Abstract
Shared decision-making is playing an increasingly large role in emergency cardiovascular care. Although there are many challenges to successfully performing shared decision-making in the emergency department, there are numerous clinical scenarios where it should be used. In this paper, we explore new research and emerging decision aids in the following emergency care scenarios: 1) low-risk chest pain, 2) new-onset atrial fibrillation, and 3) moderate-risk syncope. These decision aids are designed to engage patients and facilitate shared decision-making for specific treatment and disposition (admit versus discharge) decisions. We then offer a 3-step, practical approach to performing shared decision-making in the acute care setting, based on broad stakeholder input and prior conceptual work. Step 1 involves simply acknowledging that a clinical decision needs to be made. Step 2 involves a shared discussion about the working diagnosis and the options for care in the context of the patient’s values, preferences, and circumstances. The third and final step requires the patient and provider to agree on a plan of action regarding further medical care. The implementation of shared decision-making in emergency cardiology has the potential to shift the paradigm of clinical practice from paternalism towards mutualism and improve the quality and experience of care for our patients.
Keywords: Shared Decision-making, Emergency Cardiology
Introduction
Shared decision making (SDM) is an essential component of patient engagement. It has been defined as a joint deliberation whereby patients and clinicians consider the risks and potential benefits of various medical options to come to a mutual agreement on how to proceed.1, 2 It involves a bidirectional exchange of information with the clinician sharing research evidence and clinical expertise, and the patient sharing his/her values, preferences, and past experience. While there are many potential benefits to SDM, the fundamental goal is to improve the quality of care by promoting patient-centeredness.3 The 2001 Institute of Medicine report on quality in healthcare defined patient-centered care as “care that is respectful of and responsive to the individual patient preferences, needs, and values”.4 In emergency cardiovascular care, the increasing numbers of diagnostic and therapeutic options call for the use of SDM. Meeting this responsibility can be difficult in the emergency care setting: time constraints limiting meaningful conversations, lack of reliable evidence resulting in uncertainty between benefits and harms, variable patient decision-making ability, lack of training and tools to support SDM, all challenge the implementation of this approach in practice.5 Decision aids are “evidence-based tools designed to help patients make specific and deliberated choices among healthcare options”.6 They serve to support conversation that explores how various options make practical, intellectual, and emotional sense to patients and can help facilitate the implementation of SDM in clinical practice.7, 8
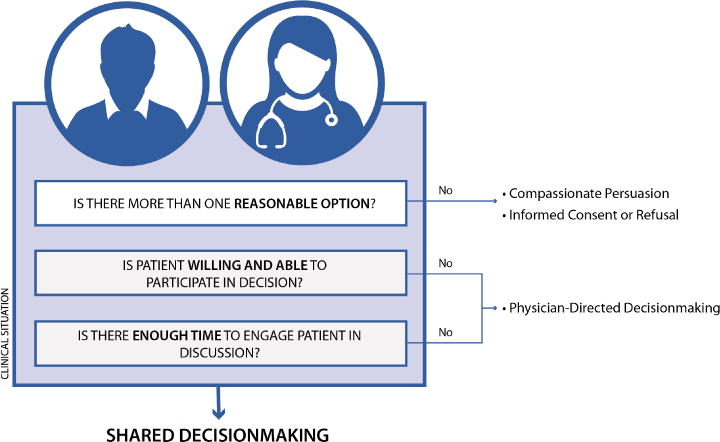
Determining whether SDM is appropriate for a particular emergency care decision depends on three factors: 1) clinical equipoise, 2) patient decision-making ability, and 3) time (see Figure 1).9 If any of these criteria are not met, then other approaches should be used such as physician-directed decision-making, compassionate persuasion, or the more standard, provision of informed consent. There are many clinical scenarios in emergency cardiology that met these three criteria. In this paper, we focus our discussion on the following three decisions: i) the disposition decision (admit versus discharge) after a negative diagnostic evaluation for low-risk chest pain, ii) choice of anticoagulation for patients with new-onset atrial fibrillation (AF), and iii) the disposition decision (admit versus discharge) after a negative diagnostic evaluation for isolated syncope. For each scenario, we discuss the recent research in these areas and SDM tools that are emerging. Finally, we propose a practical three-step approach on how to perform SDM in the emergency department (ED) (see figure 2).
Figure 1.
When is Shared Decision-Making appropriate in the Emergency Department?
Figure 2.
