Abstract
Background
The collapse of the World Trade Center (WTC) on September 11, 2001 released a dust cloud containing numerous environmental contaminants, including polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans (PCDD/Fs). PCDD/Fs are toxic and are associated with numerous adverse health outcomes including cancer, diabetes, and impaired reproductive and immunologic function. Prior studies have found adults exposed to the WTC disaster to have elevated levels of PCDD/Fs. This is the first study to assess PCDD/F levels in WTC-exposed children.
Methods
This analysis includes 110 participants, a subset of the 2014–2016 WTC Adolescent Health Study, a group of both exposed youths who lived, attended school, or were present in lower Manhattan on 9/11 recruited from the WTC Health Registry (WTCHR) and unexposed youths frequency matched on age, sex, race, ethnicity, and income. Our sample was selected to maximize the contrast in their exposure to dust from the WTC collapse. Questionnaire data, including items about chronic home dust and acute dust cloud exposure, anthropometric measures, and biologic specimens were collected during a clinic visit. Serum PCDD/F concentrations were measured according to a standardized procedure at the New York State Department of Health Organic Analytical Laboratory. We used multivariable linear regression to assess differences in PCCD/Fs between WTCHR and non-WTCHR participants. We also compared mean and median PCDD/F and toxic equivalency (TEQ) concentrations in our cohort to 2003–4 National Health and Nutrition Examination Survey (NHANES) levels for youths age 12–19.
Results
Median PCDD/F levels were statistically significantly higher among WTCHR participants compared to non-WTCHR participants for 16 out of 17 congeners. Mean and median TEQ concentrations in WTCHR participants were more than 7 times those in non-WTCHR participants (72.5 vs. 10.1 and 25. 3 vs. 3.39 pg/g lipid, respectively). Among WTCHR participants, median concentrations of several PCDD/Fs were higher than the NHANES 95th percentiles. After controlling for dust cloud exposure, home dust exposure was significantly associated with higher PCDD/F level.
Conclusions
Adolescents in lower Manhattan on the day of the WTC attack and exposed to particulate contamination from the WTC collapse had significantly elevated PCDD/F levels more than 12 years later compared to a matched comparison group, driven by chronic home dust exposure rather than acute dust cloud exposure. PCDD/F and TEQ levels substantially exceeded those in similar-aged NHANES participants. Future studies are warranted to explore associations of PCDD/Fs with health and developmental outcomes among individuals exposed to the WTC disaster as children.
Keywords: Dioxins, Endocrine disruptors, Environmental exposures, Children, Adolescents, World Trade Center
1. Introduction1
The collapse of the World Trade Center (WTC) on September 11, 2001 (9/11) resulted in the immediate release of a dust cloud containing large amounts of environmental pollutants from burning jet fuel and pulverized construction material, plastics, and electronics, which were deposited across downtown Manhattan, New York City, as well as from the continuous release of smoke from fires that burned for more than 3 months after the disaster (Landrigan, Lioy et al. 2004). Among these toxic chemicals were polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans (PCDD/Fs), byproducts of the combustion process (Rappe 1994). Elevated concentrations of PCDD/Fs were found on samples swabbed from exterior window surfaces (Rayne, Ikonomou et al. 2005) and in samples of dust, water, sediment, and sewage collected in and around the WTC site (Litten, McChesney et al. 2003). Elevated PCDD/F levels have been reported in serum samples from firefighters who responded to the disaster (Edelman, Osterloh et al. 2003) and in plasma samples from pregnant women in the immediate vicinity (Wolff, Teitelbaum et al. 2005) and from state and National Guard workers assigned to the site in the weeks after the collapse (Horii, Jiang et al. 2010). To date, no assessment of exposure to these chemicals has been conducted in local children, who may have been uniquely vulnerable to the exposures’ potential toxic effects.
PCDD/Fs are two related families of compounds produced when carbon and chlorine combine at high temperatures. Because they are lipophilic and tend to bioaccumulate in the food chain, the primary sources of human exposure are high-fat foods and breastmilk (Centers for Disease Control and Prevention). In the case of the WTC disaster, children would additionally have been exposed to PCDD/F-containing dust particles, especially those that accumulated inside their homes through contaminated ventilation systems and persisted in upholstered furniture and carpeting (U.S. Environmental Protection Agency 2002). PCDD/Fs have a half-life of approximately 7 years in adults (Michalek, Pirkle et al. 2002). Although they are reported to be eliminated more quickly in those age <18 years (Kerger, Leung et al. 2006), they may pose particular risks to this population, which is undergoing critical periods of biological development.
PCDD/Fs are associated with a wide range of adverse health outcomes. Acute toxicity commonly results in chloracne, a condition resembling severe acne that can last for years (Sorg 2014). Longitudinal studies of exposed adults have found elevated incidence of diabetes, multiple cancers, and altered reproductive and immunologic function (Pesatori, Consonni et al. 2003, Wang, Tsai et al. 2008, Kuwatsuka, Shimizu et al. 2014, Nishijo, Pham et al. 2014, Li, Chen et al. 2015), while follow-up studies of children exposed in utero or through breastfeeding have found links with impaired cognitive and behavioral function (Nakajima, Saijo et al. 2006, Neugebauer, Wittsiepe et al. 2015, Tran, Pham et al. 2016) and, among boys, altered semen quality (Mocarelli, Gerthoux et al. 2011). The biological mechanism underlying these associations involves activation of the aryl hydrocarbon receptor (AhR), which stimulates production of xenobiotic-metabolizing enzymes. By persistently activating the AhR, PCDD/Fs disrupt its normal homeostatic function, leading to disruptions in development, cell-cycle control, and tumor suppression (White and Birnbaum 2009). Toxicity of individual PCDD/Fs is determined by each congener’s binding affinity for the AhR relative to that of 2,3,7,8-TCDD, the most potent congener in the series, and these toxic equivalency quotients (TEQs) may be summed to approximate the total toxicity of the mixture (van den Berg, Birnbaum et al. 2006).
While there have been numerous studies of associations of perinatal exposure to PCDD/Fs with pregnancy and child health outcomes (e.g., (Tsukimori, Tokunaga et al. 2008, Kishi, Kobayashi et al. 2013)), as well as studies of occupationally-exposed adults (e.g., (Boers, Portengen et al. 2012, Abballe, Barbieri et al. 2013, Hu, Zheng et al. 2013, Wang, Weng et al. 2013)), ours is one of few studies to have collected data on PCDD/F exposure during childhood. Reports of children in the WTC Environmental Health Center/Survivors Health Program (Trasande, Fiorino et al. 2013) and the WTC Health Registry (WTCHR), a national monitoring program sponsored by the National Institute for Occupational Safety and Health (Thomas, Brackbill et al. 2008), suggest that more than one-third of the estimated 25,000 children living and/or attending school in lower Manhattan were exposed to the WTC dust cloud. Most returned to their residences in the week following the attack, where at least one-fourth self-reported exposure to contaminated home dust (Trasande, Fiorino et al. 2013) from the initial collapse and subsequent fires (Landrigan, Lioy et al. 2004). The current analysis draws on data from the WTC Adolescent Health Study, a 2014–16 study of youths present in lower Manhattan and age <8 years on September 11, 2001, and a matched comparison group. Our evaluation of serum PCDD/F levels in these two groups provides the first assessment of PCDD/F exposure among WTC-exposed children.
2. Materials and methods
2.1 Study population
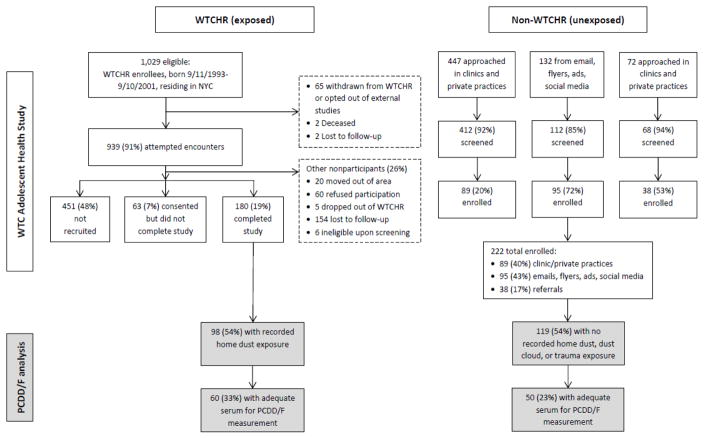
This analysis includes a subset of participants from the WTC Adolescent Health Study, details of which have been published previously (Trasande, Koshy et al. 2017). Briefly, exposed participants, born between September 11, 1993 and September 10, 2001, were recruited from the WTCHR beginning in January, 2014 by staff of the New York City Department of Health & Mental Hygiene fluent in English, Spanish, Mandarin, or Cantonese. Criteria for WTCHR enrollment included residing, working, or attending school in Manhattan south of Canal Street or being present south of Chambers Street--the areas of most concentrated exposure to contamination from the WTC collapse--on the day of the attack. Unexposed participants, who did not meet these criteria, were recruited via well visits at pediatric clinics affiliated with New York University (NYU) School of Medicine, health fairs, youth organizations, postings and advertisements at local colleges and areas where youth congregate, and social media outreach by West Coast Clinical Trials, a contract research organization. Non-WTCHR participants were frequency matched to WTCHR participants on age, sex, race, ethnicity, and income based on the distribution of these variables in the WTCHR’s most recent (2011–12) survey cycle. Potential participants were excluded if they had a serious lung or heart disease that restricted them from participating in the study procedures, heart or lung surgery, were pregnant or were unable to follow the study protocol (Figure 1). For participants age <18 years, a parent or guardian was required to schedule and attend the clinic visit. The study was reviewed and approved by the NYU School of Medicine Institutional Review Board, by research committees at Bellevue and Gouverneur Hospital Centers, and by the New York State Department of Health for the analysis of serum samples. Informed consent was obtained from all participants age ≥18 years at the clinic visit; parent/guardian consent was obtained at the clinic visit on behalf of minors, who assented to participation.
