Summary
Objective
Convulsive status epilepticus can exert profound cardiovascular effects in adults, including ventricular depolarization–repolarization abnormalities. Whether status epilepticus adversely affects ventricular electrical properties in children is less understood. Therefore, we sought to characterize ventricular alterations and the associated clinical factors in children following convulsive status epilepticus.
Methods
We conducted a 2‐year retrospective case–control study. Children between 1 month and 21 years of age were included if they were admitted to the pediatric intensive care unit with primary diagnosis of convulsive status epilepticus and had 12‐lead electrocardiogram (ECG) within 24 h of admission. Children with heart disease or ion channelopathy, or who were on vasoactive medications were excluded. Age‐matched control subjects had no history of seizures or epilepsy. The primary outcome was ventricular abnormalities represented by ST segment changes, abnormal T wave, QRS axis deviation, and corrected QT (QTc) interval prolongation. The secondary outcomes included QT/RR relationship, beat‐to‐beat QTc interval variability, ECG interval measurement between groups, and clinical factors associated with ECG abnormalities.
Results
Of 317 eligible children, 59 met the inclusion criteria. History of epilepsy was present in 31 children (epileptic) and absent in 28 children (nonepileptic). Compared with the control subjects (n = 31), the status epilepticus groups were more likely to have an abnormal ECG, with overall odds ratios of 3.8 and 7.0 for the nonepileptic and the epileptic groups, respectively. Simple linear regression analysis demonstrated that children with epilepsy exhibited impaired dependence and adaptation of the QT interval on heart rate. Beat‐to‐beat QTc interval variability, a marker of ventricular repolarization instability, was increased in children with epilepsy.
Significance
Convulsive status epilepticus can adversely affect ventricular electrical properties and stability in children, especially those with epilepsy. These findings suggest that children with epilepsy may be particularly vulnerable to seizure‐induced arrhythmias. Therefore, postictal cardiac surveillance may be warranted in this population.
Keywords: Cardiac, Children, Electrocardiogram, Epilepsy, Status epilepticus
Key Points.
Convulsive status epilepticus was associated with increased likelihood of having postictal ventricular abnormalities on ECG in children
The fundamental relationship between QT interval and heart rate was altered following status epilepticus in children with epilepsy
Children with epilepsy had higher beat‐to‐beat QTc interval variability, suggesting increased ventricular repolarization instability
Therefore, children with epilepsy may be vulnerable to seizure‐induced ventricular arrhythmias
Cardiovascular alterations associated with seizures generally are considered to reflect autonomic nervous system activation that dissipates with seizure cessation.1, 2 However, persistent QRS axis deviation, corrected QT (QTc) interval prolongation, ST segment and T wave changes, and conduction abnormalities on electrocardiogram (ECG) have been described following convulsive status epilepticus in adults, indicating that the cardiac effects of a prolonged seizure episode may extend beyond seizure cessation.3 Similar postictal ECG abnormalities have been described in adult epilepsy patients following brief seizures,4, 5, 6, 7, 8, 9, 10 suggesting that they may be more susceptible to seizure‐induced cardiac effects. Supporting increased cardiac susceptibility to seizures are interictal cardiac alterations that reflect sympathetic predominance and abnormal ventricular repolarization,11, 12, 13, 14, 15, 16 which are risk factors for stimulation‐induced arrhythmias. Furthermore, fatal and near‐fatal cases of seizure‐associated ventricular tachycardia, ventricular fibrillation, and asystole17, 18, 19 highlight the possibility that increased ventricular excitability and instability may contribute to early mortality in adult epilepsy patients.
Although convulsive status epilepticus is a common pediatric neurological condition,20 whether prolonged seizures in healthy children can lead to persistent ventricular depolarization–repolarization abnormalities remains to be studied. In contrast, studies of pediatric epilepsy patients have revealed increases in QTc interval duration and spatial variability, as well as severe heart rate (HR) oscillations and premature ventricular beats following brief seizure episodes.21, 22 Together, these observations suggest that, similar to adult epilepsy patients, abnormal ventricular depolarization–repolarization and subsequent ventricular instability may accompany seizures in children with epilepsy.
The stability of the ventricular depolarization–repolarization cycle is highly dependent on the coupling of QT interval to RR interval.23 Decreased QT/RR coupling can manifest in impaired adaptation of the QT interval to the changing HR, and in increased beat‐to‐beat QTc interval variability. Both alterations have been observed in primary cardiac conditions that are at risk for developing ventricular arrhythmias,24, 25, 26, 27 and therefore may represent candidate biomarkers for increased arrhythmogenic potential. Whether status epilepticus can disturb QT/RR coupling that is fundamental to the stability of the ventricular depolarization–repolarization cycle is unknown.
In this study, we sought to investigate whether persistent ventricular depolarization–repolarization abnormalities occurred in children following convulsive status epilepticus, determined whether epilepsy was a contributing factor, and explored additional clinical factors that may be associated with ventricular abnormalities.
