Abstract
Context:
Elevated urine net acid excretion (NAE), indicative of subclinical metabolic acidosis, has been associated with higher bone turnover. Urine citrate, which is a common clinical measure, changes in response to acid-base status but its association with bone turnover is uncertain.
Objective:
We evaluated the association between change in urine citrate and change in bone turnover and calcium excretion.
Design, Intervention, and Participants:
A total of 233 healthy men and women ≥60 years old were randomly assigned to 1.0 mmol/kg/d potassium bicarbonate (KHCO3), 1.5 mmol/kg/d KHCO3, or placebo for 84 days.
Outcome Measures:
Urine citrate, NAE, N-telopeptide of collagen type-I (NTX), calcium excretion, and serum amino-terminal propeptide of type 1 procollagen (P1NP) were measured before and after intervention.
Results:
Urine citrate increased dose dependently after KHCO3 supplementation (P trend < 0.001). The urine citrate change was significantly inversely associated with P1NP change (P = 0.021) but not with change in NTX (P = 0.051) or calcium excretion (P = 0.652). The NAE change was positively associated with change in NTX and calcium excretion (P ≤ 0.003) but not with change in P1NP (P = 0.051). When the urine citrate change and NAE change were included in the same model, the urine citrate change was not associated with change in NTX, calcium excretion, or serum P1NP (P ≥ 0.086), whereas change in NAE remained associated with change in NTX and calcium excretion (P ≤ 0.003).
Conclusion:
Urine citrate may not be a suitable alternative to NAE when assessing acid-base status in relation to bone turnover in older adults.
In older adults, urine citrate excretion increased in response to alkali supplementation, but the change in urine citrate was not a robust indicator of change in bone resorption or calcium excretion.
Slight fluctuations in acid-base balance toward a lower pH, known as mild or subclinical metabolic acidosis, have been associated with increased bone turnover and bone loss (1, 2). In healthy individuals, diet can contribute to metabolic acidosis (3–5). Typical western diets, which are high in protein and cereal grains (acid-producing foods) and low in fruits and vegetables (alkali-producing foods), promote mild metabolic acidosis. Dietary acid load can be estimated as net endogenous acid production (NEAP) or potential renal acid load (PRAL) using calculations applied to dietary intake data (4, 5). Higher NEAP and PRAL have been associated with higher bone turnover and lower bone mineral density (BMD) in some clinic- and population-based studies (6–9) but not in others (10–12). Although these calculations are straightforward, they carry limitations associated with the use of dietary intake questionnaires (13–15).
The 24-hour urine net acid excretion (NAE) is a direct measure of systemic acid load that responds to changes in diet and correlates positively with bone resorption (16–18). Because measuring urine NAE is labor intensive, its utility in large clinical and population-based studies is challenging (19). As acid load increases, urine NAE increases and citrate excretion decreases (20). Urine citrate is commonly measured in clinical laboratories, so expanding its utility as a biomarker of systemic acid-base balance linked to bone turnover could be important for clinical and research settings. Recently, Esche et al. (21) found higher urine citrate excretion was associated with higher bone strength in 6- to 18-year-old children and adolescents. Higher urine citrate excretion was also associated with significantly lower odds of fracture over 15 years of follow-up in the girls but not in the boys. The utility of urine citrate as an indicator of bone turnover in adults is not known.
The aim of this study was to evaluate urine citrate as a potential alternative to NAE as an indicator of bone resorption and calcium excretion in healthy older adults. This analysis was carried out in men and women ≥60 years old who participated in a randomized trial of alkali supplementation. In this trial, alkali supplementation reduced NAE, the bone resorption biomarker urine N-telopeptide of collagen type I (NTX), and calcium excretion (17). In the present analyses we determined (1) whether urine citrate was associated with dietary NEAP and PRAL cross-sectionally, (2) the change in urine citrate in response to two doses of alkali supplementation vs placebo, and (3) whether this change was associated with the change in bone resorption and calcium excretion over 84 days, compared with association of the change in NAE with the same outcomes.