Figure 1.
World Trade Center Health Registry (WTCHR) and non-WTCHR participants in the WTC Adolescent Health Study who were included in the current analysis.
In order to maximize the exposure contrast within the PCDD/F analysis, 98 WTCHR members who reported home dust exposure and 119 non-WTCHR members who reported no dust cloud, no home dust, and no trauma exposure (see section 2.2 for definitions) were selected as eligible for the PCDD/F comparison (54% of both the 180 WTCHR and 222 non-WTCHR participants in the WTC Adolescent Health Study). The final analytic sample included 60 WTCHR adolescents exposed to home dust and 50 non-WTCHR adolescents unexposed to home dust, dust cloud, or trauma who had adequate serum (5 mL) available for measuring PCDD/F levels.
2.2 Exposure and sociodemographic variables
Clinic visits lasting approximately 3 hours were conducted from February 20, 2014 to March 21, 2016. At these appointments, dust cloud, home dust, and trauma exposure information was collected via structured interviews with study participants and additionally with parents/guardians, if applicable. Dust cloud exposure was assessed with the question: “Were you caught in the WTC dust or debris cloud in the morning after the buildings collapsed on 9/11?” Home dust exposure was assessed with the question: “In the year after 9/11/01, did you live in an apartment or home in which WTC dust was visible on surfaces at any time, even if only briefly?” Traumatic exposure was determined by a positive response to any of the following seven items: sight of either tower collapse, sight of injured people, sight of dead bodies, sight of people jumping or falling out of buildings, physical injury to self, need to depart home/school for safety, and worry about safety of a loved one. A positive response from either child or parent/guardian was considered indicative of exposure. Demographic variables were also collected, including those used for matching purposes: age (categorized as 13–14, 15–17, or 18–22 years), sex, race (White, African-American, Asian, Other), ethnicity (Hispanic, non-Hispanic), and income (<$25,000, ≥$25,000).
2.3 Anthropometric and dietary assessment
We measured participants’ height and weight three times using calibrated stadiometers (Shorr Productions, Olney, MD) and scales (Seca model 881; Seca Corp., Hanover, MD) and averaged the results. Because diet is a possible source of dioxin exposure, participants completed a web-based version of the Diet History Questionnaire II (DHQ II) that surveyed dietary intake over the prior month and asked participants to approximate portion sizes so that energy intake could be estimated (daily kilocalories). The DHQII, a publicly-available food frequency questionnaire developed by the National Cancer Institute, is an update of the DHQ, which performed better than the then-available versions of the Willet and Bloch food frequency questionnaires at estimating nutrient intake in a 2001 validation study (Subar, Thompson et al. 2001).
2.4 Biospecimen collection and processing
Participants were instructed to fast for at least 6 hours prior to clinic visits. Saliva, urine, and blood samples were collected following informed consent/assent. Fifteen milliliters of the 28.5 mL blood samples were used to obtain serum. Samples were immediately placed in cool storage and processed within 3 hours of collection. Processed samples were then kept frozen at −80 C until further analysis.
2.5 Measurement of PCDD/Fs and tobacco exposure
PCDD/Fs were measured according to methods described by Horii et al. (Horii, Jiang et al. 2010), with some modifications. PCDD/Fs were extracted by Accelerated Solvent Extraction (ASE) using toluene as the solvent and then the extract was cleaned by multi-layer silica column and activated carbon column. Seventeen 2,3,7,8-substituted PCDD/F congeners were separated by high resolution gas chromatography (HRGC) connected to an Agilent DB 5MS Ultra inert column (60 m x 0.25 mm i.d x 0.25 μm film) and detected by high resolution mass spectrometry (HRMS; JEOL MSD 800). Quantification of PCDD/Fs in the serum samples was performed by isotope dilution mass spectrometry using 13C-labeled internal standards for each congener. The validation of the method was performed by analysis of a certified Standard Reference Material (NIST 1958). Other quality assurance and quality control protocols included analysis of matrix spikes and matrix spike duplicates and procedural blanks. 13C-labeled PCDD/F internal standards were spiked into all samples prior to the extraction step. All serum PCDD/F measures were lipid-adjusted. Level of detection (LOD) values were 0.02 pg/g lipid for all PCDD/Fs except 2,3,7,8-TCDF (LOD=0.01 pg/g lipid) and 1,2,3,7,8-PenCDD (LOD=0.04 pg/g lipid).
We analyzed salivary cotinine using a highly reliable (r=0.99 compared with serum) and sensitive (LOD=0.15 ng/mL) test from Salimetrics, Inc. (State College, PA). Tobacco exposure was categorized as low (<0.15 ng/mL salivary cotinine), medium (≥0.15 to <2.32 ng/mL), or high (≥2.32 ng/mL). For the 6 subjects missing salivary cotinine measures, we assigned those who reported neither smoking nor secondhand smoke exposure into the low cotinine category, those who reported not smoking but exposure to secondhand smoke into the medium category, and those who reported smoking into the high category.
2.6 Statistical analyses
In univariate analyses, we compared demographic characteristics and exposure levels of WTCHR vs. non-WTCHR participants, using chi-square tests for categorical variables and the nonparametric Wilcoxon rank sum test for continuous variables, as none of them was normally distributed. Among the variables hypothesized to potentially influence PCDD/F exposure, those that differed significantly between exposure groups were used as covariates in subsequent regression models. Means and distributions of PCDD/F concentrations were calculated, imputing levels below the LOD as LOD/√2 (Hornung and Reed 1990, Baccarelli, Pfeiffer et al. 2005). We performed multivariable analyses for all congeners for which <50% of the sample was below the LOD. All PCDD/F concentrations were natural log-transformed to account for skewed distribution. In simple and covariate-adjusted linear regression analyses we estimated associations between exposure status and each of the 17 PCDD/Fs, as well as the sum of all PCDDs and all PCDFs. We then estimated simultaneous associations of home dust and dust cloud exposure with each of the PCDD/Fs. In sensitivity analyses, we restricted our sample to those without any missing data (n=84), to those without missing salivary cotinine measures (n=104), and excluding those in the youngest age category (n=86), to account for potential misclassification of responses from children who were age 2 at the time of the disaster. Reported p-values are from two-sided significance tests. All data were analyzed using SAS 9.4 statistical software (SAS Institute Inc., Cary, NC).
Finally, we calculated mean and median TEQ levels for each congener listed in Table 2 using the latest (2005) World Health Organization toxic equivalency factors (TEFs) (van den Berg, Birnbaum et al. 2006) according to the formula:
and compared them to published values from a contemporary youth cohort.
Table 2.
