Abstract
In order to examine the usefulness of intratracheal instillation of nanoparticles for the screening of the harmful effects of nanoparticles, we performed intratracheal instillation studies of nanomaterials on rats using different delivery devices and postures as a basic study. Multiwall carbon nanotubes (MWCNTs) with a geometric mean length and secondary diameter of 2.16 μm and 752 nm, respectively, were used as the nanomaterials. Male F344 rats were intratracheally exposed to 0.04 or 0.2 mg/rat of MWCNT, were dissected at 1 d and 3 d, and cell analyses of the bronchoalveolar lavage fluid (BALF) were analyzed. Two delivery devices were used for the intratracheal instillation of the MWCNTs: a gavage needle and a microsprayer aerolizer. Both induced neutrophil influx in the lung at 1 and 3 d, and there were no significant differences in neutrophil inflammation between the two delivery devices. The main distribution of pulmonary inflammation by both delivery devices was in the centrilobular spaces in the lung. Two postures were used: an angle of approximately 45 degrees and a standing posture on a board, both of which also induced pulmonary influx in BALF and pulmonary inflammation mainly in the centrilobular spaces, with no large difference in pulmonary inflammation between the two postures. Taken together, the differences in the delivery devices and postures of the rats in the intratracheal instillation did not affect the acute pulmonary toxicity of the nanomaterials.
Keywords: Nanomaterial, Intratracheal instillation, Inflammation, Harmful effect, Administration
Introduction
Various manufactured nanomaterials have been produced with the increasing development of nanotechnology, but the pulmonary toxicity of these manufactured nanomaterials is not fully understood. Although the most reliable studies for exploring the pulmonary toxicity of respirable chemicals are inhalation studies, due to their having the most realistic exposure for real-life humans1), it is impossible for the pulmonary toxicity of all nanomaterials to be examined this way because of the high cost, the need for large facilities, and securing technical expertise for maintaining stable exposure and keeping a desirable dispersion of nanomaterials2, 3). Intratracheal instillation studies, on the other hand, are useful for examining the dosage dependence of nanomaterials and the clarification of the mechanism involved in exposure to nanomaterials4, 5, 6, 7), but the findings of pulmonary toxicity of nanomaterials are limited. Intratracheal instillation studies have garnered attention in the research of the pulmonary toxicity of nanomaterials because of their relatively inexpensive cost and their requirement of relatively simple equipment8).
Comparing inhalation and intratracheal instillation studies for the pulmonary response induced by nanomaterials, acute pulmonary inflammation due to a bolus effect is observed following intratracheal instillation3, 4), unlike inhalation studies. We conducted inhalation and intratracheal instillation studies of nanomaterials with high and low toxicity, and found that the pattern of pulmonary ranking of nanomaterials is the same when pulmonary inflammation in not only the acute phase but also in the chronic phase are examined3, 9, 10). There are some other studies in which the ranking of pulmonary inflammation by nanomaterials is the same in inhalation studies and intratracheal instillation studies4, 8, 11), suggesting that intratracheal instillation studies may be useful for ranking the harmful effects of nanomaterials.
There are not yet enough studies about the methodology of intratracheal instillation. Although there is a report that the results of an interlaboratory evaluation of rodent pulmonary responses to intratracheally exposed nanomaterials were similar, a detailed methodology of the intratracheal instillation studies was not shown12). Even if there are different results between laboratories, it cannot be denied that the differences might be related to a difference in the basic techniques of intratracheal instillation. Therefore, in order to examine whether or not pulmonary toxicity following intratracheal instillation of nanomaterials is a reflection of the technique used, we examined differences in pulmonary inflammation resulting from different delivery devices and different positions of animals in intratracheal instillations.
Materials and Methods
Preparation of samples of multiwall carbon nanotube (MWCNT) suspensions
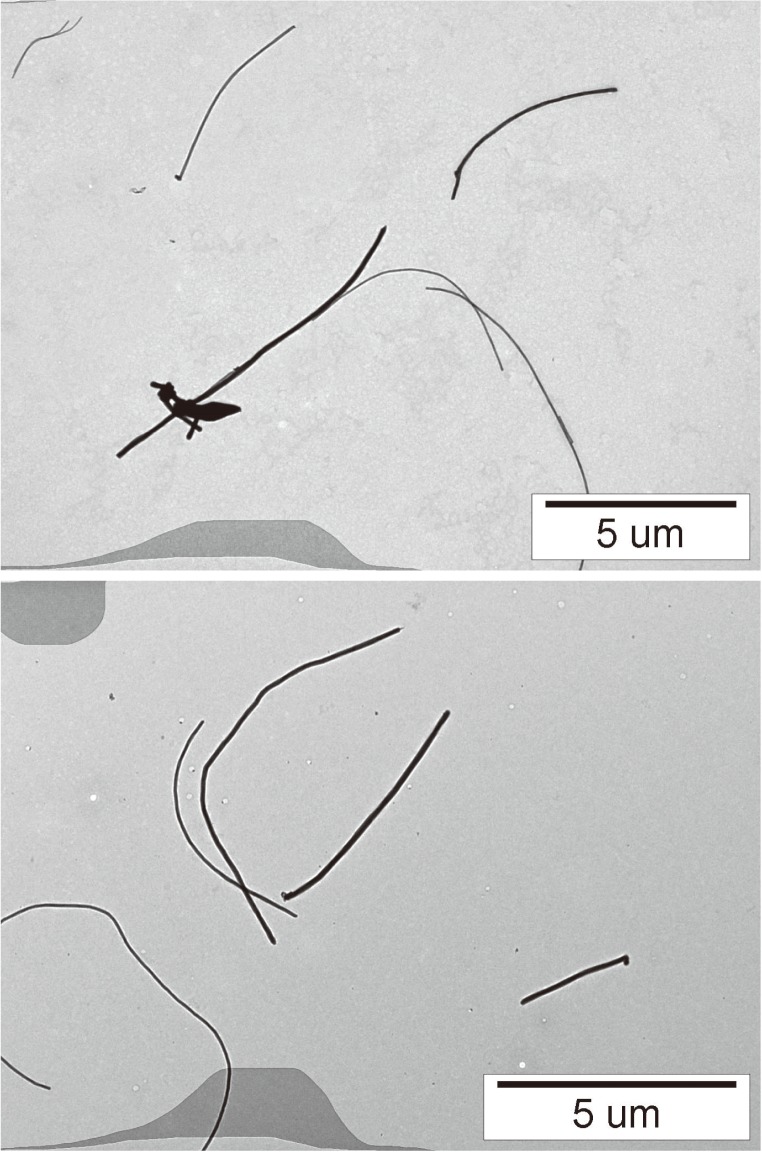
