Abstract
Context:
Immune checkpoint inhibitors, including anti–programmed cell death protein 1 (PD-1), anti–programmed cell death protein ligand 1 (PD-L1), and anti–cytotoxic T-lymphocyte antigen 4 (anti-CTLA4) monoclonal antibodies, have been widely used in cancer treatment. They are known to cause immune-related adverse events (irAEs), which resemble autoimmune diseases. Anterior pituitary hypophysitis with secondary hypopituitarism is a frequently reported irAE, especially in patients receiving anti–CTLA4 treatment. In contrast, posterior pituitary involvement, such as central diabetes insipidus (DI), is relatively rare and is unreported in patients undergoing PD-1/PD-L1 blockade.
Case Description:
We describe a case of a 73-year-old man with Merkel cell carcinoma who received the anti–PD-L1 monoclonal antibody avelumab and achieved partial response. The patient developed nocturia, polydipsia, and polyuria 3 months after starting avelumab. Further laboratory testing revealed central DI. Avelumab was held and he received desmopressin for the management of central DI. Within 6 weeks after discontinuation of avelumab, the patient’s symptoms resolved and he was eventually taken off desmopressin. The patient remained off avelumab and there were no signs or symptoms of DI 2 months after the discontinuation of desmopressin.
Conclusion:
To our knowledge, this is the first report of central DI associated with anti–PD-L1 immunotherapy. The patient’s endocrinopathy was successfully managed by holding treatment with the immune checkpoint inhibitor. This case highlights the importance of early screening and appropriate management of hormonal irAEs in subjects undergoing treatment with immune checkpoint inhibitors to minimize morbidity and mortality.
We report a case of central diabetes insipidus induced by the anti–PD-L1 drug avelumab. The patient was successfully managed by holding the immune checkpoint inhibitor.
In recent years, immune checkpoint inhibitors have emerged as powerful treatment tools in hematology and oncology. These drugs disinhibit tumor-specific immune responses by targeting repressive signals to T-cells. The programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA4) receptors are commonly involved in suppressing antitumor immune responses. Programmed cell death protein ligand 1 (PD-L1) is a ligand for PD-1 and is expressed on cancer cells and antigen-presenting cells. Activation of the PD-1 pathway generally leads to decrease T-cell function, favoring tumor survival. Avelumab is a human monoclonal antibody against PD-L1 and was recently approved by the Food and Drug Administration for management of Merkel cell carcinoma (MCC) (1) and bladder cancer (2). Avelumab blocks PD-1 signaling by inhibiting PD-1/PD-L1 interaction, which results in upregulation of antitumor immune responses.
Checkpoint inhibitors are associated with immune-related adverse events (irAEs) involving multiple endocrinology organs (3). Hypophysitis and thyroid abnormalities are the most common endocrine irAEs reported to date. Anterior pituitary hypophysitis with secondary hypopituitarism is frequently reported as an irAE, especially in patients receiving anti-CTLA4 treatment. Rates of anterior pituitary hypophysitis are lower in patients taking anti–PD-1 drugs. Posterior pituitary hypophysitis is notably rare with only a single case report of anti-CTLA4 therapy–related central diabetes insipidus (DI) (4), which was diagnosed based on clinical symptoms and response to prednisone treatment. To our knowledge, there are no cases of PD-1/PD-L1 inhibitor–associated DI reported in the literature. We herein report a case of central DI presenting with polyuria and polydipsia in a man with MCC receiving the anti–PD-L1 drug avelumab.
Case
An otherwise healthy 73-year-old white man was evaluated at the National Institutes of Health Clinical Center for multiple scalp tumors. Punch biopsy led to the histological diagnosis of MCC. Computed tomography scans of neck, chest, abdomen, and pelvis revealed bilateral cervical lymph nodes consistent with metastasis. He was enrolled onto a clinical trial (NCT02155647) investigating the clinical activity and safety of the anti–PD-L1 monoclonal antibody avelumab in MCC. His other medication included Prilosec. A thorough baseline work-up revealed normal electrolyte, hepatic, and renal function.
