Abstract
Background
While surgery offers the best curative-intent treatment, many patients with biliary tract malignancies have poor long-term outcomes. We sought to apply a non-mixture cure model to calculate the cure fraction and the time to cure after surgery of patients with peri-hilar cholangiocarcinoma (PHCC) or gallbladder cancer (GBC).
Methods
Using the Extrahepatic Biliary Malignancy Consortium, 576 patients who underwent curative-intent surgery for gallbladder carcinoma or peri-hilar cholangiocarcinoma between 1998 and 2014 at 10 major hepatobiliary institutions were identified and included in the analysis. A non-mixture cure model was adopted to compare mortality after surgery to the mortality expected for the general population matched by sex and age.
Results
The median and 5-year overall survival (OS) were 1.9 years (IQR, 0.9–4.9) and 23.9 % (95 % CI, 19.6–28.6). Among all patients with PHCC or GBC, the probability of being cured after surgery was 14.5 % (95 % CI, 8.7–23.2); the time to cure was 9.7 years and the median survival of uncured patients was 1.8 years. Determinants of cure probabilities included lymph node metastasis and CA 19.9 level (p ≤ 0.05). The cure fraction for patients with a CA 19.9 < 50 U/ml and no lymph nodes metastases were 39.0 % versus only 5.1 % among patients with a CA 19.9 ≥ 50 who also had lymph node metastasis.
Conclusions
Examining an “all comer” cohort, <15 % of patients with PHCC or GBC could be considered cured after surgery. Factors such CA 19.9 level and lymph node metastasis independently predicted long-term outcome. Estimating the odds of statistical cure following surgery for biliary tract cancer can assist in decision-making as well as inform discussions around survivorship.
Introduction
Biliary tract malignancies are uncommon gastrointestinal diseases that include cholangiocarcinoma and gallbladder carcinoma. Cholangiocarcinoma originates from the neoplastic degeneration of cholangiocytes and can be classified according to its anatomical location as intra-hepatic, perihilar (PHCC) and distal [1]. Gallbladder carcinoma (GBC) is the most common biliary tract cancer with only 10 % of all patients presenting at a stage amenable to surgical resection, as most patients present with advanced disease [2]. The biology of GBC is still largely unknown, but it appears that the cancerous transformation of PHCC and GBC epithelial cells depends on similar oncogenic mutations [3, 4]. As such, several trials have combined PHCC and GBC when assessing response to therapeutic interventions [5–9]. An example of a previous study including both PHCC and GBC is proposed by Valle et al. [5] who reported a randomized clinical trial that included patients who had both cholangiocarcinoma and GBC [5]. This study noted a comparable overall survival (OS) and progression-free survival relative to receipt of cisplatin plus gemcitabine versus gemcitabine alone.
While surgery offers the best curative-intent treatment for patients with biliary tract cancers, many patients with biliary tract malignancies relapse after surgery and have poor long-term outcomes [10]. Specifically, following resection, the 5-year OS for patients with PHCC ranges from 20 to 42 %, while the 5-year OS for patients with GBC ranges from 10 to 85 % depending on disease stage [11–13]. In addition, administration of adjuvant chemotherapy generally has a modest effect [1]. As such, while some reports have investigated long-term survival among patients undergoing resection of PHCC or GBC [14–17], whether surgery can provide a “cure” for these patients have not been investigated [18]. Statistically, a patient can be considered “cured” when his/her mortality risk returns to the same level expected in the general population [19]. Cure models have been introduced in the statistical literature and more recently have been applied in the clinical literature. One recent study investigated the probability to be cured and the time to cure among patients following surgery for a wide variety of tumors, including mouth and pharynx, esophagus, lung and trachea, ovary, bladder, non-Hodgkin lymphoma and leukemia [20]. In a separate study, investigators evaluated the cure fraction of patients with liver and pancreatic cancers [21]. Our own group has also previously assessed the probability of being statistically cured from intrahepatic cholangiocarcinoma among a large cohort of patients undergoing curative-intent surgery [22]. However, the chance of statistical cure among patients with extrahepatic biliary tract cancers remains undefined.
