Abstract
Context:
Hyperglycemia plays a key role in the pathogenesis of cardiovascular complications of diabetes. Type 2 diabetes mellitus (T2DM) is associated with high-density lipoprotein (HDL) dysfunction and increased degradation of apolipoprotein I (ApoAI). The mechanism(s) of these changes is largely unknown.
Objective:
To study the role of hyperglycemia-induced glycation on ApoAI kinetics and stability in patients with diet-controlled T2DM.
Design:
2H2O-metabolic labeling approach was used to study ApoAI turnover in patients with diet-controlled T2DM [n = 9 (5 F); 59.3 ± 8.5 years] and matched healthy controls [n = 8 (4 F); 50.7 ± 11.6 years]. The effect of Amadori glycation on in vivo ApoAI stability and the antioxidant and cholesterol efflux properties of HDL were assessed using a proteomics approach and in vitro assays.
Results:
Patients with T2DM had increased turnover of ApoAI and impaired cholesterol efflux and antioxidant properties of HDL. Glycated hemoglobin was negatively correlated with the half-life of ApoAI and cholesterol efflux function of HDL. Proteomics analysis identified several nonenzymatic early (Amadori) glycations of ApoAI at lysine sites. The kinetics analysis of glycated and native ApoAI peptides in patients with T2DM revealed that glycation resulted in a threefold shorter ApoAI half-life.
Conclusions:
The 2H2O method allowed the detection of early in vivo impairments in HDL metabolism and function that were related to hyperglycemia-induced glycation of ApoAI in T2DM.
The 2H2O method allows the detection of glycation-induced early in vivo impairments in HDL turnover and function that are related to hyperglycemia in diet-controlled patients with T2DM.
Cardiovascular disease (CVD) is the major cause of morbidity and mortality in patients with type 2 diabetes mellitus (T2DM) (1). Patients with T2DM are characterized with altered lipid and lipoprotein metabolism, including reduced high-density lipoprotein (HDL) levels (2). The inverse relationship between HDL and CVD is mediated mainly by HDL’s cholesterol transport function from the periphery to the liver by the process of reverse cholesterol transport (RCT). Apart from the removal of excess cholesterol, HDL possesses antioxidant and other functions that protect against CVD. It has been shown that the functions of HDL are altered in patients with diabetes (2–5) and the catabolism of apolipoprotein I (ApoAI), the major protein of HDL involved in RCT, is increased in patients with T2DM (6, 7). In addition, Watts et al. (8) observed that ApoA1 production was increased in patients with T2DM to compensate for the increase in HDL clearance. The accelerated degradation of ApoAI has been attributed to the increased triglyceride (TG) content of HDL and reduced plasma levels of adiponectin (9, 10); however, the mechanisms of increased ApoAI clearance in T2DM are largely unknown. It is also unclear whether hyperglycemia itself is linked to the degradation of ApoAI and that may contribute to impaired HDL function in T2DM.
Hyperglycemia is associated with increased CVD in T2DM, and intensive therapy aimed at restoring glucose levels reduces long-term CVD risk, suggesting that glycated hemoglobin (HbA1c) is a clinical biomarker for CVD risk (10). However, the relationship of HbA1c to CVD may not be direct and other intermediary mechanisms may also contribute to the outcome (11). In a hyperglycemic environment, a variety of posttranslational modifications of proteins, including glycation, lead to the glucotoxicity involved in diabetic complications. In the early stages of glycation, glucose nonenzymatically reacts with the free amino group of a protein to form Amadori glycation adducts (AGAs) (12). The degradation of AGAs further contributes to glucotoxicity through generation of advanced glycation end-products (AGEs). In contrast to the well-characterized effects of AGEs, the role of early AGAs in CVD complications of T2DM have not been characterized.
Lipoproteins, including HDL, are the primary targets of hyperglycemia-induced nonenzymatic glycation. Glycated ApoAI isolated from patients with T2DM has reduced self-association and lipid-binding capacities (13). In vitro studies demonstrated that incubation of human HDL with glucose resulted in its reduced cholesterol efflux capacity (14). Glycation may also reduce the stability of ApoAI, thereby contributing to the impaired HDL function in T2DM. Indeed, it has been shown that the clearance rate of in vitro glycated HDL is increased when injected intravenously into guinea pigs (15). However, the impact of in vivo glycation on HDL ApoAI stability in diabetes is unknown. This is in part due to the fact that it is difficult to simultaneously assess the effect of posttranslational modifications on the stability of proteins and HDL flux in vivo.
In humans, HDL flux has been probed using [2H3]-leucine as a labeled precursor of ApoAI (16, 17). Besides the discomfort related to the long-term intravenous tracer infusion and the methodological issues concerning data interpretation, these methods require large amounts of expensive tracers. As an alternative, we developed a simple method to measure integrative HDL dynamics in mice with heavy water (2H2O) as tracer in vivo (18, 19). Here, we used this method to quantify ApoAI turnover in patients with T2DM. Furthermore, we applied this approach to test the hypothesis that hyperglycemia-induced glycation reduces ApoAI stability and impairs its functions in vivo. We showed that 2H2O-metabolic labeling enabled investigation of the mechanistic link between hyperglycemia and altered HDL metabolism in diet-controlled patients with T2DM.
Methods
Subjects
The Cleveland Clinic’s Institutional Review Board reviewed and approved the protocols. All volunteers gave their informed written consent to partake in the study. T2DM was diagnosed on the basis of an oral glucose tolerance test (≥200 mg/dL after 2 hours of 75-g dextrose challenge) and/or HbA1c level (≥6.5%). Individuals were excluded if they self-reported engaging in intensive physical activity in the previous 6 months, had a history of alcohol and/or drug abuse, were smokers, or showed any evidence of cardiovascular, renal, hepatic, hypothyroid, or hematological diseases. In addition, we excluded all subjects on any lipid-lowering drugs (statins, fibrates), β-blockers, or steroids known to affect lipid metabolism. All subjects were advised to avoid strenuous exercise and to consume an isocaloric diet to prevent any diet- and exercise-induced change in HDL metabolism.