Subjects and Methods
Participants
Study participants included 233 community-dwelling men and postmenopausal women who completed an 84-day, double-blind randomized controlled trial of potassium bicarbonate (KHCO3) supplementation. All participants were at least 60 years old, had an estimated glomerular filtration rate of at least 50 mL/min/1.73 m2 and weighed between 45 and 113.5 kg. Exclusion criteria included serum potassium >5.3 mEq/L, serum bicarbonate >33 mmol/L, fasting glucose >130 mg/dL, >2 units of alcohol/d, gastroesophageal reflux disease, active malignancy, adrenal insufficiency, hyperparathyroidism, untreated thyroid disease, significant immune disorder, heart disease, salt-restricted diets, kidney stones in the last 5 years, fasting spot urine calcium/creatinine >0.38 mmol/mmol after 1 week off calcium supplements, and serum calcium outside the range of 8.3 to 10.2 mg/dL. Other exclusion criteria were current use of diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, or nonsteroidal anti-inflammatory drugs >3 times/wk; over-the-counter antacids, potassium supplements, and salt substitutes; bisphosphonates in the last 2 years; other osteoporosis medications (e.g., calcitonin, raloxifene, denosumab, and teriparatide) in the last 6 months; or glucocorticoids for >10 days in the last 3 months (17). The Institutional Review Board at Tufts Medical Center/Tufts University approved the protocol, and all participants provided written informed consent.
Intervention
Participants were randomized to receive one of three treatments for 84 days: 1.0 mmol/kg/d KHCO3, 1.5 mmol/kg/d KHCO3, or placebo (microcrystalline cellulose) as described (17). KHCO3 and placebo capsules were purchased from Life Enhancement Products, Inc. (Petaluma, CA). Covance (Princeton, NJ) verified the KHCO3 content of the capsules.
Biochemical measurements
Participants collected their urine for the 24 hours immediately preceding their study visit. Samples were aliquoted and stored at −80°C. Quest Diagnostics (Marlborough, MA) measured the 24-hour urine citrate from stored samples using enzymatic spectrophotometry. The correlation between urine citrate measured at baseline and after 15 years of frozen storage without addition of urine preservative was 0.99 and with 98% recovery, indicating long-term freezer stability (22). When the trial was completed in 2015, the following analytes were measured: 24-hour urine NAE was measured by a modification of the titration method (19, 23), urine NTX was measured by enzyme-linked immunoassay (Wampole, Princeton, NJ), urine calcium was measured by direct current plasma emission spectroscopy (Spectraspan VI Direct Plasma Emission Spectrophotometer; Beckman Instruments, Fullerton, CA), and creatinine was measured on an automated clinical chemistry analyzer (Olympus AU400; Olympus America Inc., Melville, NY) (coefficients of variation of 3% to 6%). Urine pH was measured on an Accumet Excel pH meter (Fisher Scientific, Pittsburgh, PA). Serum amino-terminal propeptide of type 1 procollagen (P1NP) was measured in fasted samples using radioimmunoassay (Orion Diagnostica Uni, Espoo, Finland). All assays’ coefficients of variation were between 3.0% and 10.1% (17). All measurements were obtained at baseline and at day 84 of the intervention.
Dietary acid load: NEAP and PRAL
An interviewer-assisted 24-hour dietary recall was administered to participants on the days that coincided with their 24-hour urine collection as described (17). The Nutrition Data System for Research software (2011 version) was used to quantify food groups and nutrients. The formulas of Frassetto et al. (4) and Remer and Manz (5) were used to calculate NEAP and PRAL, respectively, based on self-reported dietary intake at baseline.
Statistical approach
Baseline characteristics were compared across treatment groups using one-way analysis of variance, Kruskal-Wallis test, or χ2 test. Pearson correlations between baseline 24-hour citrate and 24-hour NAE and between the change in 24-hour citrate and the change in 24-hour NAE were calculated. We also explored the correlation between baseline urine citrate and renal acid excretion capacity (RAEC), which was calculated as the residual of the 24-hour NAE regressed on urine pH (a higher residual indicates higher NAE for a given urine pH) (21). General linear regression (adjusted for age, sex, and creatinine) was used to determine whether the calculated NEAP and PRAL predicted 24-hour citrate excretion and 24-hour NAE at baseline. To reduce skewness, the baseline 24-hour NAE values were natural log-transformed. Because negative values cannot be log-transformed and because the lowest baseline NAE was −6 mmol/d, a constant of 7 was added to all baseline NAE values prior to log-transformation for the cross-sectional analyses.
By design, the daily KHCO3 supplementation doses in the low- and high-dose groups were given according to participants’ body weight, which ranged from 58 to 100 kg (17). To evaluate the response of urine citrate to an absolute KHCO3 dose, we calculated the absolute daily dose (mmol KHCO3/d) according to participants’ height in meters and then divided the distribution of mmol KHCO3/height in meters/d into tertiles (the placebo group was considered as 0 mmol KHCO3/meter of height/d). The difference in the 24-hour urine citrate change across mmol KHCO3/height in meters/d groups was determined using a one-way analysis of variance. General linear models were used to evaluate whether the 84-day change in urine citrate was associated with the 84-day change in urine NTX, calcium excretion, pH, and serum P1NP. Covariates included age, sex, height, and baseline values of the outcome of interest. The same approach was used to determine if the 84-day change in 24-hour urine NAE was associated with change the same outcomes. To determine if the change in 24-hour citrate excretion and the change in 24-hour NAE were associated with change bone turnover independent of the other, both exposures were included in a model adjusted for age, sex, and baseline value of the outcome. Collinearity between change in urine citrate and change in urine NAE was assessed using the variance inflation factor but was not detected (variance inflation factor values ranged from 1.16 to 1.37). Analyses were conducted using SAS v 9.4 (SAS, Cary, NC), and statistical significance was set at α < 0.05. With 233 participants, we had 83% power to detect an unadjusted standardized β coefficient of 0.19 at a two-sided α < 0.05.
