Abstract
Apraxia of speech is a motor speech disorder characterized by combinations of slow speaking rate, abnormal prosody, distorted sound substitutions, and trial-and-error articulatory movements. Apraxia of speech is due to abnormal planning and/or programming of speech production. It is referred to as primary progressive apraxia of speech (PPAOS) when it is the only symptom of a neurodegenerative condition. Past reports suggest an association of PPAOS with primary 4-repeat (4R) tau (e.g. progressive supranuclear palsy, corticobasal degeneration), rather than amyloid, pathology. The goal of the current study was to investigate the distribution of tau tracer uptake using [18F]AV-1451 positron emission tomography (PET) imaging in patients with PPAOS. Fourteen PPAOS patients underwent [18F]AV-1451 PET (tau-PET) imaging, [C11] Pittsburgh Compound B (PiB) PET and structural MRI and were matched 3:1 by age and sex to 42 cognitively normal controls. Tau-PET uptake was assessed at the region-of-interest (ROI) level and at the voxel-level. The PPAOS group (n=14) showed increased tau-PET uptake in the precentral gyrus, supplementary motor area and Broca’s area compared to controls. To examine whether tau deposition in Broca’s area was related to the presence of aphasia, we examined a subgroup of the PPAOS patients who had predominant apraxia of speech, with concomitant aphasia (PPAOSa; n = 7). The PPAOSa patients showed tau-PET uptake in the same regions as the whole group. However, the remaining seven patients who did not have aphasia showed uptake only in superior premotor and precentral cortices, with no uptake observed in Broca’s area. This cross-sectional study demonstrates that elevated tau tracer uptake is observed using [18F]AV-1451 in PPAOS. Further, it appears that [18F]AV-1451 is sensitive to the regional distribution of tau deposition in different stages of PPAOS, given the relationship between tau signal in Broca’s area and the presence of aphasia.
Keywords: Tau imaging, Primary progressive aphasia, frontotemporal dementia, Alzheimer’s disease, primary progressive apraxia of speech
1.0 Introduction
Primary Progressive Apraxia of Speech (PPAOS) is a distinct neurodegenerative clinical syndrome whose pathophysiology is incompletely understood. PPAOS is insidious in onset and progresses over time. For the past decade, we have been studying patients with progressive apraxia of speech. We demonstrated that it can be the earliest manifestation of an underlying neurodegenerative disease and have reported that the profile of clinical characteristics and disease trajectories can differ among affected patients (Josephs et al., 2012, 2014a). In a recent longitudinal study, we observed that in some PPAOS patients, the apraxia of speech remained the most salient feature over an average duration of seven years, although a less prominent aphasia often emerged. A second subgroup developed an extrapyramidal syndrome within five years, causing significant morbidity, including inability to ambulate and a shortened life span (Josephs et al., 2014a).
On average, between two-and-one-half and three years elapse between initial symptom onset and a diagnosis of PPAOS. The delay in proper diagnosis can be attributed to a lack of awareness of this condition among both the general public, who may initially attribute its symptoms to stress or fatigue; and among health care providers, who often misdiagnose PPAOS as aphasia or dysarthria (Josephs et al., 2013). There is evidence to suggest PPAOS is not as rare as once thought; the incidence and prevalence is likely diluted because of misdiagnoses as a functional/psychogenic problem, a variant of aphasia, or another neurodegenerative disease, such as Alzheimer’s disease or amyotrophic lateral sclerosis (Josephs et al., 2013). A better understanding of its pathophysiology and associated protein deposition will facilitate more sensitive and specific diagnostic testing, patient counseling, and care.
Recent research points toward an association between PPAOS and primary 4R tauopathies, including progressive supranuclear palsy and corticobasal degeneration (Josephs et al., 2006a). Several compounds, such as [18F]AV-1451, have been developed to image tau proteins using positron emission tomography (PET; Chien et al., 2013; Xia et al., 2013). This imaging development allows for the in-vivo assessment of tau tracer uptake distribution, spread of tau tracer uptake over time, and the assessment of relationships with clinical signs and symptoms. An important caveat to this advancement is that autoradiography studies of autopsied 4R tauopathy brains have yielded mixed confusing results. Autoradiographic studies show minimal- to no in vivo binding of [18F]AV-1451 in corticobasal degeneration (Josephs et al., 2016; Lowe et al., 2016; McMillan et al., 2016; Cho et al., 2017a; Smith et al., 2017a) and progressive supranuclear palsy (Lowe et al., 2016; Marquie et al., 2017). However, autopsy studies have shown strong correlations between regional AV-1451 uptake and regional tau burden measured histologically (Josephs 2016, McMillan, 2016, Schonhaut et al., 2017). Given the possible relationship with PPAOS and both of these 4R tauopathies, we plan to follow these patients to autopsy to better understand any possible discordance between in vivo and post-mortem findings with [18F]AV-1451 and if some other process other than tau deposition may be contributing to any in vivo signal seen on tau-PET imaging. While caution is necessary in interpreting these findings, understanding the nature of tau deposition in PPAOS could allow for the development of tau-PET measurements to aid diagnosis, follow disease progression, and, ultimately, serve as a method of outcome measures for treatment efficacy. An essential first step is to determine whether tau-PET imaging is sensitive to the presence, severity, and progression of PPAOS.
The aims of this cross-sectional study were to assess tau-PET imaging using [18F]AV-1451 in a group of patients with PPAOS to 1) determine whether tau tracer uptake can be observed in PPAOS patients when compared with age-matched healthy controls, 2) characterize the regional distribution of tau-PET uptake in PPAOS, and 3) evaluate whether the [18F]AV-1451 signal is sensitive to disease progression by analyzing a sub-group of patients with PPAOS whose disease has evolved to include aphasia.
2.0 Materials and methods
2.1 Participants
Between July 2015 and March 2016, we assessed tau-PET in 14 patients with PPAOS (PPAOSall) who were enrolled in a longitudinal research study. All patients received a clinical diagnosis of PPAOS if the dominant presenting neurologic deficit was apraxia of speech, and any other neurological deficits, including aphasia, were considered no more than equivocally present (Josephs et al., 2012, 2014a). Diagnosis was made through consensus review by two expert speech-language pathologists (J.R.D. and H.M.C.). Inclusion criterion included being over 18 years of age, having a caregiver to provide collateral history and description of functional limitations, and being a native speaker of the English language. These individuals did not meet clinical criteria for any other neurologic diagnosis at initial testing [including, but not limited to, progressive supranuclear palsy (Litvan et al., 1996; Respondek et al., 2013), corticobasal syndrome (Boeve et al., 2003; Armstrong et al., 2013), or Alzheimer’s disease dementia (McKhann et al., 1984; Albert et al., 2011; Dubois et al., 2014)]. All patients underwent detailed neurological evaluation, speech and language examination, neuropsychological testing, and neuroimaging analysis that included a structural head MRI, a [C11] Pittsburgh Compound B (PiB) PET scan to assess the presence of beta-amyloid (unavailable for one patient), and a [18F]AV-1451 PET Scan. At the time that the patients underwent the [18F]AV-1451 scan, seven patients who had been previously diagnosed as PPAOS had developed mild aphasia (denoted as PPAOSa). A diagnosis of aphasia was made based on results from several language tests that assessed naming, grammar, and comprehension, as described below.
Clinical and demographic information of the patients, including age, disease duration, and pertinent neuropsychological, language, and speech findings were documented. Additionally, a cohort of 42 healthy, cognitively normal controls (24 male) was selected from the Mayo Clinic Study of Aging (MCSA). The MCSA is an epidemiological study of cognitive aging in Rochester, Olmsted County, Minnesota (Petersen et al., 2010; Roberts et al., 2008). The PPAOS cohort was matched (3 controls: 1 patient) on age and gender with MCSA participants, to serve as a control cohort for the analyses described below. All MCSA participants are PiB negative. Median age was 69.5 years old (interquartile range: 74–58.75 years old). All controls underwent [18F]AV-1451 tau-PET scans using the identical acquisition parameters to the patient cohort. The study was approved by the Mayo institutional review board and all participants consented to research.
