Abstract
Antibody Fab fragments have been exploited with significant success to facilitate the structure determination of challenging macromolecules as crystallization chaperones and as molecular fiducial marks for single particle cryo-EM approaches. However, the inherent flexibility of the “elbow” regions, which link the constant and variable domains of the Fab can introduce disorder and thus, diminish their effectiveness. We have developed a phage display engineering strategy to generate synthetic Fab variants that significantly reduces elbow flexibility, while maintaining their high affinity and stability. This strategy was validated using previously recalcitrant Fab-antigen complexes where introduction of an engineered elbow region enhanced crystallization and diffraction resolution. Furthermore, incorporation of the mutations appears to be generally portable to other synthetic antibodies and may serve as a universal strategy to enhance the success rates of Fabs as structure determination chaperones.
Keywords: Crystallization chaperone, Fab elbow angle, antibody engineering, cryo-EM fiducial mark, Fab-protein complex
Graphical abstract

Introduction
Advances in numerous technologies have led to a rapid expansion of the number of determined molecular structures of proteins, protein complexes and RNAs [1]. Nevertheless, significant bottlenecks persist and principal among these is crystallization, and in the case of cryo-EM, particle orientation and mass, and conformational rigidity. Robotics and optimized crystallization screens provide broad and systematic surveys of potential conditions, but success rates remain frustratingly low especially for highly challenging systems like membrane proteins and large macromolecular complexes [2]. Common responses to unsuccessful crystallization efforts include surface engineering [3] or changes in construct design and crystallization screening of alternate species. In many cases this involves heroic effort with no guarantee of ultimate success.
An alternative to these traditional methods has been the use of so-called crystallization “chaperones” [2, 4–6]. These come in different forms and sizes and each has its own strengths and weaknesses [7]. Chaperones promote crystallization by reducing conformational heterogeneity, by masking hydrophobic surfaces, increasing solubility and can promote crystal lattice formation through their extensive polar surface area. Their use has been particularly productive in facilitating structure determination of membrane proteins, although they have enabled structural determination of numerous recalcitrant soluble protein systems, as well. Notably, these same chaperones can be utilized directly as fiducial marks for cryo-EM applications increasing the mass of the particle, as well as facilitating its orientation.
Among the types of crystallization chaperones, the antibody Fab fragment has been the most widely exploited in part owing to the ability to generate and customize them using high throughput approaches [8, 9]. A Fab contains ~500 amino acids divided approximately equally between its variable (VHVL) and constant (CH1CK) domains. This size also makes it a very effective fiducial for cryo-EM applications [10]. Unfortunately for structural biologists, antibody frameworks have evolved to incorporate an additional spatial degree of freedom manifested through variations in the arrangements of their constant and variable Fab domains [11]. Consequently, the inter-domain flexibility due to the “elbow” linker at the VHVL-CH1CK junction is oftentimes implicated as a limiting factor in both protein complex crystallization [12, 13], as well as its efficacy in providing full benefit as a fiducial [14]. This is reflected in the structures of Fabs in the Protein Data Bank where the elbow angle between the pseudo two-fold axes of the VH-VL and the CH1-CL can vary quite significantly (Figure 1A) [15]. Indeed, multiple copies of the Fab within a single structure can exhibit drastically different elbow angles (Figure 1B), complicating crystallization and reducing their ability to orient particles accurately in cryo-EM [10].
Figure 1.
Nevertheless, the many examples of their successful application in solving highly challenging systems clearly demonstrate that the advantages of the exploiting Fabs to assist in structure determinations far outweigh any downsides [16–19]. However, it occurred to us that it might be possible to further enhance the utility of Fabs as structure determination aids by eliminating the inter-domain flexibility thereby significantly restricting or even eliminating the range of the elbow linker conformations. Indeed, engineering inter-domain linker regions has been a successful strategy to overcome this barrier for a number of structural biology targets [20, 21]. We were further encouraged by previously reported Fab structures where shorter switch residue regions resulted in intact, functional antibody fragments [22–24].
It was also apparent, however, that introducing mutations within the elbow regions is complicated by the extensive protein interface buried between VH and CH1 and VL and CL (Figure 2) [25]. The heavy chain interface region forms a “ball-and-socket” arrangement, whereby a residue in the linker inserts itself into shallow depression in the interface between domains [11]. Therefore, simple deletions and point mutations that disrupt this arrangement likely would result in producing a significant fraction of variants with diminished function or altered quaternary architecture [26]. To circumvent this problem, we coupled phage display mutagenesis with functional selection in an attempt to repack interface surrounding the ball-and-socket region. We found that there were several viable solutions for producing Fabs having a single deletion in the elbow of the heavy chain that retained full binding capacity to the antigen. These variants were used to successfully crystallize complexes that had previously failed to do so in the context of the wild-type Fab. Furthermore, a finding was that the engineered elbow variants generally led to crystal forms of higher diffraction quality than what was obtained from the wild-type Fab complex. Further, the linker variants possessed different crystallization properties, indicating that they could be treated as independent crystallization chaperones. These findings suggest that the engineered linker variants substantially broaden the landscape leading to productive crystallization. This work offers a guide for how two or three siblings of a single Fab can be easily produced through simple mutagenesis to give multiple crystallization chaperones with superior properties to their parent.
Figure 2.
