Key Points
Viruses are obligate intracellular pathogens that depend on host cellular components for replication.
Genetic screens are an unbiased and comprehensive method to uncover host cellular components that are critical for the infection with viruses.
Loss-of-function screens result in the genome-wide disruption of gene expression, whereas gain-of-function screens rely on large-scale overexpression of host genes.
Genetic knockout screens can be conducted using haploid insertional mutagenesis or the CRISPR–Cas system.
Genetic screens using the CRISPR–Cas system have provided crucial insights in the host determinants of infections with important human pathogens such as dengue virus, West Nile virus, Zika virus and hepatitis C virus.
CRISPR–Cas-based techniques additionally provide ways to generate both in vitro and in vivo models to study viral pathogenesis, to manipulate viral genomes, to eradicate viral disease vectors using gene drive systems and to advance the development of antiviral therapeutics.
Supplementary information
The online version of this article (doi:10.1038/nrmicro.2017.29) contains supplementary material, which is available to authorized users.
Subject terms: Genetic engineering, Genetic techniques, CRISPR-Cas9 genome editing, Virology, High-throughput screening
In this Review, Puschnik and colleagues discuss the technical aspects of using CRISPR–Cas technology in genome-scale knockout screens to study virus–host interactions, and they compare these screens with alternative genetic screening technologies.
Supplementary information
The online version of this article (doi:10.1038/nrmicro.2017.29) contains supplementary material, which is available to authorized users.
Abstract
Viruses depend on their hosts to complete their replication cycles; they exploit cellular receptors for entry and hijack cellular functions to replicate their genome, assemble progeny virions and spread. Recently, genome-scale CRISPR–Cas screens have been used to identify host factors that are required for virus replication, including the replication of clinically relevant viruses such as Zika virus, West Nile virus, dengue virus and hepatitis C virus. In this Review, we discuss the technical aspects of genome-scale knockout screens using CRISPR–Cas technology, and we compare these screens with alternative genetic screening technologies. The relative ease of use and reproducibility of CRISPR–Cas make it a powerful tool for probing virus–host interactions and for identifying new antiviral targets.
Supplementary information
The online version of this article (doi:10.1038/nrmicro.2017.29) contains supplementary material, which is available to authorized users.
Main
Viruses are obligate intracellular pathogens that depend on host cellular components for replication. They bind to cell surface receptors to enter cells, and they co-opt cellular functions and organelles to replicate. Host cells can counteract infections by sensing pathogen-associated molecular patterns (PAMPs), such as viral nucleic acids, and by subsequently triggering the expression of antiviral genes. The identification and characterization of host factors that promote and restrict viral replication can provide important insights into basic aspects of cellular biology and virus–host relationships, and can lead to the identification of new targets for antiviral therapeutics.
The use of forward genetic screens has provided an unbiased and comprehensive strategy to uncover host factors that promote or restrict virus replication. Originally, the use of these genetic screens was limited to genetically tractable model organisms, such as yeasts, fruit flies, roundworms and zebrafish, and relied on the use of X-rays or chemical mutagens to introduce mutations. These forward genetic screens have markedly contributed to our understanding of many fundamental biological processes1,2,3,4, but their application to cultured mammalian cells was challenging. With technological advances such as RNAi and insertional mutagenesis in human haploid cells, it became possible to disrupt gene expression on a genome scale in mammalian cell culture5,6,7. Recently, the prokaryotic CRISPR–Cas adaptive immune system has been engineered to efficiently induce knockout mutations in almost any cell type, which has revolutionized biological research8,9,10 (Box 1). In contrast to gene knockdown approaches, such as RNAi, the knockout of alleles by CRISPR–Cas often results in more marked phenotypes, a greater signal-to-noise ratio and the identification of fewer false-positives11,12,13,14. Knockout alleles are generated by the endonuclease Cas9, which is directed to a specific genomic region by a single-guide RNA (sgRNA) through Watson–Crick base pairing. Cas9 creates a double-strand break (DSB) at the target site, which is then repaired by non-homologous end joining (NHEJ). This often results in a frameshift mutation and the expression of truncated or non-functional proteins. The ease of Cas9 targeting to specific loci, combined with the design of multiplexed pools of sgRNAs that span the entire human genome14,15,16,17, has enabled the genome-scale identification of host factors that are crucial for virus replication.
In this Review, we describe how genetic screens have contributed to our understanding of virus–host biology and how CRISPR–Cas screens have been used to expand our toolkit to identify host factors that are important for virus replication. We provide practical advice on how to set up CRISPR–Cas screens and give examples of recent discoveries that have been made using CRISPR–Cas technology for viruses that cause important human diseases, including dengue virus (DENV), Zika virus (ZIKV), West Nile virus (WNV), hepatitis C virus (HCV) and noroviruses. We also discuss the potential for CRISPR–Cas technology beyond genetic screening applications, and how it could advance our understanding of viral pathogenesis and the development of antiviral therapeutics.
Box 1: CRISPR–Cas-mediated adaptive immunity.
The CRISPR–Cas system is an adaptive immune system that protects bacteria and archaea against bacteriophages and plasmids. CRISPR–Cas immunity is mediated by CRISPR RNA (crRNA) and a Cas endonculease that targets genetic elements141. The mode of action consists of three distinct steps: acquisition, expression and interference (see the figure). In the acquisition step, foreign nucleic acids are directionally integrated, as new CRISPR spacers, into a CRISPR array that is separated by repeat sequences, thus creating a memory of invading genetic elements142 (see the figure, step 1). In the expression step, the CRISPR locus is transcribed into a pre-CRISPR RNA transcript (pre-crRNA), which is then processed into a mature crRNA that contains partial CRISPR spacer sequences joined to partial CRISPR repeats132. The CRISPR locus also encodes a transactivating RNA (tracrRNA) that has complementarity to the repeat regions of crRNA transcripts143. In addition to the CRISPR array, a single or multiple Cas nucleases (for example, Cas9) are encoded by the CRISPR locus (see the figure, step 2). In the interference stage, a crRNA–tracrRNA hybrid is formed through binding of the complementary repeat region sequences, and this RNA hybrid guides the Cas nuclease towards complementary DNA sequences, which leads to the targeting and cleaving of invading genetic elements144. Most CRISPR effector proteins rely on a protospacer-adjacent motif (PAM; for example, NGG for Cas9) in the targeted nucleic acid. The PAM is essential for recognition, cleavage and the distinction between self and non-self DNA145,146,147 (see the figure, step 3). For Cas9, perfect complementarity will drive a conformational change in the endonuclease that leads to a cleavage-competent structural state148,149,150,151,152,153. The protein and RNA components of the Streptococcus pyogenes class 2 CRISPR system have been adapted to function in eukaryotes, including in human cells. A human-codon-optimized Cas9 is fused to a nuclear localization signal (NLS) to direct Cas9 to the nucleus in mammalian cells8,9,10. To generate single-guide RNAs (sgRNAs) for genome editing that mimic the natural crRNA–tracrRNA hybrid, crRNA-like sequences are fused to a partial tracrRNA through a synthetic stem–loop.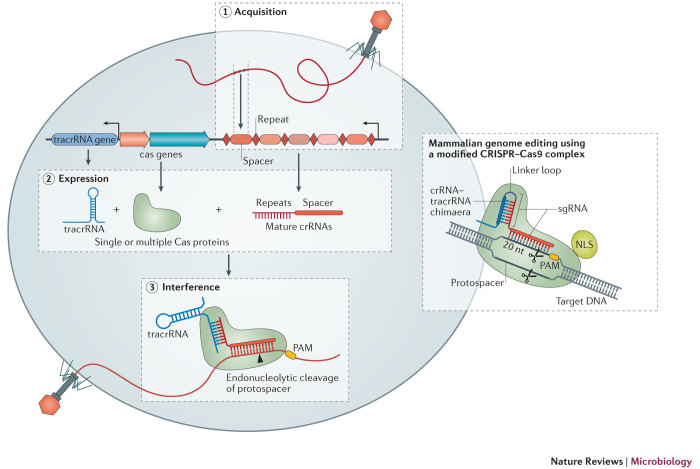
The power of unbiased genetic screens
Historically, loss-of-function screens have lagged behind gain-of-function approaches in mammalian cells owing to the lack of efficient tools that can mutate both alleles in diploid genomes in a high-throughput manner.
Gain-of-function approaches. Gain-of-function approaches rely on the ectopic overexpression of genes and have been successful in identifying cell surface receptors that are required for viral entry and host viral restriction factors. To identify entry receptors, a cell line that is refractory to infection is typically transduced with a complementary DNA library (cDNA library) derived from a cell type that is permissive to infection. For example, claudin 1 (CLDN1)18 and occludin (OCLN)19 were identified as entry receptors for HCV by transducing a non-permissive cell line with a cDNA library derived from hepatocellular carcinoma cells. In addition to discovering receptors, an unbiased expression screen also discovered that SEC14-like protein 2 (SEC14L2), which is a cytosolic lipid-binding protein, enhances the replication of clinical strains of HCV20. Furthermore, a library of ∼380 interferon-stimulated genes (ISGs) was used to identify key proteins that are important for innate immune defences against several DNA and RNA viruses21,22. In addition to these screens, in a continuing effort, comprehensive cDNA libraries that contain all annotated ORFs from humans have been cloned into lentiviral expression vectors23, generating an expression vector library that is likely to improve the utility of gain-of-function screens in the study of host–pathogen interactions.
