Abstract
Alpha-1 antitrypsin (AAT) deficiency–associated emphysema is largely attributed to insufficient inhibition of neutrophil elastase released from neutrophils. Correcting AAT levels using augmentation therapy only slows disease progression, and that suggests a more complex process of lung destruction. Because alveolar macrophages (Mɸ) express AAT, we propose that the expression and intracellular accumulation of mutated Z-AAT (the most common mutation) compromises Mɸ function and contributes to emphysema development. Extracellular matrix (ECM) degradation is a hallmark of emphysema pathology. In this study, Mɸ from individuals with Z-AAT (Z-Mɸ) have greater proteolytic activity on ECM than do normal Mɸ. This abnormal Z-Mɸ activity is not abrogated by supplementation with exogenous AAT and is likely the result of cellular dysfunction induced by intracellular accumulation of Z-AAT. Using pharmacologic inhibitors, we show that several classes of proteases are involved in matrix degradation by Z-Mɸ. Importantly, compared with normal Mɸ, the membrane-bound serine protease, matriptase, is present in Z-Mɸ at higher levels and contributes to their proteolytic activity on ECM. In addition, we identified matrix metalloproteinase (MMP)-14, a membrane-anchored metalloproteinase, as a novel substrate for matriptase, and showed that matriptase regulates the levels of MMP-14 on the cell surface. Thus, high levels of matriptase may contribute to increased ECM degradation by Z-Mɸ, both directly and through MMP-14 activation. In summary, the expression of Z-AAT in Mɸ confers increased proteolytic activity on ECM. This proteolytic activity is not rescued by exogenous AAT supplementation and could thus contribute to augmentation resistance in AAT deficiency–associated emphysema.
Keywords: macrophages, alpha-1 antitrypsin deficiency, extracellular matrix degradation, matriptase, matrix metalloproteinase-14
Clinical Relevance
The pathophysiology of Z–alpha-1 antitrypsin (Z-AAT)–related emphysema has a complex nature, but the role of macrophages (Mɸ) must still be defined. These studies show that Mɸ expressing Z-AAT have enhanced proteolytic activity against extracellular matrix through up-regulation of matriptase, and propose a new role of Mɸ in lung damage.
Alpha-1 antitrypsin (AAT) is a 52-kD circulating serine protease inhibitor produced mainly by the liver, and it is a principal inhibitor of neutrophil elastase (NE) released from activated neutrophils. AAT deficiency (AATD), manifested by abnormally low levels of AAT in circulation, was discovered in 1963, and was soon linked to lung emphysema and liver disease (1–4). AATD results from mutations in AAT gene that cause improper AAT folding, polymerization, and accumulation in the endoplasmic reticulum (ER) of hepatocytes, making an individual prone to developing fibrosis, cirrhosis, or liver carcinogenesis (1). The most common mutation leading to AATD is caused by a glutamic acid to lysine substitution at position 342 in AAT (denoted as Z-AAT). The wild-type allele is denoted as M-AAT. As a result of the Z mutation, hepatocytes secrete significantly less AAT, leading to an increased risk of developing pulmonary emphysema by the third or fourth decade of life (5). The current understanding of AATD-associated lung disease is that emphysema results from an imbalance in protease/antiproteinase activity in the lung. In AATD, the concentrations of AAT in the lung are insufficient to protect both extracellular matrix (ECM) and airway cells from the damage induced by NE released from activated neutrophils. To control NE burden, AAT replacement therapy was offered in the United States in 1989, and involves weekly injections of AAT purified from plasma of normal individuals. After more than 20 years of replacement therapy, it could be concluded that it slowed the disease progression, but did not completely halt it (6). Augmentation therapy replenishes the AAT pool in the blood, but it does not affect the expression of mutated AAT. In the lungs, both epithelial cells and macrophages (Mɸ) express AAT, and their function may be affected by intracellular accumulation of mutated AAT.
Alveolar Mɸ play a central role in lung homeostasis, host defense, response to foreign substances, and tissue remodeling (7). They are increased in patients with chronic obstructive pulmonary disease (COPD) (8) and, as suggested by recent research, could be a driving force of emphysema development (9). They can contribute on different levels: by supporting chronic inflammation; producing chemokines and cytokines; or by releasing the proteolytic activity against ECM. In patients with COPD with AATD, Mɸ release increased levels of the chemoattractants, IL-8 and leukotriene B4, in the lower respiratory tract, which consequently result in increased neutrophil recruitment (10, 11). These increased levels of IL-8 and leukotriene B4 can be corrected by administering extracellular AAT in vitro (11, 12) and, hence, should be corrected by augmentation therapy too.
Because alveolar Mɸ express AAT, we hypothesize that expression of Z-AAT would affect their function, causing them to contribute to emphysema development and resistance to augmentation therapy. ECM degradation is a hallmark of emphysema development, and we studied how expression of Z-AAT affects Mɸ ability to digest ECM. We focused on two classes of proteases involved in ECM degradation by Mɸ: matrix metalloproteinases (MMPs) and serine proteases (13–17). For our experiments, we used peripheral blood mononuclear cell (PBMC)–derived Mɸ maturated in the presence of macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage colony–stimulating factor (GM-CSF). The Mɸ produced this way express AAT, and can serve as alveolar Mɸ model at least to some extent. Among serine proteases, the urokinase plasminogen activator–plasmin cascade is a major contributor to matrix degradation by Mɸ, primarily through activation of latent MMPs (15, 18, 19). Given that there is no available plasmin for in vitro–cultured Mɸ unless it is added exogenously, we focused on a membrane-bound serine protease, matriptase. Although Mɸ express matriptase (20, 21), information about its function in Mɸ is limited.
We report here that Mɸ derived from PBMCs of individuals with Z-AAT have an increased ability to degrade gelatin ECM through the up-regulation of a membrane-bound serine protease, matriptase. These results suggest a novel role for Mɸ in lung damage and emphysema development, and promote further attention to lung Mɸ as a possible target for new therapy development.
Materials and Methods
Reagents
All cell culture reagents, unless otherwise specified, were purchased from Life Technologies, (Carlsbad, CA). All chemicals not specified are from Sigma-Aldrich (St. Louis, MO). Fluorogenic peptides and matriptase catalytic domain were purchased from R&D Systems (Minneapolis, MN).
Subjects
PBMCs expressing M-AAT or Z-AAT were obtained from outpatient volunteers (University of Florida Institutional Review Board protocol 08-2007). Subject characteristics are presented in Table 1. All patients were stable without any sign of acute exacerbation of COPD. Subjects homozygous for the normal PiM allele (PiMM) had normal pulmonary function and no history of respiratory disease.
Table 1.
Characteristics of the Subjects
| |
PiMM |
PiZZ |
|
|---|---|---|---|
| Characteristic | (n = 5) | (n = 5) | P Value (t Test) |
| Age, yr | 38.5 (24–68) | 44 (35–55) | >0.05 |
| Male sex, n | 3 | 2 | N/A |
| FEV1% predicted | 103.1 (95.5–106) | 89.9 (58–111.9) | >0.05 |
| FEV1/FVC | 86.8 (82.0–91.0) | 71.1 (55.0–83.7) | <0.05 |
| Current smoker | No | No | N/A |
Definition of abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; N/A, not applicable; PiMM, individuals homozygous for normal PiM allele; PiZZ, individuals homozygous for mutant PiZ allele.
