Abstract
Recent reports in Alzheimer’s Disease (AD) research suggests that alterations in microRNA (miRNA) expression are associated with disease pathology. Our previous studies suggest that A Disintegrin and Metalloproteinase 10 (ADAM10) expression is important in AD and could be modulated by an extended regulatory region that includes the 3’UTR. In this study we have investigated the role of trans-acting factors in ADAM10 gene regulation. Our study shows that miRNA-140-5p has enhanced expression in AD post-mortem brain hippocampus using high throughput microRNA arrays and qRT-PCR. Interestingly we have also seen that miRNA-140-5p seed sequence is present on 3’UTR of both ADAM10 and its transcription factor SOX2. The specific interaction of miRNA-140-5p with both ADAM10 and SOX2 signifies high regulatory importance of this miRNA in controlling ADAM10 expression. Thus, this investigation unravels mechanisms underlying ADAM10 down-regulation by miR-140-5p and suggests that dysfunctional regulation of ADAM10 expression is exacerbated by AD related neurotoxic effects. These findings underscore the importance of understanding the impact of trans-acting factors in the modulation of AD pathophysiology.
1. Introduction
The neurodegeneration in Alzheimer’s Disease (AD) may be caused by the accumulation of neurotoxic β-amyloid (Aβ) in the form of plaques in the brain (Hardy and Selkoe, 2002). Neurotoxic Aβ is formed through the sequential cleavage by β and γ secretase enzymes of the amyloid precursor protein (APP) (Selkoe, 1998). An alternative non-amyloidgenic pathway exists. In this pathway APP is cleaved by the α-secretase, A Disintegrin and Metalloproteinase 10 (ADAM10), in a manner that inhibits the production of the neurotoxic Aβ (Fahrenholz et al., 2000, Postina, 2008, 2012) and in turn produces the soluble amyloid precursor protein α (sAPPα), which has neuroprotective properties (Bell et al., 2008, Furukawa and Mattson, 1998). Furthermore, a major α-secretase for APP, ADAM10 plays an important role in embryonic neurogenesis and brain development (Jorissen et al., 2010, Pan and Rubin, 1997). It is also observed that ADAM10 knockout (KO) and conditional KO mice are lethal in early developmental stages (Hartmann et al., 2002, Jorissen et al., 2010)
Previous studies have shown that ADAM10 dysregulation can lead to several disease pathologies. ADAM10 upregulation can cause cancer (Kai et al., 2014, Lee et al., 2010, Mochizuki and Okada, 2007) and chronic inflammation (Crawford et al., 2009, Pruessmeyer et al., 2014) whereas its downregulation is associated with brain disorders such as AD (Bekris et al., 2012, Tanzi, 2012). ADAM10 mutations increase generation and accumulation of the neurotoxic Aβ protein in AD mouse brain and inhibit production of new neural cells in hippocampus essential to learning and memory (Suh et al., 2013). Past reports have shown association of reduced level of ADAM10 and consequent decrease of neuroprotective sAPPα with AD (Jorissen et al., 2010, Postina et al., 2004). In our previous study we have found an association between an ADAM10 promoter genetic variant and lower CSF sAPPα and ADAM10 levels in AD patients compared to cognitively normal controls as well as lower ADAM10 promoter activity in reporter experiments that included the ADAM10 3’UTR (Bekris et al., 2012). These findings suggest that ADAM10 expression is modulated according to an extended regulatory region that includes the 3’UTR.
Previous studies of the structure of human ADAM10 located on chromosome 15 reveals that highly conserved ADAM10 promoter contains regions that may control transcriptional activity and protease expression (Prinzen et al., 2005) ADAM10 promoter transactivation by retinoic acid, acitretin, PAX2 have been explored ((Doberstein et al., 2011, Prinzen et al., 2005)).
Regulation by the ADAM10 3'UTR and miRNA have been described (Chang et al., 2015, Cheng et al., 2013, Kai et al., 2014). Regulation via 3’UTR of a gene can be mediated by miRNA which can function as post transcriptional regulator of gene expression by facilitating degradation of mRNA, inhibiting translation, deadenylation and p-body localization (Bartel, 2004, Nilsen, 2007) (Fazi and Nervi, 2008). Alterations of miRNA level have been associated with neurodegenerative disorders, including AD (Femminella et al., 2015, Kumar et al., 2013), Parkinson disease (Mouradian, 2012), Huntington disease (Johnson et al., 2008), Amyptropic Lateral Sclerosis (Volonte et al., 2015). Recent studies demonstrate that dysregulation of specific miRNA contributes to amyloidogenesis in AD (Hebert et al., 2008, Liu et al., 2012, Patel et al., 2008).
Taken together, our previous studies and studies by others, suggests that both the promoter and the 3’UTR of ADAM10 are regulated by trans-acting factors. However, it is still unclear which trans-acting factors might regulate AD specific ADAM10 gene expression in brain. The aim of this investigation was to identify the factors responsible for ADAM10 regulation in AD. We used a combination of bioinformatics and experimental techniques to show that miR-140 is a negative regulator of ADAM10 and its transcription factor SOX2, which can be activated by Aβ and is implicated in AD pathogenesis.
2. Materials and Methods
2.1 Population Description
Two regions of post-mortem brain (cerebellum and hippocampus) were obtained from subjects at the Neuropathology Core of the Alzheimer’s Disease Research Center (ADRC) at the University of Washington (UW). Use of human tissue was approved by the UW and Cleveland Clinic Foundation (CCF) Institutional Review Boards. All tissue was obtained after informed consent, flash frozen at the time of autopsy and stored at –80 °C for later use. Patients with late-onset AD (n = 21) and cognitively normal controls (n = 22) were UW ADRC volunteers as previously described (Bekris et al., 2012). Further patient information can be observed in
2.2 MiRNA selection
Computational bioinformatics tool to predict putative miRNA binding on ADAM10 3’UTR was used with the help of microRNA.org (Betel et al., 2010, Betel et al., 2008). The candidate miRNA were selected using the scoring specific to this bioinformatic tool. For example, PhastCons and miRSVR score provided at microRNA.org (Betel et al., 2010, Betel et al., 2008) which considers regression method for predicting likelihood of target mRNA downregulation. Three miRNA were selected for miRNA qRT-PCR analyses: miR-140-5p, miR194, miR-182 based on seed-site pairing of miRNA with ADAM10 3’UTR, or with SOX2 (Table 2).
Table 2.
