Abstract
Objective
To examine associations of proximity to major roadways, sustained exposure to fine particulate matter (PM2.5), and acute exposure to ambient air pollutants with adipokines and measures of glucose homeostasis among participants living in the northeastern United States.
Methods
We included 5,958 participants from the Framingham Offspring cohort examination cycle 7 (1998–2001) and 8 (2005–2008) and Third Generation cohort examination cycle 1 (2002–2005) and 2 (2008–2011), who did not have type 2 diabetes at the time of examination visit. We calculated 2003 annual average PM2.5 at participants’ home address, residential distance to the nearest major roadway, and daily PM2.5, black carbon (BC), sulfate, nitrogen oxides (NOx), and ozone concentrations. We used linear mixed effects models for fasting glucose, insulin, and hemoglobin A1c which were measured up to twice, and used linear regression models for adiponectin, resistin, and leptin which were measured only once, adjusting for demographics, socioeconomic position, lifestyle, time, and seasonality.
Results
The mean age was 51 years and 55% were women. Participants who lived 64 m (25th percentile) from a major roadway had 0.28% (95% CI: 0.05%, 0.51%) higher fasting plasma glucose than participants who lived 413 m (75th percentile) away, and the association appeared to be driven by participants who lived within 50 m from a major roadway. Higher exposures to 3- to 7-day moving averages of BC and NOx were associated with higher glucose whereas the associations for ozone were negative. The associations otherwise were generally null and did not differ by median age, sex, educational attainment, obesity status, or prediabetes status.
Conclusions
Living closer to a major roadway or acute exposure to traffic-related air pollutants were associated with dysregulated glucose homeostasis but not with adipokines among participants from the Framingham Offspring and Third Generation cohorts.
Keywords: Air Pollution, Adipokines, Glucose Homeostasis, Epidemiology, Particulate Matter
1. Introduction
Higher exposure to ambient air pollution has been associated with systemic inflammation and oxidative stress, which in turn are potential underlying mechanisms for particle-induced impaired glucose tolerance and insulin resistance (Kodavanti 2015; Piya et al. 2013; Rajagopalan and Brook 2012). Elevated air pollution may also be associated with dysregulated release of a series of peptides or proteins (adipokines) secreted by adipose tissue that regulate carbohydrate metabolism (Piya et al. 2013; Rajagopalan and Brook 2012). In some (Sun et al. 2009; Xu et al. 2011; Xu et al. 2010) but not all (Haberzettl et al. 2016) controlled animal studies, mice exposed to ambient fine particulate matter (PM2.5; particles with aerodynamic diameter ≤2.5 µm) were found to have higher levels of resistin, glucose, and insulin, but lower levels of adiponectin and leptin than mice exposed to filtered air. A number of studies have found positive associations between ambient air pollution and prevalence of type 2 diabetes or impaired glucose tolerance in general populations (Eze et al. 2015; Park and Wang 2014) or women during pregnancy (Fleisch et al. 2014; Fleisch et al. 2016; Lu et al. 2017). However, only a few large-scale studies examined associations between air pollution and blood levels of fasting plasma glucose, insulin, hemoglobin A1c (HbA1c), or adipokines, which are important biomarkers of glucose homeostasis, in communities where air pollution levels are relatively low (Cai et al. 2017; Chen et al. 2016b; Honda et al. 2017; O'Donovan et al. 2017; Sade et al. 2016; Sade et al. 2015; Ward-Caviness et al. 2015; Wolf et al. 2016); and many previous human studies have been limited by small sample size, narrow age range, or high levels of ambient air pollution (Brook et al. 2016; Brook et al. 2013; Chen et al. 2016a; Chuang et al. 2010; Chuang et al. 2011; Kim and Hong 2012; Liu et al. 2016; Peng et al. 2016; Teichert et al. 2013; Wang et al. 2014).
We studied the associations of annual average PM2.5 concentration and residential proximity to the nearest major roadway with blood concentrations of adipokines (adiponectin, resistin, and leptin), fasting glucose, insulin, and HbA1c among participants from the Framingham Offspring and Third Generation cohorts. We calculated homeostasis model assessment of insulin resistance (HOMA-IR), an index that has been used to quantitatively assess insulin resistance and β-cell function (Matthews et al. 1985). We also examined the associations for short-term exposure to PM2.5, black carbon (BC), sulfate (SO42−), nitrogen oxides (NOx), and ozone (O3). Our study extends the scope of current research on the associations between ambient air pollution and measures related to glucose homeostasis by providing findings from a large sample of generally healthy middle-aged adults who lived in the Northeastern U.S., a region with relatively low levels of air pollution.
2. Methods
2.1 Study sample
We included 6,574 participants from the Framingham Offspring cohort examination 7 (1998–2001), examination 8 (2005–2008), Third Generation cohort examination 1 (2002–2005), or examination 2 (2008–2011). Detailed selection criteria and design of the two cohorts have been described previously (Kannel et al. 1979; Splansky et al. 2007). To be eligible, participants had to reside in the Northeastern U.S. at the time of examination visits, fasted overnight for at least 8 hours, and had at least one measurement of adiponectin, resistin, leptin, fasting glucose, insulin, or HbA1c. Of the 11,638 observations contributed by these 6,574 participants, we first excluded 1,002 (9%) observations contributed by participants who had diabetes at the time of the examination visits (defined as fasting glucose≥126 mg/dl (American Diabetes Association 2014) or receiving treatment), and then 247 (2%) observations that had missing data on pack years of smoking, alcohol intake, or body mass index, and leaving a total of 10,389 observations from 5,958 participants. Physical examinations were performed at the time of study visits following standardized protocols. Demographics, medication history, smoking history, and alcohol intake were collected using standard questionnaires. Census tract-level socio-economic position data were from the U.S. 2000 census. All participants provided written informed consent, and Institutional Review Boards at Beth Israel Deaconess Medical Center, Massachusetts General Hospital, and Boston University Medical Center approved the study.
2.2 Biomarker assessment
Blood samples were collected after an overnight fast. Fresh plasma samples were used for fasting glucose assessment, and blood samples for other biomarkers were stored at −80°C until assay. Detailed assessment methods have been described elsewhere (Lee et al. 2016; McManus et al. 2012; Meigs et al. 2002). Briefly, fasting glucose was measured by the hexokinase method twice in each cohort; HbA1c was measured by turbidimetric immunoassay in Offspring cohort examination 8 and Third Generation cohort examination 2; insulin was evaluated by commercially available enzyme-linked immunosorbent assay kits from Linco Research (St. Charles, MO) in Third Generation cohort examination 1, and Roche reagents (R&D Systems, Minneapolis, MN) in Offspring cohort examination 8 and Third generation cohort examination 2. Adiponectin, leptin, and resistin were measured using enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN): adiponectin was measured in Offspring cohort examination 7 and Third Generation cohort examination 1; resistin was measured in Offspring cohort examination 7; and leptin was measured in Third Generation cohort examination 1. HOMA-IR was calculated as fasting glucose (mmol/l)×insulin (µU/ml)]/22.5 (Matthews et al. 1985).
The average intra-assay coefficient of variation (CV) was 2% – 3% for fasting glucose and insulin, 4% for adiponectin, 9% for resistin, and 3% for leptin (Demissie et al. 2006; Ho et al. 2017; Thanassoulis et al. 2012). Additional information can be found at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000007.v29.p10.
2.3 Annual average concentration of PM2.5
We geocoded participants’ residential addresses using ArcGIS software and used a hybrid spatial-temporal model to estimate PM2.5 concentration at residential address (Kloog et al. 2014). The model uses satellite-based aerosol optical depth, a measure of particle abundance in the atmospheric column, to estimate daily PM2.5 at a resolution of 1×1 km2. It improved PM2.5 estimation by utilizing data from spatial predictors (such as population density and traffic density) and temporal predictors (such as meteorological parameters), as described in detail in our previous work (Kloog et al. 2014; Li et al. 2016).