Loss-of-function genetic screens. Loss-of-function screens are based on the stable knockdown or knockout of genes. Initial approaches that used RNAi have provided valuable insights into virus–host relationships24. In contrast to RNAi, which leads to the partial depletion of expression for a specific gene, recent technological advances have made it possible to completely disrupt gene expression (Table 1). One approach, termed haploid genetic screening, relies on insertional mutagenesis of genes in cultured haploid cell lines. For example, retroviral gene traps that contain a splice acceptor site can integrate into the host genome, leading to the expression of truncated mRNA transcripts7. The complete ablation of gene expression can have marked effects on virus replication and enables the identification of the most crucial host factors for virus infection. Insertional mutagenesis in haploid cells has been used to discover essential receptors for several viruses, including Ebola virus and Lassa virus25,26. Both viruses use abundant lysosomal proteins as receptors. The interaction between the Ebola virus glycoprotein and its receptor Niemann–Pick C1 protein (NPC1) is triggered by cathepsin cleavage27,28, whereas the Lassa virus glycoprotein interacts with its receptor, lysosome- associated membrane glycoprotein 1 (LAMP1), following acidification of the endosome. Subsequent structural studies defined the binding interface between the viral glycoprotein and NPC1 (Refs 29,30,31). Interestingly, several mutations arose in the host-binding site of the viral glycoprotein during the 2013–2016 Ebola virus epidemic. These mutations increased the infectivity of the virus in primate cells but not in rodent cells, which suggests that these mutations contributed to adaptation and spread in humans32,33.
Table 1. Comparison of mammalian loss-of-function screening methods.
| CRISPR–Cas | Haploid | RNAi | |
|---|---|---|---|
| Mechanism | Induced DSBs lead to error-prone NHEJ and, frequently, frameshift mutations | Integration of retroviral gene traps that contain a splice acceptor site leads to truncated mRNA transcripts | siRNAs or shRNAs bind to target mRNAs, which leads to their cleavage and degradation |
| Phenotype | Complete knockout of gene expression leads to strong phenotypes in virus infection assays | Complete knockout of gene expression leads to strong phenotypes in virus infection assays | Partial knockdown of gene expression may not produce a strong phenotype in virus infection assays |
| Selection of candidate host factors | Strong phenotypes achieved by gene knockouts result in the identification of candidate genes with higher confidence than those identified through RNAi | Strong phenotypes achieved by gene knockouts result in the identification of candidate genes with higher confidence than those identified through RNAi | Incomplete knockdown and the variability of gene expression combined with off-target effects makes the identification of candidate genes more challenging |
| Coverage | 4–12 sgRNAs per gene | ∼525 independent gene trap insertions per gene (median) | Typically 4–6 shRNAs per gene; up to 30 shRNAs per gene for pooled screening |
| Off-target effects | Mismatched base pairing may lead to off-target cleavage in the genome (usually in non-coding regions) | No described off-target mechanism | Mismatched base pairing may lead to knockdown of off-target mRNAs |
| Cell types | Wide range of cell types | Haploid and near-haploid cells (HAP1, KBM7, human and mouse embryonic stem cells) | Wide range of cell types |
| Analyses | Enrichment of multiple sgRNAs per gene | Enrichment of independent gene trap insertions per gene | Enrichment of multiple siRNAs or shRNAs per gene |
DSB, double-strand break; NHEJ, non-homologous end joining; sgRNAs, single-guide RNAs; shRNA, short hairpin RNA; siRNA, small interfering RNA.
Haploid genetic screens were important for the discovery of a cellular phospholipase that enables viral evasion of an antiviral restriction mechanism that is broadly active against many picornaviruses34. Recently, a haploid screen identified a proteinaceous receptor that enables virus entry for multiple distinct serotypes of adeno- associated virus (AAV)35, potentially affecting the use of AAV as a gene therapy vector. These and other studies have established loss-of-function screens as a reliable strategy to uncover host factors that are crucial for virus replication (Table 2).
Table 2. Genome-wide knockout screens to identify virus–host interactions.
| Viruses | Knockout screen | Critical host factors | Refs |
|---|---|---|---|
| Adeno-associated virus | Haploid | AAVR, GARP complex | 35 |
| Dengue virus | Haploid and CRISPR–Cas | OST complex (STT3A and STT3B), TRAP complex (SSR1, SSR2, SSR3), EMC, ERAD (SEL1L, AUP1, DERL2) | 61 |
| Ebola virus | Haploid | NPC1, HOPS complex | 25 |
| Enterovirus 68 | Haploid | Sialic acid | 163 |
| Hanta virus | Haploid | SREBF2, MBTPS1, MBTPS2, SCAP | 164,165 |
| Hepatitis C virus | CRISPR–Cas | CD81, CLDN1, OCLN, miR-122, CYPA, ELAVL1, RFK, FLAD1 | 61 |
| Human immunodeficiency virus | CRISPR–Cas | CD4, CCR5, ALCAM, SLC35B2, TPST2 | 88 |
| Lassa virus | Haploid | LAMP1, DAG1 | 26,166 |
| Murine norovirus | CRISPR–Cas | CD300LF | 85,86 |
| Picornaviruses | Haploid | PLA2G16 | 34 |
| Rift Valley fever virus | Haploid | Heparan sulfate, COG complex | 167 |
| West Nile virus | CRISPR–Cas | SPCS1, SPCS3, EMC, OST complex (STT3A), TRAP complex, SEL1L, HRD1 | 69,70 |
| Zika virus | CRISPR–Cas | EMC, AXL, OST complex (STT3A), TRAP complex | 71 |
AAVR, adeno-associated virus receptor; ALCAM, CD166 antigen; AXL, also known as UFO; CLDN1, claudin 1; COG, conserved oligomeric Golgi; CYPA, cyclophilin A; DAG1, dystroglycan; DERL2, derlin 2; EMC, endoplasmic reticulum membrane protein complex; ERAD, endoplasmic reticulum-associated degradation; FLAD1, FAD synthase; GARP, Golgi-associated retrograde protein; HOPS, homotypic fusion and protein sorting; HRD1, also known as SYVN1; LAMP1, lysosome-associated membrane glycoprotein 1; NPC1, Niemann–Pick C1 protein; OCLN, occludin; OST, oligosaccharyltransferase; RFK, riboflavin kinase; SCAP, SREBP cleavage-activating protein; SLC35B2, adenosine 3′-phospho 5′-phosphosulfate transporter 1; SPCS, signal peptidase complex subunit; SREBF2, sterol-regulatory-element-binding protein 2; TPST2, protein tyrosine sulfotransferase 2; TRAP, translocon-associated protein.
Practical considerations for screens
Genetic screens enable the identification of virus–host interactions without prior knowledge of the interaction and on a genomic scale. In this section, we describe the different technologies that are currently available to carry out genetic screens, and we highlight important considerations at different stages of the screen, including the generation of the library of mutant cells, the virus infection assay, phenotypic selection, next-generation sequencing and bioinformatic analyses (Fig. 1). We also consider the degree of 'saturation' in genetic screening; that is, the fraction of target genes it is possible to identify in a specific screen.
Figure 1. Genome-wide screening strategies to investigate host factors that are involved in virus infection.
Genetic screens can identify host factors that promote virus replication, as well as antiviral restriction factors. For example, in a loss-of-function screen, knockout of a viral receptor in a permissive cell line will make the cell resistant to the virus infection. By contrast, in a gain-of-function screen, overexpression of the viral receptor in a non-permissive cell line will enable virus infection. Various technologies are available for genome-wide screening. Loss-of-function screens can be carried out using a haploid mutagenesis (in a 1n cell type) or CRISPR–Cas knockout approaches, whereas gain-of-function screens use CRISPR activation (CRISPRa) or ectopic overexpression. Target cells are mutated by delivering retroviral gene trap or lentiviral expression constructs, which can either disrupt or lead to gene expression. The pooled mutagenized cell population is then subjected to a virus infection assay, in which either the cells are infected by the virus of interest, or a subgenomic viral reporter is introduced by transduction or transfection. Virus-resistant cells are selected either by surviving virus-induced cell death or by fluorescence-activated cell sorting (FACS). Next-generation sequencing and bioinformatic analyses enable the enrichment of CRISPR single-guide RNAs (sgRNAs), gene traps or complementary DNA (cDNA) insertions to be determined. CMV, cytomegalovirus; dCas9, catalytically inactive Cas9; DSB, double-strand break; LTR, long terminal repeat; NHEJ, non-homologous end joining; pA, poly(A) tail; SA, splice acceptor; VP64, herpes simplex virus VP16 activation domain.
Choice of cell line and screen. Viruses differ in their host range and tissue tropism. Whether a cell is permissive or non-permissive to virus infection is determined by the expression of genes that facilitate virus replication and genes that restrict virus infection. Genetic screens can uncover genes that promote and restrict virus replication depending on the choice of host cell type (permissive or non-permissive) and the type of screen (loss-of-function or gain-of-function; Fig. 1). CRISPR–Cas genome editing has been reported for a wide range of cell lines that can be infected with many viruses. However, large-scale genetic screens in which the sgRNAs are introduced into the cells in a pooled manner have some limitations. To ensure the appropriate representation of each of the sgRNAs in the pool, numerous cells are transduced and undergo phenotypic selection. In practice, many transformed cell lines will be suitable for generating a mutagenized cell library; however, primary cells have a limited proliferative capacity, and it is therefore more challenging to transduce and expand these cells in large numbers. Pre-arrayed sgRNA formats, in which wells contain individual synthetic sgRNA constructs for reverse transfection36, may therefore be more suitable for primary cells.
Haploid screens are limited to cell types that have a haploid or near-haploid karyotype to achieve insertional mutagenesis of the allele. Commonly used cell lines include the chronic myeloid cell line KBM7 (Ref. 7) and its derivative, HAP1 (Refs 25,37), and human38 and mouse embryonic stem cells39,40. Despite this limited choice of cell types, haploid genetic screens have been useful for studying many different virus–host interactions41,42 (Table 2).
Overall, both CRISPR–Cas and haploid screens are well suited for the identification of host factors if the cell line is permissive to the virus. The two types of screen may even be carried out in parallel for additional validation and comprehensive screening of candidate genes.
CRISPR libraries and mutagenesis. Several CRISPR sgRNA libraries are available as plasmid repositories (see the Addgene website). The libraries vary in the number of sgRNAs they contain, their target genes (genome wide or a subpool of genes only), the targeted position within the gene (for example, the ORF or the promoter), the targeted species, and their availability as a one-plasmid or two-plasmid system (such that Cas9 is encoded on the same plasmid as the sgRNA or on a second plasmid, respectively). In addition, custom libraries can be constructed for a specific class of gene (for example, kinases) or for validation screens.
