Abstract
Context:
Metabolic flexibility reflects the ability to switch from lipid to carbohydrate oxidation during insulin stimulation manifested in increased respiratory quotient (RQ). Little is known about adipose tissue metabolism and metabolic flexibility in adolescent girls with polycystic ovary syndrome (PCOS).
Objective:
We investigated whole-body lipolysis, substrate oxidation, and metabolic flexibility in obese girls with PCOS vs obese girls without PCOS.
Patients/Design:
Twenty-one obese girls with PCOS and 21 obese girls without PCOS were pair-matched for age and race. Body composition, abdominal visceral adipose tissue (VAT), sex hormones, lipid profile, and adiponectin were measured. Whole-body lipolysis ([2H5]glycerol turnover), RQ, and substrate oxidation (indirect calorimetry) were evaluated during fasting and a hyperinsulinemic-euglycemic clamp together with assessment of insulin sensitivity (IS).
Results:
Despite similar body mass index and percent body fat, girls with PCOS vs girls without PCOS had lower fasting lipolysis and fat oxidation, less increase in RQ during hyperinsulinemia with impaired suppression in lipolysis and lipid oxidation, and lower IS. In multiple regression, the best predictors of metabolic flexibility were [using clinical parameters: adiponectin, fasting triglycerides, and insulin (R2 = 0.618, P < 0.0001); using research parameters: IS, VAT, and baseline RQ (R2 = 0.756, P < 0.0001)].
Conclusions:
Obese girls with PCOS vs obese girls without PCOS have decreased lipid mobilization, diminished fat oxidation, and metabolic inflexibility. Whether this metabolic phenotype of adipose tissue dysfunction, which is conducive to fat accretion, plays a role in the induction and maintenance of obesity in adolescent girls with PCOS remains to be determined.
Obese girls with vs without PCOS have the inability to mobilize and burn fat from adipose stores in a fasting state and are metabolically inflexible during hyperinsulinemia to adjust to fuel supply.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder affecting women of reproductive age and is characterized by menstrual dysfunction, clinical and/or biochemical hyperandrogenism with or without polycystic ovaries, and insulin resistance (1). In the United States, more than half of adult patients with PCOS are overweight or obese (2), and the severity of PCOS (i.e., worse metabolic and reproductive outcomes) is exacerbated by the degree of obesity (3). Likewise, in adolescent girls, the severity of obesity is associated with greater increased odds of PCOS compared with normal-weight girls (4). As such, obesity is tightly linked to PCOS, and therefore prevention of excess weight gain is important to manage or prevent PCOS in youth and adults (1, 3).
Metabolic flexibility reflects the ability to adjust fuel oxidation to fuel availability and to switch from lipid oxidation to carbohydrate oxidation during insulin stimulation (5, 6). Obese individuals have metabolic inflexibility evidenced by diminished fat oxidation at fasting, impaired suppression of lipid oxidation, and blunted stimulation of glucose oxidation during an insulin-stimulated state, thereby reducing the magnitude of increase in respiratory quotient (ΔRQ) from fasting to the insulin-stimulated state (5, 7). Moreover, reduced postabsorptive fat oxidation appears to be a contributing factor leading to positive energy balance and, therefore, future weight gain. In a longitudinal study of adult Pima Indians, individuals with low fat oxidation were at 2.5 times greater risk for future weight gain compared with those with high fat oxidation independent of 24-hour metabolic rate (8). Additionally, adipose tissue functions as a buffer for daily lipid flux by suppressing the release of nonesterified fatty acids into the circulation and by increasing the clearance of triacylglycerol (9). Although obesity, insulin resistance, and hyperinsulinemia are common features documented in adult women and adolescents with PCOS (10, 11), data on lipid metabolism, adipose tissue function, and metabolic flexibility are limited and only available in adult women with PCOS (12, 13).
Therefore, the purposes of this study were (1) to investigate metabolic flexibility and substrate oxidation in obese adolescent girls with PCOS compared with their obese peers without PCOS, (2) to assess whole-body lipolysis measured with [2H5]glycerol tracer, and (3) to examine the relationship between metabolic flexibility; adipose tissue function; and physical, hormonal, and metabolic characteristics.
Materials and Methods
Patients
Data from 21 girls with a diagnosis of PCOS [four overweight and 17 obese; age, 10 to 17 years; body mass index (BMI), 32.7 ± 1.0 kg/m2 (mean ± standard error)], recruited from the PCOS Center at Children’s Hospital of Pittsburgh, were used in the present analysis. Subjects were pair-matched for age and race and were compared with 21 girls without PCOS (three overweight and 18 obese; age, 10 to 17 years; BMI, 33.3 ± 1.2 kg/m2) who participated in our National Institutes of Health–funded K24 grant investigating insulin resistance in childhood. All participants, recruited from March 1997 through September 2008, underwent uniform metabolic tests with identical laboratory assays throughout the studies. Data from some subjects, unrelated to metabolic flexibility and lipolysis, have been published (14–18). Eligible patients with PCOS and their families were informed about the study while being evaluated in the PCOS center and were given the opportunity to participate after the diagnosis was established and before pharmacologic therapy was initiated. Additionally, flyers were posted on the medical campus, in pediatricians’ offices, and on city bus routes inviting interested individuals to contact us to learn about the study and assess eligibility. Consistent with the Endocrine Society Clinical Practice Guidelines (1) and the Pediatric Endocrine Society Guidelines (19), caution was practiced in making a diagnosis of PCOS based on the presence of clinical signs and symptoms of hyperandrogenism (i.e., menstrual irregularities with or without acne and/or hirsutism) and with biochemical hyperandrogenemia (i.e., elevated total and/or free testosterone concentrations) after excluding other causes of hyperandrogenemia. Inclusion criteria were (1) PCOS diagnosis as described previously, (2) age 10 to 20 years and postmenarche, and (3) BMI ≥85th percentile for age and sex. Girls who were previously diagnosed with systemic or psychiatric disease and who were taking medications that affect carbohydrate or lipid metabolism (oral contraceptive pills, metformin, antiepileptics, antipsychotics, statins, and fish oil) were excluded. The study was approved by the Institutional Review Board of the University of Pittsburgh, and written informed parental consent and child assent were obtained from all participants before participation in the study in accordance with the ethical guidelines of Children’s Hospital of Pittsburgh.