Briefly, we first regressed ground PM2.5 concentration against satellite-based aerosol optical depth, adjusting for land use terms and meteorological predictors. We addressed non-random missingness of daily aerosol optical depth data by using inverse probability weighting. When compared to observed values, predictions from this model have an excellent mean out-of-sample R2 of 0.88 and little bias (slope=0.99) (Kloog et al. 2014). Second, we predicted PM2.5 concentration in 1×1 km2 if the grid cells only had aerosol optical depth measurement. Third, if the grid cells did not have aerosol optical depth measurement, we used a generalized additive model with spatial smoothing, the mean of nearby monitors, and a cell-specific random intercept to impute PM2.5 estimates (overall mean out-of-sample R2=0.88) (Kloog et al. 2014). Last, we took the differences between monitor-assessed PM2.5 and predicted PM2.5 for each cell and regressed them against monitor-specific spatial and temporal variables to generate localized daily predictions. We then added this localized daily prediction to the grid cell prediction to generate an address-specific PM2.5 prediction. We used the 2003 annual average PM2.5 concentration for all participants (Dorans et al. 2016; Li et al. 2016; Wilker et al. 2015).
2.4 Residential proximity to the nearest major roadway
In the current study, we used distance to a major roadway as a surrogate measure for traffic-related air pollution. Based on the geocoded residential addresses, we estimated the distance to the nearest major roadway, which is defined as primary highways with limited-access, primary roads without limited-access, or secondary and connecting roads. The addresses were collected and updated at each examination. For participants who lived far from major roadway, traffic on major roadways is unlikely a primary source of their ambient air pollution exposure, and the air pollution exposure profiles might be different compared to other participants, Thus, we restricted distance analysis to 5,403 participants who lived <1,000 m from the nearest major roadway (Dorans et al. 2016; Li et al. 2017b; Li et al. 2016; Wilker et al. 2015). We later included participants who lived ≥1,000 m from the nearest major roadway in a sensitivity analysis.
2.5 Short-term exposure assessment
We obtained central-site hourly measures of PM2.5, BC, and SO42− from the Harvard Supersite air pollution monitoring station located on the rooftop of the Francis A. Countway Library of Medicine (5 stories above ground and 50 m from the nearest street) in Boston, Massachusetts. We used a tapered element oscillating microbalance to measure PM2.5 and an aethalometer to measure BC. We calculated daily SO42− from elemental sulfur measured by X-Ray Fluorescence analysis of the PM2.5 filter samples, and used a SO42− analyzer on days when X-Ray Fluorescence measurements were unavailable. Detailed measurement methods have been described previously (Kang et al. 2010). Ambient levels of NOx and O3 were computed by averaging data collected from local state monitors (three for NOx and two for O3) within the Greater Boston area (Ljungman et al. 2014; Mehta et al. 2014) (Figure 1). Temperature and relative humidity were measured at the Boston Logan International Airport Weather Station, 12 km from the central-site (Ljungman et al. 2014). We included 4,116 participants who lived within 50 km from the Supersite for short-term exposure analyses (Li et al. 2017a; Ljungman et al. 2014; Rice et al. 2013).
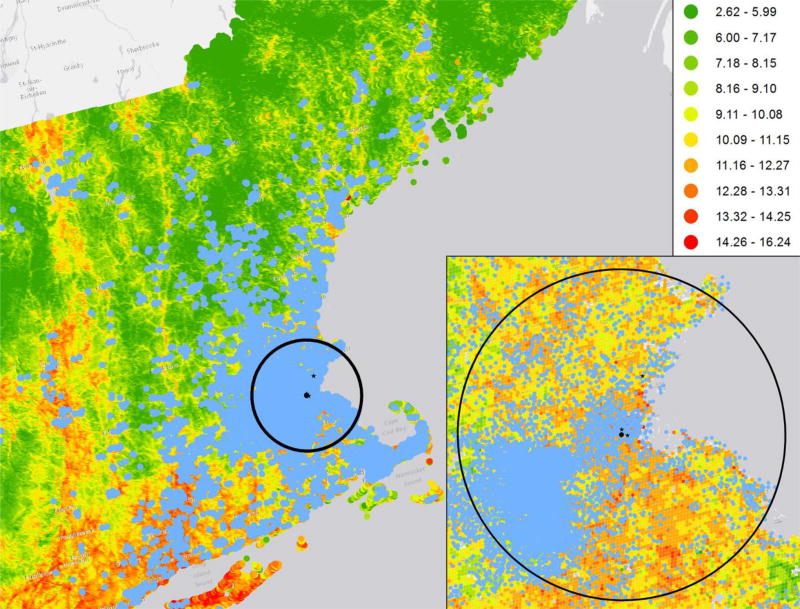
Figure 1.
Map of the study region showing the distribution of the study participants, the model-based 2003 annual average PM2.5 concentrations (µg/m3), and the locations of Supersite and local state monitors. To protect the confidentiality of the participants, the residential locations on the map were masked by altering the longitude and latitude by a small random amount. Thus, although these locations are representative of the distribution of participants’ residential locations, none of them represent actual residential locations of the study participants. Circle indicates the 50 km radius from Supersite. Blue dots: residential locations of the study participants. Black dot: Supersite. Black star: local state monitors.
2.6 Statistical methods
We used linear mixed effects models with participant-specific random intercepts for fasting glucose, insulin, and HOMA-IR to account for repeated measures, and linear regression models for adiponectin, resistin, leptin, and HbA1c, which were measured once. All outcomes were loge-transformed and modeled as continuous variables. Model assumptions were assessed by residual plots and the residuals approximate a normal distribution. We adjusted for age (centered at the mean, age and age2); sex; body mass index; smoking status (current, former, or never smoker); pack years; alcohol intake (drinks/week; standardized to 0.5 oz (15 ml) alcohol/drink) (Elias et al. 1999); educational attainment (high school or less, some college, and college graduate); physical activity (in tertiles (Kannel and Sorlie 1979)); usual occupation (Li et al. 2016; Loucks et al. 2009); census tract level median household income, median value of owner occupied housing units, and population density; and date of examination visit. Seasonality was accounted for with sine and cosine terms. We added an exam identifier to models for glucose, insulin, adiponectin, HbA1c, and HOMA-IR. Because the number of participants with missing data on educational attainment, physical activity, or smoking status were small (35 (0.34%) for smoking status, 56 (0.54%) for education status, and 126 (1.21%) for physical activity index), we created missing indicators (binary dummy variable, 1 = if the observation was missing; 0 = if the observation was non-missing) and included in the statistical analyses.
To account for the dispersion pattern of air pollution with increasing distance from a major roadway (Zhou and Levy 2007), we loge transformed distance to the nearest major roadway. We also analyzed the data using categorized distance to a major roadway (<50 m, 50 to <100 m, 100 to <200 m, 200 to <400 m, and 400 to <1,000 m).
We examined the associations for 1- to 7-day moving averages of PM2.5, BC, SO42−, NOx, and O3 using similar statistical models as in the longer-term exposure analyses, and additionally adjusted for 1-day moving average of temperature and relative humidity, and day of the week of the examination date. The 1-day moving average was calculated as the average value from 9:00 am on the day before examination visit to 9:00 am on the day of examination visit (i.e. lag 0). The 2-day moving average was calculated as the mean of lag 0 and lag 1. If missing days were more than 25% of the days for a moving average, we assigned missing to that moving average. Because HbA1c is a measure that reflects average glucose levels over a period of up to 3 months (International Expert Committee 2009), we did not include HbA1c in this analysis.
Parameter estimates were scaled by a factor that approximated the interquartile ranges: 1.5 µg/m3 for annual PM2.5, −1.9 for distance analysis, which corresponds to contrasting participants who lived 64 m (25th percentile) from a major roadway to those who lived 413 m (75th percentile) away, 5 µg/m3 for PM2.5, 0.5 µg/m3 for BC, 2 µg/m3 for SO42−, 20 ppb for NOx, and 15 ppb for O3.
