Abstract
Soil has a considerable effect on human health, whether those effects are positive or negative, direct or indirect. Soil is an important source of nutrients in our food supply and medicines such as antibiotics. However, nutrient imbalances and the presence of human pathogens in the soil biological community can cause negative effects on health. There are also many locations where various elements or chemical compounds are found in soil at toxic levels, because of either natural conditions or anthropogenic activities. The soil of urban environments has received increased attention in the last few years, and they too pose a number of human health questions and challenges. Concepts such as soil security may provide a framework within which issues on soil and human health can be investigated using interdisciplinary and transdisciplinary approaches. It will take the contributions of experts in several different scientific, medical and social science fields to address fully soil and human health issues. Although much progress was made in understanding links between soil and human health over the last century, there is still much that we do not know about the complex interactions between them. Therefore, there is still a considerable need for research in this important area.
Keywords: elemental toxicity, xenobiotic organic chemicals, essential nutrients, human pathogens, urban soil, antibiotic resistance
Introduction
Soil has a profound effect on the health and well-being of humans. Depending upon the condition of the given soil and the interactions of interest, this effect can be either positive or negative and direct or indirect. Soils that affect human health include natural soil, which usually has little anthropogenic contamination, and soils in agroecosystems, urban areas, mines, oil and gas extraction areas, landfill sites and other locations where anthropogenic contamination is more likely. People in professions that work closely with soil, such as farmers, construction workers or miners are at a greater risk of health problems that involve direct contact with soil, but everyone’s health is affected by soil to some extent. This is because soil provides many of the nutrients we require and can pass on harmful substances through the food that we eat. Some dusts generated from soil can travel thousands of miles and affect people long distances from where they originated. Although recent advances in the role soil plays in human health are being made and continue to be investigated, few people probably think about soil having an effect on their health. This paper will give a brief, general overview of the topic of soil and human health. Other excellent papers on this topic have been published recently and we encourage the reader to find additional details on many of these topics in other related publications (e.g. Pepper, 2013; Brevik & Burgess, 2015; Oliver & Gregory, 2015; Cakmak & Kutman and Li et al. in this issue).
History
Probably the first recorded depiction of the relation between human health and soil occurs in 1400 BC in the Bible in the book of Numbers where Moses directs the people to “see what the land is like….how is the soil…fertile or poor?” (Numbers 13:18–20). In 400 BCE Hippocrates published a list of things that should be considered part of a proper medical evaluation, a list that included the nature of the ground (Hippocrates, 2010) and in 60 BCE Columella wrote about hidden diseases from marshes (Sylvia et al., 1998); in each case advancing the idea that soil is important to human health. However, it was not until the early 1900s that the idea that soil could affect human health started to gain widespread acceptance. McCarrison (1921) concluded that the fertility of a soil determines the nutrient content of food crops, and therefore the health of humans who ate the crops. In the late 1930s, the USDA Yearbook of Agriculture (USDA, 1938) included discussion on the importance of soil as the origin for many of the essential elements necessary for human health in at least three of its chapters, and in 1940 the USDA established the Plant, Soil and Nutrition Research Unit (PSNRU) at Cornell University which still continues to do research into soil and human health (PSNRU, 2008). In the 1940s, individuals such as Sir Albert Howard (1940), Lady Eve Balfour (1943) and J. I. Rodale (1945) offered opinions on the links between soil and human health, in particular the effect of soil fertility on the nutrient content of foods grown in a particular soil was a common theme. The 1950s brought about the realization that soil could supply toxic amounts of elements to the human diet (USDA, 1957). André Voisin published extensively on the potential links between soil and human health in 1959, a study that was probably the most comprehensive on the subject up to that time. From these first realizations, studies into the relation between soil and human health have continued to increase and several of the areas of investigation will be described below in summary. Brevik & Sauer (2015) recently reviewed the history of the soil–human health field and we direct readers to this review for more detail.
Routes of exposure
There are three common ways that humans are exposed to soil materials: (i) ingestion, (ii) respiration and (iii) skin absorption or penetration (Brevik, 2013). Ingestion can occur deliberately, known as geophagy, or incidentally, such as during hand to mouth contact (particularly children) or when raw fruits or vegetables are consumed without adequate washing. Ingestion of soil is especially common in children (von Lindern et al., 2016) and pregnant women. Ingested soil can potentially supply essential nutrients, but it can also lead to exposure to heavy metals, organic chemicals or pathogens and in large amounts can cause an intestinal obstruction (Henry & Cring, 2013). Respiration involves inhaling soil materials. Some serious problems are linked to inhalation, such as coccidioidomycosis (Bultman et al., 2005; Stockamp & Thompson, 2016), acute inflammation of the bronchial passages, chronic bronchitis, emphysema and fibrotic changes from breathing in soil-derived dust (Zosky et al., 2014), and mesothelioma from breathing in naturally occurring asbestos minerals from soil-derived dust (Buck et al., 2016). Absorption or penetration of the skin can expose an individual to pathogens and soil chemicals (Brevik, 2013). It can also cause podoconiosis (endemic non-filarial elephantiasis), which is a non-infectious disease found in subsistence farmers who frequently go barefoot. This is due to long-term contact with volcanically-derived clay in the soil which obstructs the lymph system (Deribe et al., 2013). Prevention is as simple as wearing shoes, and the condition has ceased to occur in countries where it was once found such as in France, Ireland and Scotland once the use of shoes became commonplace (Deribe et al., 2013).
Element toxicity
There are many ways that soil can adversely affect human health. The soil may be contaminated either naturally or through anthropogenic activities with chemical elements and substances that are in toxic amounts when ingested or inhaled. A supply of any element may result in human toxicity, even elements that are essential for life. For any essential element there is an optimal range of concentration in humans, falling below this optimal range results in deficiency, whereas, concentrations above the optimal range create toxicity. Thus, the level of any essential element in humans can be deficient, adequate or toxic depending upon the concentrations of these elements in the soil and the degree of exposure. Both deficiency and toxicity can result in morbidity and in some cases mortality. There are many examples, reports and research publications on the risk of toxicity from substances in soil and the risk to human health, although some have been studied more than others. There are also elements that can be present in soil that have no known benefit for human health such as lead and mercury, but can cause problems with toxicity even at very small concentrations (Combs, 2005; Brevik & Burgess, 2015). Herein we briefly overview several elements of particular interest. The reader is referred to other papers on the supply of elements by soil, such as Steinnes (2011), Green et al. (2016) and Cakmak & Kutman (this issue), for additional information.
Lead
Lead is probably the single largest soil contaminant worldwide because it has been widely introduced into soil from anthropogenic sources such as leaded petrol (gasoline), lead-based paint, lead mining and smelting, and other industrial activities. The effects of lead, especially on children and adolescents, is well documented (Deckers & Steinnes, 2004; Balabanova et al., 2017) and has led to multiple public health problems and concerns. Lead in urban soil, where children are especially at risk for contact and contamination, is a particular problem (Filippelli & Laidlaw, 2010; Li et al., 2015). Mass lead poisoning was recently reported in Senegal (Haefliger et al., 2009) and Nigeria (Lo et al., 2012) in villages that participated in informal recycling of used lead-acid batteries and gold ore processing, respectively. The recycling and gold processing activities resulted in lead contaminated soil, with dust from such soil being inhaled, ingested or both, causing lead poisoning. Such studies demonstrate the challenges that many developing countries still face with regard to soil contamination by heavy metals and human health (Wu et al., 2015).
Arsenic
In addition to lead, arsenic poisoning remains a concern over large parts of the world. Arsenic is a naturally occurring element that can concentrate in drinking water, especially water obtained from wells (Helmke & Losco, 2013; Ayotte et al., 2015). Millions of people worldwide are exposed to potentially toxic levels of arsenic each day. Moreover, another common source of arsenic exposure is from the wood found in and around homes, especially in wood preservatives used in pressure treated timber (lumber). Arsenic can concentrate in the soil around structures made with this treated wood where it creates an exposure hazard, especially to children (Gardner et al., 2013). Another problem is the use of arsenic contaminated water to irrigate rice crops; the arsenic then accumulates in people who consume the rice (Brammer & Ravenscroft, 2009; Kwon et al., 2017). Rice is the dietary staple for about half the world’s population, and for most of these people rice also represents their primary exposure to arsenic (Zhao et al., 2010).