Profile of selected miRNA (microRNA.org)
|
|
mirSVR score: −1.0599 PhastCons score: 0.6195 |
|
|
mirSVR score: −0.9697 PhastCons score: 0.6275 |
|
|
mirSVR score: −0.6535 PhastCons score: 0.7081 |
|
|
--------- |
2.3 DNA, RNA and Protein
DNA and RNA were extracted from postmortem brain tissue using the Qiagen Allprep DNA/RNA Mini Kit (Qiagen, Valencia, CA) with a slight modification in the protocol that utilized 100% ethanol to capture small RNAs according to manufacturer’s instructions as previously described (Bekris et al., 2012). Total RNA was also DNase treated using the TURBO DNA-free™ Kit (Applied Biosystems, Austin, TX) to further deplete DNA content. Protein was extracted from control and Aβ treated human neuronal SHSY5Y cells using a modification of the Qiagen Allprep DNA/RNA Mini Kit (Qiagen, Valencia, CA). For miRNA profiling of brain tissue, DNase-treated total RNA was pooled according to disease status and brain region: (1) Control CB, (2) Control HP, (3) AD CB and (4) AD HP (Figure 1). Three mls of equal concentrations (1.35 ng/ml) from each subject sample of DNase-treated total RNA and were pooled. Four ng of DNase-treated total RNA pools were reverse-transcribed using Megaplex RT primers human pool A (Applied Biosystems, Austin, TX) as well as minus RT for detection of DNA amplification. The RT primers human pool A contains specific stem-loop primers for 377 human miRNAs, three small housekeeping RNAs (RNU44, RNU48 and MammU6) and one negative control (ath-miR159a) and are all based on miRBase v. 10.1. A pre-amplification step using equal amounts from each pool (2.5 ml) of RT or minus RT product was added to enrich for human pool A array-specific miRNA using Megaplex PreAmp Primers pool A (Applied Biosystems). Nine microliter of the resulting pre-amplification complementary DNA (cDNA) plus 450 ml of TaqMan Universal Master Mix, no AmpErase, UNG (Applied Biosystems) was transferred to a TaqMan Human miRNA A Array v2.0 (Applied Biosystems), and quantitative PCR was performed using an Applied Biosystems 7900HT Sequence Detection system. Cycling conditions were 50 C for 2 min, 94.5 C for 10 min, 40 cycles of 97 C for 30s and 59.7 C for 1 min. Cycle threshold (CT) values were recorded with SDS version 2.3 software (Applied Biosystems, Austin, TX) as previously described. Extracted protein was resuspended in immunoprecipitation buffer (150 mM NaCl, 50 mM Tris-HCl (pH 6.8), 0.5% NP-40, 0.5% sodium deoxycholate, 5 mM EDTA, 50 mg/ml leupeptin, 10 mg/ml aprotinin, and 0.25 mM PMSF) and stored at −80°C. MiRNA level was measured in triplicate by quantitative real-time PCR (qRTPCR) using the 7500 Real Time PCR System (Applied Biosystems, Foster City, CA), with RNU44 and RNU48 as loading controls (Applied Biosystems). Quantitative RT-PCR results are calculated as 2−ΔΔCT to represent fold change of experimental sample compared the cerebellum as a control, where ΔΔCT= (ΔCTCB− ΔCTHP) for human samples. Cell line experimental results are calculated as ΔΔCT=(ΔCTuntreated−ΔCTtreated). MiRNA-140-5p specific mimic (MC10205) and inhibitors (MH10205) from Ambion by Life Technologies are used to overexpress and downregulate miR-140-5p respectively. ADAM10 (Abcam) and SOX2 (Cell Signalling) antibodies along with HRP-conjugated secondary antibodies against the primary antibodies were used for protein analysis by Western blotting. Detection was done by Amersham Biosciences ECL Western blotting detection reagent, according to the manufacturer’s protocol. The intensities of protein bands were quantitated using ImageJ software (National Institutes of Health).
Figure 1. MiRNA expression levels in human AD hippocampus compared to controls.
(A) The expression profile of ADAM10 candidate miRNAs in microarray represented by 2^-ΔΔCT`. (B) Quantitative RT-PCR analysis of ADAM10 candidate miRNA-140, miRNA-182, miRNA-194 and control miRNA-126 in Control vs AD. Data represent three independent experiments with triplicate measurements. All p-values were corrected with the Bonferroni correction for multiple comparisons.
2.4 Preparation of Amyloid
Solution of oligomeric Aβ1–42 from lyophilized, HPLC-purified Ab1–42 was prepared as described previously (Barghorn et al., 2005). First, 100% 1,1,1,3,3,3 hexafluoro-2-propanol (HFIP) was used to reconstitute Aβ1–42 (1 mM), and then HFIP was removed by evaporation in a Speed Vac (Eppendorf, Hamburg, Germany). The obtained pellet was then resuspended to 5 lM in anhydrous dimethylsulfoxide. This stock was diluted with phosphate-buffered saline (PBS) to a final concentration of 400 lM and sodium dodecyl sulfate was added to a final concentration of 0.2%. The resulting solution was then incubated at 37°C for 18–24 h. The preparation was again incubated at 37°C for 18–24 h after further dilution with PBS to a final concentration of 100 µM. The nature of the Aβ1–42 oligomers of the preparation was then checked by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Akhter et al., 2015).
2.5 Generation of reporters
DNA regions of interest were amplified from genomic DNA that was PCR amplified and inserted into luciferase reporter constructs and validated by sequencing ADAM10. ADAM10 and SOX2 promoter constructs with or without mutations were inserted upstream (5’) of the luciferase gene of the pGL4.10 [luc2] vector (Promega, Madison, WI) to produce promoter-only constructs. To create promoter – 3’ UTR constructs, the ADAM10 and SOX2 3’ UTR were inserted downstream (3’) of the luciferase gene in a pre-poly A cloning site. The primers used for ADAM10 promoter construct were- 5’-gtgcttgagtaggccgaatt-3’ and 5’-ctcgcagtcgtgcctcac-3’, for ADAM10 3’UTR construct were 5’-caaatgggacacatgagacg-3’ and 5’gctgtttcaccttcaatgc-3’. The primers used to create SOX2 3’UTR constructs were 5’gccattaacggcacactgcc-3’ and 5’- tctcaaactgtgcataatgg-3’. The In-Fusion PCR Cloning System (Takara Clontech, Mountain View, CA) was used for all cloning procedures. After propagating recombinant DNA in E. coli host cells, reporter constructs were isolated and purified using an ion exchange column (Qiagen), and then fully sequenced to validate genetic content as previously described (Bekris et al., 2012)
2.6 Cell culture
For the promoter – 3’ UTR construct experiments, three human cell lines were used. Human neuroblastoma SHSY5Y and CHP212 cells (ATCC, Manassas, VA) were grown in 44.5% Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY) with 44.5% F12 (Gibco), 10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin (100 µU/ml penicillin and 100 µg/ml streptomycin). The glioblastoma U118 cells were grown in 89% DMEM, 10% FBS, and 1% penicillin/streptomycin. All cells were grown at 37 °C in a 5% CO2 atmosphere, and passaged at a concentration of 2.5 × 104 per well into 96-well tissue culture plates 48 h before transfection.