2.7 Sensitivity analyses
We examined whether associations for annual average PM2.5 or roadway proximity differed by median age, sex, educational attainment (high school or less vs. college or higher), obesity status (BMI>30 kg/m2), or prediabetes status (fasting glucose≥100 mg/dl but <126 mg/dl) by adding interaction terms. Because not all biomarkers were measured across both cohorts, we calculated median age of participants included for each biomarker. We conducted minimally adjusted analyses that only accounted for age, sex, and date of examination. We used 2003–2005 average PM2.5 concentrations to assess the impact of using 2003 as the index year, included participants who lived ≥1,000 m from the nearest major roadway to examine the influence of excluding them, and examined the associations for 2003 annual average PM2.5 only among participants who lived <1,000 m from the nearest major roadway. We additionally examined the associations within current U.S. Environmental Protection Agency national annual PM2.5 standard by excluding observations that had 2003 annual average PM2.5 >12 µg/m3 (N=1,213) in longer-term exposure analysis, and by excluding observations that had daily PM2.5 >35 µg/m3 (N=182) in any of the 7 days prior to the examination date for short-term exposure analyses. Furthermore, we assessed whether results were different after restricting short-term exposure analyses to those who lived within 40 km from the central monitor. Moreover, because NOx and O3 were moderately and negatively correlated (r=−0.54, p<0.0001), we conducted a post-hoc sensitivity analysis where we included both NOx and O3 in the same model. We restricted the annual PM2.5 analyses and proximity analyses to participants who lived within 50 km from the central site; for annual PM2.5 analyses, we further adjusted for the difference between the 1-day moving average of central site measured PM2.5 and the model-based 2003 annual average PM2.5 (ΔPM2.5). Because a different assay was used for insulin in the Third Generation cohort examination 1, we conducted a sensitivity analysis for insulin by excluding participants from examination 1. Last, we conducted analyses only among participants who had two measures for fasting glucose, insulin, and HOMA-IR.
Scaled estimates of the associations (% differences) were reported with 95% confidence intervals (CIs), and we focused on describing the association patterns between air pollutants and the biomarkers. The “% difference” was calculated as “(exp(scaled β) −1)×100%”. Analyses were performed using PROC GENMOD and PROC MIXED in SAS 9.4 (SAS Institute, Inc., Cary, NC). Figures were plotted using Stata 13 (StataCorp LP, College Station, TX).
3. Results
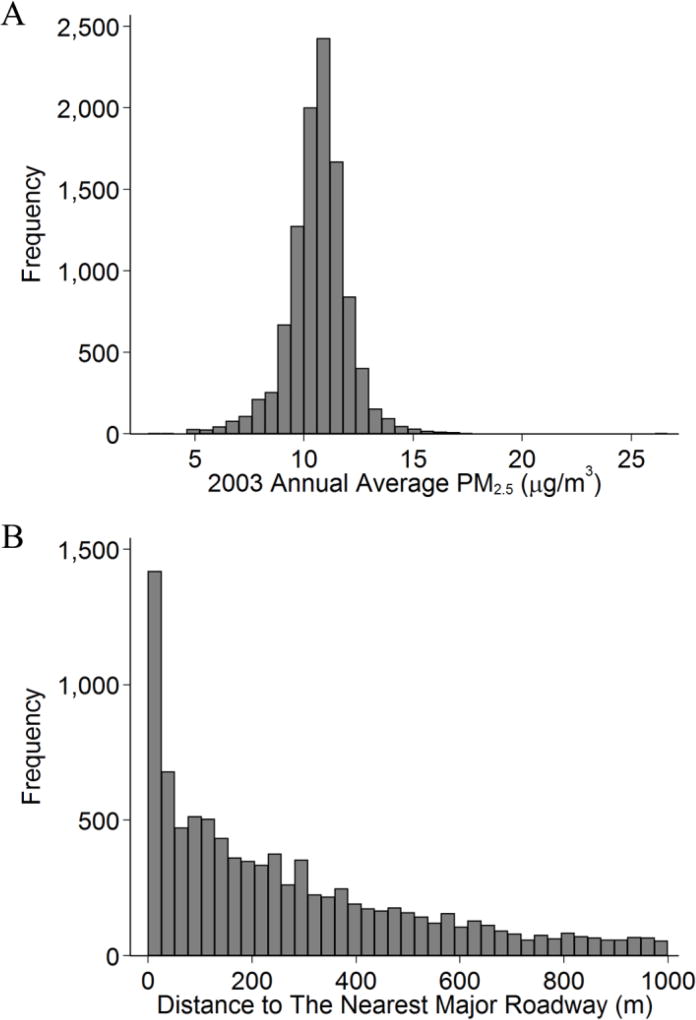
Table 1 shows the characteristics of the study sample. The model-based mean 2003 annual average PM2.5 concentration at the participant’s residential address was 10.6 µg/m3 (standard deviation: 1.4 µg/m3). Meanwhile, the 2003 annual average PM2.5 measured at central site was 11.1 µg/m3 (standard deviation: 5.5 µg/m3). About one third of the observations were from participants who lived within 100 m from a major roadway. Figure 1 shows the distribution the Framingham Heart Study participants in the Northeastern region, the 2003 annual average PM2.5 concentrations, and the locations of the local monitors. Figure 2 shows the distributions of 2003 annual average PM2.5 concentration and distance to major roadways. We included box-plots of measured biomarkers in Supplemental Figure A.
Table 1.
Summary Statistics for the 10,389 Observations (5,958 Participants) From the Framingham Offspring Cohort Examination 7 (1998–2001), Examination 8 (2005–2008), Third Generation Cohort Examination 1 (2002–2005), or Examination 2 (2008–2011).
| Characteristics | Offspring cohort Mean(SD) or N[%] |
Third Generation cohort Mean(SD) or N[%] |
||
|---|---|---|---|---|
| Examination cycle | 7 | 8 | 1 | 2 |
| No. of participants | 2,415 | 1,925 | 3,281 | 2,768 |
| Age, years | 60.4 (9.5) | 65.7 (9.0) | 39.8 (8.7) | 46.1 (8.5) |
| Women | 1,332 [55.2] | 1,093 [56.8] | 1,793 [54.7] | 1,510 [54.6] |
| BMI, kg/m2 | 27.8 (5.1) | 27.8 (5.1) | 26.8 (5.4) | 27.7 (5.5) |
| Alcohol, drinks/week | 5.2 (7.7) | 4.6 (7.1) | 4.8 (7.1) | 4.8 (6.7) |
| Smoking statusa | ||||
| Non-smoker | 893 [37.0] | 742 [38.6] | 1,862 [56.8] | 1,621 [58.6] |
| Former smoker | 1,187 [49.2] | 966 [50.2] | 835 [25.5] | 821 [29.7] |
| Current smoker | 335 [13.9] | 182 [9.5] | 584 [17.8] | 326 [11.8] |
| Pack year | 16.9 (22.2) | 15.4 (21.0) | 6.7 (11.4) | 6.8 (12.0) |
| Educational attainmentb | ||||
| High school or less | 788 [32.6] | 571 [29.7] | 541 [16.5] | 406 [14.7] |
| Some college | 729 [30.2] | 599 [31.1] | 1,063 [32.4] | 845 [30.5] |
| College graduate | 852 [35.3] | 753 [39.1] | 1,670 [50.9] | 1,516 [54.8] |
|
| ||||
| Fasting plasma glucosec,d,e, mg/dl | 97.0 (9.7) | 99.9 (9.1) | 92.6 (8.4) | 93.3 (8.7) |
| Insulinc,d,e, pmol/l | − | 57.9 (33.8) | 27.0 (13.0) | 55.7 (31.5) |
| HOMA-IRc,d,e | − | 2.1 (1.3) | 1.0 (0.5) | 1.8 (1.1) |
| HbA1cc,e, % | − | 5.5 (0.3) | − | 5.4 (0.3) |
| Adiponectinc,e, µg/ml | 8.8 (5.4) | − | 7.2 (4.8) | − |
| Leptinc,e, ng/ml | − | − | 7.3 (7.7) | − |
| Resistinc,e, ng/ml | 12.8 (5.1) | − | − | − |
|
| ||||
| 2003 annual average PM2.5, µg/m3 | 10.6 (1.3) | 10.6 (1.3) | 10.7 (1.5) | 10.6 (1.5) |
| Distance to a major roadwayf, m | 264 (244) | 268 (249) | 268 (252) | 283 (259) |
| Distance categoryf | ||||
| <50 m | 521 [23.6] | 415 [23.9] | 624 [21.6] | 514 [21.1] |
| 50-<100 m | 203 [9.2] | 161 [9.3] | 331 [11.4] | 255 [10.5] |
| 100-<200 m | 385 [17.5] | 292 [16.8] | 529 [18.3] | 418 [17.2] |
| 200-<400 m | 551 [25.0] | 430 [24.8] | 666 [23.0] | 555 [22.8] |
| 400-<1,000 m | 544 [24.7] | 437 [25.2] | 744 [25.7] | 689 [28.3] |
Abbreviation: HOMA-IR, homeostasis model assessment of insulin resistance; HbA1c, hemoglobin A1c; PM2.5, fine particulate matter; SD, standard deviation.