Cadmium
Cadmium contamination can be caused by industrial activities or by fertilization with sewage sludge or superphosphate (Nordberg et al., 2015). Cadmium is the most common heavy metal contaminant in the soils of China (Zhao et al., 2015). Large concentrations of cadmium in soil can lead to corresponding large concentrations in plant tissues (Hunter, 2008), which results in toxicity to humans when foods grown in such soil are consumed. The classic example of health problems caused by soil cadmium was the itai-itai disease outbreak in Japan in the first half of the 1900s (Nordberg et al., 2015). Mining in the Toyama Prefecture of Japan released large quantities of cadmium into the Jinzu River, which was used for rice irrigation. Rice absorbed cadmium from the water, and people who consumed the rice subsequently developed itai-itai disease. Itai-itai means “it hurts-it hurts” in Japanese, and the disease is characterized by weak, brittle bones, pain in the legs and spine, coughing, anaemia, and kidney failure. However, large cadmium concentrations in soil do not necessarily produce such symptoms because other soil and dietary conditions are important. Cadmium bioavailability is affected by soil aeration status (Zhao et al., 2015), soil pH and the concentrations of other elements present in a soil. The effect on humans is affected by the concentrations of nutrients such as iron and zinc present in the local diet (Brevik, 2013; Morgan, 2013). Residents of the village of Shipham, in England have large cadmium concentrations in their soil, but do not appear to suffer any adverse health effects because of low bioavailability of the cadmium in their soil and large soil zinc concentration (Chaney, 2015). More in-depth discussions of itai-itai soil interrelations are provided by Morgan (2013) and Brevik & Sauer (2015).
Nitrate
Soil is the primary nitrogen source for plants, and given that nitrogen is required for human health, nitrate is an essential nutrient; however, because of its importance plants can quickly diminish nitrate concentrations in soil. For production agriculture to succeed, the nitrogen consumed has to be replaced frequently, and this is usually with the use of chemical fertilizers. Properly managed, this does not endanger human health and increases crop production. However, improper use and overuse can lead to leaching of excess nitrate into groundwater or surface water (Zhang et al., 2015). Nitrate-contaminated water can cause serious toxicity when the gut microflora convert nitrate into nitrite. Nitrite then reacts with haemoglobin to form methemoglobin, which prevents oxygen from being carried throughout the body. The condition is called methemoglobinemia, and while it can occur in adults it is a bigger problem in infants (Bryan and Ivy, 2015). With the decrease in oxygen concentrations in the blood, infants can become cyanosed with a bluish colouring of the skin. Nitrate has also been identified as a risk factor in the development of stomach cancer (Nagini, 2012). Therefore, proper use of nitrogen fertilizers is vital to prevent public health concerns over nitrate (Richard, 2014).
Mercury
Mercury occurs naturally in soil formed from parent materials with a large organic content; mercury has a strong affinity for organic matter. However, anthropogenic contamination through the mining of gold, burning of coal or chlorine production can cause mercury contamination of soil over large areas. Mercury can be methylated by soil organisms causing it to become mobile in the soil leading to surface water contamination or methyl-mercury can be taken up by plants (Xu et al., 2015). Subsequent human exposure occurs through consumption of contaminated water, plants, animals or both. Therefore, the general public is more likely to encounter large concentrations of mercury through ingestion of fish when the water source is contaminated with methyl-mercury, the consumption of vegetation grown in mercury contaminated soil or the improper handling and disposal of compact fluorescent light bulbs (CFLs) (Boerleider et al., 2017; Liang et al., 2015).
Radionuclides
Soil can be contaminated with radioactive elements naturally or through anthropogenic activity. There is a wide range of radioactive elements that occur naturally and cause concern (Cygan et al., 2007); radon represents the largest natural radiation dose to humans (Appleton, 2007). Radon is a naturally occurring radioactive gas found in many parts of the world that accumulates in basements and other underground structures (Appleton, 2007). It is known to cause lung cancer in individuals (Islami et al., 2015), and because it is inherent to the soil, proper ventilation of basements to reduce the radon concentration or proper sealing to prevent the entry of radon are the only remedies (Khan & Gomes, 2017).
In addition to naturally-occurring radiation such as radon gas, the anthropogenic release of radionuclides into the environment, including soil, poses an immediate and long lasting threat to human health. Anthropogenically generated radionuclides are often the by-product of medical waste, nuclear waste, nuclear power disasters, or fallout from the testing, use or both of nuclear weapons or dirty bombs that contaminate the soil in the vicinity of or downwind from these point sources (Hu et al., 2010). These forms of radioactive pollution can occur by accident or purposefully. Probably the most publicized releases of radionuclides have been from nuclear power plant disasters, the most recent was the Fukushima Daiichi nuclear plant in Japan related to the earthquake and tsunami in 2011 (Chino et al., 2011). The Chernobyl nuclear disaster in the Ukraine (former USSR) in 1986 was another large accidental release that received considerable attention and which has several implications for human health related to radioactive fallout into soil (Brevik, 2013). The effect of radionuclides on human health can be through either direct exposure to the radioactive materials, which leads to various cancers and genetic mutations (Magill & Galy, 2005), or indirect exposure through the creation of soil nutrient imbalances because of antagonism between elemental nutrients (Brevik, 2013).
Xenobiotic organic chemicals
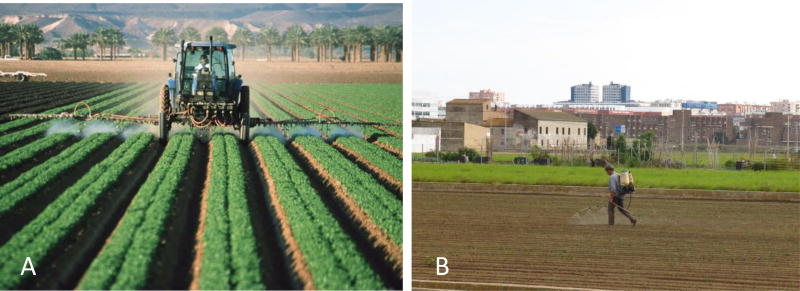
Xenobiotic organic chemicals are carbon based compounds that are synthesized and therefore unnatural. They are referred to as xenobiotic from the Greek term ‘xeno’ for strange. The differences between these synthesized organic compounds and their natural parent compounds are the common insertion of halogen atoms (chlorine, fluorine, bromine) or multivalent nonmetal atoms (such as sulphur or nitrogen) into the structures (Calabrese & Baldwin, 1998; Salem et al., 2017). Because these xenobiotic compounds contain types of atoms in locations that do not occur in natural compounds, organisms have not evolved adequate pathways of biotransformation to metabolize them. Therefore, the synthetic organic compounds are very resistant to biological decay and are usually markedly toxic to organisms even at extremely small doses. Soil contamination with organic chemicals is a serious problem in all nations (Aelion, 2009). A common hazard from these organic chemicals comes from the application of pesticides in both rural and urban areas, with a large percentage of the applied chemicals reaching the soil (Figure 1). For example, when pesticides were applied to a forest area about 25% reached the tree foliage, about 1% reached the target insect and about 30% reached the soil, with the remainder ending up in the atmosphere and surface or groundwater. Application of pesticides to crops increased the percentage of pesticide reaching the soil compared to application to the forest area (Calabrese & Baldwin, 1998).
Figure 1.
Application of agricultural chemicals can lead to considerable interaction between soil and the chemical. (a) Chemicals being applied directly to exposed soil in between the cropped rows as a side effect of treating the crops. Photograph by Jeff Vanuga, USDA–NRCS. (b) Worker sprays pesticides on a garden in an urban area. Photograph by Artemi Cerdà.
Soil pollution with organic chemicals is not limited to farming areas. Soil in urban areas is also polluted with organic chemicals from medicines, industrial activities, coal burning, motor vehicle emissions, waste incineration, sewage and deposition of solid wastes (Leake et al., 2009). Both farming and urban areas have soil contamination that includes a complex mixture of organic chemicals, metals and microorganisms from municipal and domestic septic system waste, farm animal waste and other biowastes (Pettry et al., 1973; Saha et al., 2017). A more recent health concern includes pharmaceutical waste derived from antibiotics, hormones and anti-parasitic drugs used to treat humans and domestic animals (Albihn, 2001; Aust et al., 2008; Crofts et al., 2017).