2.2 Speech and Language Analysis
Patients were followed as part of a larger study, for which several speech and language measures were acquired, as previously reported (Duffy et al., 2015, 2017; Josephs et al., 2012, 2013, 2014a). Consistent with these previous reports, judgments about motor speech abilities were based on all spoken language tasks of the Western Aphasia Battery (WAB; Kertesz, 2007) plus additional speech tasks that included vowel prolongation, speech alternating motion rates (e.g. rapid repetition of ‘puhpuhpuh’), speech sequential motion rates (e.g. rapid repetition of ‘puhtuhkuh’), word and sentence repetition tasks, and a conversational speech sample. Sixteen speech characteristics, consistent with current criteria for apraxia of speech diagnosis (Duffy, 2005; Wambaugh et al., 2006; McNeil et al., 2009), or observations of characteristics of apraxia of speech associated with neurodegenerative disease (Duffy, 2006) were rated on the Apraxia of Speech Rating Scale, which provided a quantitative index of apraxia of speech characteristics and severity (Josephs et al., 2012; Strand et al., 2014). The same speech tasks were also judged for the presence or absence of dysarthria, qualified for type, and rated on a 0–4 severity scale (0 = normal, 4 = severe). For this study, quantitative scores and video recordings of crucial aspects of the test protocol were reviewed for all patients by two of the co-authors (J.R.D and H.M.C.) who made independent judgments about the presence or absence of apraxia of speech, aphasia, dysarthria, and non-verbal oral apraxia (NVOA), and the severity of each. Consistent with past research, a cutoff of 29 was used to establish the presence of NVOA (Botha et al., 2014).
Other ratings included the following: The Communicative Effectiveness Survey (CES), which yields a measure of communicative effectiveness in various social situations (Hustad, 1999), where a lower score (out of 32 possible points) is consistent with diminished communicative effectiveness; the motor speech severity rating (MSD severity) is a 10-point functional speech severity rating adapted from Yorkston, Strand, Miller, and Hillel (1993), where 1 = nonvocal and 10 = normal speech; the Articulation Error Score (AES) was derived from the percentage of 56 words (i.e., three repetitions of 13 words plus one repetition of three sentences) in which any of the following characteristics were noted: distorted or undistorted sound substitutions, additions, or repetitions; sound omissions; sound prolongations (beyond those consistent with overall speech rate); false starts; and successful or unsuccessful attempts to correct sound errors (Duffy et al., 2015).
Several measures of language ability were obtained to quantify the presence, nature, and severity of aphasia. The Western Aphasia Battery aphasia quotient (WAB-AQ; Kertesz, 2007) served as a composite measure of global language ability; it includes measures of repetition, naming, spontaneous speech fluency, word finding, grammatical competence, verbal and reading comprehension, and writing. A WAB-AQ score of 93.8 or above was considered normal, consistent with standard test guidelines. The 15-item Boston Naming Test (BNT; Lansing et al.,1999) served as a sensitive measure of confrontation-naming ability; a score of 13 and above was considered normal. The Token Test, Part V (De Renzi and Vignolo, 1962), served as a measure of complex spoken language (syntactic) comprehension (a score greater than 18 is considered normal). The Northwestern Anagram Test (10-item version; Weintraub et al., 2009) served as a sensitive measure of grammatic expression that was independent of speech production (a score 9 or higher is considered normal). The speech-language pathologists also determined the presence versus absence of agrammatism in written or spoken language.
2.3 Neurological/neuropsychological assessment
All 14 PPAOS patients also had a neurological and neuropsychological examination to ensure that the patients did not meet criteria for another neurodegenerative disease. Handedness and highest educational attainment were also documented. All patients completed a test of general cognition [Montreal Cognitive Assessment Battery (MOCA; Nasreddine et al., 2005), with a score of 26 or above considered normal], memory [Camden Memory Test (words); Warrington, 1996, with a score of 24 or above considered normal], executive function [Frontal Assessment Battery (FAB; Dubois, Slachevsky, Litvan, and Pillon, 2000), with a score 13 or higher considered normal], and visual-perceptual function [Visual Object and Space Perception (VOSP; Warrington and James, 2002) fragmented letters, with a score of 16 or higher considered normal].
2.4 Neuroimaging Acquisition and Analysis
Tau-PET and PiB-PET scans were acquired on the same day for each subject using a 690 XTPET/CT scanner (GE Healthcare, Milwaukee, Wisconsin) operating in 3-dimensional mode. Tau-PET imaging was performed using the [18F]AV-1451 ligand. An intravenous bolus injection of approximately 370 MBq (10mCi) of [18F]AV-1451 was administered, followed by a 20-minute PET acquisition performed 80 minutes after injection. PiB-PET images were acquired by a 20-minute PET acquisition after injection of [C11] PiB (average=596MBq; range=292–729MBq) after an uptake period of 40min. For all participants, a 3T magnetization prepared rapid gradient echo (MPRAGE) T1-weighted MRI sequence was also acquired within one day of the PET scans.
Each subject’s MRI scan was segmented and corrected for intensity inhomogeneity with the unified segmentation algorithm (Ashburner and Friston, 2005) implemented in SPM12 using tissue priors from the Mayo Clinic Adult Lifespan Template (MCALT) (Schwarz et al., 2016; 2017), a publicly-available population-matched brain template (https://www.nitrc.org/projects/mcalt/). The Advanced Normalization Tools (ANTs) symmetric normalization (SyN) algorithm (Avants, Epstein, Grossman, and Gee, 2008) was used with the MCALT_T1 template image to nonlinearly transform the MCALT_ADIR122 atlas to the voxel space of each subject MRI. Each subject’s corresponding [18F]AV-1451 image was co-registered to the MRI using 6 degrees-of-freedom rigid registration in SPM12 and resampled to the MRI’s voxel space using B-spline interpolation. Partial volume correction (PVC) of the tau-PET data was performed using a two-compartment model (Meltzer et al., 1990). All voxels in the [18F]AV-1451 image were divided by the median uptake in the cerebellar crus gray matter to create SUVr images. Median SUVr values were computed over the white matter- and gray matter-segmented voxels within each transformed cortical and subcortical atlas ROI, and these were used for ROI-based analysis. A voxel-based analysis was also performed. Partial volume corrected SUVr images were normalized to the MCALT template using concatenated previously-computed rigid (PET to MRI) and nonlinear (MRI to template) normalization parameters, resampled using B-spline interpolation, and blurred with a 6mm Gaussian kernel. Voxel-level statistical comparisons were performed over all white matter- and gray matter-segmented voxels using SPM12.
Amyloid positivity was determined for each subject using previously-published methods and thresholds (Jack et al., 2008; Kantarci et al., 2011). Briefly, PiB-PET images were co-registered to the MPRAGE for each patient using 6 degrees-of-freedom rigid registration. An in-house atlas was transformed into the native space of each patient and used to calculate median PiB uptake for 6 ROIs: temporal, parietal, posterior cingulate/precuneus, anterior cingulate, prefrontal, and orbitofrontal cortex (left and right were combined for all ROIs). The median PiB uptake across these combined six regions was divided by the median PiB uptake in the cerebellar crus grey matter to create SUVR values. Patients were classified as amyloid-positive using a global cortical-to-cerebellar ratio cut-point of 1.5 (Jack et al., 2008; Kantarci et al., 2011).