Results
The linkers connecting the variable and constant domains of a Fab are about 6–7 residues long (112–118 between VH-CH1) and are involved in conserved structural motifs with defined interactions (Figure 2) [11, 15]. When the light and heavy chains are assembled together to form the intact Fab scaffold, the linkers presumably lose flexibility when they pack against elements of the folded domains. While this restricts some conformational movement between the constant and variable regions of the Fab, it is clear from structural data that considerable variation still exists. Thus, to enhance the effectiveness of Fabs as crystallization chaperones, the objective of this work was to engineer a scaffold with a reduced propensity for conformation variability without affecting other properties.
Modeling suggested that it might be feasible to delete one or two residues in the linker region, shortening it to effectively “cinch-up” the connector between the variable and constant domains. However, the modeling also indicated that such deletions would likely have to be accompanied by significant repacking of the adjacent regions surrounding the linker to retain the stability of the scaffold [25]. It was further determined that the light chain linker was much more sensitive to deleterious effects caused by deletions than the heavy chain linker. Thus, we focused our efforts on engineering the heavy chain linker exclusively. This engineering involved using phage display to identify and optimize variants that contained deletions and other amino acid changes that were required to spatially compensate for shortening of the linker length. The residues contained in the linker undergoing phage display manipulation are referred to as the “switch region”. The goal was to develop several switch region variants that could be readily ported in a universal way to other Fab scaffolds, while retaining their stability, expression levels and binding capability.
a). Library generation
Combinatorial phage display libraries encompassing the switch region of the heavy chain of a Herceptin 4D5-framework Fab were created through Kunkel mutagenesis [27]. The particular Fab (Fab 12E) used for the switch region engineering was one that had been previously generated to the histone chaperone Anti-silencing factor 1 (Asf1) [28]. Herceptin has a canonical heavy chain switch region, which is comprised largely of small hydrophilic residues (S112SASTKG118). Within this region, Ser112 forms part of the conserved ball-and-socket, while Ser113, Ala114 and Ser115 and Lys117 contain side chains largely exposed to solvent (Figure 2). Thr116 projects inward towards the inter-domain region of the Fab heavy chain, where it participates in van der Waals interactions with residues in both VH and CH1. Within the heavy chain switch region, we made a series of libraries introducing amino acid diversity through hard randomization [27] (NNK codons) coupled with the introduction of 1–3 deletions. Libraries included further diversification of Gly118 that was also mutated to proline, a conformationally restrictive residue commonly found at this position. In total, we generated six small libraries, where the largest sub-library (V111X5(G/P)118P119) had a theoretical size of ~7×107.
For phage display selections, a format was used where the Asf1 antigen was immobilized on streptavidin magnetic beads through chemical biotinylation. Three rounds of phage panning were performed, where the concentration of antigen was systematically reduced from 50 nM in round 1 to 10 nM during the last round. This increased the stringency round-to-round and ensured the engineered variants maintained the high affinity exhibited by the parent Fab (KD ~2 nM). To favor the isolation of engineered Fab variants with stable quaternary structure, we subjected the round 3 phage input pools to heat stress (65 °C for 30 min) to denature marginally stable clones of an otherwise stable scaffold (TM of Fab12E ~80 °C). Clones from each library selection were screened by phage ELISA combined with limited sequencing. A variety of sequence solutions were found when a single deletion at position 117 was introduced (X5Gly118 and X5Pro118 libraries) (Table S1). Furthermore, clones that were isolated when two deletions were introduced into the heavy chain or a single residue was removed from the light chain, displayed very low expression levels (<0.2 mg/L bacterial culture) and thus, were not pursued further. 18 Fabs with a single deletion within the switch residue region were selected for further biophysical and structural analysis (Table S2).
Biophysical Characterization of FNQIK Switch Residue Fabs
To assess the portability of the switch region variants to other Fabs with the 4D5 scaffold, the 20 switch variants were converted into protein format for expression and biophysical characterization. Expression levels can provide some insight into stability and we found that most, but not all Fabs from the selections displayed workable expression levels (> 1 mg/L). However, for in depth characterization, we decided to focus on the top three expressing Fabs (VSRRLP, FNQIKG and its derivative, FNQIKP). Here, the average protein expression yields were close to what was found for the parent Fabs (~3 mg/L). Furthermore, the Fabs generally possessed high thermal stabilities suitable for structural studies as determined by differential scanning fluorimetry (DSF) [29], with TMs ranging from 69–76 °C (compared to 71–81 °C for the parent Fab) (Table S3). Consistently lower TMs for the engineered variants is not surprising as deletion of residues linking the Fab VH and CH1 coupled with the tight packing of the linker connecting the two domains likely introduces some strain on the Fab quaternary structure. Nevertheless, the thermal stabilities observed here reflect the high thermal stability of the validated 4D5 scaffold for structural biology [30].
Fabs with engineered switch regions also maintain high affinity to their antigen. We determined the KD of several Fab-antigen complexes with three switch residue sequences (FNQIKG, FNQIKP and VSRRLP). In each instance, the affinities of the interactions were < 10 nM, effectively identical to the parent Fab from which they were derived (Table S4). The maintenance of high affinity of the engineered Fabs enabled facile purification of the Fab- antigen complexes for crystallization.