Results
At baseline, the mean ± standard deviation 24-hour urine citrate values did not differ among the placebo, low-dose, and high-dose alkali (KHCO3) supplementation groups (P = 0.426) (Table 1), nor did they differ according to groups categorized according to mmol KHCO3/meters of height/d (P = 0.194). Other participant baseline characteristics, including 24-hour urine NAE, pH, calcium excretion, and bone turnover biomarkers, did not differ among randomized groups (Table 1), as reported previously (17).
Table 1.
Participant Characteristics According to Randomized Group
| Placebo (n = 79) | Low-Dose KHCO3 (n = 79) | High-Dose KHCO3 (n = 75) | |
|---|---|---|---|
| Baseline characteristics | |||
| Female, n | 37 | 39 | 37 |
| Age, y | 67 ± 6 | 67 ± 5 | 66 ± 5 |
| BMI, kg/m2 | 25.5 ± 3.7 | 25.6 ± 4.3 | 26.0 ± 3.9 |
| Weight, kg | 72.7 ± 13.6 | 74.2 ± 13.9 | 73.8 ± 13.4 |
| Height, m | 1.68 ± 0.92 | 1.70 ± 0.95 | 1.68 ± 0.90 |
| Urine pH | 6.1 ± 0.4 | 6.2 ± 0.5 | 6.1 ± 0.4 |
| Urine calcium, mmol/da | 2.72 ± 2.05 | 2.66 ± 1.70 | 2.79 ± 2.56 |
| Urine NTX, mmol/da | 211 ± 168 | 212 ± 205 | 180 ± 162 |
| Serum P1NP, nmol/La | 1.20 ± 0.57 | 1.23 ± 0.63b | 1.30 ± 0.51c |
| Baseline acid-base status | |||
| Urine NAE, mmol/da | 8.4 ± 16.0 | 10.1 ± 18.0b | 10.3 ± 13.0 |
| Urine citrate, mmol/d | 4.0 ± 1.9 | 3.7 ± 17 | 3.9 ± 1.6 |
| Dietary NEAP, mEq/d | 46.4 ± 20.0 | 47.7 ± 25.3 | 51.6 ± 22.6 |
| Dietary PRAL, mEq/da | 3.2 ± 28.4 | 2.7 ± 1.7 | 4.8 ± 30.6 |
| KHCO3 intervention | |||
| mmol/d | 0 | 74.2 ± 18.6 | 111.0 ± 24.4 |
| mmol/meter of height/d | 0 | 43.4 ± 10.0 | 65.8 ± 13.1 |
Data are means ± standard deviation, unless indicated otherwise. Groups were not significantly different at baseline (all P > 0.180).
Median ± interquartile range.
Based on n = 78.
Based on n = 74.
At baseline, 24-hour urine citrate did not correlate with baseline (natural log) 24-hour urine NAE (Pearson r = 0.03, P = 0.706) or baseline RAEC (r = 0.10, P = 0.130). However, the change in 24-hour urine citrate was significantly inversely correlated with the change in 24-hour urine NAE (Pearson r = −0.37, P < 0.001).
Neither baseline calculated NEAP nor PRAL predicted baseline 24-hour urine citrate (NEAP: unstandardized β = −0.006, standardized β = –0.076, P = 0.223; PRAL: unstandardized β = −0.004, standardized β = −0.054, P = 0.388), whereas both NEAP and PRAL significantly predicted baseline (natural log) 24-hour urine NAE (NEAP: unstandardized β = 0.005, standardized β = 0.152, P = 0.019; PRAL: unstandardized β = 0.005, standardized β = 0.180, P = 0.005).
