Abstract
Objective
To evaluate if interruptions of external beam radiation therapy impact outcomes in men with localized prostate cancer (PCa).
Methods/Materials
We included men with localized PCa treated with three-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiation therapy (IMRT) of escalated dose (≥74 Gy in 1.8 or 2Gy fractions) between 1992–2013 at an NCI-designated cancer center. Men receiving androgen deprivation therapy were excluded. The non-treatment day ratio (NTDR) was defined as the number of non-treatment days divided by the total elapsed days of therapy. NTDR was analyzed for each National Comprehensive Cancer Network (NCCN) risk group.
Results
There were 1728 men included (839 low-risk, 776 intermediate-risk, and 113 high-risk), with a median follow up of 53.5 months (range 12–185.8). The median NTDR was 31% (range 23–71%), translating to approximately 2 breaks (each break represents a missed treatment that will be made up) for 8 weeks of RT with 5 treatments per week. The 75 percentile of NTDR was 33%, translating to approximately 4 breaks, which was used as the cutoff for analysis. There were no significant differences in freedom from biochemical failure, freedom from distant metastasis, cancer specific survival, or overall survival for men with NTDR ≥33% compared to NTDR<33% for each risk group. Multivariable analyses including NTDR, age, race, Gleason score, T stage, and PSA were performed using the proportional hazards regression procedure. NTDR≥33% was not significantly associated with increased hazard ratio for outcomes in each risk group compared to NTDR<33%.
Conclusion
Unintentional treatment breaks during dose escalated external beam radiation therapy for PCa did not cause a significant difference in outcomes, although duration of follow up limits the strength of this conclusion.
Keywords: prostate cancer, radiation therapy, outcomes, quality, treatment interruption, oncology
INTRODUCTION
Prostate cancer is the most prevalent cancer diagnosed in men in the United States, aside from skin cancer.1 External beam radiation therapy (EBRT) is a common treatment option used in patients; conventionally EBRT is typically delivered daily, Monday through Friday over about 8 weeks. During EBRT, patients may have unintentional interruptions and may miss multiple days (e.g. from holidays or unforeseeable reasons).
There is a concern that prolongation of radiation therapy (RT) from missing fractions may result in inferior outcomes because of accelerated repopulation.2 Accelerated repopulation has been show to result in inferior outcomes in cancers of the head and neck, lung, anus, and cervix.3–6 For localized prostate cancer treated with definitive EBRT, the data on the effect of treatment interruptions are limited and conflicting.7–9 In contrast, prostate cancer is generally an indolent disease: rates of freedom from biochemical failure are typically >85% at 5–10 years from diagnosis,10,11 >80% of men are alive 5–10 years from diagnosis, and over 60% of patients die of non-cancer causes.12
The purpose of the current work is to evaluate the effect of treatment breaks during definitive RT for prostate cancer. This information may aid radiation oncologists in their discussions with patients regarding the impact of unintentional treatment breaks, which is a question commonly raised by patients.
MATERIALS AND METHODS
From our institutional review board–approved, prospectively collected prostate cancer database, we selected men with clinically localized prostate cancer treated with primary definitive EBRT between 1992 and 2013. Patients treated with non-escalated RT (dose <74 Gy), androgen deprivation therapy (ADT), or with follow up <1 year were excluded. We excluded patients receiving <74 Gy because this dose is no longer a standard of care per the National Comprehensive Cancer Network (NCCN) guidelines. We excluded patients receiving ADT because this would be expected to improve biochemical outcomes among patients.13,14
The non-treatment day ratio (NTDR) was defined as the number of non-treatment days divided by the total elapsed days of therapy. This allows us to account for differences in total RT dose and planned RT duration. For example, for a radiation regimen of 80 Gy in 40 fractions delivered daily Monday through Friday, the NTDR would equal to 25.9% (14 weekend days/54 elapsed days) if beginning on Monday without any breaks, or 28.6% (16 weekend days/56 elapsed days) if beginning on any other weekday without any breaks. A ratio higher than this represents extended treatment breaks. The definition of the NTDR and its relation to our patient population is shown in Supplementary Figure 1.