Crystallization Screening of Fab Elbow Variants
To assess the role of the engineered heavy chain switch region variants on the crystallizability of the Fab-antigen complexes, we focused on two Fabs that had been generated previously to histone chaperone anti-silencing factor 1 (Asf1) (Fab12E and Fab1H). The Asf1 system was chosen for several practical reasons: 1) we were able to readily produce Fabs to multiple epitopes of this antigen; 2) the antigen is soluble, highly expressed in bacteria (>50 mg/L) and easily purified using standard procedures; 3) Asf1 itself is readily crystallized, ensuring that the antigen would not be the bottleneck in complex crystallization. Furthermore, because the isolated Fabs recognized different epitopes, we reasoned the results would be less likely to be influenced by the geometric constraints of a single Fab-antigen complex.
Switch Residue sequence is a critical determinant of Fab complex crystallization
For the first evaluation of the effects of elbow mutations on crystallization, a Fab-Asf1 system was chosen that had already yielded crystals in the wild-type Fab format. This comparison involved subjecting the complexes of Fab12E- Asf1 to crystallization screening using PEG/ION and JCSG+ screens. Previously, crystals of the wild-type Fab12E- Asf1 complex yielded a structure at 2.3 Å with 2 molecules in the ASU. For crystallization trials evaluating the crystallization properties of Fabs with engineered linkers, three distinct heavy chain switch regions containing a deletion: A1 (V111GWGGSG118), C12 (V111GPEARG118), H12 (V111FNQIKG118) and its derivative H12-P (V111FNQIKP118) were chosen. These variants encompassed a wide range of sequence variability and presumably rigidity, ranging from switch regions highly enriched in Glycine (A1), to those with larger, aliphatic side chains (H12 and H12-P).
Inspection of the crystallization results indicated that switch residue variants A1 (GWGGSG) and C12 (GPEARG) produced no or few crystal hits in the two screens after two weeks. This suggested that introduction of small, flexible residues like glycine or alanine in the A1 and C12 variants even with shortening the elbow linker probably further exacerbates the conformational flexibility of the parent sequence. On the other hand, switch residue variant H12 and H12-P (FNQIKG) crystallized in numerous conditions presumably because the two bulky hydrophobic residues at positions 112 and 115 restricted the linker conformation. The crystals generated from the FNQIKG variant diffracted to 1.7 Å; when the Gly at 118 was replaced by Pro (FNQIKP), a different crystal form was produced that diffracted to 1.5 Å (Table 1).
Table 1.
| Fab-Antigen Complex | Best Diffracting WT Fab |
Resolution (Å) | Spacegroup | Molecules in asu |
Elbow Angle (°) |
|---|---|---|---|---|---|
| Asf1-Fab1H-VSRRLP | 2.8 Å | 1.7 | P1 | 2 | 165, 186 |
| Asf1-Fab1H-FNQIKG | 2.4 | P212121 | 2 | 167, 186 | |
| Asf1-Fab1H-FNQIKG | 2.5 | P212121 | 2 | 169, 163 | |
| Asf1-Fab12E-FNQIKG | 2.3 Å | 1.7 | P21 | 1 | 171 |
| Asf1-Fab12E-FNQIKP | 1.5 | P1 | 1 | 169 | |
| CorA NTD-FabC12 FNQIKG | ~7 Å | 2.1 | C2 | 1 | 175 |
| CorA NTD-FabC12 FNQIKG | 2.0 | P21 | 1 | 174 | |
| CorA NTD-FabC12 FNQIKG | 2.5 | P1 | 2 | 174 | |
| IDE-FabIDE-FNQIKG | ~4 Å | 3.3 | P22121 | 1 | 174 |
| EbolaNP-Fab20 FNQIKG | 2.9 Å | 3.3 | P212121 | 2 | 164, 169 |
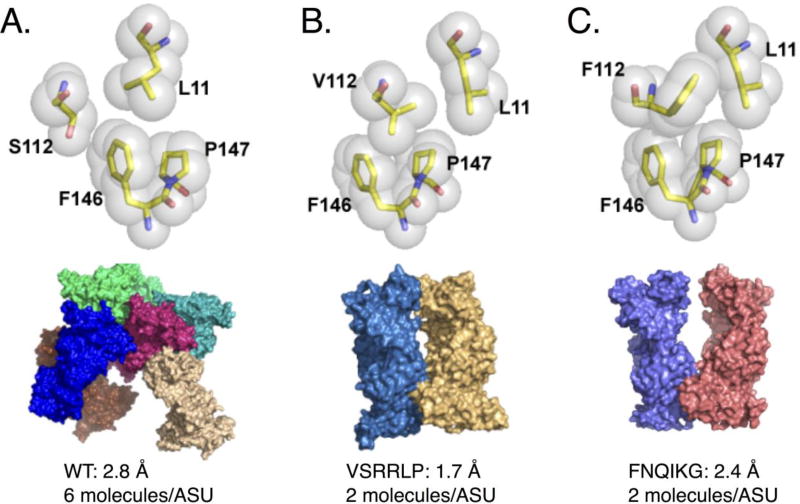
For the second test case, we chose the Fab1H-Asf1 system that had been problematic to crystallize with the wild-type Fab. Fab1H binds at an epitope on the opposite face of Asf1 compared to the Fab12E epitope. Previous efforts to crystallize this complex yielded few hits from the high-throughput screens and after much optimization resulted in a structure solved to 2.8 Å with six copies in the asymmetric unit (ASU) (pdb ID 4RRP) (Figure 3A). Notably, the Fab elbow angles varied ~40° between different copies in the ASU (Figure 1B). On the other hand, the two distinct switch residue sequences, FNQIKG and VSRRLP, yielded hits in multiple conditions and ultimately yielded crystals in different crystal forms leading to structures of 2.5 Å and 1.7 Å for each complex, respectively (Figure 3B, C, Table 1).