2.5 Statistical analysis
All analyses were performed using partial volume corrected tau-PET SUVr images (Meltzer et al., 1990). The cohort of PPAOSall patients (n=14) was compared to the age- and sex-matched controls. The sub-group of patients with PPAOS who had concomitant aphasia (PPAOSa; n=7) and those without aphasia (n=7) were also separately compared to their respective matched sub-group of controls. Voxel-level comparisons were performed using SPM12, with results assessed at p < .05 after correction for multiple comparisons using the false discovery rate correction, and an extent-threshold of 50 voxels. Significant findings were visualized using MRIcroGL (http://www.mccauslandcenter.sc.edu/mricrogl/home). Age and sex were not included in the models, as comparisons were made between age and sex matched groups.
Group differences were also assessed using an ROI-based analysis summarized by the area under the receiver operating characteristic curve (AUROC) statistic. AUROC values can be interpreted as a nonparametric measure of effect size, or group-wise differences that are independent of the underlying scale of measurement. In general, the higher the AUROC estimates, the better the discrimination between groups. An AUROC of 0.5 corresponds to chance discriminability, whereas an AUROC of 1.0 is consistent with perfect accuracy. We considered ROI values as statistically significant when their 95% confidence interval excluded 0.5. We also replicated these analyses using images without partial volume correction and the major findings were generally consistent. For brevity, these are presented only in Supplementary Material (Figures 1–3).
Tests of differences in behavioral measures and SUVr values were evaluated, with significance assessed at p ≥ 0.05, with appropriate penalties for multiple comparisons. Wilcoxon signed-rank tests were used to compare continuous data. Chi-Squared tests were used to compare categorical data. When evaluating SUVrs, if significant differences were observed across the three groups, pair-wise testing was performed using two-sided Wilcoxon rank sum test. Therefore only variables that differed across our three groups of interest were analyzed in more detail. This strategy reduces the number of statistical analyses performed and hence reduces the chance of type 1 error (false positives). Analyses were performed with R (version 3.3.1; R Foundation for Statistical Computing, Vienna, Austria) and JMP statistic software (version10. SAS Institute, Cary, NC.).
3.0 Results
3.1 Clinical designation
The PPAOSall cohort had a median age at evaluation of 69.5 years old, was 43% female, and had median disease duration of 5 years. The median disease duration was 5 years for both the PPAOS patients without aphasia and the PPAOSa patients. None of the aforementioned demographic analyses yielded statistically significant differences between groups. Median scores on the language, neuropsychological, and speech tests for all patients are included in Tables 1 and 2. Overall, performance on all speech and language batteries, and on the MOCA, was significantly poorer for each of the PPAOSa patients when compared to PPAOS patients without aphasia (p < .05). Only two PPAOSa patients performed in the unimpaired range on the MOCA, while all PPAOS patients performed normally. It is likely that language confounded performance, as verbal abilities are required for most, if not all, subtests of the MOCA. Measures such as the VOSP, Camden, and FAB, intended to test skills outside of the domains of speech and language, did not reveal differences between PPAOS sub-groups, nor between PPAOSall patients and standard test norms.
Table 1.
Demographic, neuropsychological, and neurologic information. All patients are right handed.
| Diagnosis | Sex | PiB | Disease duration (yrs) | Education (yrs) | Age (yrs) | MOCA (/30) | FAB (/18) | VOSP (/20) | Camden (/25) | CES (/32) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PPAOSa | Male | 1.18 (−) | 4 | 12 | 67 | 20 | 10 | 20 | DNT | 8 |
| 2 | PPAOSa | Female | 1.82 (+) | 4 | 12 | 69 | 14 | 8 | 20 | DNT | 8 |
| 3 | PPAOSa | Male | 1.39 (−) | 3 | 16 | 58 | 25 | 15 | 20 | 23 | 13 |
| 4 | PPAOSa | Female | 1.44 (−) | 5 | 18 | 81 | 21 | 15 | 20 | 25 | 9 |
| 5 | PPAOSa | Male | 1.23 (−) | 9 | 12 | 58 | 7 | CNC | 20 | DNT | 12 |
| 6 | PPAOSa | Male | 1.24 (−) | 6 | 24 | 74 | 28 | 16 | 20 | 24 | 13 |
| 7 | PPAOSa | Male | 1.22 (−) | 5 | 17 | 57 | 27 | 16 | 20 | 23 | 20 |
| 8 | PPAOS | Female | 1.38 (−) | 2 | 12 | 70 | 28 | 17 | 20 | 24 | 15 |
| 9 | PPAOS | Female | 1.88 (+) | 5 | 13 | 70 | 26 | 17 | 19 | 24 | 10 |
| 10 | PPAOS | Female | DNT | 7 | 16 | 57 | 29 | 18 | 20 | 25 | 14 |
| 11 | PPAOS | Male | 1.31 (−) | 7 | 17 | 64 | 28 | 15 | 20 | 25 | 13 |
| 12 | PPAOS | Male | 1.31 (−) | 2 | 17 | 81 | 29 | 17 | 20 | 25 | 16 |
| 13 | PPAOS | Female | 1.26 (−) | 6 | 16 | 77 | 30 | 16 | 20 | DNT | 14 |
| 14 | PPAOS | Male | 1.63 (+) | 4 | 20 | 72 | 27 | 14 | 19 | 24 | 18 |
| PPAOSa | Median (IQR) | 1.24 (1.44–1.22) | 5 (6–4) | 16 (18–12) | 67 (74–58) | 21 (14–27) | 15 (16–10) | 20 | 23.5 (25–23) | 12 (13–8) | |
| PPAOS without aphasia | Median (IQR) | 1.35 (1.69–1.30) | 5 (7–2) | 16 (17–13) | 70 (77–64) | 28 (29–27) | 17 (17–15) | 20 (20–19) | 24.5 (25–24) | 14 (16–13) | |
| PPAOSall | Median (IQR) | 1.31 (1.54–1.24) | 5 (6–4) | 16 (17–12) | 69.5 (75–58) | 27 (28–21) | 16 (17–15) | 20 (20–20) | 24 (25–24) | 13 (15–10) | |
Notes: [C11] Pittsburgh compound B (PiB) amyloid-PET testing, for which a global ratio of 1.5 was used to define positivity (+/−); yrs = years; disease duration indicates interval between onset of symptoms and time of testing; MOCA= Montreal Cognitive Assessment; FAB = Frontal Assessment Battery; VOSP= Visual Object and Space Perception Battery; Camden = Words Recognition Test; CES=Communication Effectiveness Survey; DNT = Did not test; CNC= could not compute; IQR = Interquartile range; maximum scores for each test are noted in the column header. A * indicates statistically significant differences between groups.
Table 2.
Patient language and speech information.