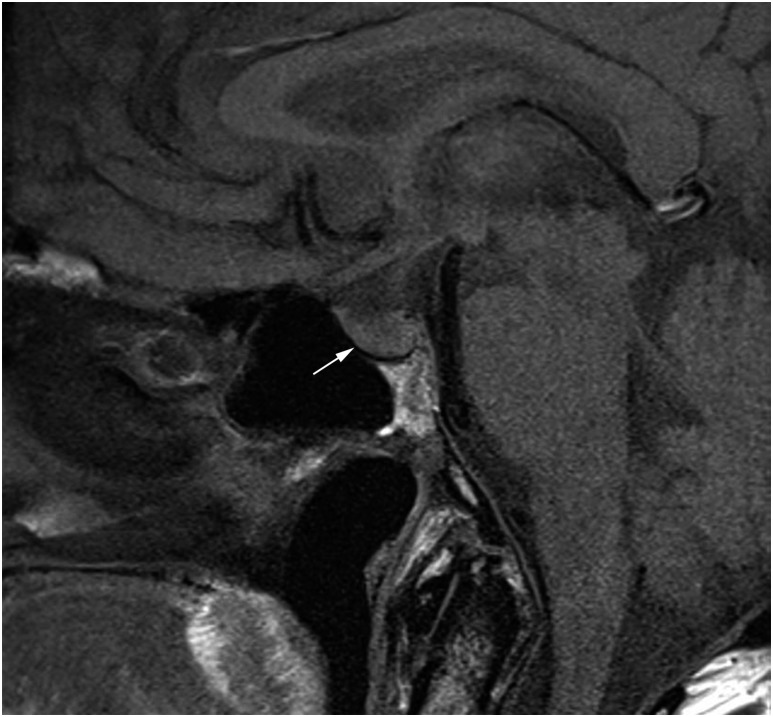
The patient received avelumab 10 mg/kg by intravenous infusion every 2 weeks and achieved a partial response (RECIST 1.1) within 3 months, which was evident by clinical resolution of his scalp tumors. Before the eighth avelumab infusion, he presented with more frequent nocturia (three to four times with increased volume each night), fatigue, polydipsia, and polyuria. Laboratory evaluation was consistent with DI: hypernatremia (sodium, 147 mmol/L), serum osmolality of 303 mOsm/kg, inappropriately low urine osmolality of 241 mOsm/kg, and urine specific gravity of 1.000 (Table 1). Further evaluations excluded other endocrinopathies, and a detailed biochemical work-up is summarized in Table 1. A cosyntropin [adrenocorticotropic hormone (ACTH)] stimulation test ruled out adrenal insufficiency with the baseline, 30-minute, and 60-minute cortisol values of 8.4, 21.2, and 23.6 μg/dL, respectively. Of note, the patient denied any use of nonsteroid anti-inflammatory drugs or other over-the-counter medications. Pituitary magnetic resonance imaging (MRI) did not show signs of hypophysitis (Fig. 1). The posterior pituitary bright spot is a manifestation of stored vasopressin. Although the posterior pituitary bright spot is missing in 20% of the general population (5), its absence on the patient’s MRI scan is consistent with central DI. The patient was started on oral desmopressin (DDAVP) at 0.1 mg once daily and avelumab was held. His fatigue, polyuria, polydipsia, and laboratory values improved on desmopressin, favoring the diagnosis of central DI. The temporal trend of the patient’s serum and urine osmolalities in relationship to desmopressin therapy is detailed in Table 2. A diagnosis of central DI was made based on the presenting symptoms of increased volume and frequency of urination with a biochemical evaluation that included elevated serum sodium concentration, normal glucose level, decreased specific gravity, inappropriately low urine osmolality for serum osmolality, and appropriate response to desmopressin.
Table 1.