The initial genome-scale CRISPR knockout (GeCKO) libraries contained 4–6 sgRNAs per gene and were designed to minimize off-target effects14,43. More recently constructed CRISPR–Cas libraries (for example, the Broad Brunello44, Toronto KnockOut13 or Sabatini–Lander libraries45) contain more sgRNAs per gene (up to 12), which increases the likelihood of statistically significant enrichment of candidate genes. However, larger sgRNA libraries also require larger-scale screening, which could be challenging to achieve, especially in cells that have a limited capacity to divide. Notably, a small sgRNA library that contains a subset of sgRNAs from a larger CRISPR–Cas library was still able to identify the majority of the same hits, albeit with less statistical significance44. This suggests that when scaling up is unfeasible owing to cost or cell number, sgRNA libraries that contain fewer sgRNAs per gene can be used in an initial screen, which can then be followed by a secondary screen and/or careful validation.
The more recent CRISPR–Cas sgRNA libraries were constructed to have greater on-target cleavage efficiency than previous sgRNA libraries, in addition to minimal off-target activity. They have shown consistent on-target cleavage efficiency, therefore reducing the chance of false-negative identifications. For example, in a genome-scale screen selecting for resistance to the toxic effect of thymidine, 11 of the 12 sgRNAs against thymidine kinase 1 (TK1) scored as hits, which indicates that the majority of these sgRNAs were active because TK1 is crucial for mediating thymidine toxicity13. In a more systematic study, ∼85% of all sgRNA constructs that target essential genes were accurately recalled without false-positive identifications11. Owing to the high efficiency of CRISPR–Cas knockouts, the marked phenotypes that are generated in knockout cells and the reproducibility of CRISPR–Cas screens, these screens have outperformed RNAi library screens for the identification of drug resistance genes14, modulators of protein stability12 and essential genes11,13. In addition to creating gene knockouts, CRISPR–Cas technology has been used to modulate the transcription levels of target genes (Box 2). This approach can be advantageous when studying essential genes because it can decrease gene expression without eliminating it completely. It also enables the role of long non-coding RNAs to be assessed, as small insertions or deletions (indels) do not typically disrupt their biological activity46.
To ensure that the sgRNA library is of sufficient quality, it is important to maintain the complexity of the sgRNA pool when expanding the sgRNA plasmid pool in Escherichia coli, during transfection or transduction of the target cells and during the extraction of genomic DNA from cells for downstream analyses. For example, we consistently found a good sgRNA representation (>99%) when the number of transduced cells was ∼500-fold higher than the total number of sgRNAs in the library. Furthermore, a low multiplicity of infection (MOI; ∼0.3) during transduction is advised to ensure that only one integration event takes place per cell47.
Phenotypic selection. Many viruses such as poliovirus or DENV are cytolytic, which enables a straightforward selection of virus-resistant cells in cell viability-based screens. This selection recovers mutant cells that do not support viral entry, translation of the viral genome, replication of the viral genome or virus-induced cell death, but typically not mutant cells that do not support virion assembly and egress. In a pooled screen, in which mutant cells are cultured together, the selection can be extremely stringent because of the requirement that resistant cells survive multiple rounds of infection. Therefore, this screening method identifies genes for which disruption causes marked phenotypes. Strong selection conditions in which >99% of cells die from infection are preferred. Although this high stringency increases the confidence in the candidate genes identified, other genes that have subtler effects on virus infection may be missed. Decreasing the stringency could help to identify these genes. Strategies to achieve this include the use of naturally attenuated virus strains or the use of antiviral compounds during selection. However, fine-tuning of the stringency is not always possible in pooled screens, and arrayed screens may be a valuable alternative.
If the virus is not efficient at inducing cell death, then a longer selection period, multiple rounds of virus challenge and larger sgRNA libraries may help to increase the signal-to-noise ratio. As an alternative strategy, fluorescence-activated cell sorting (FACS)-based selection can be used to study persistent or non-cytolytic viruses (for example, hepatitis B virus (HBV), HIV and AAV). This approach relies on genetically engineered viruses that express a fluorescent reporter or on antibody staining35. FACS-based selection enables the isolation of cells that have low or high levels of virus gene expression, making it possible to simultaneously identify factors that enhance virus infection and factors that inhibit virus infection.
It is also possible to identify host factors that are required at specific stages of the viral life cycle; for example, pseudotyped viruses48,49, viral replicons50,51 and internal ribosome entry site reporters (IRES reporters)52,53 can be used in the virus infection assay to identify host factors that are required for virus entry, genome replication and translation, respectively.
Next-generation sequencing and bioinformatics. After phenotypic selection, genomic DNA is isolated. Uninfected, mutagenized cells are used as control samples (the starting population either collected at day 0 or grown and harvested in parallel with the virus-selected population). At this step, the total amount of DNA template should be sufficiently high to maintain the complexity of the library. The sgRNA integrations are PCR-amplified and sequenced by next-generation sequencing to quantify their relative abundances. The level of sgRNA enrichment in phenotypically selected cells compared with that in unselected cells is determined by comparing the number of reads that map to specific sgRNAs in the different cell populations. To normalize for differences in sequencing depth between populations, the number of reads that map to each specific sgRNA is divided by the total number of reads. Bioinformatic tools can help to determine whether a gene is significantly enriched over background by assessing the level of enrichment of multiple sgRNAs against the same gene. Analysis tools that were developed for RNAi screens, such as RNAi gene enrichment ranking54 (RIGER) and redundant siRNA activity55 (RSA), can be repurposed for this task. More recently, scoring algorithms, such as model-based analysis of genome-wide CRISPR–Cas9 knockout (MAGeCK)56 and STARS44, have been developed to improve the bioinformatic analyses of CRISPR–Cas screen data sets, taking into account the increasing number of sgRNAs that are used per gene.
Validation of candidate genes and off-target effects. An important step after any genetic screen is the validation of the candidate genes and the consideration of off-target effects. Gene editing at off-target loci has been reported57,58,59, and if these off-target sites are within exons, they have the potential to cause false-positive results. The use of multiple sgRNAs per gene combined with the implementation of sgRNA sequence design rules can help to reduce this risk. In side-by-side comparisons with RNAi-based approaches, CRISPR–Cas screens typically have fewer false-positive identifications11,12,13,44. Nevertheless, a thorough validation of candidate genes is still essential. Individual knockout cell lines should be generated using CRISPR–Cas methods and start from a single cell clone. After confirming that the gene has been knocked out by genotyping and immunostaining, the effect of the knockout on virus replication can be measured, and genetic complementation experiments can confirm that the effect was due to the knockout.
Essential genes and genome coverage. It is challenging to identify all of the genes that affect virus replication because a proportion of them are essential for cell growth and viability and will therefore be excluded from downstream analyses. CRISPR–Cas screens and haploid screens have enabled the systematic and comprehensive identification of a core set of ∼2,000 human genes that are essential for optimal cellular growth and viability13,45,60, corresponding to ∼10% of human genes. Owing to the essential roles that these genes have in cell physiology, it is challenging to determine whether they directly influence virus replication. The remaining ∼90% of genes can be tested in genetic screens because they do not affect cell growth or viability; however, this figure is likely to be an underestimate because the list of 'essential genes' includes genes that only moderately affect cell growth and could therefore be included in the screens. For example, in haploid and CRISPR–Cas screens for host factors that are crucial for DENV replication, multiple subunits of the oligosaccharyltransferase complex (OST complex) were identified61 despite the genes that encode these subunits being classified as essential genes60.
Another important consideration is the coverage of the genome. Notably, sgRNA libraries are designed according to the presence of annotated genes in reference genomes (∼20,000 genes in humans). The increasing number of independent sgRNAs (now at 4–12 per gene) and improved sequence rules for cleavage efficiency make current sgRNA libraries more reliable than early libraries for probing the entire human genome, thus minimizing false-negative results. By contrast, haploid genetic screens rely on retroviral insertional mutagenesis and do not require genome annotation. Retroviral integration is not random and occurs more frequently in actively transcribed chromatin62, which biases the insertions towards genes. Indeed, mapping of insertion sites in gene trap screens revealed insertions in ∼70% of all annotated genes and ∼98% of expressed genes63. Recently, more extensive mapping efforts in HAP1 cells showed that >90% of all annotated genes contained insertions, with a median of 525 independent gene trap insertion events per annotated gene60. This high number of knockout events increases the power of identifying signal over noise.
Despite the fundamental differences between CRISPR–Cas and haploid genetic screens, both approaches have been equally powerful in identifying core essential human genes45,60, endoplasmic reticulum-associated protein degradation (ERAD) components64 and host factors that are required for DENV replication61. The high concordance in identified genes between the two different technologies underscores the power and reliability of knockout screens.
Box 2: CRISPR–Cas approaches to regulate transcription.
Catalytically inactive Cas9 (dCas9) can be fused to transcriptional activators or repressors to modulate gene expression without introducing irreversible mutations into the genome154,155. Approaches that use dCas9 for this purpose are commonly referred to as CRISPR activation (CRISPRa) and CRISPR interference (CRISPRi)156. To achieve transcriptional repression (that is, CRISPRi), chromatin-modifying repressor domains, such as the Krüppel-associated box (KRAB) domain, are fused to dCas9 and are recruited to transcription start sites. CRISPRi-mediated and RNAi-mediated knockdown both lead to the downregulation of gene expression, albeit through different molecular mechanisms. CRISPRi occurs by inhibiting transcription, whereas RNAi degrades mRNAs in the cytoplasm. CRISPRa relies on the fusion of dCas9 to multiple repeats of the herpes simplex virus VP16 activation domain (VP64 or VP160) to enhance transcription at target sites. Alternatively, in the synergistic activation mediator (SAM) library, MS2 RNA aptamers added to the tetraloop and second stem–loop of the sgRNA additionally recruit a fusion construct of the bacteriophage MS2 coat protein (MCP), the nuclear factor-κB subunit p65 and heat shock factor protein 1 to enhance Cas9-mediated gene expression157. CRISPRa and SAM libraries are an alternative approach for gain-of-function screens (Fig. 1), which traditionally rely on complementary DNA (cDNA) overexpression libraries23. The activation of endogenous gene expression has the advantage of not being limited by conventional molecular cloning techniques to generate cDNA constructs and can be used to increase the expression of different isoforms from the same gene. Although Cas9-mediated gene activation has not been used in the context of virus–host studies, it may be used to identify antiviral restriction factors or host factors that are expressed at very low levels and are required for virus replication.