2.7 Luciferase reporter construct assays
SHSY5Y, CHP212 and U118, cell lines were transiently transfected for 48 h with Firefly luciferase pGL4.10 [luc2] reporter constructs. A Renilla luciferase pGL4.75 [hRluc/CMV] control construct and Lipofectamine 2000 (Invitrogen) were also loaded into each well according to manufacturer’s instructions (Lynn M. Bekris, Lutz, et al., 2012). Firefly reporter constructs contained either one of the ADAM10 and SOX2 promoter region and 3’ UTR with or without mutations. Along with the reporter constructs, cells were also transfected with miR-140-5p mimics and inhibitors (Ambion) and siRNAs against SOX2 (Sigma). All transfection assays contained duplicate transfections within the same assay, and were performed at least six times. After 48 h, transiently transfected cells were harvested using the Dual-Glo® Luciferase Assay System (Promega) that allows for high throughput analysis of Firefly luciferase pGL4.10 [luc2] constructs in the first step. In the second step, Firefly luminescence was quenched and the Renilla luciferase (pGL4.75[hRluc/CMV]) was activated and analyzed. Luciferase reporter counts per second (CPS) were measured using an LMax II 384 luminometer (Molecular Devices, Sunnyvale, CA) and were presented as a ratio of Firefly/Renilla to represent reporter construct activity relative to the loading control. (Lynn M. Bekris, Lutz, et al., 2012).
2.8 Site Directed Mutagenesis
ADAM10 and SOX2 reporter constructs with mutations in promoter or 3’UTR were generated by incorporating mutations into the miR-140 binding site on ADAM10 3’UTR and SOX2 3’UTR as well as in SOX2 binding site on ADAM10 promoter by PCR-based site directed mutagenesis using Pfu Turbo DNA polymerase (Quickchange II Site Directed Mutagenesis Kit; Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer’s protocol and were verified by sequencing. The primers used for generating mutations-in miR-140 binding site on ADAM10 promoter are: 5'ctgatcattggtaaacgccaaagatgagtaatttgc-3' and SDM ADAM10 3’UTR: 5'-gcaaattactcatctttggcgtttaccaatgatcag-3'. For generating mutations-in miR-140 binding site on SOX2 3’utr are: 5'-gctcctaccgtcctaatagaacttttaaaaagtttttcgtag-3' and 5'-ctttttaaaagttctattaggacggtaggagctttgcag-3' and for generating mutations in SOX2 binding site on ADAM10 promoter are: -5'-gatgtgcgtggattcgcgttaacgatgtgacagagag-3' and 5'ctctctgtcacatcgttaacgcgaatccacgcacatc-3'
2.9 Statistical analysis
Post-mortem brain ADAM10 mRNA, protein levels, and construct luciferase activity were analyzed using SPSS Version 22 (IBM) and GraphPad Prism. Experimental results are reported as mean ± S.E and done at least in triplicates. All p-values were calculated by Student’s T-test and Tukey ANOVA for multiple comparisons. Outlier detection, FDR analysis and Bonferroni correction for multiple comparisons are done for data with human samples.
3. Results
MiRNA-140-5p is upregulated in AD compared to control
Candidate miRNA predicted to target ADAM10 were identified using bioinformatic tools (see methods section). To determine if candidate miRNA (predicted to target ADAM10) are differentially expressed in AD compared to cognitively normal control brain, miRNA levels were evaluated using miRNA arrays. MiRNA levels were measured in two regions of post-mortem brain; cerebellum and hippocampus from AD patients (n=21) and cognitively normal control (n=22) individuals. The miRNA array results suggest that a number of specific miRNAs are differentially present in AD compared to controls, including three ADAM10 candidate miRNA; miR-140-5p, miR-182, miR-194 (Fig. 1A). To validate the findings from the miRNA array results, miRNA qRT-PCR was performed. MiRNA qRT-PCR results (Fig. 1B) showed significant increased expression of miR-140 in AD hippocampus compared to other candidates miRNAs; miR-182 and miR-194 and control miRNA; miR-126 which is observed to be unchanged in AD as in other reports (Bhatnagar et al., 2014, Geekiyanage et al., 2012). Both cerebellum and hippocampus of control and AD are evaluated for the presence of miRNA to determine if a region relatively unaffected in AD might not express specific miRNA compared to a region severely affected in AD.
Validation of miR-140-5p interaction with ADAM10 3’UTR
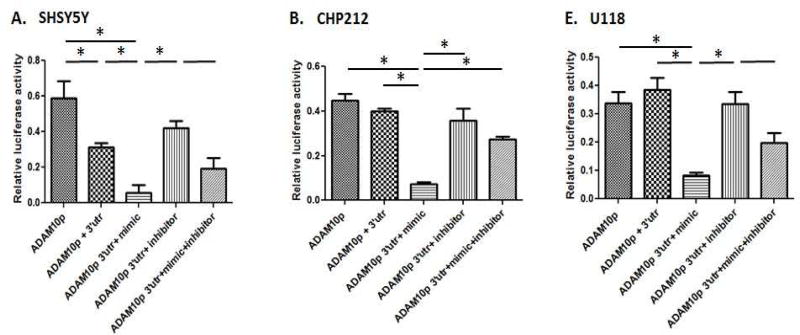
Since only miRNA-140-5p predicted to target ADAM10 was highly significantly elevated in AD compared to controls and was therefore selected for functional analyses. To validate the previous finding reporter assays are employed to provide functional evidence of an effect by miR-1405p on the ADAM10 3’UTR. Since cerebellum and hippocampus consist of a multitude of cell types but late stage hippocampus consists largely of glial cell types with large loss of most neuronal populations, experiments were performed in multiple cell lines including neuronal and astroglial cell types to help tease apart cell type specific miR-140 activity. Three cell lines were selected for reporter analyses: Two neuronal cell lines (SHSY5Y, CHP212) and one glial cell line (U118). All three cell lines express miR-1405p (data not shown). The ADAM10 promoter-3'UTR reporter constructs were transiently transfected with miRNA-1405p mimic and inhibitor and compared to ADAM10 promoter only reporter activity. The ADAM10 promoter-3'UTR luciferase reporter activity is decreased compared with ADAM10 promoter only constructs in SHSY5Y cells, but not CHP212 or U118 cells (Fig. 2A–C). The ADAM10 promoter3'UTR reporter activity decreases further upon treatment with miR-140-5p mimic in all cell lines. The downregulation by miR-140-5p mimic is nullified in the presence of miR-140-5p specific inhibitors. These results provide evidence that ADAM10 3'UTR is directly regulated by the miRNA-140-5p.