NTDR was analyzed for each NCCN risk group.15 The outcomes were freedom from biochemical failure (FFBF), freedom from distant metastasis (FFDM), and overall survival (OS). Biochemical failure was defined according to the Phoenix definition of biochemical failure, which is a rise at least 2 ng/mL above the nadir PSA following radiation. Univariable analysis used the Kaplan-Meier estimation method. The log-rank test compared the effect of NTDR on each outcome. Multivariable analysis (MVA) included NTDR, age, race, Gleason score, T stage, and PSA. Analyses were performed using the proportional hazards regression procedure using SAS 9.3.
All men were treated with either 3-dimensional conformal radiation therapy (3D-CRT) or intensity modulated radiation therapy (IMRT), 1.8–2.0 Gy per day, typically with 10-MV photons with dose prescribed to cover 95% of the planning target volume.16,17 Radiation fields included prostate and proximal seminal vesicles for low-risk patients and prostate plus proximal and distal seminal vesicles for intermediate-risk patients. High-risk patients received radiation to the prostate, seminal vesicles, and the pelvic lymph nodes using either a whole pelvic field or a small pelvic field.
RESULTS
A total of 1,728 patients, including 839 low-risk, 776 intermediate-risk, and 113 high-risk were included in this study. The characteristics of patients are listed in Table 1. Out of 1,728 patients, 260 were treated with 3DCRT, and 1,468 with IMRT. The median radiation dose was 76.2 Gy (range 74.0– 84.4 Gy). The median follow-up was 54 months (range 12–189). The median NTDR was 31% (range 23–71%), translating to approximately 2 breaks (each break represents a missed treatment that will be made up at the end of RT course) for an 8-week course of RT with 5 treatments per week. The 75 percentile of NTDR was 33%, translating to approximately 4 breaks, which was used as the cutoff to compare the outcomes with NTDR ≥33% vs <33%. We chose 33% as the cutoff to capture the number of breaks that has the potential to make a significant impact on outcomes. This cutoff value was also previously cited as being significant18.
Table 1.
Baseline patient characteristics
| All (n=1728) | Low-risk (n=839) | Intermediate-risk (n=776) | High-risk (n=113) | |
|---|---|---|---|---|
| Median age (years) | 67 | 65 | 68 | 69 |
| Median NTDR | 31% | 31% | 32% | 32% |
| n (%) | n (%) | n (%) | n (%) | |
| NTDR≥33% | 424(24.5) | 183(10.6) | 203(11.7) | 38(2.2) |
| NTDR<33% | 1304(75.5) | 656(38.0) | 573(33.2) | 75(4.3) |
| PSA<10 ng/mL | 1448(83.8) | 839(48.6) | 548(31.7) | 61(3.5) |
| PSA 10–20 ng/mL | 237(13.7) | 0 | 228(13.2) | 9(0.5) |
| PSA >20 ng/mL | 43(2.5) | 0 | 0 | 43(2.5) |
| Gleason ≤6 | 1068(61.8) | 839(48.6) | 201(11.6) | 28(1.6) |
| Gleason 7 | 611(35.4) | 0 | 575(33.3) | 36(2.1) |
| Gleason 8–10 | 49(2.8) | 0 | 0 | 49(2.8) |
| T1 | 1217(70.4) | 699(40.5) | 463(26.8) | 55(3.2) |
| T2 | 484(28.0) | 140(8.1) | 313(18.1) | 31(1.8) |
| T3 | 27(1.6) | 0 | 0 | 27(1.6) |
| T4 | 0 | 0 | 0 | 0 |
Among each risk group, there were no significant differences in FFBF, FFDM, CSS, or OS at 5 years for men with NTDR ≥33% compared to NTDR<33% (Table 2). Specifically, FFBF was 100% vs 96.8% (p=0.3), 88.6% vs 89.0% (p=0.5), 82.5% vs 67.0% (p=0.2) for low, intermediate, and high risk groups, respectively. FFDM was 99.4% vs 99.5% (p=0.2), 96.7% vs 96.4% (p=0.9), 85.6% vs 88.4% (p=0.9) for low, intermediate, and high risk groups, respectively. CSS was 100% vs 100% (p=0.4), 100% vs 98.4% (p=0.6), 100% vs 98.4% (p=0.8) for low, intermediate, and high risk groups, respectively. OS was 95.2% vs 97.6% (p=0.2), 89.6% vs 90.0% (p=0.6), 91.5% vs 95.4% (p=0.4) for low, intermediate, and high risk groups, respectively. The Kaplan-Meier FFBF estimates for men with NTDR ≥33% vs NTDR<33% are shown in Figure 1.