The tested carbon nanotubes (CNTs) were MWCNTs (Mitsui-7) purchased from Mitsui & Co., Ltd., the physico-chemical properties of which are shown in Table 1. A 50 ml bovine serum albumin (BSA)(08587-42, Nacalai Tesque, Inc ) aqueous solution of 10 mg/ml was added to the MWCNT of 50 mg sampled in a glass vial of 100 ml. The vial was placed in an ultrasonic bath (5510-MT, BRANSON) and sonication at 70 W was applied to the suspension for 1.5 h. The suspension of dispersed MWCNT was filtered through a cell strainer with a pore size of 40 μm in order to remove the coarse agglomerates. The concentration of the MWCNT in the filtered suspension was prepared to 0.1 or 0.5 mg/ml, and the suspensions were used as stock suspensions for the intratracheal instillation tests. The mean size of the secondary particles was characterized by the dynamic light scattering method (Zetasizer Nano ZS, Malvern) and the cumulant mean was 752 nm. The state of dispersing in the suspension was observed by TEM (JEM-1010, JEOL). The CNTs were well dispersed, as shown in Fig. 1. Measuring the distribution of the length of the dispersed CNTs the TEM photos, the geometric mean and the geometric SD were 2.16 μm and 4.2, respectively. The geometric mean diameter and the geometric SD were 52 nm and 1.5, respectively.
Table 1. Physico-chemical properties of MWCNTs.
| Physico-chemical Properties | Value |
|---|---|
| Maker | Mitsui & Co., Ltd |
| Sample | Mitsui-7 |
| Geometric mean diameter (SD) | 52 nm (1.5) |
| Geometric mean length (SD) | 2.16 μm (4.2) |
| Specific surface area (BET) | 23.0 m2/g |
| Purity | More than 99.6% |
| Shape | Fibrous |
| Ratio of GD* | 13 ± 3 |
| Secondary diameter(DLS) | 752 nm |
| Solubility | Low |
*Intensity ratio of G-band to D-band
Fig. 1.
TEM photograph of MWCNTs dispersed by sonication using an ultrasonic bath. The MWCNTs in the suspension were well-dispersed.
Animals
Male Fischer 344 rats (10 wk old) were purchased from Charles River Laboratories International, Inc. (Japan). The animals were kept in the Laboratory Animal Research Center of the University of Occupational and Environmental Health for two wk with access to free-feeding of commercial diet and water. All procedures and animal handling were done in accordance with the guidelines described in the Japanese Guide for the Care and Use of Laboratory Animals as approved by the Animal Care and Use Committee, University of Occupational and Environmental Health, Japan.
Intratracheal instillation of nanomaterial
The MWCNTs were suspended in 0.4 ml distilled water, and 0.04 mg (0.16 mg/kg) or 0.2 mg (0.8 mg/kg) of MWCNTs was administered to rats (12 wk old) by a single intratracheal instillation. Negative control groups received distilled water including 10% BSA. Three methods of intratracheal instillation were used after anesthetization by isoflurane inhalation: 1) Rats maintained a standing posture on a board and were exposed to MWCNTs by a gavage needle inserted into the tracheal lumen; 2) Rats were kept in a supine posture angled approximately 45 degrees to a board, and were exposed to MWCNTs by a microspray aerosolizer inserted into the tracheal lumen; and 3) Rats maintained a standing posture on a board and were exposed to MWCNTs by a microspray aerosolizer inserted into the tracheal lumen.
Animals (5 rats in each group) were dissected at 1 d and 3 d after the instillation.
There were 5 rats each in the control, low dose, and high dose groups at each time course. The right lungs were inflated with total 20 ml physiological saline under a pressure of 20 cm water, and BALF was collected and divided into two to three times. Between 15 and 18 ml of BALF was collected in collection tubes by free fall. The histopathological evaluation was performed with the left lung inflated and fixed by 10% formalin solution at 25 cm H2O pressure.
Analysis of inflammatory cells in BALF with cytospin
From 10 to 13 ml of BALF from the first subgroups was centrifuged at 400 g at 4°C for 15 min. The supernatant was transferred to a new tube and used for measuring the cytokines in the BALF. The pellets were washed by suspension with polymorphonuclear leukocyte (PMN) Buffer (137.9 mM NaCl, 2.7 mM KCl, 8.2 mM Na2HPO4, 1.5 mM KH2PO4, 5.6 mM C6H12O6) and centrifuged at 400 g at 4°C for 15 min. After the supernatant was removed, the pellets were resuspended with 1 ml of PMN Buffer. The cell number in the BALF was counted by Celltac (Nihon Kohden Corp., Tokyo, Japan), and cells were splashed on a slide glass using cytospin. After the cells were fixed and stained with Diff-Quik (SYSMEX Corp., Hyogo, Japan), the number of neutrophils were counted by microscopic observation.
