Abstract
The mammalian testis is an immunoprivileged organ where male germ cell autoantigens are immunologically ignored. Both systemic immune tolerance to autoantigens and local immunosuppressive milieu contribute to the testicular immune privilege. Testicular immunosuppression has been intensively studied, but information on systemic immune tolerance to autoantigens is lacking. In the present study, we aimed to determine the role of Axl and Mer receptor tyrosine kinases in maintaining the systemic tolerance to male germ cell antigens using the experimental autoimmune orchitis (EAO) model. Axl and Mer double-knockout (Axl−/−Mer−/−) mice developed evident EAO after a single immunization with germ cell homogenates emulsified with complete Freund’s adjuvant. EAO was characterized by the accumulation of macrophages and T lymphocytes in the testis. Damage to the seminiferous epithelium was also observed. EAO induction was associated with pro-inflammatory cytokine upregulation in the testes, impaired permeability of the blood–testis barrier and generation of autoantibodies against germ cell antigens in Axl−/−Mer−/− mice. Immunization also induced mild EAO in Axl or Mer single-gene-knockout mice. By contrast, a single immunization failed to induce EAO in wild-type mice. The results indicate that Axl and Mer receptors cooperatively regulate the systemic immune tolerance to male germ cell antigens.
A large number of immunogenic autoantigens is produced by developing male germ cells after establishment of immunocompetence.1 These germ cell antigens do not induce detrimental immune response in the testis under physiological conditions because of the testicular immunoprivileged properties. However, some stimuli may disrupt the testicular immune privilege, thereby inducing immune response against autoantigens and leading to autoimmune orchitis, which may perturb male fertility.2 Understanding the mechanisms underlying testicular immune privilege and autoimmune infertility can aid in the development of preventive and therapeutic approaches for autoimmune orchitis.
Experimental autoimmune orchitis (EAO) can be induced by immunizing murine animals with allogeneic testicular antigens emulsified with adjuvant. This model is used to investigate mechanisms underlying the pathogenesis of autoimmune orchitis.3 EAO is characterized by the generation of autoantibodies against germ cell antigens, infiltration of immune cells into the testes and the impairment of spermatogenesis.4–6 The pathogenic role of T lymphocytes is established in EAO.7 Pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), produced by the infiltrated immune cells induce germ cell apoptosis in EAO.8 Interval triple immunizations are usually required for EAO induction in rats. By contrast, single or two immunizations may induce EAO in some susceptible mouse strains.3 However, the mechanisms underlying the regulation of EAO development remain largely unknown.
Axl and Mer receptors belong to a subfamily of receptor tyrosine kinases that comprise three members: Tyro3, Axl and Mer (TAM).9 Two closely related vitamin K-dependent proteins, namely, growth arrest-specific gene 6 (Gas6) and Protein S, are common ligands of TAM receptors.10,11 Studies on gene-knockout mice provided direct insights into the physiological functions of TAM receptors. Mice lacking any single receptor or any combination of two receptors can produce healthy offspring. Apparent defects were not observed in these mice during their lifetime. However, adult TAM triple-knockout (TAM−/−) mice exhibit various severe phenotypes, such as male sterility and a broad spectrum of autoimmune diseases.12,13 Previous studies demonstrated that TAM receptors suppress innate immune response and promote the removal of apoptotic cells by phagocytes, thereby regulating autoimmune disease development.14–17
The testis is a remarkably immunoprivileged organ that tolerates immunogenic germ cell autoantigens. Substantial evidence supports the views that systemic immune tolerance to germ cell antigens and local active immunosuppressive milieu contribute to the maintenance of testicular immune privilege.18 The local immunosuppressive mechanisms within the testis have been intensively investigated.19 By contrast, mechanisms underlying systemic immune tolerance to germ cell autoantigens are poorly understood. Roles of TAM receptors in regulating testicular functions are emerging. TAM receptors and their ligand Gas6 are abundantly expressed in testicular somatic cells.20 Gas6/TAM signaling is necessary for the optimal phagocytic removal of apoptotic germ cells by Sertoli cells.21 We recently demonstrated that TAM receptors are essential for normal spermatogenesis and maintenance of testicular immune homeostasis.22,23 The present study examined the roles of Axl and Mer receptor tyrosine kinases in regulating systemic immune tolerance to male germ cell antigens.
RESULTS
EAO score
On the basis of a previously described scaling system,24 EAO scores were classified into six stages in this study (Figure 1a): stage 0, no detectable inflammatory signs in the whole testis; stage 1, focal inflammation (arrows) in the tunica albuginea; stage 2, focal inflammation adjacent to the tubuli recti; stage 3, inflammation surrounding the tubuli recti; stage 4, inflammation spreading around the seminiferous tubules and mild damage to the seminiferous epithelium; and stage 5, widespread inflammation surrounding the seminiferous tubules and severe damage to the seminiferous epithelium. The immunization of wild-type (WT) mice did not induce evident EAO (Figure 1b). Only stage 1 EAO was found in 3 of 12 WT mice 50 days after immunization. Mild EAO was observed in Axl−/−or Mer−/− mice. Notably, severe EAO at stages 4 and 5 were developed in immunized Axl−/−Mer−/− mice. According to EAO severity, testis weight significantly decreased in immunized Axl−/−Mer−/− mice (Figure 1c). Immunization failed to significantly alter testis weight in WT, Axl−/− and Mer−/− mice. In controls, the immunization of mice with complete Freund’s adjuvant (CFA) alone did not induce EAO (data not shown).
Figure 1.
EAO score. (a) Histological EAO score. Paraffin sections of the testes were stained with hematoxylin–eosin. Images represent six EAO stages based on testicular inflammation as described in the Results. Arrows, focal inflammation; BV, blood vessel; TA, tunica albuginea; RT, rete testis; ST, seminiferous tubule; TR, tubuli recti. Scale bar =20 μm. (b) EAO stages in different mice. Mice with indicated genotypes were immunized with male germ cell homogenates emulsified in CFA. EAO stage of individual mice after 50 days was determined based on histological analysis. (c) Testis size. Representative testicular images of wild-type (WT) and Axl−/−Mer−/− (AM−/−) mice at 50 days after immunization (Imm.). Mice injected with CFA alone served as the control (Ctrl). Testis weight was measured in the indicated mice (right panel). Data represent mean values ±s.e.m. (n =12). **P<0.01. A full color version of this figure is available at the Immunology and Cell Biology journal online.