Table 2.
Kaplan-Meier estimates of FFBF, FFDM, CSS, and OS for men in each risk group with NTDR≥33% vs <33%.
| At 5 years | Low-risk | Intermediate-risk | High-risk |
|---|---|---|---|
| FFBF(%) with 95%CI | 100 (100–100) vs 96.8 (94.5–98.2), p=0.3 |
88.6 (82.2–92.8) vs 89.0 (85.1–91.9), p=0.5 |
82.5 (62.1–92.5) vs 67.0 (50.8–78.9), p=0.2 |
| FFDM (%) with 95%CI | 99.4 (96.2–99.9) vs 99.5 (97.9–99.9), p=0.2 |
96.7 (92.2–98.7) vs 96.4 (94.0–97.9), p=0.9 |
85.6 (64.9–94.5) vs 88.4 (73.0–95.3), p=0.9 |
| CSS (%) with 95%CI | 100 (100–100) vs 100 (100–100), p=0.4 |
100 (100–100) vs 98.4 (95.8–99.4), p=0.6 |
100 (100–100) vs 98.4 (89.6–99.8), p=0.8 |
| OS (%) with 95%CI | 95.2 (89.5–97.8) vs 97.6 (95.5–98.8), p=0.2 |
89.6 (83.4–93.6) vs 90.0 (86.4–92.7), p=0.2 |
91.5 (70.1–97.8) vs 95.4 (81.9–98.9), p=0.4 |
Abbreviations: FFBF= freedom from biochemical failure; FFDM=freedom from distant metastasis; CSS=cancer-specific survival; OS=overall survival; CI: confidence interval
Figure 1.
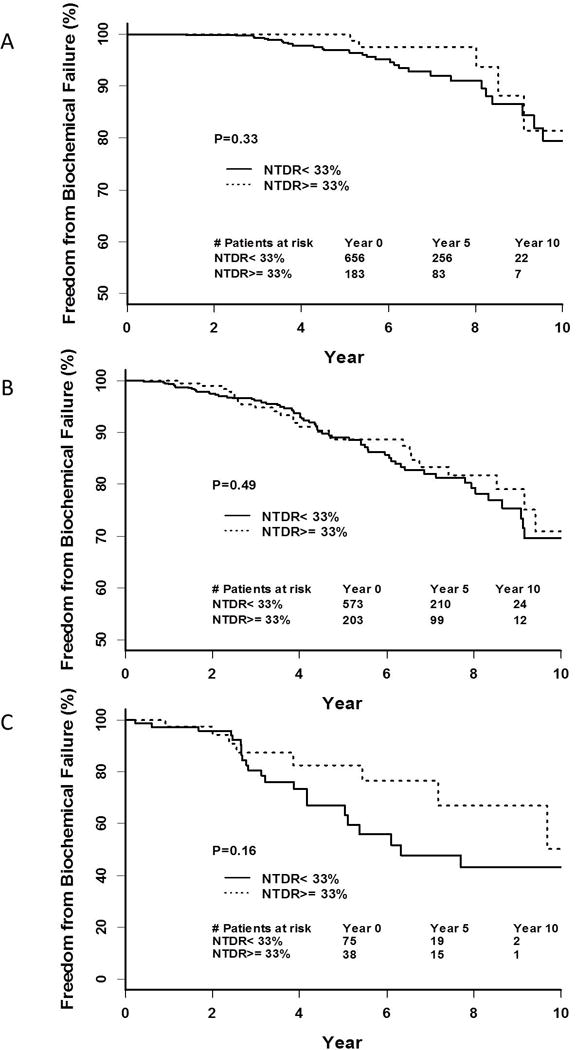
Kaplan-Meier curve of FFBF with NTDR≥33% vs <33% for (A) low-risk patients, (B) intermediate-risk patients, and (C) high-risk patients.
Multivariable analyses including NTDR, age, race, Gleason score, T stage, and PSA were performed using the proportional hazards regression procedure. NTDR≥33% was not significantly associated with increased hazard ratio for outcomes in each risk group compared to NTDR<33% (Table 3).
Table 3.