Figure 2. MiRNA-140-5p inhibition of ADAM10 promoter + 3’UTR reporter expression.
The influence of miR-140-5p on ADAM10 promoter + 3’UTR reporter expression was evaluated in neuronal SHSY5Y and CHP212, or glial U118 cell lines. ADAM10 promoter (ADAM10p) reporter constructs and ADAM10 promoter+3’ UTR constructs along with miR-140-5p mimics and inhibitors were transiently transfected into human neuronal cell lines CHP212, SHSY5Y and a glial U118 cell line. Results are presented as Firefly/Renilla luciferase activity (Relative luciferase activity). Data represent at least six independent experiments with triplicate measurements. Black line indicates Fisher exact t-test p-value < 0.05. * indicates Tukey multiple comparison correction p-value < 0.05.
ADAM10 promoter and 3’UTR activity upon Aβ treatment
Neurotoxic Aβ oligomers play an important role in the development of AD (Gilbert, 2013, Lesne et al., 2013). Neurons are severely affected by oligomeric Aβ and undergo death after exposure to oligomeric Aβ in vitro (Akhter et al., 2014, Akhter et al., 2015, Barghorn et al., 2005, Biswas et al., 2007). Therefore, it is relevant to see the effect of AD associated principal neurotoxic factor Aβ on ADAM10 promoter and 3’UTR activity. ADAM10 promoter and 3’UTR luciferase reporter constructs (Bekris et al., 2012) were transiently transfected into neuronal SHSY5Y cells, 48 h post transfection cells were treated with Aβ for 24 h. A concentration of 5 µM Aβ is used since this has been reported as an optimum level that does not induce severe toxicity or massive cell death (Akhter et al., 2015, Cheng et al., 2013). This result reveals significant downregulation of both promoter and 3’UTR activities of ADAM10 under Aβ treatment in neuronal SHSY5Y cells (Fig. 3A). This result suggests that the trans-acting factors that act upon the promoter or 3’UTR of ADAM10 may be dysregulated under Aβ treatment. Therefore, to determine if miR-140-5p might be altered under AD conditions of increased Aβ, neuronal SHSY5Y cells were treated with or without oligomeric Aβ and the level of miR-140-5p was measured by qRT-PCR (Fig. 3B). The Aβ treated cells have significantly higher miR-140-5p levels compared to the untreated control. The level of miR-140 5p under Aβ treatment is coherent with mir-140 mimic treated condition while upon co-transfection with miR-140-5p inhibitor, a decrease in miR-140-5p is observed in both miR-140 mimic and Aβ treated conditions (Fig. 3B). This result proves that miR-140-5p is upregulated under Aβ conditions and this in vitro finding is consistent with enhanced miR-140 level in AD brain as shown in Figure 1.
Figure 3. Effect of Aβ on ADAM10 and miR-140-5p.
(A) Neuronal SHSY5Y cells were transfected with ADAM10 promoter (ADAM10p) and ADAM10 promoter-3’UTR (ADAM10p+3’utr) reporter constructs, 48 h post-transfection is followed by treatment with Aβ (5 µM) for 24 hrs. Results are presented as Firefly/Renilla luciferase activity of the respective constructs with or without Aβ as control. Data represent at least six independent experiments with triplicate measurements. (B) Neuronal SHSY5Y cells with miR-140-5p expression either without or with Aβ (5 µM) treatment and transfected with miRNA-140-5p inhibitor. MiRNA-140-5p expression was measured by qRT-PCR and represented as fold change compared with untreated control. Results are represented by 2^-ΔΔCT. Black line indicates Fisher exact t-test p-value < 0.05, * Indicates Tukey multiple comparison correction p value < 0.05.
Aβ inhibits SOX2 and ADAM10 protein level
Since the ADAM10 promoter only reporter constructs as well as 3’UTR are influenced by Aβ treatment (Fig. 3A), next we addressed the question of whether miRNA regulation, specifically upregulated miR-1405p, might also influence ADAM10 promoter specific transcription factors. TFsiteScan was used as a computational tool to examine the ADAM10 promoter sequence for putative transcription factor binding sites. Transcription factor candidates were then selected based on whether miR140-5p was predicted to target the gene as well as previous evidence of transcription factor activity in neurodegenerative disorders. Among prospective ADAM10 transcription factors, we selected embryonic stem cell self-renewal regulator (SRY Sex-determining region Y)-related high mobility group (HMG) Box 2 (SOX2) for further investigation since: SOX2 expression is significantly altered in neurodegenerative conditions (Ferri et al., 2004, Sarlak et al., 2016) and the 3′-UTR of SOX2 contains a predicted 7-mer match to the miR-140-5p seed region (Table 2). The underlying molecular mechanism of ADAM10 regulation by SOX2 remains unexplored.
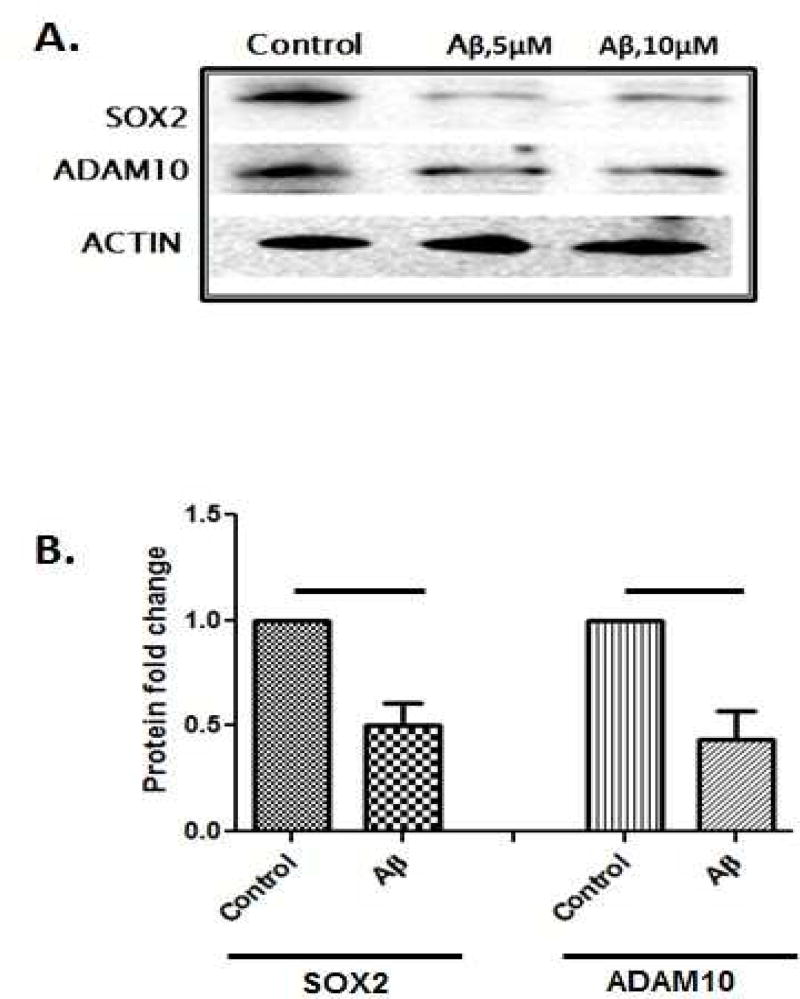
Therefore, first we evaluated SOX2 as a regulator of ADAM10 under Aβ conditions. Neuronal SHSY5Y cells were treated with neurotoxic oligomeric Aβ at a concentration of 5 µM and 10 µM for 8 h. Both SOX2 and ADAM10 protein levels were decreased upon Aβ treatment (8 h) (Fig. 4 A,B).