Methods
Patient selection
We conducted a retrospective, case–control study from February 2011 to February 2013 at the Texas Children's Hospital Pediatric Intensive Care Unit (PICU). The Baylor College of Medicine Institutional Review Board approved the study protocol with waiver of consent. Patients were screened using International Classification of Diseases, Ninth Edition codes for status epilepticus, seizure, or epilepsy. The medical records of potentially eligible subjects were subsequently reviewed to verify the diagnosis of status epilepticus according to the International League against Epilepsy guidelines.28 The inclusion criteria were: (1) primary admission diagnosis of convulsive status epilepticus, (2) age between 1 month and 21 years, and (3) 12‐lead ECG performed within 24 h of PICU admission. Patients were excluded if they met any of the following exclusion criteria: (1) use of vasoactive agents (inotropes, β‐blockers, and Ca2+ channel blockers) during PICU stay, (2) history of heart disease, or (3) history or family history of ion channelopathy. Eligible patients were further categorized into two groups: children without epilepsy (nonepileptic group) and children with epilepsy (epileptic group). Control patients were composed of a convenient sample from the same study period and were matched to the epilepsy cohort by age, had a 12‐lead ECG performed within 24 h of admission, and did not meet any of the exclusion criteria.
Data collection
A pediatric epileptologist (M.M.Q.) independently reviewed the medical records and assigned seizure semiology. A pediatric cardiologist (B.A.B.) blinded to the group assignment independently interpreted all ECG tracings. ST segment, T wave morphology, and QRS axis were classified as either normal or abnormal. Additional ECG data included HR and PR, QRS, and QT intervals. QTc interval was calculated using Bazett's formula.23 For each ECG recording, we measured 10 consecutive QT intervals with the corresponding RR intervals from lead II to assess QT/RR relationship and to calculate the beat‐to‐beat variability of QTc intervals. Clinical and laboratory data included age, gender, PICU length of stay (LOS), duration of the acute seizure episode, acute and chronic seizure semiology, antiepileptic drugs (AEDs) for acute seizure treatment, history of epilepsy, epilepsy duration, chronic AED regimen at the time of admission, and admission serum electrolytes, glucose, and blood gas values.
Primary and secondary outcomes
The primary outcomes were ventricular depolarization–repolarization abnormalities, represented by ST segment changes, abnormal T wave morphology, QRS axis deviation, and QTc interval prolongation. ST segment changes were defined as deviation > 1 mm from baseline of the ST segment that was not associated with early repolarization. The T wave was determined to be abnormal if the T wave axis was not normal for age or if T wave notching was present. An abnormal QRS axis was present if the frontal plane axis was outside the normal range for age. Prolonged QTc interval was defined as interval duration > 460 ms.29 Secondary outcomes included QT/RR relationship and beat‐to‐beat QTc interval variability to indicate the stability of ventricular depolarization–repolarization.25, 26 Additional secondary outcomes included comparisons of HR and PR, QRS, and QTc intervals between groups, and identification of factors associated with ECG abnormalities.
Statistical analysis
Continuous variables were analyzed using either Student t test or analysis of variance with post hoc Tukey test. Categorical variables were analyzed using either Fisher exact test or chi‐square test. QT/RR relationship was evaluated using simple linear regression analysis. Beat‐to‐beat QTc interval variability was quantified by short‐term variability (STV) and calculated using the following formula: STV = (∑|QTcn+1 − QTcn|)/(N × √2) where |QTcn+1 − QTcn| is the absolute difference between the two successive beats and N is the number of heartbeats. Statistical analyses were performed using GraphPad Prism 6 (La Jolla, CA, U.S.A.).
Results
Patient demographics
There were 4,681 PICU admissions during the study period, of which 422 children were admitted with a primary diagnosis of status epilepticus. All patients received continuous ECG monitoring during their PICU stay. Fifty‐nine of the 317 eligible children underwent standard 12‐lead ECG study within 24 h of admission. Of these 59 children, 28 children presented with seizure for the first time (nonepileptic groups) and 31 children had a history of epilepsy (epileptic groups; Fig. 1). There were no differences in age, gender, or PICU LOS between the control, nonepileptic, and epileptic groups (Table 1). The nonepileptic and epileptic groups needed a comparable number of AEDs to terminate status epilepticus and had similar seizure semiology, except that febrile status epilepticus was present only in the nonepileptic group (Table 1). All ECG studies were performed following the cessation of seizures. The time intervals between seizure cessation and ECG study were comparable between the nonepileptic and epileptic groups (Table 1). The admission diagnoses of the control patients were representative of our PICU population, with acute respiratory failure being the most common diagnosis (58%), followed by circulatory failure (16%). When comparing children admitted for status epilepticus who had an ECG performed with those who did not have an ECG performed, there were no significant differences in demographics, or clinical or laboratory data (Table S1).
Figure 1.
Case identification and group assignment. There were 4,681 Pediatric Intensive Care Unit (PICU) admissions during the study period, of which 422 children were admitted with a primary diagnosis of status epilepticus. Three hundred seventeen children were eligible, and 59 of these children met inclusion criteria. Twenty‐eight children presented with status epilepticus for the first time and constituted the nonepileptic group, whereas 31 children had a history of epilepsy. ECG, electrocardiogram.
Table 1.