Insights from CRISPR–Cas screens
The potential for CRISPR–Cas screens to discover host factors that are crucial for viral pathogenesis is great and may lead to the development of new antivirals65. Several viruses have been studied using CRISPR–Cas screens.
Mosquito-borne flaviviruses. The mosquito-borne flaviviruses include important pathogens such as DENV66. More recently, ZIKV has emerged in Brazil and is spreading at a rapid pace throughout South America67, causing severe congenital abnormalities in the unborn children of pregnant mothers who are infected68. The biogenesis and membrane topology of mature flavivirus proteins is complex and involves the translation of a polyprotein at the ER membrane, the co-translational and post-translational insertion of several membrane-spanning hydrophobic helices, and polyprotein cleavage by a viral protease and several host proteases into the mature viral proteins. Despite this knowledge of these processes, a detailed understanding of the host proteins that are involved is lacking.
CRISPR–Cas screens that were carried out independently using DENV61, WNV69,70 and ZIKV71 have each identified a number of ER proteins that are required for virus replication (Fig. 2). Many of these proteins are involved in the biosynthesis of membrane and secretory proteins, a core function of the ER. In particular, the proteins that were identified have described roles in N-linked glycosylation, ERAD, and signal peptide insertion and processing. Notably, the identification of these proteins was reproduced in replica screens in the same laboratory and in independent screens in different laboratories using different cell lines and different virus strains. There was also a substantial overlap with results from haploid genetic screens. This reproducibility is remarkable and a major advantage of this technology.
Figure 2. Host factors that have been identified by CRISPR–Cas screens as important for infection and replication of viruses in the family Flaviviridae.
The flaviviruses Zika virus (ZIKV), dengue virus (DENV) and West Nile virus (WNV) enter the cell by attachment to cell surface molecules, including heparan sulfate proteoglycans (HSPG) and potentially other protein receptors61,71,77. After uncoating, viral (+)RNA is translated by host ribosomes. The ribosomal subunit 40S ribosomal protein S25 (RPS25) is important for DENV infection and for translation of hepatitis C virus (HCV) RNA, but is dispensable for host mRNA translation61,158. The flavivirus polyprotein is inserted into the endoplasmic reticulum (ER) membrane and cleaved by viral and host proteases, including the host signal peptidase complex70. The viral proteins assemble a replication complex in close association with several ER-resident host protein complexes: the oligosaccharyltransferase (OST) complex, the translocon-associated protein (TRAP) complex and components of the ER-associated protein degradation (ERAD) pathway61,69,70,71. Notably, different flaviviruses have different dependencies on the two distinct OST multiprotein complexes, which contain either an STT3A or an STT3B catalytic subunit. The ERAD-related host factors belong to the classical ERAD complex and the ER membrane protein complex (EMC). HCV enters hepatocytes through the receptors CD81, occludin (OCLN) and claudin 1 (CLDN1)61,159, and the host microRNA miR-122 binds to and stabilizes the 5′ UTR of the HCV RNA61,160. FAD biosynthesis, catalysed by riboflavin kinase (RFK) and FAD synthase (FLAD1), is important for HCV RNA synthesis61. ELAVL1 binds to the 3′ UTR of HCV to circularize the viral genome by interacting with La protein (also known as SSB) and displacing polypyrimidine-tract-binding protein 1 (PTB) to stimulate virus replication61,161. Cyclophilin A (CYPA) is required for HCV replication through its interaction with NS5A61,83. UBE2J1, ubiquitin-conjugating enzyme E2 J1; YFV, yellow fever virus.
CRISPR–Cas technology also provides a reliable way to validate candidate genes and measure the effects of knockouts on virus replication. In contrast to knockdown approaches, such as RNAi, gene knockouts are absolute and do not result in the variable levels of depletion seen with RNAi. This enables a faithful comparison between genes when quantitative assays for virus replication are used, such as quantitative PCR, immunostaining or plaque assays. Remarkably, flavivirus replication was decreased 100–10,000-fold when the most significantly enriched host factors from the screens were knocked out61. This demonstrates that pooled sgRNA screens have the potential to identify host factors that are essential for virus replication.
Moreover, CRISPR–Cas knockout cells can be used to understand the molecular basis of knockout phenotypes and to help identify the stage of the virus life cycle in which the host factor is involved. For example, the OST complex was found to be required for viral RNA synthesis, but not for viral entry and translation61. The OST complex catalyses the N-linked glycosylation of newly synthesized proteins. In mammalian cells, two distinct OST multiprotein complexes are formed, each composed of a catalytic subunit (one of two paralogues, STT3A or STT3B) and accessory subunits72. Both isoforms are individually required for the replication of DENV, as knockout of either STT3A or STT3B resulted in complete abrogation of DENV replication. Other mosquito-borne flaviviruses, including ZIKV, are exclusively dependent on the STT3A isoform for viral RNA replication, which indicates a specific but divergent virus–host interaction (Fig. 2). Surprisingly, the catalytic activity of STT3A and STT3B was dispensable for virus replication, because catalytically inactive mutant proteins were able to restore DENV replication in the knockout cells, which indicates that the OST complex has an unconventional role in DENV replication. The OST complex was found to bind to multiple non-structural viral proteins that form the RNA synthesis complex at the ER61, which suggests that the OST complex acts as a scaffold to coordinate the assembly of a functional DENV RNA replication complex.
Other host factors that were found to be required for flavivirus replication include SEC61A1 and SEC63, which form the translocon channel in the ER membrane; the translocon-associated protein (TRAP) complex, which stimulates co-translational translocation of polypeptides into the ER73; and the signal peptidase complex that cleaves signal peptides in the ER lumen. Knockout of a subset of signal peptidase complex subunits (SPCSs) revealed severe defects in the polyprotein cleavage of multiple flaviviruses. In particular, cleavage of the structural proteins prM and E from the polyprotein was affected, leading to marked defects in the release of virus particles70.
Components of the ERAD pathway were also found to be important for flavivirus replication. This protein quality control mechanism targets incorrectly folded proteins in the ER lumen for retrotranslocation through the ER membrane to the cytosol, in which proteasomal degradation occurs74. Two categories of ERAD components were found in the CRISPR–Cas screens: first, components of the classical ERAD machinery, including SEL1L, derlin 2 (DERL2) and ubiquitin-conjugating enzyme E2 J1 (UBE2J1), which are part of the retrotranslocation complex75; and second, components of the ER membrane complex (EMC), an evolutionarily conserved complex that has less-well-understood roles in ERAD76. Knockout of ERAD components led to substantial decreases in viral-RNA accumulation, particle formation and virus-induced cell death for DENV, ZIKV, Japanese encephalitis virus and WNV61,69,70. However, how ERAD functions promote flavivirus replication remains to be fully understood.
It is important to note that in contrast to genetic screens with several other viruses (for example, Ebola virus), screens with WNV and DENV have not been able to identify a specific receptor that is required for viral entry into host cells. This is most probably due to redundancy in entry routes, such that knockout of one virus receptor still leaves cells susceptible through a different route. Indeed, several receptors have been reported for DENV77. Nevertheless, CRISPR–Cas screens have contributed to our understanding of flavivirus biology, revealing a central role for several ER complexes in promoting flavivirus infection.
HCV. Another important pathogen that has been investigated using CRISPR–Cas screens is HCV, which causes chronic liver disease in ∼160 million infected individuals worldwide78. Whereas mosquito-borne flaviviruses have a dependence on ER proteins, screening with HCV, which is a more distantly related member of the family Flaviviridae, revealed non-overlapping hits, including entry receptors CD81, OCLN and CLDN1, the liver-specific microRNA miR-122 and several RNA-binding proteins and metabolic enzymes61 (Fig. 2). One of the most significant hits was ELAVL1, an RNA-binding protein that is involved in mRNA stabilization79. HCV RNA replication was markedly reduced in ELAVL1-knockout cells, whereas RNA replication for other RNA viruses (for example, DENV and poliovirus) was unaffected. The HCV screens also uncovered an unexpected link between intracellular FAD levels and HCV RNA replication. The enzymes riboflavin kinase (RFK) and FAD synthase (FLAD1), which are involved in the conversion of riboflavin (vitamin B2) to FAD, were found to be crucial for the replication of HCV. Lumiflavin, an inhibitor of cellular uptake of riboflavin, potently inhibited viral RNA replication, which indicates that the modulation of intracellular FAD levels could be explored as an antiviral treatment. Host-targeted antiviral therapeutics may become an effective strategy to control virus replication because they may present a higher genetic barrier for resistant mutants to evolve than virus-targeting antivirals, and they have the potential to inhibit a broader range of viruses65. For example, cyclophilin A (CYPA) is a host factor that is required for HCV replication and also promotes HIV infection80,81,82. CYPA inhibitors have advanced to phase II/III clinical trials for the treatment of HCV infection, and their use is also being explored to treat other viral infections83.
Noroviruses. Human noroviruses are a leading cause of gastroenteritis globally. Although their mechanism of entry and cellular receptor remain unknown, carbohydrates — in particular, the histo-blood group antigens (HBGAs) — have been shown to have a role in human norovirus entry84. Unbiased genetic CRISPR–Cas screens led to the discovery of CD300LF (also known as CLM1) as a proteinaceous receptor for murine norovirus85,86. CD300LF knockout abolished murine norovirus infection in mouse cell lines and in a mouse model of murine norovirus infection. Moreover, the expression of mouse CD300LF in human cells made them susceptible to murine norovirus infection. The discovery of a key host receptor that is necessary and sufficient for the binding of murine norovirus raises the possibility that human noroviruses also require a specific proteinaceous receptor or receptors. The recent development of a more reliable in vitro infection model for human noroviruses87 combined with CRISPR–Cas technology could lead to a better understanding of the entry pathway that is used by human noroviruses and to the development of entry inhibitors.