Multivariable analysis for each risk group.
| Low-risk | BF | DM | CSM | OM | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI, p | HR | 95%CI, p | HR | 95%CI, p | HR | 95%CI, p | ||
| NTDR | ≥33% vs <33% | 0.62 | 0.26–1.51 0.30 |
1.97 | 0.40–9.80 0.41 |
0.44 | 0.02–12.3 0.63 |
1.88 | 0.93–3.80 0.08 |
| Age | ≥70 vs <70 | 0.44 | 0.20–1.01 0.052 |
0 | N/A 1 |
0 | N/A 1 |
4.06 | 2.01–8.20 <0.001 |
| Race | AA vs others | 0.77 | 0.29–2.02 0.59 |
1.21 | 0.22–6.63 0.83 |
1.71 | 0.11–27.6 0.70 |
0.97 | 0.34–2.80 0.96 |
| T stage | T2a vs T1 | 0.41 | 0.10–1.73 0.2 |
0 | N/A 1 |
0 | N/A 1 |
1.43 | 0.61–3.37 0.41 |
| PSA | continuous | 1.33 | 1.11–1.59 0.002 |
2.25 | 1.37–3.70 0.001 |
3.3 | 0.62–17.8 0.16 |
1.01 | 0.85–1.20 0.91 |
| Intermediate-risk | |||||||||
| NTDR | ≥33% vs <33% | 0.91 | 0.57–1.45 0.69 |
1.02 | 0.42–2.49 0.97 |
1.25 | 0.33–4.69 0.73 |
1.36 | 0.87–2.13 0.17 |
| Age | ≥70 vs <70 | 1.13 | 0.75–1.70 0.57 |
0.56 | 0.24–1.33 0.19 |
0.53 | 0.16–2.49 0.51 |
1.28 | 0.84–1.96 0.26 |
| Race | AA vs others | 1.11 | 0.60–2.05 0.74 |
0.89 | 0.26–3.05 0.85 |
1.92 | 0.39–9.54 0.43 |
1.07 | 0.56–2.05 0.84 |
| T stage | T2 vs T1 | 1.21 | 0.86–1.99 0.20 |
1.19 | 0.52–2.71 0.69 |
1.19 | 0.32–4.39 0.79 |
0.98 | 0.63–1.53 0.94 |
| Gleason | 7 vs 2-6 | 2.43 | 1.37–4.31 0.002 |
1.61 | 0.52–4.97 0.41 |
1.76 | 0.32–9.85 0.52 |
1.24 | 0.71–2.16 0.44 |
| PSA | 10–20 vs <10 | 1.40 | 0.87–2.23 0.17 |
0.94 | 0.33–2.70 0.90 |
0.58 | 0.11–3.14 0.52 |
0.88 | 0.52–1.49 0.63 |
| High-risk | |||||||||
| NTDR | ≥33% vs <33% | 0.58 | 0.25–1.33 0.20 |
1 | 0.31–3.25 1 |
1.02 | 0.13–7.94 0.98 |
1.69 | 0.47–6.10 0.42 |
| Age | ≥70 vs <70 | 0.64 | 0.26–1.53 0.31 |
0.25 | 0.05–1.23 0.09 |
2.16 | 0.25–18.9 0.49 |
4.36 | 1.04–18.3 0.04 |
| Race | AA vs others | 1.37 | 0.48–3.90 0.55 |
1.54 | 0.35–6.82 0.57 |
10.0 | 0.41–243 0.16 |
3.1 | 0.45–19.6 0.22 |
| T stage | T3-4 vs T1-2 | 4.78 | 1.68–13.6 0.003 |
2.16 | 0.36–12.9 0.40 |
41.9 | 1.5–1210 0.03 |
3.97 | 0.45–32.4 0.20 |
| Gleason | 7 vs 2–6 | 2.27 | 0.89–5.77 0.09 |
2.35 | 0.59–9.42 0.23 |
1.44 | 0.17–12.3 0.74 |
1.10 | 0.27–4.41 0.90 |
| 8–10 vs 2–6 | 3.88 | 1.19–12.7 0.03 |
1.68 | 0.23–12.3 0.61 |
5.51 | 0.21–146 0.31 |
1.11 | 0.09–13.7 0.94 |
|
| PSA | >20 vs <20 | 4.69 | 1.75–12.57 0.002 |
1.33 | 0.25–7.16 0.74 |
0.86 | 0.07–10.5 0.90 |
1.69 | 0.23–12.5 0.61 |
Abbreviations: HR= hazard ratio; BF= biochemical failure; DM=distant metastasis; CSM=cancer-specific mortality; OM=overall mortality; AA=African American; CI: confidence interval; N/A: not applicable due extremely low number of events
DISCUSSION
Definitive EBRT for localized prostate cancer typically takes approximately 8 weeks to complete. When patients have to take a few unintentional breaks due to various reasons, patients and providers are concerned about negative effects on treatment outcomes. We found that relatively lengthy treatment breaks (defined as ≥4 fraction breaks vs. <4 fraction breaks, with all missing fractions made up at the end of treatment) did not significantly affect FFBF, OS, FFDM, or CSM among patients receiving high dose (>74 Gy) radiation without ADT. The findings suggest that prostate cancer has an indolent disease course compared to other malignancies; for most patients, a high overall dose of RT (>74 Gy) may be sufficient to kill prostate cancer cells, despite a few treatment breaks. Notably, the follow-up time of the cohort is relatively short (median of 5 years); thus, it is unclear if the same effect would be seen in men followed for 10 or more years.