Serum PCDD/F concentrations and toxic equivalencies among WTCHR and non-WTCHR participants vs. 2003–4 NHANES.
| WTCHR (n=60) | Non-WTCHR (n=50) | p-value | NHANES | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <LOD (%)† |
mean (SD) | min | 25 %ile |
50 %ile |
75 %ile |
max | mean TEQ†† |
med TEQ |
mean (SD) | min | 25 %ile |
50 %ile |
75 %ile |
max | mean TEQ |
med TEQ |
Wilcoxon | 95 %ile††† |
|
| Serum polychlorinated dibenzo-p-dioxins (PCDDs) (pg/g lipid) | |||||||||||||||||||
| 2,3,7,8-TCDD | 0.9 | 14.2 (20.0) | 0.01 | 0.74 | 1.71 | 21.2 | 76.1 | 0.34 | 0.23 | 2.10 (5.35) | 0.31 | 0.54 | 0.68 | 0.82 | 28.1 | 0.08 | 0.04 | <0.0001 | <3.8 |
| 1,2,3,7,8-PenCDD | 0 | 41.7 (92.6) | 0.38 | 0.65 | 14.1 | 34.3 | 575 | 1.00 | 0.001 | 4.24 (8.74) | 0.24 | 0.39 | 0.51 | 0.78 | 39.6 | 0.001 | 0.001 | <0.0001 | 4.8 |
| 1,2,3,4,7,8-HexCDD | 82 | 9.99 (24.0) | 0.01 | 0.01 | 0.01 | 12.5 | 162 | 0.36 | 0.002 | 0.01 (0) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.002 | <0.0001 | <11.9 |
| 1,2,3,6,7,8-HexCDD | 53.2 | 3.56 (7.95) | 0.01 | 0.02 | 0.02 | 0.04 | 36.2 | 2.51 | 1.44 | 0.11 (0.65) | 0.01 | 0.01 | 0.02 | 0.02 | 4.63 | 0.74 | 0.65 | <0.0001 | 19.4 |
| 1,2,3,7,8,9-HexCDD | 17.1 | 25.1 (35.5) | 0.01 | 10.3 | 14.4 | 27.0 | 222 | 0.09 | 0.04 | 7.43 (6.39) | 0.01 | 3.67 | 6.47 | 11.2 | 29.7 | 0.06 | 0.02 | <0.0001 | <12.3 |
| 1,2,3,4,6,7,8-HepCDD | 25.2 | 34.3 (33.7) | 0.01 | 14.0 | 23.5 | 47.2 | 201 | 41.7 | 14.1 | 7.94 (12.1) | 0.01 | 0.01 | 3.90 | 12.5 | 66.9 | 4.24 | 0.51 | <0.0001 | 46.7 |
| OCDD | 1.8 | 287 (350) | 0.01 | 64.8 | 135 | 394 | 1812 | 14.2 | 1.71 | 200 (474) | 8.55 | 39.0 | 70.3 | 171 | 3290 | 2.10 | 0.68 | 0.003 | 352 |
| Σ PCDD | 416 (429) | 68.3 | 128 | 197 | 580 | 1921 | 60.2 | 17.5 | 222 (472) | 25.0 | 49.1 | 108 | 196 | 3290 | 7.23 | 1.90 | <0.0001 | ||
| Serum polychlorinated dibenzofurans (PCDFs) (pg/g lipid) | |||||||||||||||||||
| 2,3,7,8-TCDF | 0 | 39.9 (51.3) | 1.63 | 15.5 | 29.1 | 48.9 | 331 | 1.54 | 0.77 | 14.5 (10.9) | 1.16 | 6.84 | 12.1 | 20.7 | 52.1 | 0.25 | 0.22 | <0.0001 | <6.0 |
| 1,2,3,7,8-PenCDF | 47.7 | 21.5 (26.8) | 0.01 | 0.01 | 13.2 | 28.9 | 146 | 0.11 | 0.0001 | 4.18 (8.58) | 0.01 | 0.01 | 0.01 | 5.66 | 32.7 | 0.01 | 0.0001 | <0.0001 | <7.1 |
| 2,3,4,7,8-PenCDF | 0.9 | 18.9 (28.9) | 0.01 | 0.24 | 12.1 | 22.3 | 173 | 0.10 | 0.0003 | 2.95 (6.49) | 0.08 | 0.14 | 0.19 | 0.24 | 28.7 | 0.01 | 0.0002 | <0.0001 | <6.8 |
| 1,2,3,4,7,8-HexCDF | 27.9 | 9.72 (21.5) | 0.01 | 0.02 | 0.03 | 13.4 | 136 | 0.04 | 0.0002 | 0.87 (3.68) | 0.01 | 0.02 | 0.02 | 0.03 | 20.2 | 0.01 | 0.0002 | <0.0001 | <7.4 |
| 1,2,3,6,7,8-HexCDF | 36.0 | 4.17 (8.60) | 0.01 | 0.02 | 0.02 | 0.05 | 36.5 | 0.03 | 0.0001 | 0.55 (2.66) | 0.01 | 0.02 | 0.02 | 0.03 | 15.6 | 0.001 | 0.0001 | 0.0002 | <7.9 |
| 2,3,4,6,7,8-HexCDF | 87.4 | 3.34 (8.19) | 0.01 | 0.01 | 0.01 | 0.01 | 31.7 | 0.24 | 0.06 | 0.12 (0.73) | 0.01 | 0.01 | 0.01 | 0.01 | 5.15 | 0.11 | 0.0003 | 0.002 | <8.3 |
| 1,2,3,7,8,9-HexCDF | 18.0 | 23.7 (39.8) | 0.01 | 0.02 | 6.29 | 33.2 | 248 | 0.02 | 0.01 | 10.6 (12.8) | 0.01 | 0.02 | 0.03 | 18.5 | 41.9 | 0.01 | 0.0000 | 0.06 | <8.2 |
| 1,2,3,4,6,7,8-HepCDF | 15.3 | 154 (250) | 0.01 | 41.4 | 77.5 | 161 | 1534 | 0.64 | 0.40 | 25.2 (23.4) | 0.01 | 9.48 | 22.4 | 35.4 | 108 | 0.13 | 0.0003 | <0.0001 | 33.2 |
| 1,2,3,4,7,8,9-HepCDF | 89.2 | 10.6 (26.3) | 0.01 | 0.01 | 0.01 | 0.01 | 99.9 | 5.67 | 3.64 | 0.52 (3.57) | 0.01 | 0.01 | 0.01 | 0.01 | 25.3 | 0.89 | 0.06 | 0.01 | <8.6 |
| OCDF | 51.4 | 78.5 (146) | 0.01 | 0.01 | 26.4 | 110 | 959 | 3.99 | 2.91 | 18.8 (35.1) | 0.01 | 0.01 | 0.01 | 27.3 | 164 | 1.45 | 1.21 | 0.003 | <12.0 |
| Σ PCDF | 364 (398) | 17.2 | 127 | 232 | 449 | 2225 | 12.4 | 7.79 | 78.3 (44.0) | 17.4 | 50.0 | 71.3 | 95.4 | 250 | 2.86 | 1.49 | <0.0001 | ||
| Total TEQ (pg/g lipid) | 72.5 | 25.3 | 10.1 | 3.39 | |||||||||||||||
WTCHR: World Trade Center Health Registry; NHANES: National Health and Nutrition Examination Survey; LOD: Level of detection; TEQ: toxic equivalency quotient; min: minimum; %ile: percentile; med: median; max: maximum
LOD=0.02 pg/g lipid for all dioxins except 2,3,7,8-TCDF (LOD=0.01 pg/g lipid) and 1,2,3,7,8-PenCDD (LOD=0.04 pg/g lipid)
TEQ calculations based on 2005 World Health Organization toxic equivalency factors
2003–4, age 12–19 years
3. Results
There was no statistically significant difference between WTCHR and non-WTCHR participants in terms of sex, age, income, or exposure to tobacco smoke exposure, as measured by salivary cotinine and questionnaire. Although participants in the WTC Adolescent Health study had been frequency matched on race/ethnicity, among those selected for this analysis, a higher proportion of non-WTCHR participants self-identified as Hispanic compared to WTCHR participants (32.0% vs. 13.3%). Estimated daily kilocalorie intake also differed between the two groups, with WTCHR participants consuming more kilocalories than non-WTCHR participants (median = 1910 vs. 1480) (Table 1).
Table 1.
Characteristics of the analytic sample, World Trade Center Adolescent Health Study, 2014–2016.
