Abstract
Context:
Age at diagnosis has been identified as a major determinant of thyroid cancer–specific survival, with older patients being at higher risk for mortality, but the association of age with risk of recurrence has not been studied to date.
Objective:
To examine the effect of a patient’s age on response to therapy and disease-specific mortality in a cohort of thyroid cancer patients at high risk of recurrence, as defined by the American Thyroid Association (ATA) risk stratification system.
Design:
Retrospective cohort study of 320 patients, median age 49.3 years, with follicular cell-derived thyroid carcinoma classified at ATA high risk and followed for a median of 7 years.
Main Outcome Measures:
Association of age with response to therapy, overall mortality, disease-specific mortality, and timing of metastases.
Results:
Age was a major determinant of response to therapy. There was a significantly larger percentage of excellent responders among young patients (age <55) than among old patients (age ≥55), 40.3% vs 27.5%, P = 0.002, respectively, whereas the proportion of structural incomplete responders was higher in the old group compared with the young group, 53% vs 33%, P = 0.002, respectively. ATA high-risk young patients with a structural incomplete response to therapy had a significantly better disease-specific survival than old patients (74% vs 12%, P < 0.001, respectively).
Conclusions:
Age was a key predictor of response to therapy and disease-specific survival in ATA high-risk thyroid cancer patients. Its incorporation as a variable in the ATA risk stratification system would improve its power to predict response to therapy as well as mortality.
We found that age at diagnosis significantly predicts response to therapy and disease-specific mortality in ATA high-risk thyroid cancer patients.
Age at diagnosis has been identified as a major determinant of thyroid cancer–specific survival, with older patients being at higher risk for mortality (1–6). Whereas the association between advanced age and poorer survival is common for many types of cancer, well-differentiated thyroid carcinoma is the only human malignancy to include age as part of the American Joint Committee on Cancer (AJCC) staging system. Although it has become increasingly clear that there is no specific age cutoff that predicts prognosis (7, 8) and that the association between advancing age and worse outcomes is better represented as a continuum (9–12), most thyroid cancer-staging systems (3, 13–18) incorporate a patient’s age as a dichotomous variable. With the recognition of the overall good prognosis of well-differentiated thyroid carcinoma, the AJCC staging system recently changed the age cutoff from 45 to 55 years to provide more appropriate risk stratification (13).
The AJCC/tumor, lymph node, and metastasis system is optimized to predict survival in patients with cancer. However, the predicting of the risk of recurrence may be equally important in all stages of the disease. Advanced forms of thyroid cancer constitute between 5% and 10% of thyroid cancers, but they account for approximately one-third of all thyroid cancer-related deaths (19–21). The revised American Thyroid Association (ATA) guidelines combined surgical findings with pathologic characteristics of the tumor to create a staging system for use in all patients with thyroid carcinoma to help predict the risk of recurrent/persistent disease in this population (22). According to the ATA risk stratification system, the high-risk group comprises patients with macroscopic invasion of the tumor into perithyroidal soft tissues, incomplete tumor resection, presence of distant metastases, postoperative serum thyroglobulin (Tg) suggestive of distant metastatic disease, pathologic N1 disease with any metastatic lymph node ≥3 cm in largest dimension, and follicular thyroid carcinoma with extensive (greater than four foci) vascular invasion. Interestingly, the ATA risk stratification system does not include age as a predictor of recurrence. Previous reports demonstrate that the ATA high-risk category has a remission rate between 15% and 30% (23–25) and a 16% mortality risk (24). However, from clinical experience, it appears that young patients, even when classified initially at ATA high risk of recurrence, have much better outcomes than the statistics presented previously, specifically, better remission rates and disease-specific survival than older patients.
Therefore, because no studies to date have particularly focused on the effect of age on risk of recurrence and mortality in ATA high-risk patients, we hereby describe the natural history and prognostic factors associated with recurrence and disease-specific survival in a large cohort of high-risk thyroid cancer patients. We hypothesize that the risk of recurrence and mortality differ by age at presentation within the ATA high-risk category; specifically, that young patients have a lower risk of recurrence and better overall survival than older patients. This difference may justify the incorporation of age as a variable in the next iteration of the ATA risk stratification system with the purpose of improving its ability to predict recurrence and providing additional insight into the age-adjusted mortality in high-risk patients.
Subjects and Methods
Subjects
After obtaining Institutional Review Board approval, we used the hospital’s electronic search engine to identify patients who had a total thyroidectomy for a follicular cell-derived thyroid carcinoma between January 2005 and December 2010 and that fit the criteria for classification into ATA high risk of recurrence that included any of the following: macroscopic tumor invasion, incomplete tumor resection with gross residual disease (R2), any metastatic lymph node larger than 3 cm in size, extensive vascular invasion (more than four foci), presence of distant metastases, or a postoperative Tg level suggestive of distant metastatic disease (22). We chose to include patients operated on between 2005 and 2010 to ensure appropriate clinical follow-up when the majority of recurrences would occur. Of the 728 unique patients retrieved by the electronic search, we excluded 408 for the following reasons: 229 were intermediate or low-risk patients, 33 had inadequate information for initial staging, two had concurrent papillary and medullary or anaplastic thyroid carcinoma, and 144 had incomplete follow-up information. All 320 patients included in the study were receiving thyroid-stimulating hormone-suppressive therapy and had at least one neck ultrasound performed at our center after initial therapy during the first 2 years of follow-up (most had two or more cross-sectional imaging evaluations) and two or more serum Tg and Tg antibody (TgAb) determinations obtained while on levothyroxine suppression. A stimulated Tg value was not a requirement for inclusion in the study but was available in 82% of the patients. We used age at the time of total thyroidectomy as a cutoff to define the two groups: old ≥55 years; young <55 years.