| Diagnosis | TT (/22)* |
WAB (/100)* |
NAT (/12)* |
BNT (/15)* |
ASRS total score (/52) |
AOS Severity (/4) |
Dysarthria Type |
Dysarthria Severity (/4) |
MSD Severity (/10)* |
NVOA (/32)* |
AES (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PPAOSa | 18 | 82.2 | 6 | 10 | 22 | 2 | Spastic | 3 | 3 | 9 | 59.3 |
| 2 | PPAOSa | 19 | CNC | 8 | 10 | 37 | 4 | Spastic | 1.5 | 2 | 0 | CNC |
| 3 | PPAOSa | 13 | 84.7 | 4 | 15 | 28 | 4 | Hypokinetic | 1 | 4 | 30 | 42.8 |
| 4 | PPAOSa | 14 | 96.8 | 7 | 11 | 20 | 2.5 | Spastic-Hypokinetic | 3 | 4 | 21 | 8.9 |
| 5 | PPAOSa | 12 | CNC | CNC | CNC | CNC | 4 | Spastic | 1.5 | 1 | 4 | 34.5 |
| 6 | PPAOSa | DNT | 81.3 | 8 | DNT | 37 | 4 | Hypokinetic | 1 | 3 | 4 | 78.6 |
| 7 | PPAOSa | 19 | 96.6 | 9 | 13 | 18 | 1.5 | None | 0 | 6 | 29 | 16 |
| 8 | PPAOS | 20 | 99 | 10 | 15 | 12 | 1 | None | 0 | 8 | 31 | 12.5 |
| 9 | PPAOS | DNT | 95.2 | 10 | DNT | 23 | 3 | None | 0 | 4 | 19 | 80 |
| 10 | PPAOS | 22 | 98.8 | 10 | 15 | 24 | 3 | None | 0 | 5 | 30 | 33 |
| 11 | PPAOS | 18 | 98.2 | 10 | 15 | 20 | 2.5 | None | 0 | 6 | 32 | 14 |
| 12 | PPAOS | 18 | 97.8 | 10 | 15 | 7 | 1 | Hypokinetic | 1 | 7 | 32 | 0 |
| 13 | PPAOS | 22 | 96.8 | 7 | 15 | 25 | 3 | Spastic | 1.5 | 5 | 25 | 34.5 |
| 14 | PPAOS | 22 | 97.8 | 10 | 15 | 24 | 3 | Hypokinetic | 1 | 7 | 29 | 14 |
| PPAOSa | Median (IQR) | 16 (19–13) | 84.7 (96.7–81.8) | 7.5 (8–6) | 11 (14–10) | 25 (37–20) | 4 (4–2) | N/A | 1.5 (3–1) | 3 (4–2) | 9 (29–4) | 39 (64–14) |
| PPAOS without aphasia | Median (IQR) | 21 (22–18) | 97.8 (98.8–96.8) | 10 | 15 | 23 (24–12) | 3 (3–1) | N/A | 0 (1–0) | 6 (7–5) | 30 (32–25) | 14 (35–13) |
| PPAOSall | Median (IQR) | 19 (22–15) | 96.8 (98.1–87.3) | 9 (10–7) | 15 (15–11) | 23 (27–19) | 3 (4–2) | N/A | 1 (1.5–0) | 4.5 (6–3) | 27 (30–8) | 33 (51–13) |
Notes: TT = Token Test; WAB = Western Aphasia Battery Aphasia Quotient; NAT = Northwestern Anagram Test; BNT = Boston Naming Test, short form; ASRS = Apraxia of Speech Rating Scale 2.3; MSD Severity = Motor Speech Disorders Rating Scale Severity; NVOA = Non-Verbal Oral Apraxia; AES = Articulation Error Score (total error, percentage); DNT = Did not test; CNC= could not compute; IQR = Interquartile range; maximum scores for each test are noted in the column header. A * indicates statistically significant differences between groups.
3.2 All PPAOS patients (PPAOSall) compared with controls
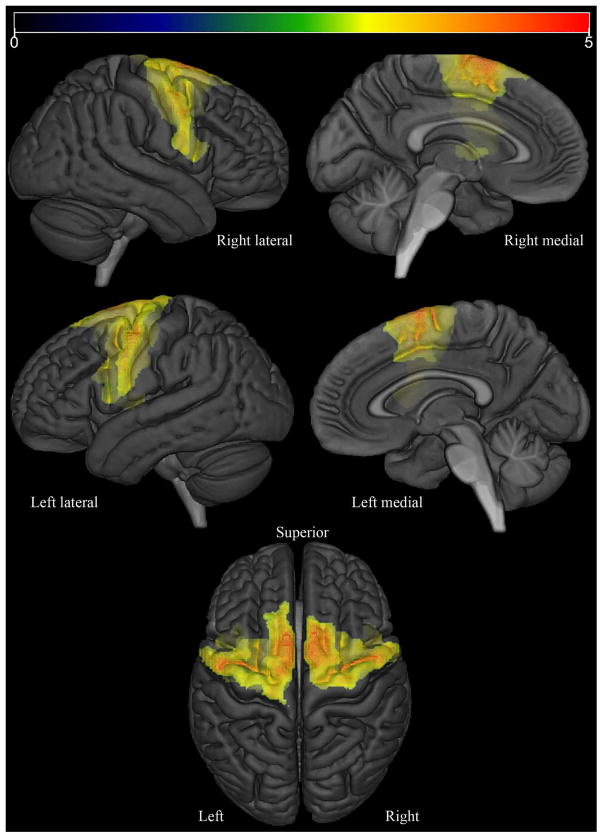
At the voxel-level, the PPAOSall cohort showed increased tau-PET uptake bilaterally throughout the premotor and precentral cortices, involving inferior, middle and superior frontal gyri and the supplementary motor areas, compared to controls (Figure 1). Increased uptake was also observed in the corpus callosum, middle cingulate gyrus, precuneus and insula. SPM values, t-values, and coordinates of peaks are provided in Supplementary Table 1. At the ROI-level, the PPAOSall cohort showed increased tau-PET uptake bilaterally in the supplementary motor areas, precentral gyri, inferior frontal operculum, middle and superior frontal gyri, and inferior frontal triangularis compared to controls. Individual SUVr values for selected ROIs, for all patients, are shown in Supplementary Table 2. AUROCs for this comparison are shown in Figure 2; confidence intervals are reported in Supplementary Table 3. The major findings were generally consistent when this analysis was replicated using images without partial volume correction (Supplementary Figure 1).
Figure 1.
Regions where partial volume-corrected AV-1451 Tau PET SUVr was significantly (FDR-corrected p<0.05; extent-threshold = 50 voxels) larger in PPAOSall than in matched controls are displayed in red on a semitransparent 3D brain render.
Figure 2.
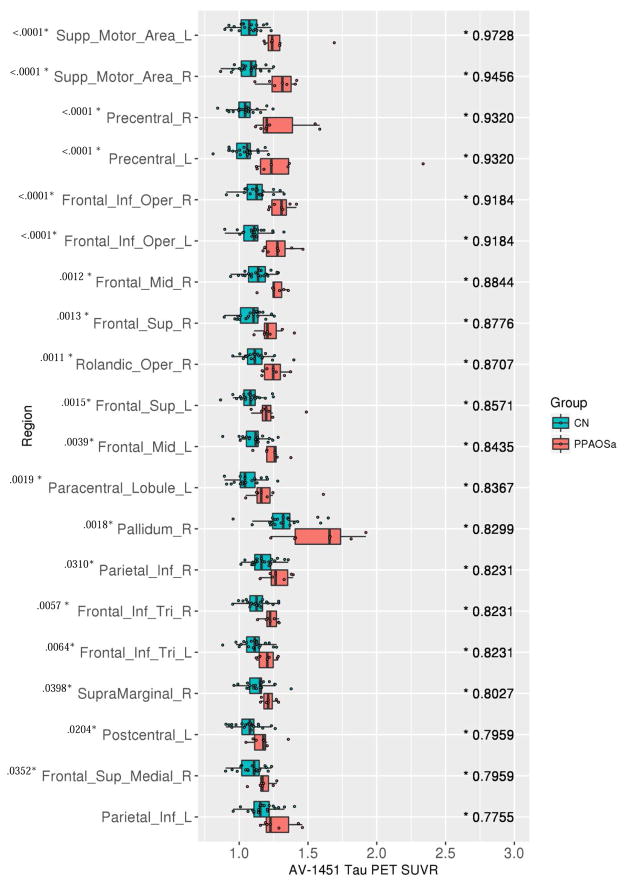
Tau-PET SUVrs (x-axis) and the associated top 20 AUROCS (y-axis), ranked from highest to lowest AUROC (value listed in the right side of each panel), for controls against PPAOSall patients. Note: A * denotes AUROC value whose 95% confidence interval excludes 0.5.