Procedures
All procedures were performed at the Pediatric Clinical and Translational Research Center of Children’s Hospital of Pittsburgh. All participants underwent medical history, physical examination, and hematologic and biochemical tests. Height and weight were assessed to the nearest 0.1 cm and 0.1 kg, respectively, and these data used to calculate BMI. Pubertal development was assessed using Tanner criteria (20). Fasting blood samples were collected for determination of sex steroid hormonal profile [total and free testosterone, sex-hormone binding globulin (SHBG), estradiol, and dehydroepiandrosterone sulfate (DHEAS)], lipid profile, glucose, insulin, free fatty acid (FFA), and adiponectin.
Body composition was evaluated with dual-energy X-ray absorptiometry with measurement of total body fat mass, fat-free mass, and percent body fat. Abdominal visceral adipose tissue (VAT) was assessed by computed tomography at the L4-5 intervertebral space (21).
Metabolic studies
All participants were admitted to the Pediatric Clinical and Translational Research Center and underwent a hyperinsulinemic-euglycemic clamp combined with stable isotope tracers and continuous indirect calorimetry after a 10- to 12-hour overnight fast (15, 17, 18). Seven girls with PCOS and eight girls without PCOS were considered to have prediabetes based on hemoglobin A1c (5.7% to 6.4%) and/or fasting glucose concentration (100 to 125 mg/dL) measured before the clamp experiment.
Fasting hepatic glucose production was measured before the start of the hyperinsulinemic-euglycemic clamp with a primed (2.2 µmol/kg) constant-rate infusion of [6,6-2H2]glucose (Isotech, Miamisburg, OH) for 2 hours as described (15, 18). Whole-body lipolysis was measured at baseline and during the hyperinsulinemic-euglycemic clamp with a primed (1.2 µmol/kg) constant-rate infusion of [2H5]glycerol (MSD Isotopes, St. Louis, MO), which was started 2 hours before the clamp (22, 23). The pyrogen-free isotopes were dissolved in 0.9% sodium chloride and sterilized by passing through a 0.22-µm filter (Millipore, Bedford, MA). After the 2-hour baseline isotope infusion period, in vivo insulin sensitivity (IS) was evaluated during a 3-hour hyperinsulinemic (80 mU/m2/min)-euglycemic clamp (15, 17, 18). Plasma glucose was clamped at approximately 100 mg/dL with a variable-rate infusion of 20% dextrose in water. The glucose infusion was adjusted based on arterialized plasma glucose measurements every 5 minutes, and blood was sampled every 10 to 15 minutes to determine insulin and FFA concentrations and plasma isotopic enrichment. Continuous indirect calorimetry (Deltatrac Metabolic Monitor; Sensormedics, Anaheim, CA) was used to measure CO2 production, O2 consumption, and RQ for 30 minutes at baseline and at the end of the 3-hour hyperinsulinemic-euglycemic clamp.
Biochemical measurements
Total testosterone was measured by high-pressure liquid chromatography-tandem mass spectroscopy, and DHEAS was measured by radioimmunoassay in dilute serum after hydrolysis (Esoterix Inc., Calabasas Hills, CA). Free testosterone was measured by equilibrium dialysis, and SHBG was measured by immunoradiometric assay. Plasma glucose was determined by the glucose oxidase method using a glucose analyzer (Yellow Springs Instrument Co., Yellow Springs, OH), and insulin was determined by a commercially available human-specific insulin radioimmunoassay kit (HI-14K; Linco/Millipore, St. Charles, MO). FFA concentration was determined using enzymatic colorimetric methods with a nonesterified fatty acid test kit (HR series; Wako, Osaka, Japan). Plasma lipid concentrations were determined using the standards of the Centers for Disease Control and Prevention (24). Adiponectin was measured using a commercially available radioimmunoassay kit (LINCO Research Inc., St. Charles, MO). HbA1c was measured by high-performance liquid chromatography (Tosoh Medics, Inc., San Francisco, CA).