| WTCHR (n=60) | Non-WTCHR (n=50) | p-value | |
|---|---|---|---|
| n (%) | n (%) | Chi-square | |
| Sex | 0.44 | ||
| Male | 32 (53.3) | 23 (46.0) | |
| Female | 28 (46.7) | 27 (54.0) | |
| Age | 0.36 | ||
| 13–14 years | 10 (16.7) | 14 (28.0) | |
| 15–17 years | 29 (48.3) | 21 (42.0) | |
| 18–22 years | 21 (35.0) | 15 (30.0) | |
| Annual income* | 0.18 | ||
| <$25,000 | 10 (21.3) | 14 (34.2) | |
| ≥$25,000 | 37 (78.7) | 27 (65.9) | |
| Race/Ethnicity | 0.03 | ||
| Non-Hispanic White | 23 (38.3) | 21 (42.0) | |
| Non-Hispanic Black | 8 (13.3) | 4 (8.0) | |
| Non-Hispanic Asian | 15 (25.0) | 9 (18.0) | |
| Non-Hispanic Other | 6 (10.0) | 0 (0) | |
| Hispanic | 8 (13.3) | 16 (32.0) | |
| WTC exposures | |||
| Dust cloud** | 30 (56.6) | 0 (0) | <0.0001 |
| Home dust | 60 (100) | 0 (0) | <0.0001 |
| Tobacco exposure | 0.60 | ||
| Low | 21 (35.0) | 19 (38.0) | |
| Medium | 29 (48.3) | 26 (52.0) | |
| High | 10 (16.7) | 5 (10.0) | |
| median (25th, 75th percentile) | median (25th, 75th percentile) | Wilcoxon | |
| Daily kilocalorie intake | 1910 (1140, 2450) | 1480 (958, 2100) | 0.02 |
| Height (cm) | 168 (163, 178) | 166 (159, 172) | 0.08 |
| Weight (kg) | 60.0 (52.5, 68.0) | 57 (50, 65) | 0.20 |
WTCHR: World Trade Center Health Registry; WTC: World Trade Center
n = 88
n = 102; 8 WTCHR participants were missing data on dust cloud exposure
Table 2 presents mean (standard deviation) and median (minimum, 25th percentile, 50th percentile, 75th percentile, maximum; interquartile range) PCDD/F levels among WTCHR and non-WTCHR participants, as well as the 95th percentile for 12–19 year olds in the 2003–4 National Health and Nutrition Examination Survey (NHANES). Median PCDD/F levels were statistically significantly higher among WTCHR participants compared to non-WTCHR participants for all chemicals except 1,2,3,7,8,9-HexCDF (p-value=0.06). Maximum values were also consistently higher among WTCHR vs. non-WTCHR participants with the exception of OCDD, for which a single non-WTCHR participant had an extremely high value (3290 pg/g lipid). Among WTCHR participants, median concentrations of several PCDD/Fs (1,2,3,7,8-PenCDD, 1,2,3,7,8,9-HexCDD, 2,3,7,8-TCDF, 1,2,3,7,8-PenCDF, 2,3,4,7,8-PenCDF, 1,2,3,4,6,7,8-HepCDF, and OCDF, ) were higher than the NHANES 95th percentiles. For 2,3,7,8-TCDF, the median exposure level among non-WTCHR participants was higher than the NHANES 95th percentile, as well.
Congener-specific and total TEQ concentrations are also shown in Table 2. Among WTCHR participants, mean and median total TEQ levels were more than 7 times those among non-WTCHR participants (mean: 72.5 vs. 10.1 pg/g lipid; median: 25.3 vs. 3.39 pg/g lipid).
Table 3 shows results from linear regression models that were run for the 5 PCDDs and 7 PCDFs for which at least 50% of participants had detectable levels. In nearly all cases, levels were statistically significantly higher among WTCHR vs. non-WTCHR participants, adjusting for race/ethnicity and daily kilocalories. The exceptions were OCDD (p-value=0.09) and 1,2,3,7,8,9-HexCDF (p-value=0.40).
Table 3.
Changes in serum PCDD/F concentrations comparing WTCHR to non-WTCHR participants (n=110).
| Covariate unadjusted | Covariate adjusted* | |||
|---|---|---|---|---|
| WTCHR vs. non-WTCHR | WTCHR vs. non-WTCHR | |||
| natural log unit increase (95% CI) | p-value | natural log unit increase (95% CI) | p-value | |
| Serum polychlorinated dibenzo-p-dioxins (PCDDs) (pg/g lipid) | ||||
| 2,3,7,8-TCDD | 1.52 (0.94, 2.11) | <0.0001 | 1.47 (0.84, 2.10) | <0.0001 |
| 1,2,3,7,8-PenCDD | 2.05 (1.34, 2.76) | <0.0001 | 1.87 (1.10, 2.63) | <0.0001 |
| 1,2,3,7,8,9-HexCDD | 1.32 (0.32, 2.32) | 0.01 | 1.37 (0.28, 2.46) | 0.01 |
| 1,2,3,4,6,7,8-HepCDD | 3.35 (2.28, 4.42) | <0.0001 | 3.21 (2.05, 4.36) | <0.0001 |
| OCDD | 0.43 (−0.21, 1.06) | 0.19 | 0.58 (−0.09, 1.26) | 0.09 |
| Σ PCDD | 0.90 (0.53, 1.26) | <0.0001 | 0.85 (0.45, 1.24) | <0.0001 |
| Serum polychlorinated dibenzofurans (PCDFs) (pg/g lipid) | ||||
| 2,3,7,8-TCDF | 0.83 (0.44, 1.22) | <0.0001 | 0.81 (0.39, 1.24) | 0.0003 |
| 1,2,3,7,8-PenCDF | 3.60 (2.38, 4.81) | <0.0001 | 3.79 (2.48, 5.09) | <0.0001 |
| 2,3,4,7,8-PenCDF | 2.44 (1.65, 3.24) | <0.0001 | 2.48 (1.60, 3.35) | <0.0001 |
| 1,2,3,4,7,8-HexCDF | 1.95 (0.96, 2.94) | 0.0002 | 1.97 (0.90, 3.05) | 0.0004 |
| 1,2,3,6,7,8-HexCDF | 1.42 (0.57, 2.28) | 0.001 | 1.55 (0.62, 2.48) | 0.001 |
| 1,2,3,7,8,9-HexCDF | 0.49 (−0.88, 1.87) | 0.48 | 0.64 (−0.85, 2.13) | 0.40 |
| 1,2,3,4,6,7,8-HepCDF | 2.06 (0.92, 3.20) | 0.001 | 1.82 (0.58, 3.06) | 0.005 |
| Σ PCDF | 1.26 (0.97, 1.55) | <0.0001 | 1.24 (0.93, 1.55) | <0.0001 |
WTCHR: World Trade Center Health Registry
adjusted for race/ethnicity and daily kilocalories
To test whether dust cloud exposure was associated with PCDD/F levels above and beyond home dust exposure, we included both variables in the models and explored their associations simultaneously. Home dust continued to be positively associated with increased levels of all PCDD/Fs, controlling for dust cloud exposure. By contrast, dust cloud was associated with decreased levels of total PCDFs as well as three individual PCDF congeners: 1,2,3,7,8-PenCDF, 1,2,3,6,7,8-HexCDF, and 1,2,3,7,8,9-HexCDF, controlling for home dust exposure (Table 4). Because of the selection protocol for the PCDD/F analysis, there was an inevitable correlation between home dust and dust cloud exposure (p-value <0.0001), so we performed two ad hoc sensitivity analyses to validate these results. First we compared the results of the simultaneously adjusted models in Table 4 to the models run in Table 3 that did not adjust for dust cloud exposure (in our analysis, WTCHR enrollment is equivalent to home dust exposure) restricted to those without missing dust cloud data (n=102), so that they reflected the same group of participants. The point estimates and confidence intervals were similar for all congeners except the three PCDFs for which we found significant associations with dust cloud exposure in the simultaneous models, indicating that adding dust cloud to the model (Table 4) did not substantially change the results except in these cases. Similarly, when we considered the relationship between dust cloud exposure and PCDD/F levels only among those exposed to home dust (n=53), we found significant negative associations for two of the three same congeners (1,2,3,6,7,8-HexCDF and 1,2,3,7,8,9-HexDCF); the third association was of borderline significance (1,2,3,7,8-PenCDF, p-value=0.07, data not shown).
Table 4.
Simultaneous associations between home dust and dust cloud exposure with serum PCDD/F concentrations, covariate-adjusted* (n=102).
| Home dust vs. no home dust | Dust cloud vs. no dust cloud | |||
|---|---|---|---|---|
| natural log unit increase (95% CI) | p-value | natural log unit increase (95% CI) | p-value | |
| Serum polychlorinated dibenzo-p-dioxins (PCDDs) (pg/g lipid) | ||||
| 2,3,7,8-TCDD | 1.31 (0.48, 2.13) | 0.002 | 0.04 (−0.84, 0.93) | 0.92 |
| 1,2,3,7,8-PenCDD | 0.81 (0.81, 2.80) | 0.001 | −0.34 (−1.42, 0.73) | 0.52 |
| 1,2,3,7,8,9-HexCDD | 2.05 (0.68, 3.43) | 0.004 | −0.72 (−2.21, 0.76) | 0.33 |
| 1,2,3,4,6,7,8-HepCDD | 3.09 (1.59, 4.59) | <0.0001 | 0.06 (−1.56, 1.68) | 0.94 |
| OCDD | 0.76 (−0.01, 1.53) | 0.05 | −0.33 (−1.16, 0.50) | 0.44 |
| Σ PCDD | 0.92 (0.39, 1.45) | 0.001 | −0.13 (−0.70, 0.44) | 0.64 |
| Serum polychlorinated dibenzofurans (PCDFs) (pg/g lipid) | ||||
| 2,3,7,8-TCDF | 0.94 (0.38, 1.51) | 0.001 | −0.36 (−0.97, 0.25) | 0.25 |
| 1,2,3,7,8-PenCDF | 4.59 (2.88, 6.30) | <0.0001 | −1.91 (−3.76, −0.07) | 0.04 |
| 2,3,4,7,8-PenCDF | 2.48 (1.33, 3.63) | <0.0001 | −0.11 (−1,35, 1.13) | 0.86 |
| 1,2,3,4,7,8-HexCDF | 2.37 (1.00, 3.75) | 0.001 | −1.06 (−2.54, 0.42) | 0.16 |
| 1,2,3,6,7,8-HexCDF | 2.57 (1.40, 3.75) | <0.0001 | −1.87 (−3.13, −0.60) | 0.004 |
| 1,2,3,7,8,9-HexCDF | 2.16 (0.27, 4.04) | 0.03 | −2.33 (−4.35, −0.30) | 0.03 |
| 1,2,3,4,6,7,8-HepCDF | 2.40 (0.80, 4.00) | 0.004 | −1.21 (−2.94, 0.52) | 0.17 |
| Σ PCDF | 1.48 (1.10, 1.86) | <0.0001 | −0.42 (−0.83, −0.007) | 0.046 |
adjusted for race/ethnicity and daily kilocalories
Results from sensitivity analyses restricted to participants with no missing data (n=84), with no missing salivary cotinine measure (n=104), and age >2 years at the time of the disaster (n=86) yielded similar results to the main analyses shown in Table 2 (Tables A.1, A.2, and A.3. repectively).