Immune cell infiltration
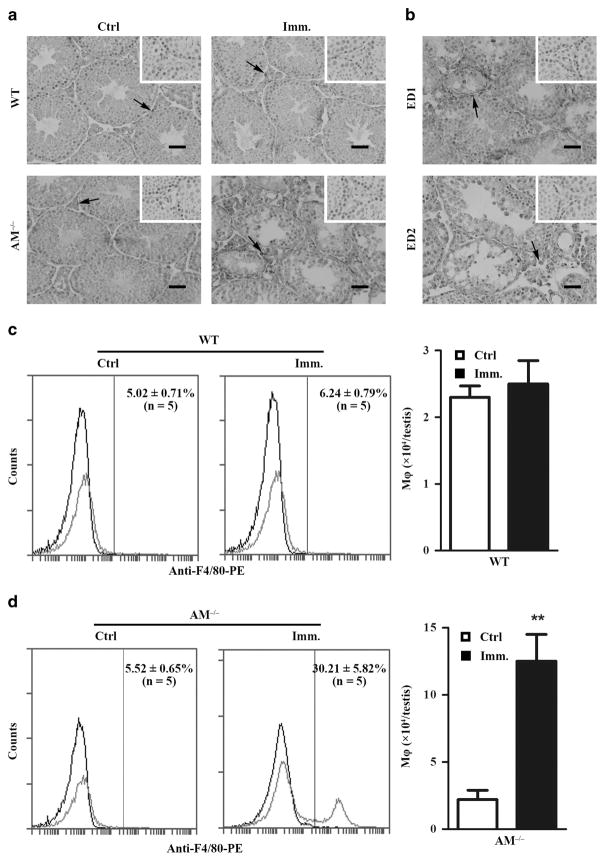
Infiltration of immune cells, including macrophages and lymphocytes, into the testis is a major EAO characteristic. Immunohistochemistry using antibody against F4/80 (a macrophage marker) showed minor F4/80+ cells (arrows) in the testicular interstitial spaces of control WT and Axl−/−Mer−/− mice (Figure 2a, left panels). Macrophages evidently accumulated in the testis of immunized Axl−/−Mer−/− mice (Figure 2a, right lower panel). By contrast, immunization did not evidently induce macrophage infiltration in the testis of WT mice (Figure 2a, right upper panel). Macrophages in Axl−/−Mer−/− testis were predominantly CD163± cells, which are circulating macrophages (Figure 2b, upper panel). CD68± cells remained in a minor population, which are resident macrophages (Figure 2b, lower panel). The total number of macrophages per testis was determined using flow cytometry after labeling of the interstitial cells with phycoerythrin (PE)-conjugated anti-F4/80. As shown in Figure 2c, immunization failed to significantly affect the macrophage number in the testis of WT mice compared with control mice (injected with CFA alone). By contrast, about approximately fivefold more macrophages were detected in the testis of immunized Axl−/−Mer−/− mice (Figure 2d) than in the control.
Figure 2.
Macrophage infiltration. (a) Immunohistochemistry for macrophages. Testicular cryosections of WT and AM−/− mice at 50 days after immunization were immunostained with anti-F4/80 antibody (right panels). Testes of mice injected with CFA alone served as the control (left panels). (b) CD68± and CD163± macrophages. Immunohistochemical analyses of testicular sections of immunized AM−/− mice were performed for discriminating CD68± and CD163 ± macrophages using ED1 and ED2 antibodies. Insets in the upper right corner are the negative controls, in which pre-immune animal sera was used as primary antibodies. Scale bar =20 μm. Images represent at least three mice. (c and d) Quantitative analyses of macrophages in the testes of WT (c) and AM−/− (d) mice. Testicular interstitial cells were labeled with PE-conjugated anti-F4/80 antibody (anti-F4/80-PE) and analyzed by flow cytometry. Left panels represent flow cytometry density plots, whereas right panels represent absolute macrophage numbers per testis based on flow cytometry data. Data are mean values ±s.e.m. of five mice. **P<0.01. A full color version of this figure is available at the Immunology and Cell Biology journal online.
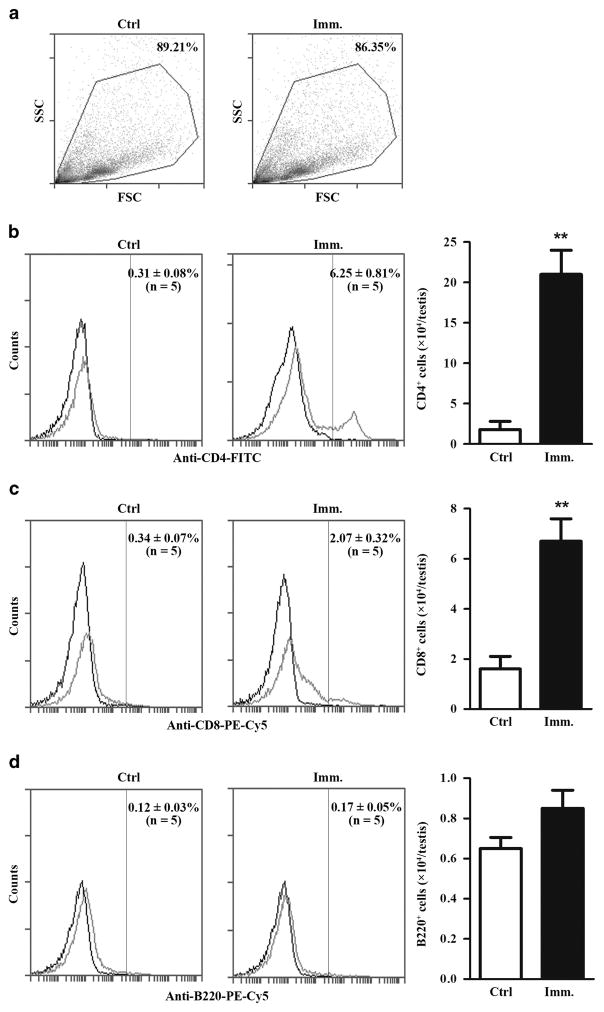
Lymphocytes in the testicular interstitium of control and immunized Axl−/−Mer−/− mice were analyzed using flow cytometry in gated cell populations (Figure 3a). Immunization significantly increased CD4+ (Figure 3b) and CD8+ (Figure 3c) cell numbers in the testis. By contrast, the immunization did not change B-cell numbers (Figure 3d). Notably, the total CD4+ cell numbers were approximately threefold higher than the total CD8+ cell numbers in the testis of immunized Axl−/−Mer−/− mice.
Figure 3.