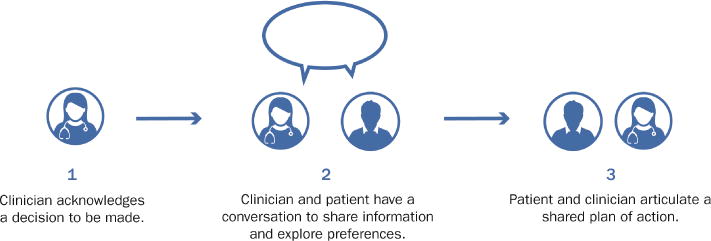
A Three-Step Practical Approach to Shared Decision-Making
Shared decision-making for low-risk chest pain
Chest pain is the one of the most common reason patients seek emergency care in North America, accounting for over 6 million ED visits in the United States (US) and over 500,000 in Canada each year.10, 11 Among these US visits, only 6–8% of patients experience a cardiac event within 30 days.12, 13 Despite the relatively low frequency of acute coronary syndrome (ACS), chest pain accounts for over 25% of US hospital admissions, 85% of which are not ultimately diagnosed with ACS.14
Current clinical evaluation, electrocardiogram (ECG), and cardiac troponin testing available in the US miss approximately 1.5% of patients with ACS, resulting in adverse medical consequences for the patient and medicolegal risk for the clinician.15 Aware of these risks, clinicians frequently admit patients at low risk for ACS for further observation and testing, leading to discordance between the magnitude of disease risk and the intensity of evaluation, unnecessary downstream procedures, and substantial healthcare expenditures.
To safely improve the value of emergency care for patients presenting with potential ACS, several investigators have developed risk stratification tools to guide clinical decision-making.16, 17 Unlike risk scores used to prognosticate outcomes among hospitalized patients with diagnosed acute myocardial infarction,18–20 these risk tools were developed and validated for use in ED patients with chest pain possibly due to ACS after other potential life-threatening causes of chest pain such as pulmonary embolism, Boerhaave’s syndrome, and aortic dissection have been ruled out. Some of these risk scores have been tested and implemented in practice and have shown to safely decrease the rate of unnecessary testing.21, 22 However, to date, none of these have explicitly educated patients regarding their short-term risk for ACS and engaged them in the decision of whether to pursue additional observation and cardiac testing during the index visit or to follow-up as an outpatient for further evaluation.
In order to both tailor the rate of testing to disease risk and to engage patients in their own healthcare decisions, we designed a decision aid, Chest Pain Choice.23 The decision aid was designed to facilitate SDM in patients with a negative initial cardiac evaluation (no life threatening non-ACS causes of chest pain identified, no ischemic changes on ECG, negative initial cardiac troponin) who were being considered for observation unit admission for further cardiac testing or referral for urgent outpatient evaluation (Appendix A). The first section describes the rationale for and results of initial cardiac testing and seeks to reassure the patient that there is currently no evidence of acute myocardial infarction. The second section describes the potential need for further cardiac testing such as stress testing or coronary CT angiography to refine prognosis, and the third section presents the patient’s 45-day risk for ACS (generated from the quantitative pretest probability instrument derived and validated by Kline and colleagues)24, 25 using prose, natural frequencies with a common denominator, and a pictogram. Finally, the fourth section lists the available management options based on these considerations, i.e. observation admission versus two forms of outpatient follow-up.
We tested the efficacy of the decision aid on decision quality (patient knowledge, decisional conflict, engagement, satisfaction) and resource use in a single center pilot trial (n=201) and found that use of the decision aid increased patient knowledge and engagement and decreased the rate of observation unit admission for cardiac testing without an increase in adverse events.26 Based on these pilot data, we subsequently tested the effectiveness of the decision aid in a larger population of patients with greater socioeconomic and geographic diversity.27 In this trial of 898 patients (451 decision aid arm, 447 usual care arm), we observed a similar magnitude and direction of effect of the decision aid. Based on these data supporting the effectiveness of the decision aid, we are currently planning an implementation study that seeks to routinize SDM in the context of a HEART score pathway21 for emergency chest pain evaluation and measure its impact on patients’ experience of care, safety, and healthcare utilization. To our knowledge, there are currently no other tools designed to facilitate SDM for ED chest pain patients.
Shared decision-making for anticoagulation for atrial fibrillation
Atrial fibrillation (AF) is a common reason for ED visits,28, 29 accounting for over 750,000 visits in the US annually.30 Recent studies using data from the Canadian provinces of Ontario and Alberta indicate that roughly 0.5% of all ED visits are related to atrial fibrillation/flutter.31, 32 The ED is often the point of a patient’s first diagnosis and provides an ideal opportunity to initiate guideline-indicated treatment. Many patients, however, leave the ED without appropriate anticoagulation,33 and, within this frenzied clinical environment, patient education can be challenging.34 Nonetheless, dedicated programs that engage ED providers in patient education at the time of discharge have been demonstrated to decrease AF-related complications at one year of follow-up.35 These studies underscore the need for systematic care for such patients.