Experimental Procedures
Details for experimental procedures are given in the online supplement.
Statistical Analysis
All results are expressed as the mean (±SE). Statistical analysis was performed using the two-tailed Student’s t test (GraphPad Prism 6.01 software; GraphPad Software, San Diego, CA), and a P value less than 0.05 was considered statistically significant. Data are plotted using GraphPad Prism 6.01.
Results
Z-AAT Is Retained in the ER
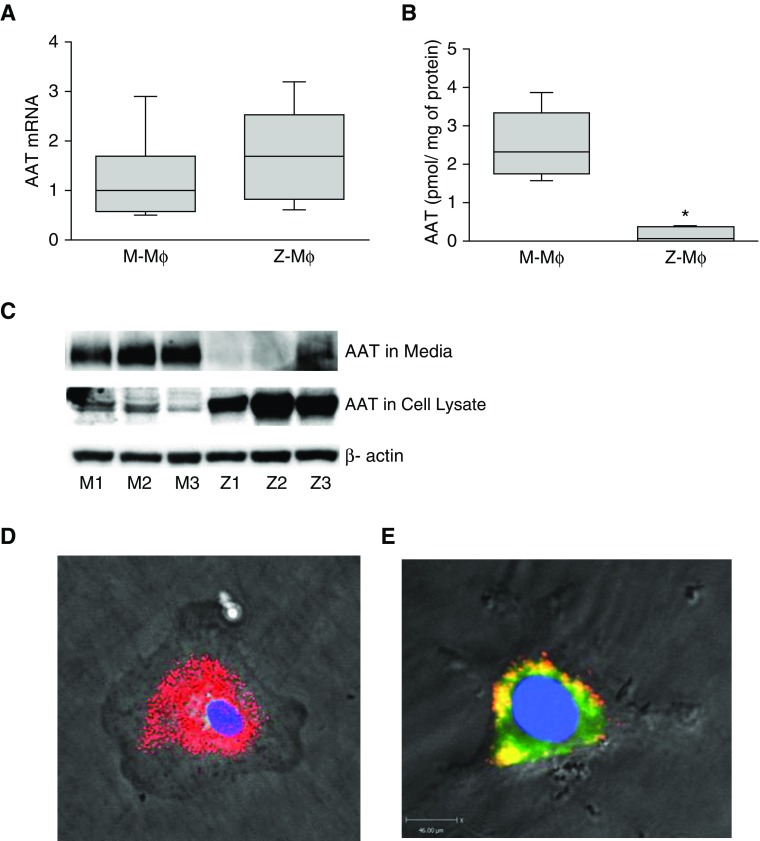
In our experiments, we use Mɸ derived from PBMCs of individuals with normal M-AAT (M-Mɸ) and Mɸ with Z-AAT mutation (Z-Mɸ), which results in AATD. To differentiate PBMCs into Mɸ, we used a combination of GM-CSF and M-CSF and characterized them by FACS and response to LPS treatment (see Figure E1 in the online supplement). Although M-Mɸ and Z-Mɸ showed comparable levels of AAT mRNA expression (Figure 1A), AAT levels in M-AAT Mɸ culture media (8 h of incubation) were significantly higher compared with Z-AAT Mɸ (1.72 ± 0.88 pmol/mg of protein versus 0.2 ± 0.082 pmol/mg of protein; P < 0.05) as measured by ELISA (Figure 1B). Western blot analysis of AAT in media and cell lysates confirmed that in Z-Mɸ AAT is retained in cells (Figure 1C). Because Mɸ autofluorescence hampered immunolocalization of intracellular AAT, we evaluated intracellular AAT distribution using Z-Mɸ infected with Z-AAT tagged with red fluorescent protein (Z-AAT-RFP) packaged in mutant AAV6 capsids (Z-AAT–RFP/AAV6-Y705-731F). Z-AAT–RFP formed in Mɸ reticular aggregates (Figure 1D) that colocalized with the ER marker protein, disulphide isomerase (Figure 1E), suggesting that reduced secretion and abnormal accumulation of Z-AAT in Z-Mɸ may represent a toxic gain-of-function phenotype on the Z- Mɸ. It is worth noting that fluorescence from cells infected with M-AAT–RFP was barely detectable without any aggregate formation, confirming that only mutated Z-AAT accumulates in cells (data not shown).
Figure 1.
Alpha-1 antitrypsin (AAT) production by M- and Z-macrophages (Mɸ). (A) Comparison of AAT mRNA expression levels in M- and Z-Mɸ. AAT mRNA was measured by real-time PCR (n = 6 individuals for each group) and normalized to 18S. (B) AAT protein levels in conditioned media from M- and Z-Mɸ (n = 3 individuals for each group) were measured by ELISA after 8 hours of incubation. (C) Immunoblot of AAT in conditioned media and cell lysates from M- and Z-Mɸ (n = 3 individuals for each group). *P < 0.05. (D and E) Images of Z-Mɸ infected with Z–alpha-1 antitrypsin Z-AAT tagged with red fluorescent protein (Z-AAT)-RFP/AAV6-Y705-731F. (E) To show ER localization of intracellular Z-AAT, cells were additionally stained with ER marker, disulphide isomerase (PDI; FITC-labeled secondary antibody). Yellow area represents colocalization of PDI and Z-AAT–RFP. Scale bar, 45 μm. Images were taken at magnification ×40.
Z-Mɸ Have Increased Protease Activity
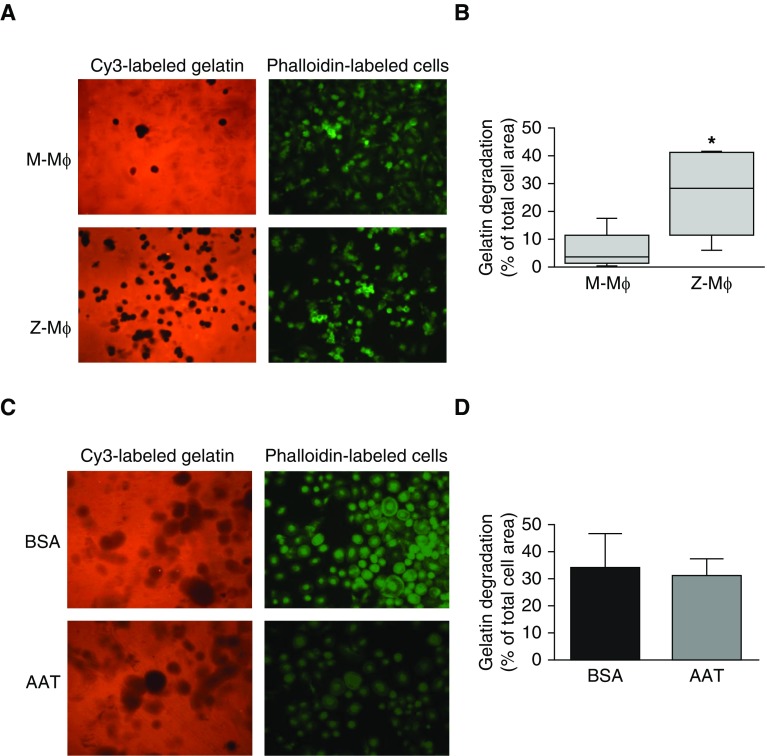
To assess whether expression of Z-AAT affects Mɸ ability to degrade ECM, M-Mɸ and Z-Mɸ were plated on fluorescent Cy3-labeled gelatin, and matrix degradation was measured by fluorescent microscopy after 24 hours. M-Mɸ seeded on the gelatin surface displayed low protease activity against the matrix. Z-Mɸ degraded significantly more gelatin (M-Mɸ degradation, 5.9 ± 6.8% of cell area; Z-Mɸ, 26.7 ± 15.3%; P < 0.01; Figures 2A and 2B), demonstrating increased extracellular proteolytic activity.
