Abstract
Context:
Unexplained infertility (UI), defined as the inability to conceive after 12 months of unprotected intercourse with no diagnosed cause, affects 10% to 30% of infertile couples. An improved understanding of the mechanisms underlying UI could lead to less invasive and less costly treatment strategies. Abnormalities in thyroid function and hyperprolactinemia are well-known causes of infertility, but whether thyrotropin (TSH) and prolactin levels within the normal range are associated with UI is unknown.
Objective:
To compare TSH and prolactin levels in women with UI and women with a normal fertility evaluation except for an azoospermic or severely oligospermic male partner.
Design, Setting, and Participants:
Cross-sectional study including women evaluated at a large academic health system between 1 January 2000 and 31 December 2012 with normal TSH (levels within the normal range of the assay and ≤5 mIU/L) and normal prolactin levels (≤20 ng/mL) and either UI (n = 187) or no other cause of infertility other than an azoospermic or severely oligospermic partner (n = 52).
Main Outcome Measures:
TSH and prolactin.
Results:
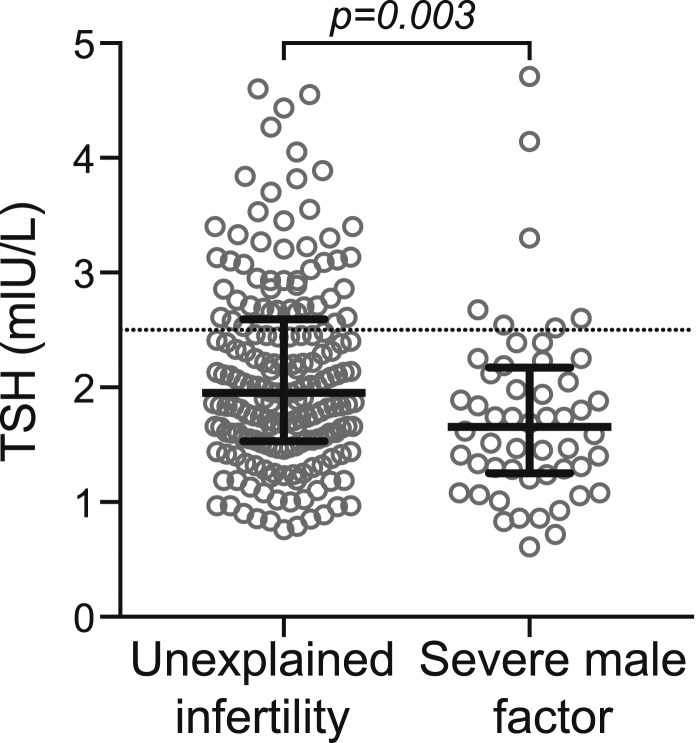
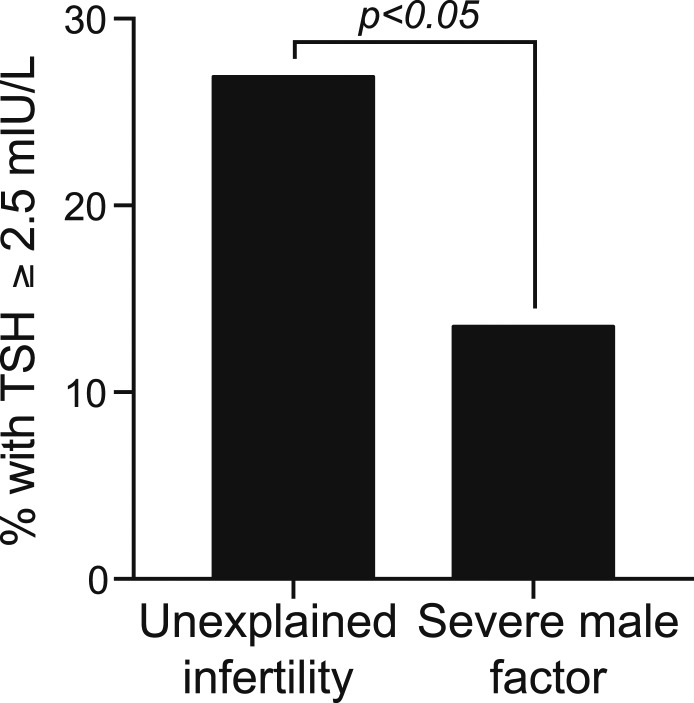
Women with UI had significantly higher TSH levels than controls [UI: TSH 1.95 mIU/L, interquartile range: (1.54, 2.61); severe male factor: TSH 1.66 mIU/L, interquartile range: (1.25, 2.17); P = 0.003]. This finding remained significant after we controlled for age, body mass index, and smoking status. Nearly twice as many women with UI (26.9%) had a TSH ≥2.5 mIU/L compared with controls (13.5%; P < 0.05). Prolactin levels did not differ between the groups.
Conclusions:
Women with UI have higher TSH levels compared with a control population. More studies are necessary to determine whether treatment of high-normal TSH levels decreases time to conception in couples with UI.