The most recent American College of Cardiology/American Heart Association/Heart Rhythm Society (AHA/ACC/HRS) guidelines36 recommend that “in patients with AF, antithrombotic therapy should be individualized based on shared decision making after discussion of the absolute and relative risks of stroke and bleeding and the patient’s values and preferences.” A challenge, however, is that many emergency providers may not be comfortable in guiding such SDM discussions because they may not have readily available stroke and bleeding risk estimates or may be not be comfortable initiating a potentially lifelong medication with significant risks. Indeed, such decisions are typically made by clinicians who have a longitudinal relationship with patients.
Structured tools, or decision aids, can be valuable in supporting these conversations. Many investigators have developed such tools that are currently being tested in various clinical practice settings (See table).37–40 For instance, the computerized antithrombotic risk assessment tool (CARAT), an online decision-support algorithm that aggregates patient stroke and bleeding risk, was developed and tested at two hospitals in Sydney, Australia. Among the 195 patients in the study, the tool recommended a change in therapy in more than half of patients, suggesting that such intervention could provide additional information to usual care.41 Similarly, the Atrial Fibrillation Decision Support Tool (AFDST) was developed by researchers at the University of Cincinnati and tested against a retrospective cohort of 1,585 adults with non-valvular AF. In comparison to usual care, the tool recommended care that was discordant to usual care in more than a third of women and elderly patients, suggesting that such a tool could improve guideline-adherent therapy in these groups.42 However, other work has shown that while AF treatment decision aids may reduce decision conflict, they may also reduce uptake of recommended therapies among hospitalized patients.40 These tools are designed to support clinicians but do not explicitly engage patients. Another interactive, online tool developed by Kasier et al., incorporated input from patients with AF and clinicians with experience treating such patients using well-established decision aid standards.38 This tool, although evidence-based, was sponsored by Janssen, a pharmaceutical company that produces rivaroxaban, an anticoagulant used in the treatment of AF. This funding source creates the potential for an actual or perceived conflict of interest. Other tools from Healthwise©, freely available online, are designed to be used by both patients and clinicians in the context of atrial fibrillation and coronary artery disease but do not provide personalized risk estimates, only generic ones. These were not designed for use in the acute care setting and may be too time-intensive to be successfully adopted by emergency clinicians. The AF tool created by Health Decision©, is freely available online and does provide personalized risk estimates with graphical presentation of stroke and bleeding risks. However, it contains little patient-directed educational content and was not designed for the acute care setting.
Table 1.
Summary of decision tools for cardiac care.
| Tool Name | Target Condition | Format | Key Features | Stakeholders Engaged | Developmental Stage | Limitations |
|---|---|---|---|---|---|---|
| Emergency Care Decision Tools | ||||||
| Chest Pain Choice (2015) | Disposition for low-risk chest pain | Paper-based, 11 × 17 inches, color | Personalized risk estimate using the quantitative pretest probability of ACS instrument. | Patient and caregiver representatives, cardiologists, ED clinicians, payer representatives | Late: single and multi-center RCTs completed | -Currently English only -Not embedded in EHR |
| Afib Choice (2016) | Anticoagulation for new-onset atrial fibrillation | Digital, tablet-based | Personalized risk assessment using CHA2DS2-VASc and HAS-BLED score | Clinicians who prescribe OAC for AF, patients with AF | Early: Ongoing validation study | -Currently English only -Not embedded in EHR -randomized data pending |
| SynDA (2016) | Disposition for isolated syncope | Paper-based, 11 × 17 inches, color | -Personalized risk assessment using the Canadian Syncope Risk Score | -ED syncope patients -ED providers -ED nurses -interaction designer |
Early: Feasibility testing underway | -English only -Not embedded in EHR -randomized data pending |
| Out-patient Decision Tools | ||||||
| Atrial Fibrillation Tool: Health Decision (2015) | Anticoagulation for Non-valvular atrial fibrillation | -Online -Paper -Potential for EHR integration |
Personalized risk assessment using CHA2DS2-VASc and HAS-BLED score | -Cardiology clinicians and researchers -SDM researchers |
Final version available online | -No published randomized data |
