Abstract
Context:
We previously reported an association between lysophosphatidylinositol (LPI) (16:1) and risk for type 2 diabetes in a Chinese population using an untargeted analysis.
Objective:
To examine the overall associations of LPIs and their related metabolites, such as nonesterified fatty acids (NEFAs) and acylcarnitines, with incident and prevalent type 2 diabetes using a targeted approach.
Design and Setting:
A case-control study was nested within the Singapore Chinese Health Study. Cases and controls were individually matched by age, sex, and date of blood collection. We used both liquid and gas chromatography tandem mass spectrometry to measure serum metabolite levels at baseline, including 8 LPIs, 19 NEFAs, and 34 acylcarnitines. Conditional logistic regression models were used to estimate the associations between metabolites and diabetes risk.
Participants:
Participants included 160 incident and 144 prevalent cases with type 2 diabetes and 304 controls.
Main Outcome Measure:
Incident and prevalent type 2 diabetes.
Results:
On the basis of a false discovery rate <0.1, we identified 37 metabolites associated with prevalent type 2 diabetes, including 7 LPIs, 18 NEFAs, and 12 acylcarnitines, and 11 metabolites associated with incident type 2 diabetes, including 2 LPIs and 9 NEFAs. Two metabolites, LPI (16:1) and dihomo-γ-linolenic acid, showed independent associations with incident type 2 diabetes and significantly enhanced the risk prediction.
Conclusions:
We found several LPIs and NEFAs that were associated with risk for type 2 diabetes and may improve our understanding of the pathogenesis. The findings suggest that lipid profiles could aid in diabetes risk assessment in Chinese populations.
Serum lysophosphatidylinositols, nonesterified fatty acids, and acylcarnitines were associated with incident and/or prevalent type 2 diabetes in Chinese adults.
Type 2 diabetes is a chronic metabolic disease characterized by hyperglycemia, insulin resistance, and relative insulin deficiency (1). The pathogenesis of diabetes is complex and involves the interaction of genetic and environmental factors. Recent research efforts on the association of circulating metabolites with risk for type 2 diabetes have provided new insights into the pathogenic mechanisms of type 2 diabetes. For instance, the increase of branched-chain amino acids, including leucine, isoleucine, and valine, has been widely reported to be related to an increased risk for type 2 diabetes in several Western and Asian populations (2–6). Moreover, the changes of fatty acids (7, 8) and acylcarnitines (9) were associated with risk for type 2 diabetes as well. Recently, in an untargeted metabolomics study of type 2 diabetes risk assessment in a Chinese population, we found a positive association between serum lysophosphatidylinositol (LPI) (16:1) and risk for type 2 diabetes (10).
LPI is a bioactive lipid produced from membrane phosphatidylinositol (PI) through the catalytic activity of the phospholipase A (PLA) family of lipases, including PLA1 and PLA2. PLA1 and PLA2 remove fatty acids from the sn-1 and sn-2 positions of PI, respectively, generating 2-acyl-LPI and 1-acyl-LPI (11). Over the past few decades, LPI has been confirmed to affect various cellular functions, such as cell growth, differentiation, and motility (12). Recently, it was proposed as the endogenous ligand of G-protein–coupled receptor 55 (GPR55) (13). The LPI/GPR55 axis has been shown to be positively associated with obesity in human (14). Notably, a recent study has demonstrated that GPR55 is expressed in the endocrine pancreas and in pancreatic β cells (15), and another study observed that the major effect of GPR55 on LPI activation on β cells is to increase insulin secretion, suggesting a role in glucose homeostasis (16). With this evidence considered together, it is still unclear whether LPI is a risk factor for type 2 diabetes. Therefore, investigation of the potential links of LPIs and their related metabolites in relation to the development of type 2 diabetes is expected to enhance our understanding of the pathogenesis of type 2 diabetes.
In this study, we thus aimed to estimate the overall associations of LPIs and their related metabolites, including nonesterified fatty acids (NEFAs) and acylcarnitines, with both incident and prevalent type 2 diabetes by using targeted metabolomics strategies. Compared with previous untargeted analysis (10), the present targeted analysis can more accurately quantify metabolites. Although several early studies focused on the relationships of fatty acids (8) and acylcarnitines (9) with type 2 diabetes, the correlations of altered fatty acids and acylcarnitines with LPIs during the development of type 2 diabetes remain unclear. This study can contribute to our understanding of the pathogenesis of type 2 diabetes.
Materials and Methods
Study design and participants
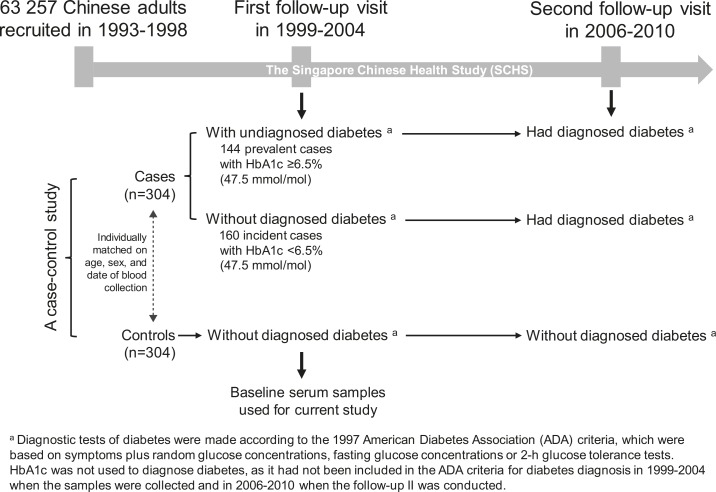
A flowchart of study design and participant selection is shown in Fig. 1. The participants were randomly selected from the Singapore Chinese Health Study (SCHS), which is a population-based study in Singapore. Detailed information on recruitment and follow-up visits for SCHS has been reported previously (10). Briefly, 63,257 Chinese men and women aged 45 to 74 years were recruited from 1993 to 1998. The first follow-up visit was carried out from 1999 to 2004, and 32,575 participants donated morning blood samples. The second follow-up visit was conducted from 2006 to 2010. In current study, we randomly selected 304 cases who were free of diagnosed diabetes, cardiovascular disease, and cancer at baseline (i.e., at the first visit when blood samples were collected) while reported to have type 2 diabetes during follow-up (i.e., at the second visit). Meanwhile, 304 controls who remained free of the aforementioned diseases at both the first and second visits were selected. The cases and controls were individually matched by age (±3 years), sex, and date of blood collection (±6 months). The baseline serum samples were used for current study. All participants gave informed consent, and the study was approved by the Institutional Review Boards at the National University of Singapore.