The objective of the current study was to estimate the probability that patients with extrahepatic biliary tract cancers can achieve “cure” following curative-intent surgery. In particular, we sought to apply a non-mixture cure model to calculate the cure fraction and the time to cure after surgery of patients with PHCC or GBC using a large, multi-institutional national cohort.
Methods
Patient demographic and clinical data
The extrahepatic biliary malignancy consortium database included patients who underwent curative-intent surgery for an extrahepatic biliary malignancy from March 1998 to December 2014 at 10 major hepatobiliary institutions (Supplementary Materials). Only patients with a histologically confirmed diagnosis of PHCC or GBC were included in the analytic study cohort. Patients who underwent palliative surgery (i.e., cholecystectomy only, biliary drainage with hepatico-jejunostomy, etc.), aborted laparotomy (i.e., biopsy only, exploratory laparotomy with no bypass, etc.), as well as those patients who underwent an incomplete resection (i.e., R2—macroscopic residual tumor) were excluded. The Institutional Review Board of the participating institutions approved the study.
Standard demographic and clinicopathologic data were collected including age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, comorbidities, tumor-related signs and symptoms, type of surgery and tumor-specific characteristics. In particular, data were collected on presence of jaundice or ascites, Carbohydrate Antigen 19.9 (CA 19.9) levels, and preoperative biliary drainage. CA 19.9 level was evaluated before surgery in non-jaundice patients and after biliary drainage in jaundice patients. Tumor-specific characteristics included median tumor size, location, Bismuth type for PHCC, and presence of vascular or perineural invasion. Data on tumor stage were also collected according to 7th edition AJCC staging system for PHCC and GBC [23]. Data on treatment-related variables, such as type of surgery and receipt of lymphadenectomy were also collected. Resection margin and nodal status were ascertained based on final pathologic assessment. Data on type of surgery and on vascular resection were collected.
Data on intraoperative estimated blood loss (EBL), length of hospital stay (LOS), reoperation, 30-day perioperative complications and mortality were obtained [24]. Complications were categorized based on the Clavien-Dindo classification system, with grade I or II being minor complications and grade III or IV being major complications [25]. Date of last follow-up and vital status was collected for all patients. All missing values (except outcome variables including patient’s status, date of last follow-up, and date of death) were imputed using the mice R CRAN package. No variable/value was missing >10 %. The survival end-point for this analysis was the overall survival defined as the time interval between the date of surgery and the date of. Time was censored at the date of the last follow-up assessment for patients who were found to be alive.
Cure fraction model
Cure models were computed using the strsmix package [19] and are of the form
where π is the proportion cured and Su(t) is the survival function for the uncured individuals. [19] Or as described by Othus et al. [26] the cure models can be defined:
Using this approach, a group of patients may be considered “cured” of a specific disease when their observed hazard rate (excess and expected hazards combined) is equal to that of the general population and, consequently, these patients are just as likely to die as a member of the general population. Application of a cure model relies on the concept that statistical plausibility of cure can be fulfilled. Among patients who underwent resection for PHCC or GBC, a subset did experience a plateauing of their survival after a period of time (Fig. 1). For this reason, [26] we applied the non-mixture cure fraction model to identify the fraction of patients who can considered to be cured after liver resection for PHCC and GBC. [19, 20, 27] As previously described, the non-mixture cure model is a parametric cure model that estimates an asymptote for the survival function at the cure proportion. [22] Moreover, time to cure was assessed. Time to cure can be interpreted as the minimum time that a patient survives before a physician can determine the patient for possible presence of a cure. Cure models define cure as occurring when time tends to infinite; in the current study, time to cure was calculated assuming a 99 % level of confidence (alpha = 0.01). To implement the cure model, expected survival and the expected hazard of death among the general population were estimated using the data of population survival from United States life tables, matched by age, race and sex [28].
Fig. 1.
Overall survival (OS) of 576 patients who underwent resection for perihilar cholangiocarcinoma (PHCC) or gallbladder cancer (GBC) (dotted lines represent 95 % confidence intervals)
Results
A total of 576 patients who underwent hepatic resection for PHCC (n = 257, 44.5 %) or GBC (n = 320, 55.5 %) met the inclusion criteria and were included in the study cohort (Table 1).