Patient demographics
| Control, n = 31 | Nonepileptic, n = 28 | Epilepsy, n = 31 | p | |
|---|---|---|---|---|
| Age, mo | 79 ± 12 | 49 ± 9 | 71 ± 11 | NS |
| Gender, M/F | 20/11 | 21/7 | 18/13 | NS |
| PICU LOS, days | 3 [0.4–28] | 2 [0.5–18] | 1 [0.5–24] | NS |
| Seizure duration, min | NA | 30 ± 9a | 31 ± 11b | NS |
| Generalized tonic–clonic seizures | 14 (50%) | 18 (58%) | NS | |
| Febrile status epilepticus | NA | 5 (18%) | 0 (0%) | <0.05 |
| Number of acute AEDs | NA | 2 [1–3] | 2 [0–3] | NS |
| Fosphenytoin use | NA | 17 (61%) | 16 (52%) | NS |
| Interval between seizure cessation and ECG, h | NA | 5 [1.5–15] | 3 [1–4.8] | NS |
| ECG indication | ||||
| Seizure etiology evaluation | 0 | 11 (39%) | 5 (16%) | NS |
| Tachycardia | 6 (19%) | 1 (4%) | 2 (7%) | |
| Bradycardia | 2 (6%) | 1 (4%) | 1 (3%) | |
| Atrial tachycardia | 2 (6%) | 0 | 0 | |
| Arrhythmia | 4 (13%) | 4 (14%) | 6 (19%) | |
| Evaluate ST segment | 0 | 6 (21%) | 8 (26%) | |
| Evaluate QT interval | 1 (3%) | 2 (7%) | 4 (13%) | |
| Evaluate heart disease | 9 (29%) | 0 | 0 | |
| Chest pain | 3 (10%) | 0 | 0 | |
| Otherc | 4 (13%) | 3 (11%) | 5 (16%) |
Values are expressed as mean ± standard error of the mean, median [min–max], or n (%).
AED, antiepileptic drug; ECG, electrocardiogram; F, female; LOS, length of stay; M, male; NS, not significant; PICU, Pediatric Intensive Care Unit.
n = 22.
n = 25.
Other indications include syncope, murmur, hypertension, and hyperkalemia.
Abnormal ventricular repolarization following status epilepticus
Control, nonepileptic, and epileptic groups exhibited comparable prevalence of sinus tachycardia (control, 11; nonepileptic, 5; epileptic, 10) and sinus bradycardia (control, 1; nonepileptic, 1; epileptic, 3). We did not observe other types of arrhythmias in the study subjects. However, compared with the control group, children without a history of epilepsy exhibited an overall odds ratio (OR) of 3.8 (95% confidence interval [CI] = 1.3–11.5, p < 0.05) of having an abnormal ECG, and an OR of 6.0 (95% CI = 1.5–24.8, p < 0.05) for abnormal T wave morphology following status epilepticus (Table 2, Fig. 2A). Children with epilepsy exhibited an overall OR of 7.0 (95% CI = 2.3–21.5, p < 0.001) of having an abnormal ECG following status epilepticus (Table 2). Specifically, they exhibited an OR of 9.3 (95% CI = 2.6–33.3, p < 0.001) for ST segment changes, 4.4 (95% CI = 1.1–18.2, p < 0.05) for abnormal T wave morphology, and 10.3 (95% CI = 1.2–89.5, p < 0.05) for QRS axis deviation (Table 2, Fig. 2A). There was no increased OR for prolonged QTc interval in the status epilepticus patients when compared with the control patients. There were no statistically significant differences in HR and interval measurements between the control, nonepileptic, and epileptic groups (Table 3).
Table 2.
Primary outcomes by group assignment
| Control, n = 31 | Nonepileptic, n = 28 | OR [95% CI] | Epilepsy, n = 31 | OR [95% CI] | |
|---|---|---|---|---|---|
| Abnormal ECG | 8 (26%) | 12 (43%) | 3.8 [1.3–11.5]a | 22 (71%) | 7.0 [2.3–21.5]b |
| ST segment changes | 4 (13%) | 9 (32%) | 3.2 [0.9–11.9] | 18 (58%) | 9.3 [2.6–33.3]b |
| Abnormal T wave morphology | 3 (10%) | 11 (39%) | 6.0 [1.5–24.8]a | 10 (32%) | 4.4 [1.1–18.2]a |
| Abnormal QRS axis | 1 (3%) | 5 (18%) | 6.5 [0.7–44.6] | 8 (26%) | 10.3 [1.2–89.5]a |
| Prolonged QTc intervalc | 0 | 1 (4%) | 3.4 [0.13–87.9] | 4 (13%) | 10.3 [0.5–200.3] |
Values are expressed as relative risk [95% CI].
CI, confidence interval; ECG, electrocardiogram; OR, odds ratio; QTc, corrected QT.
ap < 0.05, bp < 0.001 versus control group.
cAll counts adjusted by 0.5 to allow estimation of RR.
Figure 2.