3.3 PPAOS patients with aphasia (PPAOSa) and without aphasia compared to controls
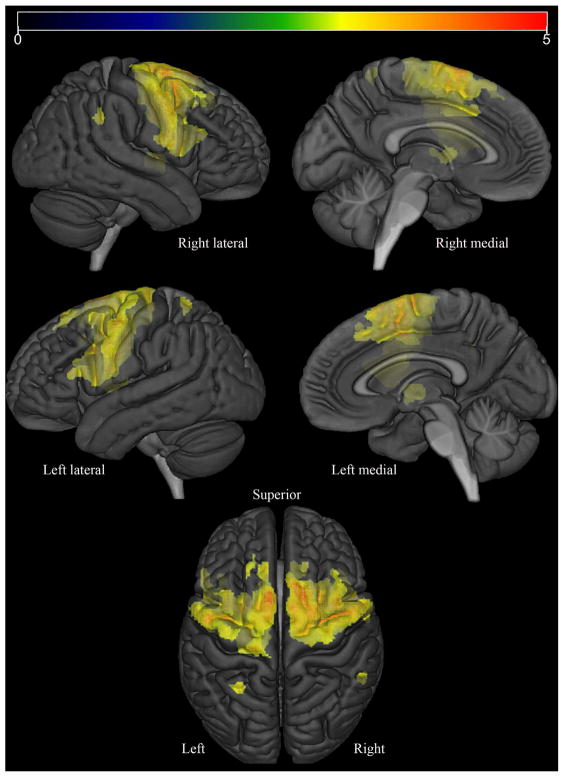
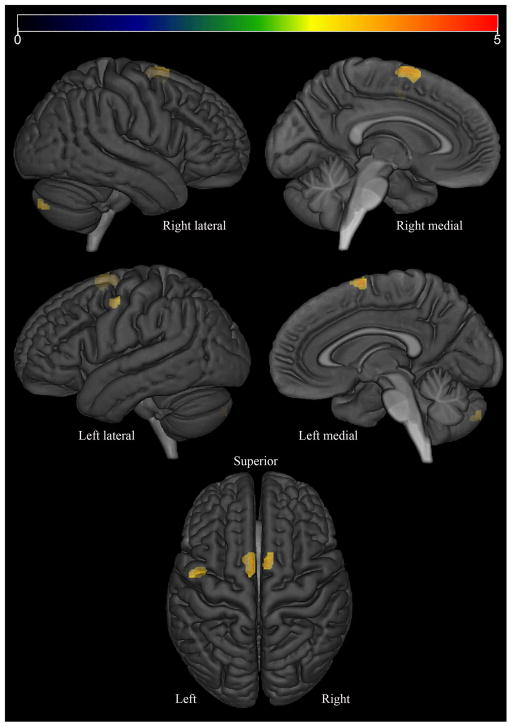
In the voxel-level analysis, the PPAOSa patients showed increased tau-PET uptake bilaterally throughout the premotor and precentral cortices, involving inferior, middle and superior frontal gyri (including Broca’s area) and the supplementary motor areas, compared to controls (Figure 3). Increased uptake was also observed in the corpus callosum, middle cingulate gyrus, insula, precuneus, left putamen and left middle temporal gyrus. In contrast, the PPAOS patients without aphasia did not show any significant differences, compared to controls, at the chosen statistical significance threshold of FDR p<0.05. Assessed instead with a threshold of uncorrected p<0.001, the PPAOS patients without aphasia showed subtle increased uptake limited to the lateral superior premotor cortex and supplementary motor areas, compared to controls (Figure 4).
Figure 3.
Regions where partial volume-corrected AV-1451 Tau PET SUVr was significantly (FDR-corrected p<0.05; extent-threshold = 50 voxels) larger in PPAOSa than in matched controls are displayed in red on a semitransparent 3D brain render.
Figure 4.
Regions where PVC AV-1451 Tau PET SUVr was significantly (uncorrected p<0.001; extent-threshold = 50 voxels) larger in PPAOS patients without aphasia than in matched controls, displayed on a semitransparent 3D brain render.
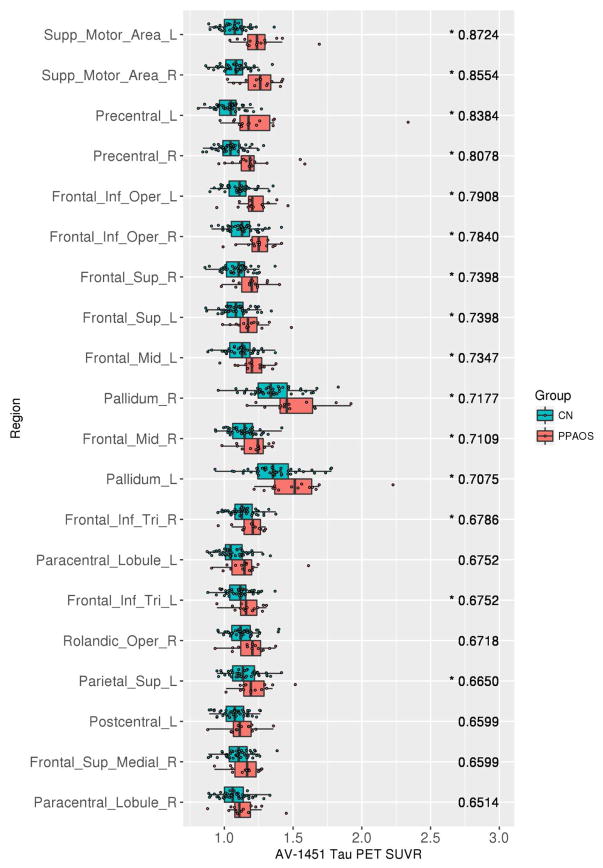
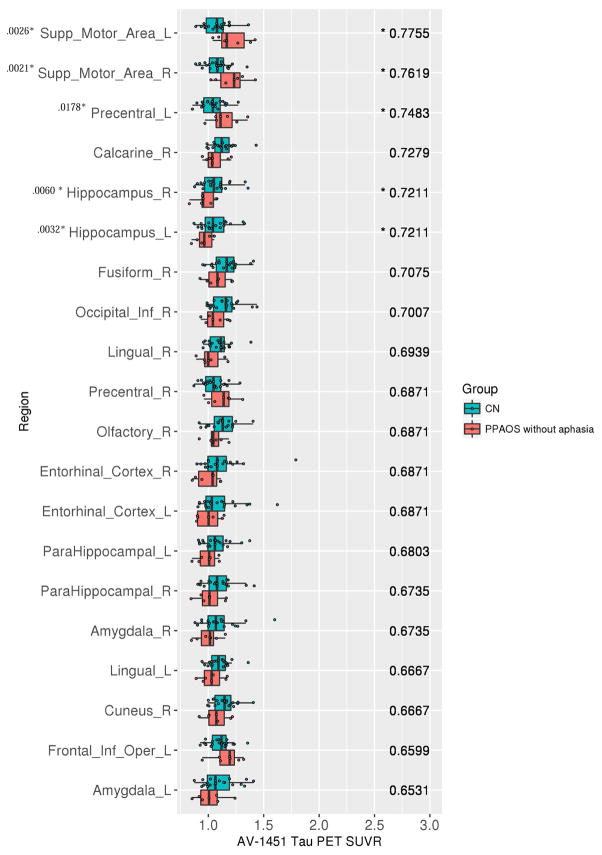
At the ROI-level, the PPAOSa patients showed increased tau-PET uptake across the same ROIs as the PPAOSall cohort. The AUROCs were, however, higher than those observed for the entire PPAOSall cohort, showing better differentiation from controls (Figure 5). In contrast, the PPAOS patients without aphasia showed increased tau-PET uptake prominently in the supplementary motor areas, and left precentral gyrus (Figure 6). These findings were generally consistent when this analysis was replicated using images without partial volume correction (Supplementary Figures 2 and 3); AUROC confidence intervals are reported in Supplementary Tables 4 and 5.