In women with no known thyroid disease and TSH levels within the normal range, we demonstrate an association between TSH levels >2.5 mIU/L and the diagnosis of UI.
Infertility, defined as the inability to conceive after 12 months of regular unprotected intercourse (1), affects ~7% to 15.5% of reproductive-aged women in the United States (2). Although a cause for infertility is identified in the majority of couples, ~10% to 30% have unexplained or idiopathic infertility, which is defined as infertility in the setting of regular ovulation, tubal patency, a normal uterine cavity, and normal semen analysis (3–6). Unexplained infertility (UI) is associated with high emotional and economic costs; annual US expenditures for all forms of infertility total $3 to $4 billion/y (7), and couples with UI have higher conception rates with in vitro fertilization (IVF), one of the most expensive forms of treatment (8). Therefore, gaining a greater understanding of potential hormonal factors that may contribute to UI may lead to more economical and effective treatment strategies for these couples.
Known causes of infertility include hyperprolactinemia and thyroid dysfunction (9, 10). Hyperprolactinemia is a common cause of amenorrhea and infertility, due to impaired gonadotropin secretion and pulsatility, likely caused by impaired gonadotropin-releasing hormone secretion (11, 12). Treatment with dopamine agonist therapy has been shown to restore ovulation and fertility in women with hyperprolactinemia (13–16). Importantly, even hyperprolactinemic women with regular menstrual cycles may experience decreased fertility because of a shortened luteal phase (17–19), but whether high-normal levels of prolactin in women with no known history of hyperprolactinemia may affect fertility remains unknown.
Murine and in vitro studies suggest that thyrotropin (TSH) and thyroid hormone are important factors during oocyte development and implantation (20–23). In humans, an in vivo model also suggests the importance of TSH for oocyte development, as TSH levels ≥2.5 mIU/L in oocyte donors inversely predict clinical pregnancy independent of the recipient’s TSH level (24). Both hyperthyroidism (25) and hypothyroidism are associated with menstrual irregularity (26), and rates of infertility have been reported to approach 50% in women with Hashimoto thyroiditis and Graves disease (27). A higher percentage of women with infertility have also been shown to have frankly abnormal TSH levels as compared with controls (28), and the recent American Thyroid Association Thyroid and Pregnancy Guidelines recommend checking TSH in all women seeking evaluation for infertility (29). However, whether higher TSH levels in a population of women with a normal TSH and no known history of thyroid disease are part of the UI phenotype remains an unanswered question.
There is no consensus on the definition of subclinical hypothyroidism in nonpregnant women who are attempting to conceive. Subclinical hypothyroidism is defined as an elevated TSH level in the setting of normal thyroid hormone levels, but what the upper limit of normal should be for TSH is controversial. Based on the National Health and Nutrition Examination III survey including a sample of 8619 girls and women (≥12 years of age) in the United States without a history of thyroid disease, the median TSH level is 1.50 mIU/L, with an upper 97.5 percentile of 6.10 mIU/L (30), whereas others argue that the upper limit of normal for TSH should be 2.5 mIU/L, based on data from the National Academy of Clinical Biochemistry that indicate that 95% of people without evidence of thyroid autoimmunity and without a personal or family history of thyroid disease have a TSH level ≤2.5 mIU/L (31) and the fact that TSH levels ≥2.5 mIU/L have been associated with adverse obstetrical outcomes including increased risk of pregnancy loss (32). Therefore, there is poor consensus regarding the normal range for nonpregnant women who are attempting to conceive, although current guidelines provide an upper limit of normal for TSH of 4 mIU/L for women in their first trimester of pregnancy, a time when TSH levels should be at their lowest because of human chorionic gonadotropin stimulation of thyroid hormone (29), suggesting that the upper limit of normal for nonpregnant women is >4 mIU/L. Furthermore, current guidelines do not recommend treating women with subclinical hypothyroidism, who are attempting to conceive naturally, with thyroid hormone replacement to improve the likelihood of conception (29, 33). Our goal in performing this study was to understand whether thyroid function, as estimated by TSH, is associated with infertility in women with a completely normal fertility evaluation who do not have a history of thyroid disease or abnormal thyroid function tests. We hypothesized that women with UI would have higher TSH and higher prolactin levels, within the normal range, as compared with a control group of women who had a similarly normal infertility evaluation but whose partners were found to be azoospermic or severely oligospermic.