Table 1.
Clinical and pathologic features of the study group (n = 576)
| Variable | N (%) |
|---|---|
| Age, years, median (IQR) | 66.5 (57.4–73.1) |
| Age, >65 years | 314 (54.5 %) |
| Female sex | 320 (55.5 %) |
| Non-caucasian race | 167 (28.9 %) |
| BMI | 26.1 (23.1–30.4) |
| ASA 1/ASA 2 | 157 (37.2 %) |
| NA | 151 |
| Comorbidities | 327 (56.7 %) |
| Jaundice, present | 246 (45.7 %) |
| NA | 39 |
| Ascites | 13 (2.5 %) |
| NA | 59 |
| CA 19-9, median (IQR) | 52 U/mL (15–236) |
| CA 19-9, < 50 | 280 (48.6 %) |
| Preoperative biliary drainage/stent, performed | 249 (43.8 %) |
| NA | 9 |
| Type of resection | |
| Bile duct resection only | 64 (12.2 %) |
| Cholecystectomy + Liver resection (4b + 5) + LN | 248 (47.1 %) |
| Bile duct resection + right hepatectomy | 116 (22.1 %) |
| Bile duct resection + left hepatectomy | 98 (18.6 %) |
| Other | 51 |
| Vascular resection, performed | 37 (6.4 %) |
| Portal vein resection, performed | 27 (4.7 %) |
| Hepatic artery resection, performed | 10 (1.7 %) |
| Portal vein embolization, performed | 18 (3.1 %) |
| EBL, ml, median (IQR) | 450 (200–800) |
| RBC transfusion, performed | 139 (26.3 %) |
| NA | 48 |
| Size, cm, median (IQR) | 2.8 (1.9–4.0) |
| Size, > 3 cm | 235 (40.8 %) |
| Margin status, R0 | 438 (76.2 %) |
| Grade, G1–G2 | 415 (72.1 %) |
| Bismuth classification* | |
| I | 28 (11.9 %) |
| II | 38 (16.2 %) |
| IIIa/IIIb | 111 (47.2 %) |
| IV | 58 (24.7 %) |
| NA | 22 |
| AJCC T stage PHCC* | |
| I–IIa–IIb | 165 (78.9 %) |
| III–IV | 44 (21.1 %) |
| NA | 48 |
| AJCC T stage gallbladder** | |
| Ia–Ib–II | 161 (53.7 %) |
| III–IV | 139 (46.3 %) |
| NA | 20 |
| Blumgart T stage | |
| I | 117 (50.2 %) |
| II | 42 (18.0 %) |
| III | 74 (31.8 %) |
| NA | 24 |
| Lymphovascular invasion, present | 174 (44.4 %) |
| NA | 185 |
| Perineural invasion, present | 271 (67.1 %) |
| NA | 173 |
| Lymph node metastasis, present | 238 (41.3 %) |
| Postoperative complication, present | 284 (53.2 %) |
| NA | 43 |
| Length of stay, days | 7 (5–13) |
| Patients status, dead | 350 (60.7 %) |
| 30-days mortality | 23 (4.2 %) |
| NA | 29 |
| Readmission | 117 (24.4 %) |
| NA | 97 |
| Neoadjuvant | |
| Chemotherapy | 23 (4.0 %) |
| Radiotherapy | 10 (1.8 %) |
| Adjuvant | |
| Chemotherapy | 239 (49.8 %) |
| Radiotherapy | 150 (32.4 %) |
NA not available
Classifications used only for Perihilar cholangiocarcinoma
Classifications used only for Gallbladder adenocarcinoma
Detailed demographic and clinicopathologic characteristics of the cohort were described in the Supplementary Materials.