HIV. To identify host factors that are required for HIV replication, a CRISPR–Cas screen was carried out in a physiologically relevant CD4+ T cell line88. In addition to the T cell surface glycoprotein CD4 and the co- receptor CC-chemokine receptor type 5 (CCR5) that are required for entry of CCR5-tropic viruses, a cell adhesion molecule named CD166 antigen (ALCAM) and two proteins, protein tyrosine sulfotransferase 2 (TPST2) and adenosine 3′-phospho 5′-phosphosulfate transporter 1 (SLC35B2), that are involved in tyrosine sulfation were found to be important for HIV infection. To validate these findings, electroporation was used to introduce Cas9–sgRNA ribonucleoprotein complexes into CD4+ T cells that were isolated from the blood of healthy human donors, and these cells were then challenged with CCR5-tropic HIV. This demonstrates that CRISPR–Cas technology can be used to study host factors in primary cells.
Bacteria, parasites and immune signalling. Genome-scale knockout screens have also been used to uncover immune-regulatory networks89, the pyroptosis pathway90 and host requirements for bacterial pathogenesis91,92,93. CRISPR–Cas screens in Toxoplasma gondii have also identified genes that are essential for the fitness of apicomplexan parasites94. In bacteria, partial knockdowns using CRISPR interference enabled the systematic phenotypic identification of essential bacterial genes in Bacillus subtilis95.
Emerging CRISPR–Cas tools
CRISPR–Cas technology has broad applications in the study of viruses, extending beyond host factor screens. CRISPR–Cas methods are being used to generate both in vitro and in vivo models to study viral pathogenesis, to edit and image viral genomes, in the development of gene drive systems that have the potential to eradicate viral disease vectors, and to advance the development of antiviral therapeutics (Fig. 3).
Figure 3. CRISPR–Cas applications beyond genetic screening.
CRISPR–Cas genome editing enables the generation of in vitro and in vivo models to study viral pathogenesis. The technology is not limited to engineering model organisms, such as mice, fruit flies and roundworms, but can also be applied to non-model organisms, such as pigs, macaques, ferrets, chickens, ticks, bats and mosquitoes. CRISPR–Cas technology is also useful for engineering the genomes of large DNA viruses, such as poxviruses. Catalytically inactive Cas9 (dCas9) proteins that are fused to fluorophores may be useful to track viral nucleic acids in cells162. CRISPR-Cas technology could lead to the development of new approaches to treat virus infections and prevent transmission, including the development of gene drive systems to eradicate viral disease vectors, the direct targeting to inactivate viral gene expression, the identification of druggable host proteins that are required for virus replication, and elucidating the mechanisms of action of antivirals. cccDNA, covalently closed circular DNA; HBV, hepatitis B virus; sgRNA, single-guide RNA.
Generation of in vitro and in vivo models to study viral disease. Traditionally, in vitro systems using cell lines have been invaluable tools to study virus infections. However, these systems have limitations in providing comprehensive insights into host physiology, immunity, pathology and transmission during infection. CRISPR–Cas technology has been used to generate advanced in vitro and in vivo knockout models to study viral pathogenesis, such as primary cells, organoids, induced-pluripotent stem cells (iPSCs)96,97,98 and animal models99. CRISPR–Cas methods have expedited the process of generating knockout animal models. In addition to genome engineering of laboratory animals, such as roundworms100,101, fruit flies102,103 and mice104,105, CRISPR–Cas approaches can be applied to non-model organisms, such as mosquitoes106, ticks, bats, pigs107,108, macaques109, ferrets110 and chickens111, which are important vectors or reservoirs of viruses. For example, bats are reservoirs for rabies virus, Nipah virus, Ebola virus and severe acute respiratory syndrome-related coronavirus, whereas mosquitoes transmit DENV, ZIKV, WNV and chikungunya virus104. Ferrets are a suitable animal model to study influenza viruses112. Previously, it was challenging to genetically engineer ferrets. Ferrets that have been genetically engineered using CRISPR–Cas technology have recently been reported and may substantially broaden the application of the ferret model in the study of influenza virus pathogenesis and transmission110.
CRISPR–Cas tools for studying large DNA viruses. Efficient genetic modification of large viral genomes has been limited by conventional molecular cloning techniques, especially for DNA viruses that belong to the proposed order Megavirales. For example, it has been challenging to edit the genomes of human poxviruses, which range from 130 kb to 375 kb in size. However, CRISPR–Cas technology has been used to efficiently edit the genomes of large DNA viruses, such as vaccinia virus, Epstein–Barr virus and adenoviral vectors113,114,115.
CRISPR–Cas antiviral strategies. There is also potential for the application of CRISPR–Cas technology in the prevention and treatment of diseases by targeting viruses and their vectors. Vector control has been used as a strategy to limit the transmission of vector-borne viruses, including ZIKV, DENV and yellow fever virus. For example, several attempts have been made to introduce genetically modified, sterile mosquitoes into the environment in an attempt to eradicate wild-type mosquito populations that transmit viral diseases116,117,118,119. CRISPR–Cas tools have been used to generate gene drives that have the potential to diminish mosquito populations120,121. Furthermore, CRISPR–Cas technology could be used to treat persistent virus infections, such as infections with HIV, HBV, HCV and herpes simplex virus122,123,124,125. Recently, HBV covalently closed circular DNA (cccDNA), the hallmark of persistent HBV infection, has been successfully targeted in cell culture and in animal models126,127,128,129. In addition, CRISPR–Cas screens can be used to understand the mode of action of antivirals. For example, CRISPR–Cas and short hairpin RNA (shRNA) screens carried out in parallel uncovered the mechanism of action of GSK983, an antiviral drug that may prove effective in the treatment of a wide range of RNA and DNA viruses130,131. GSK983 was found to block virus replication by inhibiting the cellular pyrimidine biosynthesis enzyme dihydroorotate dehydrogenase, thus reducing intracellular levels of nucleotides, which are needed for viral nucleic acid synthesis.
Conclusions and perspectives
The repurposing of the CRISPR–Cas system as a genome-engineering tool is starting to transform biomedical research in several areas, including infectious diseases, cancer and gene therapy. This new approach is also being used to gain a better understanding of how viruses exploit their host and to develop new antiviral therapeutics. Since its discovery, CRISPR–Cas technology has already advanced our understanding of the life cycles of noroviruses and flaviviruses. Future screens will undoubtedly shed light on commonalities and differences in how viruses have evolved to exploit and subvert host functions, and may provide potential targets for antiviral therapy. Together with advances in the genetic engineering of animal models using CRISPR–Cas and improvements in field applications such as gene drive systems, these new CRISPR–Cas technologies will help us to tackle current and future viral epidemics.
Continued efforts to develop and enhance CRISPR–Cas systems will expand the toolbox that enables us to gain a greater understanding of complex biological and disease processes. Engineering of Cas nucleases will make DNA and RNA targeting more versatile. For example, Staphylococcus aureus Cas9 is smaller than most Cas9 nucleases that have been used to date, making in vivo delivery of Cas9–sgRNA complexes more feasible132,133; and the CRISPR-associated endoribonuclease C2c2 could lead to the development of new RNA-targeting tools134,135. Moreover, CRISPR–Cas systems can be combined with other technologies to develop more sophisticated screening approaches. Combining CRISPR–Cas technology with advances in single-cell profiling could lead to better measurements of virus replication dynamics136,137,138,139,140. Furthermore, a screening strategy that investigates epistatic relationships (for example by combining haploid and CRISPR–Cas mutagenesis60), will enable the systematic analysis of functional interdependencies between the host genes that are most crucial for virus infection. We expect that refining and expanding the genetic toolbox for manipulating host cells will lead to novel insights into the 'arms race' between viruses and their hosts.
Acknowledgements
The authors thank M. Diamond and C. Dovey for critically reading the manuscript and for helpful comments.
Glossary
- Pathogen-associated molecular patterns
(PAMPs). Molecules that are expressed by pathogens and recognized by the host innate immune system.
- Forward genetic screens
Genetic screens in which mutant genes are identified on the basis of their phenotypes.
- Signal-to-noise ratio
The ratio of truly enriched genes (signal) versus random enrichment of genes (noise).
- Single-guide RNA
(sgRNA). An artificial fusion of CRISPR RNA (crRNA) and partial transactivating RNA (tracrRNA) through a synthetic stem–loop that mimics the natural crRNA–tracrRNA hybrid and directs Cas9 to its target DNA.
- Non-homologous end joining
A pathway that repairs double-strand breaks (DSBs) in DNA by directly ligating the break ends without the need for a homologous template.
- Frameshift mutation
A genetic mutation caused by indels (insertions or deletions) of a number of nucleotides that is not divisible by three, leading to a shift in the ORF of the gene.
- Loss-of-function
Stable suppression or disruption of gene expression in a cell or organism.
- Gain-of-function
Ectopic overexpression of genes or activation of non-expressed genes in a cell or organism.
- Transduced
Cells that are infected with a lentiviral or retroviral vector containing a DNA of interest that is integrated into the genome.
- Complementary DNA library
(cDNA library). A library that is prepared from all expressed mRNAs in a cell by reverse transcription into DNA.
- Permissive
Pertaining to a host cell: susceptible to infection with a particular virus; permissiveness usually depends on the expression of certain proviral genes and the absence of certain antiviral restriction factors.
- Lentiviral expression vectors
Gene delivery tools that are modified from HIV-1, with most of the viral genes removed and a desired gene inserted, often under the control of a cytomegalovirus (CMV) promoter. The lentiviral vector integrates into the host genomic DNA through long terminal repeats and expresses the inserted gene.