These xenobiotic organic chemicals are typically very diluted, they can form chemical mixtures in the upper layers of the soil. We have very little toxicological information about the health effects of these chemical mixtures (Carpenter et al., 2002; Fan et al., 2017). Because of the long half-lives of many organic chemicals, they are referred to as ‘persistent organic pollutants’ (POPs). Twelve POPs were identified by the Stockholm Convention in 2001 (Xu et al., 2013) and 14 additional POPs were added in amendments to the Stockholm Convention in 2009, 2011, 2013 and 2015 (Stockholm Convention, 2015) (Table 1). These POPs resist decomposition in the environment and bioaccumulate as they move up the food chain. An example of a POP is 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (DDT), which was shown to disrupt the hormonal systems of raptors causing eggshells to be too thin to support the chicks (Vega et al., 2007).
Table 1.
Persistent organic pollutants identified by the Stockholm Convention (SC). Some POPs can still be used for specific purposes as outlined in the SC. Table based on Stockholm Convention (2008; 2015).
| Chemical | Year Added |
Source* | Annex in SC |
Additional Notes |
|---|---|---|---|---|
| Aldrin | 2001 | P | A | |
| Chlordane | 2001 | P | A | |
| DDT | 2001 | P | B | DDT still used against mosquitoes in several countries to control malaria |
| Dieldrin | 2001 | P | A | |
| Endrin | 2001 | P | A | |
| Heptachlor | 2001 | P | A | |
| Hexachlorobenzene (HCB) | 2001 | P, IC, BP | A & C | |
| Mirex | 2001 | P | A | |
| Toxaphene | 2001 | P | A | |
| Polychlorinated biphenyls (PCB) | 2001 | IC, BP | A & C | Has specific exemptions under Annex A |
| Polychlorinated dibenzo-p-dioxins (PCDD) | 2001 | BP | C | |
| Polychlorinated dibenzofurans (PCDF) | 2001 | BP | C | |
| Alpha hexachlorocyclohexane | 2009 | P | A | |
| Beta hexachlorocyclohexane | 2009 | P | A | |
| Chlordecone | 2009 | P | A | |
| Hexabromobiphenyl | 2009 | IC | A | |
| Hexabromodiphenyl ether, heptabromodiphenyl ether | 2009 | IC | A | Can be used in accordance with the provisions of Part IV of Annex A |
| Lindane | 2009 | P | A | Human use for control of head lice and scabies as second line treatment |
| Pentachlorobenzene | 2009 | P, IC, BP | A & C | |
| Perfluorooctane sulfonic acid, its salts and perluorooctane sulfonyl fluoride | 2009 | IC | B | Acceptable purposes and specific exemptions in accordance with Part III of Annex B |
| Tetrabromodiphenyl ether, pentabromodiphenyl ether | 2009 | IC | A | Has specific exemptions under Part V of Annex A |
| Technical endosulfan and its related isomers | 2011 | P | A | Exemptions for crop-pest complexes in accordance with the provisions of part VI of Annex A |
| Hexabromocyclododecane | 2013 | IC | A | Expanded and extruded polystyrene in buildings in accordance with the provisions of part VII of Annex A |
| Hexachlorobutadiene | 2015 | IC | A | |
| Pentachlorophenol and its salts and esters | 2015 | P | A | Pentachlorophenol for utility poles and cross-arms in accordance with the provisions of part VIII of Annex A |
| Polychlorinated naphthalenes | 2015 | IC, BP | A & C | Can be used for production of polyfluorinated naphthalenes, including octafluoronaphthalene |
P, pesticide; IC, industrial chemicals; BP, by-products
Essential nutrients
There are 14 elements essential for plant growth that come from the soil, and many of these elements are also essential for human health (Combs, 2005). These essential nutrients end up in the human diet either directly through the consumption of plants or indirectly through the consumption of animal products (Abrahams, 2002). Hydrogen, oxygen, carbon, nitrogen, sodium, potassium, calcium, magnesium, phosphorous, sulphur and chlorine make up 99.9% of the atoms in the human body, with all but hydrogen, oxygen and carbon having soil as their major source (Brevik, 2013). However, the remaining 0.1% consists of approximately 18 additional elements known as micronutrients or trace elements that are essential in small amounts to maintain human health (Combs, 2005). Therefore, soils that provide plants with the proper nutrients for growth also contain many of the elements that are necessary for human health as well. Herein, we focus briefly on some important micronutrients that can cause fairly commonly seen health issues; major sources of selected human nutrients are given in Table 2. We refer the reader to Shetty (2009) for additional details.
Table 2.
Examples of important sources of elements essential to human life, it is important to note that this table does not give a complete list of elements essential to human health (Table based on Combs, 2005; Fraga, 2005; Shegefti et al., 2016).
| Element | Important Sources |
|---|---|
| Ca | Kale, collards, mustard greens, broccoli, dairy products |
| Cl | Dairy products, meats, eggs |
| Co | Fish, oysters, eggs, milk, green vegetables, cereals, nuts |
| Cu | Beans, peas, lentils, whole grains, nuts, peanuts, mushrooms, chocolate, organ meats, oysters, dark chocolate |
| Fe | Meats, especially red meat, cereals, legume seeds, fruits, vegetables, dairy |
| I | Vegetables, cereals, fruit |
| K | Fruits, cereals, vegetables, beans, peas, lentils, dairy products, meats |
| Mg | Seeds, nuts, beans, peas, lentils, whole grains, dark green vegetables |
| Mn | Whole grains, beans, peas, lentils, nuts, tea |
| Mo | Beans, peas, lentils, dark green leafy vegetables, organ meats |
| Na | Dairy products, meats, eggs |
| P | Nuts, beans, peas, lentils, grains, meats, eggs, dairy products |
| Se | Grain products, nuts, garlic, broccoli (if grown on high-Se soil), red meats, seafood |
| Zn | Nuts, whole grains, beans, peas, lentils, red meats, organ meats, poultry, sea food, dairy |
Iodine
Iodine deficiency has been identified as the single most preventable cause of brain damage world-wide by the World Health Organization (WHO, 2007a). Other health effects include goitre, hypothyroidism, spontaneous abortions and stillbirths, congenital anomalies, impaired mental function and delayed physical development (WHO, 2007a). Deficiencies are common in regions where soil does not supply adequate iodine to the crops grown in it, and although widespread, they are most common in the high altitude interiors of continents (Combs, 2005). Considerable progress has been made in correcting iodine deficiency disorders through the ‘Universal Salt Iodization’ programme that started in 1994 (WHO, 2007a).
Iron
Iron deficiency causes anaemia because it is an essential component of haemoglobin. About two billion people are estimated to have iron deficiency worldwide making it one of the most common nutrient deficiencies (WHO, 2007b). Iron deficient soil can lead to small iron concentrations in plants and in humans who consume them (Combs, 2005). This is especially problematic in arid soils and in populations whose diets rely on a large intake of cereal grains, but have few meat products (Deckers & Steinnes, 2004). Excessive iron, however, can lead to genetic and metabolic diseases (Fraga, 2005).
Selenium
Selenium is an essential micronutrient for humans because it has critical roles in thyroid function and immunity (Fairweather-Tait et al, 2011). Selenium concentrations in soil vary greatly throughout the world depending on geological and climatic factors and, therefore, the concentration of bioavailable selenium in plants varies considerably (Haug et al., 2007). Most of the world’s population consumes suboptimal amounts of selenium and is therefore at increased risk of cancer, heart disease, other diseases caused by increased oxidative stress (Combs, 2001) and a weakened immune system (Fraga, 2005). Conversely, selenium toxicity in humans can occur in regions where the soil or drinking water has large concentrations of selenium (Fordyce, 2013). The effects of selenium toxicity in humans are not well understood, but it is considered a probable carcinogen that targets the liver and kidneys (Hardman, 2006) and causes brittle hair and nails, hair and nail loss, gastrointestinal problems, fatigue and nerve damage (Fordyce, 2005; Fraga, 2005).
Zinc
Zinc is a critical component of several enzymes, in cellular growth and in tissues that have a rapid differentiation and turnover, such as in the gastrointestinal tract and immune system (WHO, 2006). Zinc deficiency is suspected to be common in developing countries (Fraga, 2005), but the exact level of the problem is not well known because of the lack of reliable and well-accepted indicators of zinc deficiency (WHO, 2006). Deficiency negatively affects the healing of wounds, immune system response and the ability to taste and smell (Fraga, 2005), and is also suspected of causing stunted growth (WHO, 2006). About half the world’s soils are deficient in zinc, with strongly leached acidic soil and calcareous soils being the most likely to be deficient (Combs, 2005; Abrahams, 2002).