Cure Fraction model
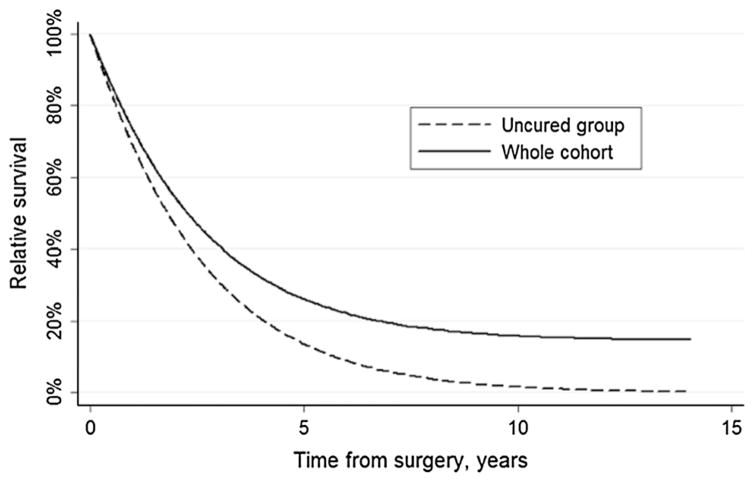
The median and 5-year overall survival (OS) were 1.9 years (IQR, 0.9–4.9) and 23.9 %, respectively (95 % Confidence Interval, 19.6–28.6; Fig. 1). The probability of being cured from PHCC or GBC by surgery was 14.5 % (95 % CI, 8.7–23.2); the time to cure was 9.7 years, and the median survival of uncured patients was 1.8 years (Table 2; Figs. 2 and 3). Cure fractions, time to cure, and median survival of uncured patients were stratified by clinical and tumor features (Table 2). Determinants of cure probabilities included preoperative CA 19.9 level, lymph node metastasis, and lymphovascular invasion (all p ≤ 0.05). After entering these variables into a multivariable cure model (Table 3), only CA 19.9 > 50 U/ml and lymph node metastasis remained as independent variables associated with cure. The cure fraction for patients with a CA 19.9 <50 U/ml and no lymph nodes metastases were 39.0 %. This value represented the highest achievable cure probability after resection for patients with PHCC or GBC. Among the 173 (30.1 %) patients with CA 19.9 < 50 U/ml and no lymph nodes metastases in the study cohort, time to cure was 4.1 years. Compared with patients who had CA 19.9 <50 U/ml and no lymph node metastasis, patients with CA 19.9 > 50 U/ml had a probability of being cured of 15.9 %, whereas patients with lymph nodes metastasis had a probability of being cured of 24.3 %. In the presence of both unfavorable prognostic factors (22.7 % of enrolled patients, n = 131), the cure fraction was 5.1 % and the time to cure was 6.8 years.
Table 2.
Univariate cure fraction calculation stratified by baseline characteristics
| Variable | Cure fraction % (95 % CI) | Time to cure years (95 % CI) | Median survival of uncured years (95 % CI) | p value |
|---|---|---|---|---|
| Study population | 14.5 % (8.7–23.2) | 9.7 years (6.5–14.5) | 1.8 years (1.5–2.2) | – |
| Gender | 0.56 | |||
| Male | 9.4 % (2.6–28.8) | 12.9 years (6.2–26.9) | 1.9 years (1.5–2.6) | |
| Female | 17.4 % (10.5–27.6) | 8.2 years (5.3–12.8) | 1.7 years (1.4–2.1) | |
| Age | 0.31 | |||
| ≤65 | 15.3 % (8.0–27.2) | 10.1 (6.1–16.8) | 2.0 years (1.6–2.5) | |
| > 65 | 14.6 % (6.4–30.0) | 8.7 (4.4–16.9) | 1.6 years (1.2–2.1) | |
| Margins status | 0.98 | |||
| R0 | 18.7 % (10.9–30.0) | 9.5 years (5.9–15.2) | 2.1 years (1.7–2.6) | |
| R1 | 0.2 % (0.1–73.5) | 11.3 years (2.5–50.6) | 1.3 years (0.9–1.8) | |
| Positive nodes | <0.001 | |||
| No | 8.3 % (0.1–48.9) | 20.3 (6.6–62.7) | 2.8 years (1.9–4.2) | |
| Yes | 10.5 % (0.6–18.3) | 6.5 (4.7–8.9) | 1.3 years (1.1–1.6) | |
| Tumor size | 0.14 | |||
| ≤3 cm | 19.8 % (12.7–29.5) | 7.5 (5.2–10.8) | 1.8 years (1.5–2.2) | |
| > 3 cm | – | – | – | |
| Perineural invasion | 0.45 | |||
| No | – | – | – | |
| Yes | 6.1 % (0.2–15.7) | 10.3 years (6.5–16.3) | 1.5 years (1.3–1.8) | |
| Lympho-vascular Invasion | 0.003 | |||
| No | 7.8 % (0.1–45.4) | 17.9 years (6.7–48.4) | 2.9 years (2.1–4.2) | |
| Yes | 14.8 % (0.9–22.6) | 5.6 years (4.1–7.7) | 1.2 years (1.0–1.4) | |
| Grade | 0.12 | |||
| G1–G2 | 13.6 % (6.4–26.5) | 11.7 years (6.7–20.3) | 2.1 years (1.7–2.6) | |
| G3–G4 | 13.6 % (6.6–25.8) | 6.9 years (4.2–11.1) | 1.4 years (1.1–1.8) | |
| CA 19.9 | 0.056 | |||
| ≤50 | 21.1 % (10.2–38.8) | 11.4 years (5.7–23.0) | 2.6 years (1.8–3.5) | |
| > 50 | 6.2 % (10.1–21.9) | 9.5 years (4.8–18.5) | 1.5 years (1.2–1.8) | |
| Adjuvant chemotherapy | 0.59 | |||
| No | 6.7 % (0.1–43.4) | 21.2 years (6.1–73.2) | 1.8 years (1.2–2.7) | |
| Yes | 19.6 % (12.9–28.6) | 5.4 years (4.3–6.8) | 2.0 years (1.8–2.3) |
Fig. 2.
Cure model results. Relative survival of entire group of patients and uncured patients. In the entire group, from sixth year after surgery onward, survival curve plateaued at about 14.5 %, which represents the cure fraction