Parameter in BALF
The concentration of Rat cytokine-induced neutrophil chemoattractant (CINC) -1 in the BALF was measured by ELISA kits, #RCN100 (R&D Systems, Minneapolis, MN). The concentration of total protein was measured using Pierce TM 660 nm Protein Assay Reagent (22660, Thermo Fisher Scientific K.K. Yokohama, Japan) by the colorimetric determination method. The concentration of rat albumin was measured by Albumin Rat ELISA Quantitation Set (E110-125) and ELISA Starter Accessory Package Kit I (E101) (Bethyl Laboratories, Inc. Montgomery, TX).
Histopathology
The lung tissue, which was inflated and fixed with a 10% formalin solution under a pressure of 25 cm water, was embedded in paraffin, and 4 μm-thick sections were cut from the lobe, then stained with hematoxylin and eosin.
Statistical analysis
Analysis of variance (ANOVA) and Dunnett’s test were applied where appropriate to determine individual differences using a computer statistical package (SPSS, SPSS Inc., Chicago, IL, U.S.A.).
Results
1) Comparison of pulmonary inflammation and delivery devices
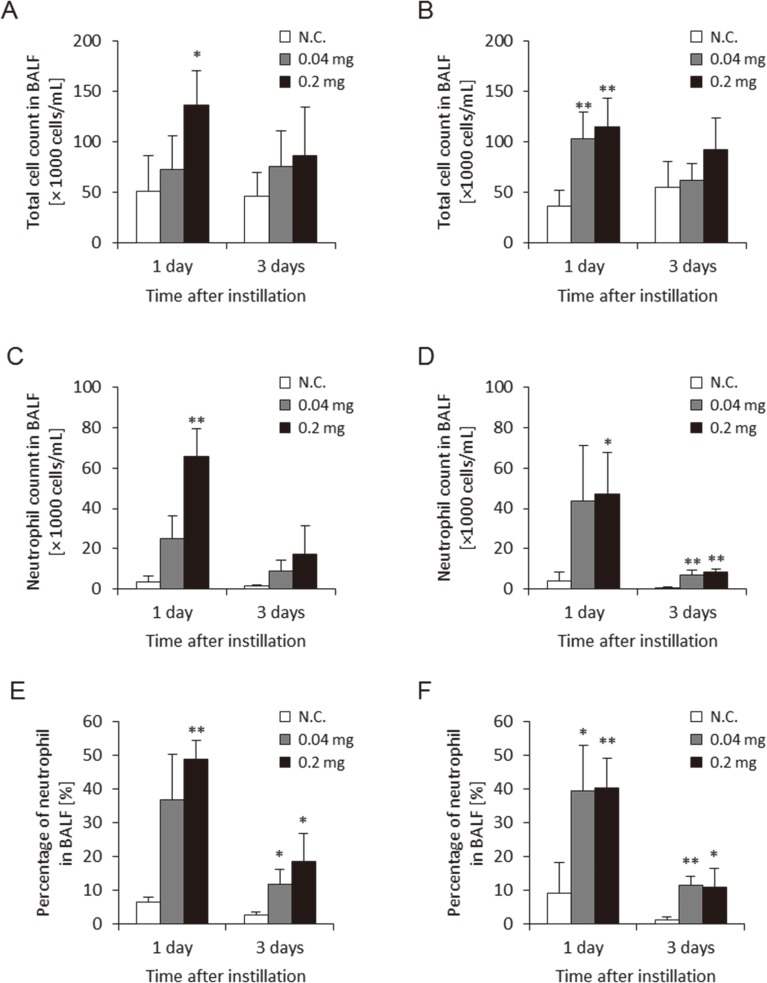
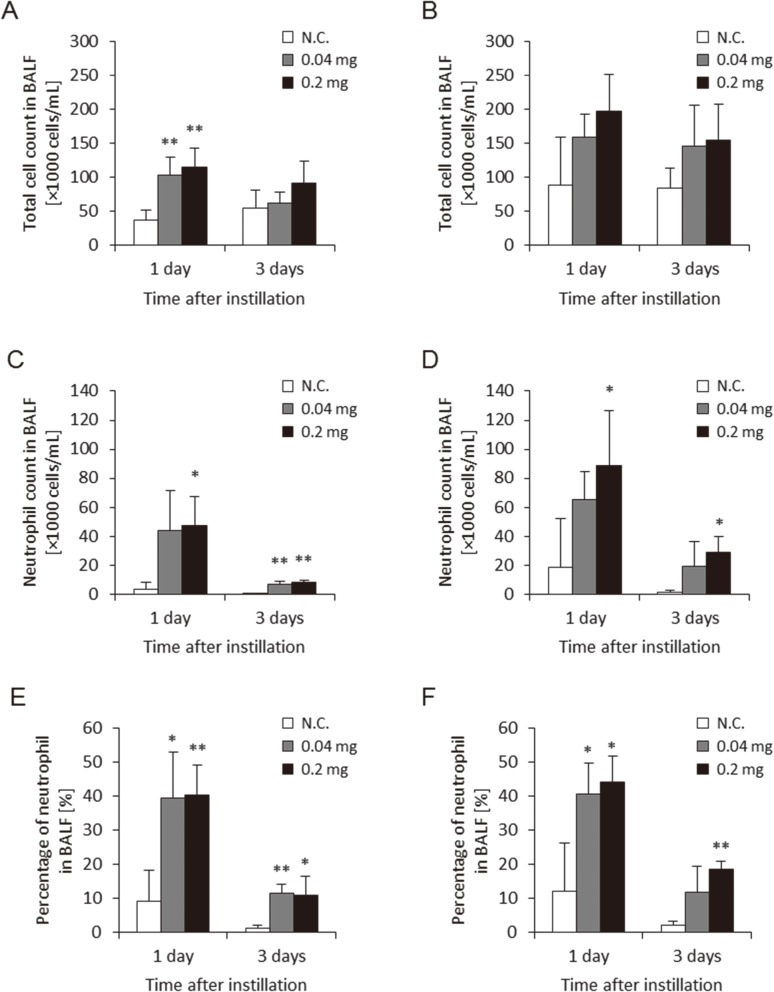
Figure 2 shows the total cell count, and the neutrophil count and percentage of neutrophils in BALF in the two approaches of gavage needle and microsprayer aerosolizer. These three parameters in BALF using both approaches increased somewhat in the 0.04 mg and 0.2 mg/rat at 1 d and 3 d postexposure. There were no persistent differences in these data between the two approaches during the observation periods. The level of these parameters at 1 d was higher than that at 3 d, but considering that MWCNTs have a high biopersistence, these phenomenon may be due to the addition of the bolus shot.