Table A.1.
Covariate-adjusted* changes in serum PCDD/F concentrations comparing WTCHR to non-WTCHR participants: complete case analysis (n=84).
| WTCHR (n=43) vs. non-WTCHR (n=41) | ||
|---|---|---|
| natural log unit increase (95% CI) | p-value | |
| Serum polychlorinated dibenzo-p-dioxins (PCDDs) (pg/g lipid) | ||
| 2,3,7,8-TCDD | 1.41 (0.66, 2.16) | 0.0003 |
| 1,2,3,7,8-PenCDD | 1.59 (0.68, 2.50) | 0.001 |
| 1,2,3,7,8,9-HexCDD | 1.25 (0.09, 2.41) | 0.04 |
| 1,2,3,4,6,7,8-HepCDD | 3.14 (1.81, 4.47) | <0.0001 |
| OCDD | 0.59 (0.09, 1.10) | 0.02 |
| Σ PCDD | 0.77 (0.34, 1.21) | 0.001 |
| Serum polychlorinated dibenzofurans (PCDFs) (pg/g lipid) | ||
| 2,3,7,8-TCDF | 0.68 (0.20, 1.17) | 0.01 |
| 1,2,3,7,8-PenCDF | 3.98 (2.50, 5.48) | <0.0001 |
| 2,3,4,7,8-PenCDF | 2.67 (1.64, 3.70) | <0.0001 |
| 1,2,3,4,7,8-HexCDF | 1.93 (0.78, 3.07) | 0.001 |
| 1,2,3,6,7,8-HexCDF | 1.58 (0.54, 2.61) | 0.003 |
| 1,2,3,7,8,9-HexCDF | 0.50 (−1.23, 2.24) | 0.57 |
| 1,2,3,4,6,7,8-HepCDF | 2.22 (0.89, 3.55) | 0.001 |
| Σ PCDF | 1.28 (0.93, 1.64) | <0.0001 |
adjusted for race/ethnicity and daily kilocalories
adjusted for race/ethnicity and daily kilocalories
Table A.2.
Covariate-adjusted* changes in serum PCDD/F concentrations comparing WTCHR to non-WTCHR participants with salivary cotinine measures (n=104).
| WTCHR (n=59) vs. non-WTCHR (n=45) | ||
|---|---|---|
| natural log unit increase (95% CI) | p-value | |
| Serum polychlorinated dibenzo-p-dioxins (PCDDs) (pg/g lipid) | ||
| 2,3,7,8-TCDD | 1.42 (0.74, 2.10) | <0.0001 |
| 1,2,3,7,8-PenCDD | 1.81 (0.99, 2.52) | <0.0001 |
| 1,2,3,7,8,9-HexCDD | 1.37 (0.24, 2.50) | 0.02 |
| 1,2,3,4,6,7,8-HepCDD | 3.03 (1.83, 4.23) | <0.0001 |
| OCDD | 0.56 (−0.15, 1.28) | 0.12 |
| Σ PCDD | 0.82 (0.41, 1.24) | 0.0002 |
| Serum polychlorinated dibenzofurans (PCDFs) (pg/g lipid) | ||
| 2,3,7,8-TCDF | 0.75 (0.30, 1.21) | 0.001 |
| 1,2,3,7,8-PenCDF | 3.92 (2.57, 5.27) | <0.0001 |
| 2,3,4,7,8-PenCDF | 2.72 (1.83, 3.62) | <0.0001 |
| 1,2,3,4,7,8-HexCDF | 1.96 (0.81, 3.12) | 0.001 |
| 1,2,3,6,7,8-HexCDF | 1.56 (0.56, 2.55) | 0.003 |
| 1,2,3,7,8,9-HexCDF | 0.84 (−0.71, 2.39) | 0.29 |
| 1,2,3,4,6,7,8-HepCDF | 1.78 (0.5, 3.01) | 0.005 |
| Σ PCDF | 1.30 (0.97, 1.63) | <0.0001 |
adjusted for race/ethnicity and daily kilocalories
WTCHR: World Trade Center Health Registry
Table A.3.
Covariate-adjusted* changes in serum PCDD/F concentrations comparing WTCHR to non-WTCHR participants excluding the youngest category (n=86).
| WTCHR (n=50) vs. non-WTCHR (n=36) | ||
|---|---|---|
| natural log unit increase (95% CI) | p-value | |
| Serum polychlorinated dibenzo-p-dioxins (PCDDs) (pg/g lipid) | ||
| 2,3,7,8-TCDD | 1.29 (0.55, 2.02) | 0.001 |
| 1,2,3,7,8-PenCDD | 1.44 (0.53, 2.34) | 0.002 |
| 1,2,3,7,8,9-HexCDD | 1.39 (0.18, 2.60) | 0.03 |
| 1,2,3,4,6,7,8-HepCDD | 2.97 (1.67, 4.27) | <0.0001 |
| OCDD | 0.66 (−0.14, 1.47) | 0.11 |
| Σ PCDD | 0.94 (0.52, 1.35) | <0.0001 |
| Serum polychlorinated dibenzofurans (PCDFs) (pg/g lipid) | ||
| 2,3,7,8-TCDF | 0.54 (0.05, 1.03) | 0.03 |
| 1,2,3,7,8-PenCDF | 3.56 (2.02, 5.10) | <0.0001 |
| 2,3,4,7,8-PenCDF | 2.33 (1.32, 3.34) | <0.0001 |
| 1,2,3,4,7,8-HexCDF | 1.93 (0.70, 3.17) | 0.003 |
| 1,2,3,6,7,8-HexCDF | 1.69 (0.62, 2.76) | 0.002 |
| 1,2,3,7,8,9-HexCDF | 0.94 (−0.78, 2.866 | 0.28 |
| 1,2,3,4,6,7,8-HepCDF | 1.53 (0.09, 2.98) | 0.04 |
| Σ PCDF | 1.11 (0.75, 1.48) | <0.0001 |
adjusted for race/ethnicity and daily kilocalories
WTCHR: World Trade Center Health Registry
4. Discussion
PCDD/F levels among adolescents who were exposed to the WTC collapse as children remain elevated more than 12 years later, in most cases well above those reported among children of similar age in the most recently available NHANES data. Median serum concentrations of 16 out of 17 congeners were significantly higher among WTCHR participants, who experienced chronic home dust exposure, compared to matched non-WTCHR participants, who did not experience chronic exposure, controlling for race/ethnicity and daily kilocalorie intake. When acute dust cloud exposure was additionally controlled for, chronic home dust exposure remained positively associated with PCDD/F levels.
Median total PCDD/F TEQ concentration among WTCHR participants (25.3 pg/g lipid) was substantially higher than the median PCDD/F TEQ concentration in general (non-exposed) populations in 155 studies analyzing individual or pooled blood/plasma/serum samples published from 1989–2010 (10.8 pg/g lipid) (Consonni, Sindaco et al. 2012). It was also higher than the 95th percentile reported among adolescents and young adults in the U.S. According to the most recent age-stratified analysis available, based on 2003–4 NHANES data, the 95th percentile of total TEQ concentration among those age 12–29 was 14.0 pg/g lipid, a measure that included both PCDD/Fs and dioxin-like polychlorinated biphenyls (PCBs) (Patterson, Wong et al. 2009), so the TEQ concentration of only PCDD/Fs would have been even lower. Both of these studies calculated TEQ concentrations using 2005 WHO TEFs.