Fig. 3.
Excess hazard rate of death of the entire study group and of uncured patients
Table 3.
Multivariate cure model in relation to clinical and tumor features
| Variable | Coefficient (95 % CI) | p |
|---|---|---|
| Constant | 39.0 % (28.2–49.8) | |
| CA 19.9, >50 | −23.1 % (−33.2–−13.2) | <0.001 |
| Lymph node status, positive | −14.7 % (−24.7–−0.5) | 0.003 |
CI confidence interval
The excess hazard of death after surgery for an extra-hepatic biliary tract cancer was plotted in Fig. 3. The excess hazard started with a 25 % increased risk of death early after resection with respect to the general population. In the first postoperative year, the excess hazard increased to approximately 30 % in the entire group and was up to 90 % in uncured patients. At the end of the first year after surgery, the hazard of uncured patients decreased until 5 years after hepatic resection to about 68 % and then progressively increased over time, whereas the entire group showed a progressive reduction until it approached zero. The excess hazard of death in the entire group of patients with biliary tract cancers decreased until a 99 % level of confidence in the general population at 9.5 years after hepatic resection, indicating that after this time point, a patient alive without tumor recurrence could be considered cured with 99 % certainty.
Discussion
PHCC and GBC are commonly classified together as biliary tract cancers [29–31]. Several previous retrospective studies, as well as prospective randomized clinical trials, have investigated combined cohorts of PHCC and GBC [5–9]. While previous studies have described factors associated with prognosis among patients undergoing surgery for biliary tract cancers, none have explicitly examined the topic of cure. Increasingly, in addition to desiring information about risk of recurrence and prognosis, patients and physicians are interested in data regarding the chance of possible cure following surgical resection [22, 31, 32]. When considering treatment options, one of the most important discussions between cancer patients and healthcare providers involves the goal of any proposed therapy [33]. Goals of care may involve whether the objective of the proposed therapy is to alleviate symptoms, prolong life, or provide a reasonable chance at cure [32]. However, most analyses on long-term survival are problematic. Not only do most reports on prognosis only consider factors assessed at the time of surgery, but also most survival analyses have been performed using only Kaplan–Meier or Cox survival methods. These statistical approaches to assess survival are limited as they assume a proportional hazard of death over time and none explicitly model the chance of cure. The assumption of proportional hazards can fail when survival curves plateaus and survival plots can have significant heterogeneity within a patient population that are note fully explained or considered in standard Kaplan–Meier analyses [26]. In contrast, cure models allow for the investigation of the varied ways in which covariates may be associated with long-term outcomes. Additionally, cure models can explicitly model survival as a mixture of two types of patients: Those who are cured and those who are not cured [34, 35]. The current study is important because, for the first time, statistical cure modeling was applied to patients with extrahepatic biliary tract tumors such as PHCC and GBC. Of note, for “all comers,” the probability of being cured from PHCC or GBC was only 14.5 %. Furthermore, the time to cure was 9.7 years, indicating that long-term surveillance of patients with PHCC and GBC is needed to ensure that cure has been achieved.