Baseline Biochemical Parameters
| Biochemical Parameter | Patient’s Value at the Time of Central DI Diagnosis | Patient’s Laboratory Value Prior to Initiating Avelumaba | Normal Range |
|---|---|---|---|
| Sodium, blood | 147 | 141 | 136–145 mmol/L |
| Potassium, blood | 3.5 (repeat, 3.9) | 4.6 | 3.4–4.6 mmol/L |
| Creatinine, blood | 1.51 | 0.94 | 0.67–1.17 mg/dL |
| Calcium, blood | 2.29 | 2.22 | 2.15–2.55 mmol/L |
| eGFR | 43 | 85 | 30–60b mL/min/1.73 m2 |
| Osmolality, blood | 303 | — | 278–298 mOsm/kg |
| Glucose, blood | 112 | 97 | 74–106 mg/dL |
| Thyrotropin | 2.92 | 2.37 | 0.27–4.20 μIU/mL |
| FT4 | 1.1 | 1.1 | 0.9–1.7 ng/dL |
| ACTH | 23.1 | 15.1 | 5–46 pg/mL |
| Prolactin | 6.8 | — | 2-25 μg/L |
| IGF-1 | 109 | — | 64–188 ng/mL |
| Cortisol | 7.0 | — | 5–25 μg/L |
| LH | 5.1 | — | 1.8–12 U/L |
| FSH | 5.8 | — | 1.3–19.3 U/L |
| Sodium, urine | 37 | — | 18–30 mmol/L |
| Osmolality, urine | 241 | — | 300–900 mOsm/kg |
Abbreviations: eGFR, estimated glomerular filtration rate; FSH, follicle-stimulating hormone; IGF-1, insulin-like growth factor-1; LH, luteinizing hormone.
When available.
Moderate decrease in eGFR.
Figure 1.
T1 sagittal section of pituitary MRI. Pituitary gland was normal with no sign of hypophysitis (white arrow). The posterior pituitary bright spot was missing.
Table 2.
Trend of Serum and Urine Osmolalities, Electrolytes, Serum Sodium, and Specific Gravity
| Day |
Days After Starting Avelumab Treatment |
Normal Range | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 126 | 132 | 148 | 168 | 170 | 182 | 196 | 224 | ||
| U Osm (mOsm/Kg) | 241 | 380 | 382 | 467 | 493 | 505 | 557 | 596 | 300–900 |
| S Osm (mOsm/Kg) | 303 | 303 | 296 | 298 | 299 | 300 | 299 | 298 | 278–298 |
| S Sodium (mmol/L) | 147 | 144 | 142 | 143 | 142 | 144 | 142 | 141 | 136–145 |
| Sp Gravity | 1.00 | 1.01 | 1.01 | 1.011 | 1.016 | 1.002–1.035 | |||
The patient presented with DI symptoms on day 126 after starting avelumab and received DDAVP from day 132 to day 167 (shaded days).
Abbreviations: S Osm, serum osmolality; Sp Gravity, urine specific gravity; S Sodium, serum sodium; U Osm, urine osmolality.
Six weeks after starting desmopressin, the patient was clinically improved with near resolution of symptoms. He no longer complained of polyuria or polydipsia even when he forgot to take desmopressin. Desmopressin therapy was discontinued without recurrence of his DI symptoms or abnormal laboratory values during a 2-month course of close follow-up (Table 2). The patient’s MCC remained stable and in partial remission since discontinuing avelumab, suggesting his antitumor immune response continued whereas his posterior hypophysitis resolved.
Discussion
In cancer patients, hypopituitarism can occur due to multiple etiologies: metastatic infiltrates of the pituitary gland, direct effect of radiotherapy, hypophysitis, and compression of the gland or hypothalamus from direct mass effect. Metastatic deposits to the pituitary gland have a predilection for the posterior lobe, which can cause central DI. However, the pituitary MRI performed at the time of diagnosis of DI in our patient did not show stalk thickening or posterior pituitary mass. The possibility of undetectable micrometastases is unlikely, as the DI resolved after discontinuing immunotherapy. Moreover, a repeat MRI after the discontinuation of avelumab did not show hypothalamic or pituitary metastases. DI can also be nephrogenic, caused by a partial or complete resistance to arginine vasopressin in the kidney. Possible etiologies of nephrogenic DI are hypokalemia and hypercalcemia. In our patient, both the potassium and calcium levels were within the normal limits (Table 1) at the time of diagnosis of DI, which makes nephrogenic DI unlikely. Therefore, this patient’s DI is most likely secondary to posterior pituitary hypophysitis precipitated by avelumab therapy.