- Gene traps
Lentiviral or retroviral constructs that predominantly integrate into the coding regions of genes to disrupt gene expression.
- Transformed cell lines
Immortalised cell lines that can proliferate indefinitely owing to one or several mutations. The cells have evaded normal cellular senescence and can be grown for prolonged periods in vitro.
- Karyotype
The number and appearance of chromosomes in the nucleus of a eukaryotic cell.
- Off-target effects
Unwanted knockout or knockdown of a gene, most often as a result of partial complementarity to an unintended target.
- Pseudotyped viruses
Viruses or viral vectors that are packaged with envelope proteins from another virus.
- Viral replicons
Self-replicating subgenomic viral RNAs that originate from viral genomes. These replicons contain viral genes that encode non-structural proteins that are critical for viral genome replication, but the genes that encode structural proteins are either deleted or replaced by foreign genes.
- Internal ribosome entry site reporters
(IRES reporters). Reporter constructs that consist of a viral IRES (an RNA element that allows for translation initiation in a cap-independent manner) fused to a reporter gene, such as a gene that encodes luciferase or a fluorescent protein.
- Background
During a CRISPR–Cas screen: random, low-level detection of single-guide RNAs that are not causal to a knockout phenotype.
- Oligosaccharyltransferase complex
(OST complex). A protein complex in the endoplasmic reticulum (ER) membrane. This complex transfers a lipid-linked oligosaccharide precursor to asparagine residues on nascent proteins in the lumen of the ER.
- Endoplasmic reticulum-associated degradation
(ERAD). A process by which the endoplasmic reticulum (ER) recognizes misfolded proteins and directs their degradation.
- Synergistic activation mediator
A CRISPR–Cas-based engineered protein complex that activates transcription from endogenous genes.
- Signal peptide
A short peptide that is present at the amino terminus of the majority of newly synthesized proteins that are targeted towards the secretory pathway.
- Translocon
A protein complex in the endoplasmic reticulum membrane that directs the translocation of nascent polypeptides from the cytosol into the endoplasmic reticulum lumen.
- Retrotranslocation complex
A membrane protein complex in the endoplasmic reticulum that mediates transport of misfolded proteins from the endoplasmic reticulum lumen into the cytosol.
- miR-122
A liver-specific microRNA that is required for hepatitis C virus replication by interacting with its 5′ UTR.
- FAD
A redox cofactor that is involved in several important reactions in metabolism.
- Pyroptosis
A caspase 1-dependent form of programmed cell death that is inflammatory and crucial for controlling microbial infections.
- Gene drive
A technique that promotes the inheritance of a particular gene to increase its prevalence in a population.
- Organoids
3D, miniaturized and simplified versions of organs, produced in vitro.
- Covalently closed circular DNA
(cccDNA). The replicative form of the hepatitis B virus DNA, which persists within the nuclei of infected liver cells and produces viral RNA transcripts.
- C2c2
A ribonuclease that is guided by a single CRISPR RNA and can be programmed to cleave single-stranded RNA targets.
- Epistatic relationships
The relationships between genes in which one gene influences the phenotypic expression of another gene.
Biographies
Andreas S. Puschnik is a graduate student in the Department of Microbiology and Immunology at Stanford University, California, USA, where he uses haploid and CRISPR–Cas genetic screens to uncover host factors that are crucial to virus infections. He was previously a Fulbright fellow at the University of California, Berkeley, USA, where he studied the antibody response to dengue virus infections.
Karim Majzoub is a postdoctoral fellow in the Department of Microbiology and Immunology at Stanford University, California, USA, where he focuses on the virus–host interactions of flaviviruses. He obtained his doctorate from the University of Strasbourg, France, where he studied virus infection and immunity.
Yaw Shin Ooi is a postdoctoral fellow in the Department of Microbiology and Immunology at Stanford University, California, USA, where he studies host factors that are crucial for dengue virus infection. He received his doctorate from Albert Einstein College of Medicine, New York, USA, where he carried out RNAi-based screens to identify alphavirus entry factors.
Jan E. Carette is Assistant Professor in the Department of Microbiology and Immunology at Stanford University, California, USA. He received his doctorate from Wageningen University, The Netherlands, and did his postdoctoral training at Vrije Universiteit Amsterdam, The Netherlands, and at the Whitehead Institute for Biomedical Research, Cambridge, Massachusetts, USA. His laboratory uses genetic approaches to understand the molecular mechanisms of virus–host interactions, ranging from pathogenic viruses to viruses used in gene therapy.
Related links
FURTHER INFORMATION
PowerPoint slides
Competing interests
J.E.C. is listed as an inventor on a patent on mutagenesis in haploid or near-haploid cells (US patent application number 2012/0190,011).
References
- 1.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartwell LH, Culotti J, Reid B. Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc. Natl Acad. Sci. USA. 1970;66:352–359. doi: 10.1073/pnas.66.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutschmann S, et al. Role of Drosophila IKKγ in a toll-independent antibacterial immune response. Nat. Immunol. 2000;1:342–347. doi: 10.1038/79801. [DOI] [PubMed] [Google Scholar]
- 4.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 5.Berns K, et al. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature. 2004;428:431–437. doi: 10.1038/nature02371. [DOI] [PubMed] [Google Scholar]
- 6.Paddison PJ, et al. A resource for large-scale RNA-interference-based screens in mammals. Nature. 2004;428:427–431. doi: 10.1038/nature02370. [DOI] [PubMed] [Google Scholar]
- 7.Carette JE, et al. Haploid genetic screens in human cells identify host factors used by pathogens. Science. 2009;326:1231–1235. doi: 10.1126/science.1178955. [DOI] [PubMed] [Google Scholar]
- 8.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mali P, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jinek M, et al. RNA-programmed genome editing in human cells. eLife. 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evers B, et al. CRISPR knockout screening outperforms shRNA and CRISPRi in identifying essential genes. Nat. Biotechnol. 2016;34:631–633. doi: 10.1038/nbt.3536. [DOI] [PubMed] [Google Scholar]
- 12.DeJesus R, et al. Functional CRISPR screening identifies the ufmylation pathway as a regulator of SQSTM1/p62. eLife. 2016;5:e17290. doi: 10.7554/eLife.17290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart T, et al. High-resolution CRISPR screens reveal fitness genes and genotype-specific cancer liabilities. Cell. 2015;163:1515–1526. doi: 10.1016/j.cell.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Shalem O, et al. Genome-scale CRISPR–Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR–Cas9 system. Science. 2014;343:80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalem O, Sanjana NE, Zhang F. High-throughput functional genomics using CRISPR–Cas9. Nat. Rev. Genet. 2015;16:299–311. doi: 10.1038/nrg3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, et al. High-throughput screening of a CRISPR/Cas9 library for functional genomics in human cells. Nature. 2014;509:487–491. doi: 10.1038/nature13166. [DOI] [PubMed] [Google Scholar]
- 18.Evans MJ, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 19.Ploss A, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saeed M, et al. SEC14L2 enables pan-genotype HCV replication in cell culture. Nature. 2015;524:471–475. doi: 10.1038/nature14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoggins JW, et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoggins JW, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ORFeome Collaboration. The ORFeome Collaboration: a genome-scale human ORF-clone resource. Nat. Methods13, 191–192 (2016). [DOI] [PubMed]
- 24.Ramage H, Cherry S. Virus–host interactions: from unbiased genetic screens to function. Annu. Rev. Virol. 2015;2:497–524. doi: 10.1146/annurev-virology-100114-055238. [DOI] [PubMed] [Google Scholar]
- 25.Carette JE, et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477:340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jae LT, et al. Lassa virus entry requires a trigger-induced receptor switch. Science. 2014;344:1506–1510. doi: 10.1126/science.1252480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller EH, et al. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J. 2012;31:1947–1960. doi: 10.1038/emboj.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cote M, et al. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature. 2011;477:344–348. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bornholdt ZA, et al. Host-primed Ebola virus GP exposes a hydrophobic NPC1 receptor-binding pocket, revealing a target for broadly neutralizing antibodies. mBio. 2016;7:e02154–15. doi: 10.1128/mBio.02154-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong X, et al. Structural insights into the Niemann-Pick C1 (NPC1)-mediated cholesterol transfer and Ebola infection. Cell. 2016;165:1467–1478. doi: 10.1016/j.cell.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, et al. Ebola viral glycoprotein bound to its endosomal receptor Niemann-Pick C1. Cell. 2016;164:258–268. doi: 10.1016/j.cell.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diehl WE, et al. Ebola virus glycoprotein with increased infectivity dominated the 2013–2016 epidemic. Cell. 2016;167:1088–1098.e6. doi: 10.1016/j.cell.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urbanowicz RA, et al. Human adaptation of Ebola virus during the West African outbreak. Cell. 2016;167:1079–1087.e5. doi: 10.1016/j.cell.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staring J, et al. PLA2G16 represents a switch between entry and clearance of Picornaviridae. Nature. 2017;541:412–416. doi: 10.1038/nature21032. [DOI] [PubMed] [Google Scholar]
- 35.Pillay S, et al. An essential receptor for adeno-associated virus infection. Nature. 2016;530:108–112. doi: 10.1038/nature16465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt T, Schmid-Burgk JL, Hornung V. Synthesis of an arrayed sgRNA library targeting the human genome. Sci. Rep. 2015;5:14987. doi: 10.1038/srep14987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Essletzbichler P, et al. Megabase-scale deletion using CRISPR/Cas9 to generate a fully haploid human cell line. Genome Res. 2014;24:2059–2065. doi: 10.1101/gr.177220.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sagi I, et al. Derivation and differentiation of haploid human embryonic stem cells. Nature. 2016;532:107–111. doi: 10.1038/nature17408. [DOI] [PubMed] [Google Scholar]
- 39.Elling U, et al. Forward and reverse genetics through derivation of haploid mouse embryonic stem cells. Cell Stem Cell. 2011;9:563–574. doi: 10.1016/j.stem.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leeb M, Wutz A. Derivation of haploid embryonic stem cells from mouse embryos. Nature. 2011;479:131–134. doi: 10.1038/nature10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wutz A. Haploid mouse embryonic stem cells: rapid genetic screening and germline transmission. Annu. Rev. Cell Dev. Biol. 2014;30:705–722. doi: 10.1146/annurev-cellbio-100913-012920. [DOI] [PubMed] [Google Scholar]