Figure 2.
Gelatin matrix degradation by Mɸ. M- and Z-Mɸ were seeded on Cy3-gelatin–coated slides for 24 hours to obtain efficient matrix degradation, then fixed and permeabilized. F-actin was stained with FITC-labeled phalloidin, and cells were examined by fluorescence microscopy. (A) Representative images and (B) quantification of images show significant degradation of gelatin (observed as black spots on Cy-3 stained gelatin) only by Z-Mɸ; M-Mɸ does not degrade a significant amount of gelatin. Data collected for Mɸ from five individuals for each group. *P < 0.01. (C) Z-Mɸ were seeded on fluorescent gelatin–coated slides in the presence of 1 mg/ml AAT or BSA (control). Representative image (C) and quantification (D) from three independent experiments. Images were taken at magnification ×20.
Increased Proteolytic Activity of Z-Mɸ Cannot Be Corrected by Addition of Exogenous AAT
To ascertain whether higher proteolytic activity of Z-Mɸ was caused by the lower levels of secreted AAT in the media, Z-Mɸ were seeded on the gelatin matrix in the presence of excess exogenous AAT. Adding AAT to culture medium in concentrations higher than usually present in lung (1 mg/ml) did not block gelatin degradation (Figures 2C and 2D), thus suggesting that increased pericellular protease activity in Z-Mɸ results from gain of proteolytic activity rather than from lower extracellular AAT antiproteinase activity.
Different Classes of Proteases Are Involved in Matrix Degradation by Z-Mɸ
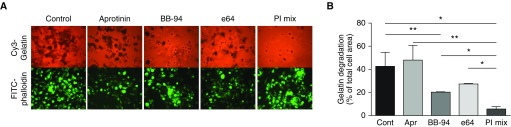
Because Mɸ express a number of proteases that can digest gelatin, we used a panel of class-specific protease inhibitors, including BB-94 (a broad-range MMP inhibitor), aprotinin (a competitive and reversible serine protease inhibitor), and e64 (an inhibitor of lysosomal cysteine proteases). Adding BB-94 or e64 to incubation media partially reduced gelatin degradation, whereas aprotinin alone did not have an effect. Combining these three inhibitors almost completely prevented gelatin matrix degradation (Figure 3), suggesting that proteases from different classes are involved in matrix degradation.
Figure 3.
Effect of protease inhibitors on matrix degradation by Z-Mɸ. Z-Mɸ were plated on Cy3-gelatin–coated slides in the presence of aprotinin (Apr; 10 μg/ml), BB-94 (5 μM), e64 (10 μM), or all three inhibitors together (protease inhibitors mix, PI mix). After 24 hours of incubation, cells were fixed and F-actin was stained with FITC-phalloidin to visualize cell areas. Images were taken at magnification ×20. Representative image (A) and quantification (B) from three independent experiments. *P < 0.01, **P < 0.05. Cont, control.
Matriptase Plays a Critical Role in Protease Activity of Z-Mɸ
To identify the proteases involved in increased Z-Mɸ gelatinolytic activity, we used fluorogenic assays that measure fluorescence released from synthetic peptides upon digestion. First, conditioned media from Mɸ did not have noticeable protease activity against fluorescent peptide, Mca-PLGL-Dpa-AR-NH2, which is a substrate for a broad range of MMPs, indicating that MMPs released into media by Mɸ were present in a nonactive, zymogen form (data not shown). The higher protease activity in Z-Mɸ may be associated with the plasma membrane cell surface. Mɸ express matriptase, a membrane-bound serine protease that plays a major role in matrix degradation in some types of cancer cells. Although matriptase is a serine protease, its membrane-associated location confers an unusual protease-inhibition profile. Interestingly, at a cellular level, matriptase is inhibited by cysteine protease inhibitors, but not by serine protease inhibitors (22).
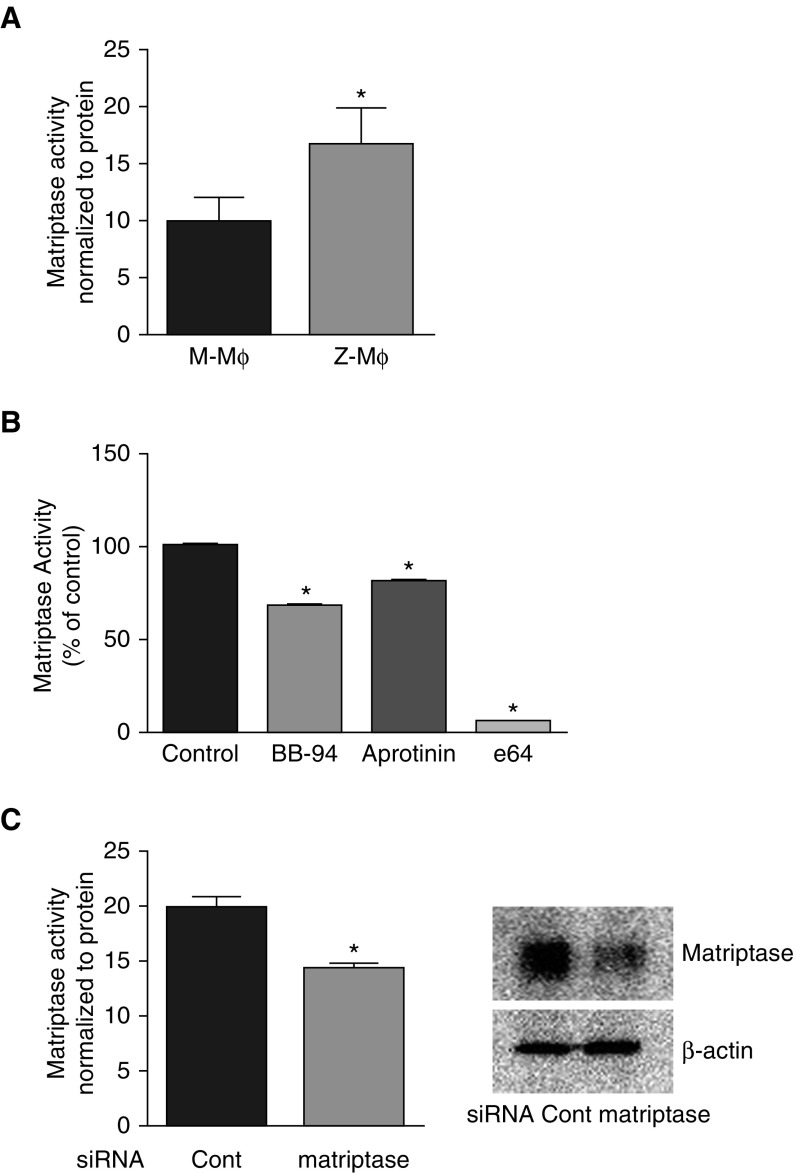
We used the Boc-QAR-AMC peptide to determine matriptase activity. M-Mɸ and Z-Mɸ displayed significant cell surface protease activity against the matriptase substrate, but, compared with M-Mɸ, Z-Mɸ activity was significantly higher (Figure 4A). To analyze how protease inhibitors affect matriptase activity on the cell surface, we pretreated cells with class-specific inhibitors for 15 minutes before the Boc-QAR-AMC peptide was added. Compared with the control, the serine protease inhibitor, aprotinin, inhibited only 20% of activity, whereas the cysteine protease inhibitor, e64, inhibited 95% of activity and MMP-inhibitor, BB-94, inhibited 30% (Figure 4B).