Fig. 2.
Cell analysis of bronchoalveolar lavage fluid (BALF) with two delivery devices. (A) Total cell count in BALF using gavage needle, (B) Total cell count in BALF using microspray aerosolizer, (C) Neutrophil count in BALF using gavage needle, (D) Neutrophil count in BALF using microspray aerosolizer, (E) Neutrophil percentage in BALF using gavage needle, (F) Neutrophil percentage in BALF using microspray aerosolizer. No difference between delivery devices was observed in neutrophil influx in the lung. (N.C.; Negative control, *p<0.05, **p<0.01)
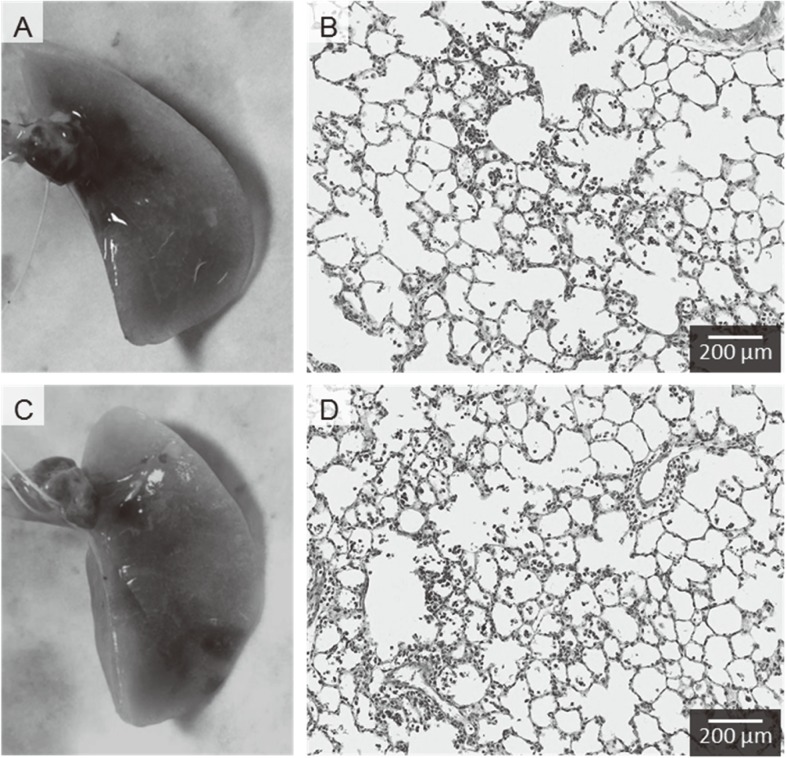
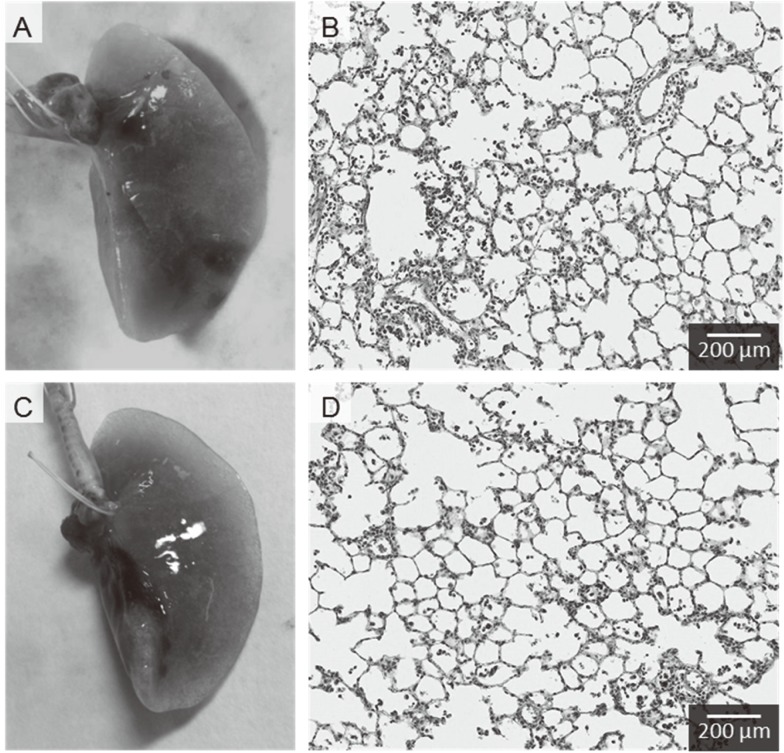
Both approaches show infiltration of inflammatory cells such as macrophages and neutrophils in the centrilobular spaces, which are neighboring alveolus lesions around the peripheral respiratory tract (Fig. 3). No pathological features such as severe fibrosis, emphysematous changes, or granulomatous changes were observed.
Fig. 3.
Representative photomicrographs of H&E-stained lung tissue in rat. (A) Macroscopic finding of lung tissue following intratracheal instillation of MWCNT using gavage needle in posture angled approximately 45 degrees, (B) Lung tissue following intratracheal instillation of MWCNT using gavage needle, (C) Macroscopic finding of lung tissue following intratracheal instillation of MWCNT using microspray aerosolizer in posture angled approximately 45 degrees, (D) Lung tissue following intratracheal instillation of MWCNT using microspray aerosolizer. Distribution of infiltration of inflammatory cells in the lung were mainly in the centrilobular lesions with both gavage needle and microspray aerosolizer.