| Atrial Firbillation Tool: Computerized antithrombotic risk assessment tool (CARAT 2016) | Anticoagulation for Non-valvular atrial fibrillation | -Online | Personalized risk assessment | Clinicians who prescribe OAC for AF, patients with AF | Tested in a prospective observational cohort study | -NOACs not included -Not patient-facing |
| Atrial Fibrillation Tool: AF decision support tool (AFDST 2017) | Anticoagulation for Non-valvular atrial fibrillation | Personalized risk assessment | Clinicians who prescribe OAC for AF, patients with AF | Tested in a retrospective cohort study | -NOACs and warfarin not distinguished -Not patient- facing |
|
| Atrial Fibrillation Tool: Healthwise (2017) | Anticoagulation for Non-valvular atrial fibrillation | -Online -Paper |
-Generic clinical information -Embedded quiz |
-Cardiology, internal medicine, and family medicine clinicians | Final version available online | -No published randomized data -No personalized risk assessment |
| Coronary Artery Disease: Healthwise (2016) | Angioplasty for stable coronary artery disease | -Online -Paper |
-Generic clinical information -Embedded quiz |
-Cardiology, internal medicine, and family medicine clinicians | Final version available online | -No published randomized data -No personalized risk assessment |
| Coronary Artery Disease: Healthwise (2016) | Angiogram for stable coronary artery disease | -Online -Paper |
-Generic clinical information -Embedded quiz |
-Cardiology, internal medicine, and family medicine clinicians | Final version available online | -No published randomized data -No personalized risk assessment |
ACS: Acute Coronary Syndrome. AF: Atrial Fibrillation. ED: Emergency Department. EHR: Electronic Health Record. OAC: Oral Anti-Coagulant. NOAC: Non-Vitamin-K Oral Anti-Coagulant. RCT: Randomized, Controlled Trial. SDM: Shared Decision-Making.
We are currently developing and validating a SDM tool and are testing it in the ED setting. After structured observation of many patient-provider anticoagulation discussions, we designed a digital tool that helps communicate risk estimates that patients and clinicians can use to work through, in a shared manner, the balance of risks that accompanies the decision to begin or forgo life-long anticoagulation. In brief, the tool allows a provider to enter a patient’s stroke risk factors in order to calculate a CHA2DS2-VASc score43 in accordance with current international practice guidelines.44–46 This score is then translated to one- and five-year stroke risks which are displayed visually (depiction of 100 people in icon form). The reduction in stroke risk with initiation of oral anticoagulation, approximately two-thirds with warfarin47 or non-vitamin K oral antagonists (NOACS),48 is depicted in Appendix B. Alternatively, the tool could be adapted for use in Canada by incorporating the CHADS2 score, as per the Canadian Cardiovascular Society guidelines for anticoagulation in AF.49 The provider then advances through the tool to explore issues surrounding anticoagulation (cost, frequency of monitoring, dosing considerations, reversibility, activity limitations) as well as average and individualized bleeding risk (based on the HAS-BLED score50, 51). After reviewing the pertinent data, the patient and provider select a treatment option that is in keeping with the patient’s goals and wishes. We anticipate that use of such a tool will increase patient engagement in the decision-making process and, ultimately, improve adoption and long-term adherence, when appropriate, to this important therapy.
For several reasons, we believe emergency providers are in an excellent position to have these discussions. First, AF is so commonly seen in the ED that to ignore this opportunity would be to leave many patients without the chance to initiate appropriate anticoagulation. Second, the initial AF diagnosis can serve as a sentinel time point, during which patients are ‘primed’ to engage in learning about the condition that has brought them to medical attention. Third, many patients may have incomplete or delayed follow-up making the ED their only contact with the medical system. Incorporating SDM in the routine ED workflow for these patients may facilitate the delivery of high-quality, patient-centered care.
Shared decision-making for moderate-risk syncope
Syncope is defined as a transient loss of consciousness, associated with an inability to maintain postural tone followed by complete, spontaneous recovery.52 It is a common reason patients seek medical care and accounts for approximately 1% of all ED visits in the US and Canada.53, 54 The etiology of syncope is often benign but can be due to an occult serious cardiac cause such as a malignant arrhythmia that may not be uncovered during the ED evaluation.55 As a result, ED syncope patients are often admitted to the hospital. Admission rates are quite variable from one provider, hospital, or region to another.56 Admission rates in the US are approximately 32%,53 while in Canada they are closer to 13%,54 potentially due to differences in financial incentives, risk tolerance among clinicians, and availability of follow-up.