Lymphocyte infiltration. (a) Flow cytometry analysis. Testicular interstitial cells were isolated from control and immunized Axl−/−Mer−/− mice and gated for lymphocyte analyses. SSC, side scattering; FSC, forward scattering. (b) CD4+ cells. Testicular interstitial cells were labeled with fluorescein isothiocyanate (FITC)-conjugated anti-CD4 antibody (anti-CD4-FITC) and analyzed by flow cytometry. (c) CD8+ cells. Testicular interstitial cells were labeled with PE-Cy5-conjugated anti-CD8 antibody (anti-CD8-PE-Cy5) and analyzed using flow cytometry. (d) B cells. Testicular interstitial cells were labeled with PE-Cy5-conjugated anti-B220 antibody (anti-B220-PE-Cy5) and analyzed using flow cytometry. Absolute numbers of lymphocytes per testis were determined based on flow cytometry data (right panels). Flow cytometry density plots represent five mice. Data are mean values ±s.e.m. of five mice. **P<0.01. A full color version of this figure is available at the Immunology and Cell Biology journal online.
Lymphocyte ratios in renal lymph nodes (RLNs)
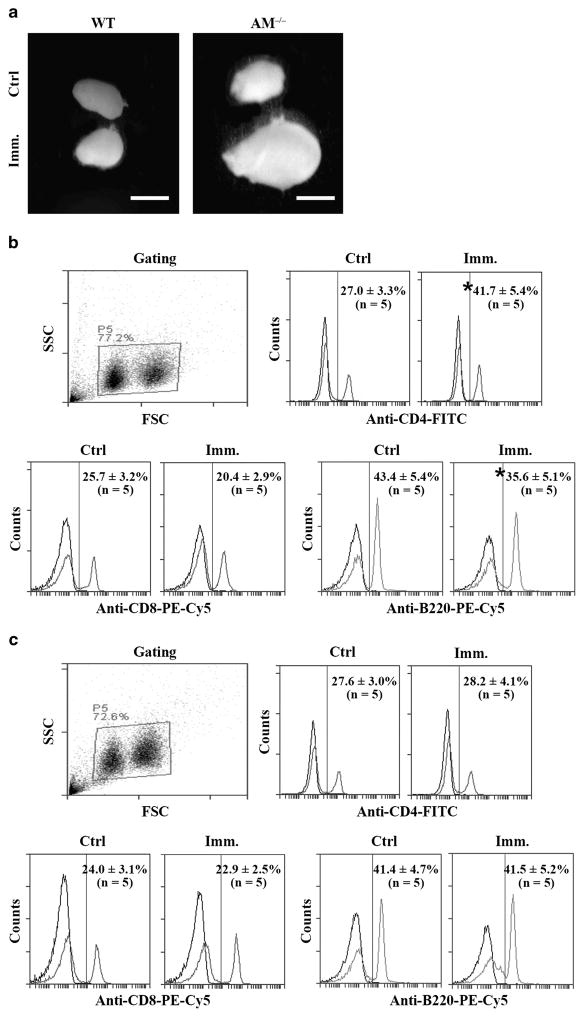
To further analyze lymphocyte cell response to immunization, we examined lymphocyte ratios in RLNs. RLN size was markedly enlarged in Axl−/−Mer−/− mice at 50 days after immunization (Figure 4a, right panel). By contrast, immunization did not alter RLN size in WT mice (Figure 4a, left panel). Ratios of lymphocyte subsets were determined using flow cytometry in gated lymphocyte populations (Figure 4b, upper left panel). Immunization significantly increased the percentages of CD4+ cells in the RLNs of Axl−/−Mer−/− mice (Figure 4b, upper right panel). By contrast, the percentages of CD8+ and B cells were decreased (Figure 4b, lower panels). The lymphocyte subsets remained consistent in the RLNs of the control and immunized WT mice (Figure 4c).
Figure 4.
Lymphocytes in renal lymph nodes (RLNs). (a) RLN size. WT and Axl−/−Mer−/− (AM−/−) mice were immunized. After 50 days, RLNs were collected for analysis. Images represent RLNs from five mice. Scale bar =1 mm. (b) Lymphocytes in RLNs of AM−/− mice. RLN cells were isolated from the control and immunized AM−/− mice. Cells were labeled with anti-CD4-FITC, anti-CD8-PE-Cy5 and anti-B220-PE-Cy5. Lymphocyte populations were gated (upper left panel). Ratios of CD4+ cells (upper right panel) and CD8+ and B cells (lower panels) were determined using flow cytometry. (c) Lymphocytes in RLNs of WT mice. RLN cells of control and immunized WT mice were analyzed as in b. Data are mean values ±s.e.m. of five mice. *P<0.05. FITC, fluorescein isothiocyanate; FSC, forward scattering; SSC, side scattering. A full color version of this figure is available at the Immunology and Cell Biology journal online.
Expression of pro-inflammatory cytokines in the testis
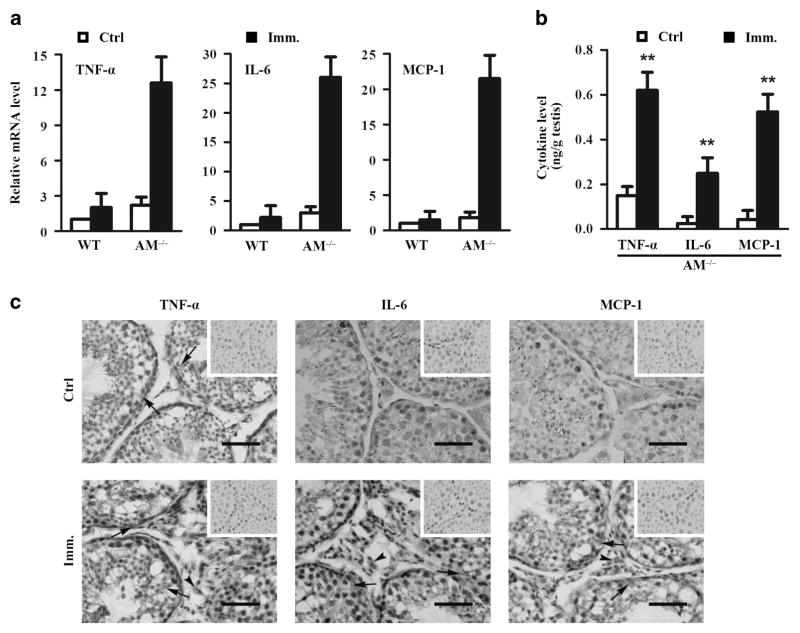
Pro-inflammatory cytokines, including IL-6, TNF-α and monocyte chemotactic protein (MCP)-1, participate in the pathogenesis of EAO.8 Thus, we examined the expression of these cytokines in the testis. Real-time quantitative reverse transcription PCR results showed that TNF-α, IL-6 and MCP-1 messenger RNA levels were evidently upregulated in the testes of Axl−/−Mer−/− mice at 50 days after immunization (Figure 5a). By contrast, immunization failed to significantly upregulate the cytokine messenger RNAs in the testes of WT mice. Enzyme-linked immunosorbent assay (ELISA) results confirmed that the cytokine levels significantly increased in the testes of immunized Axl−/−Mer−/− mice (Figure 5b). Immunohistochemistry showed that TNF-α was predominantly localized in Sertoli cells (black arrows) in the control Axl−/−Mer−/− mice (Figure 5c, upper left panel). IL-6 and MCP-1 were not detectable by immunohistochemistry in the testicular cells of the control mice (Figure 5c, upper middle and right panels). By contrast, signals of TNF-α, IL-6 and MCP-1 were evidently increased in the testes of immunized Axl−/−Mer−/− mice (Figure 5c, lower panels). Notably, signals of the three cytokines were observed in Sertoli cells (black arrows) and interstitial cells (black arrowheads). The interstitial cells expressing the cytokines are presumably macrophages.