Methods
Study population
Using the patient database registry at a large academic health system (the Research Patient Data Registry of Partners HealthCare system), we obtained data on female patients between 18 and 39 years of age who presented to the Partners HealthCare system with the diagnosis of infertility and without a disorder of menstruation between 1 January 2000 and 31 December 2012. All electronic records were then individually reviewed to ensure that the women met our inclusion and exclusion criteria. Women were included who did not conceive after ≥1 year with appropriate exposure to sperm (UI group) or who had inadequate exposure to sperm due to a male partner with azoospermia (n = 39) or severe oligospermia (n = 13) with a sperm count <1 million/mL (severe male factor). Inclusion criteria for all women included regular menstrual cycles every 21 to 35 days with ≤5 days of intercycle variability, normal uterine cavity evaluation, menstrual cycle day 3 follicle-stimulating hormone (FSH) ≤10 IU/mL with concomitant estradiol level of ≤80 pg/mL (34), TSH level within the normal range of the assay and ≤5 mIU/L, and prolactin level ≤20 ng/mL. Male partners of women with UI had semen concentration ≥15 million/mL, motility ≥40%, and normal forms ≥4% (where strict Tygerberg analysis was available), based on World Health Organization 2010 criteria (35), regardless of the year of evaluation. If strict Tygerberg (36) evaluation of sperm was not available, subjects were not excluded based on percentage of abnormal forms. Male partners of women in the severe male factor group had semen concentrations <1 million/mL on at least two occasions. We excluded women with history of hypothyroidism or hyperthyroidism (including postpartum thyroiditis or history of an abnormal TSH level), history of a high prolactin level, body mass index (BMI) <18.5 kg/m2 or ≥40 kg/m2, women with recurrent pregnancy loss (three or more miscarriages), women with abnormalities that may be associated with reproduction (e.g., complex ovarian cysts, previous ovarian surgery, cervical stenosis, endometriosis, or endometritis), or a strong suspicion by the evaluating physician of an endocrine disorder, including polycystic ovary syndrome. This study was approved by the Partners HealthCare institutional review board.
Laboratory assessment
All diagnostic and laboratory testing was performed as part of routine clinical care. TSH and prolactin levels were measured in one of four hospital laboratories within the Partners HealthCare system for >75% of patients. The remaining patients had a TSH or prolactin level checked at a known outside laboratory for which method information was available or an outside facility for which assay and method information was not available (14.6% of patients). The upper limit of normal for two of the TSH assays was <5 mIU/L, and in this case we included only patients who had a TSH within the normal range of the assay. There were two TSH assays with an upper limit of normal of >5 mIU/L, and in this case we included only patients with a TSH ≤5 mIU/L.
Statistical analysis
Statistical analysis was performed using JMP Pro 11.0 (SAS Institute, Cary, NC) software. Means and standard deviation measurements are reported and compared using the Student’s t test unless the data were nonnormally distributed, in which case medians and first and third quartile ranges are presented and compared via the Wilcoxon rank-sum test. Percentages were compared using the Fisher exact test or the Pearson χ2 test. Least-squares linear regression modeling was performed to control for clinically relevant covariates. TSH was log transformed for the regression analyses due to nonnormality. P <0.05 on a two-tailed test was used to indicate statistical significance.
Results
Clinical characteristics
A total of 239 women met our inclusion and exclusion criteria: 187 women with UI and 52 with severe male factor infertility. Subjects in the two groups were similarly distributed across the 13-year study period (P = 0.69). Characteristics of the study participants are listed in Table 1. Subjects in the UI group were slightly older than subjects in the severe male factor group (mean age ± standard deviation: UI, 31.5 ± 2.7 years; severe male factor, 30.1 ± 3.7 years; P = 0.01). Median BMI was lower in the UI group as compared with the severe male factor group [UI median BMI 23.0 kg/m2, interquartile range: (20.9, 26.2); severe male factor median BMI 24.4 kg/m2, interquartile range: (22.2, 27.0); P < 0.04], and the percentage of women with a BMI ≥25 kg/m2 was lower in the UI group than in the severe male factor group, although this difference was not statistically significant (P = 0.24). Median duration of infertility and mean day 3 FSH levels were similar in both groups.
Table 1.
Clinical Characteristics of the Study Population
| UI (n = 187) | Severe Male Factor (n = 52) | P | |
|---|---|---|---|
| Age, ya | 31.5 ± 2.7 | 30.1 ± 3.7 | 0.01 |
| BMI, kg/m2b | 23.0 (20.9, 26.2) | 24.4 (22.2, 27.0) | <0.04 |
| Tobacco | |||
| % Current use, n | 7.0% (13) | 11.5% (6) | 0.26 |
| % Past or present use, n | 15.5% (29) | 19.2% (10) | 0.53 |
| Age of menarche, yb | 13 (12, 13) | 13 (12, 13) | 0.93 |
| % Secondary infertility, n | 19.8% (37) | 28.8% (15) | 0.18 |
| Duration of infertility, mob | 16 (12, 24) | 18 (12,30) | 0.36 |
| Day 3 FSH, IU/mLa | 6.7 ± 1.7 | 6.6 ± 1.4 | 0.99 |
Mean ± standard deviation.
Median (interquartile range).
TSH and prolactin levels
TSH
Median TSH levels were significantly greater in the UI group than in the severe male factor group [UI median TSH 1.95 mIU/L, interquartile range: (1.54, 2.61); severe male factor TSH 1.66 mIU/L, interquartile range: (1.25, 2.17); P = 0.003] (Fig. 1). TSH levels remained significantly higher in the UI group when we controlled for both BMI (P < 0.02) and age (P < 0.01), variables that have been positively associated with TSH in previous studies (37–41). Although smoking status was not significantly different between the groups, both past and a current history of cigarette smoking are associated with lower TSH levels (42). Therefore, we also controlled for both current history of smoking and past or current history of smoking, and TSH remained significantly higher in the UI group than in the severe male factor group (P < 0.01 for both).