Only three other major studies have been conducted of PCDD/F-exposed children. In January, 1968, residents of Yusho, Japan, ingested rice bran oil contaminated with PCBs and PCDFs. In addition to suffering eye discharge and disfiguring dermatological symptoms, including chloracne and altered pigmentation (Urabe and Asahi 1985), exposed children experienced temporary suppression of height and weight gain in the years immediately following the poisoning (Yoshimura and Ikeda 1978). Following a major industrial accident near Seveso, Italy, in 1976, chloracne, elevated hepatic enzymes, and enhanced immunologic reactivity were reported among children exposed to high levels of pure 2,3,7,8-TCDD, the most toxic of the PCDD/F and dioxin-like compounds (Bertazzi, Bernucci et al. 1998). In a study of 499 boys who were enrolled in 2003–5 at age 8–9 and followed annually through age 18–19 in Chapaevsk, Russia, the site of a large complex of chemical plants, researchers have reported associations between serum PCDD/F and PCB levels and reduced height and BMI z-scores, delayed pubertal onset (Sergeyev, Burns et al. 2017), and lower sperm concentration, total sperm count, and total motile sperm (Minguez-Alarcon, Sergeyev et al. 2017).
At the time of the Yusho and Seveso incidents, methods to analyze PCDD/Fs in small blood samples had not yet been developed, but in recent years it has been possible to analyze stored serum collected at the time of the Seveso explosion. Analysis of 198 samples from boys who were age 1–9 at the time of the accident yielded a median lipid-adjusted 2,3,7,8-TCDD concentration of 192 ppt (Mocarelli, Gerthoux et al. 2008). Analysis of 232 samples from girls who were age 0–10 at the time of the accident yielded a median lipid-adjusted 2,3,7,8-TCDD concentration ranging from 123 ppt for those age >5–10 years to 288 for those age >0–2 years, with higher concentrations among those in the most contaminated zone: 322 ppt for those age >5–10 years to 553 ppt for those age >0–2 years (Eskenazi, Mocarelli et al. 2004). In comparison, the median 2,3,7,8-TCDD concentration in our cohort was 10 ppt 12 years after exposure (data not shown).
In the Russian Children’s Study, total lipid-adjusted serum PCDD/F concentrations at age 8–9 years (median (25th, 75th percentile): 136 pg/mL (93, 189) and 39 pg/mL (27, 57), respectively) were lower than those of our WTCHR participants (Burns, Williams et al. 2009). Congener-by-congener comparison between the Russian cohort and our WTCHR participants shows variations among the concentration distributions; in general, there was a greater interquartile range of exposure levels among the WTCHR adolescents and the median total TEQ concentrations for PCDD/Fs was higher among WTCHR participants than among the Russian boys (25.3 vs. 12.4 pg/g lipid) (Table A.4) (Burns, Williams et al. 2009). Immediately following 9/11, this discrepancy would likely have been even more pronounced. Conservatively estimating the half-life of PCDD/Fs in those age <18 as 3 years (Kerger, Leung et al. 2006) (others have reported even shorter half-life estimates (Leung, Kerger et al. 2006, Milbrath, Wenger et al. 2009)), at the time of the disaster serum concentrations among our study participants would have been approximately 16 (2 ) times higher than those shown in Table 2.
Table A.4.
Distributions and TEQs of serum PCDD/Fs in WTCHR vs. Russian Children’s Study participants.
| WTCHR (n=60) | Russian Children's Study (n=482) | |||
|---|---|---|---|---|
| median (25th, 75th percentile) | median TEQ* | median (25th, 75th percentile) | median TEQ* | |
| Serum polychlorinated dibenzo-p-dioxins (PCDDs) (pg/g lipid) | ||||
| 2,3,7,8-TCDD | 1.71 (0.74, 20.2) | 1.71 | 2.75 (1.34, 3.90) | 2.75 |
| 1,2,3,7,8-PenCDD | 14.1 (0.65, 34.4) | 14.1 | 4.10 (1.41, 7.00) | 4.10 |
| 1,2,3,4,7,8-HexCDD | 0.01 (0.01, 12.5) | 0.001 | 2.00 (0.71, 3.90) | 0.20 |
| 1,2,3,6,7,8-HexCDD | 0.02 (0.02, 0.04) | 0.002 | 8.70 (5.40, 16.6) | 0.87 |
| 1,2,3,7,8,9-HexCDD | 14.4 (10.3, 27.0) | 1.44 | 2.61 (0.85, 4.60) | 0.26 |
| 1,2,3,4,6,7,8-HepCDD | 23.5 (14.0, 47.2) | 0.23 | 12.2 (8.20, 19.5) | 0.12 |
| OCDD | 135 (64.8, 394) | 0.04 | 96.1 (69.0, 134) | 0.03 |
| Σ PCDD | 197 (128, 580) | 17.5 | 136 (93, 189) | 8.2 |
| Serum polychlorinated dibenzofurans (PCDFs) (pg/g lipid) | ||||
| 2,3,7,8-TCDF | 29.1 (15.5, 48.9) | 2.91 | 0.50 (0.42, 1.63) | 0.05 |
| 1,2,3,7,8-PenCDF | 13.2 (0.01, 28.9) | 0.40 | 0.57 (0.42, 1.91) | 0.02 |
| 2,3,4,7,8-PenCDF | 12.1 (0.24, 22.3) | 3.64 | 9.0 (6.20, 14.6) | 2.70 |
| 1,2,3,4,7,8-HexCDF | 0.03 (0.02, 13.4) | 0.0003 | 6.65 (4.10, 12.5) | 0.67 |
| 1,2,3,6,7,8-HexCDF | 0.02 (0.02, 0.05) | 0.0002 | 4.20 (2.90, 6.70) | 0.42 |
| 2,3,4,6,7,8-HexCDF | 0.01 (0.01, 0.01) | 0.0001 | 0.57 (0.42, 1.41) | 0.06 |
| 1,2,3,7,8,9-HexCDF | 6.29 (0.02, 33.2) | 0.06 | 0.57 (0.42, 1.84) | 0.06 |
| 1,2,3,4,6,7,8-HepCDF | 77.5 (41.4, 161) | 0.77 | 7.50 (5.47, 11.3) | 0.08 |
| 1,2,3,4,7,8,9-HepCDF | 0.01 (0.01, 0.01) | 0.0001 | 0.64 (0.50, 2.26) | 0.01 |
| OCDF | 26.4 (0.01, 110) | 0.01 | 2.90 (1.80, 5.00) | 0.001 |
| Σ PCDF | 232 (127, 449) | 7.79 | 39 (27, 57) | 4.2 |
WTCHR: World Trade Center Health Registry; TEQ: toxic equivalency quotient
based on 2005 World Health Organization toxic equivalency factors
Our findings are particularly robust in the light of our study’s limitations, which include its modest sample size and delayed biosample collection. In addition, levels of several PCDD/F levels in our comparison cohort were elevated relative to NHANES reference values, indicating that members of our non-WTCHR group may not have been entirely unexposed to PCDD/Fs generated by the WTC collapse. Pediatric practices from which we recruited some of our non-WTCHR participants were located just north of Canal Street and in Park Slope and Brooklyn Heights, neighborhoods over which the dust cloud is known to have blown on 9/11. Absent this potential misclassification, we would expect to see an even greater contrast in PCDD/F levels between WTCHR and non-WTCHR participants. It is also possible that background PCDD/F levels are generally higher in NYC than in the U.S. overall, although a recent analysis of regional variation in adult serum levels of persistent organic pollutants based on 2001–4 NHANES data found no difference in 1,2,3,6,7,8-HexCDD or 1,2,3,4,6,7,8-HepCDD levels (the only two PCDD/Fs measured) between the Northeast and the rest of the country (Wattigney, Irvin-Barnwell et al. 2015).
Our study has several strengths, including a sociodemographically-matched comparison group and valid and reliable blood sample analysis. Exposed participants are members of the WTCHR and have undergone three waves of follow-up surveys since 9/11, yielding a rich array of demographic, symptomological, and psychological data. In addition, as part of the WTC Adolescent Health Study, both WTCHR and non-WTCHR participants provided in-depth interviews and various biosamples, and underwent numerous assessments, including anthropometric measures as well as tests of pulmonary and cardiac function. Planned analyses will use these data to explore associations between PCDD/F exposure and an array of health outcomes. Finally, our sample is from an economically, racially, and ethnically diverse population, enhancing the generalizability of future study results.
5. Conclusion
In this first study of PCDD/F levels in WTC-exposed children, we found significantly elevated serum concentrations of 16 out of 17 congeners among participants recruited from the WTCHR compared to a matched non-WTCHR comparison group 12 years after the disaster. Total mean and median TEQ concentrations were more than 7 times higher among WTCHR vs. non-WTCHR participants. The difference between the two groups appeared to be driven by chronic home dust exposure rather than acute dust cloud exposure. Median TEQ levels among WTCHR participants were also higher than those reported by the Russian Children’s Study, a contemporary cohort of continuously exposed children. When the biological half-lives of these chemicals are considered, it is likely that this discrepancy would have been even greater at the time of the WTC collapse. Because of the known endocrine-disrupting properties of PCDD/Fs, future studies of children exposed to chronic particulate contamination from the WTC disaster should focus on metabolic and reproductive outcomes, including thyroid function, reproductive hormone levels, gynecologic disorders, and semen quality. Although individual PCDD/F levels will continue to decline as individuals age, this study provides evidence of high exposure during potentially critical periods of development that may have repercussions throughout the life course, warranting continued monitoring of this vulnerable population.