Kinetics study
To assess the effect of T2DM on ApoAI metabolism, we performed a kinetics study in newly diagnosed diet-controlled patients with T2DM and matched healthy controls. Subjects were admitted to the Clinical Research Unit at 7:00 am after a 12-hour fast. After subjects’ vital signs were recorded, an intravenous catheter was fitted into their dorsal vein for blood sampling, and a baseline sample was drawn. At 8:00 am, subjects received a 4.0-mL/kg bolus of 2H2O in five doses (given at 0, 1, 2, 3, and 4 hours) during the first day of the study and 10% of loading dose per day during the following 6 days. Additional blood samples were obtained at 2, 4, 5, 6, 7, 8, and 10 hours after the initial dose of 2H2O. Then subjects were sent home with the daily maintenance doses of 2H2O. Subjects were advised to adhere to a normal healthy diet and avoid alcohol and extraneous activities. At home, subjects were not restricted in terms of food/water intake. The subjects reported back to the Clinical Research Unit for the scheduled overnight fasted blood draws after 1, 2, 3, 4, and 7 days of initiation of the kinetics study. Serum samples were immediately processed for kinetics analyses; plasma was stored at −80°C.
Analytical procedures
Fasting plasma glucose, TG, total cholesterol, and HDL cholesterol (HDL-C) levels were measured by enzymatic analysis on an automated platform (Roche Modular Diagnostics, Indianapolis, IN). Total protein content was measured by the bicinchoninic acid protein assay method. ApoAI and ApoB100 were quantified by immunoassay methods on an ARCHITECT ci8200 Integrated Analyzer System (Abbott Laboratories, Columbus, OH). Serum levels of insulin and high-sensitivity C-reactive protein were measured using standard clinical assays. To assess the effect of T2DM on the antioxidant properties of HDL, paraoxonase-1 (PON1) activity was assayed spectrophotometrically as described (20). 2H-enrichment of body water was measured using a modified acetone exchange method (21).
Cholesterol efflux
Murine RAW264.7 cells were loaded with 3H-cholesterol (0.50 µCi/mL; American Radiolabeled Chemicals) for 24 hours at 37°C and exposed to ApoB-depleted serum (2%, volume-to-volume ratio) for 4 hours at 37°C (22). In parallel, a second set of cells was treated with 8-Br-cAMP (0.3 mM final; Sigma-Aldrich, St. Louis, MO) to induce adenosine triphosphate−binding cassette (ABC) family transporter ABCA1. Cholesterol efflux was determined as the percentage of 3H radioactivity counts in the media divided by the sum of radioactivity in the media + cells (22).
HDL isolation and particle size analysis
An anti-HDL immunoaffinity column with a covalently coupled chicken polyclonal immunoglobulin Y that recognizes total ApoAI (GenWay, San Diego, CA) was used to isolate HDL from serum according to the manufacturer’s instruction. HDL particles were separated by size using nondenaturing polyacrylamide gel electrophoresis (PAGE) on a 4% to 20% Mini-Protean TGX Precast gel (Bio-Rad, Hercules, CA). To assess ApoAI oxidation and cross-linking, immunocaptured HDL proteins were fractionated on sodium dodecyl sulfate polyacrylamide gel electrophoresis (AnyKD Mini-Protean TGX Precast gel, Bio-Rad). For Western blotting, gels were electroblotted onto the PVDF membrane and incubated with anti-ApoAI or anti-PON1 antibody.
ApoAI analysis
ApoAI turnover was assessed using the 2H2O-metabolic labeling approach as described (18). Briefly, after removal of VLDL by ultracentrifugation, the lower phase was mixed with 3 µL of HDL-precipitating reagent (STANBIO, Boerne, TX). The supernatant containing ApoB-depleted serum was used for the analysis of ApoAI. Proteins were precipitated with cold acetone (−20°C), and protein pellets were used for the analysis of ApoAI by liquid chromatography-tandem mass spectrometry (18). Briefly, the disulfide bonds of proteins were reduced, and free thiol groups were alkylated. Proteins were digested, and tryptic peptides were analyzed by liquid chromatography-tandem mass spectrometry. The data were searched with Mascot software (Version 2.5.1; Matrix Science, Boston, MA) against the National Center for Biotechnology Information Human reference sequence database (ftp://ftp.ncbi.nih.gov/refseq/). The search was performed using cysteine carbamidomethylation as a fixed modification and methionine oxidation and lysine and arginine glycation as variable modifications with trypsin as the protease. A neutral loss of 162.05 Da was used as an additional criterion to validate glycated peptides.
ApoAI glycation
ApoAI glycation was quantified based on the averaged intensity ratios of the extracted ion chromatograms corresponding to both the native and glycated peptides. The effect of glycation on ApoAI stability was assessed based on the half-lives of the glycated ApoAI and nonglycated peptides.
Calculations
Fractional catabolic rates (FCRs) of ApoAI and its glycated peptide were calculated based on the rate of 2H-incorporation (19). The FCRs were determined using a single compartmental model by fitting time course 2H-labeling [E (t)] of analytes to an exponential growth curve equation:
where E0 is asymptotical labeling; k is the rate constant, which is related to the half-life (t1/2 = ln2/k). To account for the fluctuations in body water enrichment, 2H-labeling of peptides was normalized relative to the total body water enrichment.
The production rate (PR) of ApoAI was calculated as the product of its FCR and pool size, which is the product of ApoAI concentration and plasma volume, estimated as 4.5% of the body weight.