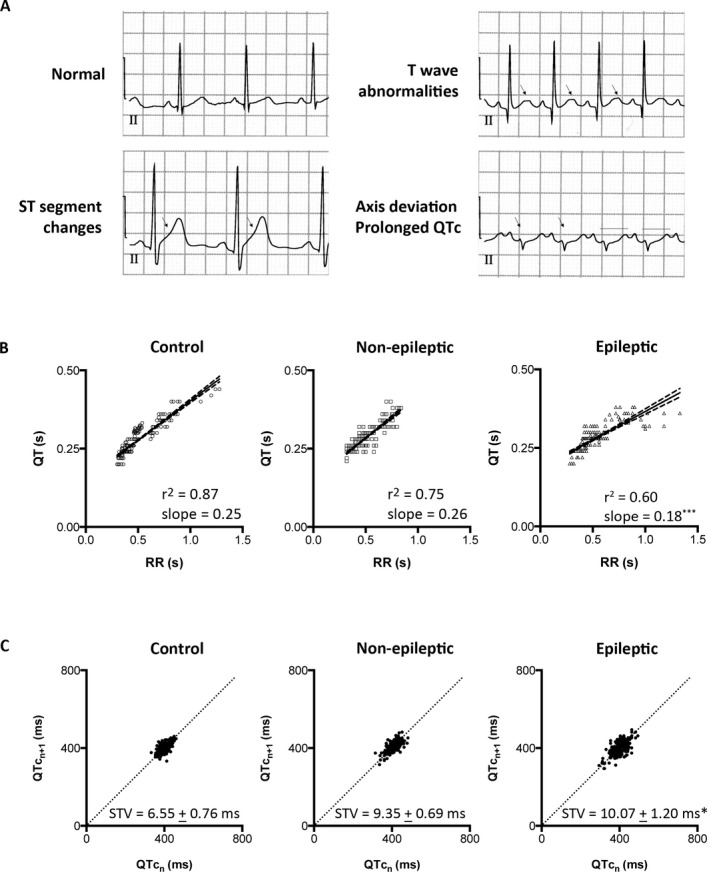
Status epilepticus–associated ventricular abnormalities and instability. (A) Sample lead II electrocardiogram tracings. Arrows indicate ST segment changes, abnormal T wave morphology, and QRS axis deviation. Lines indicate prolonged corrected QT (QTc). (B) Scatter diagrams of QT and the corresponding RR intervals from the control, nonepileptic, and epileptic groups. Linear regression (solid line) and the 95% confidence interval (dashed lines) demonstrate the best goodness‐of‐fit in the control group (r2 = 0.87). The nonepileptic group has decreased goodness‐of‐fit (r2 = 0.75), and the epileptic group has the least goodness‐of‐fit (r2 = 0.6). The epileptic group also exhibits a flatten slope as compared with the control group. (C) Poincaré plots of the QTc intervals from the control, nonepileptic, and epileptic groups. The control group had QTc values that center on the line of identity. The QTc values scatter around the line of identity in the nonepileptic and epileptic groups, with the most prominent dispersion in the epileptic group. Short‐term variability (STV) representing the mean orthogonal distance to the line of identity shows higher values in the nonepileptic and epileptic groups as compared with the control group. STV is presented as mean ± standard error of the mean. *p < 0.05, ***p < 0.001 versus control group.
Table 3.
Secondary outcomes and laboratory data by group assignment
| Control, n = 31 | Nonepileptic, n = 28 | Epilepsy, n = 31 | p | |
|---|---|---|---|---|
| HR, bpm | 132 ± 7 | 130 ± 5 | 124 ± 6 | NS |
| PR, ms | 115 ± 5 | 119 ± 4 | 123 ± 4 | NS |
| QRS, ms | 71 ± 4 | 79 ± 6 | 80 ± 10 | NS |
| QTc, ms | 414 ± 3 | 416 ± 3 | 412 ± 5 | NS |
| SaO2 | 97 ± 0.4 | 99 ± 0.5 | 98 ± 0.4 | NS |
| pH | 7.30 ± 0.02a | 7.30 ± 0.02b | 7.21 ± 0.04c | NS |
| pCO2, mmHg | 46 ± 4 | 49 ± 3 | 53 ± 4 | NS |
| HCO3, mEq/L | 22 ± 1 | 23 ± 1 | 21 ± 1 | NS |
| BE | −4.0 ± 1.1 | −3.3 ± 1.0 | −5.7 ± 1.2 | NS |
| Glucose, mg/dL | 143 ± 78 | 116 ± 47 | 131 ± 70 | NS |
| Na+, mmol/L | 138 ± 4.5 | 137 ± 3.0 | 139 ± 4.6 | NS |
| K+, mmol/L | 4.0 ± 0.7 | 4.0 ± 0.5 | 4.2 ± 0.7 | NS |
| Ca2+, mg/dL | 8.6 ± 0.3d | 9.2 ± 0.1e | 9.3 ± 0.1f , * | 0.03 |
| Mg+, mg/dL | 2.1 ± 0.3g | 2.0 ± 0.3h | 2.4 ± 1.6i | NS |
Values are expressed as mean ± standard error of the mean.
BE, base excess; HR, heart rate; NS, not significant; QTc, corrected QT; SaO2, oxygen saturation.
an = 25, bn = 24, cn = 20, dn = 27, en = 24, fn = 30, gn = 28, hn = 24, in = 29.