The data on the effect of treatment interruptions are limited and mixed.7–9,18,19 The study by Lai et al. showed lack of influence of RT duration on tumor control and treatment morbidity for stage B and C prostate cancer treated with median dose of 69.6 Gy for stage B and 70.2 Gy for stage C,8 while another study showed worse local control with RT treatment time >8 weeks when compared to ≤8 weeks in men treated with dose 65–70 Gy.7 Notably, both used non-dose escalated RT, which has been shown to be inferior to dose-escalated RT for FFBF.20,21
On the other hand, two other relatively more recent studies were published with differing results. In a study by Liauw et al., a slight prolongation of treatment time (≤7 missed days) was not associated with inferior biochemical failure, especially in men treated with escalated dose of ≥74 Gy.9 The authors found that men with 5 or more missed days had similar 4-year FFBF rates (79% vs. 83% in men with <5 missed days), especially in the subset of men receiving 74 Gy or greater (89% for both groups). Analysis of missed days was performed for the subsets of dose, ADT, and risk category. Men without ADT had a lower FFBF rate with more missed days (p = 0.003), but this association was not seen in men treated to a dose of 74 Gy or greater (p = 0.7425).
Thames et al. revealed that longer overall treatment time was significantly associated with worse biochemical failure in low- and intermediate-risk patients treated with total radiation dose ≥70 Gy but not with dose <70 Gy.19 These effects were quantified as a relative increase after 5 years follow-up of 6% in BFs for a 1-week increase in overall treatment time, a relative decrease of 15% in BFs for a 6-Gy increase in dose, and a dose equivalent of proliferation of 0.24 Gy/day. The authors concluded that meaningful improvements in outcome may be targeted by modest increases in total dose and decreases in overall treatment time. However, the dose threshold of 70 Gy in that study was lower than what we used for this current study, 74 Gy, making reconciliation of these two studies challenging.
Our study showed no significant difference in the outcomes in patients with NTDR ≥33% compared to <33% when RT dose was escalated to ≥74 Gy. These results are consistent with those reported by Liauw et al., where there was a trend towards worse biochemical failure at 4 years with ≥5 missed days (p=0.08) when all patients treated with 62–76.4Gy were included, but there was no difference in biochemical failure among those treated with escalated dose ≥74 Gy (p=0.8).9
There are a few possible reasons that may explain our results. First, biochemical outcomes have become increasingly favorable for prostate cancer because of dose escalation and stage migration.22 Thus, many of the low-risk and favorable intermediate-risk patients included in this analysis may have had favorable outcomes if observed. Next, it has been postulated that mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after high-dose radiation therapy; the high cumulative RT dose is able to kill these pericytes to prevent recurrence.23,24 Finally, given the high overall RT dose delivered (>74 Gy), which is typically not achieved in other disease sites, a different mechanism of cellular death (e.g. necroptosis25) may be responsible for further cellular kill.