Hypophysitis can be classified into primary/idiopathic or secondary based on the etiology. Primary hypophysitis refers to inflammation of the pituitary gland with no identifiable etiologic causes. Secondary hypophysitis includes cases associated with identifiable causes, including drug related (checkpoint inhibitors, interleukin-2, interferon), rupture of Rathke’s cleft cyst, and systemic inflammatory disorders. Secondary hypophysitis related to checkpoint inhibitors has a reported incidence of 8% to 13% in patients receiving anti-CTLA4 therapy (6) and 8.5% to 9.0% with anti–PD-1 therapy (7). In immunotherapy-associated hypophysitis (IH), the degree of pituitary enlargement is mild, and compression of the optic chiasm is not typically seen. Unlike other forms of hypophysitis (lymphocytic, granulomatous, xanthomatous, and plasmacytic), IH is more common in males (3). Also, compared with that of idiopathic hypophysitis, DI is extremely rare in patients with IH (only one case of DI has been reported to date) (4). IH typically occurs after an average of 2 to 3 months of therapy with immunotherapy. Older age and male sex are potential risk factors for the development of IH (3). All of these factors fit in the present case. IH mainly affects the anterior pituitary, with ACTH and thyrotropin deficiency being the most common abnormalities. It can also affect sex hormones, growth hormone, and prolactin. MRI imaging typically shows mild-to-moderate diffuse enlargement of the pituitary gland (8). However, a normal MRI does not exclude hypophysitis, and the diagnosis is made based on clinical presentation and hormone evaluation (9). In some cases of IH, the thickening of the pituitary stalk is present before symptoms occur and can resolve prior to the onset of symptoms. This could be one possible explanation of the normal MRI in our patient at the time of diagnosis of central DI. Studies have shown CTLA4 expression in the anterior pituitary gland, which may contribute to anti-CTLA4 therapy–related anterior hypophysitis (10). Avelumab, an immunoglobulin G1 monoclonal antibody, might have triggered the complement pathway, antibody-dependent cell-medicated toxicity, or unleashed an autoimmune reaction in the posterior pituitary that had been suppressed by PD-1/PD-L1. Nonetheless, outside of potentially reversing PD-1–mediated immune suppression, a specific mechanism for anti-PD-1/PD-L1 therapy–induced hypophysitis remains unclear.
Avelumab has been extensively evaluated in a phase 2 multicentered clinical trial involving 88 subjects with MCC. None of the patients exhibited hypophysitis or grade ≥3 endocrinopathies (1). Adrenal insufficiency, thyroid disorders, and type 1 diabetes with no other etiology were observed in 0.5%, 6%, and 0.1%, respectively, of the 1738 subjects who received avelumab as part of JAVELIN solid tumor and JAVELIN Merkel 200 studies (1). To our knowledge, this is the first reported case of avelumab-related central DI.
Central DI is a hypothalamus–pituitary disease due to the deficiency of arginine vasopressin synthesis from the hypothalamus and/or secretion from the neurohypophysis. The etiology of central DI includes a constitutional form and forms secondary to cranial injury, neurohypophyseal granulomatous disease, and autoimmunity. Autoimmune central DI is associated with one-third of idiopathic central DI cases and is more likely in patients with a clinical history of autoimmune disease (11). Evaluations in our patient, including anti-nuclear antibody, extractable nuclear antigen, and anti-neutrophil cytoplasmic antibody serologies, did not suggest the presence of autoimmune disease. Pituitary MRI failed to identify any mass effects and/or hypophysitis. The patient was treated with oral DDAVP, which was eventually discontinued as his DI resolved. Given his mild nature of his disease (absence of cranial nerve deficits due to mass effect and/or a severe headache), we did not start the patient on glucocorticoids. Identifying the disease early in the course and stopping avelumab promptly might have resulted in spontaneous resolution of DI. This spontaneous return of pituitary function is seen in ∼27% of primary hypophysitis cases (12).