Data presentation and statistical analysis
Continuous variables were evaluated for normality using the Shapiro-Wilk test. Only glycation measures showed strong departures from normality and were compared between groups defined by diabetic status using nonparametric Wilcoxon rank sum tests and were summarized using medians and quartiles. Normally distributed continuous measures were summarized using means and standard deviations (SDs) and were compared between groups using two-sample t tests. To evaluate the impact of demographic factors (age, sex, and fasting glucose, insulin, HbA1c, and lipid levels) on associations observed, linear regression models with and without adjustment factors were performed. When necessary, log transformations (with offsets) were performed to achieve more normally distributed measures. Mean differences between groups with 95% confidence intervals were presented. Categorical factors were compared using Fisher’s exact test. To assess correlations between continuous factors, Pearson and Spearman correlations were used. The present sample size, though small, was adequate to detect effect size differences in excess of 1.5 with 80% power. Based upon observed SDs, this corresponds to having at least 80% power to detect a mean difference of at least 0.006 (mg/dL) in HDL-C (SD = 0.004) and mean differences of at least 0.053 (mg/dL) (SD = 0.0035) in ApoAI. All calculations assumed a 0.05 significance level. Analysis was performed using SAS Software (version 9.4).
Results
Subject characteristics
Eight control subjects (age, 50.7 ± 11.6 years; four females) and nine patients with T2DM (age, 59.3 ± 8.5 years; five females) participated in the study. As shown in Table 1, the control and T2DM groups were matched for body mass index with no significant difference in age, blood pressure level, and fasting plasma lipid values (P > 0.05). Furthermore, no differences were observed in HDL-C and ApoAI levels between groups, even after adjustment for sex (Supplemental Table 1 (221.1KB, pdf) ). As expected, patients with T2DM had higher fasting glucose, HbA1c, insulin, and homeostatic model assessment of insulin resistance levels than healthy controls (P < 0.05).
Table 1.
Clinical and Biochemical Characteristics of Study Participants
| Characteristics | Control (Male:Female) | T2DM (Male:Female) | P Value |
|---|---|---|---|
| Number | 8 (4:4) | 9 (4:5) | |
| Age, y | 50.7 ± 11.6 | 59.3 ± 8.5 | 0.067a |
| BMI, kg/m2 | 28.7 ± 3.1 | 31.2 ± 3.3 | 0.15a |
| Systolic blood pressure, mm Hg | 122.3 ± 12.5 | 132.3 ± 17.2 | 0.19a |
| Diastolic blood pressure, mm Hg | 76.1 ± 9.5 | 74.1 ± 14.3 | 0.74a |
| Glucose, mg/dL | 92.5 ± 11.9 | 113.3 ± 14.3 | 0.005a |
| Insulin, mIU/dL | 7.0 (4.5, 12.0) | 16.2 (14.9, 19.9) | 0.003b |
| HOMA IR | 1.9 ± 1.04 | 5.3 ± 2.0 | <0.001a |
| HbA1c, % | 5.5 ± 0.2 | 6.3 ± 0.3 | <0.001a |
| Total cholesterol, mg/dL | 174.4 ± 42.7 | 188.7 ± 35.8 | 0.48a |
| Triglycerides, mg/dL | 96.1 ± 40.7 | 100.1 ± 37.3 | 0.84a |
| HDL-C, mg/dL | 52.3 ± 14.4 | 48.6 ± 11.1 | 0.56a |
| LDL cholesterol, mg/dL | 107.1 ± 26.3 | 120.0 ± 31.3 | 0.39a |
| ApoB100, mg/dL | 76.5 ± 12.5 | 92.4 ± 23.3 | 0.11a |
| ApoAI, mg/dL | 128.3 ± 20.4 | 132.7 ± 17.0 | 0.63a |
| ApoB100/ApoAI | 0.60 ± 0.09 | 0.70 ± 0.19 | 0.18a |
| ApoAI/HDL cholesterol | 2.5 ± 0.39 | 2.8 ± 0.45 | 0.20a |
| hsCRP, mg/L | 1.4 (0.65, 2.3) | 1.7 (1.1, 5.5) | 0.29b |
| Hypertension | 0.47c | ||
| No | 8 (100.0) | 7 (77.8) | |
| Yes | 0 (0.0) | 2 (22.2) |
Statistics are presented as means ± SD, median (P25, P75), or N (column %). The bold P values are those P < 0.05.
Abbreviations: BMI, body mass index; HOMA IR, homeostatic model assessment of insulin resistance; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein.
t test.
Wilcoxon rank sum test.
Pearson χ2 test.
ApoAI turnover
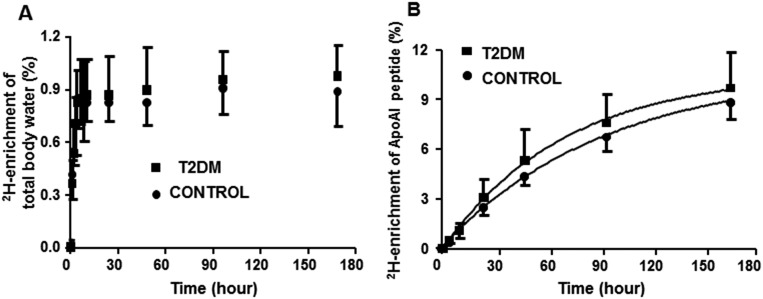
ApoAI turnover in patients with T2DM and matched controls was measured using an optimized 2H2O protocol. One hour after the last bolus dose of 2H2O, the 2H-labeling of body water was stabilized at 0.85% ± 0.03% and 0.90% ± 0.05% in controls and patients, respectively (Fig. 1A). The 2H-labeling of ApoAI increased more rapidly in the patients than in controls, indicating an increased flux of HDL in T2DM (Fig. 1B). Indeed, patients had a significantly higher clearance (FCR) of ApoAI (0.22 ± 0.01 vs 0.41 ± 0.09 pool/d; P = 0.001) than controls. This was associated with reduced half-lives of ApoAI in patients with T2DM (Table 2). Despite the increased FCR in T2DM, ApoAI pool sizes were not different. However, the PR of ApoAI was significantly higher in the patients than in controls.