Figure 1.
Flowchart of study design and participant selection.
Prevalent and incident type 2 diabetes cases: patient regrouping
In Singapore, the diagnostic tests for diabetes at the time of two visits (1999 to 2004 and 2006 to 2010) were done according to the 1997 American Diabetes Association criteria (17), which were based on symptoms plus random glucose concentrations, fasting glucose concentrations, or 2-hour glucose tolerance tests. In 2010, the American Diabetes Association proposed a hemoglobin A1c (HbA1c) value ≥6.5% (47.5 mmol/mol) as an additional diagnostic criterion (18). In considering the update of diagnostic criteria, in this study we divided the 304 cases into prevalent and incident type 2 diabetes cases according to the baseline HbA1c levels: Patients with HbA1c ≥ 6.5% (47.5 mmol/mol) at baseline were defined as prevalent or undiagnosed cases (n = 144); those with HbA1c < 6.5% (47.5 mmol/mol) at baseline were classified as incident cases (n = 160). HbA1c values in the controls were all <6.0% (42.1 mmol/mol).
Lipid assay
Blood concentrations of high-density lipoprotein (HDL) cholesterol and triglycerides were measured by using colorimetric method on a chemistry analyzer (AU5800; Beckman Coulter, Brea, CA).
Serum metabolite quantification
A targeted metabolomics assay was performed at the National University of Singapore. A total of 61 serum metabolites were quantified or semiquantified, including 8 LPIs, 19 NEFAs, and 34 acylcarnitines (Supplemental Table 1 (255.8KB, pdf) ). The methods of metabolite extraction, quantification, and validation are described in detail in the Supplemental Methods (255.8KB, pdf) . Briefly, the metabolite extraction procedure was similar to the method described previously (19, 20), with some minor modifications. All the metabolites were quantified by using an Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, CA) coupled to a 6410 Triple Quadrupole (QQQ) mass spectrometer (Agilent Technologies) except myo-inositol, which was measured using an Agilent 7890 GC system (Agilent Technologies) coupled to a 7000B QQQ mass spectrometer (Agilent Technologies). The QQQ mass spectrometer was operated in electrospray ionization negative mode for LPI and fatty acid analyses and in positive mode for acylcarnitine analyses. Mass spectra were acquired in the multiple reaction monitoring mode, and the optimized conditions are summarized in Supplemental Table 1 (255.8KB, pdf) .
All 304 paired samples were analyzed blindly in eight completely independent batches (i.e., 38 paired samples in each batch). Stable isotope–labeled internal standards were used in all the sample extractions and for calibration. Quality control (QC) was prepared by spiking a certain amount of native and labeled standards into a pooled plasma from all participants, and six QCs were analyzed along with the samples in each batch to ensure the reliability of the method and the instrument stability. The intrabatch and interbatch variations for all the targets in QCs ranged from 0.05% to 19.27% and 0.15% to 18.50%, respectively.
Statistical analysis
Demographic and clinical variables of participants are presented as mean ± standard deviation for continuous data and as proportions for categorical data. All the metabolites measured in this study were detected in all 608 serum samples except LPI (22:6), which was detected in 92.4% of all samples. Undetectable values were assigned as a proxy value of half of the lowest detected amount. Odds ratios with 95% confidence intervals and P values for the association between metabolites and type 2 diabetes were calculated by using conditional logistic regression models, with adjustment for confounding factors, including body mass index (BMI), history of hypertension, smoking, physical activity, fasting status, triglycerides, and HDL cholesterol. The P value was corrected for multiple testing via false-discovery rate (FDR) using the Benjamini-Hochberg method. The odds ratio was represented both as tertiles and per standard deviation increment. Correlations between serum metabolites were examined in controls by Pearson partial correlation analysis, adjusting for age, sex, and BMI. The c-statistic (also known as the area under the receiver-operating characteristic curve [AUCROC]) was calculated to assess the predictive utility of each metabolite by examining the improvement in discrimination (i.e., increment in AUCROC by adding metabolites to a multivariable-adjusted logistic regression model with established diabetes risk factors as the basic model). Because of a limitation of AUCROC—that it is insensitive for detecting clinically important risk differences (21)— we further evaluated the integrated discrimination improvement and category-free net reclassification improvement (NRI) indexes. Statistical analyses were performed by using SPSS Statistics 24 (IBM, Armonk, NY) and Stata software, version 14.0 (Stata Corp., College Station, TX). A two-sided P value < 0.05 and FDR < 0.1 were considered to indicate statistically significant differences.
Results
Table 1 shows baseline characteristics of the participants. Cases with prevalent and incident type 2 diabetes and their matched controls are presented separately. Both prevalent and incident cases had higher BMI and triglycerides and lower levels of HDL cholesterol than controls at baseline, and they were also more likely to report a history of hypertension (P < 0.05). In this study, only a small percentage of participants [27.3%, 166 of 608) had fasted at the time of blood collection. Although the proportion of fasting and nonfasting participants was equally distributed between cases and controls (P > 0.05), metabolic changes due to fasting status were observed in the controls (Supplemental Table 2 (255.8KB, pdf) ). We thus included fasting status in the model as a confounder. In addition, we reasoned that smoking is an important confounder and thus included it in the model.
Table 1.