Figure 3.
Structures of elbow variants FNQIK(G/P) and VSRRLP
The Fab12E-FNQIK(G/P)-Asf1 complexes readily crystallized in a number of conditions with a dominant morphology. The initial screen for Fab12E-FNQIK(P)-Asf1 produced crystals in space group P1 having one Fab-antigen complex in the ASU with an elbow angle of ~169°. Comparison of the antigen-VHVL fragments of structures determined for the native and engineered elbow gave an RMSD of ~0.3 Å2. These values are comparable to the RMSD differences between variable domains within the same asymmetric unit indicating the engineered switch residue region of Fab12E (FNQIKP) did not cause significant structural rearrangements in the antigen-binding site. Furthermore, the constant domain assembly remained unchanged relative to the native Fab with an RMSD of 0.3 Å2.
While the wild-type Fab switch residue region has a limited number of side chains directed into the protein core, the FNQIKP switch residue region possesses a more extensive network of interactions. The inward facing Phe112 and Ile115 create extensive contacts within the elbow to create a network of bulky, hydrophobic residues within this region otherwise devoid of such interactions (Fig. 3C).(Details of this structure are described in Supplementary Materials).
The second set of elbow variant structures that were determined used Fab1H, which binds to a different epitope of Asf1. In this example, switch residue sequences FNQIKG and VSRRLP were introduced into the Fab framework. The FNQIKG complex crystallized in two slightly different crystal forms that possessed two copies in the ASU and diffracted to 2.4 Å (Figure 3C). One crystal form gave elbow angles of 167° and 186° and the second form had angles of 163° and 169° (Table 1). The VSRRLP variant led to crystals that diffracted to 1.7 Å, also with two complexes in the ASU (Figure 3B). Interestingly, the elbow angles of the first form almost directly coincide with those found in the first FNQIKG variant (167°–186°) described above. In the case of VSRRLP, Val112 projects inward, making van der Waals contact with Leu11 (Figure 3B). Here, however, the different molecules in the ASU possess distinct rotameric conformations of Val112 and Leu11. The smaller aliphatic residue at the first position of the switch region likely contributes to the increased conformational heterogeneity compared to FNQIKG. Nevertheless, the VSRRLP variant alters the ball-and-socket motif of the elbow region by placing a “wedge” (Val112) between residues Leu11 and Phe146 (Figure 3B). Combined with the inward-facing Leu116, VSRRLP forms a network of buried hydrophobic residues, similar to FNQIK(G/P).
Additional Elbow Structural Examples
To more fully assess the crystallization characteristics of the switch variants, we evaluated two systems that had previously proven problematic when using wild-type Fabs as crystallization chaperones. Obviously, quantifying the superiority of the engineered elbow Fabs will require more examples; however, the following test cases furnish a framework for the usefulness of the approach.
CorA
The first example is the N-terminal domain of CorA, a Mg2+ ion channel from T. maritima. We had been successful in generating a Fab (Fab C12) that was able to selectively bind the transient open state of the channel and stabilize it [31]. To establish the mechanism for how Fab C12 accomplished this, we sought to determine the structure of the Fab bound to the monomeric N-terminal domain (NTD) of the channel; however, the wild-type Fab C12- CorA NTD complex did not yield crystals that diffracted past 10 Å. The elbow mutant FNQIKG was then introduced into Fab C12 and the screening was repeated. Over a two-week period, crystals appeared in 13 of the 480 conditions in the screen, and 19 conditions in total after three months from setting up the screening. Optimization of the six of the most promising conditions led to three distinct crystal forms with different diffraction quality: Form 1, 1.9 Å, (space group C2, elbow-175°), Form 2, (2.0 Å, space group P21, elbow-174°) Form 3, (2.5 Å space group P1, 174°). The C2 structure of the elbow engineered Fab C12 and the NTD domain of CorA is shown in Figure 4A. The results will be reported elsewhere.
Figure 4.
Insulin Degrading Enzyme
The second example was a Fab-Insulin degrading enzyme (IDE) complex. Diffraction quality crystals of Fab-bound IDE structure in complex with functionally relevant substrates e.g. insulin, have been difficult to obtain with the best resolution of ~ 4.0 Å with 8 IDE-Fab complexes in the ASU, with elbow angles of 140–199° (pdb ID 4Q5Z) (Fig 4B, C). In the case of IDE, replacing the Fab heavy chain elbow region with an engineered sequence (FNQIKG), crystals of the IDE-Fab were obtained that diffracted to 3.3 Å. Furthermore, there is only a single IDE-Fab-insulin complex within the ASU (pdb 5CJO) (Fig 4C). Again, the elbow angle of this Fab is 171°, comparable to other Fab complexes determined with this switch residue sequence.