- 42.Pillay S, Carette JE. Hunting viral receptors using haploid cells. Annu. Rev. Virol. 2015;2:219–239. doi: 10.1146/annurev-virology-100114-055119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doench JG, et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. Nat. Biotechnol. 2016;34:184–191. doi: 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang T, et al. Identification and characterization of essential genes in the human genome. Science. 2015;350:1096–1101. doi: 10.1126/science.aac7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu SJ, et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science. 2016;355:aah7111. doi: 10.1126/science.aah7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joung J, et al. Genome-scale CRISPR–Cas9 knockout and transcriptional activation screening. Nat. Protoc. 2017;12:828–863. doi: 10.1038/nprot.2017.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl Acad. Sci. USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wool-Lewis RJ, Bates P. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 1998;72:3155–3160. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong C, et al. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989;243:1188–1191. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- 51.Lohmann V, et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 52.Trono D, Pelletier J, Sonenberg N, Baltimore D. Translation in mammalian cells of a gene linked to the poliovirus 5′ noncoding region. Science. 1988;241:445–448. doi: 10.1126/science.2839901. [DOI] [PubMed] [Google Scholar]
- 53.Simoes EA, Sarnow P. An RNA hairpin at the extreme 5′ end of the poliovirus RNA genome modulates viral translation in human cells. J. Virol. 1991;65:913–921. doi: 10.1128/jvi.65.2.913-921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo B, et al. Highly parallel identification of essential genes in cancer cells. Proc. Natl Acad. Sci. USA. 2008;105:20380–20385. doi: 10.1073/pnas.0810485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konig R, et al. A probability-based approach for the analysis of large-scale RNAi screens. Nat. Methods. 2007;4:847–849. doi: 10.1038/nmeth1089. [DOI] [PubMed] [Google Scholar]
- 56.Li W, et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 2014;15:554. doi: 10.1186/s13059-014-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu Y, et al. High-frequency off-target mutagenesis induced by CRISPR–Cas nucleases in human cells. Nat. Biotechnol. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu PD, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pattanayak V, et al. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat. Biotechnol. 2013;31:839–843. doi: 10.1038/nbt.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blomen VA, et al. Gene essentiality and synthetic lethality in haploid human cells. Science. 2015;350:1092–1096. doi: 10.1126/science.aac7557. [DOI] [PubMed] [Google Scholar]
- 61.Marceau CD, et al. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature. 2016;535:159–163. doi: 10.1038/nature18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- 63.Carette JE, et al. Global gene disruption in human cells to assign genes to phenotypes by deep sequencing. Nat. Biotechnol. 2011;29:542–546. doi: 10.1038/nbt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Timms RT, et al. Genetic dissection of mammalian ERAD through comparative haploid and CRISPR forward genetic screens. Nat. Commun. 2016;7:11786. doi: 10.1038/ncomms11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bekerman E, Einav S. Combating emerging viral threats. Science. 2015;348:282–283. doi: 10.1126/science.aaa3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lessler J, et al. Assessing the global threat from Zika virus. Science. 2016;353:aaf8160. doi: 10.1126/science.aaf8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cao-Lormeau VM, et al. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma H, et al. A CRISPR-based screen identifies genes essential for West-Nile-virus-induced cell death. Cell Rep. 2015;12:673–683. doi: 10.1016/j.celrep.2015.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang R, et al. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature. 2016;535:164–168. doi: 10.1038/nature18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Savidis G, et al. Identification of Zika virus and Dengue virus dependency factors using functional genomics. Cell Rep. 2016;16:232–246. doi: 10.1016/j.celrep.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 72.Cherepanova NA, Gilmore R. Mammalian cells lacking either the cotranslational or posttranslocational oligosaccharyltransferase complex display substrate-dependent defects in asparagine linked glycosylation. Sci. Rep. 2016;6:20946. doi: 10.1038/srep20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fons RD, Bogert BA, Hegde RS. Substrate-specific function of the translocon-associated protein complex during translocation across the ER membrane. J. Cell Biol. 2003;160:529–539. doi: 10.1083/jcb.200210095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olzmann JA, Kopito RR, Christianson JC. The mammalian endoplasmic reticulum-associated degradation system. Cold Spring Harb. Perspect. Biol. 2013;5:a013185. doi: 10.1101/cshperspect.a013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christianson JC, et al. Defining human ERAD networks through an integrative mapping strategy. Nat. Cell Biol. 2011;14:93–105. doi: 10.1038/ncb2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wideman JG. The ubiquitous and ancient ER membrane protein complex (EMC): tether or not? F1000Res. 2015;4:624. doi: 10.12688/f1000research.6944.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cruz-Oliveira C, et al. Receptors and routes of dengue virus entry into the host cells. FEMS Microbiol. Rev. 2015;39:155–170. doi: 10.1093/femsre/fuu004. [DOI] [PubMed] [Google Scholar]
- 78.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 79.Brennan CM, Steitz JA. HuR and mRNA stability. Cell. Mol. Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Madan V, Paul D, Lohmann V, Bartenschlager R. Inhibition of HCV replication by cyclophilin antagonists is linked to replication fitness and occurs by inhibition of membranous web formation. Gastroenterology. 2014;146:1361–1372.e9. doi: 10.1053/j.gastro.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 81.Nag A, Robotham JM, Tang H. Suppression of viral RNA binding and the assembly of infectious hepatitis C virus particles in vitro by cyclophilin inhibitors. J. Virol. 2012;86:12616–12624. doi: 10.1128/JVI.01351-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luban J, Bossolt KL, Franke EK, Kalpana GV, Goff SP. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 83.Lin K, Gallay P. Curing a viral infection by targeting the host: the example of cyclophilin inhibitors. Antiviral Res. 2013;99:68–77. doi: 10.1016/j.antiviral.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan M, Jiang X. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 2005;13:285–293. doi: 10.1016/j.tim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 85.Orchard RC, et al. Discovery of a proteinaceous cellular receptor for a norovirus. Science. 2016;353:933–936. doi: 10.1126/science.aaf1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haga K, et al. Functional receptor molecules CD300lf and CD300ld within the CD300 family enable murine noroviruses to infect cells. Proc. Natl Acad. Sci. USA. 2016;113:E6248–E6255. doi: 10.1073/pnas.1605575113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones MK, et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014;346:755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park RJ, et al. A genome-wide CRISPR screen identifies a restricted set of HIV host dependency factors. Nat. Genet. 2017;49:193–203. doi: 10.1038/ng.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parnas O, et al. A genome-wide CRISPR screen in primary immune cells to dissect regulatory networks. Cell. 2015;162:675–686. doi: 10.1016/j.cell.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 91.Popov LM, et al. The adherens junctions control susceptibility to Staphylococcus aureus α-toxin. Proc. Natl Acad. Sci. USA. 2015;112:14337–14342. doi: 10.1073/pnas.1510265112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Virreira Winter S, Zychlinsky A, Bardoel BW. Genome-wide CRISPR screen reveals novel host factors required for Staphylococcus aureus α-hemolysin-mediated toxicity. Sci. Rep. 2016;6:24242. doi: 10.1038/srep24242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blondel CJ, et al. CRISPR/Cas9 screens reveal requirements for host cell sulfation and fucosylation in bacterial type III secretion system-mediated cytotoxicity. Cell Host Microbe. 2016;20:226–237. doi: 10.1016/j.chom.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sidik SM, et al. A genome-wide CRISPR screen in toxoplasma identifies essential apicomplexan genes. Cell. 2016;166:1423–1435.e12. doi: 10.1016/j.cell.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peters JM, et al. A comprehensive, CRISPR-based functional analysis of essential genes in bacteria. Cell. 2016;165:1493–1506. doi: 10.1016/j.cell.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hou P, et al. Genome editing of CXCR4 by CRISPR/cas9 confers cells resistant to HIV-1 infection. Sci. Rep. 2015;5:15577. doi: 10.1038/srep15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li C, et al. Inhibition of HIV-1 infection of primary CD4+ T-cells by gene editing of CCR5 using adenovirus-delivered CRISPR/Cas9. J. Gen. Virol. 2015;96:2381–2393. doi: 10.1099/vir.0.000139. [DOI] [PubMed] [Google Scholar]
- 98.Wells MF, et al. Genetic ablation of AXL does not protect human neural progenitor cells and cerebral organoids from Zika virus infection. Cell Stem Cell. 2016;19:703–708. doi: 10.1016/j.stem.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 99.Tschaharganeh DF, Lowe SW, Garippa RJ, Livshits G. Using CRISPR/Cas to study gene function and model disease in vivo. FEBS J. 2016;283:3194–3203. doi: 10.1111/febs.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Friedland AE, et al. Heritable genome editing in C. elegans via a CRISPR–Cas9 system. Nat. Methods. 2013;10:741–743. doi: 10.1038/nmeth.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dickinson DJ, Ward JD, Reiner DJ, Goldstein B. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods. 2013;10:1028–1034. doi: 10.1038/nmeth.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bassett AR, Tibbit C, Ponting CP, Liu JL. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 2013;4:220–228. doi: 10.1016/j.celrep.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gratz SJ, et al. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics. 2013;194:1029–1035. doi: 10.1534/genetics.113.152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bean AG, et al. Studying immunity to zoonotic diseases in the natural host — keeping it real. Nat. Rev. Immunol. 2013;13:851–861. doi: 10.1038/nri3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li D, et al. Heritable gene targeting in the mouse and rat using a CRISPR–Cas system. Nat. Biotechnol. 2013;31:681–683. doi: 10.1038/nbt.2661. [DOI] [PubMed] [Google Scholar]