Baseline Characteristics of Participants
| Baseline Characteristics | Prevalent Type 2 Diabetes |
Incident Type 2 Diabetes |
||||||
|---|---|---|---|---|---|---|---|---|
| Cases (n = 144) | Controls (n = 144) | OR (95% CI) | P Value | Cases (n = 160) | Controls (n = 160) | OR (95% CI) | P Value | |
| HbA1c | ||||||||
| % | 7.7 ± 1.6 | 5.6 ± 0.3 | 5.9 ± 0.4 | 5.5 ± 0.2 | ||||
| mmol/mol | 60.7 ± 12.6 | 37.7 ± 2.0 | 41.0 ± 2.8 | 36.6 ± 1.3 | ||||
| Random glucose (mmol/L) | 8.8 ± 4.2 | 4.9 ± 1.3 | 1.98 (1.54–2.55) | <0.001 | 5.8 ± 2.0 | 4.9 ± 1.2 | 1.37 (1.18–1.60) | <0.001 |
| Age (years) | 62.7 ± 6.1 | 62.7 ± 5.9 | 1.00 (0.82–1.22) | 0.99 | 61.6 ± 5.6 | 61.9 ± 6.0 | 0.96 (0.81–1.16) | 0.70 |
| Sex, n (%) | 1.00 | — | 1.00 | |||||
| Male | 62 (43.1) | 62 (43.1) | 79 (49.4) | 79 (49.4) | ||||
| Female | 82 (56.9) | 82 (56.9) | 81 (50.6) | 81 (50.6) | ||||
| BMI (kg/m2) | 24.6 ± 3.6 | 23.1 ± 3.3 | 1.15 (1.07–1.25) | <0.001 | 24.6 ± 3.4 | 22.6 ± 3.5 | 1.23 (1.11–1.37) | <0.001 |
| History of hypertension, n (%) | ||||||||
| No | 84 (58.3) | 99 (68.7) | 1.00 | — | 75 (46.9) | 118 (73.8) | 1.00 | — |
| Yes | 60 (41.7) | 45 (31.3) | 1.80 (1.01–3.26) | 0.049 | 85 (53.1) | 42 (26.3) | 5.21 (2.57–10.6) | <0.001 |
| Smoking, n (%) | ||||||||
| Never smoker | 99 (68.8) | 102 (70.8) | 1.00 | — | 106 (66.3) | 109 (68.1) | 1.00 | — |
| Past smoker | 15 (10.4) | 21 (14.6) | 0.61 (0.24–1.54) | 0.30 | 26 (16.3) | 25 (15.6) | 1.33 (0.51–3.45) | 0.56 |
| Current smoker | 30 (20.8) | 21 (14.6) | 1.93 (0.89–4.17) | 0.10 | 28 (17.5) | 26 (16.3) | 1.37 (0.61–3.10) | 0.45 |
| Alcohol consumption, n (%) | ||||||||
| <1 drink/d | 125 (86.8) | 123 (85.4) | 1.00 | — | 139 (86.9) | 140 (87.5) | 1.00 | — |
| 1–6 drinks/wk | 13 (9.0) | 17 (11.8) | 1.15 (0.46–2.89) | 0.76 | 16 (10.0) | 13 (8.1) | 2.39 (0.87–6.58) | 0.09 |
| ≥1 drink/d | 6 (4.2) | 4 (2.8) | 1.78 (0.43–7.36) | 0.43 | 3 (3.1) | 7 (4.4) | 0.99 (0.18–5.34) | 0.99 |
| Moderate to intensive physical activity, n (%) | ||||||||
| <0.5 h/wk | 118 (81.9) | 117 (81.3) | 1.00 | — | 125 (78.1) | 122 (76.3) | 1.00 | — |
| 0.5–3.9 h/wk | 16 (11.1) | 16 (11.1) | 1.13 (0.44–2.88) | 0.80 | 26 (16.3) | 15 (9.4) | 2.82 (1.14–6.95) | 0.02 |
| ≥4 h/wk | 10 (6.9) | 11 (7.6) | 1.10 (0.39–3.12) | 0.85 | 9 (5.6) | 23 (14.4) | 0.21 (0.07–0.65) | 0.007 |
| Education, n (%) | ||||||||
| None | 37 (25.7) | 31 (21.5) | 1.00 | — | 29 (18) | 26 (16.3) | 1.00 | — |
| Primary | 59 (41.0) | 66 (45.8) | 0.63 (0.29–1.37) | 0.25 | 77 (48.1) | 77 (48.1) | 1.09 (0.46–2.56) | 0.84 |
| Secondary and above | 48 (33.3) | 47 (32.6) | 0.58 (0.23–1.47) | 0.25 | 54 (33.8) | 57 (35.6) | 0.66 (0.26–1.66) | 0.38 |
| Fasting status, n (%) | ||||||||
| Nonfasting | 96 (66.7) | 103 (71.5) | 1.00 | — | 118 (73.8) | 125 (78.1) | 1.00 | — |
| Fasting | 48 (33.3) | 41 (28.5) | 1.31 (0.74–2.33) | 0.35 | 42 (26.3) | 35 (21.9) | 1.28 (0.61–2.70) | 0.52 |
| HDL cholesterol (mmol/L) | 1.1 ± 0.2 | 1.2 ± 0.3 | 0.11 (0.04–0.32) | <0.001 | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.07 (0.02–0.21) | <0.001 |
| Triglycerides (mmol/L) | 2.6 ± 1.9 | 2.0 ± 1.3 | 1.43 (1.14–1.79) | 0.002 | 2.4 ± 1.3 | 1.7 ± 0.9 | 1.96 (1.49–2.59) | <0.001 |
Data for continuous variables are presented as mean ± standard deviation for continuous variables; data for categorical variables are presented as n (%). OR with 95% CI and P values were calculated by conditional logistic regression.
Abbreviations: CI, confidence interval; OR, odds ratio.
The associations between 61 measured metabolites and the risk for prevalent and incident type 2 diabetes are shown in Supplemental Table 3 (255.8KB, pdf) . After adjustment for potential confounders, including BMI, history of hypertension, smoking, physical activity, fasting status, triglycerides, and HDL cholesterol, 37 metabolites were associated with prevalent type 2 diabetes at an FDR <0.1 (Table 2); 11 metabolites were associated with incident type 2 diabetes at an FDR <0.1 (Table 3). Although 0.05 is commonly used as a cutoff limit for FDR in the discovery of metabolic signatures, the metabolites with FDR between 0.05 and 0.1 are still considered marginally significant. To avoid missing potential biomarker candidates associated with risk for type 2 diabetes, we applied 0.1 as the cutoff value for FDR. All the 11 metabolites associated with incident type 2 diabetes were associated with prevalent type 2 diabetes as well. The associations of these 11 metabolites with prevalent type 2 diabetes were stronger than those with incident type 2 diabetes, except LPI (16:1) and (18:0), for which the associations with incident type 2 diabetes were more evident. In addition to the 11 common metabolites, we identified 26 metabolites associated only with prevalent type 2 diabetes, including 5 LPIs, 9 NEFAs, and 12 acylcarnitines. No significant associations between acylcarnitines and incident type 2 diabetes were observed. An aberrant metabolic network in relation to prevalent and incident type 2 diabetes is summarized in Fig. 2.
Table 2.