Highlights.
WTC-exposed youths have significantly elevated serum dioxin levels 12 years later.
Medians of several dioxins in exposed youth exceed 2003–4 NHANES 95th percentiles.
In our study dioxin levels reflect chronic home dust not acute dust cloud exposure.
Median dioxin TEQ is 7 times higher in exposed youth vs. matched controls.
Acknowledgments
Funding
This research was supported by the Centers for Disease Control and Prevention/National Institute of Occupational Safety and Health, through cooperative agreements U01OH01394 and U01OH01714.
The authors are grateful to staff and co-investigators, including: Jenny Lee, Hannah Wilson, Natasha Chiofalo, Rachel Simhon, Michael Ferro, Alice Trye, Kenneth Berger, Roberta Goldring, Howard Trachtman, Mikhail Kazachkov, Bret Rudy, Michael Marmor, Esther Pomares, Nomi Levy-Carrick, Robert Giusti, Claude Chemtob, Amy DiBernardo, Kun Wang, Angela Nguyen, Shengchao Yu, Sukhminder Osahan, Mark Farfel, Steve Stellman, Sean Locke, Karl Brosch, and Elaine Urbina. The authors also wish to thank volunteers who supported the study, including Nafisa Chowdhury, Frank Yuan, Eren Cetlin, Jlateh Jappah, Hannah Bava, Bomi Park, Angel Chen, Janki Jethalal, Carolena Rojas Marcos, Amy Lei, Peng Di, Christina Awada, Yunran Zhang, Shirui Zou, Paridi Bhargava, Jeffery Chen, Chloe Chan, Megan Wong, Hongjie Li, David Lin, Maureen Miller, Rosy Thapa, Sana Khan, Stanley Zhou, Natasha Nazir, Jasmine Rayner, Margaret Lee, and Paramesh Karandikar.
Footnotes
Clinical trials identifier: NCT02068181
Abbreviations
9/11: September 11, 2001,
AhR: aryl hydrocarbon receptor,
DHQ: Diet History Questionnaire,
LOD: level of detection,
NHANES: National Health and Nutrition Examination Survey,
NYU: New York University,
PCB: polychlorinated biphenyl,
PCDD: polychlorinated dibenzo-para-dioxins,
PCDF: polychlorinated dibenzofurans,
TEF: toxic equivalency factor,
TEQ: toxic equivalency quotient,
WHO: World Health Organization,
WTC: World Trade Center,
WTCHR: World Trade Center Health Registry
Competing interests
No competing interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abballe A, Barbieri PG, di Domenico A, Garattini S, Iacovella N, Ingelido AM, Marra V, Miniero R, Valentini S, De Felip E. Occupational exposure to PCDDs, PCDFs, and PCBs of metallurgical workers in some industrial plants of the Brescia area, northern Italy. Chemosphere. 2013;90(1):49–56. doi: 10.1016/j.chemosphere.2012.06.073. [DOI] [PubMed] [Google Scholar]
- Baccarelli A, Pfeiffer R, Consonni D, Pesatori AC, Bonzini M, Patterson DG, Jr, Bertazzi PA, Landi MT. Handling of dioxin measurement data in the presence of non-detectable values: overview of available methods and their application in the Seveso chloracne study. Chemosphere. 2005;60(7):898–906. doi: 10.1016/j.chemosphere.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Bertazzi PA, Bernucci I, Brambilla G, Consonni D, Pesatori AC. The Seveso studies on early and long-term effects of dioxin exposure: a review. Environ Health Perspect. 1998;106(Suppl 2):625–633. doi: 10.1289/ehp.98106625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boers D, Portengen L, Turner WE, Bueno-de-Mesquita HB, Heederik D, Vermeulen R. Plasma dioxin levels and cause-specific mortality in an occupational cohort of workers exposed to chlorophenoxy herbicides, chlorophenols and contaminants. Occup Environ Med. 2012;69(2):113–118. doi: 10.1136/oem.2010.060426. [DOI] [PubMed] [Google Scholar]
- Burns JS, Williams PL, Sergeyev O, Korrick S, Lee MM, Revich B, Altshul L, Patterson DG, Jr, Turner WE, Needham LL, Saharov I, Hauser R. Predictors of serum dioxins and PCBs among peripubertal Russian boys. Environ Health Perspect. 2009;117(10):1593–1599. doi: 10.1289/ehp.0800223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Dioxin-like chemicals: polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and coplanar and mono-ortho-substituted polychlorinated biphenyls. Biomonitoring Summary. 2016 Dec 23; Retrieved 10/9/2017, from https://www.cdc.gov/biomonitoring/DioxinLikeChemicals_BiomonitoringSummary.html.
- Consonni D, Sindaco R, Bertazzi PA. Blood levels of dioxins, furans, dioxin-like PCBs, and TEQs in general populations: a review, 1989–2010. Environ Int. 2012;44:151–162. doi: 10.1016/j.envint.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Edelman P, Osterloh J, Pirkle J, Caudill SP, Grainger J, Jones R, Blount B, Calafat A, Turner W, Feldman D, Baron S, Bernard B, Lushniak BD, Kelly K, Prezant D. Biomonitoring of chemical exposure among New York City firefighters responding to the World Trade Center fire and collapse. Environ Health Perspect. 2003;111(16):1906–1911. doi: 10.1289/ehp.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Needham L, Patterson DG, Jr, Samuels S, Turner W, Gerthoux PM, Brambilla P. Relationship of serum TCDD concentrations and age at exposure of female residents of Seveso, Italy. Environ Health Perspect. 2004;112(1):22–27. doi: 10.1289/ehp.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Y, Jiang Q, Hanari N, Lam PK, Yamashita N, Jansing R, Aldous KM, Mauer MP, Eadon GA, Kannan K. Polychlorinated dibenzo-p-dioxins, dibenzofurans, biphenyls, and naphthalenes in plasma of workers deployed at the World Trade Center after the collapse. Environ Sci Technol. 2010;44(13):5188–5194. doi: 10.1021/es100282d. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of non-detectable values. Appl Occup Environ Hyg. 1990;5:48–51. [Google Scholar]
- Hu J, Zheng M, Liu W, Li C, Nie Z, Liu G, Xiao K, Dong S. Occupational exposure to polychlorinated dibenzo-p-dioxins and dibenzofurans, dioxin-like polychlorinated biphenyls, and polychlorinated naphthalenes in workplaces of secondary nonferrous metallurgical facilities in China. Environ Sci Technol. 2013;47(14):7773–7779. doi: 10.1021/es4016475. [DOI] [PubMed] [Google Scholar]
- Kerger BD, Leung HW, Scott P, Paustenbach DJ, Needham LL, Patterson DG, Jr, Gerthoux PM, Mocarelli P. Age- and concentration-dependent elimination half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Seveso children. Environ Health Perspect. 2006;114(10):1596–1602. doi: 10.1289/ehp.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi R, Kobayashi S, Ikeno T, Araki A, Miyashita C, Itoh S, Sasaki S, Okada E, Kobayashi S, Kashino I, Itoh K, Nakajima S. Ten years of progress in the Hokkaido birth cohort study on environment and children's health: cohort profile--updated 2013. Environ Health Prev Med. 2013;18(6):429–450. doi: 10.1007/s12199-013-0357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwatsuka Y, Shimizu K, Akiyama Y, Koike Y, Ogawa F, Furue M, Utani A. Yusho patients show increased serum IL-17, IL-23, IL-1beta, and TNFalpha levels more than 40 years after accidental polychlorinated biphenyl poisoning. J Immunotoxicol. 2014;11(3):246–249. doi: 10.3109/1547691X.2013.835890. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Lioy PJ, Thurston G, Berkowitz G, Chen LC, Chillrud SN, Gavett SH, Georgopoulos PG, Geyh AS, Levin S, Perera F, Rappaport SM, Small C. Health and environmental consequences of the world trade center disaster. Environ Health Perspect. 2004;112(6):731–739. doi: 10.1289/ehp.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HW, Kerger BD, Paustenbach DJ. Elimination half-lives of selected polychlorinated dibenzodioxins and dibenzofurans in breast-fed human infants. J Toxicol Environ Health A. 2006;69(6):437–443. doi: 10.1080/15287390500246886. [DOI] [PubMed] [Google Scholar]
- Li MC, Chen PC, Tsai PC, Furue M, Onozuka D, Hagihara A, Uchi H, Yoshimura T, Guo YL. Mortality after exposure to polychlorinated biphenyls and polychlorinated dibenzofurans: a meta-analysis of two highly exposed cohorts. Int J Cancer. 2015;137(6):1427–1432. doi: 10.1002/ijc.29504. [DOI] [PubMed] [Google Scholar]
- Litten S, McChesney DJ, Hamilton MC, Fowler B. Destruction of the World Trade Center and PCBs, PBDEs, PCDD/Fs, PBDD/Fs, and chlorinated biphenylenes in water, sediment, and sewage sludge. Environ Sci Technol. 2003;37(24):5502–5510. doi: 10.1021/es034480g. [DOI] [PubMed] [Google Scholar]