- 106.Kistler KE, Vosshall LB, Matthews BJ. Genome engineering with CRISPR–Cas9 in the mosquito Aedes aegypti. Cell Rep. 2015;11:51–60. doi: 10.1016/j.celrep.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lei S, et al. Increased and prolonged human norovirus infection in RAG2/IL2RG deficient gnotobiotic pigs with severe combined immunodeficiency. Sci. Rep. 2016;6:25222. doi: 10.1038/srep25222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hai T, Teng F, Guo R, Li W, Zhou Q. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell Res. 2014;24:372–375. doi: 10.1038/cr.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niu Y, et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156:836–843. doi: 10.1016/j.cell.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 110.Kou Z, et al. CRISPR/Cas9-mediated genome engineering of the ferret. Cell Res. 2015;25:1372–1375. doi: 10.1038/cr.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oishi I, Yoshii K, Miyahara D, Kagami H, Tagami T. Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci. Rep. 2016;6:23980. doi: 10.1038/srep23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Belser JA, Eckert AM, Tumpey TM, Maines TR. Complexities in ferret influenza virus pathogenesis and transmission models. Microbiol. Mol. Biol. Rev. 2016;80:733–744. doi: 10.1128/MMBR.00022-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yuan M, et al. Efficiently editing the vaccinia virus genome by using the CRISPR–Cas9 system. J. Virol. 2015;89:5176–5179. doi: 10.1128/JVI.00339-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bi Y, et al. High-efficiency targeted editing of large viral genomes by RNA-guided nucleases. PLoS Pathog. 2014;10:e1004090. doi: 10.1371/journal.ppat.1004090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yuen KS, et al. CRISPR/Cas9-mediated genome editing of Epstein-Barr virus in human cells. J. Gen. Virol. 2015;96:626–636. doi: 10.1099/jgv.0.000012. [DOI] [PubMed] [Google Scholar]
- 116.Harris AF, et al. Field performance of engineered male mosquitoes. Nat. Biotechnol. 2011;29:1034–1037. doi: 10.1038/nbt.2019. [DOI] [PubMed] [Google Scholar]
- 117.Burt A. Heritable strategies for controlling insect vectors of disease. Phil. Trans. R. Soc. B. 2014;369:20130432. doi: 10.1098/rstb.2013.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carvalho DO, et al. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl Trop. Dis. 2015;9:e0003864. doi: 10.1371/journal.pntd.0003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adelman ZN, Tu Z. Control of mosquito-borne infectious diseases: sex and gene drive. Trends Parasitol. 2016;32:219–229. doi: 10.1016/j.pt.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hammond A, et al. A CRISPR–Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 2016;34:78–83. doi: 10.1038/nbt.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hall AB, et al. A male-determining factor in the mosquito Aedes aegypti. Science. 2015;348:1268–1270. doi: 10.1126/science.aaa2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Price AA, Sampson TR, Ratner HK, Grakoui A, Weiss DS. Cas9-mediated targeting of viral RNA in eukaryotic cells. Proc. Natl Acad. Sci. USA. 2015;112:6164–6169. doi: 10.1073/pnas.1422340112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van Diemen FR, et al. CRISPR/Cas9-mediated genome editing of herpesviruses limits productive and latent infections. PLoS Pathog. 2016;12:e1005701. doi: 10.1371/journal.ppat.1005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dong C, et al. Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antiviral Res. 2015;118:110–117. doi: 10.1016/j.antiviral.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 125.Kaminski R, et al. Excision of HIV-1 DNA by gene editing: a proof-of-concept in vivo study. Gene Ther. 2016;23:690–695. doi: 10.1038/gt.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhen S, et al. Harnessing the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated Cas9 system to disrupt the hepatitis B virus. Gene Ther. 2015;22:404–412. doi: 10.1038/gt.2015.2. [DOI] [PubMed] [Google Scholar]
- 127.Liu X, Hao R, Chen S, Guo D, Chen Y. Inhibition of hepatitis B virus by the CRISPR/Cas9 system via targeting the conserved regions of the viral genome. J. Gen. Virol. 2015;96:2252–2261. doi: 10.1099/vir.0.000159. [DOI] [PubMed] [Google Scholar]
- 128.Kennedy EM, et al. Suppression of hepatitis B virus DNA accumulation in chronically infected cells using a bacterial CRISPR/Cas RNA-guided DNA endonuclease. Virology. 2015;476:196–205. doi: 10.1016/j.virol.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ramanan V, et al. CRISPR/Cas9 cleavage of viral DNA efficiently suppresses hepatitis B virus. Sci. Rep. 2015;5:10833. doi: 10.1038/srep10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deans RM, et al. Parallel shRNA and CRISPR–Cas9 screens enable antiviral drug target identification. Nat. Chem. Biol. 2016;12:361–366. doi: 10.1038/nchembio.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Harvey R, et al. GSK983: a novel compound with broad-spectrum antiviral activity. Antiviral Res. 2009;82:1–11. doi: 10.1016/j.antiviral.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barrangou R. Diversity of CRISPR–Cas immune systems and molecular machines. Genome Biol. 2015;16:247. doi: 10.1186/s13059-015-0816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ran FA, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abudayyeh OO, et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353:aaf5573. doi: 10.1126/science.aaf5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.East-Seletsky A, et al. Two distinct RNase activities of CRISPR–C2c2 enable guide-RNA processing and RNA detection. Nature. 2016;538:270–273. doi: 10.1038/nature19802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klein AM, et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell. 2015;161:1187–1201. doi: 10.1016/j.cell.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Macosko EZ, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Adamson B, et al. A multiplexed single-cell CRISPR screening platform enables systematic dissection of the unfolded protein response. Cell. 2016;167:1867–1882.e21. doi: 10.1016/j.cell.2016.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dixit A, et al. Perturb-Seq: dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell. 2016;167:1853–1866.e17. doi: 10.1016/j.cell.2016.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jaitin DA, et al. Dissecting immune circuits by linking CRISPR-pooled screens with single-cell RNA-Seq. Cell. 2016;167:1883–1896.e15. doi: 10.1016/j.cell.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 141.Marraffini LA. CRISPR–Cas immunity in prokaryotes. Nature. 2015;526:55–61. doi: 10.1038/nature15386. [DOI] [PubMed] [Google Scholar]
- 142.Pourcel C, Salvignol G, Vergnaud G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology. 2005;151:653–663. doi: 10.1099/mic.0.27437-0. [DOI] [PubMed] [Google Scholar]
- 143.Deltcheva E, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471:602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garneau JE, et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468:67–71. doi: 10.1038/nature09523. [DOI] [PubMed] [Google Scholar]
- 145.Marraffini LA, Sontheimer EJ. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature. 2010;463:568–571. doi: 10.1038/nature08703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Almendros C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology. 2009;155:733–740. doi: 10.1099/mic.0.023960-0. [DOI] [PubMed] [Google Scholar]
- 147.Horvath P, et al. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. J. Bacteriol. 2008;190:1401–1412. doi: 10.1128/JB.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiang F, Zhou K, Ma L, Gressel S, Doudna JA. A Cas9-guide RNA complex preorganized for target DNA recognition. Science. 2015;348:1477–1481. doi: 10.1126/science.aab1452. [DOI] [PubMed] [Google Scholar]
- 149.O'Connell MR, et al. Programmable RNA recognition and cleavage by CRISPR/Cas9. Nature. 2014;516:263–266. doi: 10.1038/nature13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jinek M, et al. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science. 2014;343:1247997. doi: 10.1126/science.1247997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wiedenheft B, et al. RNA-guided complex from a bacterial immune system enhances target recognition through seed sequence interactions. Proc. Natl Acad. Sci. USA. 2011;108:10092–10097. doi: 10.1073/pnas.1102716108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, Doudna JA. Sequence- and structure-specific RNA processing by a CRISPR endonuclease. Science. 2010;329:1355–1358. doi: 10.1126/science.1192272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hale CR, et al. RNA-guided RNA cleavage by a CRISPR RNA–Cas protein complex. Cell. 2009;139:945–956. doi: 10.1016/j.cell.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gilbert LA, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gilbert LA, et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell. 2014;159:647–661. doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dominguez AA, Lim WA, Qi LS. Beyond editing: repurposing CRISPR–Cas9 for precision genome regulation and interrogation. Nat. Rev. Mol. Cell Biol. 2016;17:5–15. doi: 10.1038/nrm.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Konermann S, et al. Genome-scale transcriptional activation by an engineered CRISPR–Cas9 complex. Nature. 2015;517:583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Landry DM, Hertz MI, Thompson SR. RPS25 is essential for translation initiation by the Dicistroviridae and hepatitis C viral IRESs. Genes Dev. 2009;23:2753–2764. doi: 10.1101/gad.1832209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat. Med. 2013;19:837–849. doi: 10.1038/nm.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 161.Shwetha S, et al. HuR displaces polypyrimidine tract binding protein to facilitate La binding to the 3′ untranslated region and enhances hepatitis C virus replication. J. Virol. 2015;89:11356–11371. doi: 10.1128/JVI.01714-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen B, et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155:1479–1491. doi: 10.1016/j.cell.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Baggen J, et al. Enterovirus D68 receptor requirements unveiled by haploid genetics. Proc. Natl Acad. Sci. USA. 2016;113:1399–1404. doi: 10.1073/pnas.1524498113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kleinfelter LM, et al. Haploid genetic screen reveals a profound and direct dependence on cholesterol for hantavirus membrane fusion. mBio. 2015;6:e00801. doi: 10.1128/mBio.00801-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Petersen J, et al. The major cellular sterol regulatory pathway is required for Andes virus infection. PLoS Pathog. 2014;10:e1003911. doi: 10.1371/journal.ppat.1003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jae LT, et al. Deciphering the glycosylome of dystroglycanopathies using haploid screens for lassa virus entry. Science. 2013;340:479–483. doi: 10.1126/science.1233675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Riblett AM, et al. A haploid genetic screen identifies heparan sulfate proteoglycans supporting Rift Valley Fever virus infection. J. Virol. 2016;90:1414–1423. doi: 10.1128/JVI.02055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]