The 37 Metabolites Associated With Risk for Prevalent Type 2 Diabetes (n = 144)
| Metabolite | OR Across Tertiles (95% CI)a |
Per SD Incrementa |
||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | P Value for Trend | OR (95% CI) | P Value | |
| Inositol and lysophosphatidylinositols | ||||||
| myo-Inositol | 1.00 | 0.86 (0.43–1.74) | 0.27 (0.12–0.65) | 0.003b | 0.38 (0.23–0.61) | <0.001b |
| Lysophosphatidylinositol (16:0) | 1.00 | 1.69 (0.81–3.54) | 2.02 (0.94–4.37) | 0.041b | 1.20 (0.88–1.64) | 0.257 |
| Lysophosphatidylinositol (16:1) | 1.00 | 1.35 (0.51–3.57) | 2.53 (1.05–6.06) | 0.016b | 1.24 (0.87–1.78) | 0.228 |
| Lysophosphatidylinositol (18:0) | 1.00 | 1.84 (0.76–4.46) | 2.99 (1.05–8.52) | 0.053b | 1.51 (0.95–2.39) | 0.079 |
| Lysophosphatidylinositol (18:2) | 1.00 | 2.32 (1.07–5.01) | 2.78 (1.29–5.98) | 0.007b | 1.30 (0.98–1.71) | 0.066 |
| Lysophosphatidylinositol (20:4) | 1.00 | 1.83 (0.75–4.48) | 4.05 (1.71–9.57) | 0.001b | 2.36 (1.57–3.55) | <0.001b |
| Lysophosphatidylinositol (22:6) | 1.00 | 1.86 (0.85–4.09) | 2.02 (0.82–4.97) | 0.044b | 1.30 (0.93–1.83) | 0.129 |
| Fatty acids | ||||||
| Myristic acid (14:0) | 1.00 | 0.90 (0.35–2.28) | 3.15 (1.38–7.20) | 0.007b | 2.04 (1.37–3.04) | <0.001b |
| Palmitic acid (16:0) | 1.00 | 2.76 (1.11–6.86) | 6.45 (2.52–16.51) | 0.001b | 2.18 (1.50–3.17) | <0.001b |
| Palmitoleic acid (16:1n-7) | 1.00 | 4.43 (1.75–11.26) | 7.70 (2.69–22.02) | 0.002b | 1.90 (1.29–2.81) | 0.001b |
| Stearic acid (18:0) | 1.00 | 2.98 (1.25–7.13) | 4.74 (2.06–10.88) | 0.002b | 2.13 (1.45–3.12) | <0.001b |
| Oleic acid (18:1n-9) | 1.00 | 2.38 (0.97–5.85) | 4.44 (1.82–10.79) | 0.009b | 1.93 (1.35–2.76) | <0.001b |
| Linoleic acid (18:2n-6) | 1.00 | 2.04 (0.91–4.59) | 4.61 (1.94–10.98) | 0.004b | 1.65 (1.19–2.27) | 0.002b |
| α-Linolenic acid (18:3n-3) | 1.00 | 1.48 (0.67–3.30) | 3.20 (1.43–7.15) | 0.018b | 1.54 (1.11–2.14) | 0.009b |
| γ-Linolenic acid (18:3n-6) | 1.00 | 5.91 (2.18–15.99) | 5.46 (1.85–16.10) | 0.012b | 2.07 (1.35–3.17) | 0.001b |
| Gondoic acid (20:1n-11) | 1.00 | 1.23 (0.53–2.84) | 3.54 (1.57–8.02) | 0.008b | 2.20 (1.48–3.28) | <0.001b |
| Eicosadienoic acid (20:2n-6) | 1.00 | 3.33 (1.39–8.00) | 6.64 (2.72–16.23) | <0.001b | 2.29 (1.55–3.38) | <0.001b |
| Dihomo-γ-linolenic acid (20:3n-6) | 1.00 | 1.35 (0.57–3.19) | 5.02 (2.16–11.69) | <0.001b | 2.20 (1.52–3.20) | <0.001b |
| Mead acid (20:3n-9) | 1.00 | 1.76 (0.75–4.12) | 3.14 (1.40–7.02) | 0.031b | 2.03 (1.40–2.96) | <0.001b |
| Arachidonic acid (20:4n-6) | 1.00 | 1.82 (0.77–4.32) | 3.86 (1.70–8.75) | 0.004b | 1.84 (1.31–2.58) | <0.001b |
| Eicosapentaenoic acid (20:5n-3) | 1.00 | 3.96 (1.50–10.48) | 4.34 (1.76–10.73) | 0.009b | 1.61 (1.17–2.23) | 0.004b |
| Adrenic acid (22:4n-6) | 1.00 | 3.14 (1.23–8.02) | 6.87 (2.69–17.54) | <0.001b | 2.52 (1.66–3.84) | <0.001b |
| Clupanodonic acid (22:5n-3) | 1.00 | 1.66 (0.71–3.92) | 4.79 (2.00–11.47) | 0.001b | 2.41 (1.62–3.57) | <0.001b |
| Osbond acid (22:5n-6) | 1.00 | 1.51 (0.66–3.44) | 4.20 (1.85–9.52) | 0.003b | 2.16 (1.49–3.12) | <0.001b |
| Docosahexaenoic acid (22:6n-3) | 1.00 | 2.30 (1.01–5.21) | 4.20 (1.83–9.66) | 0.004b | 1.98 (1.41–2.78) | <0.001b |
| Carnitine and acylcarnitines | ||||||
| L-Carnitine | 1.00 | 0.39 (0.19–0.78) | 0.17 (0.06–0.44) | <0.001b | 0.57 (0.40–0.81) | 0.002b |
| 3-Hydroxybutyrylcarnitine (C4OH) | 1.00 | 1.33 (0.61–2.91) | 3.22 (1.48–7.03) | 0.011b | 1.46 (1.10–1.95) | 0.009b |
| Octanoylcarnitine (C8) | 1.00 | 1.63 (0.76–3.49) | 3.13 (1.37–7.18) | 0.050b | 1.23 (0.91–1.68) | 0.181 |
| Decanoylcarnitine (C10) | 1.00 | 1.59 (0.75–3.33) | 3.01 (1.33–6.80) | 0.052b | 1.24 (0.91–1.68) | 0.175 |
| Dodecanoylcarnitine (C12) | 1.00 | 1.43 (0.70–2.92) | 2.86 (1.28–6.41) | 0.053b | 1.35 (0.97–1.88) | 0.074 |
| 3-Hydroxydodecanoylcarnitne (C12OH) | 1.00 | 1.22 (0.58–2.56) | 2.56 (1.16–5.63) | 0.077 | 1.54 (1.12–2.12) | 0.008b |
| Tetradecanoylcarnitine (C14) | 1.00 | 1.16 (0.55–2.43) | 4.79 (1.99–11.52) | 0.005b | 1.54 (1.11–2.15) | 0.011b |
| 3-Hydroxytetradecanoylcarnitine (C14OH) | 1.00 | 1.39 (0.62–3.09) | 3.30 (1.59–6.85) | 0.004b | 1.83 (1.29–2.60) | 0.001b |
| Hexadecanoylcarnitine (C16) | 1.00 | 1.73 (0.87–3.43) | 2.02 (0.95–4.29) | 0.157 | 1.56 (1.10–2.21) | 0.013b |
| 3-Hydroxyhexadecanoylcarnitine (C16OH) | 1.00 | 1.58 (0.80–3.12) | 2.80 (1.28–6.09) | 0.029b | 1.54 (1.10–2.14) | 0.011b |
| 3-Hydroxy-9-hexadecenoylcarnitine (C16:1OH) | 1.00 | 1.81 (0.85–3.46) | 2.67 (1.16–6.16) | 0.094 | 1.58 (1.13–2.20) | 0.007b |
| C20:5-carnitine | 1.00 | 1.77 (0.92–3.43) | 5.71 (1.05–30.98) | 0.049b | 1.40 (1.03–1.91) | 0.031b |
Prevalent diabetes cases were defined as those who had HbA1c ≥ 6.5% (47.5 mmol/mol), did not report having diagnosed diabetes at baseline when blood samples were collected (1999 to 2004), but were reported to have diagnosed diabetes during the follow-up (2006 to 2010).