The diagnostic evaluation for ED syncope patients typically involves a thorough history and physical examination, and an ECG. For some patients, basic laboratory testing, including cardiac markers (such as troponin), may be ordered. Guided by the history, physical exam, and initial testing, advanced imaging may be pursued. Despite a relatively thorough work-up, the ED evaluation is often non-diagnostic. Undifferentiated syncope patients should then be risk-stratified to guide the disposition decision. This can be done using a three-tier categorization. Younger, healthier patients without concerning clinical features will generally be considered low risk and discharged with outpatient follow-up. Older patients with significant co-morbidities will generally be considered high risk and will often be admitted, either to the hospital or the observation unit, for further monitoring and inpatient testing. This leaves a moderate risk group who may be appropriate for either inpatient or outpatient work-up. Alternatively, a more formal, numerical risk-stratification may be performed using recently published tools such as the Canadian Syncope Risk Score. This score uses nine clinical variables to determine a patient’s 30-day risk of serious adverse events after an ED visit for syncope.55 Although the derivation of the Canadian Syncope Risk Score was methodologically rigorous, it is important to note that this score has not yet been externally validated.
For those patients where no serious diagnosis has been identified in the ED and who are considered moderate risk, the risks and benefits of inpatient versus outpatient evaluation may be roughly in balance. The equipoise surrounding this decision make it appropriate for SDM, assuming the patient is willing and able to participate in the decision-making process and time allows.9
The patient’s values, preferences, and circumstances should be incorporated into this decision. For example, how disruptive would an overnight admission be for this patient? What is their risk tolerance? Who do they live with and do they feel safe going home? Do they have ready access to a primary doctor or cardiologist with whom they could follow up with? What are the cost implications of being admitted for an observation stay? All of these questions should be considered when engaging patients in a shared decision around admission and further evaluation.
At the end of this process, a mutual decision should be reached through open dialogue between the patient and provider. An ED-based decision aid, named “SynDA,” has been developed for this scenario using input from emergency physicians, cardiologists, and ED syncope patients (See Appendix C). This paper-based decision aid is designed to stimulate discussion and facilitate SDM for the disposition decision for moderate-risk syncope patients who have had a negative ED evaluation. It consists for four sections. The first section explains why syncope, or fainting, occurs and then aims to reassure the patient by stating that no evidence of stroke or heart attack has been uncovered. The second section explains the possible underlying conditions that could have precipitated the syncopal event. The third section provides a 30-day personalized risk estimate based on the Canadian Syncope Risk Score.55 This risk estimate is presented both as a natural frequency and in a color-coded 100-person pictogram. The fourth and final section presents four options for future care including follow-up with the patient’s own primary physician, follow-up with a cardiologist, observation stay for monitoring and possible further testing, and an option to defer the decision to the emergency physician. A pilot randomized controlled trial is currently underway to assess the effect of the SynDA tool on patient knowledge and satisfaction as well as assess the acceptability of the tool to patients and providers.57 To our knowledge, there are currently no other tools designed to facilitate SDM for ED syncope patients.
Practical Approach
For the emergency clinician to successfully engage a patient in SDM, s/he must create a safe space where the patient feels relaxed and sufficiently empowered to ask questions, express preferences, and meaningfully engage in the decision-making process. If a patient feels anxious or is intimidated by the clinician, this will hinder their ability to truly engage in SDM. Every effort must be made to speak in clear language and avoid medical jargon to maximize patient understanding. A common misunderstanding of the application of SDM is to think that the critical challenge in determining the best treatment for the patient is a lack of information or certainty. Our experience in developing and testing decision aids has led us to understand that providing patients with information or choice alone isn’t sufficient to support SDM. The challenge lies in generating, meaningful dialogue to discover what is best for the patient based on his/her informed preferences. Once the clinician has recognized the appropriateness of SDM for a clinical scenario, the process, should include the following three steps:
Step 1) Acknowledge that clinical decision needs to be made with the patient.
Step 2) Engage in conversation with the patient to both share information about the current clinical scenario and options for future care, while exploring the patient’s values, preferences, and circumstances.
Step 3) Reach an agreement regarding the best plan of action based on the patient’s informed preferences.
The above approach is based on input from various stakeholders (ED patients, clinicians, researchers, designers) and prior conceptual work.9, 58, 59 The medium where SDM actually occurs is the conversation. If a genuine conversation is launched, the clinicians will be able to learn about the whole person they are caring for, both explicitly and implicitly. Step 2 typically happens in a dynamic, circular fashion fluidly through conversation. Exploring values and preferences is often challenging for clinicians since it requires adopting the role of listener instead of questioner/educator. For example, different patients may value time, money, and certainty differently depending on their personality and circumstances. Various questions can be asked to elicit these values. Due to social conditioning, even after being invited to join the decision-making process, patients may shy away, making statements such as the all-too-common, “I don’t know, doctor. What would you do?” Allowing the patient to process the information and contemplate the options alone for a few minutes, and then returning later to close the discussion is often helpful. The practice of SDM has been described as an “awkward dance,” but one well worth having.58
Conclusion
Shared decision-making is an effective means to improve the quality of emergency cardiovascular care by promoting patient-centeredness. There are several clinical scenarios in emergency cardiology which are potentially appropriate for SDM. In this paper, we have reviewed recent research and emerging SDM tools for three common scenarios: low-risk chest pain, new-onset atrial fibrillation, and moderate-risk syncope. These tools, with the exception of Chest Pain Choice, are still in the early stages of development and will require rigorous evaluation prior to widespread implementation. Published data indicate that these tools have the potential to improve patient satisfaction, adherence, and engagement, while decreasing low-value care.26, 27 Finally, we propose a three-step practical approach to SDM in the ED. Although the time-pressures ED clinicians face (with only minutes to spend with each patient and frequent interruptions) can make SDM difficult, we believe it is both feasible and morally indicated to pursue such an approach in select, appropriate scenarios. As an essential component of patient engagement, SDM has the potential to improve patient knowledge and decision quality, while safely leading to more sensible care. The implementation of SDM in emergency cardiology will shift the paradigm of clinical practice and improve the quality and experience of care for our patients.