KcsA K+ ion channel
Figure 5 demonstrates how elbow engineered Fabs can be exploited to orient the full length KcsA channel for single particle Cryo-EM analysis. The functional form of the channel is a ~68 kDa tetramer, which is too small to be a candidate for Cryo-EM analysis. However, with four Fabs bound to the cytoplasmic four-helix bundle domain, the size of the particle is increased to ~280 kDa putting the complex in range for Cryo-EM. Further, the Fabs provide a distinctive shape that makes the particle easy to accurately orient. Despite the modest resolution of this complex, the constant domains of the Fab a readily visible. Notably, the constant domain was not discernable in some class averages when the wild-type Fab was employed (M. Clark, personal communication). A high resolution Cryo-EM analysis is currently underway.
Figure 5.
Discussion
Fab fragments have an impressive track record as crystallization chaperones for recalcitrant protein systems [16–19, 31, 32]. They are also starting to be used as fiducial marks for single particle Cryo-EM (10). The flexibility in the elbow linkers connecting the variable and constant domains of the Fab is known to adversely affect crystallization and degrade image quality in some instances [10, 12, 13]. Based on Protein Data Bank data, Fab constant domains can suffer from disorder in the crystal lattice when not involved in extensive crystal contacts [32]. Our experience is that Fab-antigen complexes have a tendency to crystallize in lower symmetry space groups with higher numbers of molecules in the asymmetric unit cell. While it may be advantageous to have multiple copies of the molecules in the ASU in some instances to sort out structural differences due to lattice packing effects, etc., this advantage can be overwhelmed with the sheer magnitude of the undertaking when many copies are contained in the ASU. Thus, to have some consistency, we were motivated to engineer the Fab scaffold in such a way that the elbow variability could be minimized no matter what the CDR composition was while preserving its overall attributes as a chaperone.
In undertaking this endeavor, useful information can be gleaned from the pattern and extent of the elbow angle distributions observed across many Fab examples. Additionally, determining whether there are isoform-specific tendencies with respect to linker variability provided insight into how to engineer more stable elbow linkers. In this regard, Stansfield and coworkers analyzed the distribution of the elbow angles from Fab structures in the PDB circa 2006 [15]. While a large number of additional Fab structures have been deposited since then, this analysis contained sufficient examples to establish a high probability that the same trends would be followed. A finding was that the distribution was significantly different depending on the class of light chain. The light chain has two predominant isoforms: Kappa and Lambda. The Lambda light chain has an extra residue in its switch region that adds additional conformational variation to the elbow angle and this is reflected in the Stansfield analysis. However, since the Herceptin 4D5 Fab scaffold used in this study has a Kappa light chain, we limit our discussion to elements associated with this isoform, although the same strategy described above could be followed for Fabs with Lambda light chains.
The distribution of the elbow angles for Kappa light chain Fabs spanned approximately 70° and is bimodal. The most commonly occurring elbow angles fall in the 135–145° and 165–175° ranges, with the lower angle bin dominating. Notably, the engineered elbow angle variants studied here fall into the higher range of the distribution with no engineered Fab conformations for the smaller, most commonly observed angle class within the PDB. Molecular dynamics simulations of the wild-type and engineered switch residues suggested that the new variants possess less conformational flexibility. Furthermore, alignment of the variable domains of Fab12E-FNQIKG (171°) and an example of a Fab with a smaller elbow angle (130°) PDB code 1W72, points to a very different positioning of the constant domains of the two Fabs (Figure S1). This alignment suggested that the conformational changes observed in the ball-and-socket region of the engineered switch variants are incompatible with smaller elbow angles.
Interestingly, each elbow variant tested performed somewhat differently in crystallization trials. Further, some experiments where crystal seeds from one elbow variant were added into solutions containing a second elbow variant led to crystals with somewhat different space group characteristics than the introduced parent, indicating a dominant effect of the switch residue region in the inter-domain conformation of the Fab (Table 1). One interpretation of these observations is that Fabs provide potentially productive lattice contacts and that small perturbations can lead to quite different crystallization outcomes. This has important implications with regard to strategies one might take in crystallization trials. Our experience is that it is most productive to test a number of Fabs in parallel, for instance five, and from those pick the ones that look most promising. That is, the “first” Fab to crystallize is not necessarily the best Fab and much effort could be put into optimizing it, when there was a candidate that would produce much better results waiting in the queue.
However, there are situations where you do not have the luxury of trying multiple Fabs. This arises in cases where the Fab has particular properties that cannot be reproduced by alternative candidates; for instance, a Fab that exclusively captures a specific conformational state of the target molecule or binds to a desired surface epitope [31, 33]. If this Fab complex does not crystallize, there are no real alternatives. Fortunately, it is here that the elbow variants can be powerfully exploited. Our findings show that incorporating the elbow mutations into the customized Fab can generate a chaperone with completely new properties. So instead of a single opportunity limited by the wild-type Fab, the elbow mutations offer “more shots on goal”. Our current strategy for situations requiring customized Fabs is to set up crystallization trials with the wild-type along with two elbow variants. While our experience at this point is rather limited, it appears that testing three options will significantly enhance success rates for these types of situations.