Abbreviations: CI, confidence interval; OR, odds ratio; T1 tertile 1; T2, tertile 2; T3, tertile 3.
OR with 95% CI and P values were calculated by conditional logistic regression after adjustment for BMI, history of hypertension, smoking, physical activity, fasting status, triglycerides, and HDL cholesterol.
FDR < 0.1.
Table 3.
The 11 Metabolites Associated With Risk for Incident Type 2 Diabetes (n = 160)
| Metabolite | OR Across Tertiles (95% CI)a |
Per SD Incrementa |
c-Statisticsb |
IDI P Value P | NRI P Value P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | P Value for Trend | OR (95% CI) | P Value | AUC (95% CI) | P Value | |||
| Lysophosphatidylinositols | ||||||||||
| Lysophosphatidylinositol (16:1) | 1.00 | 1.70 (0.43–6.75) | 5.33 (1.53–18.59) | 0.016c | 1.96 (1.15–3.34) | 0.013c,d | 0.77 (0.71–0.83) | 0.111 | 0.002 | 0.003 |
| Lysophosphatidylinositol (18:0) | 1.00 | 1.19 (0.53–2.68) | 3.24 (1.29–8.10) | 0.008c | 1.97 (1.24–3.12) | 0.004c,d | 0.73 (0.68–0.78) | 0.549 | 0.183 | 0.044 |
| Fatty acids | ||||||||||
| Myristic acid (14:0) | 1.00 | 1.10 (0.58–2.08) | 2.10 (1.07–4.13) | 0.067c | 1.72 (1.25–2.37) | 0.001c,d | 0.75 (0.70–0.81) | 0.173 | 0.005 | 0.094 |
| Palmitic acid (16:0) | 1.00 | 1.62 (0.81–3.23) | 2.79 (1.33–5.86) | 0.014c | 1.48 (1.10–1.99) | 0.010c,d | 0.75 (0.70–0.80) | 0.176 | 0.023 | 0.074 |
| Palmitoleic acid (16:1n-7) | 1.00 | 1.20 (0.58–2.48) | 2.32 (1.19–4.55) | 0.026c | 1.44 (1.08–1.92) | 0.013c,d | 0.75 (0.70–0.80) | 0.215 | 0.027 | 0.007 |
| Stearic acid (18:0) | 1.00 | 1.68 (0.80–3.50) | 3.03 (1.37–6.71) | 0.011c | 1.59 (1.13–2.23) | 0.008c,d | 0.75 (0.69–0.80) | 0.400 | 0.024 | 0.010 |
| Eicosadienoic acid (20:2n-6) | 1.00 | 2.41 (1.13–5.11) | 3.11 (1.45–6.68) | 0.010c | 1.50 (1.12–2.02) | 0.006c,d | 0.75 (0.70–0.81) | 0.144 | 0.010 | 0.094 |
| Dihomo-gamma-linolenic acid (20:3n-6) | 1.00 | 1.56 (0.70–3.50) | 5.64 (2.38–13.34) | <0.001c,d | 1.86 (1.34–2.59) | <0.001c,d | 0.76 (0.71–0.81) | 0.099 | <0.001 | 0.001 |
| Mead acid (20:3n-9) | 1.00 | 2.66 (1.13–6.31) | 4.91 (1.95–12.32) | 0.001c,d | 1.36 (1.02–1.83) | 0.039c | 0.75 (0.69–0.80) | 0.383 | 0.082 | 0.074 |
| Arachidonic acid (20:4n-6) | 1.00 | 1.81 (0.86–3.81) | 2.21 (1.00–4.86) | 0.093c | 1.53 (1.09–2.14) | 0.014c,d | 0.75 (0.69–0.80) | 0.517 | 0.127 | 0.180 |
| Adrenic acid (22:4n-6) | 1.00 | 1.86 (0.82–4.25) | 3.41 (1.51–7.70) | 0.016c | 1.53 (1.11–2.11) | 0.010c,d | 0.75 (0.70–0.80) | 0.201 | 0.078 | 0.019 |
Incident diabetes cases were defined as those who had HbA1c < 6.5% (47.5 mmol/mol) at baseline when blood samples were collected (1999 to 2004) and were reported to have diagnosed diabetes during the follow-up (2006 to 2010).
Abbreviations: CI, confidence interval; IDI, integrated discrimination improvement; OR, odds ratio; T1 tertile 1; T2, tertile 2; T3, tertile 3.
OR with 95% CI and P values were calculated by conditional logistic regression after adjustment for BMI, history of hypertension, smoking, physical activity, fasting status, triglycerides, and HDL-cholesterol.
Area under the curve increment of individual metabolite after adding in the basic model (area under the curve = 0.74; 95% CI, 0.68 to 0.79) of BMI, history of hypertension, smoking, physical activity, fasting status, triglycerides, and HDL cholesterol.
P < 0.05 with further adjustment for random glucose and HbA1c levels.
FDR < 0.1.
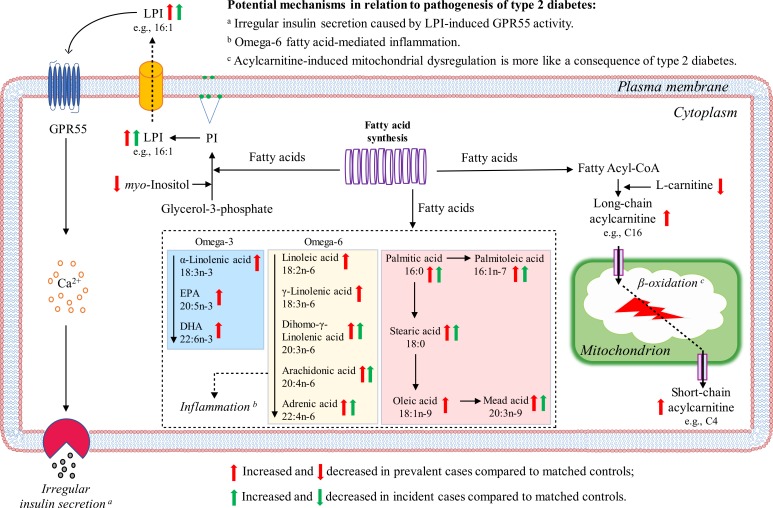
Figure 2.