Figure 4.
Matriptase activity on the cell surface of Mɸ measured with fluorescent matriptase substrate, Boc-QAR-AMC. (A) Peptide (10 μM) in Hanks’ balanced salt solution was added directly to cells. After 20 minutes of incubation at 37°C, media were removed for fluorescence measurement, and cells were lysed for protein quantification to correct for differences in cell number. Data are a summary of three independent experiments. (B) Mɸ were preincubated with indicated inhibitors for 15 minutes, then Boc-QAR-AMC peptide was added to cells. After 20 minutes, media were taken in triplicate to measure fluorescence induced by peptide degradation. Data are representative of three independent experiments. *P < 0.01 versus control. (C) Activity against Boc-QAR-AMC peptide was measured in Mɸ after 48 hours of transfection with control small interfering RNA (siRNA) or with matriptase siRNA. Efficiency of silencing was monitored by immunoblotting of cell lysates with matriptase antibody. Data are representative of three independent experiments.
To confirm that the activity against the Boc-QAR-AMC peptide on the Mɸ cell surface was related to matriptase, we measured the fluorescence generated from the peptide after silencing matriptase with specific small interfering RNA (siRNA). After 48 hours of transfection with matriptase siRNA, Mɸ had reduced matriptase protein expression by more than 50% compared with control siRNA treatment. RNA silencing reduced matriptase proteolytic activity on the Boc-QAR-AMC peptide by 25% (Figure 4C), indicating that at least 50% of anti–Boc-QAR-AMC proteolytic activity could be attributed to matriptase. Taken together, matriptase must be responsible for enhanced gelatin matrix degradation by Z-Mɸ.
Matriptase Has High Activity in Z-Mɸ
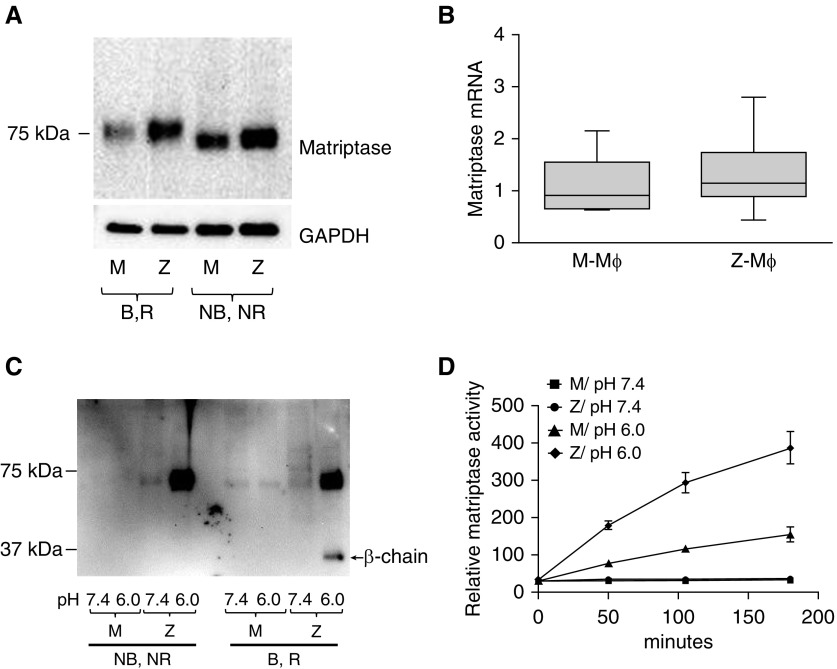
We next explored matriptase expression in Mɸ of M-AAT and Z-AAT individuals. Matriptase protein was expressed in nonstimulated Mɸ, and could be detected by immunoblotting as a single band of roughly 70 kD in nonboiled, nonreduced samples and roughly 80 kD in boiled, reduced samples (Figure 5A), corresponding to the nonactive, zymogen form of matriptase. The predicted size for matriptase is 95 kD, whereas the decreased size of 80 kD in reduced conditions is caused by its folded conformation, which is even more profound at nonreducing conditions (23).
Figure 5.
Expression and activation of matriptase in M- and Z-Mɸ. (A) Representative immunoblot of matriptase in cell lysates from M- and Z-Mɸ. Samples were prepared either without adding reducing agent and without boiling (nonboiled, nonreduced [NB, NR]) or with boiling and with reducing agent (B, R). Matriptase is a single polypeptide (B, R samples), and does not form complexes with AAT or other proteins (NB, NR samples). Z-Mɸ have much higher matriptase levels than do M-Mɸ (experiment was performed four times with Mɸ from different individuals). (B) Comparison of matriptase mRNA levels in M- and Z-Mɸ. Each group represents Mɸ from six individuals. (C) Matriptase was activated by incubation of Mɸ in phosphate buffer with mild acidity (pH 6.0) or with PBS (pH 7.4) as control for 20 minutes at 37°C. Shed matriptase was analyzed by immunoblot in NB, NR and B, R conditions. Buffer with pH 6.0 induced increased shedding of matriptase from Z-Mɸ cell surface as noncomplexed protein (NB, NR samples). Activated matriptase (30 kD) could be visualized in R, B samples. (D) Compared with M-Mɸ, Z-Mɸ exhibited increased activity of shed matriptase in conditioned media. Data are representative of three independent experiments.
Compared with M-Mɸ, Z-Mɸ consistently showed higher levels of matriptase protein (Figure 5A), despite no differences in matriptase mRNA expression between M-Mɸ and Z-Mɸ (Figure 5B). This indicates that matriptase is differentially regulated at the posttranslational level in M-Mɸ and Z-Mɸ.
Zymogen matriptase is a single polypeptide. Upon activation, it is cleaved into two chains, α and β, bound by disulfide bonds, and only in active form is it able to form complexes with its inhibitors (24). AAT is considered to be a matriptase inhibitor (21, 25), and we proposed that, upon activation of matriptase, we would see complexes between matriptase and AAT. We studied the activation of matriptase in Mɸ based on the activation scheme of previous work in epithelial cells (26), and treated Mɸ for 20 minutes with mild acidic buffer (pH 6.0) to activate matriptase, or with PBS (pH 7.4) as a control. Consistent with the notion that matriptase is shed from the cell surface upon activation, we found that the levels of matriptase shed from the surface of Z-Mɸ were considerably higher than from M-Mɸ (Figure 5C). Furthermore, the appearance of the β chain on immunoblot indicates that matriptase is shed in its active form (boiled, reduced samples), but we were unable to identify complexes with other proteins that could be detected in immunoblots of nonboiled, nonreduced samples. Protease activity assays confirmed that matriptase was shed in active form, and that Z-Mɸ shed markedly higher matriptase activity in conditioned buffer than M-Mɸ (Figure 5D).