35 (1.8%) participants in the Offspring cohort examination cycle 8 were missing smoking status and were assigned missing indicators.
46 (1.9%), 2 (0.1%), 7 (0.2%), and 1 (0.04%) participants in examination cycle 7, 8, 1, and 2, respectively, were missing educational attainment data and were assigned missing indicators.
The total number of available observations for fasting glucose, insulin, HOMA-IR, HbA1c, adiponectin, leptin, and resistin are 10,389, 7,657, 7,657, 4,690, 5,282, 3,266, and 2,030, respectively.
4,431 participants had two measurements of fasting glucose, 2,379 participants had two measurements of insulin and HOMA-IR.
Geometric mean and standard deviation.
Calculated based on participants who lived < 1,000 m from the nearest major roadway. 211 (8.7%), 190 (9.9%), 387 (11.8%), and 337 (12.2%) participants in examination cycle 7, 8, 1, and 2, respectively, lived ≥1,000 m from the nearest major roadway and were excluded in proximity analyses.
Figure 2.
Histograms of A) 2003 Annual Average Fine Particulate Matter (PM2.5) and B) Distance to Major Roadways Among Participants From the Framingham Offspring Cohort Examination 7 (1998–2001), Examination 8 (2005–2008), Third Generation Cohort Examination 1 (2002–2005), or Examination 2 (2008–2011). There were 5,958 participants (10,389 observations) with 2003 annual PM2.5, and 5,403 participants (9,264 observations) with proximity measurements.
3.1 Annual average PM2.5 and residential distance to a major roadway
The number of participants and observations in each analysis is listed in Supplemental Table A. Compared to participants who lived 413 m (75th percentile) from a major roadway, participants who lived 64 m (25th percentile) had 0.28% (95% CI: 0.05%, 0.51%) higher glucose, and the association appeared to be driven by the positive association among participants who lived within 50 m from the nearest major roadway (Table 2). There were no clear associations with other biomarkers.
Table 2.
Associations of the 2003 Annual Average Fine Particulate Matter (PM2.5) Concentrations and Residential Proximity to Major Roadways With Adipokines and Biomarkers of Glucose Homeostasis Among Participants From the Framingham Offspring Cohort Examination 7 (1998–2001), Examination 8 (2005–2008), Third Generation Cohort Examination 1 (2002–2005), or Examination 2 (2008–2011) a.
| Fasting Plasma Glucose |
HBA1c | Adiponectin | Resistin | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| % Difference |
95% CI |
% Difference |
95% CI |
% Difference |
95% CI | % Difference |
95% CI |
||
| 2003 annual PM2.5b | −0.08 | −0.28, 0.12 | −0.05 | −0.22, 0.12 | −0.30 | −1.98, 1.41 | −2.15 | −4.19, − 0.07 | |
| Living closer to major roadwaysc,d | 0.28 | 0.05, 0.51 | 0.03 | −0.17, 0.22 | −0.73 | −2.68, 1.26 | 2.20 | −0.09, 4.54 | |
| Distance categoriesc | |||||||||
| <50 | 0.72 | 0.19, 1.26 | 0.05 | −0.40, 0.50 | −1.15 | −5.60, 3.51 | 3.83 | −1.28, 9.21 | |
| 50-<100 | 0.22 | −0.44, 0.89 | 0.19 | −0.39, 0.77 | 2.10 | −3.66, 8.21 | −1.30 | −7.72, 5.58 | |
| 100-<200 | −0.03 | −0.58, 0.53 | 0.36 | −0.13, 0.85 | −0.44 | −5.16, 4.52 | 4.69 | −0.85, 10.54 | |
| 200-<400 | 0.24 | −0.27, 0.76 | 0.06 | −0.38, 0.50 | 0.24 | −4.12, 4.81 | 0.09 | −4.68, 5.09 | |
| 400-<1,000 | 0 (REF) | 0 (REF) | 0 (REF) | 0 (REF) | |||||
|
| |||||||||
| Insulin | HOMA-IR | Leptin | |||||||
|
|
|||||||||
| % Difference | 95% CI | % Difference | 95% CI | % Difference | 95% CI | ||||
|
|
|||||||||
| 2003 annual PM2.5b | −0.73 | −1.90, 0.44 | −0.81 | −2.05, 0.45 | 0.19 | −1.94, 2.38 | |||
| Living closer to major roadwaysc,d | 0.60 | −0.76, 1.99 | 0.85 | −0.62, 2.33 | 1.46 | −1.12, 4.12 | |||
| Distance categoriesc | |||||||||
| <50 m | 1.13 | −2.05, 4.42 | 1.59 | −1.82, 5.13 | 2.22 | −3.86, 8.67 | |||
| 50-<100 m | −3.02 | −6.83, 0.95 | −3.04 | −7.11, 1.20 | −1.67 | −8.76, 5.96 | |||
| 100-<200 m | −1.85 | −5.13, 1.55 | −1.92 | −5.43, 1.72 | −4.82 | −10.72, 1.48 | |||
| 200-<400 m | −0.62 | −3.67, 2.54 | −0.61 | −3.87, 2.77 | −4.22 | 9.74,1.63 | |||
| 400-<1,000 m | 0 (REF) | 0 (REF) | 0 (REF) | ||||||
Abbreviation: CI, confidence interval; PM2.5, fine particulate matter; HBA1c, hemoglobin A1c; HOMA-IR, homeostasis model assessment of insulin resistance.
Models were adjusted for centered age, (centered age)2, sex, body mass index, smoking status, pack years, alcohol intake, educational attainment, physical activity, census tract median household income, census tract median value of owner occupied housing units, census tract population density, usual occupation, date of examination visit, and sine and cosine season. An exam identifier was added for fasting glucose, insulin, HOMA-IR, HBA1c, and adiponectin.
Results were scaled to equivalent to 1.5 µg/m3 higher in PM2.5 concentrations.
Analysis was restricted to participants who lived within 1,000 m from major roadways.
Results were scaled to comparing participants who lived 64 m (25th percentile) from major roadways to those who lived 413 m (75th percentile) away.
3.2 Short-term air pollution exposure
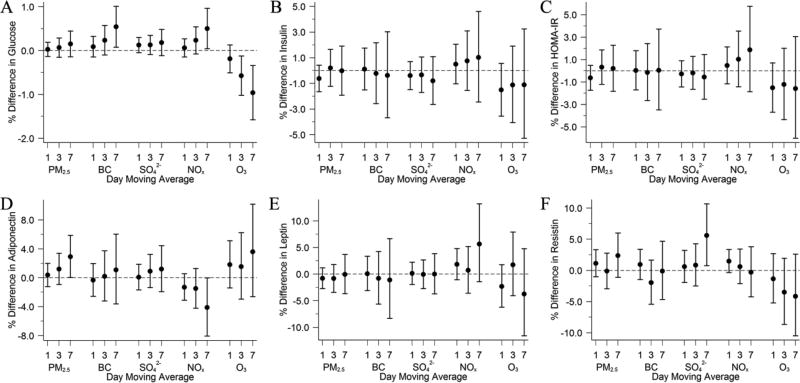
Characteristics of the 1-day moving averages of each air pollutant measured at the Harvard Supersite and the Spearman correlation coefficients are shown in Supplemental Table B. As shown in Figure 3A, both BC and NOx were positively associated with fasting glucose across several moving averages, and stronger associations were seen for longer moving averages. We also found positive associations of the 7-day moving average of PM2.5 with adiponectin and of SO42− with resistin. However, associations between O3 and glucose were negative across all moving averages, and so was the 7-day moving average of NOx with adiponectin. We did not find consistent association patterns for other biomarkers. Plots with 1- to 7-day moving averages of each pollutant are included as Supplemental Figure B.