Figure 4. Aβ treatment reduces SOX2 and ADAM10 protein levels.
(A) Decreased expression of Sox2 and ADAM10 protein upon Aβ treatment (5 µM) for 8 h compared to untreated control in human neuroblastoma SHSY5Y cell line. (B) Fold change relative to untreated control of SOX2 and ADAM10 protein level after Aβ treatment. Data represent mean ± S.E. (error bars) of at least three experiments. Black line indicates Fisher exact t-test p-value < 0.05
SOX2 regulates ADAM10
To further analyze the regulation ADAM10 by SOX2, SHSY5Y cells were transiently transfected with SOX2 overexpressing plasmids as well as SOX2 or scrambled siRNA and analyzed for ADAM10 reporter activity, 48 h post transfection. ADAM10 promoter driven luciferase reporter activity is significantly elevated upon SOX2 overexpression (SOX2 O/E) compared to ADAM10 promoter only transfection (Fig. 5A). In contrast, this enhanced ADAM10 promoter reporter activity, in response to SOX2 overexpression, is diminished due to SOX2 knockdown when cells were co-transfected with SOX2 siRNA, but not scrambled siRNA (Fig 5A).
Figure 5. SOX2 over expression induces ADAM10 promoter activity and protein expression.

(A) ADAM10 promoter activity upon SOX2 overexpression with or without SOX2 siRNA for ADAM10 promoter (ADAM10p) only reporter constructs compared to control in the human neuroblastoma SHSY5Y cell line. (B) ADAM10 protein level increases with SOX2 overexpression in human SHSY5Y cells which is inhibited in presence of specific SOX2 siRNA. (C) SOX2 and ADAM10 protein expression change upon SOX2 overexpression and knockdown by SOX2 siRNA. Data represent mean ± S.E. (error bars) of five experiments. Black line indicates p-value < 0.05 by Fisher exact t-test, * indicates Tukey multiple comparison correction p-value < 0.05.
Furthermore, when SOX2 is overexpressed in SHSY5Y cells there is an increase in endogenous ADAM10 protein level which was analyzed by western blotting of protein extracted 48 h post transfection with SOX2 plasmid (SOX2 O/E) and depleted upon SOX2 knockdown (siRNA) (Fig. 5B,C). SOX2 is overexpressed to determine whether ADAM10 protein level is changed in comparison to control cells without SOX2 overexpression as well as direct reduction of SOX2 by siRNA and therefore confirm that more SOX2 will lead to enhanced ADAM10 transcription and consequent increased expression of ADAM10 protein.
MicroRNA-140-5p targets SOX2 mRNA through a targeting sequence located at 3’-UTR
Next focus was on elucidating the functional relationship between the trans-acting factors of ADAM10 regulation; SOX2 and miR-140-5p. To study the interplay of SOX2 and miR-140 under different conditions neuronal SHSY5Y cells were transfected with SOX2 promoter reporter constructs or SOX2 promoter-3’UTR reporter constructs as well as miR-140 mimic or inhibitors (Fig. 6). The SOX2 promoter-3’UTR luciferase reporter activity was decreased compared to the SOX2 promoter only reporter activity and further decreased in the presence of miR-140-5p mimic (Fig. 6). Transfection with miR-140-5p inhibitor restored the activity to a significant extent while both mimic and inhibitor treatment is modestly changed as expected. This finding is strong evidence that miRNA-140-5p binds with SOX2-3’UTR as predicted and regulates its expression (Fig. 6). These results suggest that miRNA-140-5p has strong seed-site pairing with both ADAM10 3’UTR and the 3’UTR of its transcription factor SOX2.
Figure 6. MicroRNA-140-5p regulates SOX2 mRNA through a targeting sequence located at the SOX2 3’-UTR.
SOX2 promoter (SOX2 p) and SOX2 promoter + 3’ UTR reporter activity is influenced by miR-140-5p mimic and inhibitors upon transient transfection into the human neuronal cell line SHSY5Y. Results are presented as Firefly/Renilla luciferase activity (relative quantification). Data represent at least six independent experiments with triplicate measurements. black line indicates p < 0.05, * Indicates Tukey multiple comparison correction p-value < 0.05
Mutation in miR-140 binding site in ADAM10 3’UTR inhibits miR-140 functioning via 3’UTR
To further demonstrate miR-140-5p seed-site pairing between the 3'UTRs of both ADAM10 and SOX2, site-directed mutagenesis was used to introduce mutations in the consensus miRNA140-5p binding site of both ADAM10 3’UTR and SOX2 3’UTR reporter constructs (Fig. 7 A,B). When neuronal SHSY5Y cells were transfected with ADAM10 promoter reporter constructs together with a SOX2 overexpression vector the ADAM10 promoter driven luciferase activity increased compared to ADAM10 promoter only constructs whereas presence of SOX2 siRNA decreased the ADAM10 reporter activity (Fig. 7C). The mutations in the SOX2 binding site on ADAM10 promoter abolished SOX2 regulated ADAM10 induction evidenced by a decrease in ADAM10 promoter mutant reporter activity. Presence of SOX2 siRNA did not result in decreased ADAM10 promoter reporter activity upon mutation of the SOX2 binding site when compared to the non-mutated construct. This suggests that SOX2 is necessary for activation of the ADAM10 promoter (Fig. 7C). Next, to validate the miR-140-5p induced regulation of ADAM10 and its transcription factor SOX2, we created mutations in miRNA-140-5p seed sequence in both ADAM10-3’UTR and SOX2-3’UTR and transfected into neuronal SHSY5Y cells. As expected ADAM10 reporter activity was decreased in presence of miR-140-5p mimic and restored upon co-transfection of miR-140-5p inhibitors (Fig. 7D). The mutations in miR-140-5p binding sites on ADAM10 3’UTR inhibits miR-140-5p binding to the 3’UTR and thus cannot function in regulating the 3’UTR evidenced by unaltered ADAM10 reporter activity in presence of miR-140-5p mimic and inhibitors or both (Fig. 7D). Similarly, SOX2 promoter driven reporter activity is decreased in presence of miR-140-5p mimic whereas co-transfection of miR-140-5p inhibitors restores the downregulation by mimic significantly (Fig. 7E). The mutations in miR-140-5p binding sites on the SOX2 3’UTR inhibits miR-140-5p binding to 3’UTR. Thus, changes in SOX2 reporter activity remained unaltered in presence of miR-140-5p mimic and inhibitors or both. Taken together, these results suggest that the mutated miR-140-5p binding site lead to unaffected ADAM103’UTR and SOX2-3’UTR reporter activities in presence of miR-140-5p mimic and inhibitors (Fig. 7D,E).