In the past, using crystallization chaperones has been the choice of last resort mainly because producing them was viewed by the community as a daunting challenge. However, with high-throughput affinity reagent generation by phage display now being a part of the mainstream and with the different formats that are possible, chaperones should be considered as a go-to approach for recalcitrant structural biology problems (5,6). We have chosen to focus on Fabs as our principal chaperone format due to their versatility. For instance, because of its size (~50 kDa), Fabs can be extremely useful as an initial molecular-replacement phasing model [5]. The Fab’s size can also be exploited in single particle cryo-EM applications by providing a fiducial mark to assist in orientation and to increase the size of the molecular particle [10, 34]. We note that in single particle EM, stabilizing the elbow linker is also important to ensure that the Fab contributes its full mass to the particle being studied [14]. Taken together, chaperones will have an increasing impact on structural biology and customizing the physical characteristics that improve their usability will help stimulate their exploitation in myriad applications.
Methods
Phage display library construction
Phage display libraries of the switch region of the heavy chain of anti-Asf1 Fab12E were created through Kunkel mutagenesis. A series of libraries to the switch residue sequence (S112SASTKG118) were created. Libraries included 1, 2 or 3 residue deletions in the switch region (residues 112–117). Additionally, a library with a single switch residue deletion coupled with Gly/Pro diversity at position 118 were created. In total, four libraries were generated, where the largest sub-library (V111X5(G/P)118P119) had a theoretical size of ~1×107.
Fab Expression and Purification
Fabs were expressed as previously described (34). Briefly Fabs variants were sub-cloned into the pSFV4 vector. Protein was expressed using E. coli BL21 cell line where cells were grown in 2xYT and expression was induced at OD600 ~0.6. Induction proceeded for 5 hours at which point cell pellets were harvested. Fabs were purified as previously described using ProteinG-A1 resin for single-step purification [35].
Surface plasmon resonance
Surface plasmon resonance affinity determination was performed by a BIAcore-3000 instrument. Antigen was immobilized via a C-terminal 6× His tag to a Ni-NTA chip (GE Healthcare). Fabs served as the analyte and were used with two-fold serial dilutions. Association and dissociation times were monitored at 20°C with a 25 µL/min flowrate, and the binding response was corrected through double referencing. Traces were fit using Scrubber software.
Differential scanning fluorimetry
The melting temperatures of the Fabs were determined with SYPRO Orange dye at excitation/emission at 490/575 nm. Thermal melts were performed over a temperature range of 25–95 °C at a rate of 0.5 °C per 30 s interval. Fab concentrations of ~5–10 µM were used.
Fab complex crystallization and structure determination
Fab12E-Asf1 crystal structure (5EII)
The Fab12E-Asf1 complex was concentrated to ~10 mg/ml and crystallized in 20% PEG3350, 0.2 M ammonium acetate. Data was collected at GM/CA-CAT beamline 23ID-D. Data were processed using HKL2000 and the structure was solved using Fab structure (3PGF) and yeast Asf1 structure (1ROC). Model building and refinement were performed using Coot [36] and Phenix [37], respectively.
Fab12E(FNQIKG)-Asf1 structure (6AZ2)
Protein complex was crystallized in 20% PEG3350, 0.2 M ammonium chloride. Data were collected at GM/CA-CAT beamline 23ID-D. Data were processed using HKL3000 and the structure was solved using Fab structure (3PGF) and yeast Asf1 structure (1ROC). Model building and refinement were performed using Coot [36] and Phenix [37], respectively.
Fab12E(FNQIKP)-Asf1 structure (5UCB)
The protein preparation and crystallization conditions were identical to the Fab12E-H12-Asf1 structure complex. Data were collected on SBC beamline 19ID and processed using HKL3000. The structure was solved by molecular replacement using the Fab12E-H12-Asf1 complex as the starting model. Model building and refinement were performed using Coot [36] and Phenix [37], respectively.
Fab1H-Asf1 structure (4RRP)
The structure of the Fab1H-Asf1 complex was solved by the Northeastern Structural Genomics group and the details will be described elsewhere.
Fab1H(FNQIKG)-Asf1 structure (6AZ2)
The Fab12E-Asf1 complex was concentrated to ~8 mg/ml and crystallized in 20% PEG3350, 0.2 M ammonium acetate. Data were collected at GM/CA-CAT beamline 23ID-B. Data were processed using HKL3000 and the structure was solved using Fab structure (3PGF) and yeast Asf1 structure (1ROC). Model building and refinement were performed using Coot [36] and Phenix [37], respectively.
Fab1H(VSRRLP)-Asf1 structure (5UEA)
The protein preparation and crystallization conditions were identical to the Fab12E-H12-Asf1 structure complex. Data were collected on GM/CA-CAT beamline 23ID-D and processed using HKL3000. Data were processed using HKL3000 and the structure was solved using Fab structure (3PGF) and yeast Asf1 structure (1ROC). Model building and refinement were performed using Coot [36] and Phenix [37], respectively.
IDE-Fab(IDE) complex (5CJO)
IDE-Fab(IDE) complex was crystallized in 6% v/v Tacsimate pH 7.0, 0.1 M HEPES pH 7.0, 8% w/v Polyethylene glycol monomethyl ether 5,000, and 8% v/v tert-butanol at 18°C by hanging drop vapor diffusion. Diffraction data were collected at 100K on the SBC 19-ID beamline at Argonne National Laboratory. Data sets were processed using HKL3000, and the structure of IDE-Fab(IDE) complex was determined by molecular replacement, using the cysteine-free IDE structure (4NXO) and the Fab structure in (4IOF) as search models, and Model building and refinement were performed by using PHENIX, and Coot [36]. The final model (pdb=5CJO) has Rwork=19.6% and Rfree =24.5%.