Human pathogens
In addition to soil affecting the nutrient quality of foodstuffs and exposing humans to contaminants, it is also a vast heterogeneous habitat for millions of macroscopic and billions of microscopic organisms. Although the majority of these organisms do not cause disease in humans, several species of bacteria, fungi, protozoa, viruses and prions can cause disease depending upon many factors including the condition of the soil, climate, location, land use and other variables that upset the normal microbial biomass. A set of tables that give a good overview of pathogens found in soil, including the diseases caused, geographic distributions, problems caused and incidence is given in Loynachan (2013). Herein, we provide two examples of how soil can affect human health through contact with soil microorganisms directly or indirectly by promoting either antibiotic resistance or by producing antibiotics. Other excellent papers on this topic have been published recently and we encourage the reader to obtain additional details on many of these topics in other related publications (e.g. Adegoke et al. 2017; Baumgardner, 2012; Wall et al., 2015).
Coccidioidomycosis
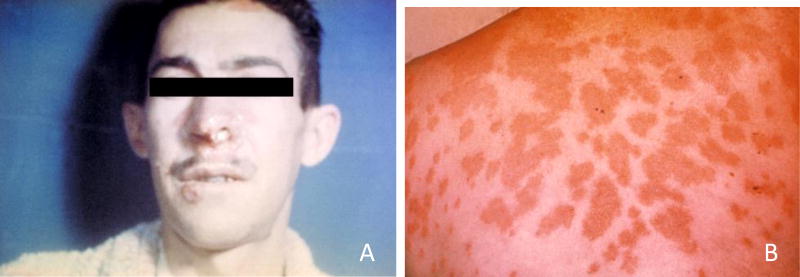
Coccidioidomycosis, also called Valley Fever (Figure 2), is caused by the fungus Coccidioides spp. which lives in soil of the southwestern United States and areas further south into Mexico and Central and South America (Brevik, 2009). The fungus usually enters humans through the respiratory route by inhalation of microscopic fungal spores. The fungus can grow under extreme environmental conditions including extreme temperatures, high salinity and very alkaline conditions that most other microorganisms cannot tolerate. However, they compete poorly with other soil fungi and bacteria, which often limits Coccidioides spp. to soils with properties that are not suitable to many other organisms (Bultman et al., 2005; Reyes-Montes et al., 2016). These fungi grow and reproduce in and above the soil. Any natural (earthquake or dust storm) or anthropogenic (construction or tillage) soil disturbance in these endemic areas can cause aerosolization of the fungus and infect humans and animals. Epidemics can occur after heavy rains that promote mycelial growth followed by drought and windy conditions. Although uncommon, the fungus can also directly infect skin or bone through contamination by a penetrating object, but inhalation is a much greater threat (Stockamp & Thompson, 2016). This disease is expected to increase in prevalence because of population growth in areas where Coccidioides is endemic. Soil maps have been used successfully in an epidemiological study that sought to identify areas where people were susceptible to contracting valley fever (Tabor et al., 2011).
Figure 2.
(a) Paracoccidioidomycosis lesions on the face of a patient and (b) erythema nodosum lesions on skin of the back because of coccidioidomycosis (valley fever) (Courtesy Centers for Disease Control and Prevention, image #4027 and #482).
Antibiotic resistance and antibiotics
Antibiotic resistance by definition occurs when an antibiotic no longer effectively controls or kills bacterial growth (an increase in the minimum inhibitory concentration); thus the bacteria are said to be resistant to the antibiotic and are difficult or impossible to treat. Antibiotic resistance continues to be a major concern in infectious disease control because of the large increase in antibiotic resistant bacteria that is being seen worldwide (Tanwir & Khiyani, 2011). Accordingly, physicians are running out of therapeutic options with which to fight these bacterial species as several of these so called superbugs are resistant to many of the most potent antibiotics available (Khan & Khan, 2016). There are several reasons why antibiotic resistance develops. First, antibiotics are often overprescribed or prescribed inappropriately by physicians or are obtained ‘over-the-counter’ when the patient does not have a bacterial infection or for the prophylactic prevention of an infection before a dental or medical procedure. Second, when prescribed appropriately, patients will begin to feel better and will discontinue the use of the antibiotic before it has completely eliminated the pathogenic bacteria. This leads to bacterial adaptations by which the organisms can survive at small concentrations of the antibiotic, potentially acquire antibiotic resistance genes, upregulate antibiotic resistance mechanisms or both. These bacteria are then able to transmit resistance genes between bacteria (intra- or inter-species transfer) leading to antibiotic resistance through a variety of mechanisms (Blair et al. 2015). The heterogeneous habitat of the soil environment favours the exchange of genetic material between organisms, many of which confer antibiotic resistance (Forsberg et al., 2012; Woolhouse & Ward, 2013). Soil is known to contain gene pools which promote the emergence of antibiotic resistance, similar to antibiotic resistant genes in a hospital setting (Nesme et al. 2014). The role of the soil is to provide an environmental niche for both the emergence and dissemination of antibiotic resistance genes (Vas-Moreira et al. 2014). Adegoke et al. (2017) and Nesme & Simonet (2015) have recently reviewed the role of soil in contributing to antimicrobial resistance and we refer the readers to these publications and others for more information. Many anthropogenic factors such as the use of organic fertilizers on crops, antibiotic use in humans and livestock and antibiotic use on crops (typically through the application of manures or waste water that contain antibiotics to crops (Palacios et al., 2017; Prigitano et al., 2017)) increase the incidence and persistence of antibiotic resistance genes and antibiotic resistant species in the soil (Adegoke et al. 2016; McManus et al. 2002; Popowska et al. 2011; Wang & Tang 2010). Whether this poses direct harm to the health of humans remains under investigation (D’Costa et al., 2011; Forsberg et al. 2012; Pepper, 2013; Udikovic-Kolic et al. 2014); however, the emerging evidence of increased antimicrobial resistance lends credence that it is and will continue to pose a risk to human health. Nevertheless, the increase in antibiotic resistance has led to a burst of research to discover new antibiotics. Not only is the soil a source of antibiotic resistance, but it is also a source of natural antibiotics. Soil organisms often increase production of antibiotic-like compounds during times of stress (Swiecilo et al. 2013) and perhaps because of the increased stresses on soil worldwide new antibiotics are being discovered, although at a slower rate than the emergence of antibiotic resistance in the same stressed soil (Martinez et al. 2008). Many of the bacteria and actinomycetes found in soil naturally secrete compounds to ward off other bacteria and actinomycetes to give them a survival advantage in the competitive soil environment. However, it is difficult to isolate and grow these organisms in the laboratory environment, which hampers the ability of scientists to discover new antibiotics quickly. The recent discovery of Teixobactin using new culture isolation methods for soil organisms (Ling et al. 2015) provides hope that more antibiotics can be discovered by soil and medical microbiologists and other fields of medicine and chemistry working together.
Urban soil
The study of urban soil has received increased attention recently, including processes that create and modify it (Lehmann & Stahr, 2007), the vertical and horizontal spatial distribution of soil properties (Howard & Orlicki, 2015) and the ability to predict such property distributions so that they can be mapped (Howard & Shuster, 2015). These studies have revealed that urban soils are very heterogeneous and strongly affected by anthropogenic activities, which supports the suggestion by some soil scientists that soil should now be considered a human–natural body rather than just a natural body (Richter et al., 2011).
The anthropogenic effects that make urban soils so heterogeneous can also introduce a number of contaminants that may have adverse effects on human health. For example, while only 2% of 0–5 year old children in the USA are poisoned with lead, it is 15–20% in Eastern and Midwestern USA city centres because of the close contact that children have with lead contaminated urban soil (Filippelli & Laidlaw, 2010). The contamination now found in urban soil came from sources such as the combustion of leaded petrol, use of lead-based paints (Filippelli & Laidlaw, 2010), smelting and other industrial operations, recycling and disposal of wastes, application of lawn chemicals (Burgess, 2013; Morgan, 2013), and even the use of pressure treated wood in the construction of playground equipment (Gardner et al., 2013) in the past. Although active contamination has been greatly reduced in many developed countries, urban soils still bear the burden of contamination from previous activities (Filippelli & Laidlaw, 2010; McClintock, 2015), and in many developing countries contamination actively continues (Trujillo-González et al., 2016).