Figure 5.
Expression of pro-inflammatory cytokines in the testis. (a) Cytokine expression at messenger RNA (mRNA) levels. Total RNAs were extracted from testes of individual control and immunized WT and Axl−/−Mer−/− (AM−/−) mice. Relative mRNA levels of major pro-inflammatory cytokines, including TNF-α, IL-6 and MCP-1, were determined using real-time qRT-PCR. (b) Protein levels of cytokines in the testis. The testes of control and immunized AM−/− mice were lysed by grinding in PBS. Cytokine levels in the lysates were measured using ELISA. (c) Distribution of cytokines in the testis. Immunohistochemistry of testis cryosections from control (upper panels) and immunized (lower panels) AM−/− mice were performed using specific antibodies against TNF-α, IL-6 and MCP-1. Arrows and arrowheads point to Sertoli cells and interstitial cells, respectively. Images are the representatives of at least three independent experiments in three mice. Scale bar =20 μm. Data represent mean values ±s.e.m. of three experiments. **P<0.01. A full color version of this figure is available at the Immunology and Cell Biology journal online.
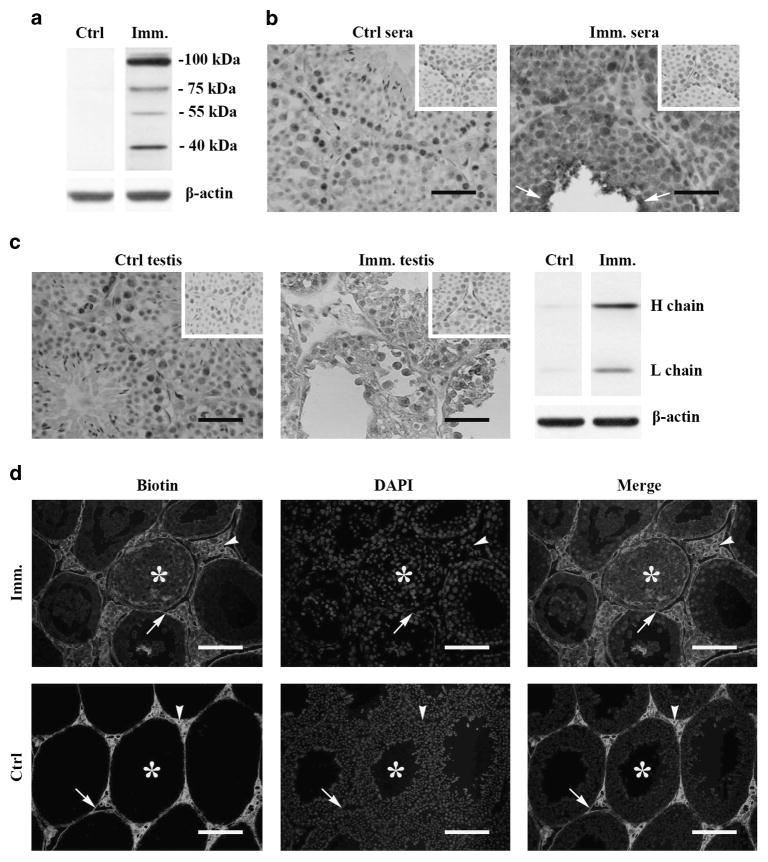
Autoantibody production and BTB permeability
The generation of autoantibodies against germ cell antigens is also a characteristic hallmark of EAO.2 Using western blot, the autoantibodies were not detected in the sera of control Axl−/−Mer−/− mice (Figure 6a, left panel). We detected autoantibodies that recognize four germ cell antigens with molecular weights between 40 and 100 kDa in the sera of mice at 50 days after immunization (Figure 6a, right panel). The four autoantigens were recognized by the sera of all immunized Axl−/−Mer−/− mice. By contrast, immunization failed to induce autoantibody production in WT mice (data not shown). Immuno histochemistry results confirmed that the sera of the immunized Axl−/−Mer−/− mice recognized most stages of male germ cells (Figure 6b, right panel). The more intensive signals were observed in elongating spermatids (arrows). By contrast, the germ cells were not stained by the sera of the control Axl−/−Mer−/− mice (Figure 6b, left panel). Further, immunostaining with horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin (Ig)G showed that the autoantibodies were deposited in the germ cells of the testes of Axl−/−Mer−/− mice at 50 days after immunization (Figure 6c, middle panel). The presence of autoantibodies in the male germ cells of the immunized Axl−/−Mer−/− mice was confirmed by western blot, showing evident heavy and light chains of IgG (Figure 6c, right panel). The autoantibody deposits were not detected in the testes of the control Axl−/−Mer−/− mice (Figure 6c, left panel).
Figure 6.
Autoantibody production and BTB permeability. (a) Autoantibodies in sera. Sera of control and immunized Axl−/−Mer−/− (AM−/−) mice were collected by tail bleeding. Male germ cells were isolated from 10-week-old WT mice. Western blots on germ cell lysates were performed using sera (1:500 dilution) of AM−/− mice as primary antibodies. (b) Distribution of autoantibodies. Testicular cryosections of 10-week-old WT mice were immunostained with sera (1:100 dilution) of control (left panel) and immunized (right panel) AM−/− mice. Insets in the upper right corners are negative controls. Negative controls were stained using sera of WT mice (without immunization) as primary antibodies. (c) Autoantibody deposition. Testicular sections of control (left panel) and immunized (middle panel) AM−/− mice were directly stained with anti-mouse IgG antibodies. Signals in germ cells (arrow) of immunized AM−/− mice indicate deposition of autoantibodies. Arrows indicate elongating spermatids. Testis of WT mice without immunization served as the negative control (upper right corners). Heavy (H) and light (L) chains of IgG in testes of immunized AM−/− mice were confirmed by Western blot using anti-mouse IgG antibodies (right panel). β-Actin was used as the loading control for western blot. (d) BTB permeability. Biotin was injected underneath the testicular capsules of control (lower panels) and immunized (upper panels) AM−/− mice. After 30 min, paraffin sections were stained with fluorescein isothiocyanate-conjugated streptavidin (left panels), after which the sections were counterstained with 4′, 6-diamidino-2-phenylindol (DAPI) (middle panels). Right panels are merged biotin and DAPI images. Arrowheads, arrows and asterisks indicate interstitial spaces, basal and adluminal compartments of the seminiferous tubules, respectively. Images represent at least three experiments of three mice. Scale bar =20 μm. A full color version of this figure is available at the Immunology and Cell Biology journal online.