Major altered lipid metabolites and related pathways in β-cell during the development of type 2 diabetes. Abbreviations: CoA, coenzyme A; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
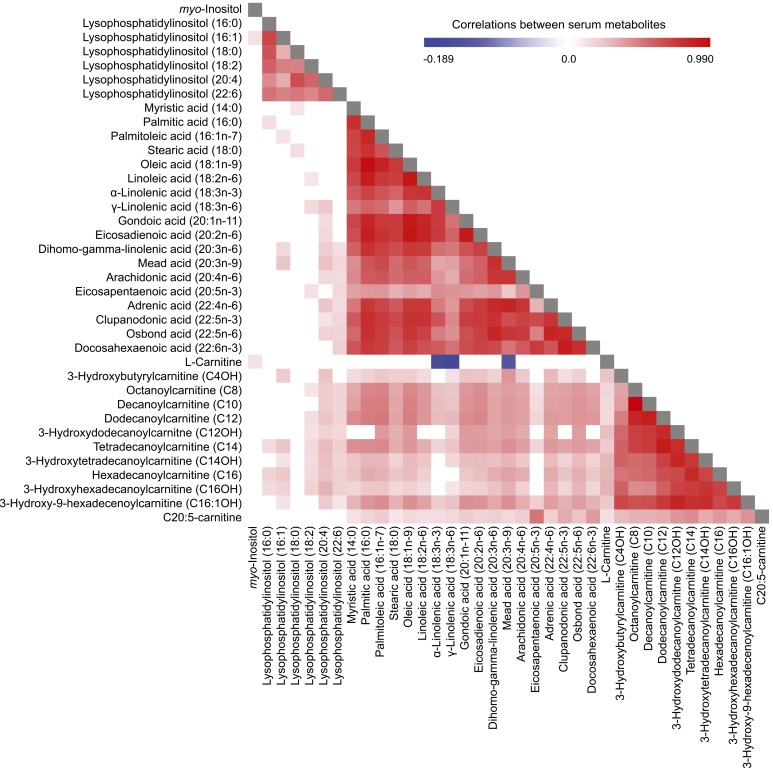
We assessed the correlations between baseline concentrations of 37 differential metabolites associated with prevalent and/or incident type 2 diabetes in controls (Fig. 3). Strong correlations were observed within groups of related metabolites, such as LPIs, NEFAs, and acylcarnitines. Moreover, NEFAs showed moderate to strong correlations with acylcarnitines. Notably, several correlations were observed between LPIs with NEFAs and acylcarnitines that had the same chain of fatty acids. For example, positive correlations between LPI (16:0) with palmitic acid (16:0) and hexadecanoylcarnitine (C16) were observed. Additionally, we found negative correlations between l-carnitine and three NEFAs (α-linolenic acid, β-linolenic acid, and mead acid), which indicated an inverse relationship between their concentrations. However, the underlying mechanisms need to be explored further.
Figure 3.
Pearson partial correlation analysis of baseline serum metabolite levels in controls, with adjustment for age, sex, and BMI.
Among 11 metabolites associated with incident type 2 diabetes, we further adjusted for random glucose and HbA1c levels and found that all metabolites remained significantly associated with type 2 diabetes risk (Table 3). We then evaluated the predictive performance of these 11 metabolites for the risk for incident type 2 diabetes (Table 3). Compared with the AUCROC value of the base model of BMI, history of hypertension, smoking, physical activity, triglycerides, and HDL cholesterol, no metabolites led to a significant increase in AUC values, whereas IDI and NRI statistics showed that dihomo-γ-linolenic acid and LPI (16:1) could significantly improve risk reclassification of incident type 2 diabetes at P < 0.01. The NRI table stratified for incident cases and matched controls is presented in Supplemental Table 4 (255.8KB, pdf) .
In addition to LPI (16:1), we found that all seven measured LPIs in prevalent type 2 diabetes cases were relatively higher than those in incident type 2 diabetes cases and controls, and they were relatively higher in incident type 2 diabetes cases than in controls (Supplemental Fig. 1 (255.8KB, pdf) ).
Discussion
Using a case-control design within the SCHS cohort aimed at estimating the association of various lipid metabolites with incident and prevalent type 2 diabetes, we found that the accumulation of certain LPIs and NEFAs was associated with an increased risk for type 2 diabetes, independently of established risk factors. Moreover, the significant correlations observed among LPIs, NEFAs, and acylcarnitines indicated potential links of them in the development of type 2 diabetes.
NEFAs can impair insulin action via the Randle cycle, accumulation of intracellular lipid derivatives (e.g., diacylglycerol), oxidative stress, inflammation, and mitochondrial dysfunction (22). In this study, we found that a series of NEFAs was associated with type 2 diabetes risk. Specifically, we observed higher levels of omega-6 fatty acids (such as linoleic acid, dihomo-γ-linolenic acid, and adrenic acid) in both prevalent and incident type 2 diabetes cases. Omega-6 fatty acids play important roles in regulation of inflammation as precursors of inflammatory mediators, such as eicosanoids (23). The increase of omega-6 fatty acids observed from incident to prevalent type 2 diabetes could have indicated an important role of omega-6 fatty acid–mediated inflammation in the pathogenesis of type 2 diabetes (Fig. 2). Indeed, Perry et al. (24) recently identified the connection between inflammation and type 2 diabetes, showing that acetyl coenzyme A was a key mediator of insulin action on the liver and linking it to inflammation-induced insulin resistance. Among these omega-6 fatty acids, dihomo-γ-linolenic acid had been reported to be associated with high insulin resistance (25) and increased risk for type 2 diabetes (26). In our study, we found it significantly improved risk reclassification of type 2 diabetes and thus could be a potential predictor of type 2 diabetes. Compared with omega-6 fatty acids, previous prospective cohort studies in relation to the association between omega-3 fatty acids and type 2 diabetes risk were inconsistent. A recent systematic review demonstrated that omega-3 fatty acids had protective associations with risk for type 2 diabetes in Asian populations but was positively associated with risk for type 2 diabetes in Western populations (27). The possible protective role of omega-3 fatty acids on type 2 diabetes in Asians, particularly Chinese, was partly supported by our findings that increased omega-3 fatty acids, such as α-linolenic acid, eicosapentaenoic acid, and docosahexaenoic acid, were observed only in prevalent type 2 diabetes, which might elevate anti-inflammatory effects for self-stress protection.