Given the large numbers of people who live in urban centres (over 54% of the world’s population as of 2014 and increasing, particularly in developing countries (WHO, 2016)) and the close contact between people and urban soil, there is large potential for negative health effects because of exposure to contaminated soil in the urban environment. There has also been a growing urban garden movement in recent years (Philpott et al., 2014), providing the potential for the transfer of containments into the human food supply through soil–plant interactions (Beniston et al., 2015; Roy & McDonald, 2015). Therefore, connections between urban soils and human health will be likely to be a growing area of research in the near future.
Soil security
The concept of soil security has been advanced recently in an effort to put policies concerning soil on to a similar level as those focused on food and water security (Koch et al., 2012). The description of soil security provided by Koch et al. (2012) coincides in many ways with commonly accepted definitions of soil quality or soil health (Karlen et al., 1997). There are definite ties between the concepts (Brevik et al., 2017), but soil security goes further than the soil quality and health concepts in that soil security integrates economic, social and political aspects (McBratney et al., 2014).
McBratney et al. (2014) identified five dimensions of soil security, and each of them have links to human health (Brevik et al., 2017) (Table 3). There are also interconnections between many of the dimensions. For example, the condition (dimension 2) of a soil affects that soil’s capability (dimension 1) to provide services, such as growing nutritious foods. Another example is how people connect (dimension 4) with soil affects how a given society tends to treat or manage their soil, which affects its condition (dimension 2). Codification (dimension 5) can also affect soil condition (dimension 2) through its effect on management choices by land managers. Therefore, the concept of soil security provides a platform that can be used to explore links between soil and human health in a transdisciplinary way, combining and merging aspects of the physical and biological sciences with those of the social and medical sciences.
Table 3.
Examples of ways that the dimensions of soil security link to human health. Some of the dimensions are interconnected. The examples are based on Brevik et al. (2017).
| Dimension of Soil Security |
Links to Human Health |
|---|---|
| 1: Capability | Production of plentiful food |
| Ability to pass essential nutrients up the food web | |
| Waste filtration function of soils, particularly in the supply of clean water | |
| 2: Condition | Ability to pass essential nutrients up the food web |
| Presence or absence of potentially harmful chemicals or organisms | |
| 3: Capital | Ecosystem services that support human health have value |
| Soil conditions that negatively influence human health have a cost | |
| Medicines developed from soils or soil organisms have economic value and save money when they shorten or prevent illness | |
| 4: Connectivity | The value that society places on soils influences how soils are managed or treated, which in turn influences soil condition |
| The terroir concept provides an example of a way to connect people to the soils that produce their food and encourage a more positive image of soil and better management | |
| Contact with healthy soil has been shown to have potential human health benefits | |
| 5: Codification | Government sponsored conservation programs can improve soil and water quality, leading to human health benefits |
| Non-binding initiatives such as the United Nations proposed Sustainable Development Goals can positively influence soil and water quality and thus human health through capability and condition |
Dimension 4, connectivity, provides an excellent example of a potential transdisciplinary approach highlighted by the concept of soil security. Societies tend to take care of things that matter to them and to neglect things that do not; the connectivity dimension includes making connections between the soil that supports society and items that matter to them (McBratney et al., 2014). In this respect, the concept of terroir may be particularly useful. Originally terroir established a connection between the soil that produces a given wine and wine connoisseurs, but more recently terroir has been expanded to include many other food products (Vaudour et al. 2015) and even the sources of water (springs, artesian aquifers, glaciers, etc.) (Capehart, 2015). Establishing connections between people and soil through products that they value, such as favourite foods, might create greater concern for the soil resource, ultimately leading to better treatment and management of our soils and through that improved human health (Karltun et al., 2013). For a more in-depth discussion of links between soil security and human health see Brevik et al. (2017).
Conclusions
Research into all of the above areas and those discussed more fully in the literature needs to continue to understand fully the effect of soil on human health. Interdisciplinary and transdisciplinary studies will be needed in the future because narrowly focused research will be inadequate to address many of the outstanding issues that still need to be understood. Many disciplines including soil science, agronomy, geology, geography (cultural and physical), biology, microbiology, ecology, public health and medicine (amongst others) will need to be involved in these collaborative studies. In addition, the field of soil and human health needs more people within scientific societies and political establishments to convey the importance of and to secure funding for these studies. This special section seeks to help achieve these goals by bringing together specialists from different fields to present original research on topics important to the study of the soil and human health connection.
Highlights.
Soil is important to human health
Effects can be positive or negative, direct or indirect
Advances have been made in recent years
Inter- and trans-disciplinary research is needed
Acknowledgments
J. Steffan and E. Brevik were partially supported by the National Science Foundation under Grant Number IIA-1355466 during this project. J. Steffan and L. Burgess were supported in part by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103442. The authors thank the editors and two anonymous reviewers for helpful comments and suggestions that improved the final manuscript.
References
- Abrahams PW. Soils: their implications to human health. Science of the Total Environment. 2002;291:1–32. doi: 10.1016/s0048-9697(01)01102-0. [DOI] [PubMed] [Google Scholar]
- Adegoke AA, Awolusi OO, Stenstrom TA. Organic fertilizers: Public health intricacies. In: Larramendy M, Soloneski S, editors. Organic Fertilizers–from Basic Concepts to Applied Outcomes. Intech, Rijeka, Croatia; 2016. pp. 343–374. [Google Scholar]
- Adegoke AA, Faleye AC, Singh G, Stenström TA. Antibiotic resistant superbugs: Assessment of the interrelationship of occurrence in clinical settings and environmental niches. Molecules. 2017;22 doi: 10.3390/molecules22010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aelion CM. Soil contamination monitoring. In: Inyang HI, Daniels JL, editors. Environmental Monitoring. Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, EOLSS Publishers; Oxford, UK: 2009. [Accessed 5 February, 2014]. www.eolss.net. [Google Scholar]
- Albihn A. Recycling biowaste―human and animal health problems. Acta Veterinaria Scandinavica Supplement. 2001;95:69–75. [PubMed] [Google Scholar]
- Appleton JD. Radon: Sources, health risks, and hazard mapping. Ambio. 2007;36:85–89. doi: 10.1579/0044-7447(2007)36[85:rshrah]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Aust MO, Godlinski F, Travis GR, Hao X, McAllister TA, Leinweber P, Thiele-Bruhn S. Distribution of sulfamethazine, chlortetracycline and tylosin in manure and soil of Canadian feedlots after subtherapeutic use in cattle. Environmental Pollution. 2008;156:1243–1251. doi: 10.1016/j.envpol.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Ayotte JD, Belaval M, Olson SA, Burow KR, Flanagan SM, Hinkle SR, Lindsey BD. Factors affecting temporal variability of arsenic in groundwater used for drinking water supply in the United States. Science of the Total Environment. 2015;505:1370–1379. doi: 10.1016/j.scitotenv.2014.02.057. [DOI] [PubMed] [Google Scholar]