Deuterium enrichment of glycerol in the plasma was determined according to previously described methods (22, 25). Plasma samples were deproteinized with barium hydroxide and zinc sulfate. The supernatant was purified through a column of mixed-bed anion and cation ion-exchange chromatography. Acetate derivatives of glycerol were prepared by adding pyridine and acetic anhydride to the dried eluted samples. Derivatized samples were analyzed for 2H enrichment on a gas chromatograph-mass spectrometer system (5985A; Hewlett Packard, Palo Alto, CA) as reported (22, 25). Selected ion monitoring software was used to monitor charge-to-mass ratio (m/z) (145) m and m/z 148 (m + 3), representing unlabeled and 2H-labeled glycerol, respectively. Standard curves of known enrichments of glycerol were performed with each assay.
Deuterium enrichment of glucose in the plasma was determined on a gas chromatograph-mass spectrometry system (5985A; Hewlett-Packard) as described previously (26). Plasma samples were deproteinized with 2-propanol, and the pentaacetate derivative of glucose was analyzed for 2H enrichment on the gas chromatograph-mass spectrometer in the electron impact mode. Selected ion monitoring of the m-190 ion (mass-to-charge ratio, 200) and the corresponding m + 2 ion (mass-to-charge ratio, 202), reflecting unlabeled and labeled glucose, was performed. Standard curves of known enrichments of glucose were performed with each assay.
Calculations
The endogenous glycerol appearance rate in plasma, indicative of whole-body lipolysis, was calculated during the last 30 minutes of the postabsorptive (basal) period and during steady-state hyperinsulinemia according to steady-state tracer dilution equations (22). A steady-state plateau of glycerol isotopic enrichment was achieved in the subjects before the start of the clamp experiment and during the last 30 minutes of the hyperinsulinemic state. Fasting endogenous glucose production was calculated during the last 30 minutes of the 2-hour isotope infusion (−30 to 0 minutes) according to steady-state tracer dilution equations (17). Insulin-stimulated glucose disposal was calculated to be equal to the rate of exogenous glucose infusion during the final 30 minutes of the hyperinsulinemic-euglycemic clamp. Peripheral IS was calculated by dividing the insulin-stimulated glucose disposal by the steady-state clamp insulin concentration multiplied by 100 (17). Insulin-stimulated carbohydrate and lipid oxidation rates were calculated according to the formulas of Frayn (27) from the indirect calorimetric data by averaging the data for 30 minutes before the beginning of the insulin infusion and for the last 30 minutes of the clamp. Metabolic flexibility was calculated as the change in RQ from fasting to the hyperinsulinemic clamp steady-state (i.e., ΔRQ).
Statistical analyses
Paired t test and χ2 test were used to compare characteristics between age- and race-matched obese girls with PCOS vs obese girls without PCOS. Spearman’s correlation analysis was used to examine bivariate relationships between metabolic flexibility (ΔRQ) and physical, hormonal, and metabolic characteristics. Multiple regression analyses were performed to estimate predictors of metabolic flexibility. Two different models were developed (clinic-based and research-based), and independent variables were selected based on the results of correlation analyses and biological plausibility. The clinic-based model included easily accessible fasting blood parameters [adiponectin, triglyceride (TG), and fasting insulin], and the research-based model consisted of parameters derived from computed tomography (VAT) and hyperinsulinemic-euglycemic clamp combined with indirect calorimetry (peripheral IS and baseline RQ). Data that did not meet the assumptions for normality were log10 transformed; untransformed data are presented for ease of interpretation. Data were analyzed using the PASW 24.0 statistical software package with significance set at P ≤ 0.05.
Results
Physical, hormonal, and metabolic characteristics of age- and race-matched obese girls with PCOS vs obese girls without PCOS
Obese girls with PCOS had similar Tanner stage, BMI, total fat mass, and percent body fat but higher VAT compared with obese girls without PCOS (Table 1). They also had higher total and free testosterone levels, higher DHEAS, lower SHBG, higher TG, higher TG/high-density lipoprotein ratio and very low-density lipoprotein, lower peripheral IS, and lower adiponectin (Table 1). HbA1c, fasting hepatic glucose production, fasting glucose, and FFA concentrations were not different, but there was a tendency for 48% higher fasting insulin concentration in girls with PCOS (Table 1).
Table 1.