The presence of autoantibodies in the germ cells of immunized Axl−/−Mer−/− mice suggest that blood–testis barrier (BTB) permeability was impaired. The biotin-tracing assay confirmed that biotin was accessed into the adluminal compartments (asterisks) of the seminiferous tubules (Figure 6d, upper panels). By contrast, the biotin was only detected in basal membranes (arrows) and interstitial spaces (arrowheads) in the testes of control Axl−/−Mer−/− mice (Figure 6d, lower panels).
DISCUSSION
Testicular immune privilege protects male germ cell autoantigens from detrimental immune responses within the testis under physiological conditions. Nevertheless, some stimuli, such as microbial infection, physical trauma and endogenous inflammation, can disrupt the testicular immune privilege and induce autoimmune orchitis, which impairs fertility.25 Both systemic immune tolerance to autoantigens and local active immunosuppression are involved in the maintenance of testicular immune privilege. Information is lacking on the mechanisms underlying the systemic immune tolerance to autoantigens. We aimed to determine the importance of Axl and Mer receptor tyrosine kinases in maintaining the systemic immune tolerance to male germ cell antigens in mice.
EAO is a tissue-specific autoimmune orchitis model that can be generated by immunizing murine with allogeneic testicular autoantigens emulsified with adjuvant. Some protocols to induce EAO in susceptible mouse strains do not require adjuvants.24 To examine the functions of Axl and Mer in the regulation of autoimmune response to male germ cell antigens, we compared the susceptibility of WT, Axl−/−, Mer−/− and Axl−/−Mer−/− mice with EAO induction. We immunized mice with homogenates of germ cells instead of whole testicular antigens to induce male germ cell-specific autoimmune responses. Severe EAO was induced in Axl−/−Mer−/− mice by a single immunization of animals with male germ cell antigens emulsified with CFA. Axl−/− or Mer−/− mice developed mild EAO after immunization. By contrast, a single immunization failed to induce EAO in WT mice. This finding agrees with the results of a previous study, which stated that three immunizations are usually required to induce EAO in rats.26,27 Although single immunization may induce EAO in some susceptible mouse strains, this protocol does not induce EAO development in C57BL/6 mice.24 Mice used in this study were obtained by backcrossing mutant mice to C57BL/6 mice for five generations, which should explain that single immunization did not induce EAO in WT mice. These results suggest that Axl and Mer cooperatively inhibit EAO induction. Notably, these data are confined to the observations at 50 days after immunization. We did not examine longer time points in this study.
TAM−/− mice developed a broad spectrum of autoimmune diseases, suggesting that TAM receptors participate in the maintenance of immune tolerance to autoantigens.13 Recent studies confirmed that TAM receptors inhibited autoimmune responses to retinal antigens.28,29 Male infertility is among the major phenotypes in TAM−/− mice.12 Therefore, we investigated the functions of TAM receptors in the mouse testes. We recently demonstrated that TAM receptors inhibit innate immune responses in Sertoli and Leydig cells, and TAM−/− mice progressively develop chronic orchitis.22,30,31 These observations suggest that TAM receptors regulate immune homeostasis within the testis. To expand the results of previous studies, we showed in the present study that Axl and Mer cooperatively participated in the maintenance of the systemic immune tolerance to male germ cell antigens. By contrast, another TAM member, Tyro3, would not participate in the systemic immune tolerance to male germ cell antigens, because Tyro3−/− mice was not susceptible to EAO induction (data not shown). This finding could be due to the expression of Axl and Mer, but not Tyro3, in antigen-presenting cells, including dendritic cells and macrophages.14 The current results provide novel insights into the mechanisms underlying the systemic immune tolerance to male germ cell antigens.
Innate immune response facilitates antigen-specific adaptive immunity to alloantigens and autoantigens.32 Toll-like receptors (TLRs)-initiated innate immune responses may induce autoimmune disease progression.33,34 TAM receptors are negative regulators of TLR signaling.14 We speculate that TAM receptors mediate immune tolerance to EAO induction through the inhibition of TLR-initiated innate immune responses. In support of this hypothesis, male germ cells abundantly express TLR agonists, including high-mobility group box 1 and several heat shock proteins.35,36 Moreover, the fact that CFA promotes EAO development suggests the involvement of TLRs in EAO induction, because major components of CFA, Mycobacteria tuberculosis, are powerful agonists of TLR2 and TLR4.37,38 The involvement of TLRs in EAO induction merit clarification, and we are investing this issue.
Leukocyte infiltration into the testis occurs during EAO induction. Significant accumulation of macrophages and T lymphocytes was observed in the testes of Axl−/−Mer−/− mice after immunization. Although both CD4+ and CD8+ cell numbers significantly increased, the increased folds in CD4+ cells were relatively higher than in CD8+ cells. This result agrees with those of previous reports that CD4+ cells are predominantly involved in EAO pathogenesis.5,7 Pro-inflammatory cytokines produced by infiltrating macrophages are involved in the pathogenesis of EAO in rats.39–41 In agreement with these previous findings, we detected the marked upregulation of major pro-inflammatory cytokines, including TNF-α, IL-6 and MCP-1, in the testes of Axl−/−Mer−/− mice after immunization. Notably, immunohistochemistry results showed that these cytokines were upregulated not only in testicular interstitial cells that are presumably macrophages, but also in Sertoli cells. These results correspond to findings in previous reports that TAM receptors inhibit TNF-α and IL-6 expression in macrophages and Sertoli cells through negatively regulating TLR signaling.14,31 Although the cytokine signals seemly appeared in some spermatogenic cells, these signals would be caused by poor resolution of the images because the germ cells are intimately embraced by Sertoli cells and do not express TAM receptors.
EAO is also characterized by the generation of autoantibodies against male germ cell antigens, which also contribute to pathogenesis.42 We demonstrated that Axl−/−Mer−/− mice significantly produced autoantibodies in response to immunization. This finding might be due to the excessive activation of innate immune responses in antigen-presenting cells of Axl−/−Mer−/− mice. Innate immune responses facilitate antigen-specific adaptive immunity.43 Mechanisms underlying the innate immune response regulation of adaptive immunity to male germ cell antigens are worthy of further study. We detected four germ cell antigens that can be recognized by autoantibodies. Identification of these autoantigens could further reveal the mechanisms of autoimmune orchitis, and facilitate the development of novel alternative diagnostic approaches for this disease. The autoantibody deposits in germ cells in immunized Axl−/−Mer−/− mice suggest that BTB permeability was impaired, which was confirmed by the biotin-tracing assay. High level of TNF-α would contribute to BTB impairment because it regulates BTB permeability through degrading extracelluar matrix.44
In conclusion, we demonstrated that Axl and Mer receptor tyrosine kinases cooperatively inhibit EAO induction in mice. The results suggest that Axl and Mer are critical for maintaining systemic immune tolerance to male germ cell antigens. The data provide novel insights into the mechanisms of testicular immune privilege.