Figure 3.
Associations of 1-, 3-, and 7-Day Moving Averages of Air Pollutants With A) Fasting Glucose, B) Insulin, C) HOMA-IR, D) Adiponectin, E) Leptin, and F) Resistin Among Participants From the Framingham Offspring Cohort Examination 7 (1998–2001), Examination 8 (2005–2008), Third Generation Cohort Examination 1 (2002–2005), or Examination 2 (2008–2011). Models were adjusted for centered age, (centered age)2, sex, body mass index, smoking status, pack years, alcohol intake, educational attainment, physical activity, census tract median household income, census tract median value of owner occupied housing units, census tract population density, usual occupation, date of examination visit, sine and cosine season, day of week, temperature, and relative humidity. An exam identifier was added for glucose, insulin, HOMA-IR, and adiponectin. Results were scaled to 5 µg/m3 for fine particulate matter (PM2.5), 0.5 µg/m3 for black carbon (BC), 2 µg/m3 for sulfate (SO42−), 20 ppb for nitrogen oxides (NOx), and 15 ppb for ozone (O3). Error bars indicate the 95% confidence intervals.
3.3 Sensitivity analyses
The associations of 2003 annual average PM2.5 concentration and proximity to major roadways with measured biomarkers did not differ by median age, sex, educational attainment, obesity status, or prediabetes status (Supplemental Tables C and D). Using 2003–2005 annual average PM2.5 concentration, including participants who lived ≥1,000 m from major roadways, restricting analyses to participants with both PM2.5 and proximity measures, or excluding observations with 2003 annual average PM2.5>12 µg/m3 did not change our results materially (Supplemental Table E). However, if we restrict the analyses to participants who lived within 50 km from the central site, living closer to a major roadway was no longer associated with higher fasting plasma glucose, but with higher leptin levels, and further adjusting for ΔPM2.5 did not change the results substantially (Supplemental Table F). Excluding participants from the Third Generation cohort examination 1 did not alter our results for insulin materially. For short-term exposure analyses, restricting to participants who lived within 40 km from the Harvard Supersite or excluding observations that had days with PM2.5>35 µg/m3 in any of the seven days before exam visit did not materially alter our results (Supplemental Figures C and D). The associations of O3 with glucose were attenuated but remained negative after adjusting for NOx (Supplemental Figure E). Restricting analyses to participants with two measurements yielded wider 95% CIs but did not alter our results materially (Supplemental Table G and Supplemental Figure F).
4. Discussion
In this large cohort of adults without diabetes, we found positive associations between living closer to a major roadway and higher levels of fasting glucose, after adjusting for demographics, individual- and area-level socioeconomic position, lifestyle factors, time, and seasonality. We further showed that short-term exposure to higher levels of BC and NOx, both correlates of local traffic-related pollution, were associated with higher levels of fasting glucose.
Reports from controlled animal studies on the associations of exposure to PM2.5 and biomarkers of glucose homeostasis or adipokines are mixed (Haberzettl et al. 2016; Sun et al. 2009; Xu et al. 2011; Xu et al. 2010). For example, in Xu et al., mice in the exposed group had higher levels of glucose and HOMA-IR than those in filtered air group, however, the differences were observed among mice fed with normal diet and were very small among those fed with highfat chow (Xu et al. 2010). In Haberzettl et al., among mice fed with high fat chow, there was no difference in levels of glucose, leptin, adiponectin, and resistin between exposed and filtered air group, however, the levels of insulin and HOMA-IR were higher among those exposed (Haberzettl et al. 2016).
A few human studies examined the associations for glucose and insulin together in the same study sample, however, the results were not always in the same direction (Brook et al. 2016; Brook et al. 2013; Chen et al. 2016b; Kim and Hong 2012; Ward-Caviness et al. 2015; Wolf et al. 2016). Generally, in short-term exposure studies, exposure to air pollution was associated with higher levels of glucose, insulin, or HOMA-IR (Brook et al. 2013; Chen et al. 2016b), whereas in longer-term exposure studies, most of the positive associations were found with glucose but not insulin or HOMA-IR (Chen et al. 2016b; Ward-Caviness et al. 2015; Wolf et al. 2016), however, the positive associations with glucose may be mainly contributed by participants with diabetes or prediabetes (Wolf et al. 2016). In some short-term exposure studies, the associations with glucose or insulin were in different directions across lagged days (Brook et al. 2016; Kim and Hong 2012). In the KORA (Cooperative Health Research in the Region Augsburg, Germany) study, Wolf et al. found positive associations of annual average PM2.5 with fasting glucose among 2,944 participants but not with insulin, and the associations was attenuated to null after restricting to participants without type 2 diabetes or prediabetes (Wolf et al. 2016). Additionally, annual average NOx was positively associated with glucose and insulin among participants without type 2 diabetes or prediabetes (Wolf et al. 2016). Ward-Caviness et al. studied the associations of distance to roadways with fasting glucose and HOMA-IR in North Carolina and found positive associations of living closer to major roadways with levels of fasting glucose but not with HOMA-IR among 2,124 individuals undergoing cardiac catheterization (Ward-Caviness et al. 2015).
In summary, findings from both controlled animal studies and human studies have three implications. First, even though acute exposures may be associated with higher levels of glucose and insulin, the associations may not be detectable or persist with longer term exposure. Second, markers of traffic such as NOx and distance to roadways may be better exposure metrics than PM2.5, suggesting that other factors associated with near-road exposure may contribute to the observed associations. Third, associations for glucose and insulin may not necessarily be in the same direction among the same study sample. Our findings in the current study are generally consistent with these implications.
Our findings were also similar to those from a large-scale study conducted in Israel, where Sade et al. found positive associations between 1- to 3-day averages of nitrogen dioxide and serum glucose among 88,351 participants (Sade et al. 2015). However, the associations between O3 and fasting glucose were consistently negative across all moving averages in the current study. For chronic exposure analyses, we found that participants who lived closer to a major roadway had higher levels of fasting glucose, which was consistent with findings by Ward-Caviness et al. and findings in the KORA study, suggesting an association between traffic-related exposures with fasting glucose.
The magnitude of the association observed between proximity to a major roadway and fasting glucose in the current study is similar to the difference in fasting glucose levels that were associated with 1 standard deviation change in the diet pattern score in a meta-analyses of over 48,000 participants in the CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) consortium (Nettleton et al. 2013), or about 110 cm3 increase in the visceral adipose tissue volume in men (60 cm3 in women) in the Framingham Multidetector Computed Tomography Study (Abraham et al. 2015). Furthermore, the 0.28% difference in fasting glucose observed in the current study (about 0.27 mg/dl) was associated with a 4% higher incidence of type 2 diabetes in the Framingham Heart Study (Wilson et al. 2007). Moreover, since only a few large scale studies have examined the associations of proximity to major roadways with biomarkers of glucose homeostasis, from a physiological perspective, our findings contribute new evidence to the current literature about the potential underlying mechanism that links air pollution to dysregulated glucose metabolism.
We did not find consistent associations between sustained or short-term exposure to ambient air pollutants and adipokines. Previous human studies and controlled animal studies have reported mixed results. For example, Wang et al. found positive associations between annual BC exposure and leptin levels among 675 elderly participants (mean age: 78.1 years), however, distance to roadways was not associated with leptin levels (Wang et al. 2014). In the KORA study, annual average NOx and nitrogen dioxides were positively associated with leptin (Wolf et al. 2016). On the other hand, controlled animal studies reported that inhalation of PM2.5 was associated with lower levels of adiponectin and leptin, and higher levels of resistin (Sun et al. 2009; Xu et al. 2011), suggesting that the physiology of energy metabolism and inflammatory response to air pollution may be different in mice and human.