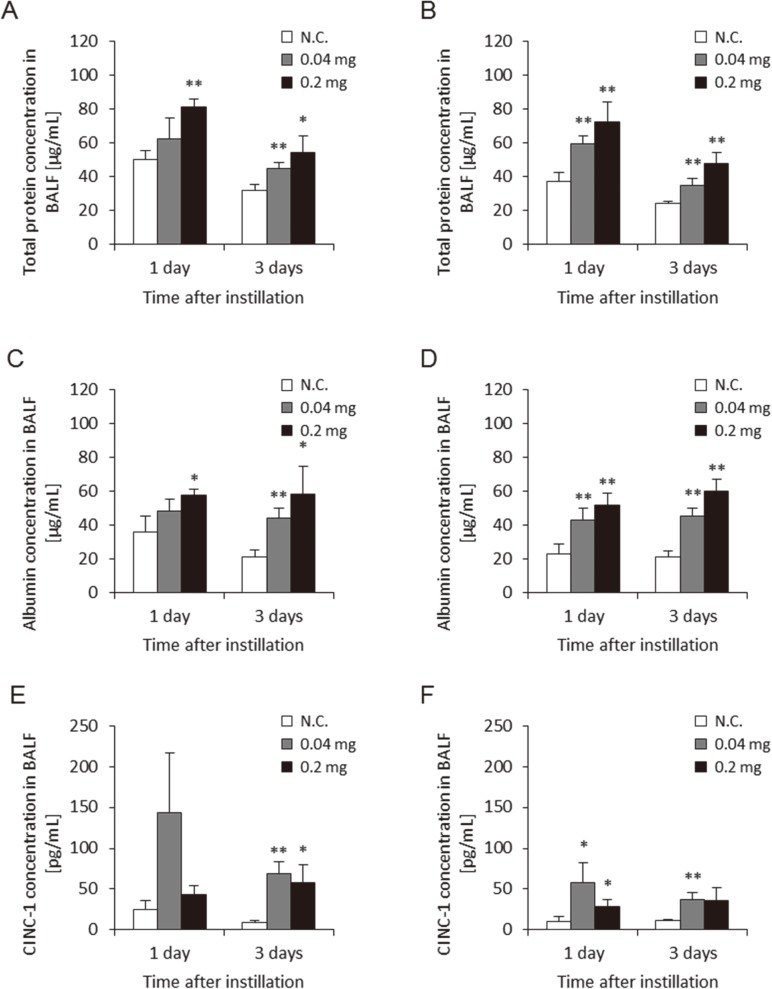
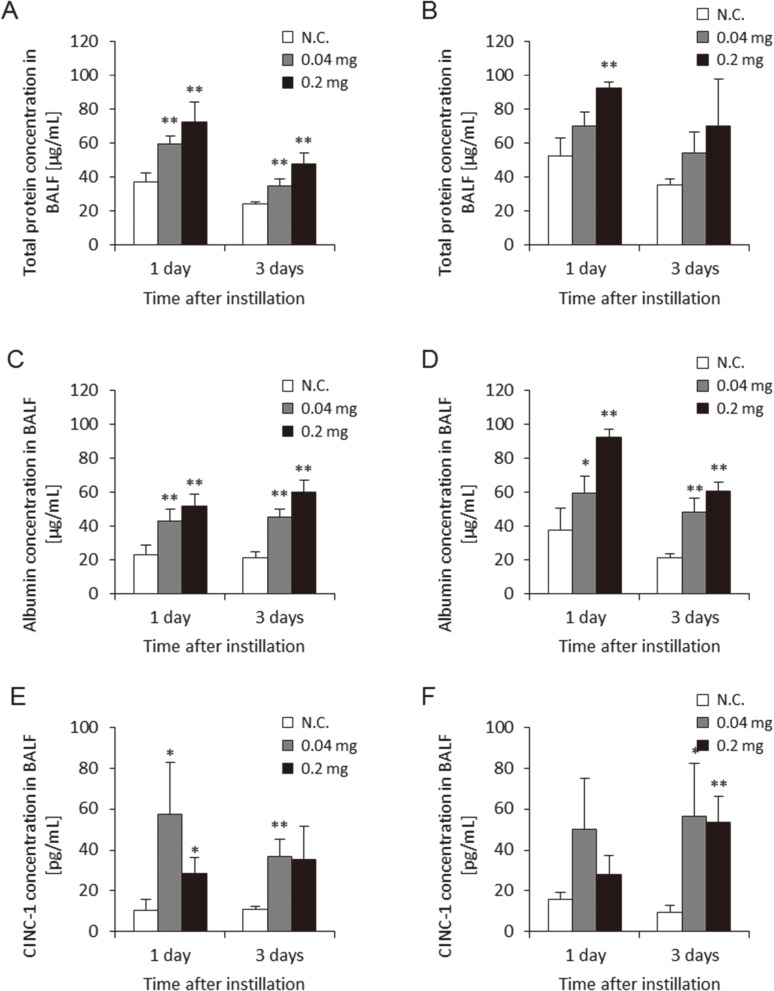
Figure 4 shows the albumin, protein, and CINC-1 concentrations in BALF by the two approaches. Albumin, protein, and CINC-1 are makers of alveolar-capillary permeability, lung injury and chemokine for neutrophil. Albumin and protein in the BALF was dose-dependently higher in the groups exposed to MWCNT at 1 d and 3 d post-exposure in both approaches. The concentration of CINC-1 in the BALF was higher in the 0.04 mg and 0.2 mg MWCNT-exposed groups during the observation period in both approaches. There were no persistent differences in these data between the two approaches during the observation periods.
Fig. 4.
