE, Ho A, et al. Color Doppler imaging: a new technique to assess orbital blood flow in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 1995;36:864–70. [PubMed] [Google Scholar]
- 11.Pechauer AD, Huang D, Jia Y. Detecting blood flow response to stimulation of the Human Eye. Biomed Res Int. 2015;2015:1–14. doi: 10.1155/2015/121973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leitgeb R, Schmetterer L, Drexler W, et al. Real-time assessment of retinal blood flow with ultrafast acquisition by color Doppler Fourier domain optical coherence tomography. Opt Express. 2003;11:3116–21. doi: 10.1364/oe.11.003116. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Chen Z, Saxer C, et al. Phase-resolved optical coherence tomography and optical Doppler tomography for imaging blood flow in human skin with fast scanning speed and high velocity sensitivity. Opt Lett. 2000;25:114–6. doi: 10.1364/ol.25.000114. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Fawzi AA, Varma R, et al. Pilot study of optical coherence tomography measurement of retinal blood flow in retinal and optic nerve diseases. Invest Ophthalmol Vis Sci. 2011;52:840–5. doi: 10.1167/iovs.10-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan O, Liu G, Liang L, et al. En face Doppler total retinal blood flow measurement with 0 kHz spectral optical coherence tomography. J Biomed Opt. 2015;20:066004. doi: 10.1117/1.JBO.20.6.066004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasan VJ, Sakadzić S, Gorczynska I, et al. Quantitative cerebral blood flow with optical coherence tomography. Opt Express. 2010;18:2477–94. doi: 10.1364/OE.18.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumann B, Potsaid B, Kraus MF, et al. Total retinal blood flow measurement with ultrahigh speed swept source/Fourier domain OCT. Biomed Opt Express. 2011;2:1539–52. doi: 10.1364/BOE.2.001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi W, Baumann B, Liu JJ, et al. Measurement of pulsatile total blood flow in the human and rat retina with ultrahigh speed spectral/Fourier domain OCT. Biomed Opt Express. 2012;3:1047–61. doi: 10.1364/BOE.3.001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee B, Choi W, Liu JJ, et al. Cardiac-Gated en Face Doppler Measurement of retinal blood flow using Swept-Source Optical Coherence Tomography at 100,000 Axial scans per second. Invest Ophthalmol Vis Sci. 2015;56:2522–30. doi: 10.1167/iovs.14-16119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson CP, Ferris FL, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 21.Sehi M, Flanagan JG, Zeng L, et al. Relative change in diurnal mean ocular perfusion pressure: a risk factor for the diagnosis of primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005;46:561–7. doi: 10.1167/iovs.04-1033. [DOI] [PubMed] [Google Scholar]
- 22.Tokayer J, Jia Y, Dhalla AH, et al. Blood flow velocity quantification using split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Biomed Opt Express. 2013;4:1909–24. doi: 10.1364/BOE.4.001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20:4710–25. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pechauer AD, Tan O, Liu L, et al. Retinal Blood Flow Response to Hyperoxia Measured With En Face Doppler Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 2016 Oct;57:141–5. doi: 10.1167/iovs.15-18917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang K-Yee, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 26.Wang Y, Fawzi A, Tan O, et al. Retinal blood flow detection in diabetic patients by Doppler Fourier domain optical coherence tomography. Opt Express. 2009;17:4061–73. doi: 10.1364/oe.17.004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JC, Wong BJ, Tan O, et al. Pilot study of Doppler optical coherence tomography of retinal blood flow following laser photocoagulation in poorly controlled diabetic patients. Invest Ophthalmol Vis Sci. 2013;54:6104–11. doi: 10.1167/iovs.13-12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tayyari F, Yusof F, Vymyslicky M, et al. Variability and repeatability of quantitative, Fourier-domain optical coherence tomography Doppler blood flow in young and elderly healthy subjects. Invest Ophthalmol Vis Sci. 2014;55:7716–25. doi: 10.1167/iovs.14-14430. [DOI] [PubMed] [Google Scholar]