Laboratory studies
Between 2005 and 2015, all Tg values were measured using the Dynotest-TgS immunoradiometric assay (Brahms Diagnostica, Berlin, Germany; functional sensitivity 0.6 ng/mL normalized to Certified Reference Material 457). Starting on 2 March 2015, all Tg tests were performed by chemiluminescent immunoassay on the Beckman Access, functional sensitivity 0.1 ng/mL (Indianapolis, IN; www.beckmancoulter.com).
Anti-TgAb were measured with immunoenzymatic assay, AIA-1800 (Tosoh, Tessenderlo, Belgium). Values ≥20 IU/mL before 2011 and ≥12 IU/mL after 2011 were considered positive.
Clinical outcomes
Response to therapy was defined as the overall treatment outcome of patients as opposed to the evaluation of response to individual treatment modalities. Patients were considered to have an excellent response to therapy or no clinical evidence of disease (NED) at final follow-up if they had a suppressed serum Tg < 0.2 ng/mL, no detectable TgAb, and no structural evidence of disease. Patients with suppressed Tg values ≥1 ng/mL; stimulated Tg values ≥10 ng/mL; or any evidence of disease on cross-sectional imaging (ultrasound, computed tomography scan, or magnetic resonance imaging), functional imaging (radioiodine scan or 18-fluorodeoxyglucose-positron emission tomography scan), or biopsy-proven disease (cytology or histology) were considered as having persistent disease. Patients with nonspecific imaging findings; nonstimulated Tg, detectable but <1 ng/mL; stimulated Tg, detectable but <10 ng/mL; or anti-Tg antibodies, stable or declining in the absence of structural or functional disease, were classified as having an indeterminate response to therapy. Patients with persistent disease were further classified as having either biochemical evidence of disease (elevated, suppressed Tg ≥1 ng/mL, stimulated Tg values ≥10 ng/mL, or rising anti-Tg antibodies without a structural correlate) or structural evidence of disease on imaging (regardless of Tg or anti-TgAb levels). Patients were considered to have structural evidence of disease if any of the following conditions were met: (1) positive cytology/histology (2) highly suspicious lymph nodes or thyroid bed nodules on the neck US (hypervascularity, cystic areas, heterogeneous content, rounded shape, or enlargement over time), or (3) evidence of metastatic disease on radioactive iodine (RAI) scans, 18-fluorodeoxyglucose-positron emission tomography scans, or other cross-sectional imaging studies. A recurrence was defined as new biochemical (suppressed Tg ≥1 ng/mL and/or stimulated Tg ≥10 ng/mL), structural, or functional evidence of disease that was detected following any period of NED.
Cause of death was attributed in the majority of patients by reviewing the patient’s death note, death certificate, or events that led to the patient’s demise. All patients with a structural incomplete response to therapy died of thyroid carcinoma (disease-specific mortality), and all patients with an excellent response at final follow-up died of causes unrelated to thyroid cancer.
Statistical methods
Statistical analyses were carried out using STATA Statistical Software v12 (StataCorp, College Station, TX). Student’s t test was used to compare continuous variables when normally distributed, and the Mann-Whitney test was used to compare medians of variables not normally distributed. Pearson’s χ2 test was used to examine categorical variables with Fischer’s exact test when appropriate. To examine the effect of age on response to therapy, we used multivariate logistic regression models. Recurrence and mortality were analyzed using survival analysis. Kaplan–Meier curves were built, and the log-rank test was used to assess for significance of the surviving function. P < 0.05 was considered statistically significant.
Results
The demographic, clinical, and pathologic characteristics of 320 patients at ATA high risk of recurrence are outlined in Table 1. The majority of patients had papillary thyroid carcinoma (67.8%), they were women (57.5%), and they had a median age of 49.3 years (range 6.8 to 82.4). Seven patients were younger than 18 years. There was a large proportion of patients with poorly differentiated thyroid carcinoma (21%), with extensive vascular invasion (56.3%), with extrathyroidal extension (94%, both minor and gross), and with incomplete tumor resection (31%; R1 and R2). Approximately one-third of the patients had N1b disease (35.1%). Even though all 320 patients were at ATA high risk of recurrence, the majority of patients (59%) were classified as having stage I disease, considering the 8th Edition of the AJCC staging system. Almost every patient was treated with RAI (98.4%), with a median cumulative activity of 150 mCi (0 to 1085 mCi), and a large percentage of them (21%) received external beam radiation therapy to the neck.
Table 1.