Figure 1.
ApoAI turnover in patients with T2DM (n = 9) and age- and body mass index−matched healthy controls (n = 8) was determined using the 2H2O-metabolic labeling approach. (A) Administration of a bolus dose (4 mL/kg) followed by maintenance doses (10% of bolus per day) of 2H2O in drinking water resulted in steady-state body water enrichment of ∼0.8% to 0.9%. (B) Time-course labeling of tryptic ApoAI peptide VSFLALEEYTK. Data are presented as mean ± SD.
Table 2.
ApoAI Kinetics Parameters in Patients With T2DM (n = 9) and Age- and BMI-Matched Healthy Controls (n = 8)
| Parameters | Control | T2DM | P Value |
|---|---|---|---|
| ApoAI pool, mg/kg | 51.6 ± 4.1 | 60.8 ± 8.0 | NS |
| ApoAI FCR, d−1 | 0.22 ± 0.01 | 0.41 ± 0.09 | 0.001 |
| ApoAI half-life, d | 3.1 ± 0.1 | 1.8 ± 0.4 | 0.002 |
| ApoAI PR, mg/kg/d | 11.6 ± 0.8 | 24.7 ± 4.9 | 0.003 |
Data are presented as mean ± SD. P values are based on t tests.
Abbreviations: BMI, body mass index; NS, nonsignificant.
Nonenzymatic glycation of ApoAI
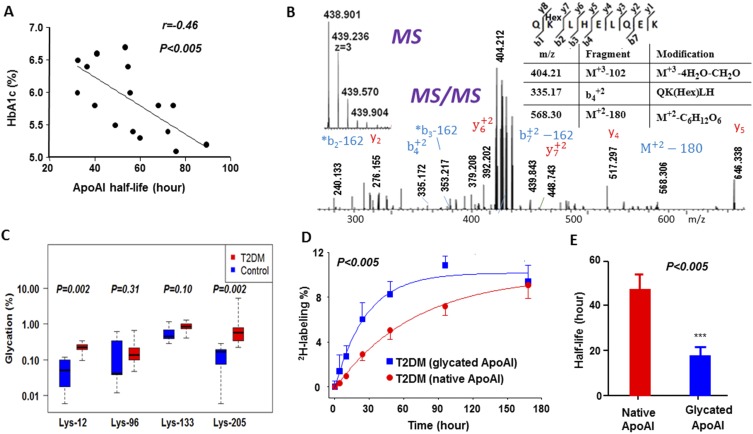
A linear regression analysis revealed that the half-life of ApoAI had a strong inverse association with HbA1c (Fig. 2A), suggesting that nonenzymatic glycation could be involved in increased degradation of ApoAI. Indeed, proteomics analysis revealed that ApoAI was glycated at the Lys-12, Lys-96, Lys-133, and Lys-205 sites. As an example, the tandem mass spectrometry spectrum of the glycated Q132KLHELQEK140 ApoAI peptide containing a +162.053 Da (C6H10O5) modification at Lys-133 is presented in Fig. 2B. Comparison of glycation measures showed that patients with T2DM had significantly higher percentage glycation of ApoAI at Lys-12 and Lys-205 sites (Fig. 2C). Because of large variabilities, glycation ratios were not significantly different between controls and patients for Lys-96 and Lys-133.
Figure 2.
Hyperglycemia-induced glycation reduced the stability of ApoAI in the patients with T2DM. (A) The relationship between ApoAI stability and hyperglycemia. ApoAI half-life was inversely associated with HbA1c in all study subjects. (B) Characterization of nonenzymatic glycation of ApoAI based on tandem mass spectrometry spectrum of the glycated ApoAI peptide QHexKLHELQEK modified at Lys-133. De novo sequencing of this peptide indicated that the neutral loss of an entire glucose moiety from the glycated parent, or its b and y ions, resulted in a 162.058-Da mass difference (see the peaks at m/z 240.133 and 568.30). Similarly, a loss of four water molecules from the parent (102.052 Da, −CH2O-4H2O) led to a residual modification of 404.212 m/z. (C) Comparison of ApoAI percent glycation (signal intensity ratios of glycated peptide over nonglycated) measures between groups. Because glycation measures showed strong departures from normality, they were compared using nonparametric Wilcoxon rank sum tests. Statistics are presented as median (P25, P75). (D) Effect of glycation on ApoA1 kinetics in patients with T2DM. The glycated ApoA1 peptide (QHexKLHELQEK) reached maximum 2H enrichment levels at a higher rate than the nonglycated (VSFLSALEEYTK) peptide, which is an indicator of its faster catabolism. (E) Glycation resulted in reduced stability of ApoAI in patients with T2DM. Half-lives of glycated (QHexKLHELQEK) and nonglycated (VSFLSALEEYTK) ApoAI peptides in patients with T2DM were quantified from 2H2O-metabolic labeling data presented in (D). Data are mean ± SD (n = 6). ***P < 0.005.
Because the mass spectrometry−based method allowed us to differentiate the glycated protein fraction from the nonglycated counterpart, we separately quantified the kinetics of the glycated and nonmodified ApoAI to assess the effect of glycation on HDL stability in patients with T2DM. Kinetics of the glycated and nonglycated ApoAI populations was determined on the basis of the rate of 2H-incorporation into the glycated and nonglycated ApoAI peptides (Supplemental Fig. 1 (221.1KB, pdf) ). Interestingly, the turnover rate of the glycated peptide was almost threefold faster than that of its nonglycated variant (Fig. 2D), which resulted in a significantly shorter half-life of the glycated peptide (Fig. 2E), suggesting that hyperglycemia-induced glycation may be involved in the reduced stability of HDL in patients with T2DM. Because of low signal intensities, we could not quantify the kinetics of glycated peptides in healthy controls. For the same reason, we could not evaluate the effects of glycation at the other Lys sites on ApoAI stability in T2DM.