Concentration of parameter in BALF with two delivery devices. (A) Concentration of albumin in BALF using gavage needle, (B) Concentration of albumin in BALF using microspray aerosolizer, (C) Concentration of protein in BALF using gavage needle, (D) Concentration of protein in BALF using microspray aerosolizer, (E) Concentration of CINC-1 in BALF using gavage needle, (F) Concentration of CINC-1 in BALF using microspray aerosolizer. No difference was observed between delivery devices for these factors. (N.C.; Negative control, *p<0.05, **p<0.01)
2) Comparison of posture and pulmonary inflammation
Figure 5 shows the cell analysis in BALF in the posture angled approximately 45 degrees and in the standing posture using the microsprayer aerosolizer. The three parameters in the BALF were higher in the two posture approaches in the 0.04 mg and 0.2 mg/rat at 1 d and 3 d post-exposure, but not significantly. There were no persistent differences in these data between the two postures during the observation periods.
Fig. 5.
Cell analysis in bronchoalveolar lavage fluid (BALF) in intratracheal instillation using microspray aerosolizer with two types of posture. (A) Total cell count in BALF in posture angled approximately 45 degrees, (B) Total cell count in BALF in vertical posture, (C) Neutrophil count in BALF in posture angled approximately 45 degrees, (D) Neutrophil count in BALF in vertical posture, (E) Neutrophil percentage in BALF in angled posture, (F) Neutrophil percentage in BALF in vertical posture. No difference was observed in neutrophil influx in the lung in the two types of posture. (N.C.; Negative control, *p<0.05, **p<0.01)
Figure 6 shows pathological features in the two posture approaches. Both postures show infiltration of inflammatory cells in the centrilobular spaces in the lung. No pathological features such as severe fibrosis, emphysematous changes, or granulomatous changes were observed. The photograph shows that in both approaches the distribution shifted to the distal sites and close to the pleura, although the distribution shifted more into the lower area than the other areas.
Fig. 6.
Representative photomicrographs of H&E-stained lung tissue in rat. (A) Macroscopic finding of lung tissue following intratracheal instillation of MWCNT in posture angled approximately 45 degrees. (B) Lung tissue following intratracheal instillation of MWCNT in posture angled approximately 45 degrees. (C) Macroscopic finding of lung tissue following intratracheal instillation of MWCNT in vertical posture. (D) Lung tissue following intratracheal instillation of MWCNT in vertical posture. Distribution of infiltration of inflammatory cells in the lung were mainly in centrilobular lesions in both of 2 types of posture.
Figure 7 shows the albumin, protein, and CINC-1 concentrations in the BALF in both approaches. The concentration of albumin and protein in the BALF in the MWCNT-exposed groups at 1 d and 3 d post-exposure was dose-dependently higher in both posture approaches, although not significantly. A higher concentration of CINC-1 in the BALF was observed in the 0.04 mg and 0.2 mg MWCNT-exposed groups during the observation period in both posture approaches. There were no persistent differences in these data between the two postures during the observation periods.
Fig. 7.
Concentration of parameters in BALF in intratracheal instillation using microspray aerosolizer with two types of posture. (A) Concentration of albumin in BALF in posture angled approximately 45 degrees. (B) Concentration of albumin in BALF in vertical posture. (C) Concentration of protein in BALF in posture angled approximately 45 degrees. (D) Concentration of protein in BALF in vertical posture. (E) Concentration of CINC-1 in BALF in posture angled approximately 45 degrees. (F) Concentration of CINC-1 in BALF in vertical posture. No difference was observed in these factors between the two types of posture. (N.C.; Negative control, *p<0.05, **p<0.01)
Discussion
We conducted an intratracheal instillation study of MWCNTs using different delivery devices and postures and examined pulmonary inflammation in order to examine whether intratracheal instillation can be useful for the screening estimation of the pulmonary toxicity of nanomaterials.
There was no difference between the delivery devices, a gavage needle and a microspray aerosolizer, in the neutrophil influx in BALF induced by the MWCNTs. MWCNTs have a potential for high inflammation, and there are many reports13, 14, 15) that exposure to MWCNTs induced neutrophil inflammation in not only inhalation but also in intratracheal instillation studies, and that inhalation of MWCNTs induced persistent pulmonary inflammation and fibrosis14, 16, 17). It has also been reported that intratracheal instillation of 1.5 mg/kg of short MWCNTs induced neutrophil inflammation in rat lung in the acute phase18). We determined 0.2 mg/rat as the maximum dose of MWCNTs because this is the maximum dose at which well-dispersed MWCNTs are kept in suspension. Silva et al.15) conducted an intratracheal instillation study at a dose of 0.2 mg of three types of MWCNTs (original, purified, carboxylic acid functionalized), and, according to the figures in their paper, the value of the PMNs in the BALF was approximately the same as those in the present study.
Considering that MWCNTs demonstrate inflammogenicity and fibrogenicity in animal studies2, 16, 17, 18), it was expected that the PMN influx in the lung in both of the approaches in the present study would persist after 3 d post-exposure, but a decrease of PMN influx was observed. Biphasic pulmonary inflammation, acute and chronic inflammation, was observed in the lung following intratracheal instillation of MWCNTs in our previous study13), and, although titanium dioxide is not a fibrous material, Kobayashi et al.7) showed that the difference between a gavage needle and a microspray aerolyzer did not yield a difference in lung inflammation and injury following intratracheal instillation of titanium dioxide nanoparticles. Taken together, the two delivery devices might not cause a difference in pulmonary inflammation, but attention should be paid to the obstruction of the suspension in the microspray aerosolizer depending on the physicochemical properties of nanomaterials, such as agglomeration (data not shown).