- Balabanova B, Stafilov T, Šajn R, Andonovska KB. Quantitative assessment of metal elements using moss species as biomonitors in downwind area of lead-zinc mine. Journal of Environmental Science and Health, Part A. 2017;52:290–301. doi: 10.1080/10934529.2016.1253403. [DOI] [PubMed] [Google Scholar]
- Balfour EB. The Living Soil. Faber and Faber Ltd; London, UK: 1943. [Google Scholar]
- Baumgardner DJ. Soil-related bacterial and fungal infections. The Journal of the American Board of Family Medicine. 2012;25:734–44. doi: 10.3122/jabfm.2012.05.110226. [DOI] [PubMed] [Google Scholar]
- Beniston JW, Lal R, Mercer KL. Assessing and managing soil quality for urban agriculture in a degraded vacant lot soil. Land Degradation & Development. 2015;27:996–1006. [Google Scholar]
- Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nature Reviews Microbiology. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- Boerleider RZ, Roeleveld N, Scheepers PT. Human biological monitoring of mercury for exposure assessment. AIMS Environmental Science. 2017;4:251–276. [Google Scholar]
- Brammer H, Ravenscroft P. Arsenic in groundwater: A threat to sustainable agriculture in South and South-east Asia. Environment International. 2009;35:647–654. doi: 10.1016/j.envint.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Brevik EC. Soil, food security, and human health. In: Verheye W, editor. Soils, Plant Growth and Crop Production. Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, EOLSS Publishers; Oxford, UK: 2009. [Accessed 7 July 2016]. http://www.eolss.net. [Google Scholar]
- Brevik EC. Soils and human health: An overview. In: Brevik EC, Burgess LC, editors. Soils and Human Health. CRC Press; Boca Raton, FL, USA: 2013. pp. 29–56. [Google Scholar]
- Brevik EC, Burgess LC. Soil: Influence on human health. In: Jorgensen SV, editor. Encyclopedia of Environmental Management. CRC Press; Boca Raton, FL, USA: 2015. pp. 1–13. [Google Scholar]
- Brevik EC, Sauer TJ. The past, present, and future of soils and human health studies. SOIL. 2015;1:35–46. [Google Scholar]
- Brevik EC, Steffan JJ, Burgess LC, Cerdà A. Links between soil security and the influence of soil on human health. In: Field DJ, Morgan CLS, McBratney AB, editors. Global Soil Security. Progress in Soil Science Series, Springer; Rotterdam, The Netherlands: 2017. pp. 261–274. [Google Scholar]
- Bryan NS, Ivy JL. Inorganic nitrite and nitrate: evidence to support consideration as dietary nutrients. Nutrition Research. 2015;35:643–654. doi: 10.1016/j.nutres.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Buck BJ, Londono SC, McLaurin BT, Metcalf R, Mouri H, Selinus O, Shelembe R. The emerging field of medical geology in brief: some examples. Environmental Earth Sciences. 2016;75:449. [Google Scholar]
- Bultman MW, Fisher ES, Pappagianis D. The ecology of soil-borne human pathogens. In: Selinus O, Alloway B, Centeno JA, Finkelman RB, Fuge R, Lindh U, Smedley P, editors. Essentials of Medical Geology. Elsevier, Amsterdam; The Netherlands: 2005. pp. 481–511. [Google Scholar]
- Burgess LC. Organic pollutants in soil. In: Brevik EC, Burgess LC, editors. Soils and Human Health. CRC Press; Boca Raton, FL, USA: 2013. pp. 83–106. [Google Scholar]
- Cakmak I, Kutman UB. Agronomic biofortification of cereals with zinc: A review. European Journal of Soil Science. this issue. [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis as a biological hypothesis. Environmental Health Perspectives. 1998;106:357–362. doi: 10.1289/ehp.98106s1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capehart KW. Fine water: a hedonic pricing approach. Journal of Wine Economics. 2015;10:129–150. [Google Scholar]
- Carpenter DO, Arcaro KF, Spink DC. Understanding the human health effects of chemical mixtures. Environmental Health Perspectives. 2002;110:25–42. doi: 10.1289/ehp.02110s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney RL. How does contamination of rice soils with Cd and Zn cause high incidence of human Cd disease in subsistence rice farmers. Current Pollution Reports. 2015;1:13–22. [Google Scholar]
- Chino M, Nakayama H, Nagai H, Terada H, Katata G, Yamazawa H. Preliminary estimation of release amounts of 131I and 137Cs accidentally discharged from the Fukushima Daiichi nuclear power plant into the atmosphere. Journal of Nuclear Science and Technology. 2011;48:1129–1134. [Google Scholar]
- Combs GF., Jr Se in global food systems. British Journal of Nutrition. 2001;85:517–547. doi: 10.1079/bjn2000280. [DOI] [PubMed] [Google Scholar]
- Combs GF., Jr . Geological impacts on nutrition. In: Selinus O, Alloway B, Centeno JA, Finkelman RB, Fuge R, Lindh U, Smedley P, editors. Essentials of Medical Geology. Elsevier, Amsterdam; The Netherlands: 2005. pp. 161–177. [Google Scholar]
- Crofts TS, Gasparrini AJ, Dantas G. Next-generation approaches to understand and combat the antibiotic resistome. Nature Reviews Microbiology. 2017 doi: 10.1038/nrmicro.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cygan RT, Stevens CT, Puls RW, Yabusaki SB, Wauchope RD, McGrath CJ, et al. Research activities at U.S. Government agencies in subsurface reactive transport modeling. Vadose Zone Journal. 2007;6:805–822. [Google Scholar]
- D’Costa VM, King CE, Kalan L, Morar M, Sung WWL, Schwarz C, et al. Antibiotic resistance is ancient. Nature. 2011;477:457–461. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- Deckers J, Steinnes E. State of the art on soil-related geo-medical issues in the world. Advances in Agronomy. 2004;84:1–35. [Google Scholar]
- Deribe K, Tomczyk S, Tekola-Ayele F. Ten years of podoconiosis research in Ethiopia. PLoS Neglected Tropical Diseases. 2013;7:e2301. doi: 10.1371/journal.pntd.0002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Liu SS, Qu R, Li K, Liu HL. Polymyxin B sulfate inducing time-dependent antagonism of the mixtures of pesticide, ionic liquids, and antibiotics to Vibrio qinghaiensis sp.-Q67. RSC Advances. 2017;7:6080–6088. [Google Scholar]
- Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, Hurst R. Selenium in human health and disease. Antioxidants & Redox Signaling. 2011;14:1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- Feron VJ, Cassee FR, Groten JP, van Vliet PW, Zorge JA. International issues on human health effects of exposure to chemical mixtures. Environmental Health Perspectives. 2002;110:893–899. doi: 10.1289/ehp.02110s6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippelli GM, Laidlaw MAS. The elephant in the playground: Confronting lead-contaminated soils as an important source of lead burdens to urban populations. Perspectives in Biology and Medicine. 2010;53:31–45. doi: 10.1353/pbm.0.0136. [DOI] [PubMed] [Google Scholar]
- Fordyce FM. Selenium deficiency and toxicity in the environment. In: Selinus O, Alloway B, Centeno JA, Finkelman RB, Fuge R, Lindh U, Smedley P, editors. Essentials of Medical Geology. Springer, Dordrecht; The Netherlands: 2013. pp. 375–416. [Google Scholar]
- Forsberg KJ, Reyes A, Wang B, Selleck EM, Sommer MOA, Dantas G. The shared antibiotic resistome of soil bacteria and human pathogens. Science. 2012;337:1107–1111. doi: 10.1126/science.1220761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga CG. Relevance, essentiality and toxicity of trace elements in human health. Molecular Aspects of Medicine. 2005;26:235–244. doi: 10.1016/j.mam.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Gardner D, Weindorf DC, Flynn M. Presence of chromium, copper, and arsenic in schoolyard soils. Soil Horizons. 2013;54 doi: 10.2136/sh12-12-0032. [DOI] [Google Scholar]