Figure 5.
Tau-PET SUVrs (x-axis) and the associated top 20 AUROCS (y-axis), ranked from highest to lowest AUROC (value listed in the right side of each panel), for controls against PPAOSa patients. Note: A * by AUROC value denotes those whose 95% confidence interval excludes 0.5. A * by region name indicates significant differences (p < .05, adjusted for multiple comparisons) on Wilcoxon rank sum test; p-value is provided. Analysis was repeated after excluding the outlier noted in the Precentral_L ROI effect; the results are unchanged.
Figure 6.
Tau-PET SUVrs (x-axis) and the associated top 20 AUROCS (y-axis), ranked from highest to lowest AUROC (value listed in the right side of each panel), for controls against PPAOS patients without aphasia. Note: A * by AUROC value denotes those whose 95% confidence interval excludes 0.5. A * by region name indicates significant group differences (p < .05, adjusted for multiple comparisons) on Wilcoxon rank sum test; p-value is provided.
3.4 Beta-amyloid burden
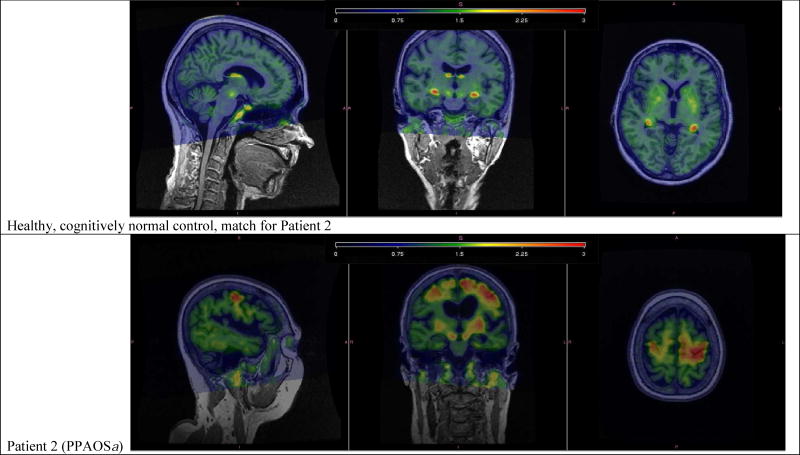
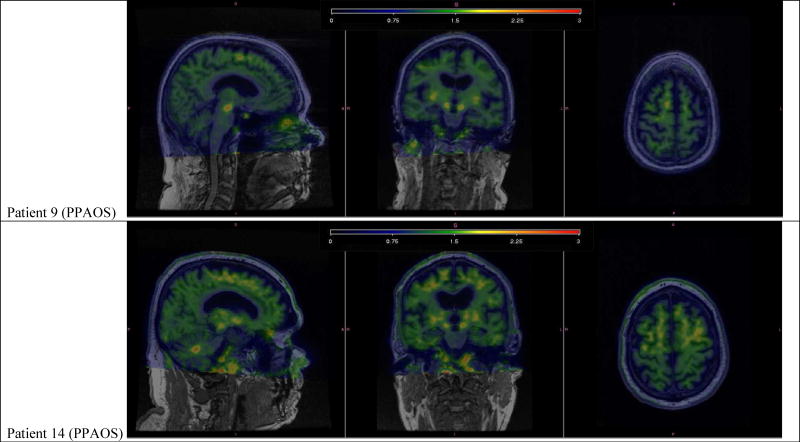
Of the fourteen patients, three were classified as amyloid-positive. Two of these three patients had the pure motor speech deficit (PPAOS); the third patient also had aphasia (i.e. PPAOSa). Figure 7 shows the tau-PET scans of these three patients. Of note, patient 2 showed marked tau uptake in the supplementary motor area and precentral cortex, while patients 9 and 14 showed subtle patterns of uptake.
Figure 7.
Individual tau-PET images, overlaid on structural MRI, for one healthy, cognitively normal control and the three PiB positive patients. The healthy, cognitively normal control shows tau tracer uptake in the pallidum, putamen, and the choroid plexus. Patient 2 shows marked tau tracer uptake noted left greater than right, spanning bilateral supplementary motor areas, precentral gyri, and inferior frontal operculum. Patient 9 shows subtle tau tracer uptake located in right greater than left supplementary motor areas. Patient 14 shows subtle tau tracer uptake in left greater than right supplementary motor areas and uptake in the dentate nucleus of the cerebellum.
4.0 Discussion
We demonstrated that an elevated [18F]AV-1451 signal was present in PPAOS patients when compared with age-matched controls, with uptake observed predominantly in premotor and precentral cortices. This pattern is consistent with regions of hypometabolism seen on 18-F fluorodeoxyglucose (FDG) PET (Josephs et al., 2006b; 2012), grey matter atrophy seen on MRI (Josephs et al., 2013), as well as white matter tract degeneration on diffusion tensor imaging (Whitwell et al., 2013a) in this population. Overall, the literature now supports consistent patterns of tau deposition, hypometabolism, and atrophy in the frontal gyri, precentral cortex, and supplementary motor areas in patients with PPAOS. Tau tracer uptake in left supplementary motor area and left precentral cortex provided the best discrimination from controls, with high sensitivity and specificity (associated with high AUROCs).
This cross-sectional study suggests that [18F]AV-1451 may be sensitive to changes later in the disease, supported by differences in regional tau uptake that were observed with our sub-analysis in a cohort of patients who had PPAOS and developed aphasia. The analysis of PPAOSa patients revealed results nearly identical to the entire cohort; however, when examining PPAOS patients without aphasia, tau uptake was seen more focally in the supplementary motor areas and not in the areas associated with the language network (such as Broca’s area). This suggests that tau in the inferior frontal operculum and triangularis may be associated with the presence of aphasia. The AUROCS appeared highest for regions in the language network when looking at the PPAOSa group compared to either the entire cohort or the subgroup of PPAOS patients without aphasia. The AUROC was highest in the supplementary motor area, as all patients, regardless of the presence of aphasia, have a dominant apraxia of speech that is likely associated with tau uptake in this region. The precentral gyrus also showed high tau uptake in PPAOS. In the stroke literature, the involvement of the precentral gyrus in apraxia of speech is controversial; however, consistent with our findings, recent data suggest that focal lesions in the precentral gyrus result in apraxia of speech, while more diffuse damage results in a combination of apraxia of speech and aphasia (Itabashi et al., 2016). Similarly, we have shown that aphasia in the context of PPAOSa is associated with atrophy and hypometabolism in a network of left hemisphere language regions, including Broca’s area (Whitwell et al., 2013b). Importantly, while aphasia developed after the initial presentation of apraxia of speech in PPAOS in the subset of PPAOSa patients, there does not appear to be a predictable relationship with disease duration.
Remarkably, the results demonstrate generally equivalent involvement in both hemispheres for both PPAOS patients with and without aphasia. While consistent with patterns of atrophy and hypometabolism previously demonstrated in PPAOS (Josephs et al., 2006b; 2012; 2013), there is reported left lateralization, or strong left predominance, of atrophy and hypometabolism seen on MRI and FDG-PET in cases of PPAOS who also have aphasia (i.e. PPAOSa) and cases of the agrammatic variant of primary progressive aphasia (Gorno-Tempini et al., 2004; Josephs et al., 2010; Josephs et al., 2006; Nestor et al., 2003; Rabinovici et al., 2008; Whitwell et al., 2013b). One possible explanation for this surprising finding is that, perhaps, tau is more widespread than either atrophy or hypometabolism in cases of PPAOS who also have aphasia. Ultimately, autopsy studies will confirm this hypothesis.