Although it is an indirect sign of distribution of MWCNTs, we think that the distribution of pathological lesions in the lung was that of MWCNTs. The main distribution of inflammation in the lung induced by MWCNTs with both the gavage needle and the microspray aerosolizer was in the centrilobular spaces, which are the neighboring alveolus lesions around the peripheral respiratory tract, and the distribution patterns in both approaches was fundamentally similar. In inhalation exposure studies of respirable chemicals, the main patterns of distribution of pulmonary inflammation are a centrilobular pattern19, 20). In a study by Bermudez et al.20), the macrophage accumulation and aggregation in the centrilobular and subpleural spaces in the rodent’s lung was induced by inhaled titanium dioxide nanoparticles, but, compared with intratracheal instillation, some inflammatory lesions following inhalation exposure were present deep in the alveolar wall and the subpleural space. There are no studies on the effect of inhaled nanoparticles on human lung, but there are some studies of exposure to welding fumes that include metal oxide nanoparticles21, 22). The distribution of opacity in the computed tomography image in patients with welding lung was compatible with that of centrilobular nodules, meaning that the deposition of nanoparticles from the welding fumes may be located in the centrilobular area21, 22).
Although there was little difference in pathological distributions between the gavage needle and the microspray aerosolizer, the distribution of inflammation in the gavage needle type shifted slightly to the proximal site, and no difference was observed between lobes in the distribution of inflammation. With the microspray aerosolizer, on the other hand, the distribution shifted to the distal sites and close to the pleura, although the distribution of inflammatory changes shifted more into the lower lobes than the other lobes. We speculated that the strong pressure of the microspray aerosolizer may have induced the lobular difference in inflammatory changes. Fujita et al.18) reported that MWCNTs following intratracheal instillation by a microspray aerosolizer penetrated into the pleura, and Xu et al.23) reported that MWCNTs were observed in the pleural cavity in the acute phase following intratracheal instillation of MWCNTs with a microspray aerosolizer.
Intratracheal instillation of MWCNTs in the posture angled approximately 45 degrees and in the standing posture induced neutrophil influx in BALF, but the difference in posture did not change the degree of neutrophil influx. The main distribution of inflammation in the lung induced by intratracheal instillation of MWCNTs in the 45 degree angled posture and in the standing posture was in the centrilobular spaces, but there was no difference in pulmonary inflammation in the rat lung in the pathological findings. Taken together, a difference of posture at this level can be ignored when we estimate the pulmonary toxicity of nanomaterials. Hasegawa-Baba et al.24) reported that the distribution of india ink in lung in the 45 degree angled posture were more dispersable than that in the standing posture in intratracheal instillation studies. There are a different tendency of distribution of materials between our and their studies, suggesting that the difference in viscosity liquid between india ink and MWCNT suspensions may connect with difference of distribution in the lung.
There may be difference between lobes in the amount of deposited nanomaterials using either posture for the intratracheal instillation of nanomaterials. Costa et al.25) performed an intratracheal instillation of oil combustion particles and found greater amounts of deposition in the inferior lobe than in the superior lobe. Although we did not measure the deposition of MWCNTs in the present study, we can speculate this tendency for downward deposition from the photography of the lung after using the microspray aerolizer. Costa et al.25) also performed an inhalation study of oil combustion particles and found a predominance of downward deposition. These results indicate that the difference in posture can be ignored.
In summary, we performed an intratracheal instillation study of MWCNTs for rat using different cannulas and postures as a basic study. The difference in delivery devices (gavage needle and microsprayer aerosolizer) did not result in a change of level and distribution of pulmonary inflammation in rat lung, and neither did the difference in posture. Taken together, intratracheal instillation studies can assess the acute pulmonary toxicity of nanomaterials within this condition.
Acknowledgments
This study is based on results obtained from a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO), Japan
References
- 1.Oberdörster G, Castranova V, Asgharian B, Sayre P (2015) Inhalation Exposure to Carbon Nanotubes (CNT) and Carbon Nanofibers (CNF): Methodology and Dosimetry. J Toxicol Environ Health B Crit Rev 18, 121–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morimoto Y, Horie M, Kobayashi N, Shinohara N, Shimada M (2013) Inhalation toxicity assessment of carbon-based nanoparticles. Acc Chem Res 46, 770–81. [DOI] [PubMed] [Google Scholar]
- 3.Morimoto Y, Izumi H, Yoshiura Y, Tomonaga T, Lee BW, Okada T, Oyabu T, Myojo T, Kawai K, Yatera K, Shimada M, Kubo M, Yamamoto K, Kitajima S, Kuroda E, Horie M, Kawaguchi K, Sasaki T (2016) Comparison of pulmonary inflammatory responses following intratracheal instillation and inhalation of nanoparticles. Nanotoxicology 10, 607–18. [DOI] [PubMed] [Google Scholar]