*p < 0.05 versus control, post hoc Tukey.
Decreased QT/RR coupling and increased ventricular repolarization instability following status epilepticus
Simple linear regression analysis revealed a strong relationship between the QT and RR interval in the control group, with an r2 = 0.87, reaffirming that RR represents a major determinant of QT interval (Fig. 2B). In contrast, we observed decreased QT/RR relationship in both status epilepticus groups, with the nonepileptic group having an r2 = 0.75, and the epileptic group having an r2 = 0.60 (Fig. 2B). In addition to exhibiting the weakest QT/RR relationship following status epilepticus, children with epilepsy demonstrated an altered QT interval adaptation to HR, reflected in a flattened QT/RR slope as compared with the control and nonepileptic groups (Fig. 2B).
To assess whether this weakened QT/RR relationship in the status epilepticus groups was associated with unstable ventricular depolarization–repolarization cycles, we examined the beat‐to‐beat variability of QTc interval. Under physiological conditions, consecutive QTc intervals are expected to remain relatively constant,24 which was observed in the control group. Poincaré plot of the nth QTc interval against the nth+1 QTc interval in the control group demonstrated QTc values clustering around the line of identity, indicating little beat‐to‐beat variability (Fig. 2C). In contrast, Poincaré plots demonstrated increased dispersion from the line of identity in the status epilepticus groups, indicating increased beat‐to‐beat variability, with the most prominent dispersion in the epileptic group (Fig. 2C). Increased beat‐to‐beat variability of the QTc interval can be quantified by STV representing deviation from the line of identity, which demonstrated a higher STV value in the epileptic group as compared with the control group (Fig. 2C).
Clinical factors associated with ECG abnormalities
There were no significant differences in oxygen saturation (SaO2) and blood gas values between groups (Table 3). Interestingly, although normal serum calcium levels were observed in all groups, children with epilepsy had statistically higher serum calcium levels compared with the control subjects (Table 3). Among the children with status epilepticus (nonepileptic and epileptic), the duration of status epilepticus was comparable between those with normal ECG and those with abnormal ECG (Table 4). Administration of fosphenytoin, benzodiazepines, levetiracetam, or phenobarbital acutely was not associated with an abnormal ECG in the seizure groups. We did not observe an association between chronic administrations of any AED and abnormal ECG in the epileptic group (Table S2). History of epilepsy was the only factor found to be associated with an abnormal ECG (Table 4).
Table 4.
Clinical factors of seizure patients by ECG findings
| Normal ECG, n = 25 | Abnormal ECG, n = 34 | p | |
|---|---|---|---|
| Gender, M/F | 16/9 | 23/11 | NS |
| Age, mo | 71 ± 58 | 52 ± 53 | NS |
| PICU LOS, days | 2 [0.8–9] | 2 [0.5–24] | NS |
| HR, bpm | 127 ± 30 | 126 ± 30 | NS |
| PR interval, ms | 121 ± 20 | 122 ± 20 | NS |
| QRS interval, ms | 80 ± 46 | 80 ± 46 | NS |
| QTc interval, ms | 414 ± 23 | 414 ± 23 | NS |
| pH | 7.29 ± 0.03 | 7.25 ± 0.03 | NS |
| pCO2, mmHg | 49 ± 3 | 53 ± 4 | NS |
| HCO3, mEq/L | 22 ± 1 | 23 ± 1 | NS |
| BE | −4.1 ± 0.9 | −4.7 ± 1.2 | NS |
| Electrolyte abnormality | 4 (16%)a | 11 (32%)b | NS |
| Serum Ca2+, mg/dL | 9.2 ± 0.2 | 9.2 ± 0.1 | NS |
| Seizure duration, min | 29 ± 6c | 38 ± 8d | NS |
| Number of acute AEDs | 2 [0–3] | 2 [0–3] | NS |
| Fosphenytoin | 16 (64%) | 19 (56%) | NS |
| Benzodiazepines | 22 (88%) | 24 (71%) | NS |
| Levetiracetam | 3 (12%) | 10 (29%) | NS |
| Phenobarbital | 3 (12%) | 1 (3%) | NS |
| History of epilepsy | 9 (36%) | 22 (65%) | 0.04 |
| Duration of epilepsy, mo | 16 [7.3–88.5]e | 16 [6.5–61]f | NS |
| Generalized tonic–clonic seizures | 6/9 (67%) | 15/22 (68%) | NS |
| Number of chronic AEDs | 1 [0–3] | 1 [0–5] | NS |
Values are expressed as mean ± standard error of the mean, median [min–max], or n (%).
AED, antiepileptic drug; BE, base excess; ECG, electrocardiogram; F, female; HR, heart rate; LOS, length of stay; M, male; NS, not significant; PICU, Pediatric Intensive Care Unit; QTc, corrected QT.
an = 21, bn = 29, cn = 18, dn = 31, en = 10, fn = 21.