Physical, Hormonal, and Metabolic Characteristics of Pair-Matched (for Age and Race) Obese Adolescent Girls With PCOS vs Without PCOS
| Variables | PCOS (n = 21) | Non-PCOS (n = 21) | P Value |
|---|---|---|---|
| Physical characteristics | |||
| Age, y | 13.6 ± 2.2 (0.5) | 13.3 ± 2.2 (0.5) | — |
| Race (AA/AW), n (%) | 9 (43)/12 (57) | 9 (43)/12 (57) | — |
| Tanner stage (III/IV/V), n (%) | 5 (24)/5 (24)/11 (52) | 2 (10)/3 (14)/16 (76) | 0.258 |
| BMI, kg/m2 | 32.7 ± 4.5 (1.0) | 33.3 ± 5.4 (1.2) | 0.593 |
| Total fat mass, kg | 36.5 ± 9.3 (2.1) | 37.1 ± 10.3 (2.3) | 0.770 |
| Percent body fat, % | 43.2 ± 4.8 (1.1) | 45.0 ± 5.3 (1.2) | 0.225 |
| Visceral adipose tissue, cm2 | 69.2 ± 29.2 (6.7) | 49.2 ± 22.1 (5.1) | 0.011 |
| Sex steroid profile | |||
| Total testosterone, ng/dL | 35.9 ± 17.0 (4.2) | 24.7 ± 11.4 (2.9) | 0.042 |
| SHBG, nmol/L | 17.5 ± 9.7 (2.5) | 31.3 ± 19.1 (4.9) | 0.026 |
| Free testosterone, pg/mL | 9.8 ± 7.1 (1.9) | 4.4 ± 2.8 (0.7) | 0.003 |
| Estradiol, pg/mL | 63.8 ± 56.9 (13.4) | 76.2 ± 57.5 (13.5) | 0.530 |
| DHEAS, μg/dL | 186.8 ± 126.8 (27.7) | 118.1 ± 81.4 (17.8) | 0.007 |
| Lipid profile | |||
| Total cholesterol, mg/dL | 161.5 ± 38.5 (8.4) | 154.0 ± 24.6 (5.4) | 0.439 |
| TG, mg/dL | 132.5 ± 67.2 (14.7) | 89.5 ± 32.7 (7.1) | 0.008 |
| HDL, mg/dL | 42.2 ± 10.8 (2.4) | 42.8 ± 6.5 (2.4) | 0.840 |
| TG/HDL ratio | 3.6 ± 2.6 (0.6) | 2.2 ± 1.0 (0.2) | 0.023 |
| LDL, mg/dL | 92.8 ± 35.4 (7.7) | 93.6 ± 24.2 (5.3) | 0.924 |
| VLDL, mg/dL | 25.5 ± 13.4 (3.1) | 16.9 ± 5.4 (1.2) | 0.013 |
| Metabolic characteristics | |||
| Hemoglobin A1c | 5.4 ± 0.4 (0.1) | 5.2 ± 0.4 (0.1) | 0.183 |
| Fasting glucose, mg/dL | 95.1 ± 7.3 (1.6) | 96.9 ± 6.0 (1.3) | 0.478 |
| Fasting insulin, µU/mL | 53.8 ± 35.6 (7.8) | 36.3 ± 17.1 (3.7) | 0.065 |
| Fasting FFA, mmol/L | 0.35 ± 0.12 (0.03) | 0.33 ± 0.13 (0.03) | 0.730 |
| Fasting HGP, mg/kg/min | 2.4 ± 0.8 (0.2) | 2.3 ± 0.5 (0.1) | 0.716 |
| Peripheral IS, (mg/kg/min per µU/mL) × 100 | 1.8 ± 1.0 (0.2) | 2.5 ± 1.2 (0.3) | 0.032 |
| Adiponectin, µg/mL | 6.1 ± 3.1 (0.8) | 9.9 ± 4.4 (1.1) | 0.015 |
Values are mean ± standard deviation (standard error of the mean) or n (%), unless otherwise indicated.
Abbreviations: AA, African American; AW, American white; HDL, high-density lipoprotein; HGP, hepatic glucose production; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
Metabolic flexibility, whole-body lipolysis, and substrate metabolism
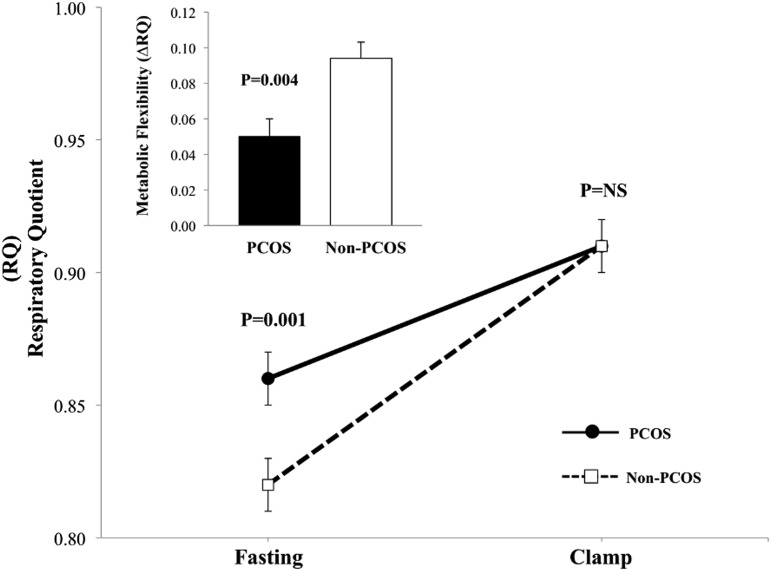
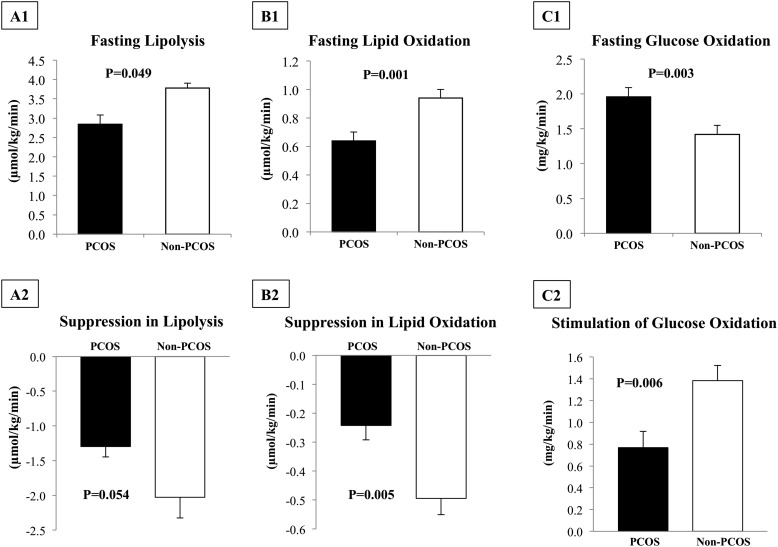
Baseline RQ was significantly higher in girls with PCOS vs girls without PCOS, and clamp RQ was not different (Fig. 1). This resulted in ∼47% lower ΔRQ during hyperinsulinemia in girls with PCOS vs girls without PCOS (0.050 ± 0.010 vs 0.095 ± 0.009, P = 0.004), which is indicative of metabolic inflexibility in girls with PCOS (Fig. 1). Fasting lipolysis and lipid oxidation were lower and glucose oxidation was higher in girls with PCOS vs girls without PCOS (Fig. 2A1–2C1). Despite higher clamp steady-state insulin concentration (PCOS: 341.1 ± 21.9 vs non-PCOS: 281.3 ± 15.5 µU/mL, P = 0.008), girls with PCOS had lower suppression in lipolysis and lipid oxidation, impaired stimulation of glucose oxidation (Fig. 2A2–2C2), and higher FFA concentrations compared with girls without PCOS (0.13 ± 0.02 vs 0.06 ± 0.01 mmol/L, P = 0.001).