As immune checkpoint inhibitors are now used to treat many cancers, clinicians should be aware of the potential risk for DI. For normoglycemic patients presenting with persistent increased thirst and frequency of urination during anti-PD-1/PD-L1 therapy, we recommend testing for DI via serum and urine specific osmolalities, urine specific gravity, and, if needed, a water deprivation test. In this patient, the symptoms of DI were controlled with DDAVP, and his DI resolved after we discontinued avelumab. As immune checkpoint inhibitors are relatively new agents, rare side effects such as DI should be reported to the Food and Drug Administration adverse event reporting system (FAERS) to better understand their side effects and effective management of drug-related adverse events.
Acknowledgments
We thank Myrna Rauckhorst and Monica Taylor for coordinating the study and collecting information. The opinions expressed in this article are the authors’ own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Financial Support: This work was supported by funding from the Intramural Research Program, Center for Cancer Research, National Cancer Institute (to I.B.).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACTH
- adrenocorticotropic hormone
- CTLA4
- cytotoxic T-lymphocyte antigen 4
- DI
- diabetes insipidus
- IH
- immunotherapy-associated hypophysitis
- irAE
- immune-related adverse event
- MCC
- Merkel cell carcinoma
- MRI
- magnetic resonance imaging
- PD-1
- programmed cell death protein 1
- PD-L1
- programmed cell death protein-ligand 1.
References
- 1.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M, Brownell I, Lewis KD, Lorch JH, Chin K, Mahnke L, von Heydebreck A, Cuillerot JM, Nghiem P. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, Mega AE, Britten CD, Ravaud A, Mita AC, Safran H, Stinchcombe TE, Srdanov M, Gelb AB, Schlichting M, Chin K, Gulley JL. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35(19):2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13(1):29–38. [DOI] [PubMed] [Google Scholar]
- 5.Brooks BS, el Gammal T, Allison JD, Hoffman WH. Frequency and variation of the posterior pituitary bright signal on MR images. AJNR Am J Neuroradiol. 1989;10(5):943–948. [PMC free article] [PubMed] [Google Scholar]
- 6.Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary. 2016;19(1):82–92. [DOI] [PubMed] [Google Scholar]
- 7.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21(2):371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98(4):1361–1375. [DOI] [PubMed] [Google Scholar]
- 9.Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, Bergmann T, Bockmeyer CL, Eigentler T, Fluck M, Garbe C, Gutzmer R, Grabbe S, Hauschild A, Hein R, Hundorfean G, Justich A, Keller U, Klein C, Mateus C, Mohr P, Paetzold S, Satzger I, Schadendorf D, Schlaeppi M, Schuler G, Schuler-Thurner B, Trefzer U, Ulrich J, Vaubel J, von Moos R, Weder P, Wilhelm T, Göppner D, Dummer R, Heinzerling LM. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T, Taverna G, Cosottini M, Lupi I. Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol. 2016;186(12):3225–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pivonello R, De Bellis A, Faggiano A, Di Salle F, Petretta M, Di Somma C, Perrino S, Altucci P, Bizzarro A, Bellastella A, Lombardi G, Colao A. Central diabetes insipidus and autoimmunity: relationship between the occurrence of antibodies to arginine vasopressin-secreting cells and clinical, immunological, and radiological features in a large cohort of patients with central diabetes insipidus of known and unknown etiology. J Clin Endocrinol Metab. 2003;88(4):1629–1636. [DOI] [PubMed] [Google Scholar]
- 12.Honegger J, Buchfelder M, Schlaffer S, Droste M, Werner S, Strasburger C, Störmann S, Schopohl J, Kacheva S, Deutschbein T, Stalla G, Flitsch J, Milian M, Petersenn S, Elbelt U; Pituitary Working Group of the German Society of Endocrinology . Treatment of primary hypophysitis in Germany. J Clin Endocrinol Metab. 2015;100(9):3460–3469. [DOI] [PubMed] [Google Scholar]