Single-particle cryo-EM analysis of Full length, wild-type KcsA
Protein was expressed and purified using standard protocols. In brief, pET28a-KcsA [38] was expressed in E. coli DE3 pLysS (Novagen Millipore), and purified in HEPES, KCl and DDM by cobalt metal affinity chromatography and size exclusion chromatography (SEC). Purified Fab4 elbow variant were added in excess to purified KcsA, and the stoichiometric complex was further purified on SEC. The peak corresponding to the complex was collected, and used directly for grid preparation without further concentration.
C-flat 1.2/1.3, 200 Mesh cryoEM grids (Protochips) were plasma cleaned for 30 sec with an air mixture in a Gatan Solarus plasma cleaner. Purified complex at ~3mg/ml in HEPES pH7.4, 200mM KCl, 0.4mM DDM was plunge-frozen using a FEI Vitrobot, operated at 100% humidity, 22°C, blot force of 3, and blot time of 3 sec. Grids were imaged on an FEI Talos 200kV microscope equipped with a Falcon II detector. Data were collected semi-automatically using Serial EM [39], with a pixel size of 1.936 Å, and a total dose of 68 e−/Å2 fractionated across 40 frames.
Whole-frame drift correction was performed using MotionCor2 [40], and data were further processed using Eman2 [41]. Approximately 6000 particles were manually picked for KcsA-Fab4 elbow variant, CTF corrected and classified in 2D. The best 1500 “top view” particles were selected and averaged.
Supplementary Material
Highlights.
Fab fragments have proven utility in structure determination of challenging macromolecular systems. In some instances, however, the flexibility in the “elbow” linker between the Fab variable and constant domains significantly reduces the effectiveness of this approach.
To eliminate this flexibility requires the linker region between the constant and variable domains of the Fab to be modified to freeze in a single conformational state without the Fab losing its binding function or stability.
Phage display was used to “cinch-up” the connector between the two domains by removing one residue in the elbow linker and then repacking the region around the removed residue to restore stability.
Two different elbow modifications that met the criterial were identified. The resulting elbow variants showed significant improvement in crystallizability and utility as fiducial markers for cryo-EM.
The elbow variants eliminate conformational heterogeneity existing in native Fab fragments, expanding their utility in enabling solving structures of challenging macromolecular systems by crystallography or single particle cryo-EM.
Acknowledgments
The work was funded by the following grants: GM117372, GM087519, GM094588, Chicago Biomedical Consortium and Pfizer, Inc. Use of the Advance Photon Source GM/CA sector 23 and the SBC sector 19 beamlines and assistance from their staffs at Argonne National Laboratory is acknowledged. We thank the Northeast Structural Genomics Consortium for providing structural information before publication. We appreciate the discussions with M. Nocula-Lugowska, B. Roux and E. Perozo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Numbers: PDB IDs 5UEA, 6AZ2, 6AYZ, 5UCB, 5CJO, 4RRP
Author’s contributions:
L.J.B. and A.A.K. conceived the study; L.J.B., K.M.S., P.K.D., W.G.L., H.R., M.C., M.J., Y.K. and D.D. performed experiments; L.J.B., K.M.S., P.K.D., W.G.L., H.R., M.C., M.J., Y.K., D.D., W.J.T. and A.A.K. analyzed data; L.J.B. and A.A.K. wrote the paper with input from all authors.
References
- 1.Garman EF. Developments in x-ray crystallographic structure determination of biological macromolecules. Science. 2014;343(6175):1102–8. doi: 10.1126/science.1247829. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter EP, et al. Overcoming the challenges of membrane protein crystallography. Curr Opin Struct Biol. 2008;18(5):581–6. doi: 10.1016/j.sbi.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derewenda ZS. Rational protein crystallization by mutational surface engineering. Structure. 2004;12(4):529–35. doi: 10.1016/j.str.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Griffin L, Lawson A. Antibody fragments as tools in crystallography. Clin Exp Immunol. 2011;165(3):285–91. doi: 10.1111/j.1365-2249.2011.04427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tereshko V, et al. Toward chaperone-assisted crystallography: protein engineering enhancement of crystal packing and X-ray phasing capabilities of a camelid single-domain antibody (VHH) scaffold. Protein Sci. 2008;17(7):1175–87. doi: 10.1110/ps.034892.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukowska MA, Grutter MG. New concepts and aids to facilitate crystallization. Curr Opin Struct Biol. 2013;23(3):409–16. doi: 10.1016/j.sbi.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Koide S. Engineering of recombinant crystallization chaperones. Curr Opin Struct Biol. 2009;19(4):449–57. doi: 10.1016/j.sbi.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellouse FA, et al. High-throughput generation of synthetic antibodies from highly functional minimalist phage-displayed libraries. J Mol Biol. 2007;373(4):924–40. doi: 10.1016/j.jmb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Hornsby M, et al. A High Through-put Platform for Recombinant Antibodies to Folded Proteins. Mol Cell Proteomics. 2015;14(10):2833–47. doi: 10.1074/mcp.O115.052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu S, et al. Fabs enable single particle cryoEM studies of small proteins. Structure. 2012;20(4):582–92. doi: 10.1016/j.str.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesk AM, Chothia C. Elbow motion in the immunoglobulins involves a molecular ball-and-socket joint. Nature. 1988;335(6186):188–90. doi: 10.1038/335188a0. [DOI] [PubMed] [Google Scholar]