Figure 1.
RQ at fasting and during the hyperinsulinemic-euglycemic clamp. Insert depicts metabolic flexibility as ΔRQ from fasting to clamp steady-state hyperinsulinemia. Data are presented as mean ± standard error of the mean.
Figure 2.
(A1) Fasting whole-body lipolysis, (B1) lipid oxidation, and (C1) glucose oxidation in girls with PCOS vs girls without PCOS. Suppression in (A2) lipolysis and in (B2) lipid oxidation and (C2) stimulation of glucose oxidation during clamp steady-state hyperinsulinemia. Data are presented as mean ± standard error of the mean.
Correlation of metabolic flexibility with physical, hormonal, and metabolic characteristics
Metabolic flexibility (i.e., ΔRQ) was positively associated with peripheral IS, biomarkers of IS such as SHBG and adiponectin, and suppression in whole-body lipolysis. On the other hand, fasting insulin concentration, baseline RQ, VAT, total cholesterol, and TG were inversely associated with ΔRQ (Table 2), which indicates an adverse relationship of these parameters with metabolic flexibility (i.e., associated with metabolic inflexibility). There was no correlation of metabolic flexibility with age, adiposity (BMI or fat mass or % body fat), total and free testosterone, and fasting glucose concentration.
Table 2.
Associations Between Metabolic Flexibility (ΔRQ) and Physical, Hormonal, and Metabolic Characteristics
| ΔRQ vs Variables | r | P Value |
|---|---|---|
| Significant associations | ||
| Adiponectin | 0.652 | <0.001 |
| Peripheral IS | 0.435 | 0.002 |
| Suppression in lipolysis | 0.368 | 0.016 |
| SHBG | 0.336 | 0.045 |
| Baseline RQ | −0.697 | <0.0001 |
| VAT | −0.521 | 0.001 |
| Total cholesterol | −0.401 | 0.009 |
| TG | −0.334 | 0.031 |
| TG/HDL ratio | −0.306 | 0.049 |
| Fasting insulin | −0.468 | 0.002 |
| Nonsignificant associations | ||
| Age | 0.039 | 0.806 |
| BMI | −0.065 | 0.684 |
| Total fat mass | −0.052 | 0.747 |
| Percent body fat | 0.094 | 0.559 |
| Total testosterone | 0.112 | 0.508 |
| Free testosterone | −0.086 | 0.623 |
| Fasting glucose | 0.112 | 0.482 |
Abbreviation: HDL, high-density lipoprotein.
In multiple regression analyses, we constructed a “clinical” model and a “research” model. The rationale for the two different models was to assess if using data that can be easily obtained in the clinical setting, such as routine blood tests, vs more sophisticated research measures, such as clamp or indirect calorimetry, can be used to predict metabolic flexibility. In the clinical model, fasting insulin, TG, and adiponectin explained 62% of the variance in metabolic flexibility, with each contributing independently and significantly (Table 3). The addition of SHBG, any of the sex hormones, or total or free testosterone to the model did not improve the R2 value. In the research model, in vivo peripheral IS, VAT, and baseline RQ explained 76% of the variance in metabolic flexibility, with each contributing independently and significantly (Table 3). The addition of change in FFA or glycerol turnover during the clamp to the model did not improve the R2 value.
Table 3.
Predictors of Metabolic Flexibility by Multiple Regression
| Models | Model R2 | R2 Change | Significance of Change | Partial R2 | Significance of Partial R2 |
|---|---|---|---|---|---|
| Model based on clinical parameters | |||||
| Adiponectin | 0.412 | 0.412 | <0.0001 | 0.135 | 0.027 |
| Triglyceride | 0.476 | 0.064 | 0.046 | 0.175 | 0.011 |
| Fasting insulin | 0.618 | 0.142 | 0.001 | 0.271 | 0.001 |
| Model based on research parameters | |||||
| Insulin sensitivity | 0.278 | 0.278 | <0.0001 | 0.204 | 0.004 |
| Visceral adipose tissue | 0.367 | 0.089 | 0.029 | 0.083 | 0.080 |
| Baseline RQ | 0.756 | 0.390 | <0.0001 | 0.615 | <0.0001 |
Clinic-based model: R2 = 0.618, P < 0.0001; research-based model: R2 = 0.756, P < 0.0001.