METHODS
Animals
Axl and Mer knockout (Axl−/− and Mer−/−) mice were kindly provided by Dr Qingxian Lu (University of Louisville, Louisville, KY, USA). Mice were progenies of an original colony with a genetic background of 50% 129/SV × 50% C57BL/6 and then backcrossed to C57BL/6 for five generations. Axl and Mer double-mutant (Axl−/−Mer−/−) mice were obtained by mating Axl−/− and Mer−/− mice and WT mice were littermates of Axl−/−Mer−/−mice. Mice were inbred under pathogen-free conditions with nutrition (food and water were provided ad libitum) and light cycle (12 h light:12 h darkness). All mice were handled according to the Guideline for the Care and Use of Laboratory Animals, and experimental procedures were approved by the Chinese Council on Animal Care.
Antibodies
Goat anti-Axl (sc-1096), goat anti-Mer (sc-6873), rabbit ED2 (sc-33560) and rabbit anti-IL-6 (sc-1265-R) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse anti-β-actin monoclonal antibody (A5316) was purchased from Sigma (St Louis, MO, USA). Rabbit ED1 (ab125212), anti-TNF-α (ab34674), anti-MCP-1 (ab7202) and rat anti-F4/80 (ab6640) antibodies were purchased from Abcam (Cambridge, UK). HRP-conjugated secondary antibodies against mouse IgG (ZB-2305), rat IgG (ZB-2307), rabbit IgG (ZB-2301) and goat IgG (ZB-2306) were purchased from Zhongshan Biotechnology Co. (Beijing, China). PE-conjugated anti-F4/80 antibody (123109) was purchased from BioLegend (San Diego, CA, USA). Fluorescein isothiocyanate-conjugated anti-CD4 (11-0041) and PE-Cy5-conjugated anti-CD8 antibodies (15-0081) were purchased from eBioscience (San Diego, CA, USA). PE-Cy5-conjugated anti-B220 antibody (553091) was purchased from BD Biosciences (San Jose, CA, USA).
EAO induction
Ten-week-old WT, Axl−/−, Mer−/− and Axl−/−Mer−/− male mice were used in EAO induction based on previously described procedures.45 Germ cell homogenates were emulsified with an equal volume of CFA (Sigma). The emulsified homogenates of 108 cells in a volume of 0.4 ml were subcutaneously injected into three sites near the popliteal lymph nodes of mice. Mice injected with an equal volume of emulsion of phosphate-buffered saline (PBS) with CFA served as the control. Fifteen mice were injected in each group. Testes were collected for EAO analysis at 50 days after the immunization.
Isolation of testicular interstitial cells and germ cells
The testes were decapsulated and incubated with 0.5 mg ml−1 type I collagenase (Sigma) at room temperature for 15 min with gentle oscillation. Cell suspensions were filtered through 80-μm copper meshes to remove the seminiferous tubules. The interstitial cells were collected by centrifugation at 600 g for 10 min and were subjected to flow cytometry.
Male germ cells were isolated from 10-week-old mice. After removal of the interstitial cells, the seminiferous tubules were cut into small pieces (~1 mm3) and incubated with 0.5 mg ml−1 of hyaluronidase (Sigma) at room temperature for 10 min with gentle pipetting to dissociate cells. After filtering through 80-μm copper meshes, cell suspensions were collected and homogenized in 1 × PBS.
Isolation of lymphocytes from RLNs
Mouse RLNs were removed and cut into small pieces in Hanks’ balanced salt solution (Sigma). The suspensions were gently pipetted to dissociate the cells and were filtered through 80-μm copper meshes. The single-cell fractions were collected for flow cytometry.
Flow cytometry
Testicular interstitial cells and RLN lymphocytes were incubated with appropriate PE- or fluorescein isothiocyanate-conjugated antibodies according to the manufacturer’s instructions and were analyzed using FACSCanto flow cytometer (BD Biosciences).
Histology and immunohistochemistry
For histological analysis, the testes were fixed in 10% neutral buffered formalin for 24 h, embedded in paraffin and cut into 5-μm-thick sections. The sections were stained with hematoxylin and eosin and mounted with neutral balsam (Zhong-shan Biotechnology Co.) for observation under a microscope. For immunohistochemical analysis, the testes were fixed in 4% paraformaldehyde for 24 h. After cryoprotection in 30% sucrose, the frozen sections were cut in a thickness of 7 μm using Leica CM1950 (Leica Biosystems, Nussloch, Germany). The sections were incubated with PBS containing 3% H2O2 for 10 min to inhibit endogenous peroxidase activity. After blocking with 5% rabbit serum in PBS for 1 h at room temperature, the sections were incubated with primary antibodies overnight at 4 ° C. After washing thrice with PBS, the sections were incubated with HRP-conjugated secondary antibodies (Zhongshan Biotechnology Co.) at room temperature for 30 min. HRP activity was visualized using the diaminobenzidine method according to the manufacturer’s instructions. The sections incubated with pre-immune animal sera as primary antibodies served as the negative control. After counterstaining with hematoxylin, the sections were mounted with neutral balsam for observation under microscope BX51 (Olympus, Tokyo, Japan).
Real-time quantitative reverse transcription PCR
Total RNA was extracted using TRIzol reagent (Life Technologies, Carlsbad, CA, USA). RNA was treated with RNase-free DNase I (Life Technologies) to remove genomic DNA contaminants. The absence of genomic DNA was confirmed by amplifing β-actin for 35 cycles using conventional PCR prior to reverse transcription. RNA (1 μg) was reverse transcribed into complementary DNA in a 20-μl reaction system containing 2.5 μM random hexamers, 2 mM deoxynucleotide triphosphates and 200 U Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA). PCR was performed in 20 μl of the reaction system containing 0.2 μl of complementary DNA, 0.5 μM forward and reverse primers each, and 10 μl of the 2 × Power SYBR Green PCR Master Mix kit (Life Technologies) using an ABI PRISM 7300 real-time cycler (Applied Biosystems, Foster City, CA, USA). Relative messenger RNA levels of target genes normalizing to β-actin were obtained by 2−ΔΔCt using a comparative threshold cycle method, as described in Applied Biosystems User Bulletin No. 2 (P/N 4303859). Reaction efficiencies were determined between 92 and 100% for amplification. The sequences of primers are listed in Table 1.