- 12.Kovari LC, Momany C, Rossmann MG. The use of antibody fragments for crystallization and structure determinations. Structure. 1995;3(12):1291–3. doi: 10.1016/s0969-2126(01)00266-0. [DOI] [PubMed] [Google Scholar]
- 13.Ostermeier C, et al. Fv fragment-mediated crystallization of the membrane protein bacterial cytochrome c oxidase. Nat Struct Biol. 1995;2(10):842–6. doi: 10.1038/nsb1095-842. [DOI] [PubMed] [Google Scholar]
- 14.Park E, Campbell EB, MacKinnon R. Structure of a CLC chloride ion channel by cryo-electron microscopy. Nature. 2016 doi: 10.1038/nature20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanfield RL, et al. Antibody elbow angles are influenced by their light chain class. J Mol Biol. 2006;357(5):1566–74. doi: 10.1016/j.jmb.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Koldobskaya Y, et al. A portable RNA sequence whose recognition by a synthetic antibody facilitates structural determination. Nat Struct Mol Biol. 2011;18(1):100–6. doi: 10.1038/nsmb.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shukla AK, et al. Structure of active beta-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497(7447):137–41. doi: 10.1038/nature12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uysal S, et al. Mechanism of activation gating in the full-length KcsA K+ channel. Proc Natl Acad Sci U S A. 2011;108(29):11896–9. doi: 10.1073/pnas.1105112108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uysal S, et al. Crystal structure of full-length KcsA in its closed conformation. Proc Natl Acad Sci U S A. 2009;106(16):6644–9. doi: 10.1073/pnas.0810663106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbaum DM, et al. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007;318(5854):1266–73. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 21.Smyth DR, et al. Crystal structures of fusion proteins with large-affinity tags. Protein Sci. 2003;12(7):1313–22. doi: 10.1110/ps.0243403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golinelli-Pimpaneau B, et al. Crystal structure of a catalytic antibody Fab with esterase-like activity. Structure. 1994;2(3):175–83. doi: 10.1016/s0969-2126(00)00019-8. [DOI] [PubMed] [Google Scholar]
- 23.Larsen NA, et al. Crystallographic and biochemical analysis of cocaine-degrading antibody 15A10. Biochemistry. 2004;43(25):8067–76. doi: 10.1021/bi049495l. [DOI] [PubMed] [Google Scholar]
- 24.Love RA, et al. How the anti-(metal chelate) antibody CHA255 is specific for the metal ion of its antigen: X-ray structures for two Fab'/hapten complexes with different metals in the chelate. Biochemistry. 1993;32(41):10950–9. doi: 10.1021/bi00092a004. [DOI] [PubMed] [Google Scholar]
- 25.Rothlisberger D, Honegger A, Pluckthun A. Domain interactions in the Fab fragment: a comparative evaluation of the single-chain Fv and Fab format engineered with variable domains of different stability. J Mol Biol. 2005;347(4):773–89. doi: 10.1016/j.jmb.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 26.Calarese DA, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300(5628):2065–71. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 27.Sidhu SS, et al. Phage display for selection of novel binding peptides. Methods Enzymol. 2000;328:333–63. doi: 10.1016/s0076-6879(00)28406-1. [DOI] [PubMed] [Google Scholar]
- 28.Schaefer ZP, Bailey LJ, Kossiakoff AA. A polar ring endows improved specificity to an antibody fragment. Protein Sci. 2016;25(7):1290–8. doi: 10.1002/pro.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niesen FH, Berglund H, Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc. 2007;2(9):2212–21. doi: 10.1038/nprot.2007.321. [DOI] [PubMed] [Google Scholar]
- 30.Deller MC, Kong L, Rupp B. Protein stability: a crystallographer's perspective. Acta Crystallogr F Struct Biol Commun. 2016;72(Pt 2):72–95. doi: 10.1107/S2053230X15024619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dominik PK, et al. Conformational Chaperones for Structural Studies of Membrane Proteins Using Antibody Phage Display with Nanodiscs. Structure. 2016;24(2):300–9. doi: 10.1016/j.str.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, et al. Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain. Nat Struct Mol Biol. 2014;21(3):244–52. doi: 10.1038/nsmb.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizk SS, et al. Allosteric control of ligand-binding affinity using engineered conformation-specific effector proteins. Nat Struct Mol Biol. 2011;18(4):437–42. doi: 10.1038/nsmb.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartesaghi A, et al. Prefusion structure of trimeric HIV-1 envelope glycoprotein determined by cryo-electron microscopy. Nat Struct Mol Biol. 2013;20(12):1352–7. doi: 10.1038/nsmb.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey LJ, et al. Applications for an engineered Protein-G variant with a pH controllable affinity to antibody fragments. J Immunol Methods. 2014;415:24–30. doi: 10.1016/j.jim.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emsley P, et al. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–21. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hulse RE, et al. Conformational dynamics at the inner gate of KcsA during activation. Biochemistry. 2014;53(16):2557–9. doi: 10.1021/bi500168u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152(1):36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Zheng SQ, et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods. 2017;14(4):331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang G, et al. EMAN2: an extensible image processing suite for electron microscopy. J Struct Biol. 2007;157(1):38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.