Interestingly, the patterns of increased tau-PET uptake in this cohort of PPAOS patients is not widely different from that reported in the literature for patients with a clinical diagnosis of progressive supranuclear palsy (PSP). In a recent study comparing a cohort of PSP patients to controls, Whitwell et al. (2017) showed elevated AV-1451 tau-PET uptake in the supplementary motor area, precentral cortex, and inferior frontal opercularis, in addition to the midbrain, pallidum, thalamus, and dentate nucleus of the cerebellum. Other studies have shown similar patterns of AV-1451 tau-PET uptake in PSP (Cho et al., 2017b; Ishiki 2017; Passamonti et al., 2017; Smith et al., 2017b). Therefore, PPAOS appears to show overlapping patterns of tau tracer uptake in the cortex, but generally lacks the subcortical and brainstem features observed in PSP. Patterns of grey matter atrophy also overlap in the supplementary motor area in PSP and PPAOS (Whitwell et al., 2013a). In fact, a pathological study has shown that the distribution of tau pathology shifts from subcortical and brainstem regions to the neocortex in pathologically confirmed PSP cases that had an apraxia of speech presentation (Josephs et al., 2005), possibly explaining our findings. However, we did observe increased tau-PET uptake in the dentate nucleus of the cerebellum in a few of our PPAOS patients, consistent with the aforementioned regional deposition seen in PSP and perhaps suggesting that these patients may later develop the clinical features of PSP.
Of the 14 PPAOS patients in this study, only three had elevated beta-amyloid on PET suggesting that Alzheimer’s disease could be present in the brain (patients 2, 9 and 14). Therefore, it is unlikely that the PPAOS phenotype is due to Alzheimer’s disease. This is consistent with past reports of PiB-PET in PPAOS (Josephs et al., 2014b). PiB positive status in PPAOS is related to the presence of the APOE4 allele, which was present in 15% of PPAOS patients (Josephs et al., 2014b). Furthermore, of the three patients with elevated beta-amyloid on PET, only one (patient 2) had cortical tau uptake to the degree typically observed in Alzheimer’s disease (Cho et al., 2016; Johnson et al., 2016; Passamonti et al., 2017; Whitwell et al., 2017). The remaining two showed low levels of tau uptake that would be unusual for Alzheimer’s disease. This is not to say that Alzheimer’s disease is definitively the cause of PPAOS in patient 2, as it is possible that Alzheimer’s disease is an accompanying pathology and that there is another primary tauopathy in the brain, as has been previously observed (Caso et al., 2013). While patient 2 is more likely than patients 9 and 14 to have clinically-symptomatic Alzheimer’s disease, the latter two may have diffuse amyloid plaques driving the elevated beta-amyloid PET signal (Lockhart et al., 2007).
There are limitations to the current study. Most importantly, a direct comparison of PPAOS patients with and without aphasia was not pursued, given the underpowered sample and lack of age and sex matched samples. Visual examination of the data suggests that with sufficient power, a cross-sectional study would support differences between these groups, primarily related to tau deposition in the language network in those with evidence of aphasia. Additionally, while statistically significant, the results for the PPAOS patients without aphasia are borderline; the aforementioned follow up studies with larger samples be necessary to confirm or reject the clinical significance of those findings. Importantly, caution is warranted in the pathological interpretation of the tau-PET findings, given that autoradiography studies have found little binding of [18F]AV-1451 to 4R tau in autopsy brains (Lowe et al., 2016; Sander et al., 2016; Marquie et al., 2015). Autopsy confirmation will ultimately be needed to determine the underlying pathology in these patients. However, a recent case report of autopsy-confirmed corticobasal degeneration associated with PPAOSa confirmed 4R tau deposition in the supplementary motor area and left Broca’s area, and [18F]AV-1451 SUVr estimates were well correlated with tau burden noted at autopsy (Josephs et al., 2016).
5.0 Conclusion
In this study, we found evidence that [18F]AV-1451 uptake is increased in specific regions in PPAOS when compared with age-matched healthy controls. The pattern is somewhat reminiscent of that observed in patients with PSP, a 4R tauopathy, suggesting that the underlying pathology in these cases could also be a 4R tauopathy. Future research will need to explore the relationship of [18F]AV-1451 binding and tau deposition in PPAOS by following PPAOS patients longitudinally to autopsy. Perhaps PPAOS is a distinctive, atypical clinical presentation of PSP; whether this is true for those patients who develop aphasia as well as those who do not is an empirical question. With longitudinal follow-up, we will be able to follow the progression and evolution of tau in PPAOS and examine associations between the detection and spread of tau and development of other clinical signs and symptoms (i.e., aphasia). This will help support the findings of this cross-sectional study, as well more clearly delineate the relationship between the [18F]AV-1451 signal and tau deposition.
Supplementary Material
Acknowledgments
We would like to thank AVID Radiopharmaceuticals, Inc., for their support in supplying AV-1451 precursor, chemistry production advice and oversight, and FDA regulatory cross-filing permission and documentation needed for this work.
The study was funded by National Institutes of Health grants [R01 DC010367 (PI: Josephs), R21-NS94684 (PI: Josephs), R01-DC12519 (PI: Whitwell), U01 AG006786 (PI: Petersen), R01-NS89757 (PI: Whitwell), R01-AG034676 (PI: Rocca), P50 AG016574 (PI: Petersen), R01 AG011378 (PI: Jack), R01 AG041851 (PIs: Jack and Knopman)]; a grant from the Department of Radiology, Mayo Clinic; the Gerald and Henrietta Rauenhorst Foundation; Elsie and Marvin Dekelboum Family Foundation; Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic; and the Schuler Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Annals of Neurology. 2003;54(Suppl 5):S15–9. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- Botha HA, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KJ. Nonverbal oral apraxia in primary progressive aphasia and apraxia of speech. Neurology. 2014;82(19):1729–1735. doi: 10.1212/WNL.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso F, Gesierich B, Henry M, Sidhu M, LaMarre A, Babiak M, et al. Nonfluent/Agrammatic PPA with In-Vivo Cortical Amyloidosis and Pick’s Disease Pathology. Behav Neurol. 2013;26:95–106. doi: 10.3233/BEN-2012-120255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34:457–468. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- Cho H, Choi JY, Hwang MS, Lee JH, Kim YJ, Lee HM, et al. Tau PET in Alzheimer disease and mild cognitive impairment. Neurology. 2016;87:375–383. doi: 10.1212/WNL.0000000000002892. [DOI] [PubMed] [Google Scholar]
- Cho H, Baek MS, Choi JY, Lee SH, Kim JS, Ryu YH, et al. 18F-AV-1451 binds to motor-related subcortical gray and white matter in corticobasal syndrome. Neurology. 2017a;89(11):1170–1178. doi: 10.1212/WNL.0000000000004364. [DOI] [PubMed] [Google Scholar]
- Cho H, Choi JY, Hwang MS, Lee SH, Ryu YH, Lee MS, et al. Subcortical 18F-AV-1451 binding patterns in progressive supranuclear palsy. Mov Disord. 2017b;32:134–140. doi: 10.1002/mds.26844. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Vignolo LA. The token test: a sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB A frontal assessment battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurology. 2014;13:614–29. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor speech disorders: substrates, differential diagnosis, and management. St Louis: Mosby; 2005. [Google Scholar]
- Duffy JR. Apraxia of speech in degenerative neurologic disease. Aphasiology. 2006;20:511–527. [Google Scholar]
- Duffy JR, Strand EA, Clark H, Machulda M, Whitwell JL, Josephs KA. Primary progressive apraxia of speech: clinical features and acoustic and neurologic correlates. Am J Speech-Lang Pat. 2015;24:88–100. doi: 10.1044/2015_AJSLP-14-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR, Hanley H, Utianski R, Clark H, Strand E, Josephs KA, et al. Temporal acoustic measures distinguish primary progressive apraxia of speech from primary progressive aphasia. Brain Lang. 2017;168:84–94. doi: 10.1016/j.bandl.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustad KC. Optimizing communicative effectiveness: bringing it together. In: Yorkston K, Beukelman D, Strand EA, Bell KR, editors. Management of motor speech disorders in children and adults. Austin, TX: Pro-Ed; 1999. pp. 483–537. [Google Scholar]