Table 1.
Primers used for qRT-PCRs
| Target genes | Primer pairs (5′→3′) | |
|---|---|---|
|
| ||
| Forward | Reverse | |
| IL-6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA |
| TNF-α | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| MCP-1 | TTAACGCCCCACTCACCTGCTG | GCTTCTTTGGGACACCTGCTGC |
| β-actin | GAAATCGTGCGTGACATCAAAG | TGTAGTTTCATGGATGCCACAG |
Abbreviations: IL-6, interleukin-6; MCP-1, monocyte chemotactic protein-1; qRT-PCR, quantitative reverse transcription PCR; TNF-α, tumor necrosis factor alpha.
Enzyme-linked immunosorbent assay (ELISA)
The testis was lysed by grinding in 1 × PBS. The lysate was centrifuged at 800 g for 5 min. The cytokine levels in the supernatant were measured using ELISA kits according to the manufacturer’s instructions. ELISA kits for TNF-α (BMS607/3), IL-6 (BMS603/2) and MCP-1 (BMS6005) were purchased from eBioscience (San Diego, CA, USA). Detection limits of ELISA kits were <7 pg ml−1, intra- and inter-assay variation were <5% and <10%, respectively.
Western blot analysis
The testis was lysed with a lysis buffer (C1053, Applygen Technologies Inc., Beijing, China). The protein concentration of lysates was determined using the bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Equal amounts of proteins (20 μg per lane) were separated on 10% SDS-polyacrylamide gel electrophoresis gel and then electrotransferred onto poly-vinyl difluoride membranes (Millipore, Bedford, MA, USA). The membranes were blocked in Tris-buffered saline (pH 7.4) containing 5% nonfat milk for 1 h, and incubated with mouse sera overnight at 4 °C. The membranes were washed twice with Tris-buffered saline containing 0.1% Tween-20 and incubated with HRP-conjugated anti-mouse IgG (Zhongshan Biotechnology Co.) at room temperature for 1 h. HRP activity was detected using an enhanced chemiluminescence detection kit (Zhongshan Biotechnology Co.).
Biotin-tracing assay
BTB permeability was assessed by the biotin-tracer assay according to previously described procedures.46 Mice were anesthetized using 50 mg kg−1 body weight pentobarbital sodium. Approximately 20 μl of 10 mg ml−1 EZ-link Sulfo-NHS-LC-Biotin (Thermo Scientific, Rockford, IL, USA) was injected underneath the testicular capsule using a 30-gauge needle. After 30 min, the testes were recovered for histochemistry. The paraffin sections were stained with fluorescein isothiocyanate-conjugated streptavidin (Vector Laboratories, Burlingame, CA, USA) at room temperature for 30 min. After washing with PBS twice, the sections were counterstained with 4′, 6-diamidino-2-phenylindol for 10 min. Slides were mounted with neutral balsam for observation under a fluorescence microscope BX51 (Olympus).
Statistical analysis
Data represent mean values ±s.e.m. Statistical significance was determined using the Student’s t-test. Calculations were performed using statistical software SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grand Nos: 31171445, 31261160491 and 31371518).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Yule TD, Montoya GD, Russell LD, Williams TM, Tung KS. Autoantigenic germ cells exist outside the blood testis barrier. J Immunol. 1988;141:1161–1167. [PubMed] [Google Scholar]
- 2.Jacobo P, Guazzone VA, Theas MS, Lustig L. Testicular autoimmunity. Autoimmun Rev. 2011;10:201–204. doi: 10.1016/j.autrev.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Naito M, Terayama H, Hirai S, Qu N, Lustig L, Itoh M. Experimental autoimmune orchitis as a model of immunological male infertility. Med Mol Morphol. 2012;45:185–189. doi: 10.1007/s00795-012-0587-2. [DOI] [PubMed] [Google Scholar]
- 4.Rival C, Theas MS, Suescun MO, Jacobo P, Guazzone V, van Rooijen N, et al. Functional and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. J Pathol. 2008;215:108–117. doi: 10.1002/path.2328. [DOI] [PubMed] [Google Scholar]
- 5.Jacobo P, Guazzone VA, Jarazo-Dietrich S, Theas MS, Lustig L. Differential changes in CD4+ and CD8+ effector and regulatory T lymphocyte subsets in the testis of rats undergoing autoimmune orchitis. J Reprod Immunol. 2009;81:44–54. doi: 10.1016/j.jri.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Rival C, Lustig L, Iosub R, Guazzone VA, Schneider E, Meinhardt A, et al. Identification of a dendritic cell population in normal testis and in chronically inflamed testis of rats with autoimmune orchitis. Cell Tissue Res. 2006;324:311–318. doi: 10.1007/s00441-005-0129-5. [DOI] [PubMed] [Google Scholar]
- 7.Mahi-Brown CA, Tung KS. Activation requirements of donor T cells and host T cell recruitment in adoptive transfer of murine experimental autoimmune orchitis (EAO) Cell Immunol. 1989;124:368–379. doi: 10.1016/0008-8749(89)90138-x. [DOI] [PubMed] [Google Scholar]
- 8.Guazzone VA, Jacobo P, Theas MS, Lustig L. Cytokines and chemokines in testicular inflammation: a brief review. Microsc Res Tech. 2009;72:620–628. doi: 10.1002/jemt.20704. [DOI] [PubMed] [Google Scholar]
- 9.Hafizi S, Dahlback B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17:295–304. doi: 10.1016/j.cytogfr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 11.Godowski PJ, Mark MR, Chen J, Sadick MD, Raab H, Hammonds RG. Reevaluation of the roles of protein S and Gas6 as ligands for the receptor tyrosine kinase Rse/Tyro 3. Cell. 1995;82:355–358. doi: 10.1016/0092-8674(95)90424-7. [DOI] [PubMed] [Google Scholar]
- 12.Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- 13.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 14.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Lemke G, Burstyn-Cohen T. TAM receptors and the clearance of apoptotic cells. Ann N Y Acad Sci. 2010;1209:23–29. doi: 10.1111/j.1749-6632.2010.05744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothlin CV, Lemke G. TAM receptor signaling and autoimmune disease. Curr Opin Immunol. 2010;22:740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fijak M, Bhushan S, Meinhardt A. Immunoprivileged sites: the testis. Methods Mol Biol. 2011;677:459–470. doi: 10.1007/978-1-60761-869-0_29. [DOI] [PubMed] [Google Scholar]