Supplementary Material
Brief Summary.
In this paper, the authors discuss recent research in the area of shared decision-making in emergency cardiology. Three clinical scenarios are examined: 1) low-risk chest pain, 2) new-onset atrial fibrillation, and 3) moderate-risk syncope. Finally, the authors propose a simple 3-step approach to shared decision-making in emergency care. Implementation of shared decision-making in emergency cardiology has the potential improve the quality and experience of care for our patients.
Acknowledgments
Funding Sources
Dr. Probst receives funding from the National Heart, Lung, and Blood Institute of the U.S. National Institutes of Health (Grant number: 5K23HL132052-02). Dr. Noseworthy receives funding from the National Heart, Lung, and Blood Institute of the U.S. National Institute of Health (R01HL131535) and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Juan P. Brito is supported by the Karl-Erivan Haub Family Career Development Award in Cancer Research at Mayo Clinic, honoring Richard F. Emslander. Dr. Hess receives funding from the Patient Centered Outcomes Research Institute (contracts 952 and 12-11-4435). None of the funding agencies were involved in the composition of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barry MJ, Edgman-Levitan S. Shared decision making–pinnacle of patient-centered care. New Eng J of Med. 2012;366:780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 2.Godolphin W. Shared decision-making. Healthcare quarterly. 2009:e186–190. doi: 10.12927/hcq.2009.20947. 12 Spec No Patient. [DOI] [PubMed] [Google Scholar]
- 3.Hargraves I, LeBlanc A, Shah ND, Montori VM. Shared Decision Making: The Need For Patient-Clinician Conversation, Not Just Information. Health affairs. 2016;35:627–629. doi: 10.1377/hlthaff.2015.1354. [DOI] [PubMed] [Google Scholar]
- 4.Medicine Io. Crossing the quality chasm: a new health system for the 21st century. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 5.Kanzaria HK, Brook RH, Probst MA, Harris D, Berry SH, Hoffman JR. Emergency physician perceptions of shared decision-making. Acad Emerg Med. 2015;22:399–405. doi: 10.1111/acem.12627. [DOI] [PubMed] [Google Scholar]
- 6.Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;(4):CD001431. doi: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hargraves I, Montori VM. Decision aids, empowerment, and shared decision making. BMJ. 2014;349:g5811. doi: 10.1136/bmj.g5811. [DOI] [PubMed] [Google Scholar]
- 8.Montori VM, Breslin M, Maleska M, Weymiller AJ. Creating a conversation: insights from the development of a decision aid. PLoS medicine. 2007;4:e233. doi: 10.1371/journal.pmed.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Probst MA, Kanzaria HK, Schoenfeld EM, et al. Shared Decisionmaking in the Emergency Department: A Guiding Framework for Clinicians. Ann Emerg Med. 2017 doi: 10.1016/j.annemergmed.2017.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. National health statistics reports. 2010:1–31. [PubMed] [Google Scholar]
- 11.Wong MK, Wang JT, Czarnecki A, et al. Factors associated with physician follow-up among patients with chest pain discharged from the emergency department. CMAJ. 2015;187:E160–168. doi: 10.1503/cmaj.141294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcoon S, Chang AM, Lee B, Salhi R, Hollander JE. HEART score to further risk stratify patients with low TIMI scores. Crit Pathw Cardiol. 2013;12:1–5. doi: 10.1097/HPC.0b013e31827377e1. [DOI] [PubMed] [Google Scholar]
- 13.Lee B, Chang AM, Matsuura AC, Marcoon S, Hollander JE. Comparison of cardiac risk scores in ED patients with potential acute coronary syndrome. Crit Pathw Cardiol. 2011;10:64–68. doi: 10.1097/HPC.0b013e31821c79bd. [DOI] [PubMed] [Google Scholar]
- 14.Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999–2008. NCHS data brief. 2010:1–8. [PubMed] [Google Scholar]
- 15.Graff LG, Chern CH, Radford M. Emergency physicians’ acute coronary syndrome testing threshold and diagnostic performance: acute coronary syndrome critical pathway with return visit feedback. Crit Pathw Cardiol. 2014;13:99–103. doi: 10.1097/HPC.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 16.Than M, Flaws D, Sanders S, et al. Development and validation of the Emergency Department Assessment of Chest pain Score and 2 h accelerated diagnostic protocol. Emerg Med Australas. 2014;26:34–44. doi: 10.1111/1742-6723.12164. [DOI] [PubMed] [Google Scholar]