Figure 1.
The UI group had a significantly higher median TSH than the severe male factor group (P = 0.003). Solid lines represent the median and first and third quartiles. Dotted line represents a TSH of 2.5 mIU/L.
After we excluded women from the UI group whose partner had a low morphology based on methods that did not use strict (Tygerberg) criteria (n = 19), TSH levels remained significantly higher in the UI group than in the severe male factor group [UI TSH 1.96 mIU/L, interquartile range: (1.54, 2.61); severe male factor TSH 1.66 mIU/L, interquartile range: (1.25, 2.17); P < 0.01] and regression analyses similarly remained significant (P ≤ 0.01 for all).
Significantly more women in the UI group had TSH values ≥2.5 mIU/L as compared with women in the severe male factor group (Fig. 2). The percentage of women in the UI group with a TSH ≥2.5 mIU/L was nearly twice the percentage in the severe male factor group (UI 26.9%, severe male factor 13.5%; P < 0.05). Of the 57 total patients who had a TSH ≥2.5 mIU/L, 22.8% (13 patients) were started on thyroid hormone replacement therapy after their initial evaluation, although it is not known whether patients were initiated on thyroid hormone replacement to prevent adverse obstetrical outcomes if they were to conceive or in an attempt to improve the likelihood of conception.
Figure 2.
A significantly higher percentage of women in the UI group (26.9%) had a TSH level ≥2.5 mIU/L compared with the severe male factor group (13.5%; P < 0.05).
To exclude the possibility that the observed differences were due to changes in assay methods or laboratory procedures, we divided patients into groups depending on where their TSH level was measured (which hospital or laboratory) and which assay was used. In one case, two laboratories used the same TSH assay, but patients who had their TSH measured with this assay were divided into two separate groups because there may have been systematic differences between the hospital laboratories that could have led to differences in TSH levels. Table 2 shows the percentage of women with UI and severe male factor in each of the laboratory and assay groupings. Although the overall χ2 test did not detect a difference between the groups with respect to where and how TSH was measured (P = 0.48), we performed individual significance testing to ensure that we were not missing differences. With individual testing, we found that a significantly higher percentage of subjects in the severe male factor group had a TSH level measured in one laboratory/assay (group 4) compared with subjects with UI (P = 0.04). When we excluded patients from laboratory/assay group 4 and those who had their TSH measured with an unknown assay, the observed differences in TSH levels remained significant; the TSH level in the UI group (n = 130) was significantly higher than that of the severe male factor group (n = 30) [UI TSH 1.94 mIU/L, interquartile range: (1.52, 2.54); severe male factor TSH 1.72 mIU/L, interquartile range: (1.32, 2.00); P = 0.01], and a significantly higher percentage of women with UI had a TSH ≥2.5 mIU/L compared with those with severe male factor infertility (25.4% vs 6.7%, P < 0.03).
Table 2.
Percentage of Women in the UI Group and Severe Male Factor Group Who Had Their TSH Measured With a Given Assay at a Specific Laboratory
| Laboratory/Assay for TSH Measurement | Reference Range (mIU/L) | UI (%) | Severe Male Factor (%) | P |
|---|---|---|---|---|
| 1 | 0.4 to 5 | 13.4% | 13.5% | 0.99 |
| 2 | 0.4 to 5 | 12.9% | 11.5% | 0.99 |
| 3 | 0.5 to 5.7 | 17.7% | 13.5% | 0.54 |
| 4 | 0.5 to 5 | 15.1% | 28.9% | 0.04 |
| 5 | 0.4 to 4.5 | 6.4% | 3.8% | 0.74 |
| 6 | 0.35 to 5.5 | 3.8% | 0 | 0.35 |
| 7 | 0.55 to 4.78 | 5.4% | 3.8% | 0.99 |
| 8 | 0.34 to 5 | 10.2% | 11.5% | 0.80 |
| Unknown | 15.1% | 13.5% | 0.99 |
“Laboratory/Assay for TSH measurement” takes into account both the assay and the specific laboratory in which TSH was measured. TSH was measured in four separate hospital laboratories and an additional outside laboratory; three of the laboratories changed their TSH assay once during the 13-year period.
Thyroid peroxidase antibodies
Only 19 of the 239 women in the study had thyroid peroxidase (TPO) antibodies assessed around the time of their infertility evaluation. Of these 19 women, 6 had an elevated TPO antibody level (3 in the UI group and 3 in the severe male factor group). Median TPO antibody levels were significantly higher in the severe male factor group than in the UI group [UI TPO antibody 13.3 IU/mL, interquartile range: (10.2, 18); severe male factor TPO antibody 90.4 IU/mL, interquartile range: (18.4, 2994.3); P = 0.03]. When the six subjects with a positive TPO antibody were excluded from the TSH analyses, the results remained significant, with a higher median TSH level in the UI group [1.95 mIU/L, interquartile range: (1.52, 2.58)] as compared with the severe male factor group [1.69 mIU/L, interquartile range: (1.22, 2.16); P < 0.01] and a significantly higher percentage of subjects with a TSH ≥2.5 mIU/L in the UI group (26%) than in the severe male factor group (12%; P < 0.04).