l-Carnitine affects insulin-mediated glucose uptake and mitochondrial utilization of fatty acids (28). It transports long-chain fatty acids from the cytosol into mitochondria in the form of acylcarnitines for β-oxidation and also facilitates the removal of short- and medium-chain fatty acids from the mitochondria. Previous studies indicated that the link of acylcarnitines with lipid-induced mitochondrial stress led to or worsened insulin resistance (29). Recently, Sun et al. (9) found that acylcarnitines, especially those with long-chain fatty acids, were associated with an increased risk for type 2 diabetes. In accordance with these studies, we observed higher levels of acylcarnitines in prevalent type 2 diabetes cases, yet no significant changes in incident cases, which indicated that acylcarnitine-induced mitochondrial dysregulation may exacerbate the progression of type 2 diabetes rather than serve as a trigger (Fig. 2).
The positive association between LPI and risk for type 2 diabetes was an emerging finding in our previous untargeted metabolomics study (10). In the current targeted metabolomics study, we observed that prevalent type 2 diabetes cases had the highest LPIs compared with incident type 2 diabetes cases and controls, and LPIs were positively associated with higher risk for both prevalent and incident type 2 diabetes. In this study, we proposed a hypothesis in relation to the pathogenic mechanisms of LPI in type 2 diabetes development (Fig. 2), but further studies are needed to confirm it. Briefly, in the development of type 2 diabetes, particularly at the early stage, LPI is accumulated, and it activates the GPR55 on β cells to secrete additional insulin in response to insulin resistance (16). The initially increase of insulin secretion actually represents a relative deficiency of insulin, which means that β-cell function starts to deteriorate. The impairment of β-cell function further results in severe insulin deficiency. When insulin secretion no longer keeps pace with insulin resistance at the onset of diabetes, more and more LPI will be accumulated to promote insulin secretion, which further aggravates the metabolic burden of β cells, leading to cell dysfunction that accelerates the development of frank diabetes. By the time diabetes is clinically diagnosed, β-cell function may be reduced by ≥50% (30). Whether as cause and/or consequence, LPI is clearly elevated in type 2 diabetes, and, importantly, it might occur earlier than glucose and HbA1c changes. Therefore, accurate quantification of LPI changes should significantly improve risk prediction of type 2 diabetes. Indeed, in this study we observed that LPI (16:1) significantly improved risk prediction of type 2 diabetes, which also confirmed our previous finding that LPI (16:1) can be an early predictor for type 2 diabetes (10).
The strength of this study is its prospective design and hence the presumed lack of recall bias in exposure data before type 2 diabetes diagnosis. Furthermore, the targeted metabolomics used in this study were precise and could quantify metabolite concentrations with a relatively low measurement variation. However, the current study had some limitations. First, although all blood was collected in the morning, ∼70% of blood samples were nonfasting, a factor that may affect the metabolite levels. However, we included fasting status in the model as a confounder. Second, ∼50% cases had undiagnosed type 2 diabetes at baseline (1999 to 2004) according to updated diagnostic criteria, with HbA1c levels ≥6.5% (47.5 mmol/mol). To avoid conflict, we redefined the status and classified these patients as prevalent and incident cases on the basis of baseline HbA1c levels and explored the association separately. Third, evidence indicates that certain fatty acids are clearly influenced by the habitual diet; in this study we cannot fully exclude confounders by dietary factors in analyzing differential metabolites because dietary data were not included. Finally, the sample size was relatively small, and the findings need to be validated externally in other ethnic populations with larger samples sizes.
In summary, we investigated the associations of LPIs, NEFAs, and acylcarnitines with both prevalent and incident type 2 diabetes in a Chinese population by using targeted metabolomics strategies. Overall, this study demonstrated the strong associations between LPIs and NEFAs with risk for type 2 diabetes and further confirms the probability of LPI (16:1) for type 2 diabetes risk prediction in this Chinese population. More important, a hypothesis in relation to the pathogenic mechanisms of LPI in type 2 diabetes development was brought forward, which further studies are needed to validate.
Acknowledgments
We thank Siew-Hong Low from the National University of Singapore for supervising the fieldwork of the Singapore Chinese Health Study and Renwei Wang from the University of Pittsburgh Cancer Institute for the maintenance of the cohort database. We also thank the founding principal investigator of the Singapore Chinese Health Study, Mimi C. Yu.
Financial Support: This study was supported by the National Medical Research Council, Singapore (W.-P.K.; NMRC/CIRG/1354/2013) and National Institutes of Health (R01 CA144034 [J.-M.Y.] and UM1 CA182876 [J-M.Y.]).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUCROC
- area under the receiver-operating characteristic curve
- BMI
- body mass index, FDR, false discovery rate
- GPR55
- G-protein–coupled receptor 55
- HbA1c
- hemoglobin A1c
- HDL
- high-density lipoprotein
- LPI
- lysophosphatidylinositol
- NEFA
- nonesterified fatty acid
- NRI
- net reclassification improvement
- PI
- phosphatidylinositol
- PLA
- phospholipase A
- QC
- quality control
- QQQ
- Triple Quadrupole
- SCHS
- Singapore Chinese Health Study.