Baseline Characteristics of 320 Included Patients at ATA High Risk of Recurrence
| Result | n = 320 | |
|---|---|---|
| Age, y; median (range) | 49.3 (6.8–82.4) | |
| Sex, female | 57.5% | 184 |
| Surgery at MSK | 63.8% | 204 |
| Median follow-up, y (range) | 7 (0.13–17) | |
| Histology | ||
| Papillary | 67.8% | 217 |
| Classical variant | 32.8% | 105 |
| Tall cell variant | 16% | 51 |
| Follicular variant | 6.25% | 20 |
| Diffuse sclerosing variant | 4.1% | 13 |
| Microcarcinoma | 2.2% | 7 |
| Other | 6.6% | 21 |
| Poorly differentiated | 20.9% | 67 |
| Hurthle cell carcinoma | 7.2% | 23 |
| Follicular carcinoma | 4.1% | 13 |
| Median tumor size, cm (range) | 2.7 (0.3–14.5) | |
| Multicentricity | 41.0% | 131 |
| Neck dissection performed | 57.8% | 185 |
| Median number of lymph nodes resected (range) | 9 (0–116) | |
| Median number of positive lymph nodes (range) | 6 (0–99) | |
| N0 | 27.5% | 88 |
| N1a | 27.5% | 88 |
| N1b | 35% | 112 |
| Nx | 10% | 32 |
| Presence of extranodal extension | 36.0% | 115 |
| Tumor capsule | ||
| Unencapsulated | 42.5% | 136 |
| Partially encapsulated | 30.3% | 97 |
| Completely encapsulated | 27.0% | 86 |
| Capsular invasion | ||
| None | 64.5% | 205 |
| Focal | 9.7% | 31 |
| Extensive | 25.6% | 82 |
| Blood vessel invasion | ||
| None | 27.5% | 88 |
| Focal | 16.3% | 52 |
| Extensive | 56.3% | 180 |
| Minor extrathyroidal extension | 61% | 195 |
| Gross extrathyroidal extension | 32.5% | 104 |
| Completeness of resection | ||
| R0 | 59.1% | 189 |
| R1 | 23.1% | 74 |
| R2 | 7.8% | 25 |
| AJCC (8th Edition) | ||
| I | 59% | 185 |
| II | 25% | 79 |
| III | 6.3% | 20 |
| IV | 9% | 29 |
| RAI therapy | 98.4% | 315 |
| Median cumulative activity, mCi (range) | 150 (0–1085) | |
| External beam radiation therapy | 20.9% | 67 |
Abbreviation: MSK, Memorial Sloan-Kettering Cancer Center.
Response to therapy
After a median follow-up of 7 years (range, 0.13 to 17 years), 113 of 320 patients (35.3%) had achieved an excellent response to therapy NED. A total of 148 patients (46.3%) had persistent/recurrent disease at final follow-up (20.3% since presentation), the majority had a structural incomplete response (n = 130; 41%), and only 6% (n = 18) had a biochemical incomplete response (Table 2).
Table 2.
Response to Therapy at Final Follow-Up Stratified by Age
| Age <55 | Age ≥55 | Total | |
|---|---|---|---|
| Excellent | 79 (40.3%) | 34 (27.5%) | 113 (35.3%) |
| Biochemical incomplete | 10 (5%) | 8 (6.5%) | 18 (5.6%) |
| Indeterminate | 43 (22%) | 16 (13%) | 59 (18.5%) |
| Structural incomplete | 64 (32.7%) | 66 (53%) | 130 (40.6%) |
| Total | 196 | 124 | 320 |
χ2 P = 0.002.
Age was a major determinant of response to therapy. There was a significantly larger percentage of excellent responders among young patients (age < 55) than among old patients (age ≥ 55), 40.3% vs 27.5%, P = 0.002, respectively, whereas the proportion of structural incomplete responders was higher in the old group compared with the young group, 53% vs 33%, P = 0.002, respectively (Table 2).
To understand the reasons underlying this different response to therapy by age, we explored demographic, clinical, and pathologic characteristics of structural incomplete responders stratified by age (Supplemental Table 1 (12.6KB, docx) ). Within the structural incomplete responders, older patients were enriched with poorly differentiated thyroid carcinomas (49% vs 30%, P = 0.002), whereas younger patients had a larger proportion of papillary thyroid carcinomas (67% vs 36%, P = 0.002). Gross extrathyroidal extension and extensive vascular invasion did not differ between the two age groups, but old patients had significantly larger tumors than young patients (4.5 vs 3.3 cm, P = 0.03). More patients in the young group had a neck dissection performed than in the old group (67% vs 36%, P < 0.001). Median cumulative dose of RAI did not differ between the two age groups, but median Tg at final follow-up was significantly higher in old patients compared with young patients (223 vs 14.5 ng/mL, P = 0.03, respectively). In a multivariate model, age was an independent predictor of structural incomplete response to therapy when adjusted by tumor type, tumor size, vascular invasion, extrathyroidal extension, metastatic disease, and neck dissection performed, odds ratio, 1.02; 95% confidence interval, 1.002 to 1.04; P = 0.029.
Natural history of ATA high-risk patients
A total of 65 out of 320 patients (20.3%) had persistent structural disease despite initial therapy (defined as surgery plus any adjuvant radiotherapy, including RAI alone and/or external beam radiation therapy): 43 patients had distant metastases (13.4%), 18 patients had persistent cervical nodal metastases (5.6%), and 4 patients had both local and distant persistent disease after initial therapy (1.25%).