Impaired cholesterol efflux and antioxidant activity of HDL in diet-controlled patients with T2DM
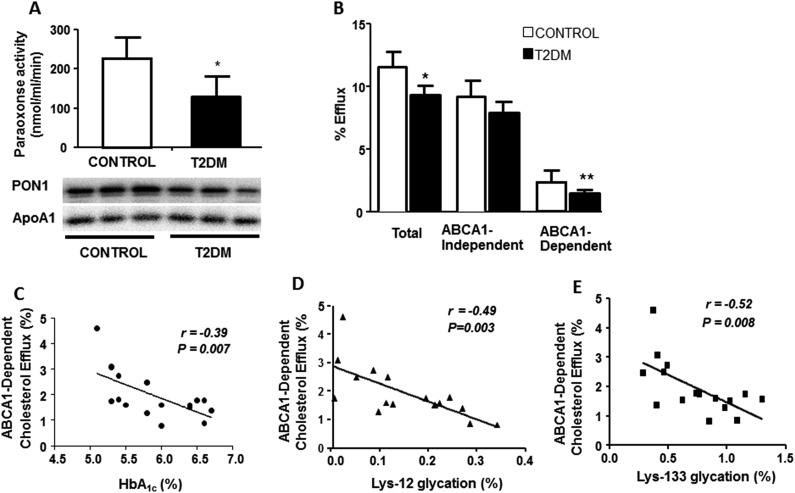
Next, we examined the effect of glycation on HDL functions. Enzymatic activity of PON1, an HDL-associated ester hydrolase that determines antioxidant activity of HDL, was significantly lower in the subjects with T2DM than in controls (P < 0.05) (Fig. 3A, top). This was associated with the reduced serum PON1 content in the patients (Fig. 3A, bottom). In addition, both total and ABCA1-dependent cholesterol effluxes from lipid-laden macrophages to ApoB-depleted serum were significantly decreased in the patients with T2DM (Fig. 3B). Although ABCA1-independent cholesterol efflux was also reduced in the patients with T2DM, the difference was not significant (P = 0.07), suggesting that hyperglycemia mainly affects the ABCA1-dependent efflux. ABCA1-dependent cholesterol efflux had an inverse correlation with HbA1c (Fig. 3C). Furthermore, strong inverse correlations were observed between ApoAI glycation at both Lys-12 and Lys-133 sites and ABCA1-dependent (Fig. 3D and 3E) but not ABCA1-independent cholesterol efflux (Supplemental Table 2 (221.1KB, pdf) ). The differences in ABCA1-dependent cholesterol efflux and several other parameters, including the FCR of ApoAI, ApoAI glycation, and PON1 activity, completely disappeared after the adjustment for HbA1c (Supplemental Table 3 (221.1KB, pdf) ). In contrast, adjustments for other clinical parameters did not show any marked difference (not shown). Overall, these results suggest that hyperglycemia-induced ApoAI glycation is involved in impaired HDL functions in T2DM.
Figure 3.
Antioxidant activity and cholesterol efflux functions of HDL were reduced in patients with T2DM compared to healthy controls, and impairment in ABCA1-dependent cholesterol efflux activity was associated with hyperglycemia (HbA1c) and ApoAI glycation. (A) Serum PON1 activity was measured spectrophotometrically using paraoxon as substrate (top). PON1 content in immunocaptured HDL was quantified by anti-PON1 immunoblotting with ApoAI as the loading control (bottom). (B) ApoB-depleted sera from patients with T2DM and healthy controls were investigated for their ability to promote [3H]cholesterol efflux from murine RAW264.7 macrophages. Correlation between ABCA1-dependent cholesterol efflux activity with (C) HbA1c, (D) ApoAI glycation at Lys-12, and (E) Lys-133 residues. ApoAI glycation was measured by mass spectrometry as described in the methods section. Data are presented as mean ± SD (n = 8 per group). All assays were performed in triplicate. *P < 0.05; **P < 0.005.
HDL particle size analysis
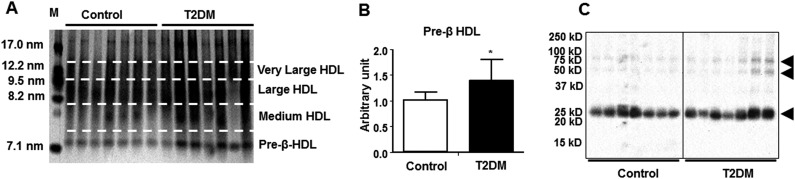
To assess the effect of T2DM on HDL particle size, HDL was isolated on an anti-HDL immunoglobulin Y column and separated by size using nondenaturing PAGE in small pre-β HDL, midsized HDL3, and large HDL2 particles. Size distribution analysis of HDL particles revealed a significant shift toward the lipid-poor pre-β content in patients with T2DM (Fig. 4A and 4B).
Figure 4.
T2DM altered HDL particle sizes and induced cross-linking of ApoAI. (A) Immunocaptured HDL particles were resolved by nondenaturing polyacrylamide gel electrophoresis (4% to 20% gradient) and silver-stained. M represents particle size marker. (B) Pre-β HDL was quantified from Western blotting, and the average band intensity was normalized to control. (C) Immunocaptured HDL proteins from controls and patients with T2DM were separated on 4% to 20% gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis under reducing conditions, and ApoAI was detected by Western blotting. Data are presented as mean ± SD. *P < 0.05. Arrowheads show ApoAI-associated bands.
ApoAI cross-links
Because T2DM is associated with accelerated oxidative damage to the plasma proteins (2) and glycation may lead to cross-linking of the proteins (12), we fractionated immunocaptured HDL proteins by SDS-PAGE. In addition to the expected monomeric ApoAI band at ∼27 kDa in both groups, ApoAI-specific Western blotting of HDL proteins pinpointed two bands of ∼50 and 75 kDa in patients with T2DM (Fig. 4C). Proteomics analysis revealed that all bands were associated with ApoAI and other HDL proteins (Supplemental Table 4 (221.1KB, pdf) ), suggesting hyperglycemia-induced inter- and intramolecular cross-linking of ApoAI in the patients with T2DM.