This study has limitations. First, we cannot comment on the reason for treatment breaks. Some patients may have had breaks because of holidays (these would be unavoidable, as most facilities do not treat on holidays); others because of urinary toxicity, though these are typically present after treatment;26 others may have had breaks because of critical obligations or medical illness. The NTDR has limitations: a treatment break of 5 consecutive days may have a different impact on outcomes compared to that of 5 interspersed days during the entire treatment. We used the NTDR because we were able to make it more generalizable to patients. There was no apparent frequency of the days missed in the entire treatment period (i.e. early vs. late), and we do not have sufficient patients to perform subset analyses.
Similarly, over the course of the 22-year period, there have been changes in dose fractionation, precision in planning and delivery, pelvis versus non-pelvis, treatment of whole pelvis versus lymph nodes, margins, doses, IMRT/IGRT and follow-up. These may affect the results of the analysis. However, given the long natural history of prostate cancer, it would likely be impossible to find the impact of these factors in a prospective setting. Additionally, the follow-up time of the cohort is relatively short (median of 5 years); thus, it is unclear if the same effect would be seen in men followed for 10 or more years.
Additionally, we did not evaluate the impact of NTDR among hypofractionated schedules, which are increasingly used among prostate cancer patients.27 Extremely hypofractionated schedules may have mechanisms for cellular death similar to brachytherapy, and it is unclear if treatment breaks of days would have significant impact on patient outcomes. Furthermore, the majority of patients included in this study had low or intermediate-risk disease, and the number of patients with high-risk disease was relatively small. It is possible that protraction of radiation course may have a negative impact on patients with high-risk disease but our data failed to demonstrate that due to the relative small number of patients with high-risk disease.
CONCLUSIONS
Unintentional treatment breaks during dose escalated external beam radiation therapy for prostate cancer did not cause a significant difference in outcomes, although duration of follow up limits the strength of this conclusion. The findings suggest that prostate cancer has an indolent disease course compared to other malignancies, and that a high overall dose of RT (>74 Gy) may be sufficient to kill prostate cancer cells, despite a few treatment breaks.
Supplementary Material
Acknowledgments
This publication was supported by grant number P30 CA006927 from the National Cancer Institute, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
This publication was supported in part by a grant from Varian Medical Systems, Inc. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of Varian Medical Systems, Inc.
Footnotes
Conflicts of Interest: None
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncologica (Stockholm, Sweden) 1988;27:131–46. doi: 10.3109/02841868809090333. [DOI] [PubMed] [Google Scholar]
- 3.Murphy CT, Galloway TJ, Handorf EA, et al. Survival Impact of Increasing Time to Treatment Initiation for Patients With Head and Neck Cancer in the United States. J Clin Oncol. 2016;34:169–78. doi: 10.1200/JCO.2015.61.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koukourakis M, Hlouverakis G, Kosma L, et al. The impact of overall treatment time on the results of radiotherapy for nonsmall cell lung carcinoma. International Journal of Radiation Oncology*Biology*Physics. 1996;34:315–22. doi: 10.1016/0360-3016(95)02102-7. [DOI] [PubMed] [Google Scholar]
- 5.Graf R, Wust P, Hildebrandt B, et al. Impact of overall treatment time on local control of anal cancer treated with radiochemotherapy. Oncology. 2003;65:14–22. doi: 10.1159/000071200. [DOI] [PubMed] [Google Scholar]
- 6.Perez CA, Grigsby PW, Castro-Vita H, Lockett MA. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. International Journal of Radiation Oncology*Biology*Physics. 1995;32:1275–88. doi: 10.1016/0360-3016(95)00220-S. [DOI] [PubMed] [Google Scholar]
- 7.Amdur RJ, Parsons JT, Fitzgerald LT, Million RR. Adenocarcinoma of the prostate treated with external-beam radiation therapy: 5-year minimum follow-up. Radiother Oncol. 1990;18:235–46. doi: 10.1016/0167-8140(90)90059-6. [DOI] [PubMed] [Google Scholar]