Prolactin
Prolactin levels were similar in the UI group and the severe male factor group [UI prolactin 10.4 ng/mL, interquartile range: (7.7, 13.4); severe male factor prolactin 11 ng/mL, interquartile range: (8.5, 13.7); P = 0.36]. Because prolactin levels may vary during the menstrual cycle (43, 44), we performed an analysis including only women who had prolactin measured on day 3 of their menstrual cycle (n = 180), and the results were similar [UI prolactin 10.8 ng/mL, interquartile range: (8.1, 13.7); severe male factor prolactin 12.5 ng/mL, interquartile range: (9.2, 14.5); P = 0.20]. There were no significant differences between the groups with respect to method of prolactin measurement (P = 0.82; Supplemental Table 1 (188.4KB, pdf) ).
Discussion
We have shown that women with UI have significantly higher TSH levels than a control group of women with a comparatively normal fertility evaluation except for an azoospermic or severely oligospermic partner. Similarly, nearly twice as many women with UI have TSH levels ≥2.5 mIU/L as compared with the control group. Importantly, all subjects in this study had TSH levels within the normal, prepregnancy reference range, suggesting that even mild variations of thyroid dysfunction within the normal range may be an important factor in fertility in women who have no known cause for their infertility.
Thyroid disease is a known cause of menstrual irregularity and infertility (9). A number of previous studies have investigated the relationship between TSH and conception rates or time to pregnancy with conflicting results; a TSH ≥2.5 mIU/L has not been associated with increased time to pregnancy in women with proven fecundity (and without a history of infertility) (45) or with adverse intrauterine insemination outcomes (46), whereas in a large population-based study including women with thyroid dysfunction, higher TSH levels were associated with fewer total pregnancies (47).
Previous studies have also investigated the percentage of women with abnormal TSH levels or subclinical hypothyroidism in different types of infertility. Abalovich et al. (48) found a higher rate of subclinical hypothyroidism (defined as a TSH >4.22 mIU/L or a TSH >26.6 mIU/L in response to 200 μg of intravenous TSH-releasing hormone stimulation) in women with premature ovarian insufficiency, tubal disease, and ovulatory dysfunction compared with fertile women, but none of the women with UI was diagnosed with subclinical hypothyroidism. A second study from Finland looked at the rate of frankly abnormal TSH levels in women diagnosed with infertility and found that 6.3% of women in the ovulatory dysfunction group and 4.8% of women in the UI group had an elevated TSH (28). Our study differs from these previous studies in that we used very strict criteria to ensure our subjects had completely normal fertility evaluations (other than azoospermia or severe oligospermia in the control group) and no known history of thyroid disease or abnormal thyroid function tests. All subjects in both groups in our study had regular menstrual cycles, <35 days in length, with ≤5 days of intercycle variability, normal uterine evaluations, and normal hormonal profiles. In the UI group, male partners had a normal semen analysis (35). The purpose of our strict inclusion and exclusion criteria was to assess whether mild variations in thyroid function or circulating prolactin levels contribute to the phenotype of UI.
Because we did not require proven fecundity as a criterion for the severe male factor group, our choice of control group probably biased our result toward the null hypothesis, because it is possible that some of the women in the severe male factor group would have been classified as having UI had they been with a partner with a normal semen profile. Therefore, the fact that we found a difference in TSH levels, despite this choice of control group, only adds to the strength of our findings. We also did not use a population of couples with less severe forms of male factor infertility as our control group, which would have yielded a much larger number of controls. The definition of male factor infertility has changed over the years, with the lower limit of most semen parameters being lower in current as compared with previous reference ranges (35). Therefore, including a population of couples over a span of 13 years, with a changing definition of male infertility, would have been problematic. Second, a previous study found that in a population of couples who underwent IVF, TSH levels were significantly higher in women with a male partner with male factor infertility as compared with other types of infertility, including ovulatory and tubal factors (49). The authors hypothesized that this is likely due to the fact that in couples diagnosed with mild male factor infertility, female factors also contribute to the diagnosis of infertility, supported by previous studies demonstrating that female partners of men with poor semen factors have lower fertilization rates using donor sperm as compared with female partners of azoospermic men (49–51). Given the complicated relationship between mild male factor infertility and female hormonal status, we included only couples with azoospermia or severe oligospermia in our analysis.