Of the 255 patients who did not have structural persistent disease after initial therapy, 32.2% had a structural disease recurrence discovered at a median of 2.2 years: 74 patients (29%) had a local recurrence discovered at a median of 2.1 years (range, 1.2 to 3.3 years), and 56 patients (22%) had distant metastatic disease discovered at a median of 2.4 years (range, 1 to 5 years). Patients who developed distant metastases were significantly older than those who did not [median age 58 (50 to 63) vs 42.3 years old (33 to 58), P < 0.001] and included a higher proportion of poorly differentiated thyroid carcinomas than those who did not (46% vs 9.2%, P < 0.001). Our cohort had 13 patients (4%) with high-risk follicular thyroid carcinoma. Despite an expected high frequency of hematogenous spread in these tumors, only two patients developed distant metastatic disease at final follow-up.
The most common site of first distant metastasis was the lung (n = 72, 70.6%), followed by the combination of lung and bone (n = 13, 13%), followed by bone (n = 12, 12%). Less common sites were brain (n = 3, 3%), orbit (n = 1, 1%), and liver (n = 1, 1%). There was no statistically significant difference in the distribution of metastases by age, although young patients tended to have more lung metastases, whereas old patients tended to have more bone metastases (P = 0.289) (Supplemental Table 2 (12.6KB, docx) ).
Table 3 shows that one-third of the patients at ATA high risk of recurrence developed distant metastatic disease (n = 102, 32%). Approximate one-half of these (46%) were discovered before or at the time of thyroid cancer diagnosis, whereas the other one-half developed metastases after initial therapy (54%) at a median time of 2.4 years. Twenty-five percent of young patients had metastases discovered at the time of their initial radioiodine scan, whereas 24% of old patients presented with metastatic disease before thyroidectomy. A larger proportion of old patients metastasized after initial therapy (59% vs 48%, P = 0.042). Older patients did so at a median time of 2 years compared with 3 years for younger patients.
Table 3.
Timing of Distant Metastatic Disease in ATA High-Risk Patients (n = 102)
| Age <55 | Age ≥55 | Total | |
|---|---|---|---|
| Before total thyroidectomy | 6 (13.6%) | 14 (24.2%) | 20 (19.6%) |
| At time of surgery | 6 (13.6%) | 1 (1.7%) | 7 (6.9%) |
| At time of initial RAI scan | 11 (25%) | 9 (15.5%) | 20 (19.6%) |
| After initial therapy | 21 (47.8%) | 34 (58.6%) | 55 (53.9%) |
| Median time, y (IQR) | 3 (1.2–5.7) | 2 (1–4.4) | 2.4 (1–5) |
| Total | 44 | 58 | 102 |
Fisher’s exact P = 0.042.
Abbreviation: IQR, interquartile range.
Mortality
A total of 57 of 320 patients (17.8%) died after a median follow-up of 7 years (range, 0.13 to 17 years). Forty-eight of these patients (84%) had a structural incomplete response at final follow-up, 3 (5%) had a biochemical incomplete response, 3 (5%) had an indeterminate response, and 3 (5%) had an excellent response to therapy. All 48 patients with a structural incomplete response to therapy died of thyroid cancer, so the disease-specific mortality was 15% in the entire cohort. Two patients with a structural incomplete response to therapy had widely metastatic, poorly differentiated thyroid carcinoma and died within 3 months of their thyroid surgery. The three patients with an excellent response to therapy died for reasons unrelated to thyroid cancer: one patient, diagnosed at age 58, died of metastatic renal cell carcinoma, one patient, diagnosed at age 68, died of a rectal bleed as a result of metastatic endometrial carcinoma, and one patient, diagnosed at age 75, died of a myocardial infarction.
High-risk patients who died (n = 57) were older (median age 63.4 vs 44.6 years, P < 0.001) and had a higher proportion of poorly differentiated thyroid carcinoma (52.6% vs 14%, P < 0.001), gross extrathyroidal extension (41% vs 31%, P < 0.001), extensive vascular invasion (81% vs 51%, P < 0.001), and distant metastatic disease (37% vs 10%, P < 0.001) than those patients who remained alive (n = 263). Interestingly, a lower proportion of patients who died had a cervical neck dissection compared with those who remained alive (31.6% vs 63.5%, P < 0.001).
Age was a key predictor of overall and disease-specific mortality. Whereas 40 of 124 (32.3%) old patients died in this high-risk cohort, only 17 of 196 (8.7%) young patients died (P < 0.001) after a median follow-up of 7 years.