- 29.Dai C, Liu X, Zhang HF, et al. Absolute retinal blood flow measurement with a dual-beam Doppler optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:7998–8003. doi: 10.1167/iovs.13-12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Bower BA, Izatt JA, et al. In vivo total retinal blood flow measurement by fourier domain Doppler optical coherence tomography. J Biomed Opt. 2007;12:041215. doi: 10.1117/1.2772871. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Lu A, Gil-Flamer J, et al. Measurement of total blood flow in the normal human retina using Doppler Fourier-domain optical coherence tomography. Br J Ophthalmol. 2009;93:634–7. doi: 10.1136/bjo.2008.150276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grunwald JE, Brucker AJ, Grunwald SE, et al. Retinal hemodynamics in proliferative diabetic retinopathy. A laser Doppler velocimetry study. Invest Ophthalmol Vis Sci. 1993;34:66–71. [PubMed] [Google Scholar]
- 33.Grunwald JE, Riva CE, Baine J, et al. Total retinal volumetric blood flow rate in diabetic patients with poor glycemic control. Invest Ophthalmol Vis Sci. 1992;33:356–63. [PubMed] [Google Scholar]
- 34.Yoshida A, Feke GT, Morales-Stoppello J, et al. Retinal blood flow alterations during progression of diabetic retinopathy. Arch Ophthalmol. 1983;101:225–7. doi: 10.1001/archopht.1983.01040010227008. [DOI] [PubMed] [Google Scholar]
- 35.Clermont AC, Aiello LP, Mori F, et al. Vascular endothelial growth factor and severity of nonproliferative diabetic retinopathy mediate retinal hemodynamics in vivo: a potential role for vascular endothelial growth factor in the progression of nonproliferative diabetic retinopathy. Am J Ophthalmol. 1997;124:433–46. doi: 10.1016/s0002-9394(14)70860-8. [DOI] [PubMed] [Google Scholar]
- 36.Cunha-Vaz JG, Fonseca JR, Abreu JF. Vitreous fluorophotometry and retinal blood flow studies in proliferative retinopathy. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978;207:71–6. doi: 10.1007/BF00414303. [DOI] [PubMed] [Google Scholar]
- 37.Cunha-Vaz JG, Fonseca JR, de Abreu JR, et al. Studies on retinal blood flow. II. Diabetic retinopathy. Arch Ophthalmol. 1978;96:809–11. doi: 10.1001/archopht.1978.03910050415001. [DOI] [PubMed] [Google Scholar]
- 38.Tayyari F, Khuu LA, Flanagan JG, et al. Retinal blood flow and retinal blood Oxygen Saturation in Mild to moderate Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2015;56:6796–800. doi: 10.1167/iovs.15-17481. [DOI] [PubMed] [Google Scholar]
- 39.Lorenzi M, Feke GT, Cagliero E, et al. Retinal haemodynamics in individuals with well-controlled type 1 diabetes. Diabetologia. 2008;51:361–4. doi: 10.1007/s00125-007-0872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grunwald JE, Brucker AJ, Petrig BL, et al. Retinal blood flow regulation and the clinical response to panretinal photocoagulation in proliferative diabetic retinopathy. Ophthalmology. 1989;96:1518–22. doi: 10.1016/s0161-6420(89)32697-2. [DOI] [PubMed] [Google Scholar]
- 41.Grunwald JE, Riva CE, Brucker AJ, et al. Effect of panretinal photocoagulation on retinal blood flow in proliferative diabetic retinopathy. Ophthalmology. 1986;93:590–5. doi: 10.1016/s0161-6420(86)33691-1. [DOI] [PubMed] [Google Scholar]
- 42.Kotoula MG, Koukoulis GN, Zintzaras E, et al. Metabolic control of diabetes is associated with an improved response of diabetic retinopathy to panretinal photocoagulation. Diabetes Care. 2005;28:2454–7. doi: 10.2337/diacare.28.10.2454. [DOI] [PubMed] [Google Scholar]
- 43.Nitta F, Kunikata H, Aizawa N, et al. The effect of intravitreal bevacizumab on ocular blood flow in diabetic retinopathy and branch retinal vein occlusion as measured by laser speckle flowgraphy. Clin Ophthalmol. 2014;8:1119–27. doi: 10.2147/OPTH.S62022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ip MS, Domalpally A, Hopkins JJ, et al. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130:1145–52. doi: 10.1001/archophthalmol.2012.1043. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan PM, Davies GE, Caldwell G, et al. Retinal blood flow during hyperglycemia. A laser Doppler velocimetry study. Invest Ophthalmol Vis Sci. 1990;31:2041–5. [PubMed] [Google Scholar]