- Green H, Broun P, Cakmak I, Condon L, Fedoroff N, Gonzalez-Valero J, et al. Planting seeds for the future of food. Journal of the Science of Food and Agriculture. 2016;96:1409–1414. doi: 10.1002/jsfa.7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger P, Mathieu-Nolf M, Lociciro S, Ndiaye C, Coly M, Diouf A, et al. Mass lead intoxication from informal used lead-acid battery recycling in Dakar, Senegal. Environmental Health Perspectives. 2009;117:1535–1540. doi: 10.1289/ehp.0900696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environmental Health Perspectives. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug A, Graham RD, Christophersen OA, Lyons GH. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microbial Ecology in Health and Disease. 2007;19:209–28. doi: 10.1080/08910600701698986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmke MF, Losco RL. Soil’s influence on water quality and human health. In: Brevik EC, Burgess LC, editors. Soils and Human Health. CRC Press; Boca Raton, FL, USA: 2013. pp. 155–176. [Google Scholar]
- Henry JM, Cring FD. Geophagy: an anthropological perspective. In: Brevik EC, Burgess LC, editors. Soils and Human Health. CRC Press; Boca Raton, FL, USA: 2013. pp. 179–198. [Google Scholar]
- Hippocrates. On Airs, Waters and Places (reprint) Kessinger Publishing, LLC; Whitefish, MT, USA: 2010. [Google Scholar]
- Howard A. An Agricultural Testament. Oxford University Press; Oxford, UK: 1940. [Google Scholar]
- Howard JL, Orlicki KM. Effects of anthropogenic particles on the chemical and geophysical properties of urban soils, Detroit, Michigan. Soil Science. 2015;180:154–166. [Google Scholar]
- Howard JL, Shuster WD. Experimental Order 1 soil survey of vacant urban land, Detroit, Michigan, USA. Catena. 2015;126:220–230. [Google Scholar]
- Hu QH, Weng JQ, Wang JS. Sources of anthropogenic radionuclides in the environment: a review. Journal of Environmental Radioactivity. 2010;101:426–437. doi: 10.1016/j.jenvrad.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Hunter P. A toxic brew we cannot live without. EMBO Reports. 2008;9:15–18. doi: 10.1038/sj.embor.7401148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Translational Lung Cancer Research. 2015;4:327–338. doi: 10.3978/j.issn.2218-6751.2015.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlen DL, Mausbach MJ, Doran JW, Cline RG, Harris RF, Schuman GE. Soil quality: a concept, definition, and framework for evaluation. Soil Science Society of America Journal. 1997;61:4–10. [Google Scholar]
- Karltun E, Lemenih M, Tolera M. Comparing farmers’ perception of soil fertility change with soil properties and crop performance in Beseku, Ethiopia. Land Degradation & Development. 2013;24:228–235. [Google Scholar]
- Khan SM, Gomes J. An interdisciplinary population health approach to the radon health risk management in Canada. [Accessed 14 May 2017];Interdisciplinary Journal of Health Sciences. 2017 6 http://riss-ijhs.ca/archives/3177. [Google Scholar]
- Khan SN, Khan AU. Breaking the spell: Combating multidrug resistant ‘superbugs’. Frontiers in Microbiology. 2016;7:174. doi: 10.3389/fmicb.2016.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A, McBratney A, Lal R. Put soil security on the global agenda. Nature. 2012;492:186. doi: 10.1038/492186d. [DOI] [PubMed] [Google Scholar]
- Kwon JC, Nejad ZD, Jung MC. Arsenic and heavy metals in paddy soil and polished rice contaminated by mining activities in Korea. Catena. 2017;148:92–100. [Google Scholar]
- Leake JR, Adam-Bradford A, Rigby JE. Health benefits of ‘grow your own food’ in urban areas: implications for contaminated land risk assessment and risk management. Environmental Health. 2009;8:1–6. doi: 10.1186/1476-069X-8-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A, Stahr K. Nature and significance of anthropogenic urban soils. Journal of Soils and Sediments. 2007;7:247–260. [Google Scholar]
- Li G, Sun G-X, Ren Y, Luo X-S, Zhu Y-G. Urban soil and human health. European Jouirnal of Soil Science. this issue. [Google Scholar]
- Li P, Lin C, Cheng H, Duan X, Lei K. Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicology and Environmental Safety. 2015;113:391–399. doi: 10.1016/j.ecoenv.2014.12.025. [DOI] [PubMed] [Google Scholar]
- Liang P, Feng X, Zhang C, Zhang J, Cao Y, You Q, et al. Human exposure to mercury in a compact fluorescent lamp manufacturing area: by food (rice and fish) consumption and occupational exposure. Environmental Pollution. 2015;198:126–132. doi: 10.1016/j.envpol.2014.12.036. [DOI] [PubMed] [Google Scholar]
- Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, et al. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517:455–59. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YC, Dooyema CA, Neri A, Durant J, Jefferies T, Medina-Marino A, et al. Childhood lead poisoning associated with gold ore processing: a village-level investigation—Zamfara State, Nigeria, October-November 2010. Environmental Health Perspectives. 2012;120:1450–1455. doi: 10.1289/ehp.1104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loynachan TE. Human disease from introduced and resident soilborne pathogens. In: Brevik EC, Burgess LC, editors. Soils and Human Health. CRC Press; Boca Raton, FL, USA: 2013. pp. 107–136. [Google Scholar]
- Magill J, Galy J. Radioactivity, Radionuclides, Radiation. Springer; Berlin, Germany: 2005. [Google Scholar]
- Martinez JL. Antibiotics and antibiotic resistance genes in natural environments. Science. 2008;321:365–67. doi: 10.1126/science.1159483. [DOI] [PubMed] [Google Scholar]
- McBratney A, Field DJ, Koch A. The dimensions of soil security. Geoderma. 2014;213:203–213. [Google Scholar]
- McCarrison R. Studies in Deficiency Disease. Hazell, Watson and Viney Ltd; Aylesbury, UK: 1921. [Google Scholar]
- McManus PS, Stockwell VO, Sundin GW, Jones AL. Antibiotic use in plant agriculture. Annual Review of Phytopathology. 2002;40:443–465. doi: 10.1146/annurev.phyto.40.120301.093927. [DOI] [PubMed] [Google Scholar]
- Morgan R. Soil, heavy metals, and human health. In: Brevik EC, Burgess LC, editors. Soils and Human Health. CRC Press; Boca Raton, FL, USA: 2013. pp. 59–82. [Google Scholar]
- Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World Journal of Gastrointestinal Oncology. 2012;4:156–169. doi: 10.4251/wjgo.v4.i7.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesme J, Cecillon S, Delmont TO, Monier J-M, Vogel TM, Simonet P. Large-scale metagenomics based study of antibiotic resistance in the environment. Current Biology. 2014;10:1096–1100. doi: 10.1016/j.cub.2014.03.036. [DOI] [PubMed] [Google Scholar]
- Nesme J, Simonet P. The soil resistome: a critical review on antibiotic resistance origins, ecology and dissemination potential in telluric bacteria. Environmental Microbiology. 2014;17:913–930. doi: 10.1111/1462-2920.12631. [DOI] [PubMed] [Google Scholar]
- Nordberg GF, Nogawa K, Nordberg M. Cadmium. In: Nordberg GF, Fowler BA, Nordberg M, editors. Handbook on the Toxicology of Metals vol. II: Specific Metals. Elsevier, Amsterdam; The Netherlands: 2015. pp. 667–716. [Google Scholar]
- Oliver MA, Gregory PJ. Soil, food security and human health: a review. European Journal of Soil Science. 2015;66:257–276. [Google Scholar]
- Pepper IL. The soil health: human health nexus. Critical Reviews in Environmental Science and Technology. 2013;43:2617–2652. [Google Scholar]
- Pettry DE, Reneau RB, Shanholtz MI, Graham SA, Jr, Weston CW. Soil pollution and environmental health. Health Services Reports. 1973;88:323–327. [PMC free article] [PubMed] [Google Scholar]
- Palacios OA, Contreras CA, Muñoz-Castellanos LN, González-Rangel MO, Rubio-Arias H, Palacios-Espinosa A, Nevárez-Moorillón GV. Monitoring of indicator and multidrug resistant bacteria in agricultural soils under different irrigation patterns. Agricultural Water Management. 2017;184:19–27. [Google Scholar]
- Philpott SM, Cotton J, Bichier P, Friedrich RL, Moorhead LC, Uno S, Valdez M. Local and landscape drivers of arthropod abundance, richness, and trophic composition in urban habitats. Urban Ecosystems. 2014;17:513–532. [Google Scholar]
- Popowska M, Rzeczycka M, Miernik A, Krawczyk-Balska A, Walsh F, Duffy B. Influence of soil use on prevalence of tetracycline, streptomycin, and erythromycin resistance and associated resistance genes. Antimicrobial Agents and Chemotherapy. 2011;56:1434–1443. doi: 10.1128/AAC.05766-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigitano A, Romanò L, Auxilia F, Castaldi S, Tortorano AM. Antibiotic resistance: Italian awareness survey 2016. Journal of Infection and Public Health. 2017 doi: 10.1016/j.jiph.2017.02.010. [DOI] [PubMed] [Google Scholar]
- PSNRU. [Accessed 7 July, 2016];Welcome to the Plant, Soil and Nutrition Research Unit. 2008 available at: http://www.ars.usda.gov/main/site_main.htm?modecode=19-07-05-05.