- Michalek JE, Pirkle JL, Needham LL, Patterson DG, Jr, Caudill SP, Tripathi RC, Mocarelli P. Pharmacokinetics of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Seveso adults and veterans of operation Ranch Hand. J Expo Anal Environ Epidemiol. 2002;12(1):44–53. doi: 10.1038/sj.jea.7500201. [DOI] [PubMed] [Google Scholar]
- Milbrath MO, Wenger Y, Chang CW, Emond C, Garabrant D, Gillespie BW, Jolliet O. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ Health Perspect. 2009;117(3):417–425. doi: 10.1289/ehp.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Sergeyev O, Burns JS, Williams PL, Lee MM, Korrick SA, Smigulina L, Revich B, Hauser R. A Longitudinal Study of Peripubertal Serum Organochlorine Concentrations and Semen Parameters in Young Men: The Russian Children's Study. Environ Health Perspect. 2017;125(3):460–466. doi: 10.1289/EHP25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarelli P, Gerthoux PM, Needham LL, Patterson DG, Jr, Limonta G, Falbo R, Signorini S, Bertona M, Crespi C, Sarto C, Scott PK, Turner WE, Brambilla P. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect. 2011;119(5):713–718. doi: 10.1289/ehp.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarelli P, Gerthoux PM, Patterson DG, Jr, Milani S, Limonta G, Bertona M, Signorini S, Tramacere P, Colombo L, Crespi C, Brambilla P, Sarto C, Carreri V, Sampson EJ, Turner WE, Needham LL. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect. 2008;116(1):70–77. doi: 10.1289/ehp.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Saijo Y, Kato S, Sasaki S, Uno A, Kanagami N, Hirakawa H, Hori T, Tobiishi K, Todaka T, Nakamura Y, Yanagiya S, Sengoku Y, Iida T, Sata F, Kishi R. Effects of prenatal exposure to polychlorinated biphenyls and dioxins on mental and motor development in Japanese children at 6 months of age. Environ Health Perspect. 2006;114(5):773–778. doi: 10.1289/ehp.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer J, Wittsiepe J, Kasper-Sonnenberg M, Schoneck N, Scholmerich A, Wilhelm M. The influence of low level pre- and perinatal exposure to PCDD/Fs, PCBs, and lead on attention performance and attention-related behavior among German school-aged children: results from the Duisburg Birth Cohort Study. Int J Hyg Environ Health. 2015;218(1):153–162. doi: 10.1016/j.ijheh.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Nishijo M, Pham TT, Nguyen AT, Tran NN, Nakagawa H, Hoang LV, Tran AH, Morikawa Y, Ho MD, Kido T, Nguyen MN, Nguyen HM, Nishijo H. 2,3,7,8-Tetrachlorodibenzo-p-dioxin in breast milk increases autistic traits of 3-year-old children in Vietnam. Mol Psychiatry. 2014;19(11):1220–1226. doi: 10.1038/mp.2014.18. [DOI] [PubMed] [Google Scholar]
- Patterson DG, Jr, Wong LY, Turner WE, Caudill SP, Dipietro ES, McClure PC, Cash TP, Osterloh JD, Pirkle JL, Sampson EJ, Needham LL. Levels in the U.S. population of those persistent organic polutants (2003–2004) included in the Stockholm Convention or in other long-range transboundary air pollution agreements. Environ Sci Technol. 2009;43:1211–1218. doi: 10.1021/es801966w. [DOI] [PubMed] [Google Scholar]
- Pesatori AC, Consonni D, Bachetti S, Zocchetti C, Bonzini M, Baccarelli A, Bertazzi PA. Short- and long-term morbidity and mortality in the population exposed to dioxin after the “S. eveso accident”. Ind Health. 2003;41(3):127–138. doi: 10.2486/indhealth.41.127. [DOI] [PubMed] [Google Scholar]
- Rayne S, Ikonomou MG, Butt CM, Diamond ML, Truong J. Polychlorinated dioxins and furans from the World Trade Center attacks in exterior window films from lower Manhattan in New York City. Environ Sci Technol. 2005;39(7):1995–2003. doi: 10.1021/es049211k. [DOI] [PubMed] [Google Scholar]
- Sergeyev O, Burns JS, Williams PL, Korrick SA, Lee MM, Revich B, Hauser R. The association of peripubertal serum concentrations of organochlorine chemicals and blood lead with growth and pubertal development in a longitudinal cohort of boys: a review of published results from the Russian Children's Study. Rev Environ Health. 2017;32(1–2):83–92. doi: 10.1515/reveh-2016-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg O. AhR signalling and dioxin toxicity. Toxicol Lett. 2014;230(2):225–233. doi: 10.1016/j.toxlet.2013.10.039. [DOI] [PubMed] [Google Scholar]
- Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America's Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- Thomas PA, Brackbill R, Thalji L, DiGrande L, Campolucci S, Thorpe L, Henning K. Respiratory and other health effects reported in children exposed to the World Trade Center disaster of 11 September 2001. Environ Health Perspect. 2008;116(10):1383–1390. doi: 10.1289/ehp.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NN, Pham TT, Ozawa K, Nishijo M, Nguyen AT, Tran TQ, Hoang LV, Tran AH, Phan VH, Nakai A, Nishino Y, Nishijo H. Impacts of Perinatal Dioxin Exposure on Motor Coordination and Higher Cognitive Development in Vietnamese Preschool Children: A Five-Year Follow-Up. PLoS One. 2016;11(1):e0147655. doi: 10.1371/journal.pone.0147655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Fiorino EK, Attina T, Berger K, Goldring R, Chemtob C, Levy-Carrick N, Shao Y, Liu M, Urbina E, Reibman J. Associations of World Trade Center exposures with pulmonary and cardiometabolic outcomes among children seeking care for health concerns. Sci Total Environ. 2013;444:320–326. doi: 10.1016/j.scitotenv.2012.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Koshy TT, Gilbert J, Burdine LK, Attina TM, Ghassabian A, Honda M, Marmor M, Chu DB, Han X, Shao Y, Kannan K. Serum perfluoroalkyl substances in children exposed to the world trade center disaster. Environ Res. 2017;154:212–221. doi: 10.1016/j.envres.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukimori K, Tokunaga S, Shibata S, Uchi H, Nakayama D, Ishimaru T, Nakano H, Wake N, Yoshimura T, Furue M. Long-term effects of polychlorinated biphenyls and dioxins on pregnancy outcomes in women affected by the Yusho incident. Environ Health Perspect. 2008;116(5):626–630. doi: 10.1289/ehp.10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Exposure and human health evaluation of airborne pollution from the World Trade Center disaster. National Center for Environmental Assessment; Research Triangle Park, NC: 2002. [Google Scholar]
- Urabe H, Asahi M. Past and current dermatological status of yusho patients. Environ Health Perspect. 1985;59:11–15. doi: 10.1289/ehp.59-1568099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization re-evaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Weng S, Wen S, Shi T, Sun G, Zeng Y, Qi C, Chen W. Polychlorinated dibenzo-p-dioxins and dibenzofurans and their association with cancer mortality among workers in one automobile foundry factory. Sci Total Environ. 2013;443:104–111. doi: 10.1016/j.scitotenv.2012.10.073. [DOI] [PubMed] [Google Scholar]
- Wang SL, Tsai PC, Yang CY, Guo YL. Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the Yucheng cohort. Diabetes Care. 2008;31(8):1574–1579. doi: 10.2337/dc07-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattigney WA, Irvin-Barnwell E, Pavuk M, Ragin-Wilson A. Regional Variation in Human Exposure to Persistent Organic Pollutants in the United States, NHANES. J Environ Public Health. 2015;2015:571839. doi: 10.1155/2015/571839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SS, Birnbaum LS. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(4):197–211. doi: 10.1080/10590500903310047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Lioy PJ, Santella RM, Wang RY, Jones RL, Caldwell KL, Sjodin A, Turner WE, Li W, Georgopoulos P, Berkowitz GS. Exposures among pregnant women near the World Trade Center site on 11 September 2001. Environ Health Perspect. 2005;113(6):739–748. doi: 10.1289/ehp.7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Ikeda M. Growth of school children with polychlorinated biphenyl poisoning or yusho. Environ Res. 1978;17(3):416–425. doi: 10.1016/0013-9351(78)90045-2. [DOI] [PubMed] [Google Scholar]