Discussion
The present investigation reveals that obese adolescent girls with PCOS compared with their equally obese peers without PCOS, pair-matched for age and race, have (1) lower fat mobilization and lower fat oxidation in the fasting state; (2) impaired suppression in whole-body lipolysis and lipid oxidation during hyperinsulinemia; (3) metabolic inflexibility manifested by a blunted increase in RQ in the insulin-stimulated state; (4) lower peripheral IS, as shown previously, together with diminished stimulation of glucose oxidation; (5) larger abdominal VAT despite similar BMI, total body fat, and percent body fat; and (6) lower adiponectin concentration.
PCOS is characterized not only by hormonal and reproductive disorders but also by a cluster of metabolic abnormalities driven by insulin resistance and hyperinsulinemia (28). Because a significant proportion of women and adolescent girls with PCOS are obese (2, 4), there is growing interest to assess adipose tissue metabolism and the capacity to switch fuel oxidation to adjust for fuel availability (i.e., metabolic flexibility) to probe the mechanisms responsible for fat accretion in PCOS. To date, however, the data are inconsistent and limited and are available only from adult women with PCOS. One study observed significantly altered metabolic flexibility with lower ΔRQ in hyperandrogenemic women with PCOS with varying BMI ranging from normal-weight to obese compared with normoandrogenemic PCOS but with significantly lower BMI and healthy normal-weight women (12). Another study compared lean and obese women with PCOS with lean and obese control subjects and reported that metabolic flexibility did not differ between women with PCOS and women in the control group, but impairment of metabolic flexibility was observed in obese vs lean control subjects (29). The contrast between these two studies could stem from differences in study design, such as the inclusion of subjects with a wide range of BMI [32.9 ± 8.0 kg/m2 (hyperandrogenemic) vs 24.7 ± 4.4 kg/m2 (normoandrogenemic)] in the former study and lean PCOS with mean BMI of 21.4 ± 2.0 kg/m2 vs obese PCOS 31.2 ± 4.1 kg/m2, controls of similar adiposity, different comparison groups, hyperandrogenemic vs normoandrogenemic PCOS, and varying sample sizes in the latter study. In the midst of these very limited and contradictory studies in adults, we investigated adipose tissue metabolism in vivo and metabolic flexibility in obese adolescent girls with PCOS and its relationship with physical, hormonal, and metabolic characteristics.
Our study demonstrates that obese girls with PCOS have 25% lower whole-body lipolysis or fat mobilization with 32% lower fat oxidation in the fasting state and ∼47% blunted ΔRQ, signifying metabolic inflexibility during hyperinsulinemia compared with their peers without PCOS. Given that adipose tissue is a metabolically active tissue in buffering daily flux of fatty acids in the postprandial period (9, 30) and given that lipids are the primary substrate oxidized by skeletal muscle in the fasting state (31), it could be postulated that decreased lipid mobilization and diminished fat oxidation in obese girls with PCOS shift lipid metabolism to lipogenesis and could contribute to metabolic inflexibility and energy imbalance conducive to fat accretion and weight gain (8). The observed lower rates of whole-body lipolysis in the fasting state in girls with PCOS vs girls without PCOS could be driven by their higher fasting insulinemia (48%), by previously reported adipose tissue catecholamine lipolytic resistance in women with PCOS (13), or both. Moreover, an adipose tissue–driven mechanistic link between androgen excess and lipid metabolism in PCOS was proposed by a recent publication (32). The authors demonstrated a functional impact of chronic and acute androgen exposure on the regulation of adipose tissue lipid metabolism, evident in suppressed lipolysis in vivo and increased lipogenesis in vitro, with the net effect of promotion of adipocyte hypertrophy.
Such observations that excess androgens, a key feature of PCOS, could influence adipocyte function and distribution either by the inhibition of adipocyte differentiation or by direct modulation of lipolysis and lipogenesis (32, 33), combined with the inherent insulin resistance in glucose metabolism in girls with PCOS (18), led us to postulate that obese adolescent girls with PCOS would have impaired suppression of whole-body lipolysis and that this would be related to metabolic inflexibility. Our data show that obese girls with PCOS have ∼36% lower suppression in lipolysis, despite ∼22% higher insulin concentrations during the clamp, compared with girls without PCOS. This impaired suppression in lipolysis is in line with the twofold higher FFA concentration during hyperinsulinemia in girls with PCOS vs girls without PCOS. Our observation of a significant and positive relationship between ΔRQ and suppression in whole-body lipolysis suggests that adipose tissue dysfunction is closely linked to metabolic inflexibility.