Discussion
In this study, we found that convulsive status epilepticus is associated with higher likelihood of having postictal ECG abnormalities that reflect altered ventricular depolarization–repolarization in children without prior seizure history and in children with epilepsy. Cardiac alterations are well described in adult epilepsy individuals that include several interictal and peri‐ictal ventricular repolarization abnormalities.3, 4, 5, 6, 7, 8, 13, 16, 30 In contrast, few studies have reported interictal and peri‐ictal ventricular abnormalities in pediatric epilepsy patients.21, 22 We have observed ST segment changes, abnormal T wave morphology, and QRS axis deviation in the status epilepticus groups, indicating altered ventricular depolarization–repolarization activation sequence following seizures. The preponderance of abnormal ECG studies in the epilepsy cohort suggests that they may be more vulnerable to status epilepticus–induced cardiac electrical instability.
Furthermore, we found that status epilepticus was associated with diminished HR influence on the QT interval, and that the magnitude of QT interval changes in response to changes in RR intervals was significantly decreased in children with epilepsy. With diminishing HR influence, the adaptation of QT interval to HR may become unpredictable and possibly lead to increased risk for ventricular arrhythmias. Impaired adaptation of the QT interval to HR has been reported in individuals with symptomatic early repolarization and Brugada syndromes,25 in which ventricular arrhythmias and sudden cardiac death constitute a significant cause of mortality. Impaired adaptation of the QT interval to HR may also adversely affect its temporal stability. Accordingly, children in both status epilepticus groups exhibited higher STV values, with the epilepsy group having the highest STV values as compared with the control subjects. Increased STV occurs in individuals with congenital long QT syndrome and may serve as a risk biomarker for ventricular arrhythmias.24, 26, 27 Therefore, impaired adaptation of the QT interval to HR that is observed following status epilepticus may represent a candidate mechanism underlying labile ventricular repolarization and suggest an increased risk for ventricular arrhythmias in these children.
QTc interval prolongation and the occurrence of ventricular dysrhythmias have been associated with ictal hypoxia and respiratory acidosis.7 Using blood gas parameters as surrogate markers for compromised respiration, we found no statistically significant differences in our status epilepticus patients when comparing children with normal ECG and those with abnormal ECG. This finding suggests that persistently impaired respiration may not play a role in postictal ECG abnormalities in our subjects; however, we cannot exclude ictal respiratory derangement as a trigger or contributing factor.
Although clinical and experimental evidence suggest that status epilepticus may be sufficient to induce abnormal ventricular repolarization,3, 31 which is also supported by our observations in the nonepileptic cohort, our analyses indicate that the presence of epilepsy significantly increases the risk for abnormal ECG. Multiple mechanisms may contribute to epilepsy‐associated cardiac changes, including persistent cardiac sympathetic predominance,11, 12, 13, 14, 15, 16, 30 recurrent seizures with associated ventricular repolarization instability,4, 5, 6, 7 effects of multiple AEDs,32 and others. In this study, the use of fosphenytoin, which has inhibitory properties on the Na+ channels, was not an independent risk factor for abnormal ECG. Furthermore, we did not observe chronic or acute administration of any AED being associated with abnormal ECG in this study. Future studies with larger numbers of subjects are required to further delineate the contribution of these potential factors to the observed postictal ventricular electrical abnormalities in pediatric epilepsy.
Variations in the activation sequence of ventricular depolarization–repolarization represented by ST segment changes, abnormal T wave morphology, and QRS axis deviation are often associated with myocardial damage. Therefore, the observed ECG abnormalities may simply reflect cardiac injury resulting from status epilepticus. Adults with comorbid cardiovascular diseases are particularly at risk for status epilepticus–associated myocardial injury, whereas individuals with a healthy cardiovascular system have normal serum troponin levels following status epilepticus.33, 34, 35, 36, 37 Given that children generally have a healthy cardiovascular system, and we have excluded children with comorbid cardiovascular diseases from this study, it is likely that pathological processes such as altered cardiac electrophysiology may play a greater role in the observed ECG abnormalities in our subjects. In support of altered cardiac electrophysiology as a candidate mechanism, a subset of adults with refractory status epilepticus also has been reported to exhibit ECG abnormalities in the absence of clinically overt cardiac injury.35 The magnitude of the ventricular depolarization–repolarization abnormalities found in our study is often considered to be of indeterminate clinical significance. Although these subtle abnormalities have been indirectly correlated with risk for ventricular arrhythmias in patients with known underlying cardiac disease and in animal models, whether these findings confer the same cardiac risk in individuals with epilepsy is unknown at the present. However, the preponderance of these changes in the status epilepticus groups nevertheless suggests that seizures may adversely influence ventricular electrical properties.