- Reyes-Montes MR, Pérez-Huitrón MA, Ocaña-Monroy JL, Frías-De-León MG, Martínez-Herrera E, Arenas R, Duarte-Escalante E. The habitat of Coccidioides spp. and the role of animals as reservoirs and disseminators in nature. BMC Infectious Diseases. 2016;16 doi: 10.1186/s12879-016-1902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard AM, Diaz JH, Kaye AD. Reexamining the risks of drinking-water nitrates on public health. The Ochsner Journal. 2014;14:392–398. [PMC free article] [PubMed] [Google Scholar]
- Richter DD, Jr, Bacon AR, Megan LM, Richardson CJ, Andrews SS, West L, et al. Human-soil relations are changing rapidly: Proposals from SSSA’s cross-divisional Soil Change Working Group. Soil Science Society of America Journal. 2011;75:2079–2084. [Google Scholar]
- Rodale JI. Pay Dirt: Farming and Gardening with Composts. Devin-Adair Company; New York, NY, USA: 1945. [Google Scholar]
- Roy M, McDonald LM. Metal uptake in plants and health risk assessments in metal-contaminated smelter soils. Land Degradation & Development. 2015;26:785–792. [Google Scholar]
- Saha JK, Selladurai R, Coumar MV, Dotaniya ML, Kundu S, Patra AK. Soil Pollution-An Emerging Threat to Agriculture. Springer; Singapore: 2017. [Google Scholar]
- Salem HM, Abdel-Salam A, Abdel-Salam MA, Seleiman MF. Soil xenobiotics and their phyto-chemical remediation. In: Hashmi MZ, Kumar V, Varma A, editors. Xenobiotics in the Soil Environment. Springer International Publishing, Cham; Switzerland: 2017. pp. 267–280. [Google Scholar]
- Shegefti S, Mehdinia A, Shemirani F. Preconcentration of cobalt (II) using polythionine-coated Fe3O4 nanocomposite prior its determination by AAS. Microchimica Acta. 2016;183:1963–1970. [Google Scholar]
- Shetty P. Fundamentals of human health and nutrition. In: Squires V, editor. The Role of Food, Agriculture, Forestry and Fisheries in Human Nutrition. Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, EOLSS Publishers; Oxford, UK: 2009. [Accessed 11 July 2015]. http://www.eolss.net. [Google Scholar]
- Steinnes E. Soils and human health. In: Sauer T, Norman JM, Sivakumar MVK, editors. Sustaining Soil Productivity in Response to Global Climate Change: Science, Policy, and Ethics. John Wiley & Sons, Inc; Chichester, UK: 2011. pp. 79–86. [Google Scholar]
- Stockamp NW, Thompson GR. Coccidioidomycosis. Infectious Disease Clinics of North America. 2016;30:229–246. doi: 10.1016/j.idc.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Stockholm Convention. The 12 Initial POPs Under the Stockholm Convention. Secretariat of the Stockholm Convention Clearing House, Châtelaine; Switzerland: 2008. [Accessed 29 April 2017]. http://chm.pops.int/TheConvention/ThePOPs/The12InitialPOPs/tabid/296/Default.aspx. [Google Scholar]
- Stockholm Convention. The new POPs under the Stockholm Convention. Secretariat of the Stockholm Convention Clearing House, Châtelaine; Switzerland: 2015. [Accessed 29 April 2017]. http://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx. [Google Scholar]
- Swiecilo A, Zych-Wezyk I. Bacterial stress response as an adaptation to life in a soil environment. Polish Journal of Environmental Studies. 2013;22:1577–1587. [Google Scholar]
- Sylvia DM, Fuhrmann JJ, Hartel PG, Zuberer DA. Principles and Applications of Soil Microbiology. Prentice Hall; Upper Saddle River, NJ, USA: 1998. [Google Scholar]
- Tabor JA, O’Rourke MK, Lebowitz MD, Harris RB. Landscape-epidemiological study design to investigate an environmentally based disease. Journal of Exposure Science and Environmental Epidemiology. 2011;21:197–211. doi: 10.1038/jes.2009.67. [DOI] [PubMed] [Google Scholar]
- Tanwir F, Khiyani F. Antibiotic resistance: A global concern. Journal of the College of Physicians and Surgeons Pakistan. 2011;21:127–129. [PubMed] [Google Scholar]
- Trujillo-González JM, Torres-Mora MA, Keesstra S, Brevik EC, Ballesta RJ. Heavy metal accumulation related to population density in road dust samples taken from urban sites under different land uses. Science of the Total Environment. 2016;553:636–642. doi: 10.1016/j.scitotenv.2016.02.101. [DOI] [PubMed] [Google Scholar]
- Udikovic-Kolic N, Wichmann F, Broderick NA, Handelsman J. Bloom of resident antibiotic-resistant bacteria in soil following manure fertilization. Proceedings of the National Academy of Sciences. 2014;111:15202–15207. doi: 10.1073/pnas.1409836111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA. Soils and Men: Yearbook of Agriculture. United States Government Printing Office; Washington, DC, USA: 1938. [Google Scholar]
- USDA. Soil: The 1957 Yearbook of Agriculture. United States Government Printing Office; Washington, DC, USA: 1957. [Google Scholar]
- Vas-Moreira I, Nune OC, Manaia CM. Bacterial diversity and antibiotic resistance in water habitats: searching the links with the human microbiome. FEMS Microbiology Reviews. 2014;38:718–761. doi: 10.1111/1574-6976.12062. [DOI] [PubMed] [Google Scholar]
- Vaudour E, Costantini E, Jones GV, Mocali S. An overview of the recent approaches to terroir functional modelling, footprinting and zoning. SOIL. 2015;1:287–312. [Google Scholar]
- Vega FA, Covelo EF, Andrade ML. Accidental organochlorine pesticide contamination of soil in Porrino, Spain. Journal of Environmental Quality. 2007;36:272–279. doi: 10.2134/jeq2006.0053. [DOI] [PubMed] [Google Scholar]
- Voisin A. Soil, Grass, and Cancer. Philosophical Library Inc; New York, NY, USA: 1959. [Google Scholar]
- von Lindern I, Spalinger S, Stifelman ML, Stanek LW, Bartrem C. Estimating children’s soil/dust ingestion rates through retrospective analyses of blood lead biomonitoring from the Bunker Hill Superfund Site in Idaho. Environmental Health Perspectives. 2016;124:1462–1470. doi: 10.1289/ehp.1510144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall DH, Nielsen UN, Six J. Soil biodiversity and human health. Nature. 2015;528:69–76. doi: 10.1038/nature15744. [DOI] [PubMed] [Google Scholar]
- Wang M, Tang JC. Research of antibiotics pollution in soil environments and its ecological toxicity. Journal of Agro-Environment Science. 2010;29:261–266. [Google Scholar]
- WHO. Evaluating the public health significance of micronutrient malnutrition. In: Allen L, de Benoist B, Dary O, Hurrell R, editors. Guidelines on Food Fortification with Micronutrients. World Health Organization; Geneva, Switzerland: 2006. pp. 39–92. [Google Scholar]
- WHO. Assessment of Iodine Deficiency Disorders and Monitoring their Elimination. 3. World Health Organization; Geneva, Switzerland: 2007a. [Google Scholar]
- WHO. [Accessed 17 July, 2016];Urban Population Growth. 2016 http://www.who.int/gho/urban_health/situation_trends/urban_population_growth_text/en/
- WHO. Assessing the Iron Status of Populations. 2. World Health Organization; Geneva, Switzerland: 2007b. [Google Scholar]
- Woolhouse MEJ, Ward MJ. Sources of antimicrobial resistance. Science. 2013;341:1460–1461. doi: 10.1126/science.1243444. [DOI] [PubMed] [Google Scholar]
- Xu J, Bravo AG, Lagerkvist A, Bertilsson S, Sjöblom R, Kumpiene J. Sources and remediation techniques for mercury contaminated soil. Environment International. 2015;74:42–53. doi: 10.1016/j.envint.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Xu W, Wang X, Cai Z. Analytical chemistry of the persistent organic pollutants identified in the Stockholm Convention: A review. Analytica Chimica Acta. 2013;790:1–13. doi: 10.1016/j.aca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Zhang X, Davidson EA, Mauzerall DL, Searchinger TD, Dumas P, Shen Y. Managing nitrogen for sustainable development. Nature. 2015;528:51–59. doi: 10.1038/nature15743. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, McGrath SP, Meharg AA. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annual Review of Plant Biology. 2010;61:535–559. doi: 10.1146/annurev-arplant-042809-112152. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Ma Y, Zhu YG, Tang Z, McGrath SP. Soil contamination in China: Current status and mitigation strategies. Environmental Science & Technology. 2015;49:750–759. doi: 10.1021/es5047099. [DOI] [PubMed] [Google Scholar]
- Zosky GR, Boylen CE, Wong RS, Smirk MN, Gutiérrez L, Woodward RC, et al. Variability and consistency in lung inflammatory responses to particles with a geogenic origin. Respirology. 2014;19:58–66. doi: 10.1111/resp.12150. [DOI] [PubMed] [Google Scholar]