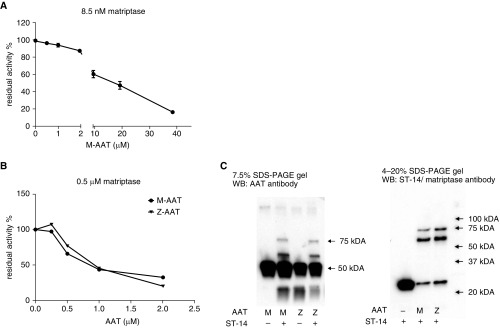
Strikingly, activated matriptase did not form complexes with its inhibitors within Mɸ. In particular, no complexes were seen between AAT and matriptase, notwithstanding that both are expressed in the same cells. AAT interacts with matriptase catalytic domain in vitro, and AAT was found to be a slow, tight-binding inhibitor of the catalytic domain of matriptase, with a second-order reaction rate constant of 3.1 × 102 M−1 s−1 (25). We were able to reproduce the findings of this work (Figure 6). For the AAT to be able to inhibit matriptase after 30 minutes of incubation, the concentration of AAT should exceed the matriptase concentration by more than 1,000-fold (Figure 6A). A prolonged incubation reduced the ratio between AAT and matriptase needed for matriptase inhibition (Figure 6B), and complexes between matriptase and AAT could be identified by immunoblot (Figure 6C). These slow kinetics of interaction were likely the reason that AAT was not considered a matriptase inhibitor in some publications (27).
Figure 6.
Inhibiting the activity of recombinant human matriptase/ST14 catalytic domain by AAT in vitro. (A) Matriptase/ST-14 (8.5 nM) catalytic domain and indicated concentrations of M-AAT were preincubated for 30 minutes at room temperature before activity measurements. (B) Matriptase/ST-14 (0.5 μM) catalytic domain and indicated concentrations of M- or Z-AAT were preincubated 20 hours at room temperature before activity measurements. (C) Immunoblotting after incubation of 0.5 μM matriptase with 2 μM AAT 20 hours at room temperature. WB, Western blotting.
Matriptase Has a Strong Catalytic Activity for MMP-14 and Regulates Its Level on the Cell Surface
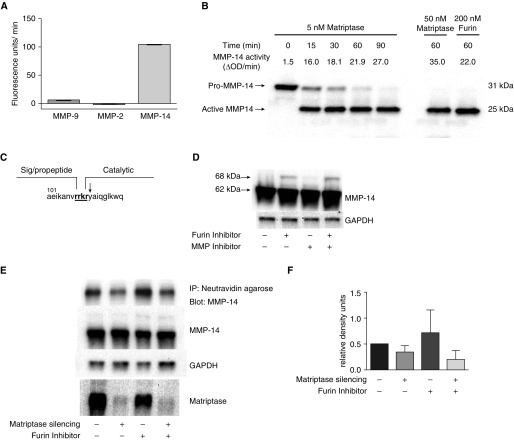
Our experiments with inhibitors to different classes of proteases clearly showed that degradation of gelatin ECM by Mɸ is a complex process. Matriptase, like many other proteases, can be a part of a protease network and contribute to ECM degradation not only through direct digestion of ECM components, but also through discrete cleavage and activation of other proproteases that target ECM proteins. To test if matriptase activates other proteases, we investigated the effect of matriptase on activation of pro–MMP-14, pro–MMP-2, and pro–MMP-9 in vitro (MMP-14, MMP-2, and MMP-9 are the most abundant MMPs in Mɸ). The matriptase catalytic domain activated MMP-14, but not MMP-2 or MMP-9 (Figure 7A). Pro–MMP-14 was activated by the matriptase catalytic domain in a concentration- and time-dependent manner (Figure 7B). At a concentration of 5 nM, matriptase processed MMP-14 with the same efficiency as furin at a concentration of 200 nM (matriptase has a much higher relative activity than does furin: 10,000 U/μg of protein versus 125 U/μg of protein, respectively), which is a known endogenous activator of MMP-14 (28). N-terminal sequence analysis of active MMP-14 processed by matriptase revealed cleavage at Arg111–Tyr112, the same cleavage site as for furin (Figure 7C). MMP-14 is a membrane-bound metalloprotease that is synthesized as a zymogen with a size of 63 kD, and, to be functionally active, the prodomain should be proteolitically removed, with the size of the mature form being 58 kD. In many types of cells, Golgi-associated proprotein convertase furin is responsible for MMP-14 activation. However, a portion of the cellular MMP-14 pool can be processed by other proteases, especially in the absence of furin (28). In addition, the part of the MMP-14 pool at the cell surfaces is able to process by autocatalysis (29). To further characterize the ability of matriptase to activate MMP-14, we used Mɸ derived from the monocytic cell line, THP-1 (THP-1–Mɸ), which expresses both MMP-14 and matriptase. In THP-1–Mɸ lysates, MMP-14 was present in the mature 58-kD form. Even after the treatment of cells with furin inhibitor or the MMP inhibitor, marimastat, to block autoprocessing, the majority of MMP-14 still appeared in its mature form, indicating alternative pathways for MMP-14 maturation in THP-1–Mɸ (Figure 7D). To assess the role of matriptase in MMP-14 activity, we silenced matriptase with siRNA and measured MMP-14 expression in THP-1–Mɸ. After 48 hours of transfection, matriptase was silenced more than 90%, but the levels of MMP-14 protein in cell lysates were not affected (Figure 7E). Inside the cells, MMP-14 is stored in microvesicles near the plasma membrane, and has functional activity only when transported to the plasma membrane. As such, we next investigated whether matriptase regulates the levels of MMP-14 on the cell surface. To identify the cell surface–associated pool of MMP-14, we used a cell surface biotinylation procedure with membrane-impermeable EZ-Link NHS-SS-biotin (Thermo Fisher Scientific, Waltham, MA). After that procedure, biotinylated proteins were pulled down with neutravidin agarose and levels of biotinylated MMP-14 were analyzed by Western blotting. The silencing of matriptase led to reduction of cell surface–associated MMP-14, especially if furin activity was inhibited (Figure 7E). Thus, matriptase mediated ECM degradation in Mɸ, at least in part, through cell surface MMP-14 activation.
Figure 7.
Activation of pro–matrix metalloproteinase (MMP)-14 with matriptase catalytic domain. (A) Pro-MMP-14, pro-MMP-2, and pro-MMP-9 were incubated with 10 nM of matriptase catalytic domain for 1 hour at 37°C, then activity against the fluorescent peptide substrate, Mca-PLGL-Dpa-AR-NH2 (10 μM), was measured. Data from a representative experiment (n = 3) are shown. (B) Pro–MMP-14 (0.5 μM) was incubated with 5 nM of matriptase catalytic domain at 37°C. Aliquots were removed at specified times for measuring activity against the fluorescent peptide. At the same time, the cleavage of pro–MMP-14 during activation was examined using immunoblot analysis. Activation of pro–MMP-14 with 0.2 U furin was used as positive control. Data from a representative experiment (n = 3) are shown. (C) Cleavage site determined by N-terminal sequence analysis after pro–MMP-14 was incubated with matriptase catalytic domain overnight at room temperature. Cleavage site is marked with downward-pointing arrow. (D) THP-1–Mɸ were incubated with furin inhibitor I, marimastat, or both inhibitors together, and the expression of MMP-14 was analyzed by immunoblot. (E) Matriptase was silenced with specific siRNA in THP-1–Mɸ, and the levels of MMP-14, matriptase, and GAPDH were analyzed by WB. Some wells were pretreated with furin inhibitor I to block furin-dependent maturation of MMP-14. To estimate the levels of the cell surface pool of MMP-14, the proteins on the surface of cells were biotinylated and then pulled down with NeutrAvidin agarose. Representative blots from three experiments. (F) Densitometric analysis of MMP-14 levels on the cell surface with correction to total level of MMP-14 in cells. Each bar represents the mean of the relative density units (±SE) from three experiments. IP, immunoprecipitation; OD, optical density.