Several factors may contribute to our findings where higher levels of fasting glucose was associated with living closer to a major roadway but not with higher annual average PM2.5 concentration. The PM2.5 estimated from the spatial-temporal model is a measure of both near-road and regional particulate matter pollution: it accounts for both particles from traffic and particles transported from distant emission sources. Meanwhile, residential proximity to major roadways is an integrated measure of small and large traffic-related particles, gaseous pollutants, other traffic-related conditions (light, vibration, and noise), or potential psychological stress associated with living closer to major roadways. It is possible that these traffic-related metrics other than air pollution contribute to the associations between living closer to a major roadway and higher levels of fasting glucose.
We unexpectedly observed negative associations between short-term exposure to O3 and fasting glucose. The reason for this observed association was unclear. O3 is a strong oxidant that may induce oxidative stress, and exposure to O3 in controlled animal studies was associated with increased levels of measures of glucose homeostasis (Bass et al. 2013; Miller et al. 2015; Vella et al. 2015). Short-term exposure to O3 was associated with higher levels of fasting glucose among a group of 560 elderly Koreans (Kim and Hong 2012) but not among 7,578 participants in Taiwan (Chuang et al. 2010). In our study, adjusting for NOx and O3 simultaneously led to attenuated associations between O3 exposure and glucose levels but did not alter the directionality. However, residual confounding, unmeasured confounding, and measurement errors in ozone levels may also contribute to the unexpected negative association pattern.
There are several strengths of our study. The study participants were from large, well-characterized cohorts; physical examinations were conducted following standardized protocols and biomarkers were assessed with quality control. We took advantage of the large sample size and adjusted for covariates including demographics, lifestyles, individual- and area-level measures of socioeconomic position, time, and seasonality. We estimated annual average PM2.5 concentration using a spatial-temporal model at a high resolution at participants’ self-reported home addresses, and we updated the address information at each examination visit. Assessments of air pollution and biomarkers were performed independently, and participants from the Framingham Heart Study scheduled their exam visits months in advance. Last, our study was conducted in a region that has ambient air pollution levels in compliance with the current U.S. air quality standards.
We note several limitations of our study. First, participants in the current analyses were middle-aged white individuals with European ancestry. Thus, findings from our study may not be generalizable to populations of different ethnicities or age groups. Second, although we have adjusted for demographics, lifestyle factors, and individual- and area-level measures of socio-economic position, we cannot exclude potential residual confounding, unmeasured confounding, or uncertainty of temporality. Thus, our findings cannot be used for inferring causality. Third, in short-term exposure analyses, we assigned the same air pollution levels measured at a central monitor site to participants who had their examination visits on the same day. The central monitor site is located 20 m above the ground level which is probably higher than usual air pollution monitors but ensures that the nearby buildings do not block the air flow (Brown et al. 2009). In our region, temporal variability contributes to the majority of the variability in shortterm air pollution exposure compared to spatial variability (Lee et al. 2011). A previous study conducted in metropolitan Boston has reported moderate correlations between central-site measured PM2.5, elemental carbon, and SO42− and outdoor concentrations (Brown et al. 2008). The assignment method may induce a Berkson type of measurement error and decrease statistical power, however, although the results may be attenuated by the non-differential measurement error, we observed positive associations of BC and NOx with fasting glucose. We assigned the annual average PM2.5 concentrations in 2003 to all participants as a surrogate of their sustained exposure. Some biomarkers were measured in Offspring examination 7 (1998–2001) during which the air pollution levels were likely higher, while air pollution levels in the Offspring examination 8 (2005–2008) and Third Generation examination 2 (2008–2011) were likely lower. Because the PM2.5 model estimates were only available starting from 2003, we used the 2003–2005 average PM2.5 in a sensitivity analysis and the results showed no substantial difference. Furthermore, our conclusions were not changed when we excluded participants whose 2003 annual average PM2.5 concentration was above 12 µg/m3, a group of participants who were likely exposed to higher levels of PM2.5 than other participants over the years. Additionally, we were not able to adjust for dietary patterns of the participants. However, we did adjust for individual-and area-level markers of socioeconomic positions, which may partially account for differences in dietary pattern that are related to place of residence. Last, annual averages of BC and NOx were not available for the current study. Although our results suggested positive associations between chronic near-road exposure and glucose homeostasis, we cannot assess whether chronic exposure to air pollutants related to near-road exposure such as BC and NOx were associated with biomarkers of glucose homeostasis.
5. Conclusions
Our findings suggested positive associations between traffic-related exposures and dysregulated carbohydrate metabolism: living closer to a major roadway or short-term exposure to traffic-related air pollutants were associated with elevated fasting glucose. Few large-scale studies have examined these associations in the United States. Future longitudinal studies with not only chronic PM2.5 assessment but also other traffic-related air pollutants such as BC and NOx are necessary to confirm or refute our findings.
Supplementary Material
Highlights.
Living closer to a major roadway was associated with higher fasting glucose.
Annual average PM2.5 was not associated with measures of glucose homeostasis.
Short-term exposure to BC and NOx were positively associated with fasting glucose.
Short-term exposure to O3 was negatively associated with fasting glucose.
Acknowledgments
We thank the Framingham Offspring and Third Generation Study participants.
Funding
This publication was made possible by US Environmental Protection Agency grant numbers RD-834798 and RD-835872. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US Environmental Protection Agency. Further, US Environmental Protection Agency does not endorse the purchase of any commercial products or services mentioned in the publication. This work was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health (grants HHSN268201500001I, N01-HC 25195, and T32HL007575), the National Institutes of Environmental Health Sciences (grant P01ES09825, K23ES026204, R00ES022243, and P30ES000002), the National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK) K24 DK080140, and the National Institute of General Medical Sciences (grant 1P20GM109036-01A1). The contents are solely the responsibility of the grantee and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Abbreviations
- BC
Black carbon
- HbA1c
Hemoglobin A1c
- HOMA-IR
Homeostasis model assessment of insulin resistance
- NOx
Nitrogen oxides
- O3
Ozone
- PM2.5
Fine particulate matter
- SO42−
Sulfate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests
W.L., K.S.D., M.B.R., I.K., J.D.S., B.A.C., P.K., D.R.G., J.B.M., and M.A.M. declare no conflicts of interest. E.H.W. received non-financial support from Servier. C.S.F. owns stock and receives salary from Merck.