Discussion
The current study used a 2H2O-labeling approach to measure ApoAI turnover in the patients with diet-controlled T2DM. We demonstrated that glycated ApoA1 FCR was significantly increased in T2DM and that was associated with impaired HDL functions. These changes were related to hyperglycemia-induced glycation of ApoAI. Finally, ApoAI glycation was associated with its rapid degradation, suggesting that hyperglycemia-induced glycation contributed to increased degradation of ApoAI in T2DM. From a clinical perspective, our data highlight that “normal” or even elevated HDL levels in T2DM do not equate with their adequate functionality. Rather, glycation of ApoAI is a marker for hyperglycemia-induced HDL dysfunction that blunts the antiatherogenic and antioxidant functions of HDL.
Although ApoAI turnover analysis is widely used to study HDL metabolism, existing methods cannot assess the effects of glycation- or oxidation-induced modifications on ApoAI kinetics and stability and HDL functionality. In this study, we simultaneously measured the turnover rates of native ApoAI and its glycated variant in humans using 2H2O tracer (18). This method in combination with a proteomics approach enabled the assessment of the effect of glycation on the stability of ApoAI. Notably, the calculated turnover rate constant of ApoAI using the 2H2O method was similar to those previously reported for healthy subjects using other isotope-labeling methods (9, 16, 24, 25). Our results show that despite no significant differences in ApoAI (and HDL-C) levels, ApoAI PR was increased, whereas its half-life was diminished, highlighting an overall increase in HDL flux in diet-controlled T2DM vs controls. Thus, in early stages of T2DM, increased production compensated for diabetes-induced accelerated clearance of HDL, keeping its level stable. This finding also marks the superiority of kinetics analysis, which can detect differences in HDL metabolism compared with common clinical static measurements that dismiss these differences on the basis of HDL levels. For example, although the pathway biology is altered but not yet to a point where it can impact the clinical phenotype.
Although the role of hypertriglyceridemia on increased clearance of ApoAI is well studied (26, 27), very little is known about the effect of hyperglycemia on ApoAI metabolism. In our study, despite hyperglycemia, patients with T2DM had normal plasma TG levels. Presumably, therefore we didn’t observe any correlation between plasma TG level and the FCR of ApoAI. In contrast, we found strong correlations between HbA1c and the FCR of ApoAI. This prompted us to investigate posttranslational glycation of ApoAI. Nonenzymatic glycation of the proteins reflects their long-term exposure to plasma glucose. Because hemoglobin’s half-life (∼90 days) is longer than that of many other plasma proteins, HbA1c levels are considered a surrogate measurement of long-term sustained hyperglycemia as a clinical marker of diabetes (28). In this study, proteomics analysis revealed that glycated ApoAI levels were increased in the patients with T2DM. Because ApoAI’s half-life in humans is 3 to 4 days, glycated ApoAI can be used as an index of short-term glycemia in prediabetes and diet-controlled diabetes (29). The kinetics analysis revealed that the half-life of glycated ApoAI is three times shorter than that of its native counterpart. Although future studies are necessary to dissect the effects of hyperglycemia and hypertriglyceridemia on HDL metabolism, this finding suggests that hyperglycemia rather than dyslipidemia could be involved in the increased FCR of ApoAI in early stages of T2DM.
Although our findings documented the role of in vivo glycation in HDL metabolism, the molecular mechanism(s) responsible for enhanced degradation of glycated ApoAI is not clear. Previous studies have shown that ApoAI glycation facilitates its dissociation from HDL (13). We speculate that glycation-induced dissociation of ApoAI from HDL particles may facilitate its degradation. Renal tubule cells could contribute to the uptake and degradation of glycated ApoAI, as the kidney is involved in the clearance of lipid-free ApoAI and pre-β HDL particles in proximal tubule cells (30). Rapid clearance of glycated ApoAI could also explain the apparent discrepancy between increased in vivo HDL flux and reduced in vitro cholesterol efflux in the patients with T2DM. It is plausible that increased HDL flux in patients with T2DM reflects inefficient cholesterol transport due to enhanced clearance of glycated ApoAI. Given the T2DM-induced changes in pre-β HDL particle distribution (Fig. 4B), it would also be interesting to know whether the kinetics of ApoAI is impaired in this population of HDL particles. Unfortunately, the sensitivity of our method precluded assessing the effect of glycation on ApoAI kinetics in distinct HDL particles.
Although the role of AGE-induced ApoAI modification in diabetes is well characterized, very little is known about the biological significance of Amadori-modified ApoAI. It has been shown that masking positive charges of ApoAI lysine residues with acetoacetate impaired ABCA1-mediated lipid efflux (31). It is possible that nonenzymatic glycation of HDL with glucose could also impair lipid-apolipoprotein interaction, thereby compromising lipidation, structural cohesion, and the cholesterol-efflux function of HDL. However, recent studies on the effect of HDL glycation on cholesterol efflux have yielded contradictory results, with both impairment (14, 32, 33) and no effect reported (34). In the current study, we demonstrated that in vivo glycation was associated with reduced cholesterol efflux and the antioxidant function of HDL. Notably, the most significant effect of hyperglycemia-induced glycation on cholesterol efflux was attributed to its ABCA1-dependent efflux. Because the Lys-133 residue is part of the lipid-binding (Asn-122 to Val-143) domain of ApoAI (35), the strong inverse correlation between ABCA1-dependent efflux and ApoAI glycation at Lys-133 (Fig. 3E) suggests that glycation at this site may impair cholesterol efflux.