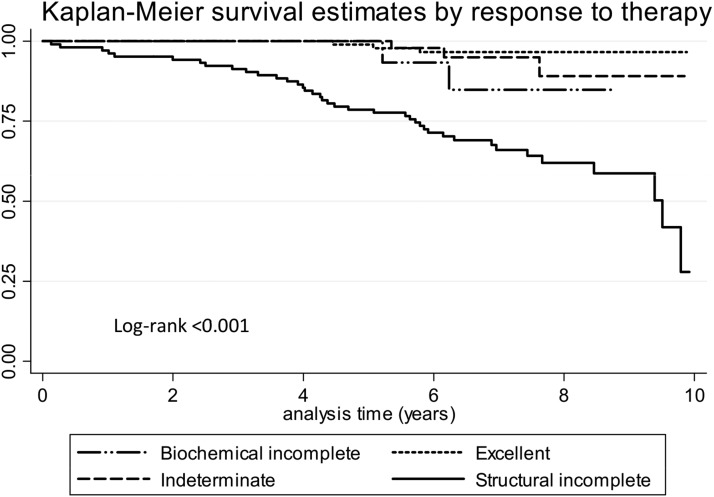
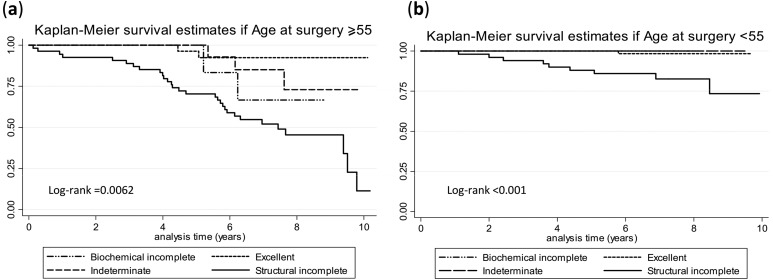
Figure 1 shows that the ATA response to therapy classification was able to predict mortality. In this sense, structural incomplete responders had a 10-year survival rate of 28%, whereas excellent responders, biochemical incomplete responders, and indeterminate responders all had survival rates at 10 years above 85%. When age was taken into account, survival of this high-risk cohort was very different for young vs old patients (Fig. 2). At 10 years, only 12% of structural incomplete responders were alive if their age at surgery was ≥55 years, compared with 74% of structural incomplete responders if their age at surgery was younger than 55 years old. There was a 65% to 95% survival rate at 10 years for excellent, biochemical incomplete, and indeterminate responders if their thyroidectomy was done after 55 years of age, compared with 99% survival for the same categories if the surgery was done before 55 years of age [Fig. 2(a) and 2(b)].
Figure 1.
Mortality by ATA response to therapy classification at 10 years.
Figure 2.
Mortality by ATA response to therapy classification at 10 years stratified by age. (a) Kaplan-Meier survival estimates if age at surgery was ≥55. (b) Kaplan-Meier survival estimates if age at surgery was <55.
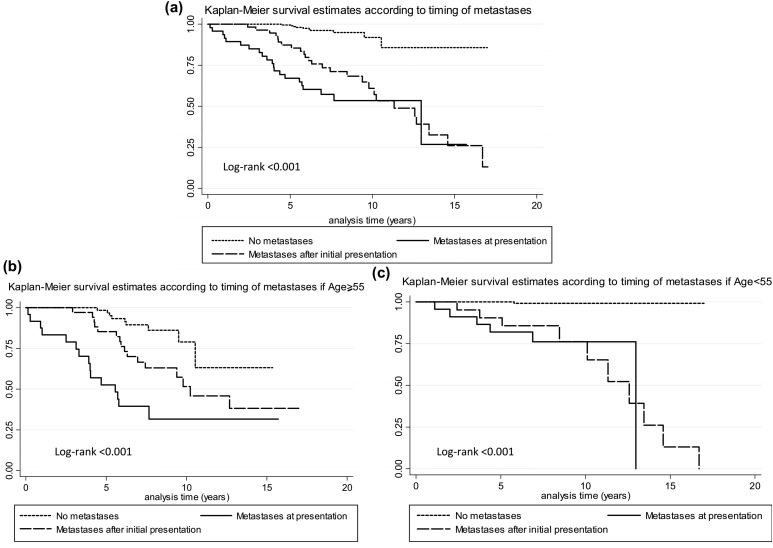
When survival was analyzed by the timing of distant metastatic disease appearance, we found that patients who presented with distant metastatic disease before or at the time of initial surgery or first RAI scan had an ∼50% survival at 10 years [Fig. 3(a)]. Those patients who had distant metastatic disease discovered after their initial therapy died later, but this difference disappeared after 10 years of follow-up. In contrast, patients at high risk of recurrence, who never developed distant metastatic disease, had an 85% survival, even after 17 years of follow-up [Fig. 3(a)].
Figure 3.
Mortality by timing of metastatic disease in (a) overall high-risk cohort and (b and c) stratified by age. Kaplan-Meier survival estimates according to the timing of metastases in the (a) entire high-risk cohort, (b) if age at surgery was ≥55, and (c) if age at surgery was <55.
When mortality by timing of metastases was stratified by age, we found that old patients who presented with metastases before thyroidectomy had a 10-year survival of 28% compared with 75% for young patients, P < 0.001; old patients who presented with metastases after initial therapy had a 10-year survival of 50% compared with 75% for young patients, P < 0.001; and old patients who never developed distant metastases had a 10-year survival rate of 77% compared with 99% for young patients, P < 0.001 [Fig. 3(b) and 3(c)].