- 8.Lai PP, Perez CA, Shapiro SJ, Lockett MA. Carcinoma of the prostate stage B and C: Lack of influence of duration of radiotherapy on tumor control and treatment morbidity. Int J Radiat Oncol. 1990;19:561–8. doi: 10.1016/0360-3016(90)90481-x. [DOI] [PubMed] [Google Scholar]
- 9.Liauw SL, Liauw SH. Prolongation of total treatment time because of infrequently missed days of treatment is not associated with inferior biochemical outcome after dose-escalated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2010.06.054. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Zaorsky NG, Keith SW, Shaikh T, et al. Impact of Radiation Therapy Dose Escalation on Prostate Cancer Outcomes and Toxicities. Am J Clin Oncol. 2016 doi: 10.1097/COC.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaorsky NG, Shaikh T, Murphy CT, et al. Comparison of outcomes and toxicities among radiation therapy treatment options for prostate cancer. Cancer Treat Rev. 2016;48:50–60. doi: 10.1016/j.ctrv.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaorsky NG, Churilla TM, Egleston BL, et al. Causes of death among cancer patients. Ann Oncol. 2016 doi: 10.1093/annonc/mdw604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolla M. Adjuvant hormonal treatment with radiotherapy for locally advanced prostate cancer. Eur Urol. 1999;35:23–5. discussion 6. [PubMed] [Google Scholar]
- 14.Bolla M, Artignan X, Chirpaz E, Balosso J, Descotes JL. [Current studies of combined radiotherapy-hormone therapy in localized and locally advanced prostatic cancers] Cancer Radiother. 1998;2:783–6. doi: 10.1016/s1278-3218(99)80024-9. [DOI] [PubMed] [Google Scholar]
- 15.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8:145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 16.Price RA, Jr, Murphy S, McNeeley SW, et al. A method for increased dose conformity and segment reduction for SMLC delivered IMRT treatment of the prostate. International Journal of Radiation Oncology*Biology*Physics. 2003;57:843–52. doi: 10.1016/s0360-3016(03)00711-9. [DOI] [PubMed] [Google Scholar]
- 17.Sharma NK, Li T, Chen DY, Pollack A, Horwitz EM, Buyyounouski MK. Intensity-Modulated Radiotherapy Reduces Gastrointestinal Toxicity in Patients Treated With Androgen Deprivation Therapy for Prostate Cancer. International Journal of Radiation Oncology*Biology*Physics. 2011;80:437–44. doi: 10.1016/j.ijrobp.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 18.D’Ambrosio DJ, Li T, Horwitz EM, Chen DYT, Pollack A, Buyyounouski MK. Does Treatment Duration Affect Outcome After Radiotherapy for Prostate Cancer? International Journal of Radiation Oncology*Biology*Physics. 2008;72:1402–7. doi: 10.1016/j.ijrobp.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thames HD, Kuban D, Levy LB, et al. The role of overall treatment time in the outcome of radiotherapy of prostate cancer: an analysis of biochemical failure in 4839 men treated between 1987 and 1995. Radiother Oncol. 2010;96:6–12. doi: 10.1016/j.radonc.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Zietman AL, Bae K, Slater JD, et al. Randomized Trial Comparing Conventional-Dose With High-Dose Conformal Radiation Therapy in Early-Stage Adenocarcinoma of the Prostate: Long-Term Results From Proton Radiation Oncology Group/American College of Radiology 95-09. Journal of Clinical Oncology. 2010;28:1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–6. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 22.Cooperberg MR, Lubeck DP, Mehta SS, Carroll PR, CaPsure Time trends in clinical risk stratification for prostate cancer: implications for outcomes (data from CaPSURE) J Urol. 2003;170:S21–5. doi: 10.1097/01.ju.0000095025.03331.c6. discussion S6-7. [DOI] [PubMed] [Google Scholar]
- 23.Wang HH, Cui YL, Zaorsky NG, et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett. 2016;375:349–59. doi: 10.1016/j.canlet.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 24.Meng MB, Zaorsky NG, Deng L, et al. Pericytes: a double-edged sword in cancer therapy. Future Oncol. 2014:1–11. doi: 10.2217/fon.14.123. [DOI] [PubMed] [Google Scholar]
- 25.Meng MB, Wang HH, Cui YL, et al. Necroptosis in tumorigenesis, activation of anti-tumor immunity, and cancer therapy. Oncotarget. 2016;7:57391–413. doi: 10.18632/oncotarget.10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson ME, Zaorsky NG, Martin JM, et al. Patient reported outcomes among treatment modalities for prostate cancer. The Canadian journal of urology. 2016;23:8535–45. [PubMed] [Google Scholar]
- 27.Arcangeli S, Greco C. Hypofractionated radiotherapy for organ-confined prostate cancer: is less more? Nature Reviews Urology. 2016;13:400–8. doi: 10.1038/nrurol.2016.106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