Due to its retrospective of the study and the relatively low prevalence of eligible subjects with 12‐lead ECG (18.6%), ascertainment bias represents a potentially significant limitation of the study. As routine practice, all children were placed on cardiopulmonary monitoring that included continuous ECG in the PICU. For this study, we have chosen to examine those who underwent additional 12‐lead ECG study because it represents a gold standard diagnostic modality for cardiac evaluation. As expected, the most common reasons for obtaining a 12‐lead ECG in all groups were related to cardiac concerns. However, we did not observe any clinical differences in the status epilepticus groups between children who had ECG performed and those who did not, suggesting that children with additional 12‐lead ECG studies may be representative of the status epilepticus groups. Even assuming that our study groups represent a skewed sample, and the remaining children had normal ECG, the estimated prevalence of ECG abnormalities still would have been approximately 11% (34 of 317 eligible subjects) following status epilepticus. We have examined only one ECG recording per subject during their PICU stay. Therefore, we were unable to determine whether the observed ECG alterations represented a preexisting condition, or how long they persisted following seizures. Additionally, due to the small sample size, we were unable to perform multivariate analyses controlling for potential confounding factors. Another limitation relates to status epilepticus possibly resulting in the most severe seizure‐associated cardiac derangements; therefore, the applicability of our findings to the general population of children following seizures remains to be determined. Our study excluded children with a known ion channelopathy, to ascertain the effects of seizures on cardiac electrical properties. However, we cannot exclude the possibility that other genetic factors may predispose children to both development of epilepsy and altered ventricular repolarization.
Conclusion
We have observed multiple ventricular depolarization–repolarization abnormalities in children following convulsive status epilepticus, suggesting that prolonged seizures may have deleterious effects on the stability of ventricular electrical properties. Children with epilepsy exhibit the highest prevalence of ECG abnormalities, the least QT/RR coupling, and the most beat‐to‐beat QTc interval variability, suggesting that these children may be at the highest risk for ventricular instability. Future prospective studies examining temporal progression of these abnormalities will lend further insight into the evolution of cardiac remodeling and its potential impact in epilepsy.
Disclosure
The authors declare no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Table S1. Patient demographics and clinical factors of seizure patients by the availability of electrocardiogram.
Table S2. Comparisons of chronic anti‐epileptic drugs (AED) in children with epilepsy by ECG findings.
Acknowledgments
National Institutes of Health National Institute of Neurological Diseases and Stroke: K08NS063117 (Y.‐C.L.), R21NS077028 (A.E.A.); Emma Bursick Memorial Fund (Y.‐C.L.).
Biography
Dr. Wail Ali is an Assistant Professor in Pediatric Critical Care Medicine at West Virginia University.

References
- 1. Meierkord H, Shorvon S, Lightman SL. Plasma concentrations of prolactin, noradrenaline, vasopressin and oxytocin during and after a prolonged epileptic seizure. Acta Neurol Scand 1994;90:73–77. [DOI] [PubMed] [Google Scholar]
- 2. Simon RP, Aminoff MJ, Benowitz NL. Changes in plasma catecholamines after tonic‐clonic seizures. Neurology 1984;34:255–257. [DOI] [PubMed] [Google Scholar]
- 3. Boggs JG, Painter JA, DeLorenzo RJ. Analysis of electrocardiographic changes in status epilepticus. Epilepsy Res 1993;14:87–94. [DOI] [PubMed] [Google Scholar]
- 4. Brotherstone R, Blackhall B, McLellan A. Lengthening of corrected QT during epileptic seizures. Epilepsia 2010;51:221–232. [DOI] [PubMed] [Google Scholar]
- 5. Surges R, Adjei P, Kallis C, et al. Pathologic cardiac repolarization in pharmacoresistant epilepsy and its potential role in sudden unexpected death in epilepsy: a case‐control study. Epilepsia 2010;51:233–242. [DOI] [PubMed] [Google Scholar]
- 6. Surges R, Scott CA, Walker MC. Enhanced QT shortening and persistent tachycardia after generalized seizures. Neurology 2010;74:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seyal M, Pascual F, Lee CY, et al. Seizure‐related cardiac repolarization abnormalities are associated with ictal hypoxemia. Epilepsia 2011;52:2105–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nei M, Ho RT, Sperling MR. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia 2000;41:542–548. [DOI] [PubMed] [Google Scholar]
- 9. Tigaran S, Molgaard H, McClelland R, et al. Evidence of cardiac ischemia during seizures in drug refractory epilepsy patients. Neurology 2003;60:492–495. [DOI] [PubMed] [Google Scholar]
- 10. Tigaran S, Rasmussen V, Dam M, et al. ECG changes in epilepsy patients. Acta Neurol Scand 1997;96:72–75. [DOI] [PubMed] [Google Scholar]