References
- Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132:1639–1647. doi: 10.1161/CIRCULATIONAHA.114.015000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- Bass V, Gordon CJ, Jarema KA, MacPhail RC, Cascio WE, Phillips PM, Ledbetter AD, Schladweiler MC, Andrews D, Miller D, Doerfler DL, Kodavanti UP. Ozone induces glucose intolerance and systemic metabolic effects in young and aged Brown Norway rats. Toxicol Appl Pharmacol. 2013;273:551–560. doi: 10.1016/j.taap.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Sun Z, Brook JR, Zhao X, Ruan Y, Yan J, Mukherjee B, Rao X, Duan F, Sun L, Liang R, Lian H, Zhang S, Fang Q, Gu D, Sun Q, Fan Z, Rajagopalan S. Extreme air pollution conditions adversely affect blood pressure and insulin resistance: The Air Pollution and Cardiometabolic Disease Study. Hypertension. 2016;67:77–85. doi: 10.1161/HYPERTENSIONAHA.115.06237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Xu X, Bard RL, Dvonch JT, Morishita M, Kaciroti N, Sun Q, Harkema J, Rajagopalan S. Reduced metabolic insulin sensitivity following sub-acute exposures to low levels of ambient fine particulate matter air pollution. Sci Total Environ. 2013;448:66–71. doi: 10.1016/j.scitotenv.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Sarnat JA, Suh HH, Coull BA, Koutrakis P. Factors influencing relationships between personal and ambient concentrations of gaseous and particulate pollutants. Sci Total Environ. 2009;407:3754–3765. doi: 10.1016/j.scitotenv.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Brown KW, Sarnat JA, Suh HH, Coull BA, Spengler JD, Koutrakis P. Ambient site, home outdoor and home indoor particulate concentrations as proxies of personal exposures. J Environ Monit. 2008;10:1041–1051. doi: 10.1039/b805991h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Hansell AL, Blangiardo M, Burton PR, de Hoogh K, Doiron D, Fortier I, Gulliver J, Hveem K, Mbatchou S, Morley DW, Stolk RP, Zijlema WL, Elliott P, Hodgson S. Long-term exposure to road traffic noise, ambient air pollution, and cardiovascular risk factors in the HUNT and lifelines cohorts. Eur Heart J. 2017;38:2290–2296. doi: 10.1093/eurheartj/ehx263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhou Y, Li S, Williams G, Kan H, Marks GB, Morawska L, Abramson MJ, Chen S, Yao T, Qin T, Wu S, Guo Y. Air pollution and fasting blood glucose: A longitudinal study in China. Sci Total Environ. 2016a;541:750–755. doi: 10.1016/j.scitotenv.2015.09.132. [DOI] [PubMed] [Google Scholar]
- Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, Habre R, Bastain TM, Lurmann F, Wilson JP, Trigo E, Gilliland FD. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in Mexican Americans. Diabetes Care. 2016b;39:547–554. doi: 10.2337/dc15-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KJ, Yan YH, Cheng TJ. Effect of air pollution on blood pressure, blood lipids, and blood sugar: a population-based approach. J Occup Environ Med. 2010;52:258–262. doi: 10.1097/JOM.0b013e3181ceff7a. [DOI] [PubMed] [Google Scholar]
- Chuang KJ, Yan YH, Chiu SY, Cheng TJ. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup Environ Med. 2011;68:64–68. doi: 10.1136/oem.2009.052704. [DOI] [PubMed] [Google Scholar]
- Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Dorans KS, Wilker EH, Li W, Rice MB, Ljungman PL, Schwartz J, Coull BA, Kloog I, Koutrakis P, D'Agostino RB, Sr, Massaro JM, Hoffmann U, O'Donnell CJ, Mittleman MA. Residential Proximity to Major Roads, Exposure to Fine Particulate Matter, and Coronary Artery Calcium: The Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2016;36:1679–1685. doi: 10.1161/ATVBAHA.116.307141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PK, Elias MF, D'Agostino RB, Silbershatz H, Wolf PA. Alcohol consumption and cognitive performance in the Framingham Heart Study. Am J Epidemiol. 1999;150:580–589. doi: 10.1093/oxfordjournals.aje.a010056. [DOI] [PubMed] [Google Scholar]
- Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Kunzli N, Schikowski T, Probst-Hensch NM. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ Health Perspect. 2015;123:381–389. doi: 10.1289/ehp.1307823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Gold DR, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, Coull BA, Zanobetti A, Gillman MW, Oken E. Air pollution exposure and abnormal glucose tolerance during pregnancy: the project Viva cohort. Environ Health Perspect. 2014;122:378–383. doi: 10.1289/ehp.1307065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Kloog I, Luttmann-Gibson H, Gold DR, Oken E, Schwartz JD. Air pollution exposure and gestational diabetes mellitus among pregnant women in Massachusetts: a cohort study. Environ Health. 2016;15:40. doi: 10.1186/s12940-016-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberzettl P, O'Toole TE, Bhatnagar A, Conklin DJ. Exposure to fine particulate air pollution causes vascular insulin resistance by inducing pulmonary oxidative stress. Environ Health Perspect. 2016;124:1830–1839. doi: 10.1289/EHP212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JE, McCabe EL, Wang TJ, Larson MG, Levy D, Tsao C, Aragam J, Mitchell GF, Benjamin EJ, Vasan RS, Cheng S. Cardiometabolic traits and systolic mechanics in the community. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.116.003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, Pun VC, Manjourides J, Suh H. Associations between long-term exposure to air pollution, glycosylated hemoglobin and diabetes. Int J Hyg Environ Health. 2017;220:1124–1132. doi: 10.1016/j.ijheh.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang CM, Koutrakis P, Suh HH. Hourly measurements of fine particulate sulfate and carbon aerosols at the Harvard-U.S. Environmental Protection Agency Supersite in Boston. J Air Waste Manag Assoc. 2010;60:1327–1334. doi: 10.3155/1047-3289.60.11.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979;139:857–861. [PubMed] [Google Scholar]
- Kim JH, Hong YC. GSTM1, GSTT1, and GSTP1 polymorphisms and associations between air pollutants and markers of insulin resistance in elderly Koreans. Environ Health Perspect. 2012;120:1378–1384. doi: 10.1289/ehp.1104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Chudnovsky AA, Just AC, Nordio F, Koutrakis P, Coull BA, Lyapustin A, Wang Y, Schwartz J. A new hybrid spatio-temporal model for estimating daily multiyear PM2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmospheric Environment. 2014;95:581–590. doi: 10.1016/j.atmosenv.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti UP. Air pollution and insulin resistance: do all roads lead to Rome? Diabetes. 2015;64:712–714. doi: 10.2337/db14-1682. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Gent JF, Leaderer BP, Koutrakis P. Spatial and temporal variability of fine particle composition and source types in five cities of Connecticut and Massachusetts. Sci Total Environ. 2011;409:2133–2142. doi: 10.1016/j.scitotenv.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Pedley A, Hoffmann U, Massaro JM, Keaney JF, Jr, Vasan RS, Fox CS. Cross-sectional associations of computed tomography (CT)-derived adipose tissue density and adipokines: The Framingham Heart Study. J Am Heart Assoc. 2016;5:e002545. doi: 10.1161/JAHA.115.002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Dorans KS, Wilker EH, Rice MB, Ljungman PL, Schwartz JD, Coull BA, Koutrakis P, Gold DR, Keaney JF, Jr, Vasan RS, Benjamin EJ, Mittleman MA. Short-term exposure to ambient air pollution and biomarkers of systemic inflammation: The Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2017a doi: 10.1161/ATVBAHA.117.309799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Dorans KS, Wilker EH, Rice MB, Long MT, Schwartz J, Coull BA, Koutrakis P, Gold DR, Fox CS, Mittleman MA. Residential Proximity to Major Roadways, Fine Particulate Matter, and Hepatic Steatosis: The Framingham Heart Study. Am J Epidemiol. 2017b doi: 10.1093/aje/kwx127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Dorans KS, Wilker EH, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Fox CS, Mittleman MA. Residential proximity to major roadways, fine particulate matter, and adiposity: The Framingham Heart Study. Obesity. 2016;24:2593–2599. doi: 10.1002/oby.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yang C, Zhao Y, Ma Z, Bi J, Liu Y, Meng X, Wang Y, Cai J, Chen R, Kan H. Associations between long-term exposure to ambient particulate air pollution and type 2 diabetes prevalence, blood glucose and glycosylated hemoglobin levels in China. Environ Int. 2016;92–93:416–421. doi: 10.1016/j.envint.