Discussion
In this study, we demonstrate that age at diagnosis is a key predictor of recurrence and mortality in ATA high-risk patients. We found that within the ATA high-risk category, young patients had a higher likelihood of achieving a complete remission (NED) (40% vs 28%, P = 0.02, respectively), whereas older patients had a higher probability of having persistent disease at final follow-up. Furthermore, age was an independent predictor of disease-specific survival. In this sense, ATA high-risk young patients with a structural incomplete response to therapy had a significantly better survival than old patients in the same group (74% vs 12%, P < 0.001, respectively).
Age has been recognized as a major determinant of cancer-specific survival in patients with follicular cell-derived thyroid carcinoma. Its prognostic ability to predict disease-specific mortality independently has led to the incorporation of a patient’s age as a dichotomous variable in all major thyroid cancer-staging systems (14–18). These staging systems have been designed to predict survival, but in an indolent disease, such as thyroid carcinoma, with generally low mortality rates, a system that predicts recurrence rather than survival becomes equally relevant at the time of evaluating overall prognosis. Interestingly, the ATA risk stratification system that is widely used to predict recurrence in all stages of the disease does not incorporate age as a predictive variable.
The aim of this study was to examine the effect of age on response to therapy. We selected a cohort of high-risk thyroid cancer patients to have an appropriate number of structural incomplete responders and death events. We found that age was a major predictor of response to therapy, such that the proportion of structural incomplete responders at final follow-up was significantly larger among old patients than among young patients (53% vs 33%, respectively). This difference, in response to therapy, likely drove the difference that we found in mortality rates by age. Whereas 32.3% of old patients died in this ATA high-risk cohort, only 8.7% of young patients died after a median follow-up of 7 years.
Previous reports that included patients at all risks of recurrence have validated the clinical applicability of the ATA risk stratification system (23–26). The high-risk group in these studies had a remission rate between 14% and 31%. We found that the overall remission rate of our high-risk cohort was compared with previous studies, with 35.3% of our patients achieving an excellent response to therapy at final follow-up. However, a patient’s age had a substantial impact on remission, such that young patients had a remission rate of 40.3% compared with 27.5% of old patients. Conversely, 53% of old patients had a structural incomplete response at final follow-up compared with only 32.6% of young patients. To attempt to explain the latter findings, we collected demographic, clinical, and pathologic characteristics of structural incomplete responders and performed a multivariate analysis. We found that old patients were enriched with poorly differentiated thyroid carcinomas, and they had larger tumors and more distant metastases at final follow-up, suggesting that even though all of the patients had been initially classified as being at high risk of recurrence, older patients seemed to have had a more aggressive disease. Despite this, age was an independent predictor of structural incomplete response to therapy in multivariate analyses. Interestingly, more neck dissections were performed in the young group with a structural incomplete response to therapy. This suggests that not only was the biology of the disease different in young vs old patients at high risk of recurrence but also that the surgical interventions were less aggressive in the old group with metastatic disease. The presence of distant metastatic disease in the overall context of more aggressive disease in elderly patients probably would have discouraged the surgeon from performing aggressive local neck resections when these would not have altered the overall prognosis.
The unique pattern of metastatic disease discovery, with approximately one-half of the patients having distant metastases discovered at diagnosis and the remaining one-half after initial therapy, is generally true for all high-risk patients (27, 28). We did not find a substantial difference in the tropism of distant metastases by age, but young patients tended to have more lung metastases, whereas old patients tended to have more bone metastases. Approximately one-quarter of old patients presented with distant metastases before thyroidectomy. These were generally bone fractures or lung metastases that resulted in the diagnosis of metastatic thyroid cancer, whereas approximately one-quarter of young patients had mostly lung metastases discovered at the time of the initial radioiodine scan. When metastases were discovered after initial therapy, young patients tended to have them discovered later (3 years) than old patients (2 years). Fifty percent of old patients who presented with distant metastases died at 5 years compared with only 20% of young patients who presented with distant metastases. This effect of age on mortality in the presence of distant metastases may be related to comorbidities of the host or to a microenvironment in old patients that allows for metastatic disease to behave more aggressively.
The mortality rate of patients at ATA high risk of recurrence has been reported at ∼16% (24). We had an overall comparable mortality rate of 17.8%. Age, however, was an important determinant of overall and disease-specific mortality risk. Whereas 32.3% of old patients died in this high-risk cohort, only 8.7% of young patients died after a median follow-up of 7 years. Old patients with a structural incomplete response to therapy had an 88% disease-specific mortality rate at 10 years compared with only 26% of young patients. The larger proportion of structural incomplete responders enriched with poorly differentiated thyroid carcinomas in the old group, and the higher percentage of older patients with distant metastatic disease at final follow-up probably contributed to this finding. However, our current findings and previous reports (7, 10, 29–32) have adjusted for these factors and still found an independent association between age and thyroid cancer mortality. The mechanism by which increasing age is associated with increased mortality in thyroid cancer is unknown, but possible hypotheses include the higher frequency of the BRAF V600E mutation in the elderly population, telomerase reverse transcriptase promoter mutations enriched in fatal nonanaplastic thyroid cancer of the elderly that can co-occur with BRAF or RAS mutations, decreased avidity and response to RAI therapy in old patients, and impaired immune response in the elderly (33–37).