- 19.Li N, Wang T, Han D. Structural, cellular and molecular aspects of immune privilege in the testis. Front Immunol. 2012;3:152. doi: 10.3389/fimmu.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Chen Y, Ge Y, Ma P, Ma Q, Ma J, et al. Immunoexpression of Tyro 3 family receptors–Tyro 3, Axl, and Mer–and their ligand Gas6 in postnatal developing mouse testis. J Histochem Cytochem. 2005;53:1355–1364. doi: 10.1369/jhc.5A6637.2005. [DOI] [PubMed] [Google Scholar]
- 21.Xiong W, Chen Y, Wang H, Wu H, Lu Q, Han D. Gas6 and the Tyro 3 receptor tyrosine kinase subfamily regulate the phagocytic function of Sertoli cells. Reproduction. 2008;135:77–87. doi: 10.1530/REP-07-0287. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Li N, Chen Q, Yan K, Liu Z, Zhang X, et al. Breakdown of immune homeostasis in the testis of mice lacking Tyro3, Axl and Mer receptor tyrosine kinases. Immunol Cell Biol. 2013;91:416–426. doi: 10.1038/icb.2013.22. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Wang H, Qi N, Wu H, Xiong W, Ma J, et al. Functions of TAM RTKs in regulating spermatogenesis and male fertility in mice. Reproduction. 2009;138:655–666. doi: 10.1530/REP-09-0101. [DOI] [PubMed] [Google Scholar]
- 24.Itoh M, De-Rooij D, Takeuchi Y. Mode of inflammatory cell infiltration in testes of mice injected with syngeneic testicular germ cells without adjuvant. J Anat. 1995;187(Pt 3):671–679. [PMC free article] [PubMed] [Google Scholar]
- 25.Rival C, Guazzone VA, Theas MS, Lustig L. Pathomechanism of autoimmune orchitis. Andrologia. 2005;37:226–227. doi: 10.1111/j.1439-0272.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 26.Doncel GF, Di Paola JA, Lustig L. Sequential study of the histopathology and cellular and humoral immune response during the development of an autoimmune orchitis in Wistar rats. Am J Reprod Immunol. 1989;20:44–51. doi: 10.1111/j.1600-0897.1989.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 27.Fijak M, Schneider E, Klug J, Bhushan S, Hackstein H, Schuler G, et al. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol. 2011;186:5162–5172. doi: 10.4049/jimmunol.1001958. [DOI] [PubMed] [Google Scholar]
- 28.Ye F, Li Q, Ke Y, Lu Q, Han L, Kaplan HJ, et al. TAM receptor knockout mice are susceptible to retinal autoimmune induction. Invest Ophthalmol Vis Sci. 2011;52:4239–4246. doi: 10.1167/iovs.10-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye F, Han L, Lu Q, Dong W, Chen Z, Shao H, et al. Retinal self-antigen induces a predominantly Th1 effector response in Axl and Mertk double-knockout mice. J Immunol. 2011;187:4178–4186. doi: 10.4049/jimmunol.1101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shang T, Zhang X, Wang T, Sun B, Deng T, Han D. Toll-like receptor-initiated testicular innate immune responses in mouse Leydig cells. Endocrinology. 2011;152:2827–2836. doi: 10.1210/en.2011-0031. [DOI] [PubMed] [Google Scholar]
- 31.Sun B, Qi N, Shang T, Wu H, Deng T, Han D. Sertoli cell-initiated testicular innate immune response through toll-like receptor-3 activation is negatively regulated by Tyro3, Axl, and mer receptors. Endocrinology. 2010;151:2886–2897. doi: 10.1210/en.2009-1498. [DOI] [PubMed] [Google Scholar]
- 32.Schenten D, Medzhitov R. The control of adaptive immune responses by the innate immune system. Adv Immunol. 2011;109:87–124. doi: 10.1016/B978-0-12-387664-5.00003-0. [DOI] [PubMed] [Google Scholar]
- 33.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 34.Li M, Zhou Y, Feng G, Su SB. The critical role of Toll-like receptor signaling pathways in the induction and progression of autoimmune diseases. Curr Mol Med. 2009;9:365–374. doi: 10.2174/156652409787847137. [DOI] [PubMed] [Google Scholar]
- 35.Zetterstrom CK, Strand ML, Soder O. The high mobility group box chromosomal protein 1 is expressed in the human and rat testis where it may function as an antibacterial factor. Hum Reprod. 2006;21:2801–2809. doi: 10.1093/humrep/del256. [DOI] [PubMed] [Google Scholar]
- 36.Biggiogera M, Tanguay RM, Marin R, Wu Y, Martin TE, Fakan S. Localization of heat shock proteins in mouse male germ cells: an immunoelectron microscopical study. Exp Cell Res. 1996;229:77–85. doi: 10.1006/excr.1996.0345. [DOI] [PubMed] [Google Scholar]
- 37.Reiling N, Holscher C, Fehrenbach A, Kroger S, Kirschning CJ, Goyert S, et al. Cutting edge: toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol. 2002;169:3480–3484. doi: 10.4049/jimmunol.169.7.3480. [DOI] [PubMed] [Google Scholar]
- 38.Drennan MB, Nicolle D, Quesniaux VJ, Jacobs M, Allie N, Mpagi J, et al. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol. 2004;164:49–57. doi: 10.1016/S0002-9440(10)63095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suescun MO, Rival C, Theas MS, Calandra RS, Lustig L. Involvement of tumor necrosis factor-alpha in the pathogenesis of autoimmune orchitis in rats. Biol Reprod. 2003;68:2114–2121. doi: 10.1095/biolreprod.102.011189. [DOI] [PubMed] [Google Scholar]
- 40.Rival C, Theas MS, Guazzone VA, Lustig L. Interleukin-6 and IL-6 receptor cell expression in testis of rats with autoimmune orchitis. J Reprod Immunol. 2006;70:43–58. doi: 10.1016/j.jri.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Theas MS, Rival C, Jarazo-Dietrich S, Jacobo P, Guazzone VA, Lustig L. Tumour necrosis factor-alpha released by testicular macrophages induces apoptosis of germ cells in autoimmune orchitis. Hum Reprod. 2008;23:1865–1872. doi: 10.1093/humrep/den240. [DOI] [PubMed] [Google Scholar]
- 42.Tung KS, Unanue ER, Dixon FJ. Pathogenesis of experimental allergic orchitis. II. The role of antibody. J Immunol. 1971;106:1463–1472. [PubMed] [Google Scholar]
- 43.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siu MK, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-alpha, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 45.Jacobo P, Perez CV, Theas MS, Guazzone VA, Lustig L. CD4+ and CD8+ T cells producing Th1 and Th17 cytokines are involved in the pathogenesis of autoimmune orchitis. Reproduction. 2011;141:249–258. doi: 10.1530/REP-10-0362. [DOI] [PubMed] [Google Scholar]
- 46.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci USA. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]