- 4.Baisch BL, Corson NM, Wade-Mercer P, Gelein R, Kennell AJ, Oberdörster G, Elder A (2014) Equivalent titanium dioxide nanoparticle deposition by intratracheal instillation and whole body inhalation: the effect of dose rate on acute respiratory tract inflammation. Part Fibre Toxicol 11, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prodan AM, Ciobanu CS, Popa CL, Iconaru SL, Predoi D (2014) Toxicity evaluation following intratracheal instillation of iron oxide in a silica matrix in rats. BioMed Res Int 2014, 134260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshiura Y, Izumi H, Oyabu T, Hashiba M, Kambara T, Mizuguchi Y, Lee BW, Okada T, Tomonaga T, Myojo T, Yamamoto K, Kitajima S, Horie M, Kuroda E, Morimoto Y (2015) Pulmonary toxicity of well-dispersed titanium dioxide nanoparticles following intratracheal instillation. J Nanopart Res 17, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi T, Oshima Y, Tsubokura Y, Hashizume N, Ajimi S, Kayashima T, Nakai M, Sasaki T, Kawaguchi K, Imatanaka N (2016) Effects of dose volume and delivery device on bronchoalveolar lavage parameters of intratracheally administered nano-sized TiO2 in rats. Regul Toxicol Pharmacol 81, 233–41. [DOI] [PubMed] [Google Scholar]
- 8.Morimoto Y, Izumi H, Yoshiura Y, Fujishima K, Yatera K, Yamamoto K (2016) Usefulness of Intratracheal Instillation Studies for Estimating Nanoparticle-Induced Pulmonary Toxicity. Int J Mol Sci 17, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morimoto Y, Izumi H, Yoshiura Y, Tomonaga T, Oyabu T, Myojo T, Kawai K, Yatera K, Shimada M, Kubo M, Yamamoto K, Kitajima S, Kuroda E, Kawaguchi K, Sasaki T (2016) Evaluation of Pulmonary Toxicity of Zinc Oxide Nanoparticles Following Inhalation and Intratracheal Instillation. Int J Mol Sci 17, 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oyabu T, Morimoto Y, Hirohashi M, Horie M, Kambara T, Lee BW, Hashiba M, Mizuguchi Y, Myojo T, Kuroda E (2013) Dose-dependent pulmonary response of well-dispersed titanium dioxide nanoparticles following intratracheal instillation. J Nanopart Res 15, 1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morimoto Y, Izumi H, Yoshiura Y, Fujisawa Y, Fujita K (2017) Significance of intratracheal instillation tests for the screening of harmful effects of nanomaterials. J UOEH 39, 123–32. [DOI] [PubMed] [Google Scholar]
- 12.Bonner JC, Silva RM, Taylor AJ, Brown JM, Hilderbrand SC, Castranova V, Porter D, Elder A, Oberdörster G, Harkema JR, Bramble LA, Kavanagh TJ, Botta D, Nel A, Pinkerton KE (2013) Interlaboratory evaluation of rodent pulmonary responses to engineered nanomaterials: the NIEHS Nano GO Consortium. Environ Health Perspect 121, 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morimoto Y, Hirohashi M, Ogami A, Oyabu T, Myojo T, Todoroki M, Yamamoto M, Hashiba M, Mizuguchi Y, Lee BW, Kuroda E, Shimada M, Wang WN, Yamamoto K, Fujita K, Endoh S, Uchida K, Kobayashi N, Mizuno K, Inada M, Tao H, Nakazato T, Nakanishi J, Tanaka I (2012) Pulmonary toxicity of well-dispersed multi-wall carbon nanotubes following inhalation and intratracheal instillation. Nanotoxicology 6, 587–99. [DOI] [PubMed] [Google Scholar]
- 14.Shvedova AA, Kisin E, Murray AR, Johnson VJ, Gorelik O, Arepalli S, Hubbs AF, Mercer RR, Keohavong P, Sussman N, Jin J, Yin J, Stone S, Chen BT, Deye G, Maynard A, Castranova V, Baron PA, Kagan VE (2008) Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol 295, L552–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva RM, Doudrick K, Franzi LM, TeeSy C, Anderson DS, Wu Z, Mitra S, Vu V, Dutrow G, Evans JE, Westerhoff P, Van Winkle LS, Raabe OG, Pinkerton KE (2014) Instillation versus inhalation of multiwalled carbon nanotubes: exposure-related health effects, clearance, and the role of particle characteristics. ACS Nano 8, 8911–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pauluhn J. (2010) Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicol Sci 113, 226–42. [DOI] [PubMed] [Google Scholar]
- 17.Ma-Hock L, Treumann S, Strauss V, Brill S, Luizi F, Mertler M, Wiench K, Gamer AO, van Ravenzwaay B, Landsiedel R (2009) Inhalation toxicity of multiwall carbon nanotubes in rats exposed for 3 months. Toxicol Sci 112, 468–81. [DOI] [PubMed] [Google Scholar]
- 18.Fujita K, Fukuda M, Endoh S, Maru J, Kato H, Nakamura A, Shinohara N, Uchino K, Honda K (2016) Pulmonary and pleural inflammation after intratracheal instillation of short single-walled and multi-walled carbon nanotubes. Toxicol Lett 257, 23–37. [DOI] [PubMed] [Google Scholar]
- 19.Bermudez E, Mangum JB, Asgharian B, Wong BA, Reverdy EE, Janszen DB, Hext PM, Warheit DB, Everitt JI (2002) Long-term pulmonary responses of three laboratory rodent species to subchronic inhalation of pigmentary titanium dioxide particles. Toxicol Sci 70, 86–97. [DOI] [PubMed] [Google Scholar]
- 20.Bermudez E, Mangum JB, Wong BA, Asgharian B, Hext PM, Warheit DB, Everitt JI (2004) Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci 77, 347–57. [DOI] [PubMed] [Google Scholar]
- 21.Akira M. (1995) Uncommon pneumoconioses: CT and pathologic findings. Radiology 197, 403–9. [DOI] [PubMed] [Google Scholar]
- 22.Chong S, Lee KS, Chung MJ, Han J, Kwon OJ, Kim TS (2006) Pneumoconiosis: comparison of imaging and pathologic findings. Radiographics 26, 59–77. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Futakuchi M, Shimizu H, Alexander DB, Yanagihara K, Fukamachi K, Suzui M, Kanno J, Hirose A, Ogata A, Sakamoto Y, Nakae D, Omori T, Tsuda H (2012) Multi-walled carbon nanotubes translocate into the pleural cavity and induce visceral mesothelial proliferation in rats. Cancer Sci 103, 2045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasegawa-Baba Y, Kubota H, Takata A, Miyagawa M (2014) Intratracheal instillation methods and the distribution of administered material in the lung of the rat. J Toxicol Pathol 27, 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa DL, Lehmann JR, Winsett D, Richards J, Ledbetter AD, Dreher KL (2006) Comparative pulmonary toxicological assessment of oil combustion particles following inhalation or instillation exposure. Toxicol Sci 91, 237–46. [DOI] [PubMed] [Google Scholar]