We also hypothesized that women with UI would have higher prolactin levels (within the normal range) as compared with the control group. A previous study demonstrated higher prolactin levels in ovulating women with infertility of an unknown cause as compared with a control group of fertile women, and treatment with a dopamine agonist resulted in conception in 16 of the 40 infertile women during the 10 months of follow-up (52). On the other hand, a more recent Cochrane review combining data from three double-blind, randomized trials of 127 women with UI treated with bromocriptine or placebo found no benefit in conception rates in the bromocriptine-treated group (53). In our study, we did not find a significant difference in prolactin levels in women with UI as compared with severe male factor infertility. Importantly, prolactin levels are exquisitely sensitive to environmental influences including stress (54) and food intake (55) and have been shown to be highest during the ovulatory and luteal phases of the menstrual cycle (43, 44). We attempted to control for some of this potential variability by including only prolactin levels measured on day 3 of the menstrual cycle, but it is possible that even if there was a difference between groups, we did not detect it because of the variability we were not able to control for. To determine whether prolactin levels contribute to the phenotype of UI, future studies will need to measure prolactin levels in a carefully controlled setting.
Strengths of our study include our very strict inclusion and exclusion criteria, which allowed us to control for other possible factors contributing to infertility. Importantly, our control group consisted of a population of women who had a similarly rigorous fertility evaluation compared with the UI group. The main limitation of this study is that we relied on health records and therefore we were limited to laboratory tests that were drawn for clinical purposes. Therefore, we could not measure thyroid antibody levels or thyroid hormone levels in our subjects, and a previous meta-analysis demonstrated an increased rate of infertility in women who were thyroid antibody positive (56). Of the 239 women included in our study, only 19 had TPO antibodies checked around the time of their infertility evaluation.
In conclusion, TSH levels are significantly higher in a population of women without known thyroid dysfunction and with UI as compared with a control group. The fact that nearly 27% of women with UI have TSH levels ≥2.5 mIU/L as compared with 13.5% of women in the control group suggests that mild abnormalities in thyroid function may contribute to some cases of UI. It also raises the question of whether treatment with thyroid hormone replacement for women with TSH levels ≥2.5 mIU/L may be an economical first step in treating UI, especially for this population for whom early use of IVF, a resource-intensive treatment, has been shown to result in higher conception rates (8). Although current practice guidelines do not recommend treating women with a TSH ≥2.5 mIU/L who are attempting to conceive naturally (29, 33), some practitioners use this lower cutoff to initiate treatment. Our data demonstrate that 22.8% of patients with a TSH ≥2.5 mIU/L were started on thyroid hormone replacement after their initial evaluation and therefore >75% of patients were not, demonstrating the great variance in clinical practice and the need for more data. Therefore, future studies will be necessary to determine whether treatment of high-normal TSH levels decreases time to conception in couples with UI.
Acknowledgments
Financial Support: This work is supported by National Institutes of Health Grants K23 DK094820 (to P.K.F.; National Institute of Diabetes and Digestive and Kidney Diseases, http://dx.doi.org/10.13039/100000062) and 1UL1TR001102 and the Claflin Distinguished Scholar Award (to P.K.F.; Massachusetts General Hospital, http://dx.doi.org/10.13039/100005294). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- FSH
- follicle-stimulating hormone
- IVF
- in vitro fertilization
- TPO
- thyroid peroxidase
- TSH
- thyrotropin
- UI
- unexplained infertility.
References
- 1.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406. [DOI] [PubMed] [Google Scholar]
- 2.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM.. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324–1331.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, Coulson C, Lambert PA, Watt EM, Desai KM. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed). 1985;291(6510):1693–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, Lopes P, Tabaste JM, Spira A. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod. 1991;6(6):811–816. [DOI] [PubMed] [Google Scholar]
- 5.Smith S, Pfeifer SM, Collins JA. Diagnosis and management of female infertility. JAMA. 2003;290(13):1767–1770. [DOI] [PubMed] [Google Scholar]
- 6.Gelbaya TA, Potdar N, Jeve YB, Nardo LG. Definition and epidemiology of unexplained infertility. Obstet Gynecol Surv. 2014;69(2):109–115. [DOI] [PubMed] [Google Scholar]
- 7.Harris Williams and Co. 2015.. Fertility market overview. Available at: www.harriswilliams.com.