Discussion
Our study was designed to answer the question: do Mɸ that express mutated Z-AAT have an altered phenotype that contributes to the development of emphysema? We focused on activities of Mɸ-derived proteases and their ability to degrade ECM, which is a key process in the progression of emphysema.
The ability of Mɸ to express AAT was established in early publications on AATD (30, 31). However, human alveolar Mɸ are not easily accessible, which may explain why the number of articles published on AAT in Mɸ is low compared with that for other cell types. As a model for alveolar Mɸ, we used PBMC-derived Mɸ cultured in the presence of GM-CSF and M-CSF growth factors, which are both present in the lung (32, 33). After characterization of Mɸ, derived in the presence of both factors, by FACS and by response to LPS (Figure E1 in the online supplement), we concluded that these Mɸ can be used as an alveolar Mɸ model, with some caveats. Next, we showed that Mɸ derived from the PBMCs of patients with Z mutation accumulate Z-AAT inside these cells, and that Z-AAT colocalized with the ER marker, disulphide isomerase. In several cell types, the accumulation of misfolded Z-AAT is capable of inducing ER stress with an ensuing unfolded protein response, ER-associated degradation, and autophagy (34). As a consequence, a number of cellular functions are affected, including constitutive activation of the NF-κB pathway, enhanced cytokine production, mitochondrial injury, and activation of caspase-12 and caspase-4 (35–38). In the present study, we demonstrate that, in Z-Mɸ, intracellular accumulation of unfolded Z-AAT results in higher proteolytic activity on ECM compared with wild-type M-Mɸ. Furthermore, supplementation of media with AAT at the concentrations higher than found in lung did not rescue this phenotype, thus indicating that increased proteolytic activity is an intrinsic feature of the Z-Mɸ. Therefore, we hypothesize that this AAT supplementation–insensitive extracellular proteolytic activity of Z-Mɸ is a key component of AATD-derived emphysema development.
We also showed that Z-Mɸ proteolytic activity against gelatin ECM could be decreased by the addition of protease inhibitors to the media. The practically complete inhibition was achieved with a mixture of several inhibitors of different classes of proteases. Thus, matrix degradation may be the result of different proteases that work as a proteolytic network (40, 41).
MMPs are the most abundant class of Mɸ-secreted proteases. However, in cell-conditioned media, MMPs remain mostly inactive due to the absence of plasmin, the major activator of MMPs (15, 39). We found no differences in MMP activities between M-Mɸ– and Z-Mɸ–conditioned media. Therefore, we hypothesized that the membrane-bound proteases may be involved in the Z-Mɸ proteolytic phenotype. The Z-Mɸ had increased activity against the substrate (Boc-QAR-AMC) for the membrane-bound serine protease, matriptase, that corresponded to increased matriptase protein levels compared with M-Mɸ. Matriptase is a serine protease with a distinctive inhibitory profile on the cell surface; the cysteine protease inhibitor, e64, was the most effective matriptase inhibitor, whereas the serine protease inhibitor, aprotinin, had a marginal effect. The ability to change sensitivity to inhibitors for enzymes associated with plasma membrane is well known. Membrane location protects NE and cathepsin G on the neutrophil cell surface and plasmin on the Mɸ cell surface (17, 40, 41). Similarly, the first study that identified matriptase’s gelatinolytic activity also demonstrated that pepstatin (an aspartic protease inhibitor), benzamidine, and PMSF (serine proteinase inhibitors) had no effect on matriptase activity, whereas the cysteine protease inhibitor, leupeptin, and EDTA completely blocked the activity (22). Indeed, the activation of matriptase is also resistant to inhibition by pharmacological inhibitors in epithelial cells (26).
In summary, these data indicate that Z-Mɸ have increased ability to degrade ECM and increased matriptase activity on the cell surface; in experiments with an e64 inhibitor, cell surface matriptase activity and degradation of ECM by Mɸ are positively correlated (Figures 3B and 4C). Together, the data strongly support the hypothesis that matriptase plays an important role in matrix degradation by Z-Mɸ.
Mechanisms for increased levels of matriptase in Z-Mɸ still need to be determined. In epithelial cells, in which it plays a key role in epithelial barrier function and tight junction assembly (42), matriptase is tightly regulated by its cognate inhibitor, hepatocyte growth factor activator inhibitor (HAI)-1. In Mɸ, the process of matriptase maturation has not been well studied. However, cells of immune origin, such as monocytes and the monocytic cell line, THP-1, express high levels of matriptase without detectable levels of HAI-1 (43). These data suggest that there may be an HAI-1–independent mechanism in matriptase regulation in these cells. In line with this suggestion, in addition to matriptase–HAI-1 complexes, human milk contains complexes of matriptase with serine antiproteases: antithrombin-III, AAT, and a2-antiplasmin (21). However, we did not find the complexes between AAT and matriptase in Mɸ, although this could have been due to technical difficulties or formation of complexes only in particular conditions. We cannot exclude that Z-AAT accumulation inside Z-Mɸ can affect matriptase levels indirectly. For example, Z-AAT accumulation in the ER may possibly affect the function of ER-resident proteins, leading to changes in the matriptase glycosylation that increases the half-life of matriptase (44). In our future studies, we will address the mechanisms of up-regulated matriptase levels in Z-Mɸ.
Although matriptase can directly digest gelatin matrix, it also activates other proteases involved in ECM degradation. Matriptase can activate urokinase-type plasminogen activator on the cell surface (43). In addition, the matriptase catalytic domain can activate pro-MMP-1 and pro-MMP-3 in vitro (45, 46). Because these proteases are not expressed in our system, we tested if matriptase can activate the most abundantly expressed MMP in Mɸ and, thus, demonstrated that MMP-14 can be processed by matriptase. MMP-14 is a membrane-associated metalloproteinase, synthesized as a zymogen and classically controlled by a proprotein convertase, furin, although a furin-independent route has also been identified (28). In our in vitro assays, matriptase processed MMP-14 at the same Arg-Arg-Lys-Arg (RRKR) site and as effectively as furin. MMP-14 has multiple levels of regulation on its functional activity (47, 48). Because matriptase is located at the plasma membrane, it may act on MMP-14 activation directly at the cell surface. The knockdown of matriptase in THP-derived Mɸ led to a significant decrease in the levels of MMP-14 on the cell surface. Interestingly, the same mechanism of recruitment of MMP-14 to the cell surface of cancer cells was shown for another membrane-bound protease, ADAM metallopeptidase domain 12 (ADAM12) (49). Thus, matriptase can mediate its effect on ECM degradation not only directly, but also through regulation of active MMP-14 levels.
In conclusion, using human PBMC-derived Mɸ, we demonstrated a novel aspect of Z-Mɸ function. Mɸ-mediated increases in protease activity against ECM offers a new explanation for lung destruction in individuals with AATD, different from the protease–antiprotease model. Additional studies are necessary to understand why matriptase is increased in Z-Mɸ, how the matriptase–MMP-14 relationship affects the larger protease milieu in AATD, and if the activation of proteolytic activity in Z-Mɸ can be modulated.