As the cure model allows for the analysis of tumor and patient variables associated with cure, cure fraction, time to cure and survival of uncured patients were estimated for different clinical scenarios. Among the entire cohort, determinants of cure probabilities included elevated preoperative CA 19.9 level >50 U/ml and the presence of lymph node metastasis. Previous studies have similarly noted that elevated CA 19.9 levels, and lymph node metastases were independent predictors of outcome and were associated with worse long-term survival for patients with PHCC and GBC [15, 36, 37]. However, unlike previous studies, we assessed the effects of these risk factors on cure probability in various clinical scenarios. For example, while patients with a preoperative CA 19.9 <50 U/ml and no lymph nodes metastases had a cure fraction of 39 % and a time to cure of 4.1 years, patients who had a CA 19.9 > 50 U/ml and lymph nodes metastases had only a cure fraction of 5.1 % and a time to cure of 6.8 years. These data serve to emphasize the marked differences in both chances of cure and the time to assuming a 99 % level of cure confidence. In general, results reported herein for patients with extrahepatic biliary tract cancers were slightly better than the 10 % overall chance of cure for patients undergoing surgery for intrahepatic cholangiocarcinoma (ICC) [22]. Moreover, when patients with ICC were compared to patients with PHCC or GBC, the cure probability was 25.8 versus 30.1 %, respectively, among patients with favorable prognostic factors. Furthermore, the overall cure proportion following resection of PHCC or GBC was 14.5 %, which was comparable to the reported long-term survival of patients with colorectal peritoneal carcinomatosis (16 %) [38], R1 resection of colorectal liver metastasis (18 %) [39], yet higher than the probability of being cured after surgery for other malignancies, such as breast cancer (48 %) [40].
Unlike most survival analyses that assume a constant hazard of death over time, survival cure modeling can account for changes in excess hazard of death as patient time is accrued. Data in Fig. 3 demonstrate how the hazard of death following surgery for PHCC and GBC varied over time. Specifically, in the first year following surgery, the excess hazard of death among the entire cohort increased to approximately 30 % and then progressively decreased. Of note, among the uncured patient mix, the hazard of death was roughly 90 % at 1 year, decreased to 68 % at 5 years, but then subsequently increased thereafter over time. These data suggest that among patients with PHCC and GBC who are not cured that the risk of death is bi-modal with the high risk of death in the first year and then later likely due to tumor recurrence. In contrast, the model suggested that a patient who was alive and disease-free at 9.5 years could be considered cured with 99 % certainty. These data on cure probability and odds of cure over time may assist the physicians in decision-making around the need for long-term surveillance, as well as help patients understand their chance to be cured after surgery.
Several limitations should be considered when interpreting the results. Given the retrospective design of the study, there may have been selection bias in how patients were chosen for surgical resection based upon tumor characteristics. In addition, patients with PHCC and GBC were combined for purposes of analyses to increase cohort size and statistical power. While other retrospective and prospective studies have similarly combined PHCC and GBC patients, the cure probability for these cohorts of patients may differ [5–9]. For this reason, future studies are needed to assess the independent specific cure probabilities of PHCC and GBC patients separately. In addition, while the statistical cure model included patients with all stages of disease, only a small subset of patients (n = 52) had stage I disease. As such, data from the current study are most applicable to patients with more advanced biliary tract cancers, which indeed constitute the majority of patients. Finally, overall survival rather than disease-free survival was utilized in the cure model. For diseases such as recurrent PHCC and recurrent GBC, the case-fatality rate is, however, extremely high (no effective salvage therapy). In instances where prevalence of recurrence virtually equates to the prevalence of death from disease, the use of overall survival is generally acceptable when assessing long-term survival outcomes.