- 8.Reindollar RH, Regan MM, Neumann PJ, Levine BS, Thornton KL, Alper MM, Goldman MB. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the Fast Track and Standard Treatment (FASTT) trial. Fertil Steril. 2010;94(3):888–899. [DOI] [PubMed] [Google Scholar]
- 9.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31(5):702–755. [DOI] [PubMed] [Google Scholar]
- 10.Fourman LT, Fazeli PK. Neuroendocrine causes of amenorrhea--an update. J Clin Endocrinol Metab. 2015;100(3):812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sauder SE, Frager M, Case GD, Kelch RP, Marshall JC. Abnormal patterns of pulsatile luteinizing hormone secretion in women with hyperprolactinemia and amenorrhea: responses to bromocriptine. J Clin Endocrinol Metab. 1984;59(5):941–948. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki T, Azuma K, Irahara M, Yasui T, Aono T. Mechanism of anovulation in hyperprolactinemic amenorrhea determined by pulsatile gonadotropin-releasing hormone injection combined with human chorionic gonadotropin. Fertil Steril. 1994;62(6):1143–1149. [DOI] [PubMed] [Google Scholar]
- 13.Seki K, Seki M. Successful ovulation and pregnancy achieved by CB-154 (2-Br-α-ergocryptine) in a woman with Chiari-Frommel syndrome. J Clin Endocrinol Metab. 1974;38(3):508–509. [DOI] [PubMed] [Google Scholar]
- 14.Thorner MO, McNeilly AS, Hagan C, Besser GM. Long-term treatment of galactorrhoea and hypogonadism with bromocriptine. BMJ. 1974;2(5916):419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd SJ, Josimovich JB, Archer DF. Amenorrhea and galactorrhea: results of therapy with 2-Brom-alpha-ergocryptine (CB-154). Am J Obstet Gynecol. 1975;122(1):85–89. [DOI] [PubMed] [Google Scholar]
- 16.Webster J, Piscitelli G, Polli A, Ferrari CI, Ismail I, Scanlon MF; Cabergoline Comparative Study Group . A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. N Engl J Med. 1994;331(14):904–909. [DOI] [PubMed] [Google Scholar]
- 17.Corenblum B, Pairaudeau N, Shewchuk AB. Prolactin hypersecretion and short luteal phase defects. Obstet Gynecol. 1976;47(4):486–488. [PubMed] [Google Scholar]
- 18.Seppälä M, Ranta T, Hirvonen E. Hyperprolactinaemia and luteal insufficiency. Lancet. 1976;307(7953):229–230. [DOI] [PubMed] [Google Scholar]
- 19.del Pozo E, Wyss H, Tollis G, Alcañiz J, Campana A, Naftolin F. Prolactin and deficient luteal function. Obstet Gynecol. 1979;53(3):282–286. [PubMed] [Google Scholar]
- 20.Wakim AN, Paljug WR, Jasnosz KM, Alhakim N, Brown AB, Burholt DR. Thyroid hormone receptor messenger ribonucleic acid in human granulosa and ovarian stromal cells. Fertil Steril. 1994;62(3):531–534. [DOI] [PubMed] [Google Scholar]
- 21.Cecconi S, Rucci N, Scaldaferri ML, Masciulli MP, Rossi G, Moretti C, D’Armiento M, Ulisse S. Thyroid hormone effects on mouse oocyte maturation and granulosa cell aromatase activity. Endocrinology. 1999;140(4):1783–1788. [DOI] [PubMed] [Google Scholar]
- 22.Aghajanova L, Stavreus-Evers A, Lindeberg M, Landgren BM, Sparre LS, Hovatta O.. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil Steril. 2011;95(1):230–237.e2. [DOI] [PubMed] [Google Scholar]
- 23.Colicchia M, Campagnolo L, Baldini E, Ulisse S, Valensise H, Moretti C. Molecular basis of thyrotropin and thyroid hormone action during implantation and early development. Hum Reprod Update. 2014;20(6):884–904. [DOI] [PubMed] [Google Scholar]
- 24.Karmon AE, Cardozo ER, Souter I, Gold J, Petrozza JC, Styer AK. Donor TSH level is associated with clinical pregnancy among oocyte donation cycles. J Assist Reprod Genet. 2016;33(4):489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krassas GE, Pontikides N, Kaltsas T, Papadopoulou P, Batrinos M. Menstrual disturbances in thyrotoxicosis. Clin Endocrinol (Oxf). 1994;40(5):641–644. [DOI] [PubMed] [Google Scholar]
- 26.Krassas GE, Pontikides N, Kaltsas T, Papadopoulou P, Paunkovic J, Paunkovic N, Duntas LH. Disturbances of menstruation in hypothyroidism. Clin Endocrinol (Oxf). 1999;50(5):655–659. [DOI] [PubMed] [Google Scholar]
- 27.Quintino-Moro A, Zantut-Wittmann DE, Tambascia M, Machado HC, Fernandes A. High prevalence of infertility among women with Graves’ disease and Hashimoto’s thyroiditis. Int J Endocrinol. 2014;2014:982705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arojoki M, Jokimaa V, Juuti A, Koskinen P, Irjala K, Anttila L. Hypothyroidism among infertile women in Finland. Gynecol Endocrinol. 2000;14(2):127–131. [DOI] [PubMed] [Google Scholar]
- 29.Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S. 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315–389. [DOI] [PubMed] [Google Scholar]
- 30.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. [DOI] [PubMed] [Google Scholar]
- 31.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Niccoli-Sire P, John R, Ruf J, Smyth PP, Spencer CA, Stockigt JR; Guidelines Committee, National Academy of Clinical Biochemistry . Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3–126. [DOI] [PubMed] [Google Scholar]