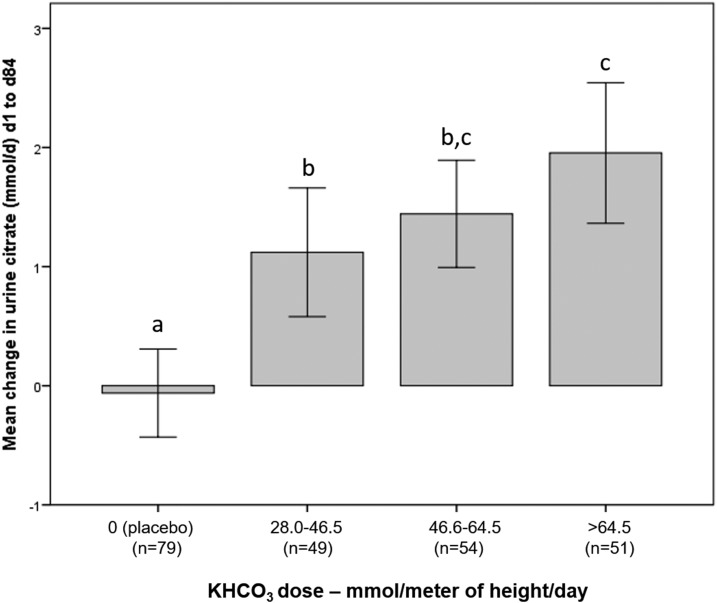
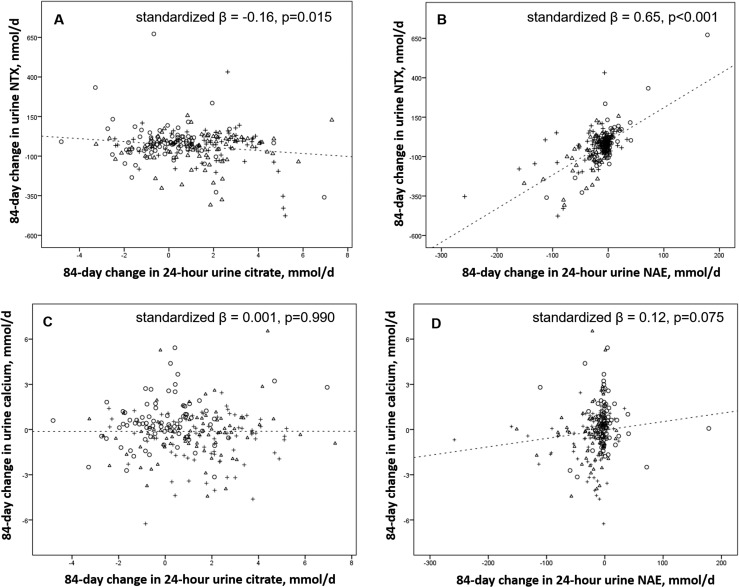
Urine citrate significantly increased as the absolute KHCO3 dose increased (P trend < 0.001) (Fig. 1). In all subjects combined, the change in 24-hour urine citrate was inversely associated with change in serum P1NP but not with change in urine calcium (Table 2; Fig. 2). The association between change in urine citrate and change in urine NTX did not reach statistical significance in the fully adjusted model (Table 2). The change in 24-hour urine NAE was positively associated with change in 24-hour urine NTX and change in urine calcium (Fig. 2). The association between change in urine NAE and change in serum P1NP did not reach statistical significance in the fully adjusted model (Table 2). When the change in 24-hour urine citrate and the change in 24-hour urine NAE were both included as exposures in the same model adjusted for baseline value, age, sex, and height, the change in urine citrate was no longer significantly associated with the change in serum P1NP, whereas the change in urine NAE remained positively associated with the changes in urine NTX and urine calcium but not with change in P1NP (Table 3).
Figure 1.
Change in urine citrate excretion in response to KHCO3 dose per meter of height per day. Error bars represent 95% confidence intervals. Overall P trend < 0.001. Groups with different lowercase letters differ at P < 0.022.
Table 2.