As a result of the retrospective nature of this study, our data may have been incomplete or could have suffered from treatment bias by physicians regarding the selection of surgical or adjuvant therapies. However, only 33 of 728 initial patients had incomplete information for initial staging, and 144 patients had incomplete follow-up information. Both of these groups were excluded from the study. We believe that such a small proportion of patients with incomplete data would probably not have altered the overall results. Regarding the treatment selection bias, endocrinologists and surgeons at Memorial Sloan-Kettering Cancer Center work in a multidisciplinary team and share a unified approach to the management of high-risk patients, which would limit the treatment bias. A second limitation relates to the known referral bias of advanced patients to a tertiary referral center that may have resulted in higher metastatic and mortality events than seen in other settings. Even if this was the case, both young and old patients would have had the same standard of care, and the effect of age on response to therapy and on mortality in high-risk patients is likely independent of quality of care and generalizable to other high-risk cohorts. Finally, the overall number of deaths was limited. Despite this small number of death events, we had the power to build Kaplan-Meier curves stratified by response to therapy, age, and timing of metastases that proved very informative.
In conclusion, in this study, we report the effect of age on response to therapy and disease-specific mortality in a cohort of thyroid cancer patients within the ATA high-risk category. We describe that young patients achieve a higher rate of complete remission at final follow-up and a lower mortality rate than old patients, even in the presence of structural disease. Conversely, older patients have a larger proportion of structural incomplete responders to therapy that results in overall increased mortality of this group. We appreciate that a continuum of risk exists within all of the ATA categories, but given that age has such a strikingly different effect on response to therapy and disease-specific mortality in the high-risk group, we propose that age be incorporated as a variable in the ATA high-risk category to integrate prognostic information in a single stratification system and improve its power to predict response to therapy as well as mortality.
Acknowledgments
We thank Drs. James A. Fagin and R. Michael Tuttle for providing helpful comments and critically reviewing this manuscript.
Financial Support: Support for this paper was provided by National Institutes of Health/National Cancer Institute Grant P30 CA 008748.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AJCC
- American Joint Committee on Cancer
- ATA
- American Thyroid Association
- NED
- no clinical evidence of disease
- RAI
- radioactive iodine
- Tg
- thyroglobulin
- TgAb
- thyroglobulin antibody.
References
- 1.Cady B, Sedgwick CE, Meissner WA, Bookwalter JR, Romagosa V, Werber J. Changing clinical, pathologic, therapeutic, and survival patterns in differentiated thyroid carcinoma. Ann Surg. 1976;184(5):541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzaferri EL, Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981;70(3):511–518. [DOI] [PubMed] [Google Scholar]
- 3.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114(6):1050–1057, discussion 1057–1058. [PubMed] [Google Scholar]
- 4.Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis MC, Steward DL, Maxon HR, Sherman SI; National Thyroid Cancer Treatment Cooperative Study Group . The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab. 2012;97(6):E878–E887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito Y, Miyauchi A, Kihara M, Takamura Y, Kobayashi K, Miya A. Relationship between prognosis of papillary thyroid carcinoma patient and age: a retrospective single-institution study. Endocr J. 2012;59(5):399–405. [DOI] [PubMed] [Google Scholar]
- 6.Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ, Aniss A, Morris L, Vaisman F, Corbo R, Momesso D, Vaisman M, Carvalho A, Learoyd D, Leslie WD, Nason RW, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gonen M, Pathak KA, Shen WT, Sywak M, Kowalski L, Freeman J, Perrier N, Shah JP. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid. 2016;26(3):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orosco RK, Hussain T, Brumund KT, Oh DK, Chang DC, Bouvet M. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the Surveillance, Epidemiology, and End Results database. Thyroid. 2015;25(1):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Londero SC, Krogdahl A, Bastholt L, Overgaard J, Pedersen HB, Hahn CH, Bentzen J, Schytte S, Christiansen P, Gerke O, Godballe C; Danish Thyroid Cancer Group-DATHYRCA (part of the DAHANCA organization) . Papillary thyroid carcinoma in Denmark, 1996–2008: outcome and evaluation of established prognostic scoring systems in a prospective national cohort. Thyroid. 2015;25(1):78–84. [DOI] [PubMed] [Google Scholar]
- 9.Oyer SL, Smith VA, Lentsch EJ. Reevaluating the prognostic significance of age in differentiated thyroid cancer. Otolaryngol Head Neck Surg. 2012;147(2):221–226. [DOI] [PubMed] [Google Scholar]