- 32.Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab. 2010;95(9):E44–E48. [DOI] [PubMed] [Google Scholar]
- 33.Practice Committee of the American Society for Reproductive Medicine Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545–553. [DOI] [PubMed] [Google Scholar]
- 34.Practice Committee of the American Society for Reproductive Medicine Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9–e17. [DOI] [PubMed] [Google Scholar]
- 35.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. [DOI] [PubMed] [Google Scholar]
- 36.Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5(5):586–592. [DOI] [PubMed] [Google Scholar]
- 37.Nyrnes A, Jorde R, Sundsfjord J. Serum TSH is positively associated with BMI. Int J Obes. 2006;30(1):100–105. [DOI] [PubMed] [Google Scholar]
- 38.Fox CS, Pencina MJ, D’Agostino RB, Murabito JM, Seely EW, Pearce EN, Vasan RS. Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med. 2008;168(6):587–592. [DOI] [PubMed] [Google Scholar]
- 39.Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92(12):4575–4582. [DOI] [PubMed] [Google Scholar]
- 40.Bremner AP, Feddema P, Leedman PJ, Brown SJ, Beilby JP, Lim EM, Wilson SG, O’Leary PC, Walsh JP. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab. 2012;97(5):1554–1562. [DOI] [PubMed] [Google Scholar]
- 41.Vadiveloo T, Donnan PT, Murphy MJ, Leese GP. Age- and gender-specific TSH reference intervals in people with no obvious thyroid disease in Tayside, Scotland: the Thyroid Epidemiology, Audit, and Research Study (TEARS). J Clin Endocrinol Metab. 2013;98(3):1147–1153. [DOI] [PubMed] [Google Scholar]
- 42.Asvold BO, Bjøro T, Nilsen TI, Vatten LJ. Tobacco smoking and thyroid function: a population-based study. Arch Intern Med. 2007;167(13):1428–1432. [DOI] [PubMed] [Google Scholar]
- 43.Franchimont P, Dourcy C, Legros JJ, Reuter A, Vrindts-Gevaert Y, Van Cauwenberge JR, Gaspard U. Prolactin levels during the menstrual cycle. Clin Endocrinol (Oxf). 1976;5(6):643–650. [DOI] [PubMed] [Google Scholar]
- 44.Vekemans M, Delvoye P, L’Hermite M, Robyn C. Serum prolactin levels during the menstrual cycle. J Clin Endocrinol Metab. 1977;44(5):989–993. [DOI] [PubMed] [Google Scholar]
- 45.Plowden TC, Schisterman EF, Sjaarda LA, Zarek SM, Perkins NJ, Silver R, Galai N, DeCherney AH, Mumford SL. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J Clin Endocrinol Metab. 2016;101(6):2358–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karmon AE, Batsis M, Chavarro JE, Souter I.. Preconceptional thyroid-stimulating hormone levels and outcomes of intrauterine insemination among euthyroid infertile women. Fertil Steril. 2015;103(6):258–263.e1. [DOI] [PubMed] [Google Scholar]
- 47.Feldthusen AD, Pedersen PL, Larsen J, Toft Kristensen T, Ellervik C, Kvetny J. Impaired fertility associated with subclinical hypothyroidism and thyroid autoimmunity: the Danish General Suburban Population Study. J Pregnancy. 2015;2015:132718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abalovich M, Mitelberg L, Allami C, Gutierrez S, Alcaraz G, Otero P, Levalle O. Subclinical hypothyroidism and thyroid autoimmunity in women with infertility. Gynecol Endocrinol. 2007;23(5):279–283. [DOI] [PubMed] [Google Scholar]
- 49.Cramer DW, Sluss PM, Powers RD, McShane P, Ginsburgs ES, Hornstein MD, Vitonis AF, Barbieri RL. Serum prolactin and TSH in an in vitro fertilization population: is there a link between fertilization and thyroid function? J Assist Reprod Genet. 2003;20(6):210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emperaire JC, Gauzere E, Audebert A. Female fertility and donor insemination. Lancet. 1980;1(8183):1423–1424. [DOI] [PubMed] [Google Scholar]
- 51.Albrecht BH, Cramer D, Schiff I. Factors influencing the success of artificial insemination. Fertil Steril. 1982;37(6):792–797. [DOI] [PubMed] [Google Scholar]
- 52.Lenton EA, Sobowale OS, Cooke ID. Prolactin concentrations in ovulatory but infertile women: treatment with bromocriptine. BMJ. 1977;2(6096):1179–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hughes E, Collins J, Vandekerckhove P. Bromocriptine for unexplained subfertility in women. Cochrane Database Syst Rev. 2000; (2):CD000044. [DOI] [PubMed]
- 54.Sobrinho LG. Prolactin, psychological stress and environment in humans: adaptation and maladaptation. Pituitary. 2003;6(1):35–39. [DOI] [PubMed] [Google Scholar]
- 55.Quigley ME, Ishizuka B, Ropert JF, Yen SS. The food-entrained prolactin and cortisol release in late pregnancy and prolactinoma patients. J Clin Endocrinol Metab. 1982;54(6):1109–1112. [DOI] [PubMed] [Google Scholar]
- 56.van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JA, Goddijn M, Bisschop PH. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2011;17(5):605–619. [DOI] [PubMed] [Google Scholar]