Associations Between Change (Δ) in Measures of Acid-Base Status and Change in Bone Turnover Markers, Urine Calcium, and Urine pH
|
Δ Urine Citrate, mmol/d |
Δ Urine NAE, mmol/d |
|||||||
|---|---|---|---|---|---|---|---|---|
| Unstandardized β | Standardized β | P Value | Model R2 | Unstandardized β | Standardized β | P Value | Model R2 | |
| Δ Urine NTX, nmol/d | ||||||||
| Unadjusted | −9.373 | −0.160 | 0.015 | 0.026 | 2.134 | 0.653 | <0.001 | 0.427 |
| Baseline adjusted | −6.051 | −0.103 | 0.071 | 0.269 | 1.748 | 0.535 | <0.001 | 0.518 |
| + Age and sex adjusted | −6.938 | −0.118 | 0.040 | 0.279 | 1.761 | 0.539 | <0.001 | 0.507 |
| + Height adjusted | −6.588 | −0.112 | 0.051 | 0.285 | 1.762 | 0.540 | <0.001 | 0.515 |
| Δ Urine calcium, mmol/d | ||||||||
| Unadjusted | −0.026 | −0.001 | 0.991 | 0.0004 | 0.223 | 0.117 | 0.075 | 0.014 |
| Baseline adjusted | −0.522 | −0.015 | 0.793 | 0.244 | 0.309 | 0.162 | 0.005 | 0.270 |
| + Age and sex adjusted | −0.857 | −0.025 | 0.672 | 0.247 | 0.325 | 0.170 | 0.003 | 0.275 |
| + Height adjusted | −0.917 | −0.027 | 0.652 | 0.248 | 0.326 | 0.171 | 0.003 | 0.276 |
| Δ Serum P1NP, nmol/L | ||||||||
| Unadjusted | −0.739 | −0.154 | 0.019 | 0.024 | 0.028 | 0.104 | 0.116 | 0.011 |
| Baseline adjusted | −0.740 | −0.154 | 0.007 | 0.281 | 0.033 | 0.110 | 0.029 | 0.273 |
| + Age and sex adjusted | −0.653 | −0.136 | 0.017 | 0.292 | 0.030 | 0.112 | 0.048 | 0.287 |
| + Height adjusted | −0.635 | −0.132 | 0.021 | 0.294 | 0.030 | 0.111 | 0.051 | 0.290 |
| Δ Urine pH | ||||||||
| Unadjusted | 0.223 | 0.589 | <0.001 | 0.347 | −0.012 | −0.568 | <0.001 | 0.323 |
| Baseline adjusted | 0.197 | 0.519 | <0.001 | 0.465 | −0.010 | −0.482 | <0.001 | 0.423 |
| + Age and sex adjusted | 0.197 | 0.519 | <0.001 | 0.469 | −0.010 | −0.479 | <0.001 | 0.429 |
| + Height adjusted | 0.197 | 0.520 | <0.001 | 0.470 | −0.010 | −0.479 | <0.001 | 0.429 |
Based on linear regression in which change in bone turnover markers, urine calcium, and pH were outcomes, and change in urine citrate and NAE were exposures in separate models.
Figure 2.
Unadjusted associations between (A) change in 24-hour urine citrate and change in urine NTX, (B) change in 24-hour urine NAE and change in urine NTX, (C) change in 24-hour urine citrate and change in urine calcium, and (D) change in 24-hour urine NAE and change in urine calcium in older adults randomized to low- (triangles) or high- (plus symbols) dose KHCO3 supplementation or placebo (circles) for 84 days.
Table 3.
Independent Association of Change (Δ) in 24-Hour Urine Citrate and Δ NAE With Δ Bone Turnover Markers, Urine Calcium, and Urine pH
|
Δ Urine Citrate, mmol/d |
Δ Urine NAE, mmol/d |
Model R2 | |||||
|---|---|---|---|---|---|---|---|
| Unstandardized β | Standardized β | P Value | Unstandardized β | Standardized β | P Value | ||
| Δ Urine NTX, nmol/d | |||||||
| Baseline adjusted | 4.630 | 0.078 | 0.121 | 1.846 | 0.565 | <0.001 | 0.501 |
| + Age and sex adjusted | 3.795 | 0.064 | 0.206 | 1.840 | 0.564 | <0.001 | 0.511 |
| + Height adjusted | 4.260 | 0.072 | 0.155 | 1.852 | 0.567 | <0.001 | 0.519 |
| Δ Urine calcium, mmol/d | |||||||
| Baseline adjusted | 1.769 | 0.051 | 0.409 | 0.345 | 0.181 | 0.003 | 0.272 |
| + Age and sex adjusted | 1.432 | 0.041 | 0.501 | 0.353 | 0.185 | 0.003 | 0.277 |
| + Height adjusted | 1.372 | 0.040 | 0.521 | 0.353 | 0.185 | 0.003 | 0.277 |
| Δ Serum P1NP, nmol/L | |||||||
| Baseline adjusted | −0.603 | −0.124 | 0.041 | 0.021 | 0.078 | 0.200 | 0.286 |
| + Age and sex adjusted | −0.528 | −0.109 | 0.075 | 0.020 | 0.073 | 0.225 | 0.297 |
| + Height adjusted | −0.510 | −0.105 | 0.086 | 0.020 | 0.073 | 0.226 | 0.299 |
| Δ Urine pH | |||||||
| Baseline adjusted | 0.156 | 0.409 | <0.001 | −0.007 | −0.345 | <0.001 | 0.565 |
| + Age and sex adjusted | 0.157 | 0.410 | <0.001 | −0.007 | −0.346 | <0.001 | 0.569 |
| + Height adjusted | 0.157 | 0.411 | <0.001 | −0.007 | −0.346 | <0.001 | 0.569 |
Based on linear regression in which change in bone turnover markers, urine calcium, and urine pH were outcomes and change in urine citrate and NAE were exposures in the same model.