- 10.Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. Exploring the relationship between patient age and cancer-specific survival in papillary thyroid cancer: rethinking current staging systems. J Clin Oncol. 2016;34(36):4415–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Shen W, Sakamoto N. Population-based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. J Clin Oncol. 2013;31(4):468–474. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee M, Muenz DG, Chang JT, Papaleontiou M, Haymart MR. Tree-based model for thyroid cancer prognostication. J Clin Endocrinol Metab. 2014;99(10):3737–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuttle MM, Haugen B, Shah J, Sosa JA, Rohren E, Subramaniam RM, Hunt JLPN. Thyroid differentiated and anaplastic carcinoma. In: Amin MB Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer International; 2017. [Google Scholar]
- 14.Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA, Mayer M, Sylvester RJ, van Glabbeke M. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer. 1979;15(8):1033–1041. [DOI] [PubMed] [Google Scholar]
- 15.Sherman SI, Brierley JD, Sperling M, Ain KB, Bigos ST, Cooper DS, Haugen BR, Ho M, Klein I, Ladenson PW, Robbins J, Ross DS, Specker B, Taylor T, Maxon HR III; National Thyroid Cancer Treatment Cooperative Study Registry Group . Prospective multicenter study of thyroiscarcinoma treatment: initial analysis of staging and outcome. Cancer. 1998;83(5):1012–1021. [DOI] [PubMed] [Google Scholar]
- 16.Hay ID, Grant CS, Taylor WF, McConahey WM. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102(6):1088–1095. [PubMed] [Google Scholar]
- 17.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104(6):947–953. [PubMed] [Google Scholar]
- 18.Dean DS, Hay ID. Prognostic indicators in differentiated thyroid carcinoma. Cancer Control. 2000;7(3):229–239. [DOI] [PubMed] [Google Scholar]
- 19.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2017;317(13):1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jegerlehner S, Bulliard JL, Aujesky D, Rodondi N, Germann S, Konzelmann I, Chiolero A, Group NW; NICER Working Group . Overdiagnosis and overtreatment of thyroid cancer: a population-based temporal trend study. PLoS One. 2017;12(6):e0179387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136(9):2187–2195. [DOI] [PubMed] [Google Scholar]
- 22.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA, Shaha A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20(12):1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaisman F, Momesso D, Bulzico DA, Pessoa CH, Dias F, Corbo R, Vaisman M, Tuttle RM. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf). 2012;77(1):132–138. [DOI] [PubMed] [Google Scholar]
- 25.Pitoia F, Bueno F, Urciuoli C, Abelleira E, Cross G, Tuttle RM. Outcomes of patients with differentiated thyroid cancer risk-stratified according to the American Thyroid Association and Latin American Thyroid Society risk of recurrence classification systems. Thyroid. 2013;23(11):1401–1407. [DOI] [PubMed] [Google Scholar]
- 26.Castagna MG, Maino F, Cipri C, Belardini V, Theodoropoulou A, Cevenini G, Pacini F. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol. 2011;165(3):441–446. [DOI] [PubMed] [Google Scholar]
- 27.Sabet A, Binse I, Dogan S, Koch A, Rosenbaum-Krumme SJ, Biersack HJ, Biermann K, Ezziddin S. Distinguishing synchronous from metachronous manifestation of distant metastases: a prognostic feature in differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2017;44(2):190–195. [DOI] [PubMed] [Google Scholar]
- 28.Lin JD, Hsueh C, Chao TC. Long-term follow-up of the therapeutic outcomes for papillary thyroid carcinoma with distant metastasis. Medicine (Baltimore). 2015;94(26):e1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A, Sywak M, Eskander AE, Freeman JL, Campbell MJ, Shen WT, Vaisman F, Momesso D, Corbo R, Vaisman M, Shaha A, Tuttle RM, Shah JP, Patel SG. Survival from differentiated thyroid cancer: what has age got to do with it? Thyroid. 2015;25(10):1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SJ, Myong JP, Suh H, Lee KE, Youn YK. Optimal cutoff age for predicting mortality associated with differentiated thyroid cancer. PLoS One. 2015;10(6):e0130848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nixon IJ, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gönen M, Shah JP. Defining a valid age cutoff in staging of well-differentiated thyroid cancer. Ann Surg Oncol. 2016;23(2):410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazurat A, Torroni A, Hendrickson-Rebizant J, Benning H, Nason RW, Pathak KA. The age factor in survival of a population cohort of well-differentiated thyroid cancer. Endocr Connect. 2013;2(3):154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, Romei C, Miccoli P, Pinchera A, Basolo F. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93(10):3943–3949. [DOI] [PubMed] [Google Scholar]
- 34.Haymart MR. Understanding the relationship between age and thyroid cancer. Oncologist. 2009;14(3):216–221. [DOI] [PubMed] [Google Scholar]
- 35.Ronga G, Filesi M, Montesano T, Di Nicola AD, Pace C, Travascio L, Ventroni G, Antonaci A, Vestri AR. Lung metastases from differentiated thyroid carcinoma. A 40 years’ experience. Q J Nucl Med Mol Imaging. 2004;48(1):12–19. [PubMed] [Google Scholar]
- 36.Mihailovic J, Stefanovic L, Malesevic M, Markoski B. The importance of age over radioiodine avidity as a prognostic factor in differentiated thyroid carcinoma with distant metastases. Thyroid. 2009;19(3):227–232. [DOI] [PubMed] [Google Scholar]
- 37.Ibrahimpasic T, Xu B, Landa I, Dogan S, Middha S, Seshan V, Deraje S, Carlson DL, Migliacci J, Knauf JA, Untch B, Berger MF, Morris L, Tuttle RM, Chan T, Fagin JA, Ghossein R, Ganly I. Genomic alterations in fatal forms of non-anaplastic thyroid cancer: identification of MED12 and RBM10 as novel thyroid cancer genes associated with tumor virulence. Clin Cancer Res. 2017;23(19):5970–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]