- 11. Dutsch M, Hilz MJ, Devinsky O. Impaired baroreflex function in temporal lobe epilepsy. J Neurol 2006;253:1300–1308. [DOI] [PubMed] [Google Scholar]
- 12. Lotufo PA, Valiengo L, Bensenor IM, et al. A systematic review and meta‐analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia 2012;53:272–282. [DOI] [PubMed] [Google Scholar]
- 13. Nei M, Ho RT, Abou‐Khalil BW, et al. EEG and ECG in sudden unexplained death in epilepsy. Epilepsia 2004;45:338–345. [DOI] [PubMed] [Google Scholar]
- 14. Poh MZ, Loddenkemper T, Reinsberger C, et al. Autonomic changes with seizures correlate with postictal EEG suppression. Neurology 2012;78:1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toth V, Hejjel L, Fogarasi A, et al. Periictal heart rate variability analysis suggests long‐term postictal autonomic disturbance in epilepsy. Eur J Neurol 2010;17:780–787. [DOI] [PubMed] [Google Scholar]
- 16. Neufeld G, Lazar JM, Chari G, et al. Cardiac repolarization indices in epilepsy patients. Cardiology 2009;114:255–260. [DOI] [PubMed] [Google Scholar]
- 17. Espinosa PS, Lee JW, Tedrow UB, et al. Sudden unexpected near death in epilepsy: malignant arrhythmia from a partial seizure. Neurology 2009;72:1702–1703. [DOI] [PubMed] [Google Scholar]
- 18. Ferlisi M, Tomei R, Carletti M, et al. Seizure induced ventricular fibrillation: a case of near‐SUDEP. Seizure 2013;22:249–251. [DOI] [PubMed] [Google Scholar]
- 19. Lanz M, Oehl B, Brandt A, et al. Seizure induced cardiac asystole in epilepsy patients undergoing long term video‐EEG monitoring. Seizure 2011;20:167–172. [DOI] [PubMed] [Google Scholar]
- 20. Chin RF, Neville BG, Peckham C, et al. Incidence, cause, and short‐term outcome of convulsive status epilepticus in childhood: prospective population‐based study. Lancet 2006;368:222–229. [DOI] [PubMed] [Google Scholar]
- 21. Akalin F, Tirtir A, Yilmaz Y. Increased QT dispersion in epileptic children. Acta Paediatr 2003;92:916–920. [DOI] [PubMed] [Google Scholar]
- 22. Kandler L, Fiedler A, Scheer K, et al. Early post‐convulsive prolongation of QT time in children. Acta Paediatr 2005;94:1243–1247. [DOI] [PubMed] [Google Scholar]
- 23. Bazett HC. An analysis of the time‐relations of electrocardiograms. Heart 1920;7:353–370. [Google Scholar]
- 24. Hinterseer M, Beckmann BM, Thomsen MB, et al. Relation of increased short‐term variability of QT interval to congenital long‐QT syndrome. Am J Cardiol 2009;103:1244–1248. [DOI] [PubMed] [Google Scholar]
- 25. Talib AK, Sato N, Kawabata N, et al. Repolarization characteristics in early repolarization and Brugada syndromes: insight into an overlapping mechanism of lethal arrhythmias. J Cardiovasc Electrophysiol 2014;25:1376–1384. [DOI] [PubMed] [Google Scholar]
- 26. Varkevisser R, Wijers SC, van der Heyden MA, et al. Beat‐to‐beat variability of repolarization as a new biomarker for proarrhythmia in vivo. Heart Rhythm 2012;9:1718–1726. [DOI] [PubMed] [Google Scholar]
- 27. Hinterseer M, Beckmann BM, Thomsen MB, et al. Usefulness of short‐term variability of QT intervals as a predictor for electrical remodeling and proarrhythmia in patients with nonischemic heart failure. Am J Cardiol 2010;106:216–220. [DOI] [PubMed] [Google Scholar]
- 28. Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus—report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015;56:1515–1523. [DOI] [PubMed] [Google Scholar]
- 29. Van Hare GF, Dubin AM. Moss and Adams' Heart Disease in Infants, Children and Adolescents. Philadephia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 30. Drake ME, Reider CR, Kay A. Electrocardiography in epilepsy patients without cardiac symptoms. Seizure 1993;2:63–65. [DOI] [PubMed] [Google Scholar]
- 31. Brewster AL, Marzec K, Hairston A, et al. Early cardiac electrographic and molecular remodeling in a model of status epilepticus and acquired epilepsy. Epilepsia 2016;57:1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shorvon S, Tomson T. Sudden unexpected death in epilepsy. Lancet 2011;378:2028–2038. [DOI] [PubMed] [Google Scholar]
- 33. Manno EM, Pfeifer EA, Cascino GD, et al. Cardiac pathology in status epilepticus. Ann Neurol 2005;58:954–957. [DOI] [PubMed] [Google Scholar]
- 34. Chatzikonstantinou A, Ebert AD, Hennerici MG. Temporal seizure focus and status epilepticus are associated with high‐sensitive troponin I elevation after epileptic seizures. Epilepsy Res 2015;115:77–80. [DOI] [PubMed] [Google Scholar]
- 35. Hocker S, Prasad A, Rabinstein AA. Cardiac injury in refractory status epilepticus. Epilepsia 2013;54:518–522. [DOI] [PubMed] [Google Scholar]
- 36. Mehrpour M, Hajsadeghi S, Fereshtehnejad SM, et al. Serum levels of cardiac troponin I in patients with status epilepticus and healthy cardiovascular system. Arch Med Res 2013;44:449–453. [DOI] [PubMed] [Google Scholar]
- 37. Soundarya N, Lawrence D, Samip J, et al. Elevation of cardiac troponins in prolonged status epilepticus: a retrospective chart analysis. SOJ Neurol 2014;1:1–4. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient demographics and clinical factors of seizure patients by the availability of electrocardiogram.
Table S2. Comparisons of chronic anti‐epileptic drugs (AED) in children with epilepsy by ECG findings.