Discussion
In older community-dwelling adults, 24-hour urine citrate increased as the absolute dose of alkali supplementation (per meter of height) increased, but the increase in urine citrate did not significantly predict the decrease in the bone resorption biomarker urine NTX. In contrast, the decrease in NAE (the gold-standard measure of systemic acid-base status) significantly predicted a decrease in urine NTX. When the change in urine citrate and change in urine NAE were both included as predictors of change in bone resorption in the same fully adjusted model, the change in urine NAE remained an important predictor of change in urine NTX and calcium excretion. This finding suggests that, in older men and women, urine NAE is a more robust indicator of short-term changes in bone resorption than urine citrate. In the combined model, neither changes in citrate nor NAE remained an important predictor of change in P1NP, likely reflecting the secondary role of P1NP compared with NTX, related to coupling bone resorption and formation. In our trial, low- and high-dose alkali supplementation reduced urine calcium excretion, as previously reported (17). However, the change in urine citrate was not associated with the change in urine calcium. In contrast, the decrease in NAE was significantly associated with a decrease in urine calcium. In subclinical metabolic acidosis, urine calcium excretion increases because renal calcium reabsorption is reduced, which can lead to an increase in bone turnover (24). That the change in urine citrate was not associated with the change in calcium excretion in older adults is consistent with our finding that change in urine citrate did not independently predict change in bone turnover. These findings are not consistent with those of Esche et al. (21), who reported that higher urine citrate was associated with higher bone mineral content and lower long-term fracture risk in children and adolescents. It is possible that urine citrate predicts changes in BMD or fracture risk, which we did not measure, more so than it predicts changes in bone turnover biomarkers in older adults. However, it is likely a weaker predictor than urine NAE because urine NTX reflects bone remodeling and independently predicts BMD and fracture (25, 26). It is also plausible that urine citrate is associated with bone remodeling differently in children [as studied by Esche et al. (21)] than in older adults (studied here). Nonetheless, in older adults, change in urine NAE appears to be a better predictor of change in bone resorption and calcium excretion than change in urine citrate.
The increase in urine citrate in response to alkali supplementation was significantly correlated with the decrease in urine NAE. At baseline, however, urine citrate did not correlate with urine NAE, indicating that a single urine citrate measure is not a suitable marker of acid-base status in older adults. In children and adolescents, urine citrate was significantly positively correlated with the RAEC (21), but we did not find a similar association in older adults. Furthermore, at baseline, neither the calculated NEAP nor PRAL (4, 5) predicted the 24-hour urine citrate excretion, whereas they are important positive predictors of 24-hour NAE. NEAP and PRAL are higher when intakes of protein and cereal grains are high relative to fruits and vegetables, which together increase acid load and therefore acid excretion (4, 5, 27). Esche et al. (21) reported that lower PRAL was significantly correlated with higher urine citrate in children and adolescents. Under controlled feeding conditions, urine citrate increased when a vegetarian diet (alkali-producing) was consumed and decreased when a high-protein western-type diet (acid-producing) was consumed for 5 days by healthy young men (28). Conversely, NAE increased after consumption of a high-protein diet for 5 days and decreased when a diet higher in fruits and vegetables was consumed (27). It is possible that changes in dietary acid load are associated with changes in urine citrate, but we were unable to evaluate this because the calculated dietary acid load did not change appreciably during our intervention (e.g., the mean ± standard deviation NEAP at baseline was 48.5 ± 22.7 mEq/d, whereas on day 84 it was 49.4 ± 22.4 mEq/d). This was not unexpected because our study did not incorporate dietary changes. Nonetheless, our findings confirm that urine citrate responds to changes in alkali intake, as others have reported (20, 29). However, our findings also suggest that in older adults 24-hour urine NAE may be a more useful biomarker of dietary acid load than 24-hour urine citrate because the NEAP and PRAL predicted NAE but did not predict urine citrate at baseline.