- 17.Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191–196. doi: 10.1007/BF03086144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 19.Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation. 2000;101:2557–2567. doi: 10.1161/01.cir.101.22.2557. [DOI] [PubMed] [Google Scholar]
- 20.Fox KA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE) BMJ. 2006;333:1091. doi: 10.1136/bmj.38985.646481.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8:195–203. doi: 10.1161/CIRCOUTCOMES.114.001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Than MP, Pickering JW, Aldous SJ, et al. Effectiveness of EDACS Versus ADAPT Accelerated Diagnostic Pathways for Chest Pain: A Pragmatic Randomized Controlled Trial Embedded Within Practice. Ann Emerg Med. 2016;68:93–102 e101. doi: 10.1016/j.annemergmed.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Anderson RT, Montori VM, Shah ND, et al. Effectiveness of the Chest Pain Choice decision aid in emergency department patients with low-risk chest pain: study protocol for a multicenter randomized trial. Trials. 2014;15:166. doi: 10.1186/1745-6215-15-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kline JA, Johnson CL, Pollack CV, Jr, et al. Pretest probability assessment derived from attribute matching. BMC Med Inform Decis Mak. 2005;5:26. doi: 10.1186/1472-6947-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell AM, Garvey JL, Chandra A, Diercks D, Pollack CV, Kline JA. Prospective multicenter study of quantitative pretest probability assessment to exclude acute coronary syndrome for patients evaluated in emergency department chest pain units. Ann Emerg Med. 2006;47:447. doi: 10.1016/j.annemergmed.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Hess EP, Knoedler MA, Shah ND, et al. The chest pain choice decision aid: a randomized trial. Circ Cardiovasc Qual Outcomes. 2012;5:251–259. doi: 10.1161/CIRCOUTCOMES.111.964791. [DOI] [PubMed] [Google Scholar]
- 27.Hess EP, Hollander JE, Schaffer JT, et al. Shared decision making in patients with low risk chest pain: prospective randomized pragmatic trial. BMJ. 2016;355:i6165. doi: 10.1136/bmj.i6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coll-Vinent B, Fuenzalida C, Garcia A, Martin A, Miro O. Management of acute atrial fibrillation in the emergency department: a systematic review of recent studies. Eur J Emerg Med. 2013;20:151–159. doi: 10.1097/MEJ.0b013e328359588f. [DOI] [PubMed] [Google Scholar]
- 29.McDonald AJ, Pelletier AJ, Ellinor PT, Camargo CA., Jr Increasing US emergency department visit rates and subsequent hospital admissions for atrial fibrillation from 1993 to 2004. Ann Emerg Med. 2008;51:58–65. doi: 10.1016/j.annemergmed.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Barrett TW, Vermeulen MJ, Self WH, Jenkins CA, Ferreira AJ, Atzema CL. Emergency department management of atrial fibrillation in the United States versus Ontario, Canada. J Am Coll Cardiol. 2015;65:2258–2260. doi: 10.1016/j.jacc.2015.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosychuk RJ, Graham MM, Holroyd BR, Rowe BH. Emergency department presentations for atrial fibrillation and flutter in Alberta: a large population-based study. BMC Emerg Med. 2017;17:2. doi: 10.1186/s12873-016-0113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atzema CL, Austin PC, Miller E, Chong AS, Yun L, Dorian P. A population-based description of atrial fibrillation in the emergency department, 2002 to 2010. Ann Emerg Med. 2013;62:570–577 e577. doi: 10.1016/j.annemergmed.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Coll-Vinent B, Martin A, Malagon F, et al. Stroke prophylaxis in atrial fibrillation: searching for management improvement opportunities in the emergency department: the HERMES-AF study. Ann Emerg Med. 2015;65:1–12. doi: 10.1016/j.annemergmed.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Koponen L, Rekola L, Ruotsalainen T, Lehto M, Leino-Kilpi H, Voipio-Pulkki LM. Patient knowledge of atrial fibrillation: 3-month follow-up after an emergency room visit. J Adv Nurs. 2008;61:51–61. doi: 10.1111/j.1365-2648.2007.04465.x. [DOI] [PubMed] [Google Scholar]