In addition to the adipose tissue dysfunction in girls with PCOS, our data suggest that altered secretion of adipokine and abdominal VAT could be linked to metabolic inflexibility. In the current study, metabolic inflexibility correlated significantly with adiponectin concentration, which was ∼40% lower in girls with PCOS. Because adiponectin is shown to stimulate lipid oxidation via activation of adenosine 5′-monophosphate–activated protein kinase (34), it is possible that the observed lower lipid oxidation (∼32%) at baseline in girls with PCOS is related to their lower adiponectin levels. Further, our data show that metabolic inflexibility correlates directly with enlarged VAT. Obese girls with PCOS had significantly larger abdominal VAT than girls without PCOS despite similar BMI, total body fat, and percent body fat. It is possible that the larger visceral adipose compartment might contribute to their metabolic inflexibility or that their metabolic inflexibility contributes to their visceral fat accretion.
Based on the bivariate relationship of metabolic flexibility with physical, hormonal, and metabolic characteristics, we explored two different regression models to assess the predictors of metabolic flexibility. One model used data that can be easily obtained in the clinical setting, and the other used sophisticated data obtained in a research setting. In the clinical model, fasting insulin, TG, and adiponectin explained 62% of the variance in metabolic flexibility. In the research model, in vivo peripheral IS, VAT, and baseline RQ explained 76% of the variance in metabolic flexibility. It is not surprising that the more precise research measurements obtained under carefully controlled experimental conditions would explain a larger proportion of metabolic flexibility. In our study, in contrast to the previous study in adult PCOS (12), ΔRQ was not correlated with free testosterone, and although SHBG showed a bivariate relationship to ΔRQ, it was not an independent and significant contributor in the multiple regression models. This is most likely because SHBG is a biomarker of IS/hyperinsulinemia; once either IS or fasting insulin is entered into the model, SHBG loses its significance due to multicollinearity (35).
The strengths of the present investigation include (1) an evaluation of adipose tissue metabolism and metabolic flexibility in obese girls with PCOS vs obese girls without PCOS; (2) the comprehensive assessment from physical, to hormonal, to state-of-the-art metabolic tests including hyperinsulinemic-euglycemic clamp combined with stable isotope tracers and indirect calorimetry; and (3) a pair-matched analysis with respect to age and race and no differences in Tanner stage. Potential limitations would be the continued uncertainty about the diagnosis of PCOS in adolescent girls and the absence of data on gynecological age. However, our diagnostic criteria were based on the Endocrine Society and the Pediatric Endocrine Society Guidelines (1, 19) to avoid under- or over-diagnosis of PCOS. In addition, we did not study lean youth with PCOS. It would be of scientific interest to determine if adipose tissue dysfunction and metabolic inflexibility is also a feature of lean girls with PCOS or if it is modulated by obesity. Unfortunately, few nonobese girls have been diagnosed with PCOS. Another possible weakness could be that, to minimize participant burden, we did not use a multistep hyperinsulinemic clamp to measure suppression of whole-body lipolysis in response to a lower insulin infusion rate to achieve submaximal suppression of lipolysis (36). However, a recent study showed a strong correlation between one-step hyperinsulinemic-euglycemic clamp vs a multistep clamp (37). Given the current experimental design and the data at hand, one cannot empirically assess for possible ceiling or floor effects with respect to the observed changes in RQ, lipolysis, and lipid and glucose oxidation from fasting to insulin-stimulated state. The cross-sectional nature of our evaluation does not allow us to examine causal relationships. It remains to be determined if adipose tissue dysfunction and metabolic inflexibility are inherent in girls with PCOS, predisposing them to obesity—particularly abdominal visceral obesity—or if abdominal visceral adiposity together with hypoadiponectinemia and insulin resistance/hyperinsulinemia result in metabolic inflexibility and adipose dysfunction.
In summary, the present investigation reveals that obese girls with PCOS are characterized by decreased mobilization of fat from adipose stores and diminished capacity to burn fat in the fasting state. During hyperinsulinemia they manifest metabolic inflexibility with an impaired ability to switch from lipid to carbohydrate oxidation to meet fuel availability. It remains to be determined if this metabolic phenotype plays a role in fat accretion and weight regulation in girls with PCOSs.
Acknowledgments
The authors thank the children who participated in this study and their parents; Nancy Guerra, CRNP, for assistance; Resa Stauffer for laboratory expertise; and the nursing staff of the Pediatric Clinical and Translational Research Center for outstanding care of the participants and meticulous attention to the research.
Financial Support: This study was supported by National Institute of Child Health and Human Development Grants K24-HD01357 and R01-HD27503 (to S.A.), National Center for Advancing Translational Sciences Clinical and Translational Science Award UL1-TR000005, and National Center for Research Resources Grant UL1-RR024153 to the General Clinical Research Center.
Author Contributions: J.Y.K. and S.A. designed the study, analyzed the data, and wrote the manuscript. H.T. contributed data and reviewed the manuscript, S.F.M. collected and maintained the database and reviewed the manuscript. All authors approved the manuscript in its final version. S.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CT
- computed tomography
- DHEAS
- dehydroepiandrosterone sulfate
- FFA
- free fatty acid
- IS
- insulin sensitivity
- PCOS
- polycystic ovary syndrome
- RQ
- respiratory quotient
- SHBG
- sex-hormone binding globulin
- TG
- triglyceride
- VAT
- visceral adipose tissue.