This study was not designed to evaluate the association between the change in urine citrate and change in bone turnover and urine calcium, which is a limitation. However, by measuring urine citrate in a randomized placebo-controlled dose-finding trial of alkali supplementation in which bone turnover was reduced in the supplementation groups (17), we were able to efficiently test the hypothesis that changes in urine citrate predict changes in bone turnover in older adults. We previously demonstrated that alkali supplementation reduced urine NAE (17) and showed here that urine citrate increased concomitantly. This allowed us to evaluate whether the change in urine citrate and NAE (both objective indicators of systemic acid-base status) similarly predicted change in bone turnover and calcium excretion. Our study participants were generally healthy older adults and were primarily white, so generalizability to dissimilar groups is uncertain. However, it is important to evaluate the utility of urine citrate with respect to bone turnover in this demographic group because they are at risk for age-related metabolic acidosis, bone loss, and fracture (30, 31).
In summary, two dietary estimates of acid-base status, NEAP and PRAL, predicted urine NAE but not citrate, suggesting that NAE is a more robust indicator of acid-base status than urine citrate in older adults. Furthermore, the change in urine citrate was not as robust an indicator of change in bone-turnover or calcium excretion as change in NAE. Therefore, urine citrate does not appear to be a suitable alternative to urine NAE when assessing acid-base status in relation to bone turnover in older adults.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01AR0602261 and US Department of Agriculture Agreement 58-1950-0-014. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the US Department of Agriculture.
Clinical Trial Information: ClinicalTrials.gov no. NCT01475214 (registered 21 November 2011).
Author Contributions: M.K.S. conducted statistical analyses, contributed to data interpretation, and drafted the manuscript. B.D.-H. designed the study, contributed to data interpretation, revised the manuscript for intellectual content, and approved the manuscript’s final version.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- bone mineral density
- KHCO3
- potassium bicarbonate
- NAE
- net acid excretion
- NEAP
- net endogenous acid production
- NTX
- N-telopeptide of collagen type-I
- P1NP
- amino-terminal propeptide of type 1 procollagen
- PRAL
- potential renal acid load
- RAEC
- renal acid excretion capacity.
References
- 1.Chen W, Melamed ML, Abramowitz MK. Serum bicarbonate and bone mineral density in US adults. Am J Kidney Dis. 2015;65(2):240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabatabai LS, Cummings SR, Tylavsky FA, Bauer DC, Cauley JA, Kritchevsky SB, Newman A, Simonsick EM, Harris TB, Sebastian A, Sellmeyer DE; Health, Aging, and Body Composition Study . Arterialized venous bicarbonate is associated with lower bone mineral density and an increased rate of bone loss in older men and women. J Clin Endocrinol Metab. 2015;100(4):1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carnauba RA, Baptistella AB, Paschoal V, Hübscher GH. Diet-induced low-grade metabolic acidosis and clinical outcomes: a review. Nutrients. 2017;9(6):E538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frassetto LA, Todd KM, Morris RC Jr, Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998;68(3):576–583. [DOI] [PubMed] [Google Scholar]
- 5.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95(7):791–797. [DOI] [PubMed] [Google Scholar]
- 6.Welch AA, Bingham SA, Reeve J, Khaw KT. More acidic dietary acid-base load is associated with reduced calcaneal broadband ultrasound attenuation in women but not in men: results from the EPIC-Norfolk cohort study. Am J Clin Nutr. 2007;85(4):1134–1141. [DOI] [PubMed] [Google Scholar]
- 7.New SA, MacDonald HM, Campbell MK, Martin JC, Garton MJ, Robins SP, Reid DM. Lower estimates of net endogenous non-carbonic acid production are positively associated with indexes of bone health in premenopausal and perimenopausal women. Am J Clin Nutr. 2004;79(1):131–138. [DOI] [PubMed] [Google Scholar]
- 8.Rahbar A, Larijani B, Nabipour I, Mohamadi MM, Mirzaee K, Amiri Z. Relationship among dietary estimates of net endogenous acid production, bone mineral density and biochemical markers of bone turnover in an Iranian general population. Bone. 2009;45(5):876–881. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald HM, New SA, Fraser WD, Campbell MK, Reid DM. Low dietary potassium intakes and high dietary estimates of net endogenous acid production are associated with low bone mineral density in premenopausal women and increased markers of bone resorption in postmenopausal women. Am J Clin Nutr. 2005;81(4):923–933. [DOI] [PubMed] [Google Scholar]