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman PL, Wilker EH, Rice MB, Schwartz J, Gold DR, Koutrakis P, Vita JA, Mitchell GF, Vasan RS, Benjamin EJ, Mittleman MA, Hamburg NM. Short-term exposure to air pollution and digital vascular function. Am J Epidemiol. 2014;180:482–489. doi: 10.1093/aje/kwu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks EB, Lynch JW, Pilote L, Fuhrer R, Almeida ND, Richard H, Agha G, Murabito JM, Benjamin EJ. Life-course socioeconomic position and incidence of coronary heart disease: the Framingham Offspring Study. Am J Epidemiol. 2009;169:829–836. doi: 10.1093/aje/kwn403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MC, Wang P, Cheng TJ, Yang CP, Yan YH. Association of temporal distribution of fine particulate matter with glucose homeostasis during pregnancy in women of Chiayi City, Taiwan. Environ Res. 2017;152:81–87. doi: 10.1016/j.envres.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McManus DD, Lyass A, Ingelsson E, Massaro JM, Meigs JB, Aragam J, Benjamin EJ, Vasan RS. Relations of circulating resistin and adiponectin and cardiac structure and function: the Framingham Offspring Study. Obesity. 2012;20:1882–1886. doi: 10.1038/oby.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AJ, Zanobetti A, Koutrakis P, Mittleman MA, Sparrow D, Vokonas P, Schwartz J. Associations between short-term changes in air pollution and correlates of arterial stiffness: The Veterans Affairs Normative Aging Study, 2007–2011. Am J Epidemiol. 2014;179:192–199. doi: 10.1093/aje/kwt271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigs JB, D'Agostino RB, Sr, Nathan DM, Rifai N, Wilson PW. Longitudinal association of glycemia and microalbuminuria: the Framingham Offspring Study. Diabetes Care. 2002;25:977–983. doi: 10.2337/diacare.25.6.977. [DOI] [PubMed] [Google Scholar]
- Miller DB, Karoly ED, Jones JC, Ward WO, Vallanat BD, Andrews DL, Schladweiler MC, Snow SJ, Bass VL, Richards JE, Ghio AJ, Cascio WE, Ledbetter AD, Kodavanti UP. Inhaled ozone (O3)-induces changes in serum metabolomic and liver transcriptomic profiles in rats. Toxicol Appl Pharmacol. 2015;286:65–79. doi: 10.1016/j.taap.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleton JA, Hivert MF, Lemaitre RN, McKeown NM, Mozaffarian D, Tanaka T, Wojczynski MK, Hruby A, Djousse L, Ngwa JS, Follis JL, Dimitriou M, Ganna A, Houston DK, Kanoni S, Mikkila V, Manichaikul A, Ntalla I, Renstrom F, Sonestedt E, van Rooij FJ, Bandinelli S, de Koning L, Ericson U, Hassanali N, Kiefte-de Jong JC, Lohman KK, Raitakari O, Papoutsakis C, Sjogren P, Stirrups K, Ax E, Deloukas P, Groves CJ, Jacques PF, Johansson I, Liu Y, McCarthy MI, North K, Viikari J, Zillikens MC, Dupuis J, Hofman A, Kolovou G, Mukamal K, Prokopenko I, Rolandsson O, Seppala I, Cupples LA, Hu FB, Kahonen M, Uitterlinden AG, Borecki IB, Ferrucci L, Jacobs DR, Jr, Kritchevsky SB, Orho-Melander M, Pankow JS, Lehtimaki T, Witteman JC, Ingelsson E, Siscovick DS, Dedoussis G, Meigs JB, Franks PW. Meta-analysis investigating associations between healthy diet and fasting glucose and insulin levels and modification by loci associated with glucose homeostasis in data from 15 cohorts. Am J Epidemiol. 2013;177:103–115. doi: 10.1093/aje/kws297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G, Chudasama Y, Grocock S, Leigh R, Dalton AM, Gray LJ, Yates T, Edwardson C, Hill S, Henson J, Webb D, Khunti K, Davies MJ, Jones AP, Bodicoat DH, Wells A. The association between air pollution and type 2 diabetes in a large cross-sectional study in Leicester: The CHAMPIONS Study. Environ Int. 2017;104:41–47. doi: 10.1016/j.envint.2017.03.027. [DOI] [PubMed] [Google Scholar]
- Park SK, Wang W. Ambient air pollution and type 2 diabetes: a systematic review of epidemiologic research. Curr Environ Health Rep. 2014;1:275–286. doi: 10.1007/s40572-014-0017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Bind MC, Colicino E, Kloog I, Byun HM, Cantone L, Trevisi L, Zhong J, Brennan K, Dereix AE, Vokonas PS, Coull BA, Schwartz JD, Baccarelli AA. Particulate air pollution and fasting blood glucose in nondiabetic individuals: associations and epigenetic mediation in the Normative Aging Study, 2000–2011. Environ Health Perspect. 2016;124:1715–1721. doi: 10.1289/EHP183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piya MK, McTernan PG, Kumar S. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol. 2013;216:T1–T15. doi: 10.1530/JOE-12-0498. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes. 2012;61:3037–3045. doi: 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice MB, Ljungman PL, Wilker EH, Gold DR, Schwartz JD, Koutrakis P, Washko GR, O'Connor GT, Mittleman MA. Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am J Respir Crit Care Med. 2013;188:1351–1357. doi: 10.1164/rccm.201308-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade M, Kloog I, Liberty IF, Schwartz J, Novack V. The association between air pollution exposure and glucose and lipids levels. J Clin Endocrinol Metab. 2016;101:2460–2467. doi: 10.1210/jc.2016-1378. [DOI] [PubMed] [Google Scholar]
- Sade MY, Kloog I, Liberty IF, Katra I, Novack L, Novack V. Air pollution and serum glucose levels: a population-based study. Medicine (Baltimore) 2015;94:e1093. doi: 10.1097/MD.0000000000001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert T, Vossoughi M, Vierkotter A, Sugiri D, Schikowski T, Schulte T, Roden M, Luckhaus C, Herder C, Kramer U. Association between traffic-related air pollution, subclinical inflammation and impaired glucose metabolism: results from the SALIA study. PloS one. 2013;8:e83042. doi: 10.1371/journal.pone.0083042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanassoulis G, Massaro JM, Corsini E, Rogers I, Schlett CL, Meigs JB, Hoffmann U, O'Donnell CJ, Fox CS. Periaortic adipose tissue and aortic dimensions in the Framingham Heart Study. J Am Heart Assoc. 2012;1:e000885. doi: 10.1161/JAHA.112.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella RE, Pillon NJ, Zarrouki B, Croze ML, Koppe L, Guichardant M, Pesenti S, Chauvin MA, Rieusset J, Geloen A, Soulage CO. Ozone exposure triggers insulin resistance through muscle c-Jun N-terminal kinase activation. Diabetes. 2015;64:1011–1024. doi: 10.2337/db13-1181. [DOI] [PubMed] [Google Scholar]
- Wang Y, Eliot MN, Kuchel GA, Schwartz J, Coull BA, Mittleman MA, Lipsitz LA, Wellenius GA. Long-term exposure to ambient air pollution and serum leptin in older adults: results from the MOBILIZE Boston study. J Occup Environ Med. 2014;56:e73–77. doi: 10.1097/JOM.0000000000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward-Caviness CK, Kraus WE, Blach C, Haynes CS, Dowdy E, Miranda ML, Devlin RB, Diaz-Sanchez D, Cascio WE, Mukerjee S, Stallings C, Smith LA, Gregory SG, Shah SH, Hauser ER, Neas LM. Association of roadway proximity with fasting plasma glucose and metabolic risk factors for cardiovascular disease in a cross-sectional study of cardiac catheterization patients. Environ Health Perspect. 2015;123:1007–1014. doi: 10.1289/ehp.1306980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker EH, Preis SR, Beiser AS, Wolf PA, Au R, Kloog I, Li W, Schwartz J, Koutrakis P, DeCarli C, Seshadri S, Mittleman MA. Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke. 2015;46:1161–1166. doi: 10.1161/STROKEAHA.114.008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino RB., Sr Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167:1068–1074. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- Wolf K, Popp A, Schneider A, Breitner S, Hampel R, Rathmann W, Herder C, Roden M, Koenig W, Meisinger C, Peters A, Group KO-S. Association between long-term exposure to air pollution and biomarkers related to insulin resistance, subclinical inflammation, and adipokines. Diabetes. 2016;65:3314–3326. doi: 10.2337/db15-1567. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu C, Xu Z, Tzan K, Zhong M, Wang A, Lippmann M, Chen LC, Rajagopalan S, Sun Q. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci. 2011;124:88–98. doi: 10.1093/toxsci/kfr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yavar Z, Verdin M, Ying Z, Mihai G, Kampfrath T, Wang A, Zhong M, Lippmann M, Chen LC, Rajagopalan S, Sun Q. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol. 2010;30:2518–2527. doi: 10.1161/ATVBAHA.110.215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Levy JI. Factors influencing the spatial extent of mobile source air pollution impacts: a meta-analysis. BMC Public Health. 2007;7:89. doi: 10.1186/1471-2458-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.