References
- 1.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK, Endocrine S; Endocrine Society . Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749. [DOI] [PubMed] [Google Scholar]
- 3.Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14(2):95–109. [DOI] [PubMed] [Google Scholar]
- 4.Christensen SB, Black MH, Smith N, Martinez MM, Jacobsen SJ, Porter AH, Koebnick C. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril. 2013;100(2):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49(5):677–683. [DOI] [PubMed] [Google Scholar]
- 6.Færch K, Vaag A. Metabolic inflexibility is a common feature of impaired fasting glycaemia and impaired glucose tolerance. Acta Diabetol. 2011;48(4):349–353. [DOI] [PubMed] [Google Scholar]
- 7.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94(6):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, Ravussin E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259(5 Pt 1):E650–E657. [DOI] [PubMed] [Google Scholar]
- 9.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45(9):1201–1210. [DOI] [PubMed] [Google Scholar]
- 10.Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren-Ulanch J, Arslanian S. Treatment of PCOS in adolescence. Best Pract Res Clin Endocrinol Metab. 2006;20(2):311–330. [DOI] [PubMed] [Google Scholar]
- 12.Di Sarra D, Tosi F, Bonin C, Fiers T, Kaufman JM, Signori C, Zambotti F, Dall’Alda M, Caruso B, Zanolin ME, Bonora E, Moghetti P. Metabolic inflexibility is a feature of women with polycystic ovary syndrome and is associated with both insulin resistance and hyperandrogenism. J Clin Endocrinol Metab. 2013;98(6):2581–2588. [DOI] [PubMed] [Google Scholar]
- 13.Ek I, Arner P, Bergqvist A, Carlström K, Wahrenberg H. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab. 1997;82(4):1147–1153. [DOI] [PubMed] [Google Scholar]
- 14.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87(4):1555–1559. [DOI] [PubMed] [Google Scholar]
- 15.Tfayli H, Ulnach JW, Lee S, Sutton-Tyrrell K, Arslanian S. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96(5):1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JY, Tfayli H, Michaliszyn SF, Lee S, Nasr A, Arslanian S. Anti-mullerian hormone in obese adolescent girls with polycystic ovary syndrome. J Adolesc Health. 2017;60(3):333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab. 2001;86(1):66–71. [DOI] [PubMed] [Google Scholar]
- 18.Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138(1):38–44. [DOI] [PubMed] [Google Scholar]
- 19.Witchel SF, Oberfield S, Rosenfield RL, Codner E, Bonny A, Ibáñez L, Pena A, Horikawa R, Gomez-Lobo V, Joel D, Tfayli H, Arslanian S, Dabadghao P, Garcia Rudaz C, Lee PA. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr. 2015;83(6):376–389. [DOI] [PubMed] [Google Scholar]
- 20.Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39(2):43–55. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Kuk JL, Hannon TS, Arslanian SA. Race and gender differences in the relationships between anthropometrics and abdominal fat in youth. Obesity (Silver Spring). 2008;16(5):1066–1071. [DOI] [PubMed] [Google Scholar]
- 22.Arslanian SA, Kalhan SC. Correlations between fatty acid and glucose metabolism. Potential explanation of insulin resistance of puberty. Diabetes. 1994;43(7):908–914. [DOI] [PubMed] [Google Scholar]
- 23.Danadian K, Lewy V, Janosky JJ, Arslanian S. Lipolysis in African-American children: is it a metabolic risk factor predisposing to obesity? J Clin Endocrinol Metab. 2001;86(7):3022–3026. [DOI] [PubMed] [Google Scholar]
- 24.Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care. 2009;32(1):100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel D, Kalhan S. Glycerol metabolism and triglyceride-fatty acid cycling in the human newborn: effect of maternal diabetes and intrauterine growth retardation. Pediatr Res. 1992;31(1):52–58. [DOI] [PubMed] [Google Scholar]
- 26.Arslanian S, Suprasongsin C. Glucose-fatty acid interactions in prepubertal and pubertal children: effects of lipid infusion. Am J Physiol. 1997;272(4 Pt 1):E523–E529. [DOI] [PubMed] [Google Scholar]
- 27.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55(2):628–634. [DOI] [PubMed] [Google Scholar]
- 28.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adamska A, Karczewska-Kupczewska M, Nikołajuk A, Otziomek E, Górska M, Kowalska I, Strączkowski M. Normal metabolic flexibility despite insulin resistance women with polycystic ovary syndrome. Endocr J. 2013;60(9):1107–1113. [DOI] [PubMed] [Google Scholar]
- 30.Romacho T, Elsen M, Röhrborn D, Eckel J. Adipose tissue and its role in organ crosstalk. Acta Physiol (Oxf). 2014;210(4):733–753. [DOI] [PubMed] [Google Scholar]
- 31.Dagenais GR, Tancredi RG, Zierler KL. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J Clin Invest. 1976;58(2):421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, Semple RK, Hebenstreit D, Dunn WB, Tomlinson JW, Arlt W. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(9):3327–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dicker A, Rydén M, Näslund E, Muehlen IE, Wirén M, Lafontan M, Arner P. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia. 2004;47(3):420–428. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–1295. [DOI] [PubMed] [Google Scholar]
- 35.Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, Clore JN, Blackard WG. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72(1):83–89. [DOI] [PubMed] [Google Scholar]
- 36.Jensen MD, Nielsen S. Insulin dose response analysis of free fatty acid kinetics. Metabolism. 2007;56(1):68–76. [DOI] [PubMed] [Google Scholar]
- 37.Søndergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD. How to Measure Adipose Tissue Insulin Sensitivity. J Clin Endocrinol Metab. 2017;102(4):1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]