- 10.Jia T, Byberg L, Lindholm B, Larsson TE, Lind L, Michaëlsson K, Carrero JJ. Dietary acid load, kidney function, osteoporosis, and risk of fractures in elderly men and women. Osteoporos Int. 2015;26(2):563–570. [DOI] [PubMed] [Google Scholar]
- 11.Garcia AH, Franco OH, Voortman T, de Jonge EA, Gordillo NG, Jaddoe VW, Rivadeneira F, van den Hooven EH. Dietary acid load in early life and bone health in childhood: the Generation R Study. Am J Clin Nutr. 2015;102(6):1595–1603. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald HM, Black AJ, Aucott L, Duthie G, Duthie S, Sandison R, Hardcastle AC, Lanham New SA, Fraser WD, Reid DM. Effect of potassium citrate supplementation or increased fruit and vegetable intake on bone metabolism in healthy postmenopausal women: a randomized controlled trial. Am J Clin Nutr. 2008;88(2):465–474. [DOI] [PubMed] [Google Scholar]
- 13.Dijkstra SC, Neter JE, Brouwer IA, Huisman M, Visser M. Misperception of self-reported adherence to the fruit, vegetable and fish guidelines in older Dutch adults. Appetite. 2014;82:166–172. [DOI] [PubMed] [Google Scholar]
- 14.Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol. 1995;24(2):389–398. [DOI] [PubMed] [Google Scholar]
- 15.Miller TM, Abdel-Maksoud MF, Crane LA, Marcus AC, Byers TE. Effects of social approval bias on self-reported fruit and vegetable consumption: a randomized controlled trial. Nutr J. 2008;7(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell JA, Whiting SJ. Effect of fruit on net acid and urinary calcium excretion in an acute feeding trial of women. Nutrition. 2004;20(5):492–493. [DOI] [PubMed] [Google Scholar]
- 17.Dawson-Hughes B, Harris SS, Palermo NJ, Gilhooly CH, Shea MK, Fielding RA, Ceglia L. Potassium bicarbonate supplementation lowers bone turnover and calcium excretion in older men and women: a randomized dose-finding trial. J Bone Miner Res. 2015;30(11):2103–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jehle S, Hulter HN, Krapf R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2013;98(1):207–217. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen K. Titrimetric determination of the net excretion of acid/base in urine. Scand J Clin Lab Invest. 1957;9(3):287–291. [DOI] [PubMed] [Google Scholar]
- 20.Simpson DP. Citrate excretion: a window on renal metabolism. Am J Physiol. 1983;244(3):F223–F234. [DOI] [PubMed] [Google Scholar]
- 21.Esche J, Johner S, Shi L, Schönau E, Remer T. Urinary citrate, an index of acid-base status, predicts bone strength in youths and fracture risk in adult females. J Clin Endocrinol Metab. 2016;101(12):4914–4921. [DOI] [PubMed] [Google Scholar]
- 22.Remer T, Montenegro-Bethancourt G, Shi L. Long-term urine biobanking: storage stability of clinical chemical parameters under moderate freezing conditions without use of preservatives. Clin Biochem. 2014;47(18):307–311. [DOI] [PubMed] [Google Scholar]
- 23.Chan JC. The rapid determination of urinary titratable acid and ammonium and evaluation of freezing as a method of preservation. Clin Biochem. 1972;5(2):94–98. [DOI] [PubMed] [Google Scholar]
- 24.Bushinsky DA. Acid-base imbalance and the skeleton. Eur J Nutr. 2001;40(5):238–244. [DOI] [PubMed] [Google Scholar]
- 25.Baxter I, Rogers A, Eastell R, Peel N. Evaluation of urinary N-telopeptide of type I collagen measurements in the management of osteoporosis in clinical practice. Osteoporos Int. 2013;24(3):941–947. [DOI] [PubMed] [Google Scholar]
- 26.Cauley JA, Danielson ME, Greendale GA, Finkelstein JS, Chang YF, Lo JC, Crandall CJ, Neer RM, Ruppert K, Meyn L, Prairie BA, Sowers MR. Bone resorption and fracture across the menopausal transition: the Study of Women’s Health Across the Nation. Menopause. 2012;19(11):1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remer T, Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 1994;59(6):1356–1361. [DOI] [PubMed] [Google Scholar]
- 28.Siener R, Hesse A. The effect of different diets on urine composition and the risk of calcium oxalate crystallisation in healthy subjects. Eur Urol. 2002;42(3):289–296. [DOI] [PubMed] [Google Scholar]
- 29.Sakhaee K, Alpern R, Jacobson HR, Pak CY. Contrasting effects of various potassium salts on renal citrate excretion. J Clin Endocrinol Metab. 1991;72(2):396–400. [DOI] [PubMed] [Google Scholar]
- 30.Amodu A, Abramowitz MK. Dietary acid, age, and serum bicarbonate levels among adults in the United States. Clin J Am Soc Nephrol. 2013;8(12):2034–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eastell R, O’Neill TW, Hofbauer LC, Langdahl B, Reid IR, Gold DT, Cummings SR. Postmenopausal osteoporosis. Nat Rev Dis Primers. 2016;2:16069. [DOI] [PubMed] [Google Scholar]