In conclusion, less than the 15 % of patients with PHCC and GBC can be considered cured after surgery. Factors such CA 19.9 level and lymph node metastasis independently predicted long-term outcome. In fact, the chance of statistical cure among patients with both versus neither adverse factor was 5.1 versus 30.1 %, respectively. In turn, the current study provides data for patients and physicians on the odds of statistical cure following surgery for biliary tract cancer and therefore can assist in decision-making as well as inform discussions around survivorship.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00268-016-3691-y) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest None.
References
- 1.Valero V, 3rd, et al. Management of perihilar cholangiocarcinoma in the era of multimodal therapy. Expert Rev Gastroenterol Hepatol. 2012;6(4):481–495. doi: 10.1586/egh.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanthan R, et al. Gallbladder cancer in the 21st century. J Oncol. 2015;2015:967472. doi: 10.1155/2015/967472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansel DE, et al. Identification of novel cellular targets in biliary tract cancers using global gene expression technology. Am J Pathol. 2003;163(1):217–229. doi: 10.1016/S0002-9440(10)63645-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tannapfel A, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52(5):706–712. doi: 10.1136/gut.52.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valle J, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 6.Friend E, et al. Development of a questionnaire (EORTC module) to measure quality of life in patients with cholangiocarcinoma and gallbladder cancer, the EORTC QLQ-BIL21. Br J Cancer. 2011;104(4):587–592. doi: 10.1038/sj.bjc.6606086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Khoueiry AB, et al. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs. 2012;30(4):1646–1651. doi: 10.1007/s10637-011-9719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Khoueiry AB, et al. S0941: a phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br J Cancer. 2014;110(4):882–887. doi: 10.1038/bjc.2013.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Josef E, et al. SWOG S0809: a Phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol. 2015;33(24):2617–2622. doi: 10.1200/JCO.2014.60.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarnagin WR, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–517. doi: 10.1097/00000658-200110000-00010. (discussion 517–519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akamatsu N, Sugawara Y, Hashimoto D. Surgical strategy for bile duct cancer: advances and current limitations. World J Clin Oncol. 2011;2(2):94–107. doi: 10.5306/wjco.v2.i2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao K, et al. Patterns and prognostic value of lymph node dissection for resected perihilar cholangiocarcinoma. J Gastroenterol Hepatol. 2016;31(2):417–426. doi: 10.1111/jgh.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, et al. Evaluation of adjuvant radiation therapy for resected gallbladder carcinoma: a multi-institutional experience. Ann Surg Oncol. 2015;22(Suppl 3):1100–1106. doi: 10.1245/s10434-015-4685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hueman MT, Vollmer CM, Jr, Pawlik TM. Evolving treatment strategies for gallbladder cancer. Ann Surg Oncol. 2009;16(8):2101–2115. doi: 10.1245/s10434-009-0538-x. [DOI] [PubMed] [Google Scholar]
- 15.Amini N, et al. Lymph node status after resection for gallbladder adenocarcinoma: prognostic implications of different nodal staging/scoring systems. J Surg Oncol. 2015;111(3):299–305. doi: 10.1002/jso.23813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss MJ, et al. Multimodal treatment strategies for advanced hilar cholangiocarcinoma. Langenbecks Arch Surg. 2014;399(6):679–692. doi: 10.1007/s00423-014-1219-1. [DOI] [PubMed] [Google Scholar]
- 17.Poruk KE, Pawlik TM, Weiss MJ. Perioperative management of hilar cholangiocarcinoma. J Gastrointest Surg. 2015;19(10):1889–1899. doi: 10.1007/s11605-015-2854-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maetani S, Gamel JW. Evolution of cancer survival analysis. Surg Oncol. 2010;19(2):49–51. doi: 10.1016/j.suronc.2010.03.002. (discussion 61) [DOI] [PubMed] [Google Scholar]