References
- 1.Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27(4):269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Floegel A, Stefan N, Yu Z, Mühlenbruch K, Drogan D, Joost HG, Fritsche A, Häring HU, Hrabě de Angelis M, Peters A, Roden M, Prehn C, Wang-Sattler R, Illig T, Schulze MB, Adamski J, Boeing H, Pischon T. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62(2):639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menni C, Fauman E, Erte I, Perry JR, Kastenmüller G, Shin SY, Petersen AK, Hyde C, Psatha M, Ward KJ, Yuan W, Milburn M, Palmer CN, Frayling TM, Trimmer J, Bell JT, Gieger C, Mohney RP, Brosnan MJ, Suhre K, Soranzo N, Spector TD. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes. 2013;62(12):4270–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu F, Tavintharan S, Sum CF, Woon K, Lim SC, Ong CN. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J Clin Endocrinol Metab. 2013;98(6):E1060–E1065. [DOI] [PubMed] [Google Scholar]
- 6.Walford GA, Ma Y, Clish C, Florez JC, Wang TJ, Gerszten RE; Diabetes Prevention Program Research Group . Diabetes Prevention Program Research G. Metabolite profiles of diabetes incidence and intervention response in the diabetes prevention program. Diabetes. 2016;65(5):1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Wang M, Yang X, Bi M, Na L, Niu Y, Li Y, Sun C. Fasting serum lipid and dehydroepiandrosterone sulfate as important metabolites for detecting isolated postchallenge diabetes: serum metabolomics via ultra-high-performance LC-MS. Clin Chem. 2013;59(9):1338–1348. [DOI] [PubMed] [Google Scholar]
- 8.Yi LZ, He J, Liang YZ, Yuan DL, Chau FT. Plasma fatty acid metabolic profiling and biomarkers of type 2 diabetes mellitus based on GC/MS and PLS-LDA. FEBS Lett. 2006;580(30):6837–6845. [DOI] [PubMed] [Google Scholar]
- 9.Sun L, Liang L, Gao X, Zhang H, Yao P, Hu Y, Ma Y, Wang F, Jin Q, Li H, Li R, Liu Y, Hu FB, Zeng R, Lin X, Wu J. Early prediction of developing type 2 diabetes by plasma acylcarnitines: a population-based study. Diabetes Care. 2016;39(9):1563–1570. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Wang Y, Ong CN, Subramaniam T, Choi HW, Yuan JM, Koh WP, Pan A. Metabolic signatures and risk of type 2 diabetes in a Chinese population: an untargeted metabolomics study using both LC-MS and GC-MS. Diabetologia. 2016;59(11):2349–2359. [DOI] [PubMed] [Google Scholar]
- 11.Ruban EL, Ferro R, Arifin SA, Falasca M. Lysophosphatidylinositol: a novel link between ABC transporters and G-protein-coupled receptors. Biochem Soc Trans. 2014;42(5):1372–1377. [DOI] [PubMed] [Google Scholar]
- 12.Piñeiro R, Falasca M. Lysophosphatidylinositol signalling: new wine from an old bottle. Biochim Biophys Acta. 2012;1821(4):694–705. [DOI] [PubMed] [Google Scholar]
- 13.Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun. 2007;362(4):928–934. [DOI] [PubMed] [Google Scholar]
- 14.Moreno-Navarrete JM, Catalán V, Whyte L, Díaz-Arteaga A, Vázquez-Martínez R, Rotellar F, Guzmán R, Gómez-Ambrosi J, Pulido MR, Russell WR, Imbernón M, Ross RA, Malagón MM, Dieguez C, Fernández-Real JM, Frühbeck G, Nogueiras R. The L-α-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes. 2012;61(2):281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero-Zerbo SY, Rafacho A, Díaz-Arteaga A, Suárez J, Quesada I, Imbernon M, Ross RA, Dieguez C, Rodríguez de Fonseca F, Nogueiras R, Nadal A, Bermúdez-Silva FJ. A role for the putative cannabinoid receptor GPR55 in the islets of Langerhans. J Endocrinol. 2011;211(2):177–185. [DOI] [PubMed] [Google Scholar]
- 16.Liu B, Song S, Ruz-Maldonado I, Pingitore A, Huang GC, Baker D, Jones PM, Persaud SJ. GPR55-dependent stimulation of insulin secretion from isolated mouse and human islets of Langerhans. Diabetes Obes Metab. 2016;18(12):1263–1273. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183–1197. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Y, Fang J, Ong CN, Chen S, Li N, Cui L, Huang C, Ling Q, Chia SE, Chen M. Targeted analysis of omega-6-derived oxylipins and parent polyunsaturated fatty acids in serum of hepatitis B virus-related hepatocellular carcinoma patients. Metabolomics. 2017;13(1):6. [Google Scholar]
- 20.Liu Y, Su J, van Dam RM, Prem K, Hoong JY, Zou L, Lu Y, Ong CN. Dietary predictors and plasma concentrations of perfluorinated alkyl acids in a Singapore population. Chemosphere. 2017;171:617–624. [DOI] [PubMed] [Google Scholar]
- 21.McGeechan K, Macaskill P, Irwig L, Liew G, Wong TY. Assessing new biomarkers and predictive models for use in clinical practice: a clinician’s guide. Arch Intern Med. 2008;168(21):2304–2310. [DOI] [PubMed] [Google Scholar]
- 22.Charles MA, Eschwège E, Thibult N, Claude JR, Warnet JM, Rosselin GE, Girard J, Balkau B. The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia. 1997;40(9):1101–1106. [DOI] [PubMed] [Google Scholar]
- 23.Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metab. 2012;2012:539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry RJ, Camporez JP, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM, Ruan HB, Yang X, Caprio S, Kaech SM, Sul HS, Birnbaum MJ, Davis RJ, Cline GW, Petersen KF, Shulman GI. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160(4):745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurotani K, Sato M, Ejima Y, Nanri A, Yi S, Pham NM, Akter S, Poudel-Tandukar K, Kimura Y, Imaizumi K, Mizoue T. High levels of stearic acid, palmitoleic acid, and dihomo-gamma-linolenic acid and low levels of linoleic acid in serum cholesterol ester are associated with high insulin resistance. Nutr Res. 2012;32(9):669–675 e663. [DOI] [PubMed] [Google Scholar]
- 26.Forouhi NG, Imamura F, Sharp SJ, Koulman A, Schulze MB, Zheng J, Ye Z, Sluijs I, Guevara M, Huerta JM, Kröger J, Wang LY, Summerhill K, Griffin JL, Feskens EJ, Affret A, Amiano P, Boeing H, Dow C, Fagherazzi G, Franks PW, Gonzalez C, Kaaks R, Key TJ, Khaw KT, Kühn T, Mortensen LM, Nilsson PM, Overvad K, Pala V, Palli D, Panico S, Quirós JR, Rodriguez-Barranco M, Rolandsson O, Sacerdote C, Scalbert A, Slimani N, Spijkerman AM, Tjonneland A, Tormo MJ, Tumino R, van der A DL, van der Schouw YT, Langenberg C, Riboli E, Wareham NJ. van der AD, van der Schouw YT, Langenberg C, Riboli E, Wareham NJ. Association of plasma phospholipid n-3 and n-6 polyunsaturated fatty acids with type 2 diabetes: the EPIC-InterAct Case-Cohort Study. PLoS Med. 2016;13(7):e1002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng JS, Huang T, Yang J, Fu YQ, Li D. Marine N-3 polyunsaturated fatty acids are inversely associated with risk of type 2 diabetes in Asians: a systematic review and meta-analysis. PLoS One. 2012;7(9):e44525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol. 2007;581(Pt 2):431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. [DOI] [PubMed] [Google Scholar]
- 30.Kendall DM, Cuddihy RM, Bergenstal RM. Clinical application of incretin-based therapy: therapeutic potential, patient selection and clinical use. Am J Med. 2009;122(6, Suppl):S37–S50. [DOI] [PubMed] [Google Scholar]