Figure 7. Mutation of the miR-140-5p binding site reduces miR-140-5p inhibition of promoter + 3’UTR reporter activity.
(A,B) Schematic representation of Luciferase reporter constructs those were transiently transfected into human neuronal SHSY5Y- human ADAM10 Promoter constructs with SOX2 binding site mutation, 3’ UTR reporter constructs of ADAM10 and SOX2 with miR-140 binding site mutation, miR-140 mimic and. (C,D,E) Human neuronal SHSY5Y cells were transiently transfected with the specific reporter constructs along with SOX2 overexpression (OE) and siRNA or miR-140 mimic and inhibitors as depicted. Luciferase reporter assay are presented as Firefly/Renilla luciferase activity of the respective constructs (relative quantification). Data represent at least six independent experiments with triplicate measurements. Black line indicates Fisher exact t-test p-value < 0.05, * indicates Tukey multiple comparison correction p-value < 0.05
4. Discussion
The accumulation of Aβ as the result of altered metabolism and clearance is central to AD pathogenesis (Hardy and Selkoe, 2002, Huang and Mucke, 2012). Decreased levels of ADAM10 in AD are associated with enhanced accumulation of Aβ, loss of neuroprotective sAPPα and subsequent neurodegeneration (Colciaghi et al., 2002, Zhang et al., 2011). Little is known about the underlying mechanisms involved in decreased ADAM10 in AD patients. Two types of gene regulatory factors; transcription factors and miRNA can greatly influence gene expression. Transcription factors regulate transcription either positively or negatively whereas miRNAs can regulate gene expression mostly through repression. Thus, transcription factors and miRNAs are crucial candidates for understanding disrupted gene regulation in AD.
The results of this study suggest that miRNA-140-5p acts as an important regulator of ADAM10. Both cerebellum and hippocampus were evaluated for the presence of miRNA. However, given that end stage AD hippocampus has lost much of the neuronal population and consists largely of glial cells (Serrano-Pozo et al., 2013) it is still unknown which cell types or if early stage AD (i.e. MCI) hippocampus expresses high miR-140 levels compared to cognitively controls. Therefore, these results should be approached with careful consideration of disease stage. In this study AD brain samples as well as Aβ treated neuronal SHSY5Y show elevated expression of miR-140 (Fig. 1, Fig. 3b). Overexpression of this miRNA in SHSY5Y leads to ADAM10 downregulation (Fig. 2a). Others have also described miRNA-140-5p as a regulator of ADAM10 in cancer cells (Jing et al., 2016, Kai et al., 2014). Interestingly, miR-140-5p is upregulated in blood from patients with acute ischemic stroke suggesting it may be upregulated in response to neurological injury (Sorensen et al., 2014). Furthermore, miR-140-5p expression in hippocampal CA1 neurons from prion infected mice significantly correlates with genes involved in neuronal projection and dendrite development implicating a critical role by miR-140-5p in neuronal processes (Majer et al., 2012).
Another interesting finding of this study is the role of miR-140-5p in suppressing ADAM10 expression via an important interaction with ADAM10-3’UTR and with its transcription factor SOX2. Thus, the elevated levels of miR-140-5p in AD patients may also contribute to lower ADAM10 protein levels via SOX2. In support of these results a recent study reports that SOX2 is a positive regulator of ADAM10 transcription (Sarlak et al., 2016). Another study, reports that SOX2 co-localizes with the α-secretase ADAM10 in neural stem cells of the subventricular zone. (Demars et al., 2010). SOX2 deficiency not only inhibits neurogenesis but also causes neurodegeneration in the adult mouse brain (Ferri et al., 2004). SOX2 level is significantly decreased in brain of AD patients as well as in an AD transgenic mouse model (Crews et al., 2010). Taken together, this study and reports by others suggest that a decrease in SOX2 may play a critical role in AD neurodegeneration.
In summary, miR-140-5p represses expression of ADAM10 and SOX2 in vitro and miR-140 is elevated in AD brain implicating miR-140-5p in the pathogenesis of AD. The presence of a miR140-5p binding site in both the ADAM10 and the SOX2 3’UTR suggests a high regulatory impact by miRNA-140-5p in ADAM10 regulation. From these findings it can also be hypothesized that decrease in ADAM10 level by miR-140-5p may lead to reduced neuroprotective sAPP-α, a product of ADAM10 cleavage of full length APP. Decline in the level of sAPP-α expedites AD pathogenesis (Van Nostrand et al., 1992)
This study may provide a unique approach for AD therapeutics that includes the pharmacological inhibition of miR-140-5p in vivo to increase SOX2 and ADAM10 level. This approach may enhance the level of soluble APP-α and reduce Aβ levels. Our findings highlight combinatorial regulation of transcription factor and microRNA in the targeted regulation of disease associated gene like ADAM10 and provide a strong basis for future research aimed at annotating transcription factors and miRNAs as potential diagnostic and prognostic biomarkers as well as therapeutic targets of AD.
Figure 8.
Schematic representation of miR-140-5p regulation of ADAM10
Table 1.