Our T2DM cohort had unusual normal concentrations of HDL and TG. This may be related to the very early stage of the disease and a variety of unmeasured clinical factors, including menopausal status and concentration of sex steroids, body fat composition and distribution, and lifestyle factors (such as diet), which should be controlled in future studies as they may attenuate the results. However, despite the normal concentration of HDL, the metabolism and function of ApoAI were clearly abnormal, and we expect even more alterations in advanced T2DM, which is associated with more severe hyperglycemia and dyslipidemia. Thus, consistent with previous studies demonstrating that serum HDL-C level is not a marker of RCT (36), our results indicate that neither HDL-C nor ApoAI level reflected HDL functionality. In contrast, ApoAI turnover analysis presented here provides a surrogate measure of HDL functionality in vivo. Therefore, the method presented in this study provides a useful and robust tool to assess the effect of the disease and therapeutic modalities on HDL functionality in vivo.
In conclusion, the 2H2O-based metabolic labeling approach allows for early detection of hyperglycemia-induced changes in HDL metabolism and ApoAI stability. As a simple and safe approach, the 2H2O method could be applied to study HDL metabolism in other diseases to provide further insight into in vivo HDL functionality.
Acknowledgments
Financial Support: This study was supported by the Cleveland Clinic Center for Translational Science Activities, American Diabetes Association grant 1-15IN-31, National Institutes of Health Grant RO1GM112044, and American Heart Association grant 15GRNT25500004 (T.K.).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 2H2O
- heavy water
- ABC
- adenosine triphosphate−binding cassette
- AGA
- Amadori glycation adduct
- AGE
- advanced glycation end-product
- ApoAI
- apolipoprotein I
- CVD
- cardiovascular disease
- FCR
- fractional catabolic rate
- HbA1c
- glycated hemoglobin
- HDL
- high-density lipoprotein
- HDL-C
- high-density lipoprotein cholesterol
- PAGE
- polyacrylamide gel electrophoresis
- PON1
- paraoxonase-1
- PR
- production rate
- RCT
- reverse cholesterol transport
- SD
- standard deviation
- T2DM
- type 2 diabetes mellitus
- TG
- triglyceride.
References
- 1.American Diabetes Association Economic costs of diabetes in the U.S. in 2012 [published correction appears in Diabetes Care. 2013;36(6):1797]. Diabetes Care. 2013;36(4):1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaleel A, Henderson GC, Madden BJ, Klaus KA, Morse DM, Gopala S, Nair KS. Identification of de novo synthesized and relatively older proteins: accelerated oxidative damage to de novo synthesized apolipoprotein A-1 in type 1 diabetes. Diabetes. 2010;59(10):2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon SM, Davidson WS, Urbina EM, Dolan LM, Heink A, Zang H, Lu LJ, Shah AS. The effects of type 2 diabetes on lipoprotein composition and arterial stiffness in male youth. Diabetes. 2013;62(8):2958–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorrentino SA, Besler C, Rohrer L, Meyer M, Heinrich K, Bahlmann FH, Mueller M, Horváth T, Doerries C, Heinemann M, Flemmer S, Markowski A, Manes C, Bahr MJ, Haller H, von Eckardstein A, Drexler H, Landmesser U. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 2010;121(1):110–122. [DOI] [PubMed] [Google Scholar]
- 5.Manjunatha S, Distelmaier K, Dasari S, Carter RE, Kudva YC, Nair KS. Functional and proteomic alterations of plasma high density lipoproteins in type 1 diabetes mellitus. Metabolism. 2016;65(9):1421–1431. [DOI] [PubMed] [Google Scholar]
- 6.Golay A, Zech L, Shi MZ, Chiou YA, Reaven GM, Chen YD. High density lipoprotein (HDL) metabolism in noninsulin-dependent diabetes mellitus: measurement of HDL turnover using tritiated HDL. J Clin Endocrinol Metab. 1987;65(3):512–518. [DOI] [PubMed] [Google Scholar]
- 7.Duvillard L, Pont F, Florentin E, Gambert P, Vergès B. Inefficiency of insulin therapy to correct apolipoprotein A-I metabolic abnormalities in non-insulin-dependent diabetes mellitus. Atherosclerosis. 2000;152(1):229–237. [DOI] [PubMed] [Google Scholar]
- 8.Watts GF, Barrett PH, Ji J, Serone AP, Chan DC, Croft KD, Loehrer F, Johnson AG. Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes. 2003;52(3):803–811. [DOI] [PubMed] [Google Scholar]
- 9.Vergès B, Petit JM, Duvillard L, Dautin G, Florentin E, Galland F, Gambert P. Adiponectin is an important determinant of apoA-I catabolism. Arterioscler Thromb Vasc Biol. 2006;26(6):1364–1369. [DOI] [PubMed] [Google Scholar]
- 10.Pietzsch J, Julius U, Nitzsche S, Hanefeld M. In vivo evidence for increased apolipoprotein A-I catabolism in subjects with impaired glucose tolerance. Diabetes. 1998;47(12):1928–1934. [DOI] [PubMed] [Google Scholar]
- 11.Nathan DM, Cleary PA, Backlund JYC, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56(1):1–21. [DOI] [PubMed] [Google Scholar]
- 13.Calvo C, Talussot C, Ponsin G, Berthézène F. Non enzymatic glycation of apolipoprotein A-I. Effects on its self-association and lipid binding properties. Biochem Biophys Res Commun. 1988;153(3):1060–1067. [DOI] [PubMed] [Google Scholar]
- 14.Duell PB, Oram JF, Bierman EL. Nonenzymatic glycosylation of HDL and impaired HDL-receptor-mediated cholesterol efflux. Diabetes. 1991;40(3):377–384. [DOI] [PubMed] [Google Scholar]
- 15.Witztum JL, Fisher M, Pietro T, Steinbrecher UP, Elam RL. Nonenzymatic glucosylation of high-density lipoprotein accelerates its catabolism in guinea pigs. Diabetes. 1982;31(11):1029–1032. [DOI] [PubMed] [Google Scholar]