- 35.Fuenzalida C, Hernandez G, Ferro I, Siches C, Ambros A, Coll-Vinent B. Long-term benefits of education by emergency care nurses at discharge of patients with atrial fibrillation. Int Emerg Nurs. 2017 doi: 10.1016/j.ienj.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 36.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson C, Hendriks J. Partnering with patients in shared decision-making for stroke prevention in atrial fibrillation. Eur J Cardiovasc Nurs. 2017;16:178–180. doi: 10.1177/1474515116685193. [DOI] [PubMed] [Google Scholar]
- 38.Kaiser K, Cheng WY, Jensen S, et al. Development of a shared decision-making tool to assist patients and clinicians with decisions on oral anticoagulant treatment for atrial fibrillation. Curr Med Res Opin. 2015;31:2261–2272. doi: 10.1185/03007995.2015.1096767. [DOI] [PubMed] [Google Scholar]
- 39.Eckman MH, Wise RE, Naylor K, et al. Developing an Atrial Fibrillation Guideline Support Tool (AFGuST) for shared decision making. Curr Med Res Opin. 2015;31:603–614. doi: 10.1185/03007995.2015.1019608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson RG, Eccles MP, Steen IN, et al. A patient decision aid to support shared decision-making on anti-thrombotic treatment of patients with atrial fibrillation: randomised controlled trial. Qual Saf Health Care. 2007;16:216–223. doi: 10.1136/qshc.2006.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandya E, Masood N, Wang Y, Krass I, Bajorek B. Impact of a Computerized Antithrombotic Risk Assessment Tool on the Prescription of Thromboprophylaxis in Atrial Fibrillation. Clin Appl Thromb Hemost. 2016 doi: 10.1177/1076029616670031. 1076029616670031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eckman MH, Lip GY, Wise RE, et al. Using an Atrial Fibrillation Decision Support Tool for Thromboprophylaxis in Atrial Fibrillation: Effect of Sex and Age. J Am Geriatr Soc. 2016;64:1054–1060. doi: 10.1111/jgs.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 44.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 45.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 46.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 48.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 49.Macle L, Cairns J, Leblanc K, et al. 2016 Focused Update of the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2016;32:1170–1185. doi: 10.1016/j.cjca.2016.07.591. [DOI] [PubMed] [Google Scholar]
- 50.LaHaye SA, Gibbens SL, Ball DG, Day AG, Olesen JB, Skanes AC. A clinical decision aid for the selection of antithrombotic therapy for the prevention of stroke due to atrial fibrillation. Euro Heart J. 2012;33:2163–2171. doi: 10.1093/eurheartj/ehs167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 52.Sun BC, Costantino G, Barbic F, et al. Priorities for emergency department syncope research. Ann Emerg Med. 2014;64:649–655 e642. doi: 10.1016/j.annemergmed.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Probst MA, Kanzaria HK, Gbedemah M, Richardson LD, Sun BC. National trends in resource utilization associated with ED visits for syncope. Am J Emerg Med. 2015;33:998–1001. doi: 10.1016/j.ajem.2015.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thiruganasambandamoorthy V, Taljaard M, Stiell IG, et al. Emergency department management of syncope: need for standardization and improved risk stratification. Intern Emerg Med. 2015;10:619–627. doi: 10.1007/s11739-015-1237-1. [DOI] [PubMed] [Google Scholar]
- 55.Thiruganasambandamoorthy V, Kwong K, Wells GA, et al. Development of the Canadian Syncope Risk Score to predict serious adverse events after emergency department assessment of syncope. CMAJ. 2016;188:E289–298. doi: 10.1503/cmaj.151469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cook OG, Mukarram MA, Rahman OM, et al. Reasons for Hospitalization Among Emergency Department Patients With Syncope. Acad Emerg Med. 2016;23:1210–1217. doi: 10.1111/acem.13053. [DOI] [PubMed] [Google Scholar]
- 57.Syncope Decision Aid for Emergency Care (SynDA) U.S. National Institutes of Health; 2016. [Google Scholar]
- 58.Hess EP, Grudzen CR, Thomson R, Raja AS, Carpenter CR. Shared Decision-making in the Emergency Department: Respecting Patient Autonomy When Seconds Count. Acad Emerg Med. 2015;22:856–864. doi: 10.1111/acem.12703. [DOI] [PubMed] [Google Scholar]
- 59.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