- Ishiki A, Harada R, Okamura N, Tomita N, Rowe CC, Villemagne VL, et al. Tau imaging with [18F]THK-5351 in progressive supranuclear palsy. Eur J Neurol. 2017;24:130–136. doi: 10.1111/ene.13164. [DOI] [PubMed] [Google Scholar]
- Itabashi R, Nishio Y, Kataoka Y, Yazawa Y, Furui E, Matsuda M, et al. Damage to the left precentral gyrus is associated with apraxia of speech in acute stroke. Stroke. 2016;47:31–36. doi: 10.1161/STROKEAHA.115.010402. [DOI] [PubMed] [Google Scholar]
- Jack CR, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Boeve BF, Duffy JR, Smith GE, Knopman DS, Parisi JE, et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase. 2005;11:283–296. doi: 10.1080/13554790590963004. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006a;66:41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006b;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Fossett TR, Strand EA, Claassen DO, Whitwell JL, et al. Fluorodeoxyglucose F18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Arch Neurol. 2010;67(5):596–605. doi: 10.1001/archneurol.2010.78. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135:1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013;81:337–345. doi: 10.1212/WNL.0b013e31829c5ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, et al. The evolution of primary progressive apraxia of speech. Brain. 2014a;137:2783–2765. doi: 10.1093/brain/awu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, et al. APOE ε4 influences β-amyloid deposition in primary progressive aphasia and speech apraxia. Alzheimer Dement. 2014b;10:630–636. doi: 10.1016/j.jalz.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Tacik P, Duffy JR, Senjem ML, Tosakulwong N, et al. [18F] AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta Neuropathol. 2016;132:931–933. doi: 10.1007/s00401-016-1618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Lowe V, Przybelski SA, Senjem ML, Weigand SD, Ivnik RJ, et al. Magnetic resonance spectroscopy, β-amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology. 2011;77:951–958. doi: 10.1212/WNL.0b013e31822dc7e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery (Revised) San Antonio: PsychCorp; 2007. [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston naming test. Arch Clin Neuropsych. 1999;14:481–487. [PubMed] [Google Scholar]
- Litvan I, Agin Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Lockhart A, Lamb JR, Osredkar T, Sue LI, Joyce JN, Ye L, et al. PIB is a non-specific imaging marker of amyloid-beta (Aβ) peptide-related cerebral amyloidosis. Brain. 2007;130:2607–2615. doi: 10.1093/brain/awm191. [DOI] [PubMed] [Google Scholar]
- Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016;4:58. doi: 10.1186/s40478-016-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann neurol. 2015;78:787–800. doi: 10.1002/ana.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McMillan CT, Irwin DJ, Nasrallah I, et al. Multimodal evaluation demonstrates in vivo 18F-AV-1451 uptake in autopsy-confirmed corticobasal degeneration. Acta Neuropathol. 2016;132:935–937. doi: 10.1007/s00401-016-1640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil MR, Robin RA, Schmidt RA. Apraxia of speech: definition and differential diagnosis. In: McNeil MRE, editor. Clinical management of sensorimotor speech disorders. New York: Thieme; 2009. [Google Scholar]
- Meltzer CC, Leal JP, Mayberg HS, Wagner HNJ, Frost JJ. Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. J Comput Assist Tomo. 1990;14:561–570. doi: 10.1097/00004728-199007000-00011. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126(Pt 11):2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Rodríguez PV, Hong YT, Allinson KSJ, Williamson D, Borchert RJ, et al. 18F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain. 2017;140:781–791. doi: 10.1093/brain/aww340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. The Mayo Clinic Study of Aging: prevalence of mild cognitive impairment is higher in men. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64(4):388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Respondek G, Roeber S, Kretzschmar H, Troakes C, Al-Sarraj S, Gelpi E, et al. Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Movement Disorders. 2013;28:504–9. doi: 10.1002/mds.25327. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander K, Lashley T, Gami P, Gendron T, Lythgoe MF, Rohrer JD, et al. Characterization of tau positron emission tomography tracer [F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other dementias. Alzheimers Dement. 2016;12:1116–11124. doi: 10.1016/j.jalz.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Schonhaut DR, McMillan CT, Spina S, Dickerson BC, Siderowf A, Devous MD, et al. 18F-flortaucipir tau PET distinguishes established progressive supranuclear palsy from controls and Parkinson’s disease: A multicenter study. Annals of Neurology. 2017 doi: 10.1002/ana.25060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz CG, Gunter JL, Wiste HJ, Przybelski SA, Weigand SD, Ward CP, et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. NeuroImage Clin. 2016;11:802–812. doi: 10.1016/j.nicl.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz CG, Gunter JL, Ward CP, Vemuri P, Senjem ML, Wiste HJ, et al. The Mayo Clinic Adult Lifespan Template (MCALT): Better Quantification across the Lifespan. Alzheimer’s Association International Conference Proceedings. 2017 [Google Scholar]
- Smith R, Schöll M, Widner H, van Westen D, Svenningsson P, Hägerström D, et al. In vivo retention of 18F-AV-1451 in corticobasal syndrome. Neurology. 2017a;89(8):845–853. doi: 10.1212/WNL.0000000000004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Schain M, Nilsson C, Strandberg O, Olsson T, Hägerström D, et al. Increased basal ganglia binding of 18F-AV-1451 in patients with progressive supranuclear palsy. Mov Disord. 2017b;32:108–114. doi: 10.1002/mds.26813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand EA, Duffy JR, Clark HM, Josephs K. The apraxia of speech rating scale: a tool for diagnosis and description of apraxia of speech. J Commun Disord. 2014;51:43–50. doi: 10.1016/j.jcomdis.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Gunter JL, Senjem ML, Whitwell JL, Kantarci K, Knopman DS, et al. Alzheimer’s disease diagnosis in individual subjects using structural MR images: validation studies. NeuroImage. 2008;39:1186–97. doi: 10.1016/j.neuroimage.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh JL, Duffy JR, McNeil MR, Robin DA, Rogers MA. Treatment guidelines for acquired apraxia of speech: a synthesis and evaluation of the evidence. J Med Speech Lang Pathol. 2006;14:35–67. [Google Scholar]
- Warrington EK, James M. The Visual Object and Space Perception Battery (VOSP) Bury St Edmunds, England: Thames Valley Test Company; 1991. [Google Scholar]
- Warrington EK. The Camden Memory Tests. Hove, UK: Psychology Press; 1996. [Google Scholar]
- Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis. 2009;24:408–416. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, et al. Neuroimaging comparison of primary progressive apraxia of speech and progressive supranuclear palsy. Eur J Neurol. 2013a;20:629–637. doi: 10.1111/ene.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Xia R, Mandrekar J, Machulda MM, et al. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang. 2013b;125:245–252. doi: 10.1016/j.bandl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Lowe VJ, Tosakulwong N, Weigand SD, Senjem ML, Schwarz CG, et al. [18F]AV-1451 tau positron emission tomography in progressive supranuclear palsy. Mov Disord. 2017;32:124–133. doi: 10.1002/mds.26834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, et al. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement. 2013;9:666–76. doi: 10.1016/j.jalz.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Yorkston KM, Strand E, Miller R, Hillel A, Smith K. Speech deterioration in amyotrophic lateral sclerosis: Implications for the timing of intervention. J Med Speech-Lang Pa. 1993;1:35–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.