Acknowledgments
Acknowledgments
The authors thank Drs. D. Avram, A. Bryant, and C. Saltini (University of Florida, Gainesville, FL) for critically reading and commenting on the manuscript.
Footnotes
Author Contributions: K.K. and M.L.B. contributed to the conception and design of the study; K.K., R.L.W., G.A., B.E.H., and N.K. contributed to the acquisition of data; K.K., G.W.M., F.N.R., and M.L.B. contributed to the analysis and interpretation of data; K.K., G.W.M., F.N.R., and M.L.B. contributed to the drafting of the article or critical revision for important intellectual content.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0366OC on March 31, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gooptu B, Dickens JA, Lomas DA. The molecular and cellular pathology of α1-antitrypsin deficiency. Trends Mol Med. 2014;20:116–127. doi: 10.1016/j.molmed.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Laurell C-B, Eriksson S. The electrophoretic α1-globulin pattern of serum in α1-antitrypsin deficiency. 1963. COPD. 2013;10:3–8. doi: 10.3109/15412555.2013.771956. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson S. Pulmonary emphysema and alpha1-antitrypsin deficiency. Acta Med Scand. 1964;175:197–205. doi: 10.1111/j.0954-6820.1964.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 4.Sharp HL, Bridges RA, Krivit W, Freier EF. Cirrhosis associated with alpha-1–antitrypsin deficiency: a previously unrecognized inherited disorder. J Lab Clin Med. 1969;73:934–939. [PubMed] [Google Scholar]
- 5.Crystal RG. Alpha 1–antitrypsin deficiency, emphysema, and liver disease: genetic basis and strategies for therapy. J Clin Invest. 1990;85:1343–1352. doi: 10.1172/JCI114578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman KR, Burdon JGW, Piitulainen E, Sandhaus RA, Seersholm N, Stocks JM, Stoel BC, Huang L, Yao Z, Edelman JM, et al. RAPID Trial Study Group. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:360–368. doi: 10.1016/S0140-6736(15)60860-1. [DOI] [PubMed] [Google Scholar]
- 7.Lambrecht BN. Alveolar macrophage in the driver’s seat. Immunity. 2006;24:366–368. doi: 10.1016/j.immuni.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Tetley TD. Macrophages and the pathogenesis of COPD. Chest. 2002;121(5) suppl:156S–159S. doi: 10.1378/chest.121.5_suppl.156s. [DOI] [PubMed] [Google Scholar]
- 9.Ueno M, Maeno T, Nishimura S, Ogata F, Masubuchi H, Hara K, Yamaguchi K, Aoki F, Suga T, Nagai R, et al. Alendronate inhalation ameliorates elastase-induced pulmonary emphysema in mice by induction of apoptosis of alveolar macrophages. Nat Commun. 2015;6:6332. doi: 10.1038/ncomms7332. [DOI] [PubMed] [Google Scholar]
- 10.Woolhouse IS, Bayley DL, Stockley RA. Sputum chemotactic activity in chronic obstructive pulmonary disease: effect of α(1)-antitrypsin deficiency and the role of leukotriene B(4) and interleukin 8. Thorax. 2002;57:709–714. doi: 10.1136/thorax.57.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubbard RC, Fells G, Gadek J, Pacholok S, Humes J, Crystal RG. Neutrophil accumulation in the lung in alpha 1-antitrypsin deficiency: spontaneous release of leukotriene B4 by alveolar macrophages. J Clin Invest. 1991;88:891–897. doi: 10.1172/JCI115391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer LT, Paone G, Krein PM, Rouhani FN, Rivera-Nieves J, Brantly ML. Role of human neutrophil peptides in lung inflammation associated with alpha1-antitrypsin deficiency. Am J Physiol Lung Cell Mol Physiol. 2004;286:L514–L520. doi: 10.1152/ajplung.00099.2003. [DOI] [PubMed] [Google Scholar]
- 13.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke–induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 14.Filippov S, Caras I, Murray R, Matrisian LM, Chapman HA, Jr, Shapiro S, Weiss SJ. Matrilysin-dependent elastolysis by human macrophages. J Exp Med. 2003;198:925–935. doi: 10.1084/jem.20030626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleetwood AJ, Achuthan A, Schultz H, Nansen A, Almholt K, Usher P, Hamilton JA. Urokinase plasminogen activator is a central regulator of macrophage three-dimensional invasion, matrix degradation, and adhesion. J Immunol. 2014;192:3540–3547. doi: 10.4049/jimmunol.1302864. [DOI] [PubMed] [Google Scholar]
- 16.Werb Z, Banda MJ, Jones PA. Degradation of connective tissue matrices by macrophages. I. Proteolysis of elastin, glycoproteins, and collagen by proteinases isolated from macrophages. J Exp Med. 1980;152:1340–1357. doi: 10.1084/jem.152.5.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman HA., Jr Role of enzyme receptors and inhibitors in regulating proteolytic activities of macrophages. Ann N Y Acad Sci. 1991;624:87–96. doi: 10.1111/j.1749-6632.1991.tb17009.x. [DOI] [PubMed] [Google Scholar]
- 18.Van den Steen PE, Opdenakker G, Wormald MR, Dwek RA, Rudd PM. Matrix remodelling enzymes, the protease cascade and glycosylation. Biochim Biophys Acta. 2001;1528:61–73. doi: 10.1016/s0304-4165(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 19.Jones PA, Werb Z. Degradation of connective tissue matrices by macrophages. II. Influence of matrix composition on proteolysis of glycoproteins, elastin, and collagen by macrophages in culture. J Exp Med. 1980;152:1527–1536. doi: 10.1084/jem.152.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberst MD, Singh B, Ozdemirli M, Dickson RB, Johnson MD, Lin C-Y. Characterization of matriptase expression in normal human tissues. J Histochem Cytochem. 2003;51:1017–1025. doi: 10.1177/002215540305100805. [DOI] [PubMed] [Google Scholar]
- 21.Tseng IC, Chou FP, Su SF, Oberst M, Madayiputhiya N, Lee MS, Wang JK, Sloane DE, Johnson M, Lin CY. Purification from human milk of matriptase complexes with secreted serpins: mechanism for inhibition of matriptase other than HAI-1. Am J Physiol Cell Physiol. 2008;295:C423–C431. doi: 10.1152/ajpcell.00164.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi YE, Torri J, Yieh L, Wellstein A, Lippman ME, Dickson RB. Identification and characterization of a novel matrix-degrading protease from hormone-dependent human breast cancer cells. Cancer Res. 1993;53:1409–1415. [PubMed] [Google Scholar]
- 23.Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem. 2000;275:26333–26342. doi: 10.1074/jbc.M002941200. [DOI] [PubMed] [Google Scholar]
- 24.Benaud C, Dickson RB, Lin C-Y. Regulation of the activity of matriptase on epithelial cell surfaces by a blood-derived factor. Eur J Biochem. 2001;268:1439–1447. doi: 10.1046/j.1432-1327.2001.02016.x. [DOI] [PubMed] [Google Scholar]