Population Description
| Demographic information |
Brain mRNA and protein | |
|---|---|---|
| Controls | AD | |
|
| ||
| n | 22 | 21 |
| % Female | 50 | 55 |
| % APOE ε4 | 18 | 68 |
| Mean age (standard deviation) | 87 (5) | 82 (7) |
| Plaque score | Absent–moderate | Sparse–frequent |
| Braak stage | I–IV | IV–VI |
-
Highlights
MiR-140-5p is upregulated in human AD hippocampus compared to control.
-
➢
Both ADAM10 and SOX2 reporter construct activity is influenced by miR-140-5p.
-
➢
Aβ induces miR-140-5p expression and reduces ADAM10 and SOX2 reporter and protein levels.
-
➢
Mutagenesis of ADAM10 and SOX2 miR-140-5p seed sequence abolishes reporter inhibition.
-
➢
SOX2 and miR-140-5p may regulate ADAM10 in the microenvironment of the AD brain.
Acknowledgments
This work was supported by grants from the National Institutes of Health (K99/R00 AG034214 and P50 AG05136) and the Jane and Lee Seidman Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declare that they have no actual or potential conflicts of interest related to the publishing of this work.
References
- Akhter R, Sanphui P, Biswas SC. The essential role of p53-up-regulated modulator of apoptosis (Puma) and its regulation by FoxO3a transcription factor in beta-amyloid-induced neuron death. The Journal of biological chemistry. 2014;289(15):10812–22. doi: 10.1074/jbc.M113.519355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter R, Sanphui P, Das H, Saha P, Biswas SC. The regulation of p53 up-regulated modulator of apoptosis by JNK/c-Jun pathway in beta-amyloid-induced neuron death. Journal of neurochemistry. 2015;134(6):1091–103. doi: 10.1111/jnc.13128. [DOI] [PubMed] [Google Scholar]
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, et al. Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer's disease. Journal of neurochemistry. 2005;95(3):834–47. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):28197. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bekris LM, Lutz F, Li G, Galasko DR, Farlow MR, Quinn JF, et al. ADAM10 expression and promoter haplotype in Alzheimer's disease. Neurobiology of aging. 2012;33(9):2229, e1–e9. doi: 10.1016/j.neurobiolaging.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KF, Zheng L, Fahrenholz F, Cuello AC. ADAM-10 over-expression increases cortical synaptogenesis. Neurobiology of aging. 2008;29(4):554–65. doi: 10.1016/j.neurobiolaging.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome biology. 2010;11(8):R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic acids research. 2008;36(Database issue):D149–53. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Chertkow H, Schipper HM, Yuan Z, Shetty V, Jenkins S, et al. Increased micro RNA- 34c abundance in Alzheimer's disease circulating blood plasma. Frontiers in molecular neuroscience. 2014;7:2. doi: 10.3389/fnmol.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SC, Shi Y, Vonsattel JP, Leung CL, Troy CM, Greene LA. Bim is elevated in Alzheimer's disease neurons and is required for beta-amyloid-induced neuronal apoptosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(4):893–900. doi: 10.1523/JNEUROSCI.3524-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Jan CI, Peng CY, Lai YC, Hu FW, Yu CC. Activation of microRNA-494-targeting Bmi1 and ADAM10 by silibinin ablates cancer stemness and predicts favourable prognostic value in head and neck squamous cell carcinomas. Oncotarget. 2015;6(27):24002–16. doi: 10.18632/oncotarget.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Li W, Zhang Z, Yoshimura S, Hao Q, Zhang C, et al. MicroRNA-144 is regulated by activator protein-1 (AP-1) and decreases expression of Alzheimer disease-related a disintegrin and metalloprotease 10 (ADAM10) The Journal of biological chemistry. 2013;288(19):13748–61. doi: 10.1074/jbc.M112.381392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colciaghi F, Borroni B, Pastorino L, Marcello E, Zimmermann M, Cattabeni F, et al. [alpha]Secretase ADAM10 as well as [alpha]APPs is reduced in platelets and CSF of Alzheimer disease patients. Molecular medicine. 2002;8(2):67–74. [PMC free article] [PubMed] [Google Scholar]
- Crawford HC, Dempsey PJ, Brown G, Adam L, Moss ML. ADAM10 as a therapeutic target for cancer and inflammation. Current pharmaceutical design. 2009;15(20):2288–99. doi: 10.2174/138161209788682442. [DOI] [PubMed] [Google Scholar]
- Crews L, Adame A, Patrick C, Delaney A, Pham E, Rockenstein E, et al. Increased BMP6 levels in the brains of Alzheimer's disease patients and APP transgenic mice are accompanied by impaired neurogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(37):12252–62. doi: 10.1523/JNEUROSCI.1305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. Journal of neuroscience research. 2010;88(10):2103–17. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doberstein K, Pfeilschifter J, Gutwein P. The transcription factor PAX2 regulates ADAM10 expression in renal cell carcinoma. Carcinogenesis. 2011;32(11):1713–23. doi: 10.1093/carcin/bgr195. [DOI] [PubMed] [Google Scholar]
- Fahrenholz F, Gilbert S, Kojro E, Lammich S, Postina R. Alpha-secretase activity of the disintegrin metalloprotease ADAM 10. Influences of domain structure. Annals of the New York Academy of Sciences. 2000;920:215–22. doi: 10.1111/j.1749-6632.2000.tb06925.x. [DOI] [PubMed] [Google Scholar]
- Fazi F, Nervi C. MicroRNA: basic mechanisms and transcriptional regulatory networks for cell fate determination. Cardiovascular research. 2008;79(4):553–61. doi: 10.1093/cvr/cvn151. [DOI] [PubMed] [Google Scholar]
- Femminella GD, Ferrara N, Rengo G. The emerging role of microRNAs in Alzheimer's disease. Frontiers in physiology. 2015;6:40. doi: 10.3389/fphys.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131(15):3805–19. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Mattson MP. Secreted amyloid precursor protein alpha selectively suppresses Nmethyl-D-aspartate currents in hippocampal neurons: involvement of cyclic GMP. Neuroscience. 1998;83(2):429–38. doi: 10.1016/s0306-4522(97)00398-9. [DOI] [PubMed] [Google Scholar]