- 19.Lambert PC, et al. Estimating and modeling the cure fraction in population-based cancer survival analysis. Biostatistics. 2007;8(3):576–594. doi: 10.1093/biostatistics/kxl030. [DOI] [PubMed] [Google Scholar]
- 20.Cvancarova M, et al. Proportion cured models applied to 23 cancer sites in Norway. Int J Cancer. 2013;132(7):1700–1710. doi: 10.1002/ijc.27802. [DOI] [PubMed] [Google Scholar]
- 21.Dal Maso L, et al. Long-term survival, prevalence, and cure of cancer: a population-based estimation for 818 902 Italian patients and 26 cancer types. Ann Oncol. 2014;25(11):2251–2260. doi: 10.1093/annonc/mdu383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spolverato G, et al. Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer. 2015;121(22):3998–4006. doi: 10.1002/cncr.29619. [DOI] [PubMed] [Google Scholar]
- 23.Edge SB, Byrd D, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7. Springer; New York: 2010. [Google Scholar]
- 24.Mayo SC, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB (Oxford) 2011;13(7):473–482. doi: 10.1111/j.1477-2574.2011.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Othus M, et al. Cure models as a useful statistical tool for analyzing survival. Clin Cancer Res. 2012;18(14):3731–3736. doi: 10.1158/1078-0432.CCR-11-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsodikov AD, Ibrahim JG, Yakovlev AY. Estimating cure rates from survival data: an alternative to two-component mixture models. J Am Stat Assoc. 2003;98(464):1063–1078. doi: 10.1198/01622145030000001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/NVSR/62_07/
- 29.Miyazaki M, et al. Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition. J Hepatobiliary Pancreat Sci. 2015;22(4):249–273. doi: 10.1002/jhbp.233. [DOI] [PubMed] [Google Scholar]
- 30.Chan E, Berlin J. Biliary tract cancers: understudied and poorly understood. J Clin Oncol. 2015;33(16):1845–1848. doi: 10.1200/JCO.2014.59.7591. [DOI] [PubMed] [Google Scholar]
- 31.Park JO, et al. Gemcitabine plus cisplatin for advanced biliary tract cancer: a systematic review. Cancer Res Treat. 2015;47(3):343–361. doi: 10.4143/crt.2014.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim Y, et al. Patient perceptions regarding the likelihood of cure after surgical resection of lung and colorectal cancer. Cancer. 2015;121(20):3564–3573. doi: 10.1002/cncr.29530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawlik TM, et al. What are patients’ expectations about the effects of chemotherapy for advanced cancer? J Am Coll Surg. 2014;219(3):588–590. doi: 10.1016/j.jamcollsurg.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Cai C, et al. smcure: an R-package for estimating semi-parametric mixture cure models. Comput Methods Progr Biomed. 2012;108(3):1255–1260. doi: 10.1016/j.cmpb.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Angelis R, et al. Mixture models for cancer survival analysis: application to population-based data with covariates. Stat Med. 1999;18(4):441–454. doi: 10.1002/(sici)1097-0258(19990228)18:4<441::aid-sim23>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 36.Cai WK, et al. Preoperative serum CA19-9 levels is an independent prognostic factor in patients with resected hilar cholangiocarcinoma. Int J Clin Exp Pathol. 2014;7(11):7890–7898. [PMC free article] [PubMed] [Google Scholar]
- 37.Goetze TO. Gallbladder carcinoma: prognostic factors and therapeutic options. World J Gastroenterol. 2015;21(43):12211–12217. doi: 10.3748/wjg.v21.i43.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goere D, et al. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013;257(6):1065–1071. doi: 10.1097/SLA.0b013e31827e9289. [DOI] [PubMed] [Google Scholar]
- 39.Hosokawa I, et al. Long-term survival benefit and potential for cure after R1 resection for colorectal liver metastases. Ann Surg Oncol. 2016;23(6):1897–1905. doi: 10.1245/s10434-015-5060-8. [DOI] [PubMed] [Google Scholar]
- 40.Rama R, Swaminathan R, Venkatesan P. Cure models for estimating hospital-based breast cancer survival. Asian Pac J Cancer Prev. 2010;11(2):387–391. [PubMed] [Google Scholar]