- 16.Ouguerram K, Krempf M, Maugeais C, Maugère P, Darmaun D, Magot T. A new labeling approach using stable isotopes to study in vivo plasma cholesterol metabolism in humans. Metabolism. 2002;51(1):5–11. [DOI] [PubMed] [Google Scholar]
- 17.Turner S, Voogt J, Davidson M, Glass A, Killion S, Decaris J, Mohammed H, Minehira K, Boban D, Murphy E, Luchoomun J, Awada M, Neese R, Hellerstein M. Measurement of reverse cholesterol transport pathways in humans: in vivo rates of free cholesterol efflux, esterification, and excretion. J Am Heart Assoc. 2012;1(4):e001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasumov T, Willard B, Li L, Li M, Conger H, Buffa JA, Previs S, McCullough A, Hazen SL, Smith JD. 2H2O-based high-density lipoprotein turnover method for the assessment of dynamic high-density lipoprotein function in mice. Arterioscler Thromb Vasc Biol. 2013;33(8):1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Bebek G, Previs SF, Smith JD, Sadygov RG, McCullough AJ, Willard B, Kasumov T. Proteome dynamics reveals pro-inflammatory remodeling of plasma proteome in a mouse model of NAFLD. J Proteome Res. 2016;15(9):3388–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy DJ, Tang WH, Fan Y, Wu Y, Mann S, Pepoy M, Hazen SL. Diminished antioxidant activity of high-density lipoprotein-associated proteins in chronic kidney disease. J Am Heart Assoc. 2013;2(2):e000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah V, Herath K, Previs SF, Hubbard BK, Roddy TP. Headspace analyses of acetone: a rapid method for measuring the 2H-labeling of water. Anal Biochem. 2010;404(2):235–237. [DOI] [PubMed] [Google Scholar]
- 22.Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, Smith JD, Hazen SL. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33(7):1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashyap S, Kheniser K, Li L, Bena J, Kasumov T. The therapeutic efficacy of intensive medical therapy in ameliorating high-density lipoprotein dysfunction in subjects with type two diabetes [published correction appears in Lipids Health Dis. 2016;15:191] Lipids Health Dis. 2016;15(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welty FK, Lichtenstein AH, Barrett PHR, Dolnikowski GG, Schaefer EJ. Interrelationships between human apolipoprotein A-I and apolipoproteins B-48 and B-100 kinetics using stable isotopes. Arterioscler Thromb Vasc Biol. 2004;24(9):1703–1707. [DOI] [PubMed] [Google Scholar]
- 25.Pont F, Duvillard L, Florentin E, Gambert P, Vergès B. High-density lipoprotein apolipoprotein A-I kinetics in obese insulin resistant patients: an in vivo stable isotope study. Int J Obes Relat Metab Disord. 2002;26(9):1151–1158. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg IJ, Blaner WS, Vanni TM, Moukides M, Ramakrishnan R. Role of lipoprotein lipase in the regulation of high density lipoprotein apolipoprotein metabolism: studies in normal and lipoprotein lipase-inhibited monkeys. J Clin Invest. 1990;86(2):463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horowitz BS, Goldberg IJ, Merab J, Vanni TM, Ramakrishnan R, Ginsberg HN. Increased plasma and renal clearance of an exchangeable pool of apolipoprotein A-I in subjects with low levels of high density lipoprotein cholesterol. J Clin Invest. 1993;91(4):1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care. 2011;34(2):518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaleel A, Halvatsiotis P, Williamson B, Juhasz P, Martin S, Nair KS. Identification of Amadori-modified plasma proteins in type 2 diabetes and the effect of short-term intensive insulin treatment. Diabetes Care. 2005;28(3):645–652. [DOI] [PubMed] [Google Scholar]
- 30.Kozyraki R, Fyfe J, Kristiansen M, Gerdes C, Jacobsen C, Cui S, Christensen EI, Aminoff M, de la Chapelle A, Krahe R, Verroust PJ, Moestrup SK. The intrinsic factor-vitamin B12 receptor, cubilin, is a high-affinity apolipoprotein A-I receptor facilitating endocytosis of high-density lipoprotein. Nat Med. 1999;5(6):656–661. [DOI] [PubMed] [Google Scholar]
- 31.Brubaker G, Peng DQ, Somerlot B, Abdollahian DJ, Smith JD. Apolipoprotein A-I lysine modification: effects on helical content, lipid binding and cholesterol acceptor activity. Biochim Biophys Acta. 2006;1761(1):64–72. [DOI] [PubMed] [Google Scholar]
- 32.Matsuki K, Tamasawa N, Yamashita M, Tanabe J, Murakami H, Matsui J, Imaizumi T, Satoh K, Suda T. Metformin restores impaired HDL-mediated cholesterol efflux due to glycation. Atherosclerosis. 2009;206(2):434–438. [DOI] [PubMed] [Google Scholar]
- 33.Nobécourt E, Tabet F, Lambert G, Puranik R, Bao S, Yan L, Davies MJ, Brown BE, Jenkins AJ, Dusting GJ, Bonnet DJ, Curtiss LK, Barter PJ, Rye KA. Nonenzymatic glycation impairs the antiinflammatory properties of apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 2010;30(4):766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown BE, Nobecourt E, Zeng J, Jenkins AJ, Rye KA, Davies MJ. Apolipoprotein A-I glycation by glucose and reactive aldehydes alters phospholipid affinity but not cholesterol export from lipid-laden macrophages. PLoS One. 2013;8(5):e65430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank PG, Marcel YL. Apolipoprotein A-I: structure-function relationships. J Lipid Res. 2000;41(6):853–872. [PubMed] [Google Scholar]
- 36.Blum CB, Dell RB, Palmer RH, Ramakrishnan R, Seplowitz AH, Goodman DS. Relationship of the parameters of body cholesterol metabolism with plasma levels of HDL cholesterol and the major HDL apoproteins. J Lipid Res. 1985;26(9):1079–1088. [PubMed] [Google Scholar]