- Geekiyanage H, Jicha GA, Nelson PT, Chan C. Blood serum miRNA: non-invasive biomarkers for Alzheimer's disease. Experimental neurology. 2012;235(2):491–6. doi: 10.1016/j.expneurol.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert BJ. The role of amyloid beta in the pathogenesis of Alzheimer's disease. Journal of clinical pathology. 2013;66(5):362–6. doi: 10.1136/jclinpath-2013-201515. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alphasecretase activity in fibroblasts. Human molecular genetics. 2002;11(21):2615–24. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(17):6415–20. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):120422. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing P, Sa N, Liu X, Liu X, Xu W. MicroR-140-5p suppresses tumor cell migration and invasion by targeting ADAM10-mediated Notch1 signaling pathway in hypopharyngeal squamous cell carcinoma. Experimental and molecular pathology. 2016;100(1):132–8. doi: 10.1016/j.yexmp.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Johnson R, Zuccato C, Belyaev ND, Guest DJ, Cattaneo E, Buckley NJ. A microRNA-based gene dysregulation pathway in Huntington's disease. Neurobiology of disease. 2008;29(3):438–45. doi: 10.1016/j.nbd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Jorissen E, Prox J, Bernreuther C, Weber S, Schwanbeck R, Serneels L, et al. The disintegrin/metalloproteinase ADAM10 is essential for the establishment of the brain cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(14):4833–44. doi: 10.1523/JNEUROSCI.5221-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai Y, Peng W, Ling W, Jiebing H, Zhuan B. Reciprocal effects between microRNA-140-5p and ADAM10 suppress migration and invasion of human tongue cancer cells. Biochemical and biophysical research communications. 2014;448(3):308–14. doi: 10.1016/j.bbrc.2014.02.032. [DOI] [PubMed] [Google Scholar]
- Kumar P, Dezso Z, MacKenzie C, Oestreicher J, Agoulnik S, Byrne M, et al. Circulating miRNA biomarkers for Alzheimer's disease. PloS one. 2013;8(7):e69807. doi: 10.1371/journal.pone.0069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Schramme A, Doberstein K, Dummer R, Abdel-Bakky MS, Keller S, et al. ADAM10 is upregulated in melanoma metastasis compared with primary melanoma. The Journal of investigative dermatology. 2010;130(3):763–73. doi: 10.1038/jid.2009.335. [DOI] [PubMed] [Google Scholar]
- Lesne SE, Sherman MA, Grant M, Kuskowski M, Schneider JA, Bennett DA, et al. Brain amyloid-beta oligomers in ageing and Alzheimer's disease. Brain : a journal of neurology. 2013;136(Pt 5):1383–98. doi: 10.1093/brain/awt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Liu C, Zhu J, Shu P, Yin B, Gong Y, et al. MicroRNA-16 targets amyloid precursor protein to potentially modulate Alzheimer's-associated pathogenesis in SAMP8 mice. Neurobiology of aging. 2012;33(3):522–34. doi: 10.1016/j.neurobiolaging.2010.04.034. [DOI] [PubMed] [Google Scholar]
- Majer A, Medina SJ, Niu Y, Abrenica B, Manguiat KJ, Frost KL, et al. Early mechanisms of pathobiology are revealed by transcriptional temporal dynamics in hippocampal CA1 neurons of prion infected mice. PLoS pathogens. 2012;8(11):e1003002. doi: 10.1371/journal.ppat.1003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki S, Okada Y. ADAMs in cancer cell proliferation and progression. Cancer science. 2007;98(5):621–8. doi: 10.1111/j.1349-7006.2007.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouradian MM. MicroRNAs in Parkinson's disease. Neurobiology of disease. 2012;46(2):27984. doi: 10.1016/j.nbd.2011.12.046. [DOI] [PubMed] [Google Scholar]
- Nilsen TW. Mechanisms of microRNA-mediated gene regulation in animal cells. Trends in genetics : TIG. 2007;23(5):243–9. doi: 10.1016/j.tig.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Pan D, Rubin GM. Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell. 1997;90(2):271–80. doi: 10.1016/s0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- Patel N, Hoang D, Miller N, Ansaloni S, Huang Q, Rogers JT, et al. MicroRNAs can regulate human APP levels. Molecular neurodegeneration. 2008;3:10. doi: 10.1186/1750-1326-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postina R. A closer look at alpha-secretase. Current Alzheimer research. 2008;5(2):179–86. doi: 10.2174/156720508783954668. [DOI] [PubMed] [Google Scholar]
- Postina R. Activation of alpha-secretase cleavage. Journal of neurochemistry. 2012;120(Suppl 1):46–54. doi: 10.1111/j.1471-4159.2011.07459.x. [DOI] [PubMed] [Google Scholar]
- Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, et al. A disintegrin metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. The Journal of clinical investigation. 2004;113(10):1456–64. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinzen C, Muller U, Endres K, Fahrenholz F, Postina R. Genomic structure and functional characterization of the human ADAM10 promoter. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19(11):1522–4. doi: 10.1096/fj.04-3619fje. [DOI] [PubMed] [Google Scholar]
- Pruessmeyer J, Hess FM, Alert H, Groth E, Pasqualon T, Schwarz N, et al. Leukocytes require ADAM10 but not ADAM17 for their migration and inflammatory recruitment into the alveolar space. Blood. 2014;123(26):4077–88. doi: 10.1182/blood-2013-09-511543. [DOI] [PubMed] [Google Scholar]
- Sarlak G, Htoo HH, Hernandez JF, Iizasa H, Checler F, Konietzko U, et al. Sox2 functionally interacts with betaAPP, the betaAPP intracellular domain and ADAM10 at a transcriptional level in human cells. Neuroscience. 2016;312:153–64. doi: 10.1016/j.neuroscience.2015.11.022. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends in cell biology. 1998;8(11):447–53. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Gomez-Isla T, Growdon JH, Frosch MP, Hyman BT. A phenotypic change but not proliferation underlies glial responses in Alzheimer disease. The American journal of pathology. 2013;182(6):2332–44. doi: 10.1016/j.ajpath.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen SS, Nygaard AB, Nielsen MY, Jensen K, Christensen T. miRNA expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Translational stroke research. 2014;5(6):711–8. doi: 10.1007/s12975-014-0364-8. [DOI] [PubMed] [Google Scholar]
- Suh J, Choi SH, Romano DM, Gannon MA, Lesinski AN, Kim DY, et al. ADAM10 missense mutations potentiate beta-amyloid accumulation by impairing prodomain chaperone function. Neuron. 2013;80(2):385–401. doi: 10.1016/j.neuron.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2012;2(10) doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nostrand WE, Wagner SL, Shankle WR, Farrow JS, Dick M, Rozemuller JM, et al. Decreased levels of soluble amyloid beta-protein precursor in cerebrospinal fluid of live Alzheimer disease patients. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(7):2551–5. doi: 10.1073/pnas.89.7.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonte C, Apolloni S, Parisi C. MicroRNAs: newcomers into the ALS picture. CNS & neurological disorders drug targets. 2015;14(2):194–207. doi: 10.2174/1871527314666150116125506. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Thompson R, Zhang H, Xu H. APP processing in Alzheimer's disease. Molecular brain. 2011;4:3. doi: 10.1186/1756-6606-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]