- 25.Janciauskiene S, Nita I, Subramaniyam D, Li Q, Lancaster JR, Jr, Matalon S. α1-antitrypsin inhibits the activity of the matriptase catalytic domain in vitro. Am J Respir Cell Mol Biol. 2008;39:631–637. doi: 10.1165/rcmb.2008-0015RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MS, Tseng IC, Wang Y, Kiyomiya K, Johnson MD, Dickson RB, Lin CY. Autoactivation of matriptase in vitro: requirement for biomembrane and LDL receptor domain. Am J Physiol Cell Physiol. 2007;293:C95–C105. doi: 10.1152/ajpcell.00611.2006. [DOI] [PubMed] [Google Scholar]
- 27.Béliveau F, Désilets A, Leduc R. Probing the substrate specificities of matriptase, matriptase-2, hepsin and DESC1 with internally quenched fluorescent peptides. FEBS J. 2009;276:2213–2226. doi: 10.1111/j.1742-4658.2009.06950.x. [DOI] [PubMed] [Google Scholar]
- 28.Yana I, Weiss SJ. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol Biol Cell. 2000;11:2387–2401. doi: 10.1091/mbc.11.7.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remacle AG, Rozanov DV, Fugere M, Day R, Strongin AY. Furin regulates the intracellular activation and the uptake rate of cell surface–associated MT1-MMP. Oncogene. 2006;25:5648–5655. doi: 10.1038/sj.onc.1209572. [DOI] [PubMed] [Google Scholar]
- 30.Mornex JF, Chytil-Weir A, Martinet Y, Courtney M, LeCocq JP, Crystal RG. Expression of the alpha-1-antitrypsin gene in mononuclear phagocytes of normal and alpha-1-antitrypsin–deficient individuals. J Clin Invest. 1986;77:1952–1961. doi: 10.1172/JCI112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perlmutter DH, Cole FS, Kilbridge P, Rossing TH, Colten HR. Expression of the alpha 1-proteinase inhibitor gene in human monocytes and macrophages. Proc Natl Acad Sci USA. 1985;82:795–799. doi: 10.1073/pnas.82.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouhani FN, Brantly ML, Markello TC, Helip-Wooley A, O’Brien K, Hess R, Huizing M, Gahl WA, Gochuico BR. Alveolar macrophage dysregulation in Hermansky-Pudlak syndrome type 1. Am J Respir Crit Care Med. 2009;180:1114–1121. doi: 10.1164/rccm.200901-0023OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibata Y, Berclaz P-Y, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15:557–567. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 34.Kroeger H, Miranda E, MacLeod I, Pérez J, Crowther DC, Marciniak SJ, Lomas DA. Endoplasmic reticulum–associated degradation (ERAD) and autophagy cooperate to degrade polymerogenic mutant serpins. J Biol Chem. 2009;284:22793–22802. doi: 10.1074/jbc.M109.027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hidvegi T, Schmidt BZ, Hale P, Perlmutter DH. Accumulation of mutant alpha1-antitrypsin Z in the endoplasmic reticulum activates caspases-4 and -12, NFkappaB, and BAP31 but not the unfolded protein response. J Biol Chem. 2005;280:39002–39015. doi: 10.1074/jbc.M508652200. [DOI] [PubMed] [Google Scholar]
- 36.Perlmutter DH. The role of autophagy in alpha-1-antitrypsin deficiency: a specific cellular response in genetic diseases associated with aggregation-prone proteins. Autophagy. 2006;2:258–263. doi: 10.4161/auto.2882. [DOI] [PubMed] [Google Scholar]
- 37.Lawless MW, Greene CM, Mulgrew A, Taggart CC, O’Neill SJ, McElvaney NG. Activation of endoplasmic reticulum–specific stress responses associated with the conformational disease Z α 1-antitrypsin deficiency. J Immunol. 2004;172:5722–5726. doi: 10.4049/jimmunol.172.9.5722. [DOI] [PubMed] [Google Scholar]
- 38.Carroll TP, Greene CM, O’Connor CA, Nolan AM, O’Neill SJ, McElvaney NG. Evidence for unfolded protein response activation in monocytes from individuals with alpha-1 antitrypsin deficiency. J Immunol. 2010;184:4538–4546. doi: 10.4049/jimmunol.0802864. [DOI] [PubMed] [Google Scholar]
- 39.Overall CM, Blobel CP. In search of partners: linking extracellular proteases to substrates. Nat Rev Mol Cell Biol. 2007;8:245–257. doi: 10.1038/nrm2120. [DOI] [PubMed] [Google Scholar]
- 40.Korkmaz B, Attucci S, Jourdan ML, Juliano L, Gauthier F. Inhibition of neutrophil elastase by alpha1-protease inhibitor at the surface of human polymorphonuclear neutrophils. J Immunol. 2005;175:3329–3338. doi: 10.4049/jimmunol.175.5.3329. [DOI] [PubMed] [Google Scholar]
- 41.Owen CA, Campbell MA, Sannes PL, Boukedes SS, Campbell EJ. Cell surface–bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J Cell Biol. 1995;131:775–789. doi: 10.1083/jcb.131.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friis S, Godiksen S, Bornholdt J, Selzer-Plon J, Rasmussen HB, Bugge TH, Lin C-Y, Vogel LK. Transport via the transcytotic pathway makes prostasin available as a substrate for matriptase. J Biol Chem. 2011;286:5793–5802. doi: 10.1074/jbc.M110.186874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kilpatrick LM, Harris RL, Owen KA, Bass R, Ghorayeb C, Bar-Or A, Ellis V. Initiation of plasminogen activation on the surface of monocytes expressing the type II transmembrane serine protease matriptase. Blood. 2006;108:2616–2623. doi: 10.1182/blood-2006-02-001073. [DOI] [PubMed] [Google Scholar]
- 44.Ihara S, Miyoshi E, Nakahara S, Sakiyama H, Ihara H, Akinaga A, Honke K, Dickson RB, Lin C-Y, Taniguchi N. Addition of β1-6 GlcNAc branching to the oligosaccharide attached to Asn 772 in the serine protease domain of matriptase plays a pivotal role in its stability and resistance against trypsin. Glycobiology. 2004;14:139–146. doi: 10.1093/glycob/cwh013. [DOI] [PubMed] [Google Scholar]
- 45.Milner JM, Patel A, Davidson RK, Swingler TE, Desilets A, Young DA, Kelso EB, Donell ST, Cawston TE, Clark IM, et al. Matriptase is a novel initiator of cartilage matrix degradation in osteoarthritis. Arthritis Rheum. 2010;62:1955–1966. doi: 10.1002/art.27476. [DOI] [PubMed] [Google Scholar]
- 46.Jin X, Yagi M, Akiyama N, Hirosaki T, Higashi S, Lin C-Y, Dickson RB, Kitamura H, Miyazaki K. Matriptase activates stromelysin (MMP-3) and promotes tumor growth and angiogenesis. Cancer Sci. 2006;97:1327–1334. doi: 10.1111/j.1349-7006.2006.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 48.Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP) J Cell Physiol. 2004;200:2–10. doi: 10.1002/jcp.20064. [DOI] [PubMed] [Google Scholar]
- 49.Albrechtsen R, Kveiborg M, Stautz D, Vikeså J, Noer JB, Kotzsh A, Nielsen FC, Wewer UM, Fröhlich C. ADAM12 redistributes and activates MMP-14, resulting in gelatin degradation, reduced apoptosis and increased tumor growth. J Cell Sci. 2013;126:4707–4720. doi: 10.1242/jcs.129510. [DOI] [PubMed] [Google Scholar]